Abstract
Background
Nonvitamin A apocarotenoids occur in foods. Some function as retinoic acid receptor antagonists in vitro, though it is unclear if apocarotenoids are absorbed or accumulate to levels needed to elicit biological function.
Objective
The aim of this study was to quantify carotenoids and apocarotenoids (β-apo-8′-, -10′-, -12′-, and -14′-carotenal, apo-6′-, -8′-, -10′-, -12′-, and -14′-lycopenal, retinal, acycloretinal, β-apo-13-carotenone, and apo-13-lycopenone) in human plasma after controlled consumption of carotenoid-rich tomato juices.
Design
Healthy subjects (n = 35) consumed a low-carotenoid diet for 2 wk, then consumed 360 mL of high-β-carotene tomato juice (30.4 mg of β-carotene, 34.5 μg total β-apocarotenoids/d), high-lycopene tomato juice (42.5 mg of lycopene, 119.2 μg total apolycopenoids/d), or a carotenoid-free control (cucumber juice) per day for 4 wk. Plasma was sampled at baseline (after washout) and after 2 and 4 wk, and analyzed for carotenoids and apocarotenoids using high-pressure liquid chromatography (HPLC) and HPLC-tandem mass spectrometry, respectively. The methods used to analyze the apocarotenoids had limits of detection of ∼ 100 pmol/L.
Results
Apocarotenoids are present in tomato juices at 0.1–0.5% of the parent carotenoids. Plasma lycopene and β-carotene increased (P < 0.001) after consuming high-lycopene and β-carotene tomato juices, respectively, while retinol remained unchanged. β-Apo-13-carotenone was found in the blood of all subjects at every visit, although elevated (P < 0.001) after consuming β-carotene tomato juice for 4 wk (1.01 ± 0.27 nmol/L) compared with both baseline (0.37 ± 0.17 nmol/L) and control (0.46 ± 0.11 nmol/L). Apo-6′-lycopenal was detected or quantifiable in 29 subjects, while β-apo-10′- and 12′-carotenal were detected in 6 and 2 subjects, respectively. No other apolycopenoids or apocarotenoids were detected.
Conclusions
β-Apo-13-carotenone was the only apocarotenoid that was quantifiable in all subjects, and was elevated in those consuming high-β-carotene tomato juice. Levels were similar to previous reports of all-trans-retinoic acid. Other apocarotenoids are either poorly absorbed or rapidly metabolized or cleared, and so are absent or limited in blood. β-Apo-13-carotenone may form from vitamin A and its presence warrants further investigation. This trial was registered at clinicaltrials.gov as NCT02550483.
Keywords: β-apocarotenoids; apolycopenoids; carotenals; lycopenals; β-carotene 15,15′-oxygenase 1 (BCO1); β-carotene 9,10′-oxygenase 2 (BCO2); β-apo-13-carotenone; tomato
INTRODUCTION
Elevated plasma carotenoids have been associated with decreased risk for a number of chronic diseases, including various cancers (1, 2) and cardiovascular disease (3). Given that provitamin A carotenoids can be cleaved by mammalian enzymes to produce vitamin A, it has been suggested that other cleavage products (apocarotenoids) of both provitamin A and nonprovitamin A carotenoids may in fact be the compounds responsible for the protective effects noted in those with high blood carotenoids (4, 5). Two mammalian enzymes can cleave intact carotenoids: β-carotene 15,15′-oxygenase 1 (BCO1), which cleaves centrally to produce retinal (6, 7), and β-carotene 9,10′-oxygenase 2 (BCO2), which cleaves eccentrically to produce apo-10′-carotenal (8). Other apocarotenoids can be produced via nonenzymatic or oxidative cleavage (9, 10). Research has shown that feeding animals preformed carotenoid cleavage products can modulate biological action (11, 12), demonstrating that these compounds have biological functions when present.
Recent in vitro work has demonstrated that BCO1 not only cleaves β-carotene, but can also cleave lycopene (to form acycloretinal, also called apo-15′-lycopenal) (13). β-Apo-13-carotenone, β-apo-14′-carotenal, and β-apo-14′-carotenoic acid have been shown to be retinoid receptor antagonists (14), so their presence in plasma could have significant implications for vitamin A signaling. Some β-apocarotenals can be converted directly to vitamin A by BCO2 (13) and thus maintain growth at 72–78% of the rate of β-carotene as determined by the curative-rat growth assay (15).
Plants can also produce apocarotenoids via numerous carotenoid cleavage dioxygenases. The resulting apocarotenoids play important roles as phytohormones (16), aroma compounds (17), and growth regulators (18). As a result, foods often contain small amounts of apocarotenoids, typically at 0.1–1% of the parent carotenoid, and could possibly be absorbed directly in the intestine (19, 20). Apocarotenoids can also be produced nonenzymatically, via oxidation reactions (10, 21). Multiple routes of production of apocarotenoids complicate interpretation of current mammalian apocarotenoid data in the literature.
The primary objective of this study was to better understand how plasma carotenoid and apocarotenoid concentrations are correlated when humans consume carotenoid-rich foods. To meet this aim, putative carotenoid metabolites were accurately and precisely measured in both foods and in human plasma samples using a newly developed HPLC-tandem mass spectrometry (MS/MS) method. This will inform future work on carotenoids and their metabolites in studies of chronic disease.
