Abstract
Cell behavior is strongly influenced by physical, mechanical contacts between cells and their extracellular matrix. We review how the transcriptional regulators YAP/TAZ integrate mechanical cues with the response to soluble signals and metabolic pathways to control multiple aspects of cell behavior, including proliferation, cell plasticity and stemness essential for tissue regeneration. Corruption of cell-environment interplay leads to aberrant YAP/TAZ activation that is instrumental for multiple diseases, including cancer.
Introduction
Fundamental aspects of cell behaviour in living organisms - morphogenesis, collective migration and self-organization - are not genetically encoded but emergent properties of cells interconnected within a tissue network. Furthermore, most disease states either do not have a genetic origin, or, as is the case in cancer, are preceded or accompanied by a corruption of the tissue environment that fuels aberrant cell behaviour1,2.
Our current knowledge on signaling pathways regulating gene expression, proliferation and fate decisions does not fully account for these emergent properties, failing to explain how these events are exquisitely localized in living tissues. Understanding the spatiotemporal control of cell behavior thus requires incorporation of information on how structural and architectural complexity of tissues is transmitted to their constituent cells.
YAP/TAZ, two highly related transcriptional regulators sense how cells, perceive themselves and their tissue environment and communicate with it. YAP/TAZ activity in a cell is under direct control of its shape and polarity, which is in turn dictated by the cytoskeletal structure3. Further, the tensional state and organization of the cytoskeleton reflect the local pattern of geometrical and mechanical strains associated with the position of the cell within the global 3D tissue architecture and surrounding stromal composition4,5. These inputs are essentially physical, and include the pattern forces emanating from the cell's attachment to other cells and from the topology and rigidity of the extracellular matrix (ECM)6. YAP/TAZ convert such inputs into gene expression signatures and coherent, context-dependent biological responses3,7.
The structural features of a cell must also be linked to its metabolism and to its responsiveness to other environmental cues, such as morphogen signals and nutrient availability. For example, epithelial budding starts with a localized distortion in the shape of few cells that precedes their proliferation, suggesting that localized changes in mechanics can determine the response to mitogens8. Notably, cells respond to mechanical stresses by adjusting composition and fine 3D organization of the surrounding ECM and by reorganizing cell-cell adhesion. Thus, inward directed forces (generated by cytoskeletal contractility), deformation of organelles and external forces (pulling from ECM rigidity, other cells or fluid flow) are always in perfect balance5,6.
Here we review recent data providing an emerging picture in which YAP/TAZ serve as nexus orchestrating the interplay between cellular mechanics, metabolism and developmental signaling cascades, allowing for cell and context-specific responses. We also discuss that the YAP/TAZ transcriptional program may be devoted to the installment of positive feedback systems promoting - YAP/TAZ-mediated mechanical and metabolic signaling. Through such mechanisms, YAP/TAZ shape organs during development, forge self-organization phenomena and adapt cell behavior to tissue needs, as in tissue repair or regeneration. When elements of this control fail or are imbalanced, abnormal YAP/TAZ activation causes multiple diseases, including fibrosis, cancer, atherosclerosis3.. We finally outline some exciting open issues in the burgeoning field of YAP/TAZ regulation and biology.
Sensing and responding to biomechanical signals through YAP/TAZ
In order harmonize their behavior to the surrounding mechanical niches, cells must translate external cues into signaling conduits that impact on gene expression, a process referred to as mechanotransduction4. Mechanotransduction starts with the ability of cells to probe the physical features of the microenvironment through integrins and other adhesive proteins, and to counterbalance extracellular forces by adjusting their own tensional state through actomyosin contractility and organization of the F-actin cytoskeleton4. For years, these models left unaddressed the question of how mechanical instructions are executed in the nucleus through specific transcription factors (TFs). Strikingly, recent discoveries are converging to a model in which multiple types of mechanical inputs in a variety of cellular settings rely on the regulation of two transcriptional regulators, YAP and TAZ (reviewed in3,9). Note that we here depict YAP and TAZ (naming them YAP/TAZ throughout this contribution) as equivalent proteins; this is based their structural similarities and genetic redundancy in a host of experimental conditions and biological functions. However these proteins display different phenotypes in individual mouse knockout lines10,11, and dissecting individual functions from overlapping ones is an open issue for the field.
YAP and TAZ activity is regulated by the conformation and tension of the F-actin cytoskeleton, which in turn depend primarily on the substrate on which cells adhere. Indeed, cells spread when plated on large and stiff substrates; in these conditions, they display high ROCK- and non-muscle myosin II-driven cytoskeletal tension ultimately turning on YAP/TAZ. Conversely softer or smaller substrates, imposing cell rounding and adhesion, cause cytoplasmic retention and inhibition of YAP and TAZ12,13 (FIG. 1). Importantly, this is an evolutionary conserved mechanism, shared by multiple cell types, attesting the generality of YAP/TAZ mechanotransduction3,7. Indeed, a potent means to induce YAP/TAZ activity is raising F-actin reorganization and stress fiber formation by experimental depletion of capping and severing proteins, such as ADF/Cofilin and CapZ13. Different sub-pools of F-actin can, however, have opposite roles on YAP/TAZ activity and in highly polarized cell cultures, as contraction of the apical circumferential actin belt is required to inhibit YAP/TAZ14. This phenomenon possibly involves the formation of an apical F-actin network by the spectrin family of scaffolding proteins. Indeed, depletion of spectrins results in aberrant YAP/TAZ nuclear localization both in Drosophila wing and ovary follicle cells and in human MDCK cells, possibly due to abnormal restructuring of F-actin into basal stress fibers15,16.
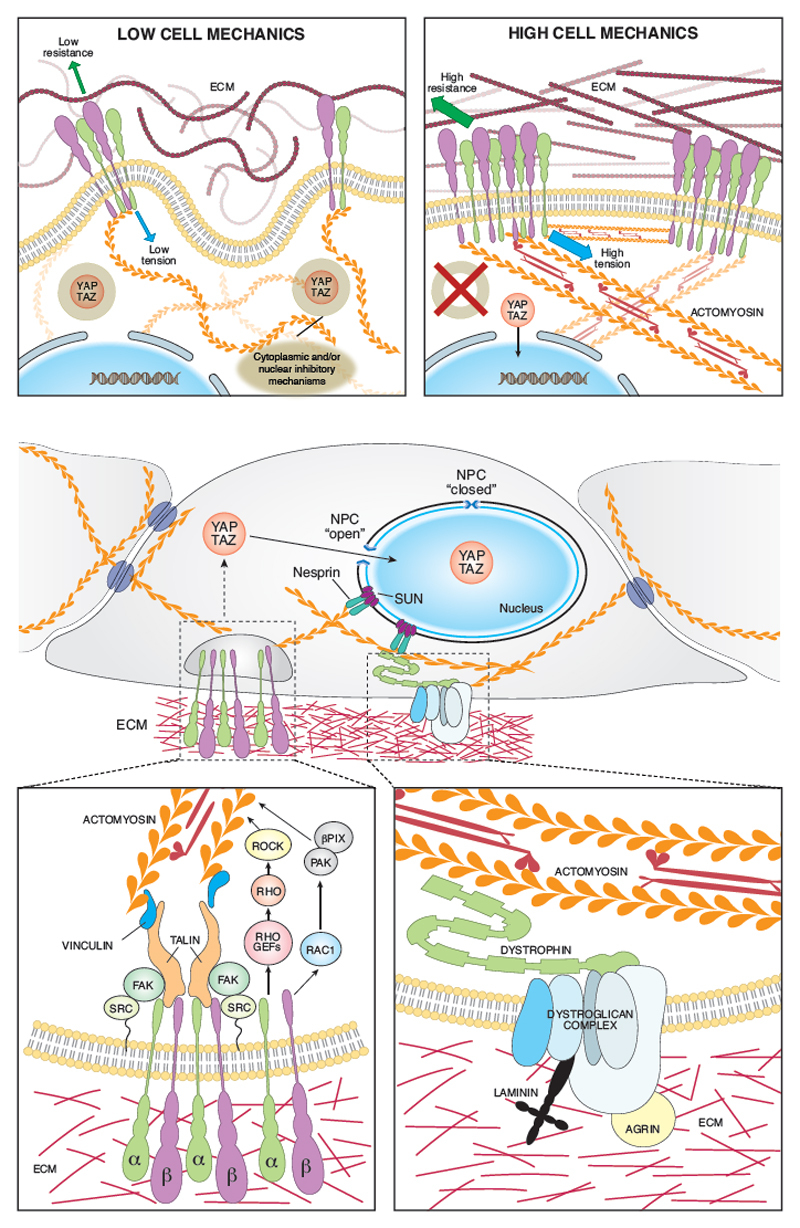
Figure 1. Biomechanical signal transduction to YAP/TAZ.
