Abstract
Objectives. To evaluate whether adding Web-based cognitive behavioral treatment (CBT) to standard outpatient psychiatric or addiction treatment improved substance use outcomes.
Methods. We conducted a randomized clinical trial in New Haven, Connecticut, between 2014 and 2017 comparing 8 weeks of standard outpatient treatment to the same treatment with access to a culturally adapted version of Web-based CBT with a 6-month follow-up. Participants were 92 treatment-seeking individuals with Spanish as their primary language and current substance use disorder, with few other restrictions.
Results. Treatment completion and data availability were high (98% of the randomized sample). For the primary outcome (change in frequency of primary substance used), there was a significant effect of treatment condition by time (t 1, 718 = −2.64; 95% confidence interval = −0.61, 0.09; P = .01), indicating significantly greater reductions for those assigned to Web CBT, which were durable through the 6-month follow-up. The knowledge test indicated significantly greater increases for those assigned to Web CBT.
Conclusions. Adding a culturally adapted version of Web-based CBT to standard treatment improved substance use outcomes.
Public Health Implications. This approach has high potential to address health disparities by providing an easily accessible, inexpensive form of evidence-based treatment to a range of Latinos with substance use disorders.
There is ample evidence of health disparities affecting Latinos, the largest minority group in the United States.1,2 Limited access to behavioral health treatments is a particular concern among Latinos, who often experience heightened vulnerability because of racism and discrimination, poverty, unemployment, lack of health insurance, social exclusion, and physical comorbidities.3 These disparities extend to substance use disorders: Latinos tend to have elevated rates of substance use and related problems and experience disproportionate levels of adverse consequences related to substance use.4 They are also much less likely to receive mental health and substance abuse treatment services than are non-Hispanic Whites and African Americans.4 Improving access to evidence-based therapies that are culturally and linguistically appropriate is critical in addressing this significant health disparity.5
As increasing numbers of Latinos seek out Internet-delivered health information,6 Web-based therapies that offer standardized and affordable access to evidence-based interventions are promising strategies for reducing health inequities.7,8 Many Web sites offer health-related substance abuse and mental health information, but few are evidence based, and to date there are no validated approaches for Latinos with substance use disorders.9
We describe primary outcomes of a randomized clinical trial comparing a culturally adapted version of computer-based training for cognitive behavioral therapy (CBT4CBT-Spanish) to standard outpatient mental health and addiction treatment in a heterogeneous population of treatment-seeking Latino adults. Considering previous findings of the efficacy, safety, and durability of the existing English-language versions of CBT4CBT when added to standard outpatient treatment (treatment as usual [TAU]),10–13 we anticipated that the addition of access to CBT4CBT-Spanish would improve substance use outcomes relative to standard treatment alone. We also anticipated that exposure to CBT4CBT-Spanish would be associated with improved knowledge of cognitive and behavioral concepts.
METHODS
We recruited participants from individuals seeking treatment at 1 of 3 settings offering outpatient services to Latinos in the New Haven, Connecticut, area: the Hispanic Clinic and the Substance Abuse Treatment Unit of the Connecticut Mental Health Center, and Multicultural Ambulatory Addiction Services. Participants were individuals aged 18 years or older who met current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; Washington, DC: American Psychiatric Association; 1994) criteria for cocaine, marijuana, opioid, alcohol, or other stimulant abuse or dependence and who spoke Spanish as their preferred or principal language. We excluded individuals if they had an untreated bipolar or schizophrenic disorder or were not sufficiently stable for 8 weeks of outpatient treatment. There were no requirements regarding literacy.