METHODS
Chemicals
Optima-grade water and methanol, HPLC-grade methyl tert-butyl ether (MTBE), hexanes, ethanol, acetone, and toluene were purchased from Fisher Scientific. Ammonium acetate was from J.T. Baker. Butylated hydroxytoluene, β-carotene, β-apo-8′-carotenal, retinol, and retinal were purchased from Sigma Aldrich, whereas apo-6′-, -8′-, and -12′-lycopenal were purchased from CaroteNature. Lycopene was isolated from tomato paste (20), and phytoene and phytofluene were isolated from tangerine tomatoes (22). Other β-apocarotenoids [β-apo-10′-, -12′-, and -14′-carotenal, β-apo-13-carotenone (14)] and apolycopenoids [apo-15′-lycopenal and apo-13-lycopenone (20, 23)] were synthesized as described elsewhere.
Subjects and experimental design
Healthy, nonsmoking males and females (aged 21–75 y) were recruited near Beltsville, MD, and the study was completed between September and November 2015 at the Beltsville Human Nutrition Research Center (BHNRC, USDA-Agricultural Research Service). Subjects were required to have blood glucose <126 mg/dL and triglycerides <300 mg/dL, and were excluded if any of the following were present: cancer, type 2 diabetes, gastrointestinal surgeries or malabsorptive disorders, metabolic disease, pregnancy or currently lactating, or use of blood thinning medications. In total, 36 subjects were enrolled. Subjects were assigned to treatment groups (n = 12/group; 6 men, 6 women) with a stratified randomization scheme by the investigator at the BHNRC. Subjects were stratified first by sex, then by BMI, then subjects were sequentially assigned to study groups 1–3. Independently, the treatment associated with each study group was assigned by the supervisory dietician of the BHNRC. For 2 wk (washout phase), all subjects maintained a free-living diet with limited carotenoids (target <1 mg/d). For the next 4 wk (intervention phase), all subjects ate only food provided by the BHNRC, but were allowed to drink water, coffee (≤2 cups/d), tea (≤2 cups/d), and diet soda. Subjects were maintained on a low carotenoid diet (<1 mg/d). Diets were scaled for weight maintenance and portion sizes were adjusted in 200-kcal increments so that the nutrient composition of the diets was the same for all subjects, regardless of energy intake. Sample size was determined to detect an increase in plasma β-carotene from 0.3 to 0.6 μmol/L (SD = 0.2 μmol/L) with α = 0.05 and 80% power; 9 subjects/group were required, thus 12 subjects/group were enrolled to account for possible attrition (24). Blood was collected by a trained phlebotomist after the 2-wk carotenoid washout (week 0), after 2 wk of controlled feeding plus intervention (week 2), and after 4 wk of controlled feeding plus intervention (week 4). The blood was then centrifuged to yield plasma, divided into aliquots, and stored at –80°C until analysis. This clinical trial was approved by the Institutional Review Board of MedStar Health Research Institute (protocol #2015-118) and was registered at clinicaltrials.gov as NCT02550483.
Treatments
Three different juices were administered during this intervention. Orange-colored, high-β-carotene tomatoes were derived from Ohio 8245 and contain the beta allele from LA317. The red, high-lycopene tomatoes OH2461, contain the old gold crimson (ogc) allele. Both Solanum lycopersicum inbred lines were produced using conventional breeding practices and were grown at The Ohio State University's North Central Agricultural Research Station in Fremont, OH. Tomatoes were harvested and processed into juice at The Ohio State University's Food Industries Center Pilot Plant, Columbus, OH, as described previously (22). Carotenoid and apocarotenoid quantities in the tomato juices are listed as the doses administered (2 cans of 180 mL each, or 360 mL) per day in Table 1. A cucumber-based placebo beverage (lacking carotenoids) was used as a macronutrient-matched control and produced at the BHNRC.
TABLE 1.
Carotenoids and apocarotenoids in 360 mL tomato juices administered daily
| High-β-carotene tomato juice1 | High-lycopene tomato juice2 | |
|---|---|---|
| Carotenoid, mg/d | ||
| Phytoene | 1.55 | 7.09 |
| Phytofluene | 0.23 | 2.62 |
| β-Carotene | 30.4 | 0.65 |
| Other cis-lycopene | 0.46 | 4.66 |
| All-trans- + 5-cis-lycopene | 0.46 | 37.8 |
| Total lycopene | 0.92 | 42.5 |
| Total carotenoids | 33.1 | 52.9 |
| Apocarotenoid, μg | ||
| 6′-Aldehyde | — | 49.4 |
| 8′-Aldehyde | 18.3 | 45.3 |
| 10′-Aldehyde | 1.28 | 2.04 |
| 12′-Aldehyde | 4.99 | 9.82 |
| 14′-Aldehyde | 1.39 | 1.78 |
| 15′-Aldehyde | 1.78 | 2.91 |
| 13′-Ketone | 6.79 | 7.93 |
| Total apocarotenoids | 34.5 | 119 |
High-β-carotene tomato juice contained no detectable apolycopenoids.
High-lycopene tomato juice contained no detectable apocarotenals, but did contain 0.62 μg β-apo-13-carotenone.
Extraction of carotenoids and apocarotenoids from tomato juices and blood plasma
Carotenoids and apocarotenoids were extracted from ∼1.5 mL of tomato juice as previously described (25). Frozen plasma was thawed in cold water and 1 mL extracted for determination of carotenoids and apocarotenoids as previously described (20).