Let top panel: cells respond to ECM rigidity by adjusting their tensional state through Integrin-mediated cell-ECM adhesions. Low ECM resistance corresponds to reduced adhesion and focal adhesion maturation, low actomyosin contractility and YAP/TAZ inactivation by yet unclear cytoplasmic or nuclear inhibitors (brown ring). Right panel: High ECM resistance favors integrin clustering and intracellular tension, disabling YAP/TAZ mechanically-regulated inhibitory mechanisms (red cross), allowing for YAP/TAZ function. Center: The F-actin cytoskeleton integrates mechanical strain arising from cell-ECM and cell-cell adhesions. These physical inputs are transduced into YAP/TAZ function. YAP/TAZ activation is mediated by release of YAP/TAZ from inhibitors but also entails modulation of YAP/TAZ nuclear-cytoplasmic shuttling. The F-actin cytoskeleton impacts on the mechanics and shape of the nucleus through Nesprin and SUN complexes, favoring YAP/TAZ nuclear entry by inducing nuclear deformation and increased permeability of the NPC. Bottom panels: Schematic representation of the signaling cascade that triggers actomyosin deposition and contractility at Integrin-mediated focal adhesions (left). ECM components Laminin and Agrin can signal to the actomyosin cytoskeleton through the dystroglycan-dystrophin complex (right). βPIX: PAK-interacting exchange factor; ECM: extracellular matrix; FAK: Focal adhesion kinase; NPC: nucelar pore complex; PAK: p-21 activated kinase; Rho GEFs: Rho Guanidine nucleotide exchange factors; ROCK: Rho-associated protein kinase; SUN: SUN domain-containing protein.
The finding that molecular components of focal adhesions (FAs), namely integrins, focal adhesion kinase (FAK) and Src, are required for YAP/TAZ activity is consistent with the fact that the degree of cell distortion and spreading are key signals impinging on the arrangement of the F-actin cytoskeleton and YAP/TAZ activity (FIG. 1). The relationship between cell shape, F-actin and YAP/TAZ activity impacts on hepatic stellate cell conversion to myofibroblasts during liver fibrosis17–19, proliferation of basal layer skin keratinocytes20,21, activation and proliferation of cancer associated fibroblasts22, self-renewal and chemoresistance of breast cancer stem cells (CSCs) induced by glucocorticoids23, the atherogenic effect of disturbed blood flow on endothelial cells24,25, and post-damage intestinal regeneration26.
Mechanistically, YAP/TAZ activation by integrin-dependent FA formation may be linked to the activation of the RhoGEF β-PIX, the small GTPase Rac1 and its effector PAK27,28, which are known F-actin modulators, suggesting a role for integrin-dependent cytoskeletal modifications in mechanical regulation of YAP/TAZ (FIG. 1). Moreover, Yes/Src family proteins directly phosphorylate YAP to promote YAP transcriptional activity21,29. Still unclear, however, is to what extent Src impacts on YAP/TAZ function directly, through phosphorylation, rather than indirectly, though its effects on the cytoskeleton. Intriguingly, the dynamic formation of FAs is itself influenced by substrate stiffness; indeed, a molecular clutch model for FAs implies that sufficient substrate stiffness is required for integrin-bound talins to unfold and expose a vinculin-binding domain to promote full FA maturation and YAP/TAZ nuclear accumulation30. This suggests that FA components might not only be required for adhesion to the substrate but can act as mechanotransducing units31 (FIG. 1). Notably, the main YAP-regulating mechanosensitive adhesive platforms are those exerting the highest force, that is the β1-integrin clusters more proximal to the nucleus connecting F-actin filaments to the nuclear lamina, but not the peripheral FAs, which appear inconsequential for YAP/TAZ regulation32 (FIG. 1). Altogether, it appears that YAP/TAZ regulation is not guided by the total levels of F-actin but its subcellular organization, fine structure, tension and, likely, resistance offered by molecular struts within the cytoskeleton (such as microtubules33 and potentially intermediate filaments) and by the whole nucleus (see below). Therefore YAP/TAZ mechanotransduction differs profoundly from actin regulation of the TF MAL, a sensor of F-actin/G-actin ratio that binds directly free, nuclear G-actin in a manner inhibiting its association to its DNA binding partner Serum Response Factor (SRF) 34.
Integrin signaling is critical for modulation of YAP/TAZ activity in 3D, and relevant to instruct differentiation of cultured skeletal stem cells and to drive proliferation of malignant cells13,35. Yet, regulation of YAP/TAZ in normal, primary epithelial cells growing within physiological matrices as organoids requires an additional level of complexity. When intestinal organoids are cultured in non-deformable 3D hydrogels of defined stiffness and composition, YAP/TAZ are induced at high stiffness triggering proliferation of stem cells, but only transiently26,36. As the organoid expands, YAP/TAZ are turned off, possibly as an effect of increased cell crowding, causing loss of stemness and organoid demise. In contrast, when the hydrogel is designed to be physically plastic - allowing its proteolytic degradation by metalloproteases - fate plasticity and self-organization emerge. Under these conditions, YAP/TAZ activity remains localized at the tip of the organoid's crypt-like, budding protrusions, a location characterized by elevated cellular strain where YAP/TAZ preserve the intestinal stem cells of the organoid36. Similarly, skeletal stem cells require the activity of matrix metalloproteinase Mmp14 to remodel the surrounding collagen matrix in order to form integrin clustering, raising F-actin cytoskeletal tension that ultimately results in YAP/TAZ activation35. What remains mysterious is what determines the spatial specificity of crypt formation in developing growing organoids. Is it stochastic variegation in the Matrigel hydrogel or is it intrinsic to cell heterogeneity within the organoids, for example arising from differential MMP expression?
It must be noted, however, that cell spreading can lead to YAP/TAZ activation independently of the formation of FAs33. Signaling by diverse ECM components can trigger YAP/TAZ activity in different tissue types. For example, in the neonatal mouse heart, the ECM proteoglycan Agrin binds the dystrophin/glycoprotein complex (DGC) and induces YAP nuclear accumulation, promoting cardiomyocyte proliferation37–39 (FIG. 1). Mechanical signaling by Agrin regulates also HCC cells, in which Agrin is bound by the receptor LRP4/MUSK to enhance Integrinβ1-FAK1 signaling and enhance YAP/TAZ mechanotransduction40. Other membrane bound proteins, which are not necessarily adhesion molecules, can transduce mechanical signals to YAP/TAZ, as the Ca2+ ion channel Piezo1 in human neural stem cells (NSCs), which is activated when NSCs are experiencing a stiff substrate, resulting in YAP/TAZ activation41.
A major open question in the field is how, mechanistically, signaling information generated by the F-actin cytoskeleton turns YAP/TAZ subcellular localization and activity ON or OFF. One possibility is that Angiomotin family proteins (AMOTs) act as mechanical mediators between F-actin structures and YAP/TAZ. AMOTs directly bind and potentially inhibit YAP/TAZ42; moreover, AMOTs interact with F-actin, in manner mutually exclusive with their YAP/TAZ association43. However, validation of AMOTs as mechanotransducers and bona fide YAP/TAZ inhibitors is lacking81.
The nucleus itself can act as a mechanosensitive subcellular compartment by means of the interaction between F-actin and the LINC protein complex, as extensively reviewed elsewhere44. Recent work has proposed that YAP/TAZ entry in the nucleus occurs by increasing permeability through nuclear pores45 through LINC-regulated nuclear stretching (FIG. 1). At the same time, disruption of F-actin anchoring to LINC causes a global collapse of the stress fibers spanning the nucleus, with ensuing YAP/TAZ inhibition32.
