Abstract
In its most basic conception, a novelty is simply something new. However, when many previously proposed evolutionary novelties have been illuminated by genetic, developmental, and fossil data, they have refined and narrowed our concept of biological “newness.” For example, they show that these novelties can occur at one or multiple levels of biological organization. Here, we review the identity of structures in the avian vocal organ, the syrinx, and bring together developmental data on airway patterning, structural data from across tetrapods, and mathematical modeling to assess what is novel. In contrast with laryngeal cartilages that support vocal folds in other vertebrates, we find no evidence that individual cartilage rings anchoring vocal folds in the syrinx have homology with any specific elements in outgroups. Further, unlike all other vertebrate vocal organs, the syrinx is not derived from a known valve precursor, and its origin involves a transition from an evolutionary “spandrel” in the respiratory tract, the site where the trachea meets the bronchi, to a target for novel selective regimes. We find that the syrinx falls into an unusual category of novel structures: those having significant functional overlap with the structures they replace. The syrinx, along with other evolutionary novelties in sensory and signaling modalities, may more commonly involve structural changes that contribute to or modify an existing function rather than those that enable new functions.
Keywords: bioacoustics, vocal communication, tracheal rings, birds, tetrapods
Several novel traits have played key roles in the evolution of birds, including, for example, feathers, flight, and song (1). While analyses have traced the deep origins of the genes involved in feather development, and fossils have illuminated the evolutionary paths to branched feathers and to the origin of flight, little similar work has been undertaken to approach the complex novelty of bird vocal production. Further, while avian vocalization has evolved through changes in both the neural control of vocalization and the vocal structures themselves, comparatively little attention has been paid to the latter. Here, we focus on one critically understudied and undertheorized aspect of avian evolutionary novelty, the origin of the evolutionarily novel sound-producing organ of birds, the syrinx. We will discuss the evolution of the avian syrinx at multiple levels of biological organization—developmental, structural, and functional—and consider on which of these levels, if any, the syrinx has homologs in nonavian taxa. We will go on to show how an integrated approach incorporating data and perspectives from developmental biology, vocal physiology, and paleontology defines a promising agenda for understanding the evolution of the syrinx.
A New Structure Without a New Function
Assessing novelty in a structure requires knowledge of potentially homologous structures present in an individual, in outgroups, or estimated in ancestral taxa (2–4). The extant sister taxon of birds, crocodilians, communicates with sounds produced via vocal folds in the larynx (5). Outgroup reptiles also are capable of vocal communication using the same structures (6, 7). However, sometime before the origin of crown birds, the vocal function of the larynx was lost, and a novel structure, the syrinx, was gained at the tracheobronchial juncture (Figs. 1 and 2) (8). Given that the larynx is not involved in sound production in birds, there are two scenarios for syrinx evolution. Novel selective regimes unrelated to sound production could have led to the loss of sound production in the larynx, and subsequently a new vocal organ arose in a new position under selection for acoustic function. Alternatively, vocal folds could have remained viable in the larynx (perhaps with reduced or diminishing efficacy), but a syrinx evolved to supplement sound production, which was followed by loss of the larynx sound source.
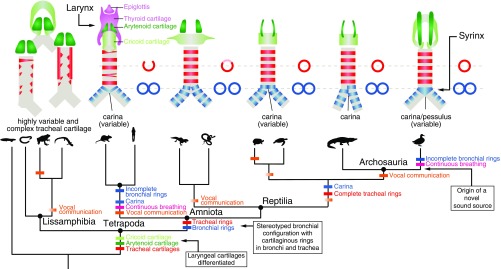
Fig. 1.
Tetrapod phylogeny showing the sequence of the acquisition of different airway traits through evolutionary time. (Upper, Left to Right) Schematic diagrams depict airway morphology in caecilians, frog, salamander, rat, gecko, tortoise, alligator, and a duck. Paired arytenoid cartilages (dark green) are present in most tetrapods (26). Cricoid cartilage (light green) is absent in some lissamphibians. Tracheal cartilage morphologies (red) are highly irregular (i.e., not ring-shaped) in lissamphibians (29), and irregular, forked rings are also sometimes observed in mammals (88). Stereotyped configuration of trachea and paired bronchi is common to all amniotes, and cartilaginous rings are observed in both the bronchi and trachea (49). Fusion of bronchial rings (blue) at the tracheobronchial juncture forms a carina, or a pessulus in birds (26). (Lower) Colored dashes indicate the branches along which distinct morphological and behavioral innovations (15, 61) may have evolved, with uncertainty (i.e., variability among species in a group) indicated by color gradients. Boxes describe major transitions leading to a syrinx in modern birds.
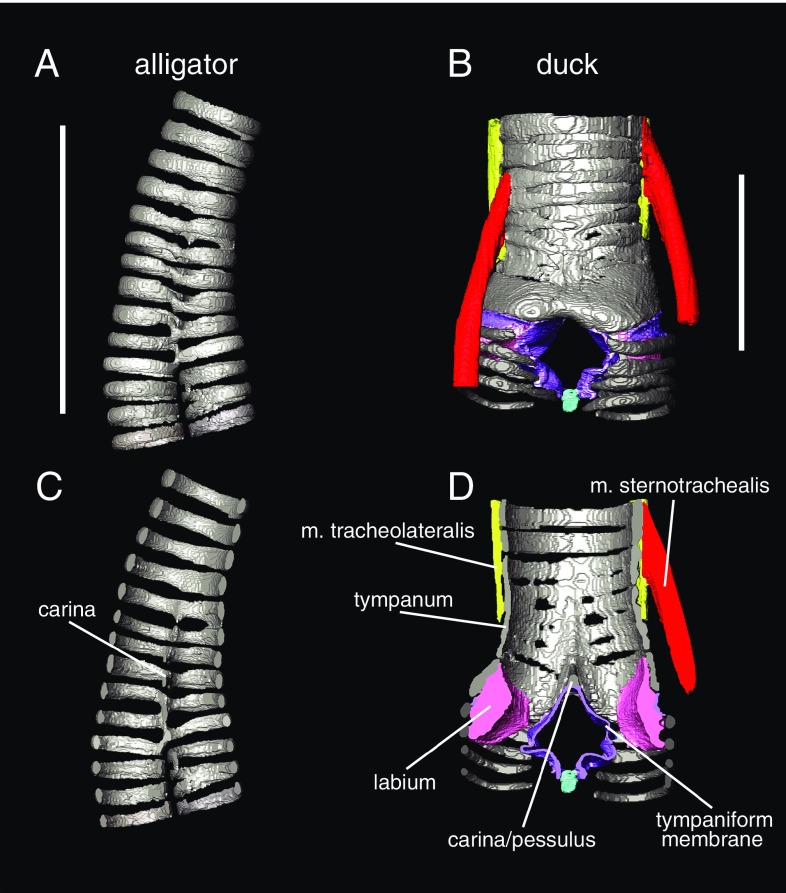
Fig. 2.