Analysis of carotenoids and apocarotenoids from tomato juices and blood plasma
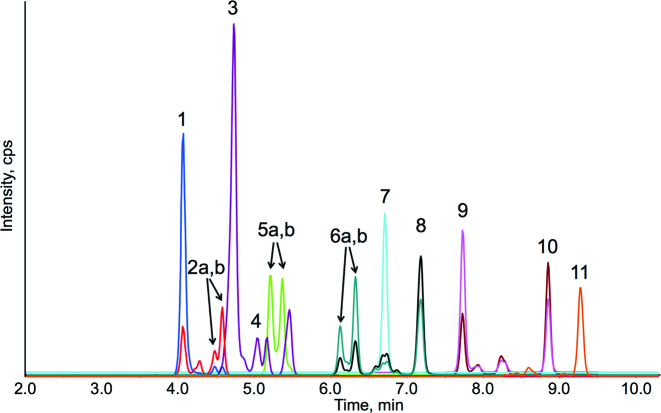
Carotenoids were analyzed in plasma using HPLC-photodiode array detection according to published methods (22). Apocarotenoids were measured using HPLC-photodiode array-MS/MS (Agilent 1260, interfaced with a QTRAP 5500 triple quadrupole mass spectrometer, AB Sciex) using atmospheric pressure chemical ionization operated in positive ion mode. Dried extracts were redissolved in 200 μL of 1:1 methanol:MTBE and 5 μL was injected. The injection volume was chosen to maximize the signal without matrix suppression. Apocarotenoids were separated on a C30 column (4.6 × 150 mm, 5 μm particle size, YMC Inc.) using a gradient of 1) 80:18:2 methanol:water:2% aqueous ammonium acetate and 2) 78:20:2 MTBE:methanol:2% aqueous ammonium acetate as follows: begin at 0% B, increase linearly to 100% B over 9 min, hold at 100% B for 2 min, and return to the initial conditions for 2 min, for a total run time of 13 min. The column was held at 40°C and the flow was 1.3 mL/min. Multiple reaction monitoring and MS parameters are presented in Table 2 and a representative mass chromatogram is shown in Figure 1.
TABLE 2.
HPLC-MS/MS parameters for analysis of apocarotenoids from tomato juices and blood plasma1
| Peak label | Retention time, min | Compound identity | HPLC-APCI+-MS/MS transitions2 | CE2 (eV) | DP (V) |
|---|---|---|---|---|---|
| 1 | 4.05 | β-Apo-13-carotenone | 259.2 > 175.13, 123.1, 119.1, 69.0 | 21, 27.5, 31, 27 | 60 |
| 2a, b | 4.5, 4.6 | Apo-13-lycopenone | 259.2 > 175.1, 123.1, 119.1, 69.03 | 21, 27.5, 31, 27 | 60 |
| 3 | 4.75 | β-Apo-15′-carotenal (retinal) | 285.2 > 161.13, 123.1, 119.1, 69.0 | 15, 27.5, 15, 20 | 60 |
| 4 | 5.05 | Apo-15′-lycopenal (acycloretinal) | 285.2 > 161.1, 123.1, 119.1, 69.04 | 15, 27.5, 15, 20 | 60 |
| 5a, b | 5.2, 5.35 | β-Apo-14′-carotenal | 311.2 > 159.13, 123.1, 95.1, 69.0 | 15, 27.5, 27, 27 | 60 |
| —4 | 6.3 | Apo-14′-lycopenal | 311.2 > 159.13, 123.1, 95.1, 69.0 | 15, 27.5, 27, 27 | 60 |
| 6a, b | 6.1, 6.4 | β-Apo-12′-carotenal | 351.2 > 161.13, 123.1, 119.1, 69.0 | 27, 27.5, 27, 27 | 80 |
| 7 | 6.7 | β-Apo-10′-carotenal | 377.3 > 123.1, 119.13, 95.1, 69.0 | 27.5, 27, 27, 27 | 80 |
| 8 | 7.2 | Apo-12′-lycopenal | 351.2 > 161.1, 123.1, 119.1, 69.03 | 27, 27.5, 27, 27 | 80 |
| —4 | 7.8 | Apo-10′-lycopenal | 377.3 > 123.1, 119.1, 95.1, 69.03 | 27.5, 27, 27, 27 | 80 |
| 9 | 7.75 | β-Apo-8′-carotenal | 417.3 > 123.1, 119.13, 95.1, 69.0 | 27.5, 37, 37, 37 | 80 |
| 10 | 8.85 | Apo-8′-lycopenal | 417.3 > 123.1, 119.1, 95.1, 69.03 | 27.5, 37, 37, 37 | 80 |
| 11 | 9.3 | Apo-6′-lycopenal | 443.3 > 157.13, 105.03, 69.03 | 45, 60, 55 | 80 |
CE, collision energy; DP: declustering potential; MRM, multiple reaction monitoring; MS/MS, tandem mass spectrometry.
MRM experiments were scheduled to maximize dwell times for each apocarotenoid.
Quantifier ion; other MRMs are qualifier ions. The sum of the three MRMs was used for apo-6′-lycopenal.
Apo-10′- and -14′-lycopenal are not in the chromatogram because of the lack of authentic standards. Their retention times were determined from tomato samples as these analytes were absent in blood plasma. All analytes were run with the following parameters: entrance potential: 10 V; collision cell exit potential: 11 V; curtain gas: 45 psi; and source temperature: 400°C.
FIGURE 1.
HPLC-MS/MS chromatograms of apocarotenoid and apolycopenoid standards. Colors represent different MS/MS transitions and peaks are annotated in Table 2.