YAP/TAZ regulation by cell polarity through Hippo signaling
The Hippo pathway is an evolutionary conserved kinase cascade initially uncovered by genetic screens for tissue overgrowth mutants in Drosophila melanogaster46–50. The core of the mammalian Hippo pathway is composed by a two-kinase module in which MST1 and MST2 (orthologues of Hippo in Drosophila), in cooperation with their binding partner Sav1, phosphorylate and activate LATS1 and LATS2 (orthologues of Warts in Drosophila); in turn, activated LATS proteins, aided by the co-factor MOB-1, directly phosphorylate YAP and TAZ inhibiting their activity through cytoplasmic retention and protein degradation51 (FIG. 2A). Alternatively to MST1/2, members of the MAPK4K family of mitogen activated protein kinases phosphorylate and activate LATS, resulting in YAP/TAZ inhibition52,53. A potent upstream inducer of the Hippo pathway is Merlin which serves as scaffold for the core Hippo kinase module at cell-cell junctions in epithelial cells54,55. Indeed, epithelial architecture, with the engagement of cell-cell junctions and apicobasal polarity, is a strong inhibitor of YAP/TAZ activity. Mechanistically, cell polarity is associated to the membrane localization of Scribble, which serves as scaffold for a MST/LATS/TAZ complex that favors YAP/TAZ inhibition56. Other polarity proteins also impact on YAP/TAZ function, such as the apical crumbs complex (CRB), which binds to YAP/TAZ and favors their cytoplasmic localization57 (FIG. 2A). Loss of cell polarity is a general feature of EMT, a change in cell morphology associated with changes in cell fate and acquisition of stemness traits. Consistently, YAP/TAZ are activated by EMT and are required downstream of EMT to confer cancer stem cell attributes56,58.
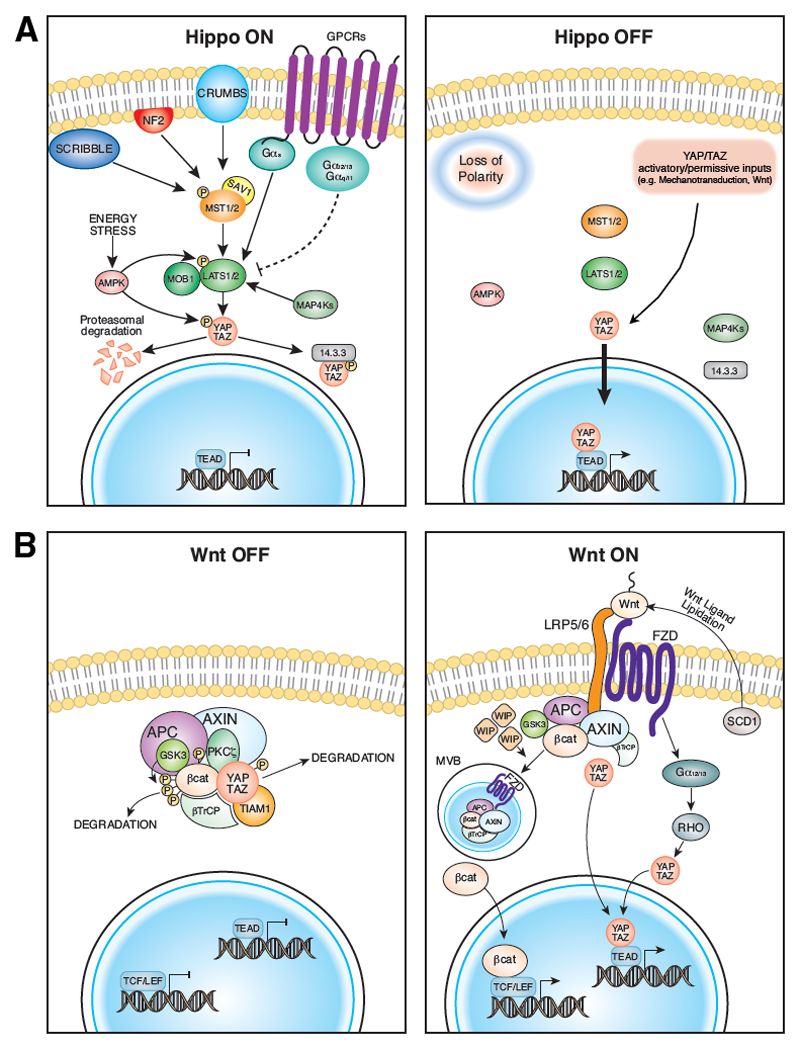
Figure 2. YAP/TAZ regulation by Hippo and Wnt signaling.
A) Schematic representation of the upstream components that activate the core module of the Hippo pathway, composed by the kinases MST1/2 and LATS1/2. The “Hippo ON” state results in YAP/TAZ phosphorylation with ensuing proteasomal degradation and/or cytoplasmic sequestration (left panel). In the “Hippo OFF” state YAP/TAZ are set free of inhibitory phosphorylations; in presence of other YAP/TAZ inducing/permissive extrinsic or intrinsic signals, YAP/TAZ activity is unleashed (right panel). B) YAP/TAZ - Wnt signaling interplay. The destruction complex is a multiprotein machinery at the core of the canonical Wnt pathway, controlling the intracellular levels of β-catenin. In the “Wnt OFF” state, YAP/TAZ associate with Axin to be incorporated in the destruction complex together with β-catenin. As such, the complex restrains both β-catenin and YAP/TAZ activity regulating their sequestration and/or degradation in the cytoplasm (left panel). In the presence of a “Wnt ON” state, the destruction complex is inhibited as Axin and GSK3 bind to the Wnt receptors LRP5/6 being internalized into MVBs. The disassembly of the complex by Wnt ligands abolishes both β-catenin and YAP/TAZ cytosolic inhibition, triggering their nuclear accumulation with consequent activation of transcriptional responses81 (right panel). The role of the destruction complex as checkpoint coordinating YAP/TAZ activity has been further corroborated by recent reports, including the findings that: i) SCD1 activity promotes the secretion of active (i.e., lipid-modified) Wnt ligands, leading to YAP/TAZ activation86; ii) cytosolic TIAM1 is incorporated in the destruction complex to promote β-TrCP-dependent YAP/TAZ degradation and sequestration87; iii) the actin regulator WIP stabilizes YAP/TAZ proteins by inhibiting the destruction complex into MVBs85; iv) the tumor suppressor PKCζ associates with the destruction complex to inhibit YAP nuclear localization88. APC: adenomatous polyposis coli; AMPK: AMP-activated protein kinase; βcat: β-catenin; β-TrCP: beta-transducin repeat containing protein; FZD: Frizzled; GSK3: Glycogen synthase kinase 3; LATS: large tumor suppressor kinase; LRP5/6: low-density lipoprotein receptor-related protein 5/6; MAP4K: MAP kinase kinase kinase kinase; MVB: multi-vesicular body; MST: mammalian Ste20-like kinase; NF2: neurofibromatosis type 2; PKCζ: protein kinase C, zeta; SAV1: Salvador 1; SCD1: stearoyl-CoA-desaturase 1; WIP: WASP-interacting protein.