3D morphology of airway cartilage in archosaurs visible with diffusible iodine-based contrast-enhanced computed tomography (diceCT). 3D models of tracheal cartilage structure in the alligator (Alligator mississippiensis) (A and C) and Muscovy duck (Cairina moschata) (B and D) in gray. Panels show both external (A and B) and cross-sectional views of the tracheobronchial juncture (C and D). Soft tissue anatomy is clearly visible in B and D, including both intrinsic syringeal muscles (red and yellow), membranes (purple), and labia/vocal folds (pink). Specimens were dissected out, stained following ref. 109, and scanned at The University of Texas High-Resolution Computed Tomography Facility. Image segmentation was done in Avizo 6.3 (FEI Visualization Sciences Group). See ref. 8 for further details on staining and scanning parameters. (Scale bars: 2 cm.)
The fossil record presently does not allow definitive assessment of whether there was a period in the evolution of stem bird-line taxa, including dinosaurs, when ability for acoustic communication via the larynx was lost before sound production via a syrinx was gained. Senter (9) proposed that, because the vocal organs of crocodilians and birds are different, they evolved independently from a nonvocal ancestor. If this were true, sound production in the bird lineage would involve a new structure arising in a different location that converged on the same function (i.e., sound production via vocal folds) that is present in other tetrapods. In this view, Crocodilians are inferred to represent an additional case of evolution of sound production via a larynx, separate from the evolution of the larynx in other tetrapods. What evidence we do have, however, does not appear to support this scenario. Among extant tetrapods, vocal communication may or may not be ancestral to lepidosaurs, but there is increasing evidence that it was present in many turtles, the closest outgroup of crocodilians and birds (10, 11). Other behaviors in which vocal communication is deployed, such as posthatching parental care and feeding of young, have consistently been inferred to be homologous in turtles, birds, and crocodilians (12–14).
Apparent independent gains in the auditory capability early in all major lineages of dinosaurs (15) seem to suggest continued selection for improved hearing, most often linked to communication with congeners (as well as predator evasion and prey detection). The development of apparent bony resonating cavities linked to the nasal cavities of some ornithischian dinosaurs (16) and proposed auditory roles for sinuses in theropod dinosaurs (17) also support selection for the maintenance, if not for elaboration, of acoustic communication. Data on dinosaurian outgroups, including pterosaurs, is more limited. It is possible that auditory capabilities were more limited but not dissimilar to the relatively vocal extant crocodilians (15). However, it is clear that extant crocodilians have an auditory system adequate for the use of acoustic communication (18). The only Mesozoic fossil record of a syrinx is from a crown avian species related to Anseriformes (8) and thus cannot speak to this question.
In other major transitions, such as the origin of weight-bearing limbs from fins and the modification of limbs into wings, the function of one or more structures changes (2). Embedded in some concepts of evolutionary novelty is the notion that a feature at its origin enables a novel function (e.g., ref. 19). However, in the origin of the syrinx there does not appear to be an origin of a novel function. There is no evidence that the ancestral syrinx, a simple structure (discussed further below), could produce calls with a larger frequency range or longer or louder calls than an alligator larynx. Furthermore, across all other tetrapods the larynx has been modified to produce a wide range of complex sounds and calls. The core question posed in this context, then, is why would a novel structure evolve to maintain the same function, acoustic communication? The answer may inform our understanding of novelty in distinct systems (e.g., in locomotory, sensory, or signaling contexts).
In acoustic signaling, the addition of supplementary sound-producing mechanisms is not uncommon. Mechanical sound production (e.g., in flight or via vibration of water or stamping or shaking) can supplement vocal fold-produced acoustic signals (20). Supralaryngeal vibratory sound-producing tissues have arisen in the palate in koalas (21) and in the nasal region of odontocete whales (22–24), while the larynx is retained as a sound source. Only in birds has the ancestral larynx sound source been lost. Phenotypes that arise within dinosaurs such as neck elongation or proposed respiratory, metabolic, and ecological shifts associated with flight should be considered as potential drivers behind both syrinx gain and loss of a sound source in the larynx.
Phylogeny and Identity in the Respiratory Tract
In 1872 T. H. Huxley wrote “Birds possess a larynx in the ordinary position, but it is another apparatus, the lower larynx or syrinx, developed at the end of the trachea that is their great vocal organ” (25, p. 93). This sentence, in which Huxley first gives the name “syrinx” to the avian vocal organ, introduces two fundamental issues of homology at the level of adult morphology: First, what is a syrinx without reference to a vocal function? Or perhaps more simply: How does one define a syrinx morphologically? Second, and more important for our discussion of homology: How is a syrinx related to the larynx, the major vocal organ in all other tetrapods?
The larynx and syrinx do not appear to be homologous at a structural level, as there is no evidence of historical continuity between these two organs, and they are copresent in the same organism in different anatomical positions. (Birds possess both a larynx and a syrinx, but birds use only the latter to vocalize.) The larynx is a valve closing the terminus of the airway in air-breathing vertebrates. Unsupported by cartilage in its simplest form (i.e., a fissure in air-breathing nontetrapod sarcopterygians, or lungfish), at least one or two pairs of cartilage elements ancestrally support the valve-closing tissues and associated muscles in tetrapods (Fig. 1) (26). While its primary function in the earliest stem tetrapod has been assumed to be as a valve, it was already co-opted for a vocal function in multiple groups of lissamphibians (27). In these taxa, cutaneous gas exchange remains important (contrasted with gas exchange in the lungs), and the juncture(s) between the trachea and bronchi that extend to the lungs are not highly stereotyped; from one to three lungs can be present with a trachea variable in length (28). Cartilaginous support of the airway in lissamphibians is also highly varied and does not generally extend to the bronchi (Fig. 1) (29–31). Larynx cartilages, by contrast, are always present and have distinct shapes and topology-based identities (cricoid, arytenoid, and, to a lesser extent, the thyroid cartilage in mammals) across tetrapods, including birds and mammals (Fig. 1) (26). Muscles arising from the hyoid and larynx cartilages that extend to the softer folds of the larynx valve (regardless of a vocal function) also are broadly homologous even in the highly modified larynges of frogs, baleen whales, and primates (26).
As potential serial homologs at a structural level, the syrinx and larynx fail even superficial criteria of similarity of parts (Fig. 1), although they share a similar function as vocal organs where such facility is present. A syrinx is never located at the terminus of the airway where a larynx is situated (Fig. 1). Tracheobronchial (and thus, syrinx-associated) airway cartilages have a separate and later phylogenetic origin (in the ancestral amniote) (Fig. 1) than the larynx-associated cartilages (in the ancestral tetrapod) (Fig. 1). Attempts to homologize individual cartilage elements in the syrinx just across crown birds (Aves) have been largely unsuccessful (32–34).
The vocal folds of the larynx, when present, are modifications of connective tissues of the valve closing off the airway during swallowing (35, 36). Although the exact histological composition of vocal folds is species-specific (36–39), vocal folds across amniotes are multilayered structures, regardless of whether they are located in a syrinx or larynx. The similarity of sound-generation mechanisms (40–42), and thus the physical requirements of compliance and stiffness, likely constrain vocal-fold morphologies (43).