Statistical analysis
Two-way ANOVA (modeling treatment, visit, and the interaction of treatment by visit) with Tukey's post-hoc test, α = 0.05, and P < 0.05 was used to assess statistical differences between the primary outcomes to this study—plasma carotenoid and apocarotenoid concentrations—while linear regression was used to establish a relation between plasma β-carotene and plasma β-apo-13-carotenone. All statistical analyses were conducted within RStudio (Version 0.99.903). Data are presented as means ± SDs.
RESULTS
A total of 36 subjects began the intervention; 35 completed it (Figure 2). One subject was dropped owing to development of a medical condition requiring an excluded medication. The overall study population had the following characteristics: age: 58.1 ± 9.7 y, BMI (kg/m2): 27.1 ± 4.2, fasting glucose: 5.28 ± 0.45 mmol/L, fasting triglycerides: 1.20 ± 0.57 mmol/L, HDL cholesterol: 1.48 ± 0.39 nmol/L, and LDL cholesterol: 3.24 ± 0.67 nmol/L (Table 3).
FIGURE 2.
Subject selection CONSORT flow diagram.
TABLE 3.
Baseline subject characteristics1
| Age, y | BMI, kg/m2 | Plasma triglycerides, mmol/L | HDL cholesterol, mmol/L | LDL cholesterol, mmol/L | Blood glucose, mmol/L | |
|---|---|---|---|---|---|---|
| All subjects (n = 36) | 58.1 ± 9.7 | 27.1 ± 4.2 | 1.20 ± 0.57 | 1.48 ± 0.39 | 3.24 ± 0.67 | 5.28 ± 0.45 |
| Control (n = 12: 6 M, 6 F) | 59.6 ± 7.3 | 27.8 ± 5.3 | 1.10 ± 0.45 | 1.47 ± 0.42 | 3.51 ± 0.85 | 5.28 ± 0.31 |
| High-β-carotene tomato juice (n = 12: 6 M, 6 F) | 58.8 ± 11.1 | 26.5 ± 3.3 | 1.18 ± 0.55 | 1.54 ± 0.38 | 3.03 ± 0.66 | 5.39 ± 0.53 |
| High-lycopene tomato juice (n = 12: 6 M, 6 F) | 56.1 ± 10.6 | 27.1 ± 4.2 | 1.30 ± 0.72 | 1.44 ± 0.39 | 3.21 ± 0.44 | 5.17 ± 0.47 |
Values are means ± SDs.
Given that apocarotenoids can be generated via auto-oxidation, special care was taken to ensure that β-apocarotenoids and apolycopenoids were not created during sample handling, extraction, or chromatographic analysis. Samples were kept frozen at –80°C prior to analysis and extracted quickly with small volumes of cold solvents to minimize the drying time. Samples were redissolved in injection solvent ≤30 min before analysis by HPLC–MS/MS, a time frame confirmed not to generate apocarotenoids in human plasma extracts. Subjects receiving the high-β-carotene tomato juice consumed 30.4 mg β-carotene/d, while subjects receiving the high-lycopene tomato juice consumed 42.5 mg lycopene/d (Table 1). Previously formed apocarotenoids were also detected in both tomato juices, with 34.5 μg of β-apocarotenoids and 119.2 μg of apolycopenoids delivered daily from the high-β-carotene and high-lycopene tomato juices, respectively. Total β-apocarotenoids were found at 0.11% of β-carotene, while apolycopenoids were found at 0.28% of lycopene.
In subjects consuming the high-β-carotene tomato juice, plasma β-carotene increased significantly (P < 0.001) from 675 ± 484 nmol/L at week 0 to 3180 ± 1300 nmol/L after the 4-wk intervention (Table 4). Plasma β-carotene remained unchanged at weeks 0, 2, and 4 in subjects consuming the high lycopene and control diets, and was significantly lower than subjects consuming the high-β-carotene tomato juice at both 2 and 4 wk. In subjects consuming the high lycopene tomato juice, plasma lycopene increased from 437 ± 123 nmol/L during week 0 to 1400 ± 352 nmol/L at week 4 (Table 4). Plasma lycopene was unchanged at weeks 0, 2 and 4 in subjects consuming the high-β-carotene and control diets, and was significantly lower than subjects consuming the high-lycopene diet at weeks 2 and 4. Retinol concentrations were normal (1.80 ± 0.42 μmol/L) and did not change during the study in any of the treatments at any time point.
TABLE 4.
Plasma β-carotene, lycopene and β-apo-13-carotenone at baseline (week 0), and 2 and 4 wk after control, high-β-carotene, or high-lycopene tomato juice interventions1
| Time of testing | Control (n = 11), nmol/L | High-β-carotene tomato juice (n = 12), nmol/L | High-lycopene tomato juice (n = 12), nmol/L |
|---|---|---|---|
| Plasma β-carotene | |||
| Week 0 | 451 ± 267a | 675 ± 484a | 484 ± 259a |
| Week 2 | 399 ± 174a | 2840 ± 1090b | 508 ± 272a |
| Week 4 | 356 ± 163a | 3180 ± 1300b | 616 ± 711a |
| Plasma lycopene | |||
| Week 0 | 436 ± 143a | 469 ± 151a | 437 ± 123a |
| Week 2 | 356 ± 74.3a | 503 ± 138a | 1310 ± 290b |
| Week 4 | 253 ± 67.4a | 580 ± 503a | 1400 ± 352b |
| Plasma β-apo-13-carotenone | |||
| Week 0 | 0.28 ± 0.10a | 0.37 ± 0.17a,b | 0.30 ± 0.07a |
| Week 2 | 0.28 ± 0.10a | 0.51 ± 0.20b | 0.30 ± 0.08a |
| Week 4 | 0.46 ± 0.11a,b | 1.01 ± 0.27c | 0.53 ± 0.13b |
Values are means ± SDs. Different superscript letters denote statistically significant differences (P < 0.05), according to 2-way ANOVA with Tukey's post-hoc test, of treatment and time within each carotenoid or apocarotenoid.