The Hippo cascade is a prime input for YAP/TAZ activity and the first mechanisms discovered for YAP/TAZ regulation. Therefore “Hippo signaling” is often used as a surrogate term for “YAP/TAZ activity”. This would seem incorrect both because the Hippo cascade regulates downstream effectors other than YAP/TAZ 59, and because the Hippo pathway might not be the main player of YAP/TAZ modulation in diverse physiological and pathological settings. In fact, there are several LATS-independent cases of YAP/TAZ regulation, including mechanotransduction, Rho-GTPase signaling, inflammation and metabolism12,13,21,60–71. Worth noting is that, in mammary gland, lung, heart, kidney, pancreas, epidermis and nervous system, tissues in which tumor emergence is associated to high YAP/TAZ activity, genetic depletion of Hippo pathway components does not result in tumor formation60,68,72–76. This highlights how a combination of events, Hippo-dependent and -independent must accumulate to activate YAP/TAZ above the threshold required for tumorigenesis7. Moreover, direct phosphorylation of YAP by LATS may not be sufficient to inhibit YAP nuclear accumulation: First, YAP phosphorylation on the prime LATS target residue S127 does not prevent YAP nuclear localization77; second, inhibition of nuclear export results in nuclear accumulation of phosphorylated YAP12,78; and third, mechanical deformation of the nucleus can modulate subcellular localization of YAP in a phosphorylation-independent manner45. That said, although cell mechanics can regulate YAP/TAZ in a manner uncoupled from their phosphorylation by LATS1/2, LATS1/2 kinase activity can also be modulated by F-actin levels, and contribute to YAP/TAZ mechanotransduction13,33,79,80. We thus envision Hippo signaling as one segment of a broader framework of molecular events by which the cell structure act as overarching determinant of YAP/TAZ function. The reader may refer to other reviews for a more detailed discussion on this topic9,81.
Wnt-YAP/TAZ signaling
Besides their roles as effectors of the Hippo and mechanotransduction pathways, YAP and TAZ are also biochemical and transcriptional mediators of Wnt signaling, providing a mechanistic link to the observed overlap between Wnt and YAP/TAZ biology. At the core of the canonical Wnt pathway, the destruction complex is a multiprotein machinery that controls the intracellular levels of β-catenin, but is also a critical checkpoint coordinating both β-catenin and YAP/TAZ activity7,81 (FIG. 2B and see its legend for details). Strikingly, in the mouse small intestine, YAP/TAZ are not only massively activated by inactivation of destruction complex components (as in APC mutant cells) but also essential for the protumorigenic effects of aberrant Wnt signaling82–84. Similarly, YAP/TAZ mediate the effects of Wnt signaling in promoting proliferation and tumorigenesis of cancer stem cells (CSCs)61,82,85–89, to regulate differentiation of MSC61, and to sustain the intestinal crypt regeneration either of organoids in vitro or after tissue damage in vivo26,36,82,88.
Notably, cytoplasmic YAP/TAZ favors the recruitment of β-TrCP to the destruction complex, and as such facilitate β-catenin degradation82 (FIG. 2B). Therefore YAP/TAZ nuclear translocation induced by mechanical signals or Hippo inactivation can lead to β-catenin nuclear localization73,82,90,91. This suggests a coherent regulation of YAP/TAZ and β-catenin, that explains why activation of the Hippo pathway, by controlling YAP/TAZ cytosolic localization, also restrains Wnt/β-catenin signalling81. Moreover YAP/TAZ can inhibit Wnt signaling by inhibiting Dishevelled (DVL)92,93. β-catenin itself is regulated by mechanical cues as well94. This mechanism raises the possibility that mechanical regulation of YAP/TAZ and β-catenin may be connected. Indeed, in epithelial monolayers, mechanical strains induce nuclear translocation of YAP followed by that of β-catenin, collectively leading to transcriptional activation required for cycle reentry95. Finally, an additional layer of interconnection between YAP/TAZ and β-catenin occurs on chromatin as outlined below.
In addition to the canonical Wnt/β-catenin pathway, the activation of the Wnt-FZD/ROR pathway promote YAP/TAZ signalling required for cell migration and osteogenic differentiation, acting through a Ga12/13-RhoGTPases96 (FIG. 2B).
Metabolic control of YAP/TAZ
A number of reports indicate a close connection between glucose homeostasis and YAP/TAZ activity97. Glucose availability has been recently found to sustain YAP/TAZ activity by feeding the hexosamine biosynthesis pathway (HBP). Mechanistically, high levels of glucose lead to production of UDP-GlcNAc and this results in increased YAP O-GlcNAcylation which can stabilize YAP protein levels98,99. Moreover, phosphofructokinase 1 (PFK1), responsible for the most rate limiting step of the glycolytic cascade, promotes the YAP/TAZ transcriptional program by stabilizing their interaction with TEAD transcription factors63.
A metabolic shift from oxidative respiration to aerobic glycolysis, the “Warburg effect”, is a hallmark of cancer. Under these conditions, glycation of Hsp90 impairs its chaperone activity on LATS1 ultimately contributing to YAP/TAZ activation100. On the other hand, shortage of glucose availability inhibit YAP/TAZ activity mainly through the activation of the cellular energy sensor AMP-activated protein kinase (AMPK), which in turn regulates YAP/TAZ activity through multiple parallel phosphorylation events, either directly or through LATS197. Collectively, these studies suggest that the increased glucose consumption supporting cell proliferation in cancer cells also sustains a pro-tumorigenic transcriptional program through YAP/TAZ.
The metabolism of lipids, through the mevalonate/cholesterol biosynthetic pathway, is linked to the regulation YAP/TAZ, by promoting proliferation and migration of mammalian cells or tissue overgrowth in Drosophila embryos. Mechanistically, mevalonic acid sustains YAP/TAZ activity through the production of geranylgeranyl-pyrophosphate, which is covalently attached to the RhoA GTPase and required for membrane localization and activation of this protein. In turn, membrane active RhoA activates YAP/TAZ in a LATS1/2 independent manner70,101.
The mTOR pathway represents a prototypical mechanism for control of cell size and proliferation in response to nutrients, growth factors and cellular energy levels102. The mTORC1 kinase complex sustains YAP/TAZ activity in a model of perivascular epithelioid tumors, by inhibiting the autophagosome-dependent lysosomal degradation of YAP protein in response to nutrients availability103. Moreover, the mTORC2 kinase complex can activate YAP/(TAZ) through inhibitory phosphorylation of AMOT proteins104, or MST1105, although the physiological relevance remains unknown.
YAP/TAZ regulation by GPCR signaling
Several metabolic regulators, hormones and growth factors act as extracellular signals through different classes of G-protein-coupled receptors (GPCRs), that, in turn, are able to induce or repress YAP/TAZ activity51,97. Specifically, stimulation of Gα-coupled receptors by multiple ligands, including lysophospholipids sphingosine 1-phosphate (S1P), lysophosphatidic acid (LPA), thrombin, estrogens and acetylcholine, results in YAP/TAZ activation through the promotion of a RhoGTPase-regulated F-actin cytoskeleton in a manner that is either LATS1/2-dependent or independent51,65,79,97. Conversely, Gαs-coupled GPCR signaling elicited by glucagon or epinephrine represses YAP/TAZ activity through cAMP/PKA-dependent inhibition of RhoA-GTPase79,106 (FIG. 2A).
Constitutive YAP/TAZ activation by aberrant expression of GPCRs or by genetic mutations affecting their associated heterotrimeric G-proteins is implicated in the malignant promotion of uveal melanoma107, skin basal-cell carcinoma108 and in sarcomas associated with Kaposi herpesvirus (KSHV)109.
Crosstalk with growth factor and inflammatory signaling
YAP/TAZ are interconnected with signaling pathways initiated by soluble growth factors through reciprocal mechanisms of regulation. As reviewed elsewhere, these include transforming growth factor beta (TGFβ), bone morphogenetic protein (BMPs), Hedgehog (Hh) and EGFR pathways51. Following intestinal injury, during which inflammation contributes to cell proliferation110, the release of IL-6 and IL-11 activates YAP/TAZ-dependent proliferation in the intestinal crypt. Mechanistically, interleukin-mediated gp130 signalling induces the activation of Src family kinases (SFKs), that trigger YAP activity by direct tyrosine phosphorylation71. Moreover, increased YAP/TAZ activity can upregulate gp130 signaling through a transcriptional mechanism, resulting in an amplification loop, as observed during tumor growth initiated by APC loss111. TAZ also regulates the differentiation of CD4+ T cells in the TH17 pro-inflammatory subset and promotes the expression of TH17 cell–associated genes, including IL17A112. This may be relevant for colorectal cancer (CRC), as a high level of IL17A and inflammatory T helper 17 (TH17) cells in the tumor microenvironment promote CRC progression170.