The muscles that move these vocal folds attach to the cartilaginous framework of the larynx. Only two muscles associated with the syrinx, the musculus tracheolateralis and musculus sternotrachealis, are inferred to be present in the crown avian ancestor (Fig. 2) (8). These muscles are homologs of muscles that in nonavian outgroup taxa extend from the tongue elements to the pectoral girdle (musculus sternohyoideus) (44, 45). The presence of these muscles alongside laryngeal muscles supports a lack of homology between muscles controlling laryngeal and syringeal sound production. No muscles extend to the tracheobronchial juncture in any outgroup; that is, any taxon lacking a syrinx. As noted above, the proposed homology of these muscles with a muscle that spans from the hyoid apparatus to the sternum (45) is consistent with this shift (insertion on the trachea or at the tracheobronchial juncture is a novelty uniquely associated with syrinx origin) (Fig. 2). Within birds, more novel intrinsic muscles arise (e.g., ref. 32), but these appear to represent later novelties in the crown clade.
New biological structures are generated by tinkering with precursors. Some notions of evolutionary innovation consider all derived conditions (46) to be novelties. Other, more stringent definitions of novelty require that a putatively novel structure have neither homology (presence in a common ancestor) with any structure in an outgroup nor serial homology (homonymy) to any structure within the body. Regardless of the conditions under which the syrinx evolved, it passes these tests of novelty, at least at the structural level.
To identify what is structurally new in the case of syrinx evolution, we can compare the homologous region of the airway, the tracheobronchial juncture, in outgroup taxa. These comparisons require estimation of the ancestral condition within Aves to parse what changed at the origin of a syrinx as opposed to later morphological diversification. Because the syrinx was originally defined functionally as a vocal organ situated at the tracheobronchial juncture, it followed that features of this juncture have been treated as vocal organ-specific tissues rather than being considered in the light of outgroup airway features. Comparing broader variation in the airway across tetrapods (Fig. 1) informs what changes in shape observed in the avian airway relative to close reptilian relatives may be unrelated to syrinx origin but potentially related to changes in respiration and metabolic rate also known to have occurred at the origin of birds (1).
Modifications of the cartilaginous ring structures and the spaces between them have been given unique names (e.g., “pessulus,” “tympanum,” and “medial tympaniform membrane”) (Fig. 2) that have obscured their relationship with similar structures unrelated to vocal production in other tetrapods (i.e., the carina and the bronchial wall). As shown in Fig. 1, the pessulus, a keeled midline element that occurs where the airway branches into the bronchi, was named for its proposed function as a support to certain vocal-fold conformations, but it is not unique to birds, nor is it inferred to be present in the ancestral syrinx (8). Stein (47) assumed that the presence of a pessulus in songbirds was important for the ability to generate two independent sounds simultaneously. However, two-voice vocalization is possible without a pessulus (e.g., in larks) (32). Crocodilians also show midline fusion of rings (not differentially mineralized) at the tracheobronchial juncture (Fig. 2). This fusion has been named the “carina” (48) when present in other vertebrates, including crocodilians and humans (Fig. 1). Both the pessulus and the carina have been proposed to provide stabilization for the cartilaginous framework of the bronchi as they merge into the trachea (e.g., refs. 47, 49, and 50).
Differentially mineralized airway cartilages at the tracheobronchial juncture, connective tissue between these cartilages, two associated external muscles (m. tracheolateralis and m. sternotrachealis), and incomplete bronchial half rings are structures novel in birds relative to outgroups (8). Whether two paired sound sources (located within the bronchi) or one (at the tracheobronchial juncture or in the trachea) occurred first is ambiguously optimized given variation in early-branching lineages of living birds (8), and the sound production via thin membranes or thickened labia is similarly unclear given homoplasy among these basally divergent extant lineages.
Parsing which structures are minimally present when the tracheobronchial juncture is first modified for sound production must remain tentative. Further fossil data and comparisons with outgroup taxa may inform whether initial modifications for other purposes (e.g., support during continuous breathing or related to flight) may have been exapted in the formation of a vocal organ at this location. For example, the only unpaired air sac in birds (51), the interclavicular air sac, consistently surrounds the syrinx in crown birds and has been described as essential to its vocal function (e.g., ref. 9). However, vocal production can occur in its absence, although in living birds it does help maintain pressure around the phonating syrinx (e.g., ref. 52). Whether the air sac arose before, at, or after syrinx origin is unclear. Finally, despite limited data, the open space between the endpoints of bronchial half rings (the medial tympaniform membrane) (Fig. 2B) that may be involved in sound production in birds (40, 53) was recently found in other distantly related taxa (e.g., snakes) in contexts unrelated to sound production.
The syrinx appears to be unique among tetrapod vocal organs in one key respect: No valve-like or vibratory membranes (e.g., labia) are known to be present in the tracheobronchial juncture of any tetrapods before syrinx origin. Indeed, in the only known instances of syrinx loss in birds (54), labia or compliant membranes in the airway are also lost, suggesting that such structures have no function in regular avian respiration. However, the presence of incomplete bronchial rings and associated medial membranes is maintained. The persistence of these compliant or potentially distensible walls in the absence of a vocal function suggests the possibility of an identity unrelated to that function, but it does not appear to have been as a valve. The syrinx origin from a nonvalve precursor is in stark contrast to the modifications of narial valves in marine mammals into the phonic lips of odontocetes (55) or the modification of a choana (internal nares), which may be a functional valve (56, 57), into additional vibratory folds in the soft palate of koalas (21). As we discuss below, this distinction may be key to understanding both structural and developmental differences in the syrinx.
From Spandrel to Evolutionary Key Innovation
The nature of airway patterning creates a fundamentally different set of questions about the identity and potential selective regimes for the syrinx compared with other tetrapod vocal organs. The region homologous to a syrinx in outgroups, the tracheobronchial juncture, has identity as only one part of a continuous airway. Its presence is the consequence of the bifurcation of the airway from the single tube of the trachea into the two tubes of the bronchi, and it does not appear to possess an independent character. We argue that in the origin of the syrinx, the tracheobronchial juncture goes from a “spandrel” (58) of sorts, a structure shaped by the requirements of a functioning airway but without a specific function, into a key site of novel, vocal function-selective regimes.
Because the tracheobronchial juncture is a structural necessity in a bifurcated airway, the first juncture was present at the origin of multiple lungs in ancient tetrapods (28). However, when continuous breathing and an increase in metabolic rate evolved in bird-lineage archosaurs (Fig. 1 and see Fig. 4) (1), airflow patterns changed. Discontinuous and continuous breathing expose the airway walls to substantially different amounts of wall shear stress, which is a function of flow velocity characteristics adjacent to the surface (59, 60). In continuous breathers, including birds and mammals (61), the airway lumen is subjected to dynamic fluctuations in the magnitude and direction of wall shear stress with inspiration and expiration (Fig. 3A and SI Appendix, Methods S1) (62).