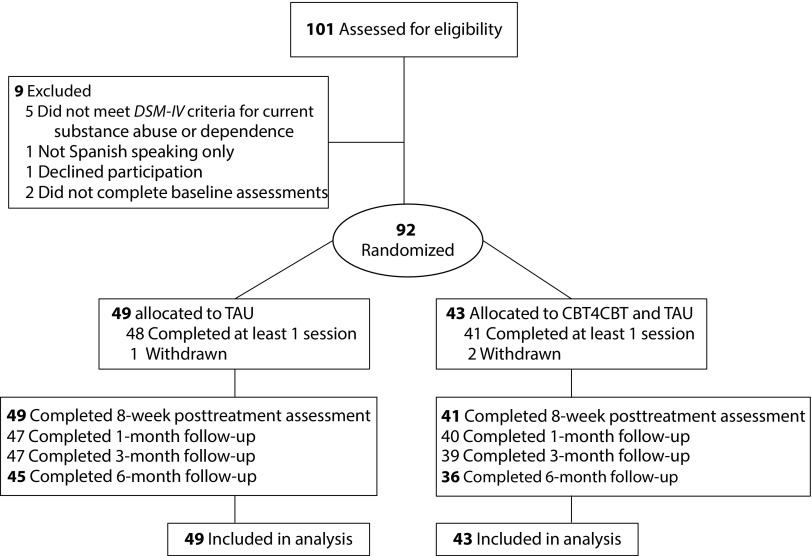
As shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 1), we determined that 92 of the 101 individuals screened were eligible for the study. They provided written informed consent. After we described the study to them, we randomized participants in equal proportion to 1 of the 2 conditions, using a computerized urn randomization program,14 which concealed the sequence until treatments were assigned, to balance treatment groups with respect to gender, education level (less than high school, high school graduate, or above), primary drug used (cocaine, marijuana, alcohol, other), self-reported familiarity with computers (yes vs no), and level of acculturation (Marin Short Acculturation Scale for Hispanics [SASH]).15
FIGURE 1—
CONSORT Diagram, Flow of Participants Through the Trial
Note. CBT4CBT = computerized cognitive behavioral therapy plus treatment as usual; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Washington, DC: American Psychiatric Association; 1994); TAU = treatment as usual.
Treatments
Standard TAU.
We offered participants standard care at each of the clinics, which typically consisted of supportive counseling delivered via weekly group or individual sessions, with access to other services as needed. For all conditions, we monitored receipt of medical, legal, psychological, and social support services both within and outside the program at each assessment visit.
CBT4CBT plus TAU.
In this condition, we added CBT4CBT-Spanish to standard care, and it was accessible at each clinic. CBT4CBT-Spanish is a cultural adaptation of a 7-session Web-based program for cognitive behavioral treatment (CBT) that has been validated in multiple trials.10–13,16 The process of cultural adaptation is described in detail elsewhere (Silva ML, Anez L, Gordon MA, Ball SA, Carroll KM, Paris M, unpublished). Briefly, while retaining focus on teaching 7 core CBT skills and strategies using multimedia tools, adaptations at the program and content level focused on the integration of cultural values in storyline and character development.
A telenovela format was the platform for teaching skills to facilitate engagement with the program, and the storyline included multiple culturally relevant experiences, such as immigration-related family separation. The narrator and characters were developed to resonate with Latino cultural values and concepts, including respeto (respect), confianza (trust), machismo, caballerismo, marianismo (gender-specific values), familismo (family orientation), fatalismo (fatalism), sabiduría (wisdom), and personalismo (value of interpersonal relationships).17,18
As in the English versions of CBT4CBT, each of the 7 modules included a practice exercise to facilitate generalization of each of the 7 core CBT skills (functional analysis, coping with craving, problem solving, assertiveness, decision-making, and recognizing and changing thoughts) and used a variety of strategies (graphics, voice-overs, interactive exercises, and true–false questions) to convey the intended skills. The program collected no protected health information; participants accessed the CBT4CBT program through an identification and password system to protect confidentiality. The program has an underlying data structure that tracks, for each user, the number of times each participant logs in, the specific pages visited, responses to quiz questions, and completion of homework. Participants could access the Web-based content as much as they wished during the active phase of treatment only.
Assessments.
