Abstract
Despite higher rates of hospitalization and mortality following traumatic brain injury (TBI) in patients over 65 years old, older patients remain underrepresented in drug development studies. Worse outcomes in older individuals compared to younger adults could be attributed to exacerbated injury mechanisms including oxidative stress, inflammation, blood-brain barrier disruption, and bioenergetic dysfunction. Accordingly, pleiotropic treatments are attractive candidates for neuroprotection. Taurine, an endogenous amino acid with antioxidant, anti-inflammatory, anti-apoptotic, osmolytic, and neuromodulator effects, is neuroprotective in adult rats with TBI. However, its effects in the aged brain have not been evaluated. We subjected aged male rats to a unilateral controlled cortical impact injury to the sensorimotor cortex, and randomized them into four treatment groups: saline or 25mg/kg, 50mg/kg, or 200mg/kg i.p. taurine. Treatments were administered 20 minutes post-injury and daily for 7 days. We assessed sensorimotor function on post-TBI days 1 – 14 and tissue loss on day 14 using T2-weighted magnetic resonance imaging. Experimenters were blinded to the treatment group for the duration of the study. We did not observe neuroprotective effects of taurine on functional impairment or tissue loss in aged rats after TBI. These findings in aged rats are in contrast to previous reports of taurine neuroprotection in younger animals. Advanced age is an important variable for drug development studies in TBI, and further research is required to better understand how aging may influence mechanisms of taurine neuroprotection.
Keywords: aging, scientific rigor, magnetic resonance imaging, sensorimotor function, therapy, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a critical public health concern. In the USA alone, TBI affects approximately 1.4 million each year and 5.3 million survivors live with a TBI-related disability (Faul and Coronado 2015; Langlois et al. 2006). TBI-related hospitalizations and deaths are higher in elderly patients compared to other age groups (Coronado et al. 2011; Taylor et al. 2017), with falls and motor vehicle accidents being the leading causes of TBI in patients over 65 years of age (Thompson et al. 2006). Despite the disproportionately high mortality, there is a paucity of research to develop treatment options for this vulnerable population. Worse outcomes in aged versus younger patients have been attributed to exacerbated injury mechanisms including edema, inflammation, oxidative stress, blood-brain barrier disruption and mitochondrial dysfunction in the aging brain (Gilmer et al. 2010; Onyszchuk et al. 2008; Sandhir et al. 2008; Timaru-Kast et al. 2012; Unterberg et al. 1994). Given this complexity, pleiotropic treatments targeting one or more of these injury mechanisms would therefore be attractive candidates to mediate neuroprotection in older survivors of TBI.
Taurine is an abundant endogenous amino acid synthesized by neurons and astrocytes in the central nervous system (Vitvitsky et al. 2011), where it functions as an osmolyte, neuromodulator, trophic factor, stabilizer of membrane integrity, and regulator of intracellular calcium homeostasis (Wu and Prentice 2010). A common component of nutritional supplements and energy drinks, taurine has an excellent safety profile with doses as high as 10 g per day deemed safe in human clinical trials (Shao and Hathcock 2008). Intracellular taurine concentrations are normally higher than those in the extracellular compartment. Under pathologic osmotic stress taurine is released into extracellular fluid to limit cell swelling (Pasantes-Morales et al. 2002). Taurine’s ability to modulate inhibitory neurotransmission, along with its anti-inflammatory, anti-oxidant and anti-apoptotic properties have supported several reports of neuroprotection in preclinical models of ischemic stroke, epilepsy, and spinal cord injury (Oja and Saransaari 2013; Ricci et al. 2009; Wu and Prentice 2010; Ye et al. 2013; Ripps and Shen 2012; Gupta et al. 2006).
Significant reductions in endogenous brain taurine levels have been reported across varying severities and models of TBI (Harris et al. 2012; Schuhmann et al. 2003; Xu et al. 2011; Singh et al. 2016; Pascual et al. 2007). Brain taurine levels may also be affected in normal aging, with some studies reporting lower levels in aged rats compared to young rats (Benedetti et al. 1991; Zhang et al. 2009; Banay-Schwartz et al. 1989) while others indicated no effect of older age on taurine content (Harris et al. 2015). Long-term supplementation with exogenous taurine improved memory retention in aged mice (El Idrissi et al. 2013). Exogenous taurine administration also showed promising neuroprotective effects following TBI in young adult rats where it preserved blood-brain barrier integrity, cerebral blood flow, and mitochondrial function while reducing edema, oxidative damage and inflammation (Gu et al. 2015; Su et al. 2014; Sun et al. 2015; Wang et al. 2016). However, the effects of taurine supplementation on neurological outcomes following TBI in older animals has not been studied. Therefore, the goal of this study was to evaluate neuroprotection by exogenous taurine administration in aged rats with TBI. Our primary outcome measures of neuroprotection were tissue loss quantified on MRI and behavioral performance assessed by the beam walk and bilateral adhesive removal tests.
Failure in translating numerous seemingly promising drug candidates for TBI from bench to bedside has called for a paradigm shift in the planning and execution of preclinical studies (Kochanek et al. 2016). Conducting preclinical studies in adherence with stringent standards of scientific rigor used in human clinical trials is one way to minimize false positive results, ultimately saving time and money by ensuring that only the most promising candidates make it to patient trials (Steward and Balice-Gordon 2014). To this end, our study included clearly defined outcome measures, appropriately powered sample sizes based on anticipated effect size, randomization of subjects to treatment groups, experimenter blinding, pre-determined inclusion and exclusion criteria, and use of appropriate controls (Begley 2013).
Materials and Methods
Animals
Aged male Fischer rats (20–22 month old F344; National Institute on Aging colony housed at Charles River Laboratories, Wilmington, MA) weighing 350 to 400 g were used for this study. Animals were housed in pairs on a 12 hour light-dark cycle with free access to standard rat chow and water. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center and are consistent with standards of animal care set forth in the guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
A total of 49 rats were ordered for this study. Animals were visually inspected for healthy appearance and behavior. All animals also received baseline MRI scans before the start of the study. These screening criteria excluded 7 rats from the study due to baseline health issues such as liver dysfunction and brain tumors. One rat in each group died after TBI, all on D0 except 1 in the 50 mg/kg taurine group that died on D14 post-TBI. Behavioral analysis included data from the rat that died on D14.
Controlled Cortical Impact
CCI is a well-characterized model of contusive injury in rodents and was performed as we have previously described (Harris et al. 2012). Briefly, animals were anesthetized with inhaled isoflurane (4% for induction, 2 – 3% for maintenance), placed on a heating pad for thermoregulation and immobilized with ear bars on a stereotaxic frame. Their heads were shaved, a local long-acting anesthetic (0.25% bupivacaine) administered subcutaneously, and skulls exposed using a single midline incision. A 6 mm-diameter craniotomy was performed over the right sensorimotor cortex, directly lateral to bregma and centered midway between bregma and the temporal ridge. Cortical impact was delivered with an electromagnetically controlled impact device (Leica, St. Louis, MO) mounted on a stereotaxic manipulator (impactor tip size=5 mm; velocity = 5 m/s; depth = 2.5 mm; contact time = 300 ms). The bone flap was then replaced, sealed in place using dental cement, and the incision sutured closed. A 0.25 % lidocaine/prilocaine topical anesthetic was applied to the incision site and animals were maintained under isoflurane anesthesia for an additional hour before transfer to a heated recovery cage.