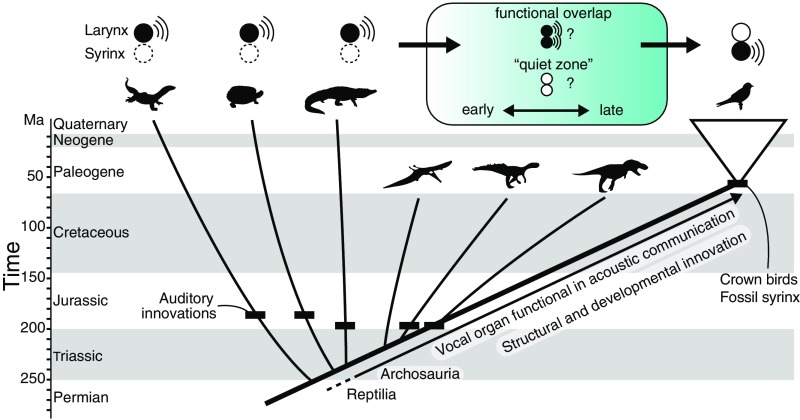
Fig. 4.
Evolution of sound-producing mechanisms in archosaurs. There are two primary hypotheses for the evolutionary transition from a laryngeal sound source to a syringeal sound source (blue-shaded box). Auditory innovations shown as black dashes (15) suggest a sustained role for acoustic communication in archosaurs. Understanding whether the shift to a syringeal sound source occurred early or late in bird-lineage archosaurs will require further comparative genomic and paleontological work.
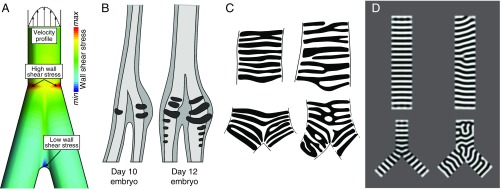
Fig. 3.
Models inform hypotheses of airway cartilage evolution and development. (A) Airflow through tubes exposes tube walls to a wall shear stress (WSS) that is proportional to the flow velocity gradient (shown schematically at the top of the airway). Steep velocity gradients at the wall give rise to high WSS levels. Flow velocity throughout an airway bifurcation can be predicted by computational fluid dynamics simulations (SI Appendix, Methods S1). Spatial distribution of WSS derived from computed velocity profiles during exhalation is shown for a bifurcation, with regions of elevated WSS denoted. Regions of elevated WSS near the tracheobronchial juncture, such as those shown here, suggest different structural requirements than the rest of the airway, which may have influenced the evolution of vibrating sound sources and/or cartilage structure. (B) Earliest stages of syrinx cartilage formation in the male duck (adapted from ref. 77). The derived (larger, left–right asymmetric) morphology of the syrinx cartilage is present from its initiation and does not form by later shaping or differential growth of the airway cartilage. (C) Examples of human airway cartilage patterns (redrawn from ref. 49) in the trachea and the tracheobronchial juncture. (D) Simulation of a Turing system (with a gradient to orient stripes). Diverse cartilage patterns are predicted at the tracheobronchial juncture. Each panel in D corresponds to a different parameter value (SI Appendix, Methods S2). For computational ease, we solve on a flat, 2D domain, which we argue is a reasonable assumption given the low Gaussian curvature of the system.
Simulations using simplified airways show that airflow is characterized by flow separation and concomitant zones of increased circulation and altered flow patterns (possibly including turbulence) that lead to localized regions of elevated wall shear stress (Fig. 3A and SI Appendix, Methods S1). Although accurately predicting sites of increased wall shear stress is difficult, requiring estimates of the velocity fields, in the simplified airway simulations the highest stress during exhalation is predicted to be at the tracheobronchial juncture (Fig. 3A and SI Appendix, Methods S1). This localized increase in shear stress may have provided a selective pressure for elaboration of the airway support specifically at the juncture to maintain airway patency. Understanding whether and how aerodynamic forces may have favored the evolution of novel soft tissue or cartilage morphologies at the tracheobronchial juncture will require further physical modeling of airway dynamics across a range of morphologies.
More generally, however, the onset of continuous breathing is not sufficient to produce a novel vocal organ: Mammals are also continuous breathers but did not evolve a syrinx. Thus, additional factors should be considered. For example, body size, relative neck length, and larynx position relative to the hyoid apparatus are known to have changed across the evolution of dinosaurs and, combined with the changing dynamics of a shift in respiratory behavior, may have favored the evolution of the syrinx. The ancestral airway geometries in Mammalia and Archosauria may also be distinct: Crocodilians and humans, for example, have bronchi that diverge at different angles.
Any syrinx precursor conditions are limited by strong selection for maintaining respiratory function. Compliant membranes in birds occur in areas where cartilaginous rings became reduced (tympaniform membranes) (Fig. 2) or by soft-tissue accumulations on the inner wall surface (labia) (Fig. 2) (39). Sound is produced in the syrinx by airflow-induced, self-sustained oscillations of these membranes and labia (e.g., refs. 40, 42, and 63). Thus, as soon as precursor vibratory membranes or labia evolve, there must be a mechanism to abduct them from the airway during normal breathing. Airflow in tubes with distensible walls (i.e., airway walls or membranes capable of stretching under conditions of differential pressure such as incipient vibratory tissues) could lead to collapse. The appearance of vibratory tissue precursors must have, at most, only briefly predated the attachment of the first muscles to the trachea to abduct them. Although extant birds possess a varying number of extrinsic and intrinsic syrinx muscles, two pairs of ancestrally present extrinsic muscles (Fig. 2B) (8, 34) control the movements of cartilaginous components of the syrinx to regulate the position of membranes and labia during vocal production, thus keeping the airway from collapsing during normal (nonvocal) respiration (64). The origin of the interclavicular air sac may have occurred before or after the origin of these tissues; its earliest fossil correlates are presently known only well after the origin of flight and the inferred origin of continuous respiration (8). Further study of tetrapod airways in continuous and discontinuous breathers is needed to understand potential constraints on airway morphology evolution.
Development and Novelty in the Respiratory Tract—How Did the Syrinx Evolve?
The unique stresses experienced at the tracheobronchial juncture suggest one possibility for why the syrinx arose there—but how did the syrinx evolve? Because the main components of the syrinx—cartilage, muscle, and soft tissue—form before a bird hatches, a developmental perspective may shed light on syrinx origins and especially on questions of novelty and homology. Despite the lack of structural homology between the larynx and syrinx, it is not known to what extent, if any, their morphogenesis shares underlying developmental or genetic mechanisms.
The larynx and the syrinx differ significantly in how the vocal folds and associated structures arise during development. Laryngeal cartilages and vocal folds are derived from a combination of mesoderm and neural crest (65). In contrast, syrinx vocal folds do not appear to be neural crest derived. Muscles surrounding the laryngeal opening and attaching to the vocal folds in amniotes are derived from paraxial mesoderm (65–67) and have homologies identified in lissamphibians (68). They have different somite and cell-lineage progenitors from the m. sternohyoideus (Fig. 2) (69, 70), the presumptive precursor to the two ancestral muscles associated with the syrinx in extant birds (Fig. 2) (44). In sum, at the developmental level, some individual larynx cartilages are homologous across tetrapod taxa, including birds. These structures are formed through the interaction of mesodermal and neural crest cells during development, and the evolution of these interactions may produce the diverse morphologies of laryngeal vocal folds and their supporting cartilage elements (65, 68, 71).