A bilingual research assistant assessed participants before treatment, weekly during treatment, at the 8-week treatment termination point, and 1, 3, and 6 months after the termination point. Assessments included those with Spanish translations already available, such as the Structured Clinical Interview for DSM-IV,19 the Addiction Severity Index,20 and the Marin SASH,15 or assessments validated in previous work with similar populations.21 We administered the Structured Clinical Interview for DSM-IV19 to participants before randomization to establish substance use and other psychiatric diagnoses. We administered the Substance Use Calendar, similar to the Timeline Follow Back,22 at each assessment to collect day-by-day self-reports of drug and alcohol use for the 28-day period before randomization, as well as throughout the 56-day treatment phase and the 6-month follow-up. We evaluated the receipt of medical, legal, psychological, and other services at each assessment visit.
We verified participant self-reports of drug and alcohol use through urine toxicology (ToxCup Drug Screen Cup 5 with adulterant checks, TestCountry, San Diego, CA) and breathalyzer screens obtained at every assessment visit. The correspondence between recent drug use self-reports and qualitative urine toxicology screen results was excellent: of the 579 urine samples collected during treatment (mean 6.5 per participant randomized), only 28 (4.8%) indicated recent cocaine use when the participant denied use in the past 3 days. Self-report and urine results matched in 94% of urine specimens collected: 19.2% of all urine specimens were positive for cocaine. For the 23 participants with primary cocaine use, 11.7% of the 154 urine specimens collected were positive for cocaine metabolites when the participant denied recent use. For marijuana use, of the 579 urine specimens collected, 26 (4.5%) were inconsistent with the urine toxicology screen: 93.1% were consistent with self-report. For the 33 participants with primary marijuana use, of 218 urine specimens collected, 5 (6.9%) indicated recent marijuana use when the participant denied it. Opioid use was rare: of the 579 urine specimens collected, only 8 (1.4%) were positive for opioid metabolites and in only 4 cases (0.7%) did the participant deny recent opioid use. Finally, of 570 breathalyzer samples collected, 18 (3.5%) indicated recent alcohol use. These data suggest excellent consistency with self-report in this sample relative to English-speaking participants in recent similar trials.11,13
Data Analyses
The primary outcome measure was change in self-reported frequency of substance use (operationalized as days of primary substance use by week), evaluated using random effects regression analyses23,24; we used piecewise regression25 to analyze follow-up data. Because many participants used both drugs and alcohol, we evaluated days of any drug or alcohol use as a secondary outcome indicator. Because of significant differences across the 3 sites in rates of psychiatric diagnoses, we added site to the model as a cluster variable to account for this variability. Secondary outcomes also included static substance use outcomes (e.g., percentage days of primary substance use, percentage of urine specimens negative for drug metabolites), and we analyzed these using analysis of variance (ANOVA). Because of the intended heterogeneity in the sample regarding primary substance used (some participants used alcohol only), as well as differences in detectability of different drugs in urine specimens,26,27 we used urine toxicology screen results primarily to validate self-reports of substance use. We used repeated measures ANOVA for secondary outcomes evaluated over time (knowledge of cognitive and behavioral concepts).
RESULTS
Table 1 presents baseline demographic data by treatment condition. The sample was 33% women with a mean age of 43 years. Most (65%) were unemployed, 41% reported completing high school, and 76% reported being unmarried or living alone. The majority (72%) reported that they were born in Puerto Rico, 4% indicated that they were born in the continental United States, and the remainder reported that they were born in Mexico (9%), Central America (10%), or South America (2.2%). Participants reported that they had lived in the United States for an average of 17 years, and almost all (99%) reporting speaking only or mostly Spanish as a child. In terms of acculturation, the Marin SASH15 indicated a low level of acculturation for participants as a group, with 95% having a mean score of 3.0 or less on the SASH. Acculturation did not vary significantly by treatment condition, gender, or site.