HPLC-MS/MS methods were developed to separate and quantitate apocarotenoids and apolycopenoids in human plasma. Figure 1 shows the chromatographic separation of the β-apocarotenoids and apolycopenoids analyzed in this study in a process taking 13 min, with peaks annotated in Table 2. Of all of the apocarotenoids in our panel (β-apo-8′-, -10′-, -12′-, -14′-, and -15′-carotenal, apo-6′-, -8′-, -10′-, -12′-, -14′-, and -15′-lycopenal, β-apo-13-carotenone, and apo-13-lycopenone), only β-apo-13-carotenone was detectable and quantifiable in the plasma of all 35 subjects at each of the 3 visits. β-Apo-13-carotenone was found in the high-β-carotene tomato juice group at 0.37 ± 0.17 nmol/L at week 0 and increased significantly to 1.01 ± 0.27 nmol/L after 4 wk of high-β-carotene tomato juice consumption (Table 4). β-Apo-13-carotenone was unchanged with the intervention in the control group and elevated to a lesser extent in the group on the high-lycopene tomato juice (from 0.30 ± 0.07 nmol/L at week 0 to 0.53 ± 0.13 nmol/L at week 4). β-Apo-13-carotenone was significantly higher in subjects consuming high-β-carotene tomato juice than in those consuming high-lycopene or control diets at both 2 and 4 wk. Figure 3 shows a scatterplot of the concentration of plasma β-carotene compared with plasma β-apo-13-carotenone and, based on the line of best fit, we would predict subjects to have 0.29 nmol/L β-apo-13-carotenone when plasma β-carotene is absent. β-Apo-10′-carotenal was detected in the plasma samples of 6 subjects and apo-12′-carotenal was detected in 2 subjects, but not above our limit of quantitation of  pmol/L. The other 2 β-apocarotenals (β-apo-8′- and -14′-carotenal) were not detected in any subjects. Apo-6′-lycopenal was detected in 15 subjects and was quantifiable (0.82 ± 0.10 nmol/L) in 14 subjects after 4 wk. Other apolycopenoids (apo-8′-, -10′-, -12′-, -14′-, and -15′-lycopenal and apo-13-lycopenone) were not detected in the plasma of any subjects.
pmol/L. The other 2 β-apocarotenals (β-apo-8′- and -14′-carotenal) were not detected in any subjects. Apo-6′-lycopenal was detected in 15 subjects and was quantifiable (0.82 ± 0.10 nmol/L) in 14 subjects after 4 wk. Other apolycopenoids (apo-8′-, -10′-, -12′-, -14′-, and -15′-lycopenal and apo-13-lycopenone) were not detected in the plasma of any subjects.
FIGURE 3.
Linear regression of plasma β-carotene concentrations (n = 35) vs. plasma β-apo-13-carotenone concentrations (n = 35) from all visits (n = 3). Green squares are subjects on control, orange circles are those on high-β-carotene tomato juice, and red triangles are those on high-lycopene tomato juice, P < 0.001.
DISCUSSION
This work has quantified both β-apocarotenoids and apolycopenoids in plasma after prolonged consumption of carotenoid-rich foods, via controlled feeding, to elucidate the relation between dietary and circulating carotenoid and apocarotenoid levels. Concentrations of both carotenoids and apocarotenoids were quantified in the study agents (tomato juices) and plasma. The controlled feeding ensured that all of the subjects consumed the same food for the duration of the study, to ensure consistent and low background carotenoid or apocarotenoid intake. Both plasma β-carotene and lycopene increased significantly in blood when volunteers consumed high-β-carotene or high-lycopene tomato juices for 4 wk, but despite reaching >3 μmol/L and 1.4 μmol/L, respectively, β-apocarotenals or apolycopenals were not consistently found in quantifiable levels in plasma.
β-Apocarotenoids have been demonstrated to be produced in vitro or ex vivo when exposed to intestinal homogenates (26), though when antioxidants were added, β-apocarotenoids were no longer detectable (9), suggesting that these β-apocarotenoids are being produced by nonenzymatic oxidative cleavage. In addition, apocarotenoids can be produced in plants (and could presumably be absorbed by those consuming plants) by carotenoid cleavage dioxygenases (27), or by chemical means (i.e., in the absence of enzymes via auto-oxidation) (10, 28–30). Distinguishing apocarotenoids produced in vivo via enzymatic and nonenzymatic processes from those absorbed from a diet containing preformed apocarotenoids (19, 20) is challenging. Additionally, the ease with which these apocarotenoid products are produced incidentally from sample handling during laboratory extraction prior to analysis is a complicating confounder (31). During development of the method for conducting the analyses described here, we observed that thawing blood plasma at room temperature for 3 h was sufficient to artifactually produce apolycopenals.