Airway morphogenesis appears to be stereotyped across tetrapods and seems to mirror the pattern of accrual of respiratory novelty, first in tetrapods and then in amniotes (Fig. 1). The tetrapod trachea forms when the ventral segment of the anterior foregut tube buds to form a Y-shaped diverticulum (72). This tube elongates, and the two tips of the tube become the bronchi and lungs (73). Signaling between the endodermal epithelium and the surrounding mesodermal mesenchyme is essential for branching and for the proper formation of the cartilaginous support structures (74, 75), which form after the trachea, bronchi, and lungs are apparent. The extrinsic muscles of the trachea in birds migrate from their paraxial mesoderm sources as the tracheobronchial cartilages form (67).
Development of syringeal cartilage has been described at the histological level in chicken (76) and duck (77), but the molecular mechanisms behind syrinx morphogenesis are mostly unknown. Derived syringeal cartilage morphologies in birds are present at the earliest formation of airway cartilage in the embryo (Fig. 3B) (76, 77). Importantly, these morphologies do not form through the alteration of regularly spaced, ring-shaped anlagen like those seen in outgroup taxa or those that form in the rest of the avian airway. By contrast, they are already distinct when airway cartilage formation is initiated. There is no evidence that any individual airway cartilage elements in amniotes, including birds, have distinct identities, unlike laryngeal structures. Rather, what appears to be molecularly specified in the airway is a program generating a series of spaced cartilage rings from a contiguous sleeve of undifferentiated mesenchyme. This is in contrast to the vertebrae, for example, in which individual identities are established through expression of defined sets of Hox genes (78, 79).
Several secreted signaling molecules have been identified affecting tracheal cartilage formation in the mouse, including bone morphogenetic proteins Bmp4 and Bmp7 (80–82), Wnt family members (83), Sonic hedgehog and the fibroblast growth factor Fgf10 (84, 85), and the T-box transcription factors Tbx4 and Tbx5 (86). A patterning system may be modified by changing the size of the progenitor field or altering the timing or level of production of the regulatory signals driving the formation of the cartilage rings. Modifications could alter the number and/or spacing of cartilage rings. However, as a full series of rings emerges de novo from each set of starting conditions, it becomes impossible to homologize individual cartilage rings between species. In other words, there is a homologous mechanism by which the series of elements is produced, but the elements themselves are not individuated (87).
Consistent with a proposed lack of homology among individual airway cartilage elements, patterns of airway cartilage (49, 88) resemble configurations produced by simple Turing models (reviewed in 89). A generic Turing system using the Swift–Hohenberg equation (90), which generates periodic patterns of many types (e.g., stripes, spots, zigzags), produces patterns akin to those observed in the trachea (Fig. 3D and SI Appendix, Methods S2). Simulations predict that, under certain combinations of parameters, the cartilage rings will be single or bifurcating resembling “forked” cartilage rings present in the upper airways of a number of organisms [e.g., mouse and human (49)] (depicted schematically in Fig. 1). Disorganized patterns tend to be recovered at the tracheobronchial juncture in the simulations and in many nonavian amniotes, including humans (Fig. 3 C and D and SI Appendix, Methods S2) (49, 88). The Swift–Hohenberg equation does not require a known mechanism and encompasses patterns generated by reaction-diffusion and mechanical models (e.g., via buckling). These simple models can inform testable predictions concerning Turing-type patterning and suggest mechanisms to ensure the proper spacing and circumferential orientation of airway cartilage in vivo.
From the simulation results and available developmental data, we propose identity of syrinx structures based only on relative location with respect to the topology of the airway (i.e., split of the bronchi) but no fixed identities for individual cartilages (8). Even though morphological investigation of the syrinx dates back more than 200 years (25), identifying one-to-one homology between cartilage elements in different species has previously proven problematic. Naming schemes conferring identity to specific syrinx cartilage elements have attempted to ascribe homology to individual elements across birds, but at the same time researchers recognized that these identity schemes did not seem to work among major clades of birds (32, 33).
Previous naming schemes for parts of the syrinx assigned identities to particular rings (e.g., A1, B2) (32), but fusion or loss of elements led to nonhomology of these named elements (e.g., ref. 91). For example, the number of cartilage elements identified as fused into a tympanum (Fig. 2) are known to vary within species of hawks, falcons, owls, alcids, parrots, and songbirds (from four to seven can be identified), while the shape, identity, and vocal production from the resultant organ are not known to show associated variation (32, 91–93). In the few cases in which it has been detailed, the total number of tracheal rings in the airway varies independently of the structural and functional similarity in the syrinx in the adult (92, 93). For example, in ducks, the relative shape and size of a part of the syrinx, the bulla, did not show significant intraspecific variation, while the number of tracheal rings varied with neck length in males and females (92). There seems generally little to no evidence of selection for a certain number of rings constituting a syrinx or airway, although factors presumably affecting acoustic production (e.g., ring diameter in the cranial or caudal part of the airway, size of the labia where present) may vary (92, 94).
We propose that syrinx origin must involve changes in development affecting, at minimum, the cartilage pattern at the juncture, differential mineralization of these juncture rings, the formation of vibratory soft tissue, and novel muscle associations with the airway. However, the mechanisms of change behind these shifts are as yet undescribed. The genic underpinnings of airway cartilage diversity across amniotes, especially in ring diameter, shape, thickness, and the presence or absence of complete bronchial rings, remains little explored. We must assess whether the programs that underpin the unique development of vibratory tissue or the creation of spaces among rings for vibratory membranes are new. For example, it is possible that the vocal folds in the syrinx and larynx have converged on the same genetic means of morphogenesis: Co-option of the same gene networks that form the vocal folds of the larynx could be expressed in syringeal labia where present, thereby constituting an example of deep homology (3, 95). To determine whether deep homology exists for structures such as labia, more work is needed to understand syrinx morphogenesis and its underlying developmental genetic mechanisms.
Insights into Biological Novelty from the Origin of the Syrinx
The recognition that structural novelty evolved via changes in development prompts a return to some of the key questions raised earlier in this essay and proposes new ones. Despite presumed sustained selection for a function in acoustic signaling, why has the larynx lost its role as a sound-producing organ in crown birds? If vocal functions were copresent for some duration, could sound sources in a larynx and early syrinx be coupled or complementary, or would the presence of novel vocal folds in the syrinx severely impact or inhibit sound production in a larynx (Fig. 4)? When novel vibratory structures have evolved in other tetrapods, they occur structurally downstream from the larynx, for instance in the internal or external nares. How do precursors of vibratory structures arise deep in the airway, and how might these structures at the tracheobronchial juncture come under novel selective regimes?
Consideration of the syrinx prompts broader questions about the nature of structural and functional novelty in organs and tissues. In key examples of biological innovation in locomotor systems, novel function is associated with novel selective regimes for changes in structure (e.g., the shifts in function in the evolution of the tetrapod limb) (3, 96). However, in novel vocal organs in mammals, and likely in the syrinx, a novel structure evolved while acoustic communication via a vocal organ was maintained.
Organs related to signaling and sensory modalities may be enriched for this type of elaborative novelty. For example, the repertoires of pigments and coloration regimes used in visual signaling, and thus available to sexual selection, are largely combinatorial; that is, they are copresent with other plesiomorphic coloration systems. Within vertebrates, olfactory sensitivity has been extended with the addition of a novel vomeronasal organ, and mechanical sound production is copresent with other mechanisms of acoustic signaling. Sexual selection has been implicated in many of these innovations (e.g., refs. 97 and 98).