TABLE 1—
Baseline Characteristics Overall and by Group: New Haven, CT, March 2015–December 2016
| Variable | CBT4CBT plus TAU (n = 43), No. (%) or Mean ±SD | TAU Only (n = 49), No. (%) or Mean ±SD | Total (n = 92), No. (%) or Mean ±SD |
| Categorical variables | |||
| Female | 12 (27.9) | 18 (36.7) | 30 (32.6) |
| Place of birth | |||
| Puerto Rico | 31 (72.1) | 35 (71.4) | 66 (71.7) |
| US mainland | 2 (4.7) | 2 (4.1) | 4 (4.3) |
| South America | 1 (2.3) | 1 (2.0) | 2 (2.2) |
| Mexico | 4 (9.3) | 4 (8.2) | 8 (8.7) |
| Central America | 3 (7.0) | 6 (12.2) | 9 (9.8) |
| Other | 2 (4.7) | 1 (2.0) | 3 (3.3) |
| Completed high school, yes | 16 (37.2) | 22 (44.9) | 38 (41.3) |
| Never married/living alone | 32 (74.4) | 38 (77.6) | 70 (76.1) |
| Unemployed | 28 (65.1) | 32 (65.3) | 60 (65.2) |
| Referred by criminal justice system | 6 (14.0) | 5 (10.2) | 11 (12.0) |
| Receiving public assistance | 27 (62.8) | 34 (69.4) | 61 (66.3) |
| Current and lifetime DSM-IV disorders | |||
| Lifetime posttraumatic stress disorder | 19 (44.2) | 24 (49.0) | 43 (46.7) |
| Current posttraumatic stress disorder | 18 (42.9) | 20 (40.8) | 38 (41.8) |
| Lifetime any depressive disordera | 26 (60.5) | 34 (69.4) | 60 (65.2) |
| Current any depressive disorder | 18 (41.9) | 27 (55.1) | 45 (48.9) |
| Lifetime any anxiety disorderb | 32 (74.4) | 41 (83.7) | 73 (79.3) |
| Current any anxiety disorder | 31 (72.1) | 40 (81.6) | 71 (77.2) |
| Lifetime any psychotic disorder | 15 (34.9) | 18 (36.7) | 33 (35.9) |
| Current any psychotic disorder | 8 (18.6) | 13 (26.5) | 21 (22.8) |
| Lifetime psychotic or bipolar disorder | 21 (48.8) | 21 (42.9) | 42 (45.7) |
| Current psychotic or bipolar disorder | 13 (30.2) | 16 (32.7) | 29 (31.5) |
| At least 1 current nondrug disorder | 34 (79.1) | 43 (87.8) | 77 (83.7) |
| Lifetime alcohol abuse or dependence | 34 (79.1) | 41 (83.7) | 75 (81.5) |
| Current alcohol abuse or dependence | 24 (55.8) | 24 (49.0) | 48 (52.2) |
| Lifetime cocaine abuse or dependence | 26 (60.5) | 20 (40.8) | 46 (50.0) |
| Current cocaine abuse or dependence | 15 (34.9) | 11 (22.4) | 26 (28.3) |
| Lifetime cannabis abuse or dependence | 29 (67.4) | 26 (53.1) | 55 (59.8) |
| Current cannabis abuse or dependence | 21 (48.8) | 23 (46.9) | 44 (47.8) |
| Primary substance used, self-reported | |||
| Alcohol | 14 (32.6) | 18 (36.7) | 32 (34.8) |
| Cocaine | 12 (27.9) | 11 (22.4) | 23 (25.0) |
| Marijuana | 15 (34.9) | 18 (36.7) | 33 (35.9) |
| Opiates | 1 (2.3) | 1 (2.0) | 2 (2.2) |
| Benzodiazepines | 0 (0) | 1 (2.0) | 1 (1.1) |
| Heroin | 1 (2.3) | 0 (0) | 1 (1.1) |
| Continuous variables | |||
| Age, y | 42.3 ±11.5 | 43.4 ±11.5 | 42.9 ±11.5 |
| Years living in US mainland | 16.8 ±13.5 | 18.6 ±13.2 | 17.7 ±13.3 |
| Days paid for work in past 28 d | 2.4 ±6.1 | 3.3 ±7.5 | 2.9 ±6.8 |
| Days of primary substance use, past 28 d | 10.1 ±9.3 | 13.7 ±10.2 | 12.0 ±9.9 |
| Age first used primary substance, y | 20.2 ±10.3 | 19.2 ±9.3 | 19.7 ±9.7 |
| Years primary substance use | 20.0 ±12.3 | 23.0 ±11.9 | 21.6 ±12.1 |
| Total no. of lifetime nonsubstance DSM-IV psychiatric disorders | 3.3 ±2.2 | 3.9 ±2.7 | 3.6 ±2.5 |
| Total no. of current nonsubstance DSM-IV psychiatric disorders | 2.3 ±1.8 | 2.8 ±2.1 | 2.6 ±2.0 |
Note. CBT4CBT = computerized cognitive behavioral therapy plus treatment as usual; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Washington, DC: American Psychiatric Association; 1994); TAU = treatment as usual.