Much of what has been inferred about the in vivo biological effects of eccentric carotenoid cleavage products has been deduced from in vitro and animal knockout studies. However, recent evidence demonstrates that BCO2 knockout animals also have altered lipid metabolism (32), mitochondrial dysfunction, and increased cellular oxidative stress (33), suggesting that the absence of eccentric carotenoid cleavage is not the only difference between BCO2−/− and wild-type mice. This metabolic dysfunction noted in BCO2−/− animals occurs without altering hepatic vitamin A metabolism, retinoic acid, or retinyl esters in the liver, suggesting a mechanism independent of vitamin A (34). Chickens (35), cows (36), and sheep (37) with BCO2 deficiency have higher levels of carotenoids in skin, milk, and adipose tissue, respectively, though this accumulation does not explicitly demonstrate that the increase in carotenoids is a result of less carotenoid cleavage by BCO2, as it could be a result of altered lipid metabolism (32). Radiolabeled β-carotene (38) and lycopene (39) have also been used with the aim of distinguishing mammalian in vivo eccentric carotenoid cleavage. In these studies, β-carotene and lycopene metabolites were tentatively, but not definitively, identified.
Previously, it was thought that in vitro, recombinant BCO1 cleaves provitamin A but not nonprovitamin A carotenoids (40). However, more-recent experiments have demonstrated that purified BCO1 can catalyze cleavage of lycopene and β-apocarotenals (13). Purified chicken BCO2 cleaves provitamin A carotenoids, but with 10-fold less catalytic efficiency than BCO1, as well as lutein, zeaxanthin, and 9-cis-β-carotene, but not all-trans-lycopene or β-apo-8′-, -10′-, -12′-, or -14′-carotenal (41). Newer data have shown that BCO2 is compartmentalized in the inner mitochondrial membrane, whereas β-carotene is present predominantly in the cytoplasm, suggesting that enzyme and substrate may not interact in a physiologically relevant system (42, 43). It has been proposed by Palczewski and colleagues (42) that physical separation of BCO1 and BCO2 prevents competition for β-carotene, as well as the production of β-apocarotenoids, which can interfere with retinoid signaling (14). This is consistent with our findings here, with little or no β-apocarotenals circulating in plasma despite >30 mg β-carotene being consumed per day. Single-nucleotide polymorphisms at 2 locations within BCO2 have been shown to be associated with risk for age-related macular degeneration, though the BCO2 variants were not related to plasma or macular lutein and zeaxanthin concentrations, perhaps suggesting that BCO2 is acting via a carotenoid-independent mechanism (44).
Here, we found that β-apo-13-carotenone is the only apocarotenoid present in concentrations that could be quantified in each blood sample in all subjects, and its concentration is consistent with previous work (14,45). This is despite other β-apocarotenals and apolycopenals being present in higher concentrations than β-apo-13-carotenone in our tomato juices. Interestingly, of the compounds investigated here, β-apo-13-carotenone is the only apocarotenoid smaller than retinol and could thus be produced via oxidative cleavage of vitamin A in addition to cleavage of provitamin A carotenoids. This idea is consistent with our finding that β-apo-13-carotenone is found in all subjects, regardless of carotenoid consumption (Figure 3). It is possible that β-apocarotenals and lycopenals are biologically important cleavage products produced via human BCO2 but that they do not circulate in plasma, instead being formed in tissues. Little is known about the pharmacokinetics of the production of these compounds, and it is possible that they are produced in, and rapidly cleared from, the blood stream, forming as-yet-unknown metabolites, or are shuttled to organs (e.g., liver) for storage or further metabolism. It is possible that β-apocarotenals and lycopenals are handled similarly to retinal, as studies in rats have demonstrated that retinal is distributed to peripheral tissues within 2 h, suggesting rapid clearance from plasma (46). Here, we found that β-apo-13-carotenone circulated at levels similar to that of all-trans retinoic acid [which circulates at  nmol/L in healthy individuals (47)].
nmol/L in healthy individuals (47)].
The levels of parent carotenoids circulating after our intervention with β-carotene- or lycopene-rich food products are consistent with previous reports of feeding carotenoid-containing foods (48–50) or supplements (51). Some previous reports found higher percentages of β-apocarotenoids relative to β-carotene, compared to lycopenoids relative to lycopene (19, 20), though we found the inverse in the tomato juices we fed in this study (i.e., more lycopenoids as a percentage of lycopene as compared with β-apocarotenoids as a percentage of β-carotene). Regardless of the lower proportion of β-apocarotenoids to β-carotene found in our high-β-carotene tomato juice, more β-apocarotenoids (β-apo-13-carotenone, β-apo-10′ and β-apo-12′-carotenal) were detected in the plasma of our volunteers than apolycopenoids (apo-6′-lycopenal).
In conclusion, with a tightly controlled background diet, feeding large doses of β-carotene ( mg/d) or lycopene (
mg/d) or lycopene (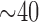 mg/d) for 4 wk did not lead to detectable concentrations (>100 pmol/L plasma) of most β-apocarotenals or lycopenals, respectively, in human plasma. This leads us to conclude that eccentric aldehyde cleavage products of carotenoids are not primary carotenoid metabolites that circulate in plasma.
mg/d) for 4 wk did not lead to detectable concentrations (>100 pmol/L plasma) of most β-apocarotenals or lycopenals, respectively, in human plasma. This leads us to conclude that eccentric aldehyde cleavage products of carotenoids are not primary carotenoid metabolites that circulate in plasma.
β-Apo-13-carotenone was the only apocarotenoid found in similar concentrations to retinoic acid in all subjects, and its presence is not dependent on continuous carotenoid consumption. Given that β-apo-13-carotenone is shorter than retinal, it is possible that it is an oxidative cleavage product from vitamin A. Indeed, there is evidence for the formation of β-apo-13-carotenone in vitamin A supplements devoid of carotenoid, in addition to its presence in carotenoid-containing foods (31). Given the demonstration of retinoic acid receptor anatagonism, the implications of circulating β-apo-13-carotenone require further investigation.