However, there are instances in other systems where a novel structure has been proposed to have evolved alongside a separate, arguably functionally overlapping structure. Teeth and baleen in mysticete whales are one such example. Keratinous baleen allows extant mysticetes to filter feed efficiently. Baleen may have arisen after the origin of filter feeding in a toothed ancestor (99). Although baleen and teeth are not redundant structures, there may have been overlap in their role in feeding. It has been debated whether functional teeth and baleen were present in the same animal (100) or whether teeth were lost, producing something similar to the proposed “silent period” in syrinx evolution, and baleen subsequently evolved in a toothless lineage (101, 102).
Developmental and genic examples may shed light on the mechanism of the evolution of novel structures for overlapping functions. At the developmental level, there are many instances of a function being maintained while the processes underlying the function have diverged. For example, Wnt3a plays a key role in forming the apical ectodermal ridge, a key structure in the chicken limb bud, but plays no role in the formation of the same structure in the mouse, being replaced in that function by the distinct gene, Wnt3 (103, 104). Similarly, Snail and Slug are paralogous transcription factors involved in key roles during vertebrate development. However, the sites of expression of Snail and Slug are swapped between mouse and chick, and so, correspondingly, are their roles in development (105). Likewise, the Notch pathway transcription factors Hairy2 and Hes1 have replaced one another as key cycling genes during somite segmentation in the chick and mouse, respectively (106). It appears to be common at the genetic level to produce functional redundancy via duplication, after which one member of the redundant pair is often lost (107, 108). In these cases, it is thought that loss is usually due to relaxed selection on the duplicate. While possibly comparatively rare in vertebrate structure, cases of novelty in the face of functional similarity may merit a new focus of study.
The syrinx and other evolutionary novelties in sensory and signaling modalities may be distinct from those related to the locomotor system but similar to known examples at other levels of organization. Specifically, all these cases may generally involve a duplicate or overlapping role or elaboration on a single function rather than being driven by a selective environment favoring a new function. As such, a better knowledge of syrinx evolution may contribute importantly to our understanding of evolutionary novelties more generally.
Supplementary Material
Acknowledgments
This work was funded by Gordon and Betty Moore Foundation Grant 4498 (to J.A.C., F.G., T.R., and C.J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804586115/-/DCSupplemental.
References
- 1.Xu X, et al. An integrative approach to understanding bird origins. Science. 2014;346:1253293. doi: 10.1126/science.1253293. [DOI] [PubMed] [Google Scholar]
- 2.Muller GB, Wagner GP. Novelty in evolution: Restructuring the concept. Annu Rev Ecol Syst. 1991;22:229–256. [Google Scholar]
- 3.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 4.Hall BK, Kerney R. Levels of biological organization and the origin of novelty. J Exp Zoolog B Mol Dev Evol. 2012;318:428–437. doi: 10.1002/jez.b.21425. [DOI] [PubMed] [Google Scholar]
- 5.Riede T, Li Z, Tokuda IT, Farmer CG. Functional morphology of the Alligator mississippiensis larynx with implications for vocal production. J Exp Biol. 2015;218:991–998. doi: 10.1242/jeb.117101. [DOI] [PubMed] [Google Scholar]
- 6.Russell AP, Rittenhouse DR, Bauer AM. Laryngotracheal morphology of Afro-Madagascan geckos: A comparative survey. J Morphol. 2000;245:241–268. doi: 10.1002/1097-4687(200009)245:3<241::AID-JMOR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Sacchi R, Galeotti P, Fasola M, Gerzeli G. Larynx morphology and sound production in three species of Testudinidae. J Morphol. 2004;261:175–183. doi: 10.1002/jmor.10236. [DOI] [PubMed] [Google Scholar]
- 8.Clarke JA, et al. Fossil evidence of the avian vocal organ from the Mesozoic. Nature. 2016;538:502–505. doi: 10.1038/nature19852. [DOI] [PubMed] [Google Scholar]
- 9.Senter P. Voices of the past: A review of Paleozoic and Mesozoic animal sounds. Hist Biol. 2008;20:255–287. [Google Scholar]
- 10.Colafrancesco KC, Gridi-Papp M. 2016. Vocal sound production and acoustic communication in amphibians and reptiles. Vertebrate Sound Production and Acoustic Communication, Springer Handbook of Auditory Research (Springer, Cham, Switzerland), pp 51–82.
- 11.Ferrara CR, Vogt RC, Eisemberg CC, Doody JS. First evidence of the pig-nosed turtle (Carettochelys insculpta) vocalizing underwater. Copeia. 2017;105:29–32. [Google Scholar]
- 12.Farmer CG. Parental care: The key to understanding endothermy and other convergent features in birds and mammals. Am Nat. 2000;155:326–334. doi: 10.1086/303323. [DOI] [PubMed] [Google Scholar]
- 13.Meng Q, Liu J, Varricchio DJ, Huang T, Gao C. Palaeontology: Parental care in an ornithischian dinosaur. Nature. 2004;431:145–146. doi: 10.1038/431145a. [DOI] [PubMed] [Google Scholar]
- 14.Carl NJ, Darlington J. Extended parental care in broad-snouted caiman Caiman latirostris (Daudin, 1801) (Crocodylia, Alligatoridae) Herpetol Notes. 2017;10:127–129. [Google Scholar]
- 15.Sobral G, Müller J. Archosaurs and their kin: The ruling reptiles. In: Clack JA, Fay RR, Popper AN, editors. Evolution of the Vertebrate Ear: Evidence from the Fossil Record. Springer International Publishing; Cham, Switzerland: 2016. pp. 285–326. [Google Scholar]
- 16.Weishampel DB. Acoustic analyses of potential vocalization in lambeosaurine dinosaurs (Reptilia: Ornithischia) Paleobiology. 1981;7:252–261. [Google Scholar]
- 17.Witmer LM, Ridgely RC. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat Rec (Hoboken) 2009;292:1266–1296. doi: 10.1002/ar.20983. [DOI] [PubMed] [Google Scholar]
- 18.Vergne AL, Pritz MB, Mathevon N. Acoustic communication in crocodilians: From behaviour to brain. Biol Rev Camb Philos Soc. 2009;84:391–411. doi: 10.1111/j.1469-185X.2009.00079.x. [DOI] [PubMed] [Google Scholar]
- 19.Mayr E. The emergence of evolutionary novelties. In: Tax S, editor. Evolution After Darwin. Vol 1. Univ of Chicago Press; Chicago: 1960. pp. 349–380. [Google Scholar]
- 20.Bradbury JW, Vehrencamp SL. Principles of Animal Communication. 2nd Ed Sinauer Associates, Oxford Univ Press; Sunderland, MA: 2011. [Google Scholar]
- 21.Charlton BD, et al. Koalas use a novel vocal organ to produce unusually low-pitched mating calls. Curr Biol. 2013;23:R1035–R1036. doi: 10.1016/j.cub.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 22.Reidenberg JS, Laitman JT. Existence of vocal folds in the larynx of Odontoceti (toothed whales) Anat Rec. 1988;221:884–891. doi: 10.1002/ar.1092210413. [DOI] [PubMed] [Google Scholar]
- 23.Cranford TW, Amundin M, Norris KS. Functional morphology and homology in the odontocete nasal complex: Implications for sound generation. J Morphol. 1996;228:223–285. doi: 10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Madsen PT, Jensen FH, Carder D, Ridgway S. Dolphin whistles: A functional misnomer revealed by heliox breathing. Biol Lett. 2012;8:211–213. doi: 10.1098/rsbl.2011.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huxley TH. A Manual of the Anatomy of Vertebrated Animals. D. Appleton and Co.; New York: 1872. [Google Scholar]
- 26.Negus VE. The Comparative Anatomy and Physiology of the Larynx. Hafner Pub. Company; New York: 1949. [Google Scholar]
- 27.Fitch WT. 2016. Vertebrate bioacoustics: Prospects and open problems. Vertebrate Sound Production and Acoustic Communication, Springer Handbook of Auditory Research (Springer, Cham, Switzerland), pp 297–328.