Drug treatment
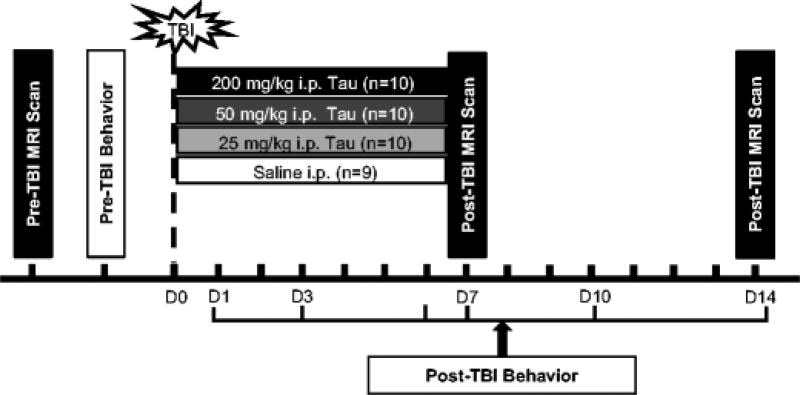
Taurine (Sigma-Aldrich, St. Louis, MO) solution was prepared on the day animals received CCI by dissolving it in sterile saline. Following surgery, animals were randomized into four groups and administered 200 mg/kg (n = 10), 50 mg/kg (n = 10) or 25 mg/kg (n = 10) taurine or vehicle saline (n = 9) intraperitoneally 20 min post-impact and then daily for 7 days (Fig. 1). Experimenters were blinded to the treatment group for the entire duration of the study.
Fig. 1.
Schematic of study design. Baseline magnetic resonance imaging (MRI) was performed on 20–22 month old male F344 rats prior to receiving a traumatic brain injury (TBI). Rats were trained on behavioral tasks for 5 days immediately prior to the day of injury. TBI was induced by controlled cortical impact and animals were randomized into four treatment groups (saline and 25 mg/kg, 50 mg/kg, or 200 mg/kg taurine). Behavioral tests of sensorimotor function were performed on post-TBI day 1, 3, 7, 10 and 14 for each group. MRI was performed on post-injury day 14 to quantify brain tissue loss.
Magnetic resonance imaging (MRI)
MRI scans were performed before TBI (up to 2 weeks) to ensure normal brain morphology, followed by scans 14 days post-TBI to quantify brain tissue loss. All MRI acquisitions were performed in a 9.4-Tesla horizontal bore magnet (Varian Inc., Palo Alto, CA, USA) equipped with a 12-cm gradient coil (40 G/cm, 250 µs) and interfaced to a Varian INOVA console, as previously described (Harris et al. 2012). During imaging, anesthesia was maintained with 1.5% to 3% isoflurane in a 2:1 mixture of air/O2 delivered via nosecone. Respiration was monitored with a pressure pad (SA Instruments, Stony Brook, NY, USA) and maintained at a rate of 40 to 80 cycles/min. Animals were placed on a heating pad in the scanning cradle, and maintained at 37°C via a feedback control rectal probe (Cole Parmer, Vernon Hills, IL, USA). Coronal and sagittal gradient echo multi-slice localizing images were acquired to check the animal’s positioning in the magnet (repetition time = 100 ms, echo time = 2.8 ms, number of slices = 10, slice thickness = 1 mm). Coronal and sagittal rapid acquisition with relaxation enhancement T2-weighted images were then acquired (repetition time = 4,000 ms, echo time = 18 ms, echo train length = 8; averages = 2; field of view = 2.56 × 2.56 cm2; resolution = 256 × 256 pixels; number of slices = 20; slice thickness = 1 mm) for estimation of tissue loss.
Estimation of tissue loss
Tissue loss was estimated from MRI scans performed on D14 post-TBI. The slice with the largest visible lesion was identified and two slices before and after (total 5) were included in the analysis. A vertical midline was traced onto each of the images in ImageJ to distinguish brain hemispheres ipsilateral and contralateral to CCI. A horizontal line was drawn through the ear canals to identify the lower border for quantification in order to eliminate signal drop-off further away from the radiofrequency surface coil. For each hemisphere, the outer borders of visually intact tissue were traced and the total area determined using ImageJ. Then, the ventricles were traced using the Paxinos rat brain atlas for reference (Paxinos), and the area measured. The area of the ventricles was subtracted from intact tissue area for each hemisphere in every slice to quantify tissue loss as follows:
Area of tissue loss thus obtained was multiplied by slice thickness (1 mm) and all slices summed to estimate total tissue loss for each rat.
Behavioral assessment
Rats were handled for 5 mins every day for 2 weeks prior to beginning behavioral experiments. Beam walk and bilateral adhesive removal tests were used to measure sensorimotor performance. Animals were trained on both tests for 5 days to establish a reliable pre-injury baseline performance. Behavioral testing was performed on D1, D3, D7, D10, and D14 after TBI (Fig. 1). On D14 when both behavioral assessment and imaging were conducted, behavior was tested first to avoid residual anesthesia effects. Behavioral scores after injury were expressed as a percent of pre-injury scores so that each animal served as its own control.
Beam walk: The protocol was modified from previously published methods (Harris et al. 2012) to accommodate the larger aged animals. Rats were trained to traverse a custom-made 1 m long horizontal beam supported at a height of 50 cm to arrive at their home cage positioned at the opposite end. On each day of testing, animals were given one trial each on beams of increasing difficulty (flat planks 5 cm, 4 cm, 3.5 cm, and 3 cm in width) with a minimum inter-trial interval of 2 min. The beam walk performance was scored on a 0- to 12-point scale, with each of four trials assigned a score of 0 to 3 points: 3 points = no impairment, crosses with 0–1 faults (i.e. paw slips off and falls below the plane of the beam); 2 points = mildly impaired, crosses with 2–6 faults; 1 point = moderately impaired, crosses with 7+ faults, or falls upside down on the beam 1–3 times; 0 points = severely impaired; falls upside down on the beam 4+ times, falls off the beam, or is unable to cross.
Bilateral adhesive removal: The protocol was modified from previously published methods (Bouzat et al. 2011; Leasure and Grider 2010). A round adhesive sticker (0.5 inch diameter; Avery) was applied with even pressure on each forepaw, the animal was returned to its home cage and filmed for subsequent blinded analysis. The time taken by the animal to shake either forepaw or touch it with its mouth was recorded as the contact time and the time taken by the animal to remove adhesive from each forepaw was recorded as the removal time. Animals received three successive trials (of 2 min duration) on each day, with a minimum inter-trial interval of 2 min. For each day, the best of the three trials (fastest time recorded for contact and removal) was used for analysis.