Controlling YAP/TAZ in the nucleus
Following nuclear translocation, YAP/TAZ elicit their biological functions mainly by regulating gene transcription through their association with the DNA-binding TEA domain family members (TEAD 1-4)113–115. Genome-wide, the mammalian complex of YAP or TAZ with TEAD (YAP/TAZ-TEAD) is directly recruited to promoter regions only for a minority of YAP/TAZ targets, as most associations to chromatin occur at distant enhancers, that contact their cognate promoters through DNA looping114. Enhancer-bound YAP/TAZ-TEAD complexes regulate gene transcription by inducing a p300-dependent acetylation at lysine 27 of histone H3 (H3K27ac)113, by recruiting the Mediator complex and by inducing transcriptional elongation through the pause release of RNA Pol-II115 (FIG. 3A).
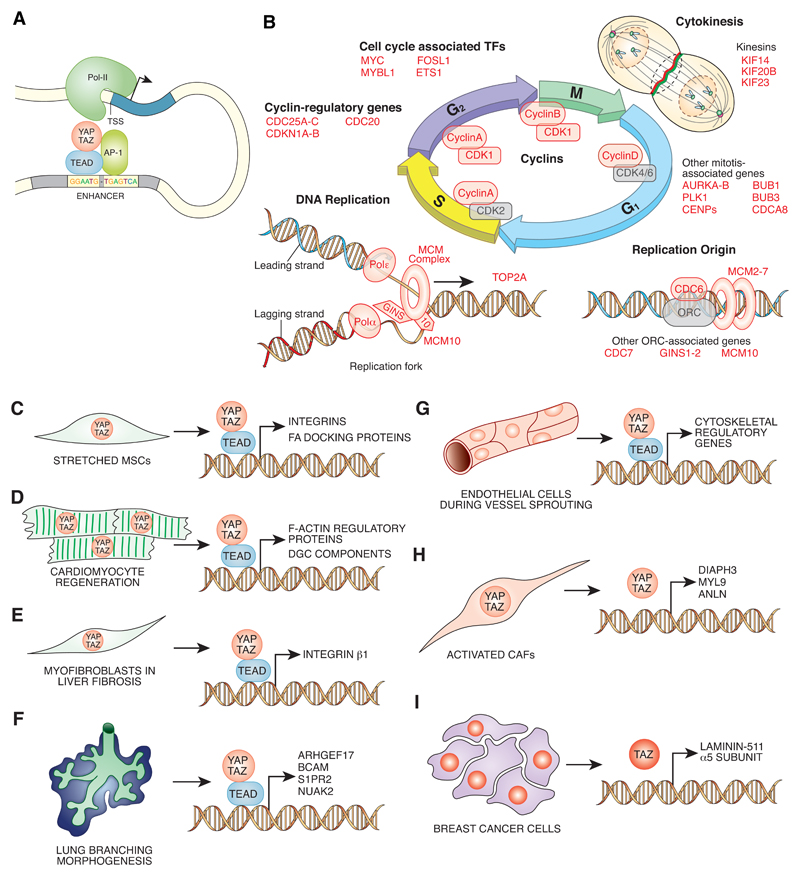
Figure 3. YAP/TAZ nuclear activities and positive feedback mechanisms.
A. Active YAP/TAZ in the nucleus bind preferentially to TEAD co-transcription factors at cognate TEAD/AP-1 binding sequences. Complexes formed by YAP or TAZ with TEAD are in most cases formed at enhancer regions distal to the TSS, at which Pol-II is activated by chromatin looping. B. YAP/TAZ promote cell cycle progression by directly activating the transcription of different genes required for DNA replication, mitosis and checkpoint progression and indirectly by activating the transcription of other master transcription factors, as indicated. YAP/TAZ direct transcriptional target genes are shown in red.. C-I. Schematic representation of different physio-pathological and developmental conditions in which YAP/TAZ transcriptional program leads to the installment of a positive mechanical feedback. C) Mechanically activated MSCs152; D) regenerating cardiomyocytes38; E) activated myofibroblasts during liver fibrosis17; F) lung branching morphogenesis153; G) endothelial cells during vascular development154,155; H-I) activated CAFs and breast cancer cells22,156., AP-1: activator protein 1; CAFs: cancer associated fibroblasts; DGC: dystrophin-associated glycoprotein; FA: focal adhesions; MCM: mini chromosome maintenance; MSCs: mesenchymal stem cells; Pol-II: RNA polymerase II; Polα: DNA polymerase alpha; Polε : DNA polymerase epsilon; TEAD: TEA-domain family member; TSS: transcription start site.
On DNA, YAP/TAZ-TEAD usually act combination with other TFs bound at neighboring cis-regulatory elements. Analysis of ChIP-seq data revealed that the AP-1 (Activator Protein 1) TFs are recruited on a large set of enhancers bound by YAP/TAZ-TEAD, allowing synergistic cooperation in the control of tumor proliferation, migration and invasion, to drive epigenetic reprogramming of cancer cells into a stably chemoresistant state, and for bone metabolism114,116–118. Other TFs acting in combination with YAP/TAZ on the cis-regulatory elements of individual genes are listed in Table 1 (for example RUNX2119,120), which also includes work on the potential role of YAP/TAZ as transcriptional repressors.
Table 1. Main transcription factors cooperating with YAP/TAZ.
The biological model in which the ChIP experiments were performed is indicated in brackets, when available. ND: Not determined, hESC: human embryonic stem cell, PDAC: pancreatic ductal adenocarcinoma, SSC: skeletal stem cells, TF: transcription factor.
| YAP/TAZ-TEAD cooperation with other transcription factors. | ||||
|---|---|---|---|---|
| TF | cis-Regulatory element | Biological role and tissue context | Transcriptional effect | Ref |
| AP1 | Enhancer | Tumor proliferation, migration and invasion (A549, HCT116, SK-N-SH, ECC1, MDA-MB-231); chemoresistance; bone metabolism | Activation | 114,116–118 |
| PITX2 | Enhancer/Promoter | Mouse myocardial regeneration upon ischemic injury (P2 mouse ventricle) | Activation | 136 |
| NICD/RBPJ | Enhancer | Trophectoderm lineage specification in mouse blastocyst | Activation | 160 |
| ZEB1 | Promoter | Breast cancer aggressiveness (MDA-MB-231) | Activation | 161 |
| ICD-ERBB4 | ND | Breast cancer cell migration | Activation | 162 |
| E2F | Promoter | Cell cycle progression (S2R+); PDAC proliferation (KrasG12D-driven pancreatic tumors and primary cell cultures) | Activation | 125,127 |
| MYC | Promoter | Cell cycle entry and cell proliferation (3T9MycER fibroblasts) | Activation | 128 |
| SNAIL/SLUG | Promoter | Osteoprogenitors proliferation (SSC-derived osteoblast progenitors) | Activation | 119 |
| p65 | Promoter | Breast cancer cell migration (MCF7) | Activation | 138 |
| NuRD complex | Enhancer | Mesoderm specification of hESCs (hESC) | Inhibition | 163 |
| Promoter | Epithelial cells growth and migration (MCF-10A) | Inhibition | 164,165 | |
| Promoter | Maintenance/Preservation of smooth muscle mesenchymal progenitors (C3H10T1/2) | Inhibition | 166 | |
| SMADs | Enhancer | Mesoderm specification of hESCs (hESC) | Inhibition | 163 |
| YAP/TAZ cooperation with DNA binding platforms | ||||
| RUNX2 | Promoter | Osteogenic differentiation (SSC-derived osteoblast progenitors; C2C12) | Activation | 119,120 |
| p73 | Promoter | DNA-damaged induced apoptosis (HCT119, HEK293) | Activation | 167,168 |
| p53 Mutant | Promoter | Cancer cell proliferation (MDA-MB-468) | Activation | 169 |
| FoxO1 | Promoter | Myocardial response to ischemic injury (H9C2) | Activation | 135 |
| NICD/RBPJ | Enhancer | Embryonic differentiation of neural crest precursors (MDA-231) | Activation | 145 |
| β-catenin/TCF | Promoter/ND | Heart development (E14.5 hearts) | Activation | 73 |
| β-catenin/TBX5 | Promoter | Cancer cell lines pro-survival genes (HuTu80) | Activation | 29 |
YAP/TAZ can interact with nuclear proteins that compete with their association with TEAD. Vgl-like-4 (VGLL4) counteracts the YAP/TAZ-dependent tumor growth by disrupting their interaction with TEADs in gastric, lung and breast cancer121–123. Notably, Vgll proteins can also serve as binding partners to TEAD, alternative to YAP/TAZ, promoting anchorage independent growth and cell proliferation. The structural determinants of this association were recently reported124. Another example of a nuclear inhibitor is TIAM1, which prevents the interaction between TEAD and YAP or TAZ in the nucleus, probably by sequestering YAP/TAZ, inhibiting migration and invasion of CRC cancers87.