- 28.Farmer CG. The Biology of the Avian Respiratory System. Springer; Cham, Switzerland: 2017. Pulmonary transformations of vertebrates; pp. 99–112. [Google Scholar]
- 29.Hilton WA. The pulmonary respiratory system of salamanders. Herpetologica. 1952;8:87–92. [Google Scholar]
- 30.Duellman WE. Biology of Amphibians. Johns Hopkins Univ Press; Baltimore: 1994. [Google Scholar]
- 31.Kuehne B, Junqueira LC. Histology of the trachea and lung of Siphonops annulatus (Amphibia, Gymnophiona) Rev Bras Biol. 2000;60:167–172. doi: 10.1590/s0034-71082000000100019. [DOI] [PubMed] [Google Scholar]
- 32.Ames PL. The Morphology of the Syrinx in Passerine Birds. Peabody Museum of Natural History, Yale University; New Haven CT: 1971. [Google Scholar]
- 33.Warner RW. The anatomy of the syrinx in passerine birds. J Zool. 1972;168:381–393. [Google Scholar]
- 34.King AS. Functional anatomy of the syrinx. In: King AS, McLelland J, editors. Form and Function in Birds. Vol 4 Academic; New York: 1989. [Google Scholar]
- 35.Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel) 1974;26:89–94. doi: 10.1159/000263771. [DOI] [PubMed] [Google Scholar]
- 36.Klemuk SA, Riede T, Walsh EJ, Titze IR. Adapted to roar: Functional morphology of tiger and lion vocal folds. PLoS One. 2011;6:e27029. doi: 10.1371/journal.pone.0027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurita S, Nagata K, Hirano M. A comparative study of the layer structure of the vocal fold. In: Bless DM, Adds JH, editors. Vocal Fold Physiology. Contemporary Research and Clinical Issues. College Hill Press; San Diego: 1983. pp. 3–21. [Google Scholar]
- 38.Riede T, Lingle S, Hunter EJ, Titze IR. Cervids with different vocal behavior demonstrate different viscoelastic properties of their vocal folds. J Morphol. 2010;271:1–11. doi: 10.1002/jmor.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riede T, Goller F. Morphological basis for the evolution of acoustic diversity in oscine songbirds. Proc Biol Sci. 2014;281:20132306. doi: 10.1098/rspb.2013.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goller F, Larsen ON. In situ biomechanics of the syrinx and sound generation in pigeons. J Exp Biol. 1997;200:2165–2176. doi: 10.1242/jeb.200.16.2165. [DOI] [PubMed] [Google Scholar]
- 41.Riede T, Goller F. Peripheral mechanisms for vocal production in birds–Differences and similarities to human speech and singing. Brain Lang. 2010;115:69–80. doi: 10.1016/j.bandl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elemans CPH, et al. Universal mechanisms of sound production and control in birds and mammals. Nat Commun. 2015;6:8978. doi: 10.1038/ncomms9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Titze I, Riede T, Mau T. Predicting achievable fundamental frequency ranges in vocalization across species. PLoS Comput Biol. 2016;12:e1004907. doi: 10.1371/journal.pcbi.1004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgeworth FH. The Cranial Muscles of Vertebrates. Cambridge Univ Press; London: 1935. [Google Scholar]
- 45.Diogo R, Abdala V. Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development. CRC Press, Boca Raton, FL; Science Pub.; Enfield, NH: 2010. [Google Scholar]
- 46.Pigliucci M. What, if anything, is an evolutionary novelty? Philos Sci. 2008;75:887–898. [Google Scholar]
- 47.Stein RC. Modulation in bird sounds. Auk. 1968;85:229–243. [Google Scholar]
- 48.Luschka H. Die Anatomie der Brust des Menschen. Laupp & Siebeck; Tübingen, Germany: 1863. [Google Scholar]
- 49.Vanpeperstraete F. The Cartilaginous Skeleton of the Bronchial Tree. Springer; Berlin: 1973. [PubMed] [Google Scholar]
- 50.Gaunt AS, Wells MK. Models of syringeal mechanisms. Integr Comp Biol. 1973;13:1227–1247. [Google Scholar]
- 51.Maina J. The Lung-Air Sac System of Birds: Development, Structure, and Function. Springer; Berlin: 2005. [Google Scholar]
- 52.Brackenbury J. Control of sound production in the syrinx of the fowl Gallus gallus. J Exp Biol. 1980;85:239–251. [Google Scholar]
- 53.Goller F, Larsen ON. New perspectives on mechanisms of sound generation in songbirds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:841–850. doi: 10.1007/s00359-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 54.Beddard FE. The Structure and Classification of Birds. Longmans, Green, and Company; London: 1898. [Google Scholar]
- 55.Reidenberg JS, Laitman JT. Discovery of a low frequency sound source in Mysticeti (baleen whales): Anatomical establishment of a vocal fold homolog. Anat Rec (Hoboken) 2007;290:745–759. doi: 10.1002/ar.20544. [DOI] [PubMed] [Google Scholar]
- 56.Reiss JO, Eisthen HL. Comparative anatomy and physiology of chemical senses in amphibians. In: Thewissen JGM, Nummela S, editors. Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates. Univ California Press; Berkeley, CA: 2008. pp. 43–63. [Google Scholar]
- 57.Jankowski R. The Evo-Devo Origin of the Nose, Anterior Skull Base and Midface. Springer; Paris: 2013. A theory of secondary palate formation; pp. 63–71. [Google Scholar]
- 58.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 59.White FM. Viscous Fluid Flow. McGraw-Hill; New York: 2006. [Google Scholar]
- 60.Xia G, Tawhai MH, Hoffman EA, Lin C-L. Airway wall stiffening increases peak wall shear stress: A fluid-structure interaction study in rigid and compliant airways. Ann Biomed Eng. 2010;38:1836–1853. doi: 10.1007/s10439-010-9956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milsom WK. Intermittent breathing in vertebrates. Annu Rev Physiol. 1991;53:87–105. doi: 10.1146/annurev.ph.53.030191.000511. [DOI] [PubMed] [Google Scholar]
- 62.Pidaparti RM, Swanson J. Effect of mechanical ventilation waveforms on airway wall shear. J Med Eng Technol. 2015;39:1–8. doi: 10.3109/03091902.2014.968675. [DOI] [PubMed] [Google Scholar]
- 63.Fee MS. Measurement of the linear and nonlinear mechanical properties of the oscine syrinx: Implications for function. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:829–839. doi: 10.1007/s00359-002-0349-z. [DOI] [PubMed] [Google Scholar]
- 64.Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol. 1996;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- 65.Tabler JM, et al. Cilia-mediated hedgehog signaling controls form and function in the mammalian larynx. eLife. 2017;6:e19153. doi: 10.7554/eLife.19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- 67.Huang R, Zhi Q, Izpisua-Belmonte JC, Christ B, Patel K. Origin and development of the avian tongue muscles. Anat Embryol (Berl) 1999;200:137–152. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt J, Piekarski N, Olsson L. Cranial muscles in amphibians: Development, novelties and the role of cranial neural crest cells. J Anat. 2013;222:134–146. doi: 10.1111/j.1469-7580.2012.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laitman JT, Noden DM, Van De Water TR. Formation of the larynx: From Hox genes to critical periods. In: Rubin JS, Sataloff RT, Korovin GS, editors. Diagnosis and Treatment of Voice Disorders. Plural Publishing; San Diego: 2014. pp. 3–20. [Google Scholar]