Necropsy
At the end of the study on D14, animals were euthanized via ketamine/xylazine overdose and transcardial perfusion, and necropsied to identify potential underlying co-morbidities. Subjects were presented to the veterinarian with the head decapitated and the brain removed. Following external examination, a midline incision was made and extended cranially and caudally, exposing the abdominal and thoracic cavities, and the testicles. All major organs were examined externally and incised for observation of any internal abnormalities as previously published (Fiette and Slaoui 2011). All gross necropsy findings were reported. Necropsy was not performed on the first 4 animals in the study and on those that died spontaneously before the end of the study, resulting in group sizes of 6, 9, 8, and 9 in the saline, 25 mg/kg, 50 mg/kg and 200 mg/kg groups, respectively.
Data Management and Statistical Analysis
Due to the limited data available on TBI in aged rats, we estimated group sizes a priori based on the literature in adult rats. Preliminary data in our aged rats (n=8; unpublished) showed approximately 80% impairment in function on the beam walk test on post-TBI day 7. Based on a published report showing 50% improvement in neurological severity score by taurine in young adult rats with TBI, we predicted that taurine would show similar degree of improvement in our study. Using G*Power software we computed that a sample size of n=10 per group and 30% standard deviation would yield significance of 0.05 with 89% power on a one-tailed two-sample t-test. All study procedures (TBI, drug treatments, imaging, behavioral training and testing) were then completed by individuals who were blinded to the treatment group. All analyses/data summaries were completed, results recorded and the data set locked, prior to unblinding and statistical analysis.
Preliminary plots suggested that tissue loss was normally distributed and variances were equal across treatment groups. Therefore, an overall one-way ANOVA test was performed to determine if tissue loss was associated with taurine dose. An ANOVA with contrast coefficients of −3, −1, 1, 3 was used to test for a linear trend in dose response; 1, 0, 0, −1 was used to test saline versus 200 mg/kg dose; and 3, −1, −1, −1 was used to test for difference between saline and all taurine treatment groups combined. Multivariate normality was not reasonable for adhesive removal and beam test data. Therefore, permutation multivariate analysis of variance (MANOVA) profile analysis using Wilk’s lambda statistic was used to determine if trends (profiles) in the adhesive removal and beam walk tests differed across treatment groups. The elements of the contrast matrix for the profile analysis were 1 – 1 0 0 0, 1 0 −1 0 0, 1 0 0 −1 0, 1 0 0 0 −1 in rows 1 through 4 respectively. Since overall MANOVA suggested no significant difference in trends across treatment groups, we pooled all data to determine post hoc injury effects across days using nonparametric Wilcoxon’s signed ranked test. Statistical analyses were performed using SPSS (for tissue loss and post hoc analyses) and SAS (for adhesive removal and beam walk tests). Data are presented as mean ± standard error of mean (SEM) and p < 0.05 is considered to be statistically significant.
Results
Brain tissue loss
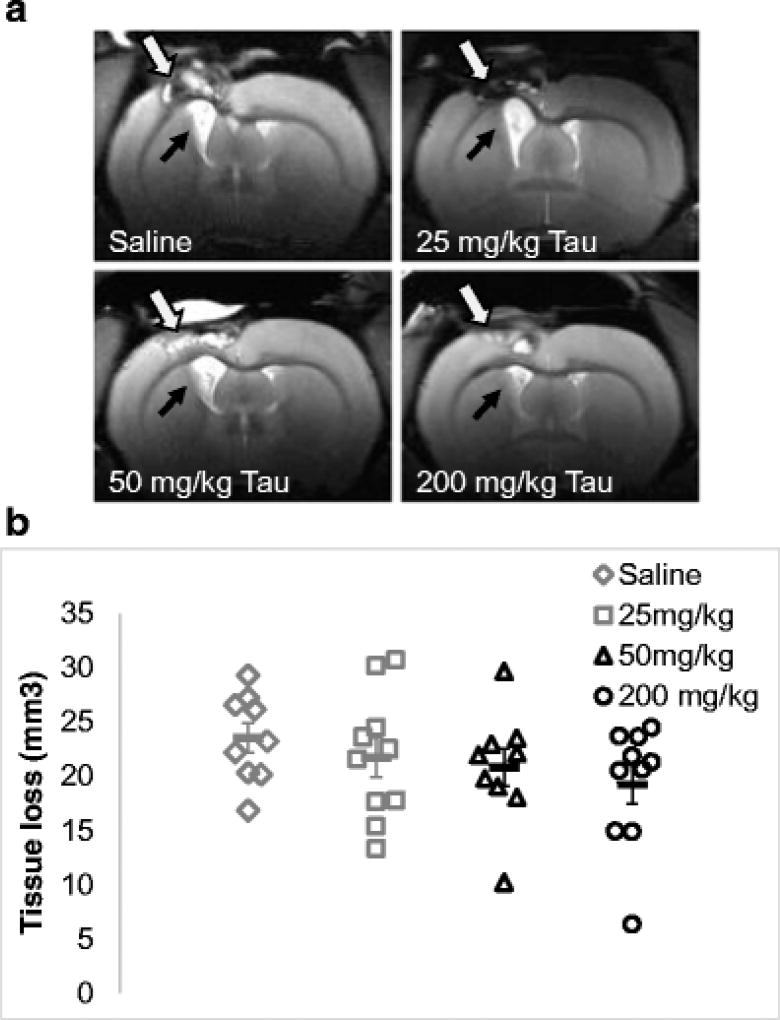
All animals showed varying degrees of ipsilateral ventricular expansion and cortical damage (considered together as tissue loss) on T2-weighted MRI 14 days after TBI (Fig. 2a). Aged rats administered saline had average tissue loss of 23.54 ± 1.35 mm3 whereas 25 mg/kg, 50 mg/kg and 200 mg/kg taurine groups had tissue loss of 21.73 ± 1.85 mm3, 20.8 ± 1.74 mm3 and 19.22 ± 1.78 mm3, respectively. Taurine treatment had no significant effect on overall TBI-mediated tissue loss. Although rats administered taurine showed evidence of a dose-dependent reduction in tissue loss, it was not statistically significant (p=0.078; Fig. 2b). Post hoc analyses found no significant differences in tissue loss between saline and the highest dose of taurine tested (200 mg/kg; p = 0.083) nor between saline and all taurine groups combined (p = 0.15).
Fig. 2.
Taurine effect on brain tissue loss. a) Representative T2-weighted coronal magnetic resonance images of rat brains on post-TBI day 14 show cortical tissue loss at the site of impact (white arrows) and ventricular expansion (black arrows) in the hemisphere ipsilateral to the injury. b) Scatter plot depicts tissue loss in each rat within each group. An apparent dose-dependent reduction in tissue loss in taurine treated groups did not reach significance (p = 0.078). Horizontal bars represent group mean ± standard error of mean.