Meets criteria for major depression or dysthymic disorder.
Meets criteria for general anxiety disorder, specific phobia, social anxiety disorder, posttraumatic stress disorder, obsessive compulsive disorder, or anxiety disorder, not otherwise specified.
In terms of substance use, 36% reported their primary substance was marijuana, 35% reported alcohol, and 25% reported cocaine; the remainder reported opioids (3%) or benzodiazepines (1%). Participants reported using their primary drug an average of 12 days of the 28 before screening. As shown in Table 1, both lifetime and current rates of substance use disorders were high (lifetime alcohol abuse or dependence 82%, current 52%; lifetime cannabis abuse or dependence 60%, current 49%; lifetime cocaine abuse or dependence 50%, current 28%). Rates of other psychiatric disorders were also high, including current major depression (47%), generalized anxiety disorder (41%), posttraumatic stress disorder (42%), and serious mental illness (SMI; schizophrenia or bipolar disorder, 32%). The mean number of current nonsubstance use psychiatric disorders was 2.6 (SD = 2.0). None of these demographic, substance use, or psychiatric variables differed significantly by treatment condition at baseline. Rates of psychiatric disorders did vary significantly by site, reflecting the fact that 2 of the sites provided both mental health and substance use services and the other was a specialty substance use clinic. Rates of participants with at least 1 current nonsubstance psychiatric disorder by site were 96.7%, 73.3%, and 37.5% (χ2 = 32.0; P ≤ .001). Thus, we included site as a cluster variable in outcome analyses.
Treatment Exposure and Serious Adverse Events
Treatment exposure and adherence were excellent across conditions (Table 2). Participants completed an average of 45 of 56 days in the protocol, submitted 6.5 urine specimens (of 8.0 maximum), and completed 2.9 individual treatment sessions, with no statistically significant differences by treatment condition for any of these variables or other services received (medical, psychiatric, or case management). Although those assigned to TAU completed significantly more group sessions (mean 5.2 vs 2.4), those assigned to CBT4CBT-Spanish completed a large proportion of the 7.0 possible modules (mean 5.3), thus balancing treatment exposure overall. Fifty-six percent of the participants assigned to CBT4CBT-Spanish completed all 7 modules, which compares favorably to previous studies,10,11,13,28 as does the number of homework assignments completed (mean 4 of 6 possible). Rates of serious adverse events that warranted hospitalization are also shown in Table 2. These included hospitalization for suicidal ideation, detoxification, or medical issues (e.g., pneumonia, chest pains). Rates did not differ across conditions, and none of these events were determined to be related to treatment.