- 70.White S, Danowitz M, Solounias N. Embryology and evolutionary history of the respiratory tract. Edorium J Anat Embryol. 2016;3:54–62. [Google Scholar]
- 71.Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 73.Qi BQ, Beasley SW. Stages of normal tracheo-bronchial development in rat embryos: Resolution of a controversy. Dev Growth Differ. 2000;42:145–153. doi: 10.1046/j.1440-169x.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- 74.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 75.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BLM. Morphogenesis of the trachea and esophagus: Current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 76.Tymms AOV. The syrinx of the common fowl, its structure and development. Proc R Soc Victoria. 1913;25:286–304. [Google Scholar]
- 77.Wolff E. La différenciation sexuelle normale et le conditionnement hormonal des caractères sexuels somatiques précoces, tubercule génital et syrinx chez l’embryon de canard. Bull Biol Fr Belg. 1950;84:119–193. [PubMed] [Google Scholar]
- 78.Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- 79.Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236:2454–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: A novel mouse model for tracheal agenesis. Dev Biol. 2008;322:145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zouvelou V, Luder H-U, Mitsiadis TA, Graf D. Deletion of BMP7 affects the development of bones, teeth, and other ectodermal appendages of the orofacial complex. J Exp Zool B Mol Dev Evol. 2009;312B:361–374. doi: 10.1002/jez.b.21262. [DOI] [PubMed] [Google Scholar]
- 82.Geng Y, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci USA. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Snowball J, Ambalavanan M, Whitsett J, Sinner D. Endodermal Wnt signaling is required for tracheal cartilage formation. Dev Biol. 2015;405:56–70. doi: 10.1016/j.ydbio.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller L-AD, et al. Role of sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 85.Sala FG, et al. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development. 2011;138:273–282. doi: 10.1242/dev.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8:e1002866. doi: 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gilbert SF, Bolker JA. Homologies of process and modular elements of embryonic construction. J Exp Zool. 2001;291:1–12. doi: 10.1002/jez.1. [DOI] [PubMed] [Google Scholar]
- 88.Heller R, Von Kristelli Schrötter H. Die carina tracheae. Ein Beitrag zur Kenntniss der Bifurcation der Luftröhre, nebst vergleichend anatomischen Bemerkungen über den Bau derselben. Denkschr Kais Akad Wiss/Math-Naturwiss Cl. 1897;64:397–438. [Google Scholar]
- 89.Kondo S, Miura T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science. 2010;329:1616–1620. doi: 10.1126/science.1179047. [DOI] [PubMed] [Google Scholar]
- 90.Swift J, Hohenberg PC. Hydrodynamic fluctuations at the convective instability. Phys Rev A. 1977;15:319–328. [Google Scholar]
- 91.Gaban-Lima R, Höfling E. Comparative anatomy of the syrinx in the tribe Arini (Aves: Psittacidae) Braz J Morphol Sci. 2006;23:501–512. [Google Scholar]
- 92.Miller EH, Williams J, Jamieson SE, Gilchrist HG, Mallory ML. Allometry, bilateral asymmetry and sexual differences in the vocal tract of common eiders Somateria mollissima and king eiders S. spectabilis. J Avian Biol. 2007;38:224–233. [Google Scholar]
- 93.Miller EH, Seneviratne SS, Jones IL, Robertson GJ, Wilhelm SI. Syringeal anatomy and allometry in murres (Alcidae: Uria) J Ornithol. 2008;149:545–554. [Google Scholar]
- 94.Hardouin LA, Thompson R, Stenning M, Reby D. Anatomical bases of sex- and size-related acoustic variation in herring gull alarm calls. J Avian Biol. 2014;45:157–166. [Google Scholar]
- 95.Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457:818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 96.Coates MI, Jeffery JE, Rut M. Fins to limbs: What the fossils say. Evol Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- 97.Haga S, et al. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- 98.Seddon N, et al. Sexual selection accelerates signal evolution during speciation in birds. Proc Biol Sci. 2013;280:20131065. doi: 10.1098/rspb.2013.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitchell ED. A new cetacean from the late Eocene La Meseta Formation Seymour Island, Antarctic Peninsula. Can J Fish Aquat Sci. 1989;46:2219–2235. [Google Scholar]
- 100.Deméré TA, McGowen MR, Berta A, Gatesy J. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst Biol. 2008;57:15–37. doi: 10.1080/10635150701884632. [DOI] [PubMed] [Google Scholar]
- 101.Peredo CM, Pyenson ND, Boersma AT. Decoupling tooth loss from the evolution of baleen in whales. Front Mar Sci. 2017;4:67. [Google Scholar]
- 102.Fordyce RE, Marx FG. Gigantism precedes filter feeding in baleen whale evolution. Curr Biol. 2018;28:1670–1676.e2. doi: 10.1016/j.cub.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 103.Kengaku M, et al. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- 104.Barrow JR, et al. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Locascio A, Manzanares M, Blanco MJ, Nieto MA. Modularity and reshuffling of Snail and Slug expression during vertebrate evolution. Proc Natl Acad Sci USA. 2002;99:16841–16846. doi: 10.1073/pnas.262525399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jouve C, et al. Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development. 2000;127:1421–1429. doi: 10.1242/dev.127.7.1421. [DOI] [PubMed] [Google Scholar]
- 107.Long M, Betrán E, Thornton K, Wang W. The origin of new genes: Glimpses from the young and old. Nat Rev Genet. 2003;4:865–875. doi: 10.1038/nrg1204. [DOI] [PubMed] [Google Scholar]
- 108.Taylor JS, Raes J. Duplication and divergence: The evolution of new genes and old ideas. Annu Rev Genet. 2004;38:615–643. doi: 10.1146/annurev.genet.38.072902.092831. [DOI] [PubMed] [Google Scholar]
- 109.Gignac PM, et al. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): An emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. J Anat. 2016;228:889–909. doi: 10.1111/joa.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.