Behavioral outcomes
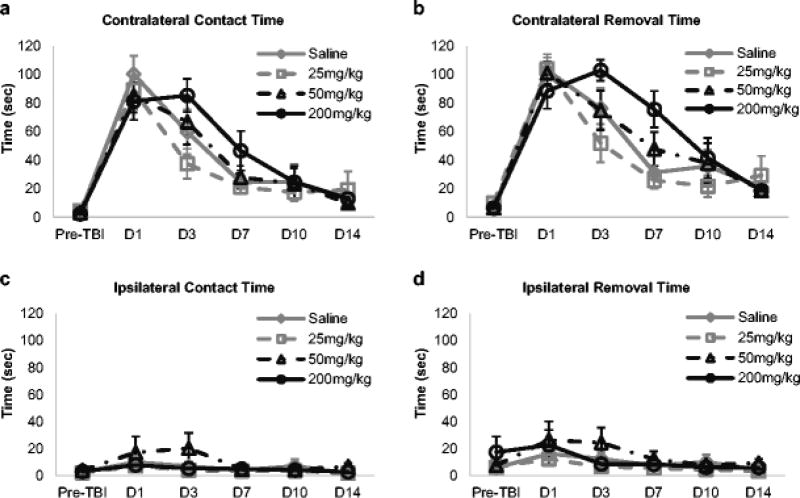
Aged rats showed impaired performance on sensorimotor tests post-TBI that recovered over time. On the bilateral adhesive removal test, TBI resulted in a significant increase in the contralateral limb contact and removal times in all groups on all days post-injury (Fig. 3a, 3b). Contralateral limb contact times increased approximately 20- to 50- fold from pre-injury performance to D1 for all treatment groups (Fig. 3a; 2.4 ± 0.6 to 100.3 ± 12.9 sec for saline; 4.6 ± 2.5 to 89.2 ± 13.7 sec for 25 mg/kg taurine; 3.3 ± 1.2 to 85.2 ± 11.6 sec for 50 mg/kg taurine; 2.3 ± 0.7 to 81.3 ± 13.1 for 200 mg/kg taurine). A similar pattern emerged for removal times (Fig. 3b). Although post-TBI differences were smaller in magnitude, ipsilateral limb contact times on D1, D3, D7 and D10 and ipsilateral removal times on D1 and D3 post-injury were also significantly greater than pre-injury (Fig. 3c, 3d). Ipsilateral limb contact times increased approximately 2- to 5- fold from pre-injury performance to D1 (Fig. 3c; 2.7 ± 1.3 to 10 ± 5.74 sec for saline; 2.1 ± 1.1 to 7.1 ± 3.2 sec for 25 mg/kg taurine; 4.0 ± 1.9 to 17.2 ± 11.8 sec for 50 mg/kg taurine; 3.1 ± 1 to 7.9 ± 3.0 sec for 200 mg/kg taurine). A similar pattern emerged for removal times (Fig. 3d).
Fig. 3.
Taurine effects on bilateral adhesive removal performance. Time taken by rats to contact (a, c) and remove (b, d) a circular adhesive stuck on the contralateral left paw and ipsilateral right paw was measured. Traumatic brain injury (TBI) produced significant delay in contralateral limb contact and removal times in all treatment groups at post-TBI day 1 (p < 0.001), which recovered partially over time. Significant increase in ipsilateral limb contact and removal time was also observed at post-TBI day 1 (p < 0.001), although its magnitude was lower than the contralateral side. Taurine did not improve TBI-mediated impairment in adhesive removal performance at any dose tested. Data are expressed as group mean ± standard error of mean for each time point.
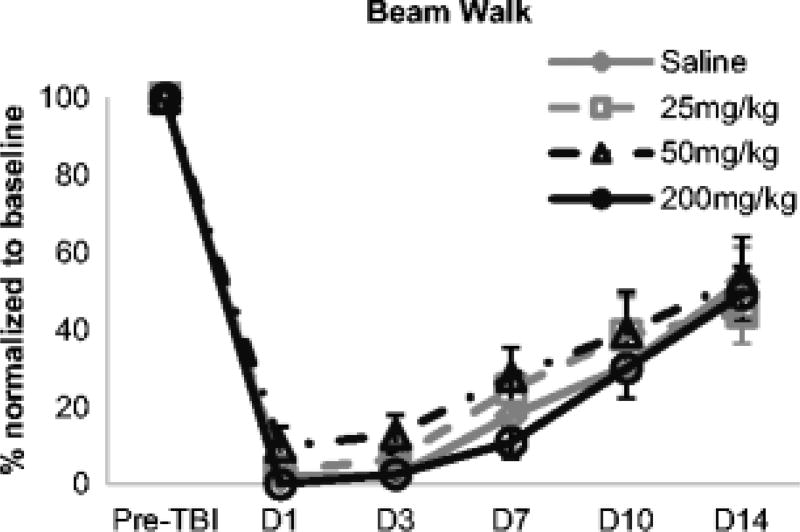
On the beam walk test, TBI impaired performance in all groups on all days post-injury (Fig. 4). An approximately 90% impairment in beam walk performance was observed at D1 for all groups. Injury effects on adhesive removal and beam walk scores recovered significantly between D1 and D14 post-TBI (p<0.0001), although scores at D14 remained significantly different from pre-injury scores. There were no significant differences among the four treatment groups on the adhesive removal test (p = 0.46 and p = 0.09 for contralateral limb contact and removal times, respectively) or the beam walk test (p = 0.64).
Fig. 4.
Taurine effects on beam walk performance. Post-injury scores on beam walk were normalized to pre-injury baseline scores for each animal. Traumatic brain injury significantly impaired beam walk performance in all groups (p < 0.001), although performance recovered partially over time. Taurine did not significantly improve post-injury beam walk performance at any dose tested. Data are expressed as group mean ± standard error of mean for each time point.
Health outcomes
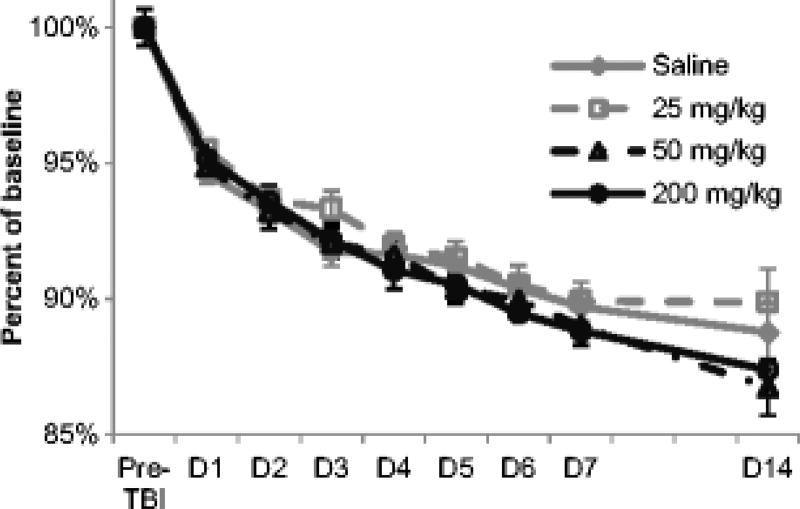
All animals had similar weights at baseline and experienced similar weight loss following TBI, irrespective of treatment group (Fig. 5). One rat in each of the four treatment groups died before the end of the study resulting in a mortality of 9% in 200 mg/kg and 25 mg/kg taurine groups and 10% in the saline and 50 mg/kg taurine groups. Group sizes were insufficient for statistical analysis of mortality. All groups showed similar incidence of co-morbidities on post-mortem necropsy, with testicular tumors being the most common (Table 1).