YAP/TAZ downstream genes and effector pathways
Sustained cell proliferation is probably one of the best characterized YAP/TAZ-dependent biological responses. Mechanistically, YAP/TAZ stimulate cell proliferation by controlling the expression of genes directly involved in the cell cycle, such as proteins involved in DNA replication and repair, cyclins and their regulators, mitotic kinases and factors required for completion of mitosis114,125–127. Moreover, YAP/TAZ indirectly control cell proliferation through the induction transcriptional regulators of the cell cycle114,127 (FIG. 3B). Intriguingly, Myc-dependent transcription requires the recruitment of YAP to MYC/TEAD-loaded promoters, potentially amplifying the effects of YAP/TAZ on cell proliferation128. Thus, Myc-driven transcription is substantially a YAP/TAZ-driven gene expression program. This expands the long-neglected chromatin layer as a pivotal element of the integration of YAP/TAZ with other oncogenic or lineage specific transcription factors. YAP/TAZ further controls the expression of the CTGF, CYR61 and AXL and anti-apoptotic genes of the Bcl2 and IAP family. These targets may support YAP/TAZ-driven chemoresistance129–131 and protection against apoptosis7,132. In some experimental contexts, YAP may display poorly understood tumor suppressive responses. Intriguingly, individual cells retaining elevated YAP levels undergo apoptosis, when they are no longer enmeshed into a YAP-supporting microenvironment133. Similar tumor suppressive responses exist in colorectal cancer92. Exciting evidence in Drosophila suggests that part of these tumor suppressive effects of YAP are connected to "cell competition" phenomena134. Whether cell competition occurs in adult mammalian tissues, and, if so, in a YAP/TAZ regulated manner, remains unknown.
YAP/TAZ also activate a transcriptional program required for cardiomyocyte protection from reactive oxygen species (ROS) generated after cardiac injury and ischemic damage135,136. In these conditions, active YAP fosters cardiomyocyte survival and partial heart regeneration either by cooperating with TEAD/Pitx2 to regulate the expression of a large subset of Pitx2 targets136, or by using FoxO1 as DNA-binding platform to induce the expression of antioxidant genes135.
As outlined above, several metabolic pathways can regulate YAP/TAZ. In response, YAP/TAZ activation reprograms cell metabolism to sustain massive cell proliferation. Mechanistically, YAP/TAZ modify metabolism through direct transcriptional regulation of glycolysis enzymes137–139, HBP99, glutamine metabolism and nucleotide biosynthesis140,141. In addition, YAP/TAZ can induce the BCAR4 lncRNA, leading to upregulation of the glycolytic PFKFB3139, supporting YAP/TAZ transcriptional responses63. Furthermore, YAP/TAZ upregulation through EphA2-RhoA-ROCK signaling is connected to raised glutamine metabolism141.
YAP/TAZ activate mTOR signaling by promoting the transcription of several regulators of the mTOR pathway, such as the mTORC1 activator Ras homolog enriched in brain (RHEB), required for proliferation of transient-amplifying (TA) cells during tissue renewal of mouse incisors67; the L-type amino acid transporter 1 (LAT1)142, which enhances amino acid uptake and consequent mTORC1 activation and the microRNA miR-29, which inhibits PTEN translation and PI3K-mediated activation of mTORC1 and mTORC2 kinases143. However, large gaps remain in our understanding of the potential overlap between Hippo, mTOR and YAP/TAZ for control of cell and organ size.
YAP/TAZ impact on Notch
Work in various cellular context has revealed the importance of functional and molecular connections between YAP/TAZ and Notch signaling144. YAP/TAZ-dependent regulation of Notch ligands in one cell triggers activation of Notch signaling in neighboring cells. For example, YAP/TAZ-induced JAG1 expression activate Notch signaling to promote differentiation of neural crest progenitors into smooth muscle cells during arterial wall development145. Moreover, contraction of muscle fibers triggers YAP mechanotransduction to induce JAG2 and turn on Notch signaling in neighboring satellite cells, preventing their differentiation146.
Besides inducing Notch activity in trans (non-cell autonomously), YAP/TAZ-driven expression of Notch ligands can protect the cells endowed with the highest YAP/TAZ levels from receiving Notch signaling, so called “cis-inhibition”147. Indeed epidermal progenitors are maintained in an undifferentiated state by mechanical activation of YAP/TAZ that upregulate DLL1, JAG2 and DLL3 ligands, resulting in cell-autonomous cis-inhibition of Notch signaling148. Thus the structural features of the microenvironment are translated into dynamic changes of YAP/TAZ activity to orchestrate spatial control of self-renewal vs. differentiation of stem cells.
Another exciting discovery is that YAP mechanotransduction feeding on Notch signaling is at the centerpiece of the enigmatic somitic "segmentation clock" (periodic waves of gene function that travel through the presomitic mesoderm)149. It appears that what regulates this oscillatory system are the extensive biomechanical changes occurring in cells exiting gastrulation, including collective movement and compaction, patterning YAP activity leading to repression of Notch150.
The influence of YAP/TAZ on cell mechanics
In light of the overarching role of cell mechanics in controlling YAP/TAZ activity, it should be mentioned that an important subset of the YAP/TAZ transcriptional program is devoted to the installment of a positive feedback loop that promotes F-actin remodeling and high mechanical signaling that further supports YAP/TAZ activity. Such a feedback mechanism is illustrated by the medaka fish hirame mutant, in which a loss-of function mutation of YAP causes flattening of the entire animal body, due to impaired development of actomyosin-mediated tissue tension151. Further, ChIP-seq analysis on MSCs cultured on substrates of different rigidities has revealed that mechanically-activated YAP/TAZ can enhance the assembly of FA complexes by directly promoting the transcription of integrins and FAs docking proteins152 (FIG. 3C). During cardiomyocyte regeneration, YAP/TAZ directly promote the expression of F-actin regulatory proteins and adhesion molecules, including components of the DGC38 (FIG. 3D). During liver fibrosis, YAP/TAZ promote the sustained expression of Integrinβ1 by activated myofibroblasts17 (FIG. 3E).
Branching morphogenesis of the lung is impaired in YAP conditional knockout mice, and a number of YAP/TAZ targets modulate actomyosin contractility153 (FIG. 3F). YAP/TAZ also crosstalk with the vascular endothelial growth factor (VEGF) during angiogenesis. During vascular development, cytoskeletal rearrangement of endothelial cells induced by VEGF triggers the activation of YAP/TAZ which feeds back on cytoskeletal gene expression to sustain endothelial remodeling and to ensure correct VEGF receptor trafficking154,155 (FIG. 3G).
Another “inside-out” positive feedback loop is found in breast cancer associated fibroblasts (CAFs), in which YAP/TAZ directly promote the expression of modulators of F-actin contractility. Increased contractility fosters the deposition of fibrous collagen, that, by enhancing ECM stiffness, sustains YAP/TAZ activity in both CAFs and epithelial cancer cells22 (FIG. 3H). In breast cancer cells, expression of TAZ can induce cancer stem cell properties56 but also sustain its own activity by inducing transcription of laminin 511, a pro-YAP/TAZ ECM component156 (FIG. 3I). In cancer cell lines, YAP/TAZ-TEAD and AP1 cooperate to activate Dock proteins, a family of small GTPases that regulate Rac1 and Cdc42 to drive cytoskeletal organization, cell adhesion and motility116.
Outstanding questions
The interest in YAP/TAZ biology and regulation was initially inspired by pioneering work on the Hippo cascade and its roles in controlling organ size and tumorigenesis. Subsequently we learned that the Hippo kinase cassette is a segment of a broader framework in which YAP/TAZ are regulated by the structural features of cells and tissues, in Hippo-dependent and -independent ways. New data have shed light on the molecular mechanisms by which YAP/TAZ act as sensors and effectors of the cell’s behavior in a tissue context.