ACKNOWLEDGEMENTS
The authors acknowledge Harold Seifried and Sharon Ross from the Division of Cancer Prevention at the National Cancer Institute for useful discussions related to project planning. The authors’ responsibilities were as follows—JLC, JAN, SJS and EHH: designed the research; JLC, JAN, KMR, and MJC: conducted the research; DMF and RWC: provided essential research materials; JLC: performed the statistical analyses; JLC, JAN, and EHH: analyzed the data; JLC: wrote the manuscript; JLC and EHH: had responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors had any conflicts of interest related to this study.
Notes
Supported by NCI-USDA-IAA #ACN15005-001-00000, the Nutrient and Phytochemical Analytic Shared Resource of The Ohio State University's Comprehensive Cancer Center (NIH P30 CA016058), Foods for Health, a focus area of the Discovery Themes Initiative at The Ohio State University.
Abbreviations used:
- BCO1
β-carotene 15,15′-oxygenase 1
- BCO2
β-carotene 9,10′-oxygenase 2
- BHNRC
Beltsville Human Nutrition Research Center
- MS
mass spectrometry
- MTBE
methyl tert-butyl ether
REFERENCES
- 1. Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao Y, Goodman MT et al.. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104:1905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, Giovannucci E. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;35:2154–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr. 2005;81:990–7. [DOI] [PubMed] [Google Scholar]
- 4. Carail M, Caris-Veyrat C. Carotenoid oxidation products: from villain to saviour?, Pure Appl Chem. 2006;78:1493–503. [Google Scholar]
- 5. Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr. 2012;96:1214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wyss A, Wirtz G, Woggon W-D, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W. Cloning and expression of β,β-carotene 15,15′-dioxygenase. Biochem Biophys Res Commun. 2000;271:334–6. [DOI] [PubMed] [Google Scholar]
- 7. von Lintig J, Vogt K. Filling the gap in vitamin A research. J Biol Chem. 2000;275:11915–20. [DOI] [PubMed] [Google Scholar]
- 8. Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6. [DOI] [PubMed] [Google Scholar]
- 9. Mordi RC, Walton JC, Burton GW, Hughes L, Keith IU, David LA, Douglas MJ. Oxidative degradation of β-carotene and β-apo-8′-carotenal. Tetrahedron. 1993;49:911–28. [Google Scholar]
- 10. Caris-Veyrat C, Schmid A, Carail M, Böhm V. Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J Agric Food Chem. 2003;51:7318–25. [DOI] [PubMed] [Google Scholar]
- 11. Ip BC, Hu K-Q, Liu C, Smith DE, Obin MS, Ausman LM, Wang X-D. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila). 2013;6:1304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costabile BK, Kim Y-KK, Iqbal J, Zuccaro MV, Wassef L, Narayanasamy S, Curley RW, Harrison EH, Hussain MM, Quadro L. β-Apo-10′-carotenoids modulate placental microsomal triglyceride transfer protein expression and function to optimize transport of intact β-carotene to the embryo. J Biol Chem. 2016;291:18525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. dela Seña C, Narayanasamy S, Riedl KM, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1). J Biol Chem. 2013;288:37094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW Jr., Harrison EH. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J Biol Chem. 2012;287:15886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma RV, Mathur SN, Ganguly J. Studies on the relative biopotencies and intestinal absorption of different apo-beta-carotenoids in rats and chickens. Biochem J. 1976;158:377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, MCcarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–4. [DOI] [PubMed] [Google Scholar]
- 17. Schwartz SH, Qin X, Zeevaart JAD. Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem. 2001;276:25208–11. [DOI] [PubMed] [Google Scholar]
- 18. Booker J, Auldridge M, Wills S, Mccarty D, Klee H, Leyser O. MAX3/CCD7 Is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol. 2004;14:1232–8. [DOI] [PubMed] [Google Scholar]
- 19. Fleshman MK, Lester GE, Riedl KM, Kopec RE, Narayanasamy S, Curley RW, Schwartz SJ, Harrison EH. Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of β-carotene bioaccessibility and bioavailability. J Agric Food Chem. 2011;59:4448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim SJ, Nara E, Kobayashi H, Terao J, Nagao A. Formation of cleavage products by autoxidation of lycopene. Lipids. 2001;36:191–9. [DOI] [PubMed] [Google Scholar]
- 22. Cooperstone JL, Ralston RA, Riedl KM, Haufe TC, Schweiggert RM, King SA, Timmers CD, Francis DM, Lesinski GB, Clinton SK et al.. Enhanced bioavailability of lycopene in humans when consumed as cis-isomers from tangerine tomatoes compared to red tomato juices, a randomized, cross-over clinical trial. Mol Nutr Food Res. 2015;59:658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Narayanasamy S, Sun J, Pavlovicz RE, Abdulkerim E, Rush CE, Sunkel BD, Li C, Harrison EH, Curley RW Jr. Synthesis of apo-13- and apo-15-lycopenoids, cleavage products of lycopene that are retinoic acid antagonists. J Lipid Res. 2017;58:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson RR, Prabhala RH, Plezia PM, Alberts DS. Effect of beta-carotene on lymphocyte subpopulations in elderly humans: evidence for a dose-response relationship. Am J Clin Nutr. 1991;53:90–4. [DOI] [PubMed] [Google Scholar]