Fig. 5.
Taurine effect on post-TBI weight loss. Animal weights were recorded prior to injury and then every day for 7 days post-injury as well as on D14. Post-injury weights were normalized to the baseline weight for each animal. Although all animals lost weight after TBI, no significant differences in weight loss were observed among the treatment groups. Data are expressed as mean ± standard error of mean.
Table 1.
Co-morbidities in aged F344 rats with TBI
| Testicular tumor (%) |
Nephropathy (%) |
Splenomegaly (%) |
Hepatic issues (%) |
Pneumonia (%) |
Other (%) |
|
|---|---|---|---|---|---|---|
| Saline (n=6) | 6 (100) | 4 (67.7) | 1 (16.7) | 1 (16.7) | 0 (0) | 1 (16.7) |
| 25 mg/kg (n=9) | 8 (88.9) | 1 (11.1) | 1 (11.1) | 2 (22.2) | 1 (11.1) | 1 (11.1) |
| 50 mg/kg (n=8) | 8 (100) | 0 (0) | 0 (0) | 3 (37.5) | 1 (12.5) | 2 (25) |
| 200 mg/kg (n=9) | 8 (88.9) | 3 (33.3) | 0 (0) | 3 (33.3) | 1 (11.1) | 0 (0) |
Incidence of various co-morbidities observed in post-mortem gross necropsy is expressed in absolute numbers and as a percent of the total number of rats necropsied in each group. Necropsy was not performed on the first 4 rats in the study nor on those that died spontaneously before the end of the study on day 14. “Other” co-morbidities included dermal abscess, pancreatic tumors, and abdominal icterus.
Discussion
This is the first study evaluating neuroprotective effects of taurine following injury to the aged brain. Normal aging is associated with worse TBI outcomes due to exacerbated injury mechanisms including edema, inflammation, oxidative stress, blood-brain barrier disruption and mitochondrial dysfunction (Gilmer et al. 2010; Onyszchuk et al. 2008; Sandhir et al. 2008; Timaru-Kast et al. 2012; Unterberg et al. 1994). We selected taurine as a promising therapeutic candidate for TBI in older individuals due to its osmoregulatory, anti-inflammatory, antioxidant, anti-apoptotic and neurotrophic properties. Moreover, several preclinical studies have reported neuroprotective effects of taurine in TBI (Su et al. 2014; Sun et al. 2015; Wang et al. 2016), as well as in cerebral stroke, retinal degeneration, epilepsy, and spinal cord injury (Froger et al. 2014; Gupta et al. 2006; Oja and Saransaari 2013; Ricci et al. 2009; Ripps and Shen 2012; Wu and Prentice 2010; Ye et al. 2013). However, these studies used young adult male rats or mice. Research using older animal models to study cerebral injury and disease remains scarce, but two studies in experimental stroke showed that therapies demonstrating neuroprotection in young rats failed to confer similar benefits in aged rats (Tan et al. 2009; Won et al. 2006). In this study we sought to determine whether taurine, a promising therapeutic candidate in young rodents, was also neuroprotective in older rats with TBI.
We performed a dose-response study of the effects of taurine on tissue loss and functional outcomes following CCI in aged rats using best practices of rigor and reproducibility including use of an appropriate preclinical model, sample sizes determined a priori, randomization of subjects, experimenter blinding, and pre-determined exclusion criteria. We found no significant brain tissue sparing by taurine in aged rats following TBI. Using our data on D14 tissue loss in saline-treated and 200 mg/kg taurine-treated aged rats, we can calculate an effect size of 0.88, which would require a sample size of 20 to yield significance with 80% power according to our current study design. This suggests that future studies of neuroprotective therapies in aged animals might require larger sample sizes than those performed in young adults.
None of the doses of taurine we tested significantly improved performance on the adhesive removal or beam walk tests. On the adhesive removal test, unilateral CCI resulted in a large and significant increase in contact and removal times of the contralateral limb that persisted to 14 days post-TBI. In contrast, the ipsilateral limb contact and removal times were faster and resolved more rapidly to baseline. Although not frequently reported in animal studies of TBI, separating ipsilateral from contralateral limb performance distinguishes effects specific to the unilateral injury from non-specific effects of the anesthesia or surgery (Peterson et al. 2015a; Peterson et al. 2015b; Simon-O’Brien et al. 2016; Hoane et al. 2011; Kuypers and Hoane 2010).
In contrast to a previous study demonstrating functional improvements in aged mice following 8 months of oral supplementation with 0.05% taurine (El Idrissi et al. 2013), our study in aged rats with TBI did not find behavioral improvements with one week of intraperitoneal taurine administration. Longer-term administration may be required, or it is possible that the pathophysiological stresses of TBI may exacerbate functional impairment beyond that of normal aging to the point that taurine neuroprotection is limited.
Our finding that taurine does not confer significant neuroprotection in aged rats also contrasts with previous studies of TBI in young adult rats that all demonstrated neuroprotection (Gu et al. 2015; Su et al. 2014; Sun et al. 2015; Wang et al. 2016). This could be attributed to: 1) differences in age of the animals, 2) methodological differences in injury model, outcome measures, dose and/or route of taurine administration, or 3) different experimental standards of scientific rigor, as discussed below.
Effects of aging on neuroprotection
Exacerbated injury mechanisms including edema, inflammation, oxidative stress, blood-brain barrier disruption and mitochondrial dysfunction may contribute to the aging-dependent decline in TBI outcomes. Aging could also affect the absorption, distribution, metabolism and excretion of drugs, potentially contributing to differences in neuroprotection. The doses of taurine we tested were based on previous reports in young adult rats (Su et al. 2014; Sun et al. 2015; Wang et al. 2016). Dose-response studies found that an intravenous (i.v.) 50 mg/kg dose of taurine mediated optimal neuroprotection compared to saline controls (Sun and Xu 2008; Sun et al. 2015), whereas additional studies reported that a 200 mg/kg i.v. dose conferred significant tissue and functional benefits in adult male rats (Su et al. 2014; Wang et al. 2016). In contrast, not even our highest dose (200 mg/kg i.p.) significantly reduced brain tissue loss, improved sensorimotor function, or prevented weight loss following TBI in aged rats. It is possible that aged rats may require longer treatment and/or higher doses than young adults to see significant benefit (Mitchell and Anderson 1998; Undie et al. 1995). This underscores the need to understand the effects of age on therapeutic efficacy and pharmacokinetics that will allow a more accurate estimate of the effective dose range and treatment duration of taurine for future evaluation.