TABLE 2—
Treatment Exposure, Serious Adverse Events, and Outcomes by Treatment Condition: New Haven, CT, March 2015–December 2016
| Variable | CBT4CBT plus TAU (n = 43), No. (%) or Mean ±SD | TAU Only (n = 49), No. (%) or Mean ±SD | Total (n = 92), No. (%) or Mean ±SD | df | f or χ2 | P |
| Treatment exposure | ||||||
| D in treatment (max = 56) | 47.7 (14.4) | 41.7 (16.8) | 44.5 (15.9) | 1,90 | 3.45 | .07 |
| No. of urine specimens collected (max = 8) | 6.3 (2.3) | 6.7 (2.1) | 6.5 (2.2) | 1,87 | 0.50 | .48 |
| % of expected urine specimens collected | 79.3 (28.5) | 83.3 (25.8) | 81.5 (27.0) | 1,87 | 0.50 | .48 |
| No. of individual sessions | 2.8 (2.6) | 2.9 (2.5) | 2.9 (2.5) | 1,90 | 0.01 | .91 |
| No. of group sessions | 2.4 (3.9) | 5.2 (6.6) | 3.9 (5.7) | 1,90 | 6.04 | .02 |
| Total no. of case management sessions | 0.6 (1.4) | 1.1 (3.4) | 0.9 (2.7) | 1,83 | 0.60 | .44 |
| No. of medical services | 1.0 (1.0) | 1.0 (1.0) | 1.0 (1.0) | 1,83 | 0.04 | .85 |
| No. of psychiatric services | 1.3 (1.2) | 0.9 (0.8) | 1.1 (1.0) | 1,83 | 3.51 | .07 |
| No. d took medication for psychiatric problems | 39.2 (22.3) | 38.3 (22.5) | 38.7 (22.3) | 1,83 | 0.03 | .86 |
| Serious adverse events | ||||||
| Participants with ≥ 1 SAEs, psychiatric or substance use related | 3 (7) | 2 (4.1) | 5 (5.4) | 1 | 0.37 | .54 |
| Participants with ≥ 1 SAEs, medical issues | 1 (2.3) | 3 (6.1) | 4 (4.3) | 1 | 0.79 | .37 |
| Secondary substance use outcomes | ||||||
| % days abstinent from primary drug during treatment, self-report | 76.9 (24.7) | 62.2 (37.6) | 69.0 (32.9) | 1,87 | 4.54 | .04 |
| % urine specimens negative for all drugs | 42.9 (44.5) | 37.4 (41.4) | 39.9 (42.7) | 1,87 | 0.37 | .54 |
| % positive breathalyzer tests | 2.8 (15.8) | 6.3 (19.6) | 4.7 (17.9) | 1,87 | 0.84 | .36 |
Note. CBT4CBT = computerized cognitive behavioral therapy plus treatment as usual; SAE = National Institutes of Health–defined serious adverse event within treatment; TAU = treatment as usual.
Within-Treatment and Follow-Up Outcomes
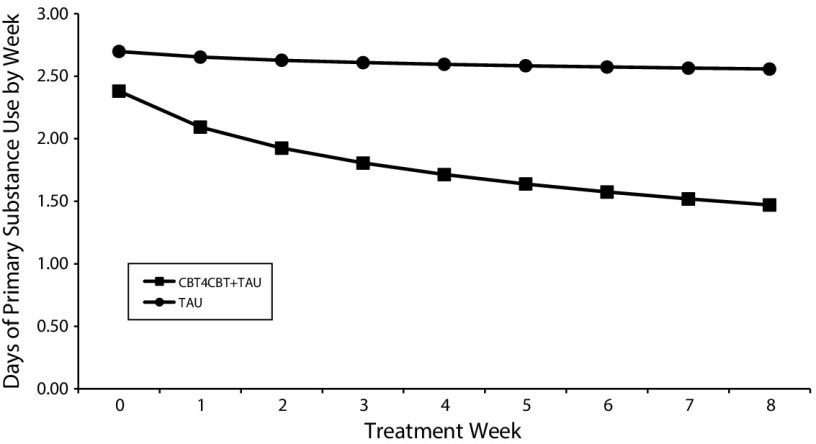
Results of random regression analyses for the primary outcome (days of primary substance use by week) are illustrated in Figure 2 and presented, including confidence intervals, in Table A (available as a supplement to the online version of this article at http://www.ajph.org). For the intent-to-treat sample, the model indicated no significant change for the sample as a whole during the 8-week treatment period (effect for time, t1715 = 0.71; P = .48) but confirmed the primary hypothesis, indicating significantly greater reduction in frequency of primary substance use over time for CBT4CBT plus TAU compared with TAU alone (treatment by week, t1718 = −2.64; P = .01). Sensitivity analyses, also shown in Table A, indicated that results were similar regardless of sample (treatment initiators, treatment exposed) and how site or primary substance were modeled (e.g., included as a clustering variable or ignored) and extended to the secondary outcome of days of any drug or alcohol use by week.