- 25. Kopec RE, Cooperstone JL, Schweiggert RM, Young GS, Harrison EH, Francis DM, Clinton SK, Schwartz SJ. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-β-carotene tomato sauce and from carrots. J Nutr. 2014;144:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang GW, Wang XD, Russell RM, Krinsky NI. Characterization of beta-apo-13-carotenone and beta-apo-14′-carotenal as enzymatic products of the excentric cleavage of beta-carotene. Biochemistry. 1991;30:9829–34. [DOI] [PubMed] [Google Scholar]
- 27. Hou X, Rivers J, León P, McQuinn RP, Pogson BJ. Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 2016;21:792–803. [DOI] [PubMed] [Google Scholar]
- 28. Handelman GJ, van Kuijk FJGM, Chatterjee A, Krinsky NI. Characterization of products formed during the autoxidation of β-carotene. Free Radic Biol Med. 1991;10:427–37. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez EB, Rodriguez-Amaya DB. Lycopene epoxides and apo-lycopenals formed by chemical reactions and autoxidation in model systems and processed foods. J Food Sci. 2009;74:674–82. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez EB, Rodriguez-Amaya DB. Formation of apocarotenals and epoxycarotenoids from β-carotene by chemical reactions and by autoxidation in model systems and processed foods. Food Chem. 2007;101:563–72. [Google Scholar]
- 31. Cichon MJ. Investigating the role of tomato phytochemicals through targeted and untargeted metabolomics [dissertation]. Columbus (OH): The Ohio State University; 2015. [Google Scholar]
- 32. Guo X, Wu L, Lyu Y, Chowanadisai W, Clarke SL, Lucas EA, Smith BJ, He H, Wang W, Medeiros DM et al.. Ablation of β,β-carotene-9′,10′-oxygenase 2 remodels the hypothalamic metabolome leading to metabolic disorders in mice. J Nutr Biochem. 2017;46:74–82. [DOI] [PubMed] [Google Scholar]
- 33. Wu L, Guo X, Hartson SD, Davis MA, He H, Medeiros DM, Wang W, Clarke SL, Lucas EA, Smith BJ et al.. Lack of β, β-carotene-9′, 10′-oxygenase 2 leads to hepatic mitochondrial dysfunction and cellular oxidative stress in mice. Mol Nutr Food Res. 2017;61:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu L, Guo X, Lyu Y, Clarke SL, Lucas EA, Smith BJ, Hildebrand D, Wang W, Medeiros DM, Shen X et al.. Targeted metabolomics reveals abnormal hepatic energy metabolism by depletion of β-carotene oxygenase 2 in mice. Sci Rep. 2017;7:14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Strömstedt L, Wright D, Jungerius A, Vereijken A, Randi E et al.. Identification of the Yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008;4:e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE et al.. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics. 2009;182:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Våge DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ho CC, De Moura FF, Kim SH, Clifford AJ. Excentral cleavage of beta-carotene in vivo in a healthy man. Am J Clin Nutr. 2007;85:770–7. [DOI] [PubMed] [Google Scholar]
- 39. Gajic M, Zaripheh S, Sun F, Erdman JW Jr. Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat lier. J Nutr. 2006;136:1552–7. [DOI] [PubMed] [Google Scholar]
- 40. Lindqvist A, Andersson S. Biochemical properties of purified recombinant human β-carotene 15,15′-monooxygenase. J Biol Chem. 2002;277:23942–8. [DOI] [PubMed] [Google Scholar]
- 41. dela Seña C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant chicken β-carotene 9′,10′-oxygenase (BCO2). J Biol Chem. 2016;291:14609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palczewski G, Amengual J, Hoppel CL, von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014;28:4457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raghuvanshi S, Reed V, Blaner WS, Harrison EH. Cellular localization of β-carotene 15,15′ oxygenase-1 (BCO1) and β-carotene 9′,10′ oxygenase-2 (BCO2) in rat liver and intestine. Arch Biochem Biophys. 2015;572:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyers KJ, Mares JA, Igo RP, Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK et al.. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Investig Opthalmology Vis Sci. 2014;55:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eroglu A, Hruszkewycz DP, Curley RW, Harrison EH. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch Biochem Biophys. 2010;504:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sass JO, Tzimas G, Elmazar MMA, Nau H. Metabolism of retinaldehyde isomers in pregnant rats: 13-cis- and all- trans-retinaldehyde, but not 9-cis-retinaldehyde, yield very similar patterns of retinoid metabolites. Drug Metab Dispos. 1999;27:317–21. [PubMed] [Google Scholar]
- 47. Arnold SLM, Amory JK, Walsh TJ, Isoherranen N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J Lipid Res. 2012;53:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bohn T, Blackwood M, Francis DM, Tian Q, Schwartz SJ, Clinton SK. Bioavailability of phytochemical constituents from a novel soy fortified lycopene rich tomato juice developed for targeted cancer prevention trials. Nutr Cancer. 2013;65:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grainger EM, Hadley CW, Moran NE, Riedl KM, Gong MC, Pohar K, Schwartz SJ, Clinton SK. A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br J Nutr. 2015;114:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Micozzi MS, Brown ED, Edwards BK, Bieri J, Taylor PR, Khachik F, Beecher GR, Smith JC Jr. Plasma carotenoid response to chronic intake of selected foods and β-carotene supplements in men. Am J Clin Nutirtion. 1992;55:1120–5. [DOI] [PubMed] [Google Scholar]
- 51. Nierenberg DW, Stukel TA, Baron JA, Dain BJ, Greenberg ER, Group TSCPS . Determinants of increase in plasma concentration of ß-carotene after chronic oral supplementation. Am J Clin Nutr. 1991;53:1443–9. [DOI] [PubMed] [Google Scholar]