In the clinical setting, older individuals suffer higher mortality and poorer outcomes from TBI (Utomo et al. 2009). Elderly individuals often also suffer from other non-neurological co-morbidities including respiratory, cardiovascular and hematologic complications, and acute kidney injury that correlate with worse outcomes following TBI (D. Zygun 2005; D. A. Zygun et al. 2003; Corral et al. 2012). Similar co-morbidities also occur in aged F344 rats, contributing to their clinical relevance as a model to study therapeutic candidates for TBI with aging. Since co-morbidities can affect TBI outcomes, it is possible that they might mask any neuroprotective effects of taurine. However, in this study, the incidence of the various co-morbidities was similar across treatment groups.
Methodological differences in taurine studies
Previous studies that demonstrated neuroprotective efficacy in adult rats used intravenous injection, which raises the possibility that our chosen route of delivery, intraperitoneal, might be a limitation. However, intraperitoneally injected taurine crosses the blood-brain barrier resulting in increased brain levels of taurine in adult mice and rats (Junyent et al. 2009; Lallemand and De Witte 2004). Moreover, magnetic resonance spectroscopy studies in our laboratory have confirmed increases in brain taurine levels of uninjured adult rats within an hour of i.p. taurine injection (unpublished data). Accordingly, we do not believe that target engagement is a problem although it is possible that slower delivery of taurine during the acute post-injury period due to intraperitoneal delivery might have tempered its neuroprotective effect.
Previous taurine studies have used different models of TBI from the current study (Su et al. 2014; Sun et al. 2015; Wang et al. 2016). The weight drop model results in a diffuse injury and lateral fluid percussion produces a mixed focal cortical injury and diffuse subcortical injury, whereas our CCI model produces a focal contusion (Xiong et al. 2013), a frequent feature in accidents and fall-related TBI suffered by the elderly (Aghakhani et al. 2015). Each of these TBI models elicits a unique pattern of molecular, cellular and pathophysiological responses (Mondello et al. 2016) that may affect outcomes. Outcome measures that confirmed taurine neuroprotection in young adult rats included neurological severity score, brain water content, blood-brain barrier integrity, expression of anti-oxidant enzymes and inflammatory cytokines as well as histopathological analysis of neuronal tissue loss. Although it is possible that ex vivo histopathology is a more sensitive measure of tissue sparing than our analysis of T2-weighted MRI, this seems unlikely since previous studies have demonstrated very high correlation between histological and MRI tissue loss (Onyszchuk et al. 2007).
Contrary to published reports on taurine-mediated improvement in neurological function in young adults, we did not observe behavioral improvements in this study. Behavioral tests for aged rodents often need to be adapted from those used for young adults and must account for physiological and motivational differences arising from age (Hanell and Marklund 2014; Kennard and Woodruff-Pak 2011). Whether current methods of testing functional outcomes in aged rodents are adequately sensitive to TBI severity and therapeutic benefits remains an open question.
Scientific rigor in preclinical studies
Our use of a highly rigorous study design might lead to conservative estimates of neuroprotection, as has been seen previously: one study that applied optimal study design to retest eight promising compounds in a murine model of amyotrophic lateral sclerosis found much lower non-significant effects on lifespan extension than was previously reported (Scott et al. 2008). Although previous studies of taurine in young adult rats employed randomization, experimenter blinding was only reported for certain measurements such as neurological severity scores but not for histopathological or immunocytochemical analyses (Su et al. 2014; Sun et al. 2015). Applying the same standards of rigor and methodological approaches in a randomized study of both young and aged rats would be the most direct way to confirm the effects of aging on taurine neuroprotection.
Study Limitations and Future Directions
Given the number of groups involved in this study, we elected to use intra-animal controls to compare pre- and post-injury measures in the same subjects over time, instead of additional sham-injured groups. Additionally, this study did not determine whether taurine alters the expression of molecular targets within cell death, inflammation, oxidative stress or neurotransmission cascades that may affect neuroprotection in aged animals. Whether there are age-related alterations in the taurine transporter TauT at the blood-brain barrier remains to be determined (Kang 2006; Kang et al. 2002). Also, although we focused on sensorimotor functional outcomes because our TBI model causes structural damage to sensorimotor cortex but not hippocampus (Harris et al. 2012), possible effects of injury and treatment on hippocampal-dependent cognitive function in aged rats should be explored in future studies.
Similar to all the previous taurine studies in young adults, we only used male rats based on the higher incidence of TBI in men. However, there is growing evidence that TBI outcomes may vary based on sex (Farace and Alves 2000; Kraus et al. 2000; Slewa-Younan et al. 2004). Furthermore, estrogen receptors may regulate TauT-mediated taurine uptake, and brain taurine concentration is lower in female mice compared to age-matched males (Duarte et al. 2014; Shennan and Thomson 2007). Thus, additional research is needed to evaluate taurine neuroprotection in females.
We tested three doses in this study based on previous reports in young adults. Two of the doses were lower, while one exceeded the highest dose of 10 g/day safely tested in humans (Durelli et al. 1983). Different doses based on appropriate pharmacokinetic and safety studies, and different routes of administration of taurine, need to be tested in future studies with aged animals.
Conclusion
Using best practices for scientific rigor, this study did not demonstrate significant structural or functional neuroprotection by taurine in aged rats with TBI, in contrast to previous findings in younger animals. Advanced age is an important, but often overlooked, variable in TBI drug development given the higher incidence, poorer outcomes, and complex mechanisms involved in TBI in older individuals. Preclinical studies that better reflect the demographics, such as age and sex, of human TBI will be critical for successfully translating therapies from bench to bedside.
Acknowledgments
We thank Dr. Allison Neely at the Laboratory Animal Resources facility at the University of Kansas Medical Center for performing animal necropsies.
Funding: This study was supported by funding from the National Institutes of Health (R21 NS091920 to Dr. Janna Harris and P30 AG035982 to the University of Kansas Alzheimer’s Disease Center) and a KUMC Lied Basic Science grant awarded to Dr. Harris. The Hoglund Brain Imaging Center is supported by Forrest and Sally Hoglund and the University of Kansas School of Medicine. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors
Contributor Information
Raeesa Gupte, Hoglund Brain Imaging Center, University of Kansas Medical Center, KS 66160, USA, 913-588-3519, rgupte@kumc.edu.
Sarah Christian, Hoglund Brain Imaging Center, University of Kansas Medical Center, KS 66160, USA, 913-588-9070, schristian@kumc.edu.
Paul Keselman, Hoglund Brain Imaging Center, University of Kansas Medical Center, KS 66160, USA, 913-588-9079, pkeselman@kumc.edu.