A model is emerging in which cytoskeletal organization reflecting tissue-level mechanical forces, serves as an overarching determinant of YAP/TAZ activation. In turn, through feedback and crosstalk mechanism, YAP/TAZ impact on a variety of cellular events, from growth factors responsiveness to metabolism. Here we tried to integrate data from different model systems to offer a comprehensive picture, but it should be noted that the relative relevance of each step may be different in distinct cells and in vivo conditions. YAP/TAZ activation can endow cell plasticity, reprogramming primary, genetically normal differentiated cells into their corresponding tissue-specific stem or progenitors157. There are obvious implications for regenerative medicine, but many questions remain. What are the constituents of this program? What are the partner transcription factors by which YAP/TAZ operate in various histotypes? Are these tissue-specific or universal? What are the downstream genes? Can we use YAP/TAZ to epigenetically explore the so far enigmatic self-renewal properties of stem cells?
Furthermore, the mechanisms by which YAP/TAZ are regulated by cell mechanics remain poorly understood. YAP/TAZ mechanotransduction can be independent from LATS, still, LATS itself can be regulated by F-actin potentially reinforcing the mechanotransduction pathway. Moreover, the Hippo pathway feeds back on cytoskeletal organization by directly phosphorylating cytoskeletal components43,158. However, it’s unclear how this interplay is orchestrated.
The Hippo pathway has additional targets other than YAP/TAZ, including regulation of chromosome segregation, the spindle checkpoint, p53, hormonal receptors and immune cell function59,171. Ironically, it is still unclear whether YAP/TAZ are instrumental or dispensable for organ size control that made the Hippo pathway famous.
Finally, YAP/TAZ activation is instrumental for both cancer and regeneration. The essential role of YAP/TAZ for the emergence of solid tumors resembles the “transcriptional addiction” phenomenon observed in cancer cells, through the disproportional use of enhancer elements7,159 It’s currently unclear whether the knowledge outlined above can be used to combat excess of YAP/TAZ activity in cancer. Addressing these issues might have immediate clinical implications for an anti-YAP/TAZ based cancer therapy.
Acknowledgments
The authors thank all members of the S.P. laboratory for discussion. This work is supported by AIRC Special Program Molecular Clinical Oncology ‘5 per mille’, by an AIRC PI-Grant to S.P., and by Epigenetics Flagship project CNR-MIUR grants to S.P, and from a donation in memoriam of Liana Simonutti. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 670126-DENOVOSTEM).
Footnotes
Author Contributions
All authors contributed equally to all aspects of the article (researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission).
Competing interests statement
The authors declare no competing interests.
References
- 1.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B, et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149:1207–1220. doi: 10.1016/j.cell.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 5.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–8. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Makita R, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–53. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 11.Morin-Kensicki EM, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 13.Aragona M, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa KT, Yamashita K, Sakurai N, Ohno S. The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell Rep. 2017;20:1435–1447. doi: 10.1016/j.celrep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Deng H, et al. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. Elife. 2015;4:e06567. doi: 10.7554/eLife.06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher GC, et al. The Spectrin cytoskeleton regulates the Hippo signalling pathway. The EMBO Journal. 2015;34:940–954. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin K, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun. 2016;7 doi: 10.1038/ncomms12502. 12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caliari SR, et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep. 2016;6 doi: 10.1038/srep21387. 21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachowski D, et al. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci Rep. 2017;7 doi: 10.1038/s41598-017-02689-x. 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbediwy A, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, et al. αE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev. 2016;30:798–811. doi: 10.1101/gad.274951.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorrentino G, et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8 doi: 10.1038/ncomms14073. 14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima H, et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Developmental Cell. 2017;40:523–536.e6. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Yui S, et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22:35–49.e7. doi: 10.1016/j.stem.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabra H, et al. β1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. J Biol Chem. 2017;292:19179–19197. doi: 10.1074/jbc.M117.808063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sero JE, Bakal C. Multiparametric Analysis of Cell Shape Demonstrates that β-PIX Directly Couples YAP Activation to Extracellular Matrix Adhesion. Cell Syst. 2017;4:84–96.e6. doi: 10.1016/j.cels.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbluh J, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elosegui-Artola A, et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016;18:540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 31.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiu J-Y, Aires L, Lin Z, Vogel V. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nat Cell Biol. 2018;20:262–271. doi: 10.1038/s41556-017-0030-y. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster CT, Gualdrini F, Treisman R. Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics. Genes Dev. 2017;31:2361–2375. doi: 10.1101/gad.304501.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Developmental Cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 37.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14:529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morikawa Y, et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal. 2015;8:ra41–ra41. doi: 10.1126/scisignal.2005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassat E, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakraborty S, et al. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 41.Pathak MM, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mana-Capelli S, Paramasivam M, Dutta S, McCollum D. Angiomotins link F-actin architecture to Hippo pathway signaling. Mol Biol Cell. 2014;25:1676–1685. doi: 10.1091/mbc.E13-11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol. 2018;20:373–381. doi: 10.1038/s41556-018-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elosegui-Artola A, et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171:1397–1410.e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 47.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 48.Tapon N, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 49.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 50.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 51.Meng Z, Moroishi T, Guan K-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Developmental Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng Z, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6 doi: 10.1038/ncomms9357. 8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin F, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 57.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Developmental Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Diepenbruck M, et al. Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition. J Cell Sci. 2014;127:1523–1536. doi: 10.1242/jcs.139865. [DOI] [PubMed] [Google Scholar]
- 59.Furth N, Aylon Y. The LATS1 and LATS2 tumor suppressors: beyond the Hippo pathway. Cell Death Differ. 2017;24:1488–1501. doi: 10.1038/cdd.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azzolin L, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 62.Das A, Fischer RS, Pan D, Waterman CM. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J Biol Chem. 2016;291:6096–6110. doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enzo E, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. The EMBO Journal. 2015;34:1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gailite I, Aerne BL, Tapon N. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc Natl Acad Sci USA. 2015;112:E5169–78. doi: 10.1073/pnas.1505512112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng X, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mo J-S, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu JK-H, et al. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice. Cell Stem Cell. 2017;21:91–106.e6. doi: 10.1016/j.stem.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reginensi A, et al. Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development. PLoS Genetics. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silvis MR, et al. α-Catenin Is a Tumor Suppressor That Controls Cell Accumulation by Regulating the Localization and Activity of the Transcriptional Coactivator Yap1. Sci Signal. 2011;4:ra33–ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorrentino G, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 71.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Q, et al. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lavado A, et al. Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators. Development. 2013;140:3323–3334. doi: 10.1242/dev.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin C, Yao E, Chuang P-T. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev Biol. 2015;403:101–113. doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.George NM, Day CE, Boerner BP, Johnson RL, Sarvetnick NE. Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol. 2012;32:5116–5128. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 78.Ren F, Zhang L, Jiang J. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol. 2010;337:303–312. doi: 10.1016/j.ydbio.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu F-X, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Codelia VA, Sun G, Irvine KD. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014;24:2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 82.Azzolin L, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. β-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 85.Gargini R, et al. WIP Drives Tumor Progression through YAP/TAZ-Dependent Autonomous Cell Growth. Cell Rep. 2016;17:1962–1977. doi: 10.1016/j.celrep.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 86.Noto A, et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene. 2017;36:4573–4584. doi: 10.1038/onc.2017.75. [DOI] [PubMed] [Google Scholar]