Joshua Habiger, Department of Biostatistics, University of Kansas Medical Center, KS 66160, USA, 405-744-9657, jhabige@okstate.edu.
William M. Brooks, Department of Neurology, Director, Hoglund Brain Imaging Center, Director, University of Kansas Alzheimer’s Disease Center Neuroimaging Core, University of Kansas Medical Center, KS 66160, USA, 913-588-9075, wbrooks@kumc.edu.
Janna L. Harris, Department of Anatomy & Cell Biology, Director, Animal Magnetic Resonance Imaging Core, Hoglund Brain Imaging Center, University of Kansas Medical Center, KS 66160, USA, 913-588-9076, jharris2@kumc.edu.
References
- Aghakhani K, Heidari M, Ameri M, Mehrpisheh S, Memarian A. Characteristics of Traumatic Brain Injury among Accident and Falling Down Cases. Acta Med Iran. 2015;53(10):652–655. [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. II. Taurine and small neutral amino acids. Neurochem Res. 1989;14(6):563–570. doi: 10.1007/BF00964919. [DOI] [PubMed] [Google Scholar]
- Begley CG. Six red flags for suspect work. Nature. 2013;497(7450):433–434. doi: 10.1038/497433a. [DOI] [PubMed] [Google Scholar]
- Benedetti MS, Russo A, Marrari P, Dostert P. Effects of ageing on the content in sulfur-containing amino acids in rat brain. J Neural Transm Gen Sect. 1991;86(3):191–203. doi: 10.1007/BF01250705. [DOI] [PubMed] [Google Scholar]
- Bouzat P, Francony G, Thomas S, Valable S, Mauconduit F, Fevre MC, et al. Reduced brain edema and functional deficits after treatment of diffuse traumatic brain injury by carbamylated erythropoietin derivative. Crit Care Med. 2011;39(9):2099–2105. doi: 10.1097/CCM.0b013e31821cb7b2. [DOI] [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, et al. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. MMWR Surveill Summ. 2011;60(5):1–32. [PubMed] [Google Scholar]
- Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Manez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care. 2012;16(2):R44. doi: 10.1186/cc11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JM, Do KQ, Gruetter R. Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging. 2014;35(7):1660–1668. doi: 10.1016/j.neurobiolaging.2014.01.135. [DOI] [PubMed] [Google Scholar]
- Durelli L, Mutani R, Fassio F. The treatment of myotonia: evaluation of chronic oral taurine therapy. Neurology. 1983;33(5):599–603. doi: 10.1212/wnl.33.5.599-a. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Shen CH, L’Amoreaux WJ. Neuroprotective role of taurine during aging. Amino Acids. 2013;45(4):735–750. doi: 10.1007/s00726-013-1544-7. [DOI] [PubMed] [Google Scholar]
- Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93(4):539–545. doi: 10.3171/jns.2000.93.4.0539. [DOI] [PubMed] [Google Scholar]
- Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015;127:3–13. doi: 10.1016/B978-0-444-52892-6.00001-5. [DOI] [PubMed] [Google Scholar]
- Fiette L, Slaoui M. Necropsy and sampling procedures in rodents. Methods Mol Biol. 2011;691:39–67. doi: 10.1007/978-1-60761-849-2_3. [DOI] [PubMed] [Google Scholar]
- Froger N, Moutsimilli L, Cadetti L, Jammoul F, Wang QP, Fan Y, et al. Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Prog Retin Eye Res. 2014;41:44–63. doi: 10.1016/j.preteyeres.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Gilmer LK, Ansari MA, Roberts KN, Scheff SW. Age-related mitochondrial changes after traumatic brain injury. J Neurotrauma. 2010;27(5):939–950. doi: 10.1089/neu.2009.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Zhao Y, Qian K, Sun M. Taurine attenuates hippocampal and corpus callosum damage, and enhances neurological recovery after closed head injury in rats. Neuroscience. 2015;291:331–340. doi: 10.1016/j.neuroscience.2014.09.073. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Seki Y, Yosida J. Role of taurine in spinal cord injury. Curr Neurovasc Res. 2006;3(3):225–235. doi: 10.2174/156720206778018776. [DOI] [PubMed] [Google Scholar]
- Hanell A, Marklund N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front Behav Neurosci. 2014;8:252. doi: 10.3389/fnbeh.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JL, Choi IY, Brooks WM. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci. 2015;7:202. doi: 10.3389/fnagi.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, et al. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J Cereb Blood Flow Metab. 2012;32(12):2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Swan AA, Heck SE. The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav Brain Res. 2011;223(1):119–124. doi: 10.1016/j.bbr.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junyent F, Utrera J, Romero R, Pallas M, Camins A, Duque D, et al. Prevention of epilepsy by taurine treatments in mice experimental model. J Neurosci Res. 2009;87(6):1500–1508. doi: 10.1002/jnr.21950. [DOI] [PubMed] [Google Scholar]
- Kang YS. The effect of oxidative stress on the transport of taurine in an in vitro model of the blood-brain barrier. Adv Exp Med Biol. 2006;583:291–298. doi: 10.1007/978-0-387-33504-9_32. [DOI] [PubMed] [Google Scholar]
- Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T. Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J Neurochem. 2002;83(5):1188–1195. doi: 10.1046/j.1471-4159.2002.01223.x. [DOI] [PubMed] [Google Scholar]
- Kennard JA, Woodruff-Pak DS. Age sensitivity of behavioral tests and brain substrates of normal aging in mice. Front Aging Neurosci. 2011;3:9. doi: 10.3389/fnagi.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Bramlett HM, Shear DA, Dixon CE, Mondello S, Dietrich WD, et al. Synthesis of Findings, Current Investigations, and Future Directions: Operation Brain Trauma Therapy. J Neurotrauma. 2016;33(6):606–614. doi: 10.1089/neu.2015.4133. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Peek-Asa C, McArthur D. The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation. Neurosurg Focus. 2000;8(1):e5. doi: 10.3171/foc.2000.8.1.156. [DOI] [PubMed] [Google Scholar]
- Kuypers NJ, Hoane MR. Pyridoxine administration improves behavioral and anatomical outcome after unilateral contusion injury in the rat. J Neurotrauma. 2010;27(7):1275–1282. doi: 10.1089/neu.2010.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F, De Witte P. Taurine concentration in the brain and in the plasma following intraperitoneal injections. Amino Acids. 2004;26(2):111–116. doi: 10.1007/s00726-003-0058-0. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Grider M. The effect of mild post-stroke exercise on reactive neurogenesis and recovery of somatosensation in aged rats. Exp Neurol. 2010;226(1):58–67. doi: 10.1016/j.expneurol.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Anderson KJ. Age-related changes in [3H]MK-801 binding in the Fischer 344 rat brain. Neurobiol Aging. 1998;19(3):259–265. doi: 10.1016/s0197-4580(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Mondello S, Shear DA, Bramlett HM, Dixon CE, Schmid KE, Dietrich WD, et al. Insight into Pre-Clinical Models of Traumatic Brain Injury Using Circulating Brain Damage Biomarkers: Operation Brain Trauma Therapy. J Neurotrauma. 2016;33(6):595–605. doi: 10.1089/neu.2015.4132. [DOI] [PubMed] [Google Scholar]
- Oja SS, Saransaari P. Taurine and epilepsy. Epilepsy Res. 2013;104(3):187–194. doi: 10.1016/j.eplepsyres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160(2):187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25(2):153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Ordaz B, Ochoa LD. Mechanisms counteracting swelling in brain cells during hyponatremia. Arch Med Res. 2002;33(3):237–244. doi: 10.1016/s0188-4409(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Pascual JM, Solivera J, Prieto R, Barrios L, Lopez-Larrubia P, Cerdan S, et al. Time course of early metabolic changes following diffuse traumatic brain injury in rats as detected by (1)H NMR spectroscopy. J Neurotrauma. 2007;24(6):944–959. doi: 10.1089/neu.2006.0190. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; [DOI] [PubMed] [Google Scholar]
- Peterson TC, Hoane MR, McConomy KS, Farin FM, Bammler TK, MacDonald JW, et al. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery following Traumatic Brain Injury. J Neurotrauma. 2015a;32(11):765–779. doi: 10.1089/neu.2014.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TC, Maass WR, Anderson JR, Anderson GD, Hoane MR. A behavioral and histological comparison of fluid percussion injury and controlled cortical impact injury to the rat sensorimotor cortex. Behav Brain Res. 2015b;294:254–263. doi: 10.1016/j.bbr.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci L, Valoti M, Sgaragli G, Frosini M. Protection by taurine of rat brain cortical slices against oxygen glucose deprivation- and reoxygenation-induced damage. Eur J Pharmacol. 2009;621(1–3):26–32. doi: 10.1016/j.ejphar.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213(2):372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann MU, Stiller D, Skardelly M, Bernarding J, Klinge PM, Samii A, et al. Metabolic changes in the vicinity of brain contusions: a proton magnetic resonance spectroscopy and histology study. J Neurotrauma. 2003;20(8):725–743. doi: 10.1089/089771503767869962. [DOI] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9(1):4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol. 2008;50(3):376–399. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Shennan DB, Thomson J. Estrogen regulation and ion dependence of taurine uptake by MCF-7 human breast cancer cells. Cell Mol Biol Lett. 2007;12(3):396–406. doi: 10.2478/s11658-007-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-O’Brien E, Gauthier D, Riban V, Verleye M. Etifoxine improves sensorimotor deficits and reduces glial activation, neuronal degeneration, and neuroinflammation in a rat model of traumatic brain injury. J Neuroinflammation. 2016;13(1):203. doi: 10.1186/s12974-016-0687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Trivedi R, Haridas S, Manda K, Khushu S. Study of neurometabolic and behavioral alterations in rodent model of mild traumatic brain injury: a pilot study. NMR Biomed. 2016;29(12):1748–1758. doi: 10.1002/nbm.3627. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Green AM, Baguley IJ, Gurka JA, Marosszeky JE. Sex differences in injury severity and outcome measures after traumatic brain injury. Arch Phys Med Rehabil. 2004;85(3):376–379. doi: 10.1016/j.apmr.2003.05.007. [DOI] [PubMed] [Google Scholar]
- Steward O, Balice-Gordon R. Rigor or mortis: best practices for preclinical research in neuroscience. Neuron. 2014;84(3):572–581. doi: 10.1016/j.neuron.2014.10.042. [DOI] [PubMed] [Google Scholar]
- Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, et al. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience. 2014;266:56–65. doi: 10.1016/j.neuroscience.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Sun M, Xu C. Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol. 2008;28(4):593–611. doi: 10.1007/s10571-007-9183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zhao Y, Gu Y, Zhang Y. Protective effects of taurine against closed head injury in rats. J Neurotrauma. 2015;32(1):66–74. doi: 10.1089/neu.2012.2432. [DOI] [PubMed] [Google Scholar]
- Tan Z, Li X, Kelly KA, Rosen CL, Huber JD. Plasminogen activator inhibitor type 1 derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res. 2009;1281:84–90. doi: 10.1016/j.brainres.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54(10):1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timaru-Kast R, Luh C, Gotthardt P, Huang C, Schafer MK, Engelhard K, et al. Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One. 2012;7(8):e43829. doi: 10.1371/journal.pone.0043829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undie AS, Wang HY, Friedman E. Decreased phospholipase C-beta immunoreactivity, phosphoinositide metabolism, and protein kinase C activation in senescent F-344 rat brain. Neurobiol Aging. 1995;16(1):19–28. doi: 10.1016/0197-4580(95)80004-b. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Schneider GH, Gottschalk J, Lanksch WR. Development of traumatic brain edema in old versus young rats. Acta Neurochir Suppl (Wien) 1994;60:431–433. doi: 10.1007/978-3-7091-9334-1_117. [DOI] [PubMed] [Google Scholar]
- Utomo WK, Gabbe BJ, Simpson PM, Cameron PA. Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury. 2009;40(9):973–977. doi: 10.1016/j.injury.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V, Garg SK, Banerjee R. Taurine biosynthesis by neurons and astrocytes. J Biol Chem. 2011;286(37):32002–32010. doi: 10.1074/jbc.M111.253344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Fan W, Cai Y, Wu Q, Mo L, Huang Z, et al. Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids. 2016;48(9):2169–2177. doi: 10.1007/s00726-016-2244-x. [DOI] [PubMed] [Google Scholar]
- Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, et al. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006;1123(1):237–244. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Prentice H. Role of taurine in the central nervous system. J Biomed Sci. 2010;17(Suppl 1):S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Zhuo J, Racz J, Shi D, Roys S, Fiskum G, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. J Neurotrauma. 2011;28(10):2091–2102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye HB, Shi HB, Yin SK. Mechanisms underlying taurine protection against glutamate-induced neurotoxicity. Can J Neurol Sci. 2013;40(5):628–634. doi: 10.1017/s0317167100014840. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu H, Wu J, Zhang X, Liu M, Wang Y. Metabonomic alterations in hippocampus, temporal and prefrontal cortex with age in rats. Neurochem Int. 2009;54(8):481–487. doi: 10.1016/j.neuint.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zygun D. Non-neurological organ dysfunction in neurocritical care: impact on outcome and etiological considerations. Curr Opin Crit Care. 2005;11(2):139–143. doi: 10.1097/01.ccx.0000155356.86241.c0. [DOI] [PubMed] [Google Scholar]
- Zygun DA, Doig CJ, Gupta AK, Whiting G, Nicholas C, Shepherd E, et al. Non-neurological organ dysfunction in neurocritical care. J Crit Care. 2003;18(4):238–244. doi: 10.1016/j.jcrc.2003.10.007. [DOI] [PubMed] [Google Scholar]