- 87.Diamantopoulou Z, et al. TIAM1 Antagonizes TAZ/YAP Both in the Destruction Complex in the Cytoplasm and in the Nucleus to Inhibit Invasion of Intestinal Epithelial Cells. Cancer Cell. 2017;31:621–634.e6. doi: 10.1016/j.ccell.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Llado V, et al. Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of β-Catenin and Yap by PKCζ. Cell Rep. 2015;10:740–754. doi: 10.1016/j.celrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oudhoff MJ, et al. SETD7 Controls Intestinal Regeneration and Tumorigenesis by Regulating Wnt/β-Catenin and Hippo/YAP Signaling. Developmental Cell. 2016;37:47–57. doi: 10.1016/j.devcel.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 90.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 91.Nowell CS, et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat Cell Biol. 2016;18:168–180. doi: 10.1038/ncb3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varelas X, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Developmental Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 94.Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez M-E, Farge E. Mechanotransduction in tumor progression: The dark side of the force. J Cell Biol. 2018 doi: 10.1083/jcb.201701039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park HW, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santinon G, Pocaterra A, Dupont S. Control of YAP/TAZ Activity by Metabolic and Nutrient-Sensing Pathways. Trends in Cell Biology. 2016;26:289–299. doi: 10.1016/j.tcb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Peng C, et al. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Molecular Cell. 2017;68:591–604.e5. doi: 10.1016/j.molcel.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, et al. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun. 2017;8 doi: 10.1038/ncomms15280. 15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nokin M-J, et al. Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP-mediated tumor growth and metastasis. Elife. 2016;5:260. doi: 10.7554/eLife.19375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proceedings of the National Academy of Sciences. 2013;111:E89–E98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang N, et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Artinian N, et al. Phosphorylation of the Hippo Pathway Component AMOTL2 by the mTORC2 Kinase Promotes YAP Signaling, Resulting in Enhanced Glioblastoma Growth and Invasiveness. J Biol Chem. 2015;290:19387–19401. doi: 10.1074/jbc.M115.656587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sciarretta S, et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 2015;11:125–136. doi: 10.1016/j.celrep.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu F-X, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu F-X, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iglesias-Bartolome R, et al. Inactivation of a Gα(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu G, et al. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2015;34:3536–3546. doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taniguchi K, et al. YAP-IL-6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc Natl Acad Sci USA. 2017;114:1643–1648. doi: 10.1073/pnas.1620290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geng J, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18:800–812. doi: 10.1038/ni.3748. [DOI] [PubMed] [Google Scholar]
- 113.Stein C, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genetics. 2015;11:e1005465. doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galli GG, et al. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Molecular Cell. 2015;60:328–337. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu X, et al. Tead and AP1 Coordinate Transcription and Motility. Cell Rep. 2016;14:1169–1180. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shaffer SM, et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546:431–435. doi: 10.1038/nature22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao L, et al. YAP1 is essential for osteoclastogenesis through a TEADs-dependent mechanism. Bone. 2018;110:177–186. doi: 10.1016/j.bone.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 119.Tang Y, Feinberg T, Keller ET, Li X-Y, Weiss SJ. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat Cell Biol. 2016;18:917–929. doi: 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hong J-H, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 121.Zhang W, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, et al. VGLL4 Selectively Represses YAP-Dependent Gene Induction and Tumorigenic Phenotypes in Breast Cancer. Sci Rep. 2017;7 doi: 10.1038/s41598-017-06227-7. 6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiao S, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 124.Pobbati AV, Chan SW, Lee I, Song H, Hong W. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure. 2012;20:1135–1140. doi: 10.1016/j.str.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Nicolay BN, Bayarmagnai B, Islam ABMMK, Lopez-Bigas N, Frolov MV. Cooperation between dE2F1 and Yki/Sd defines a distinct transcriptional program necessary to bypass cell cycle exit. Genes Dev. 2011;25:323–335. doi: 10.1101/gad.1999211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mizuno T, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–5122. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 127.Kapoor A, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Croci O, et al. Transcriptional integration of mitogenic and mechanical signals by Myc and YAP. Genes Dev. 2017;31:2017–2022. doi: 10.1101/gad.301184.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schoumacher M, Burbridge M. Key Roles of AXL and MER Receptor Tyrosine Kinases in Resistance to Multiple Anticancer Therapies. Curr Oncol Rep. 2017;19:19. doi: 10.1007/s11912-017-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 131.Lin C-H, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2013;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Su T, et al. Two-signal requirement for growth-promoting function of Yap in hepatocytes. Elife. 2015;4 doi: 10.7554/eLife.02948. 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suijkerbuijk SJE, Kolahgar G, Kucinski I, Piddini E. Cell Competition Drives the Growth of Intestinal Adenomas in Drosophila. Curr Biol. 2016;26:428–438. doi: 10.1016/j.cub.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shao D, et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5 doi: 10.1038/ncomms4315. 3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tao G, et al. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature. 2016;534:119–123. doi: 10.1038/nature17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao Y, et al. TNFα-YAP/p65-HK2 axis mediates breast cancer cell migration. Oncogenesis. 2017;6:e383. doi: 10.1038/oncsis.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng X, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. The EMBO Journal. 2017 doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cox AG, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18:886–896. doi: 10.1038/ncb3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Edwards DN, et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci Signal. 2017;10:eaan4667. doi: 10.1126/scisignal.aan4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hansen CG, Ng YLD, Lam W-LM, Plouffe SW, Guan K-L. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–1313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tumaneng K, et al. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Totaro A, Castellan M, Di Biagio D, Piccolo S. Crosstalk between YAP/TAZ and Notch Signaling. Trends in Cell Biology. 2018 doi: 10.1016/j.tcb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manderfield LJ, et al. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development. 2015;142:2962–2971. doi: 10.1242/dev.125807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Esteves de Lima J, Bonnin M-A, Birchmeier C, Duprez D. Muscle contraction is required to maintain the pool of muscle progenitors via YAP and NOTCH during fetal myogenesis. Elife. 2016;5 doi: 10.7554/eLife.15593. 3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Totaro A, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:1–13. doi: 10.1038/ncomms15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hubaud A, Pourquié O. Signalling dynamics in vertebrate segmentation. Nat Rev Mol Cell Biol. 2014;15:709–721. doi: 10.1038/nrm3891. [DOI] [PubMed] [Google Scholar]
- 150.Hubaud A, Regev I, Mahadevan L, Pourquié O. Excitable Dynamics and Yap-Dependent Mechanical Cues Drive the Segmentation Clock. Cell. 2017;171:668–682.e11. doi: 10.1016/j.cell.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Porazinski S, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nardone G, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8 doi: 10.1038/ncomms15321. 15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin C, et al. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. Elife. 2017;6 doi: 10.7554/eLife.21130. 14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim J, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127:3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X, et al. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Developmental Cell. 2017;42:462–478.e7. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 156.Chang C, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α 6Bβ 1 integrin to sustain breast cancer stem cells. Genes Dev. 2015;29:1–6. doi: 10.1101/gad.253682.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Panciera T, et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell. 2016;19:725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lucas EP, et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol. 2013;201:875–885. doi: 10.1083/jcb.201210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bradner JE, Hnisz D, Young RA. Transcriptional Addiction in Cancer. Cell. 2017;168:629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rayon T, et al. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental Cell. 2014;30:410–422. doi: 10.1016/j.devcel.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lehmann W, et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7 doi: 10.1038/ncomms10498. 10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haskins JW, Nguyen DX, Stern DF. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci Signal. 2014;7:ra116–ra116. doi: 10.1126/scisignal.2005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beyer TA, et al. Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep. 2013;5:1611–1624. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 164.Kim M, Kim T, Johnson RL, Lim D-S. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep. 2015;11:270–282. doi: 10.1016/j.celrep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 165.Valencia-Sama I, et al. Hippo Component TAZ Functions as a Co-repressor and Negatively Regulates ΔNp63 Transcription through TEA Domain (TEAD) Transcription Factor. J Biol Chem. 2015;290:16906–16917. doi: 10.1074/jbc.M115.642363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cotton JL, et al. YAP/TAZ and Hedgehog Coordinate Growth and Patterning in Gastrointestinal Mesenchyme. Developmental Cell. 2017;43:35–47.e4. doi: 10.1016/j.devcel.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Strano S, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Molecular Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 168.Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Molecular Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 169.Di Agostino S, et al. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2015;17:188–201. doi: 10.15252/embr.201540488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 171.Du X, et al. Hippo/Mst signalling couples metabolic state and immune function of CD8alpha(+) dendritic cells. Nature. 2018;558:141–145. doi: 10.1038/s41586-018-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]