FIGURE 2—
Rates of Change in Frequency of Primary Substance Use Across Time, Results of Random Effects Analyses: New Haven, CT, March 2015–December 2016
Note. CBT4CBT = computerized cognitive behavioral therapy plus treatment as usual; TAU = treatment as usual.
Secondary Outcomes
As shown in Table 2, analyses of secondary outcomes were consistent with analyses of primary outcomes—in that there were significant differences in self-reported days of abstinence from the primary drug used during treatment (77% for CBT4CBT plus TAU vs 62% for TAU). Results of urine and breath samples both favored CBT4CBT plus TAU over TAU alone but did not reach statistical significance, which was anticipated because of the marked heterogeneity in substance use type and patterns in the sample.
As a measure of the extent to which the CBT4CBT-Spanish program conferred basic knowledge of cognitive and behavioral concepts, a 28-item true–false test, with items drawn from the Web site as well as the CBT manual (e.g., “everyone’s triggers are the same”), indicated low scores for both groups at baseline (CBT4CBT plus TAU 59.8% correct [SD = 11.2]; TAU 62.1% [SD = 8.9]). There was a significant effect for time (f1,78 = 19.1; P < .01) as well as a group by time effect, indicating greater increase in scores for those assigned to CBT4CBT (group by time f1,78 = 24.9; P < .01). Posttreatment scores were 71.2% correct for CBT4CBT plus TAU (SD = 11.6) and 61.4% (SD = 11.3) for TAU.
Effects of Psychiatric Diagnoses
Given the high level of current psychiatric comorbidity in the sample, exploratory analyses evaluated the effect of major diagnostic classes (depressive disorders, anxiety disorders, and SMI) on treatment utilization and substance use outcomes by including them as random factors in the random regression models we have described. Overall, participants with current depressive disorders, anxiety disorders, and SMI reported receiving significantly more psychiatric services and psychiatric medication than did those without these disorders, but there was no significant effect of these categories on treatment retention, CBT4CBT-Spanish modules, or data availability (data available on request). For mood and anxiety disorders, there was neither any main effect of these disorders on days of primary substance use nor any interaction of diagnostic category with treatment condition.
However, as shown in Table B (available as a supplement to the online version of this article at http://www.ajph.org), there was a significant interaction of treatment condition and current SMI status over time, such that those who had a current diagnosis of SMI who were assigned to TAU had less change in frequency of primary drug use over time than did the 3 other groups (participants without SMI assigned to TAU or CBT4CBT plus TAU and those with SMI assigned to CBT4CBT plus TAU), suggesting that CBT4CBT was effective in reducing substance use even among individuals with current psychotic or bipolar disorder (group by week by SMI status, f1714 = 7.91; P < .01).
Follow-up Outcomes
Analysis of 6-month follow-up outcomes indicated continuation of the benefits of adding CBT4CBT to TAU on the primary outcome. Using piecewise analyses, we found that for days of drug or alcohol use by week, there was a significant effect of group by time (f1,2706 = 4.2; P = .04) over the 6-month follow-up, as well as significant effect of group by time by phase (within treatment vs follow-up, f1,2706 = 6.29; P = .01), suggesting that the slopes (rate of change) of the groups changed from within treatment to follow-up. Results were similar regardless of how variance attributable to site or primary drug type was modeled (as a cluster variable or ignored) as well as when days of any drug or alcohol use was used as the dependent variable. Overall self-reported days of abstinence from the participants’ primary drug was lower for those assigned to CBT4CBT plus TAU than TAU alone throughout follow-up (83.4 vs 65.6 days, respectively; f1,73 = 6.41; P = .01), as was reported days of abstinence from all drugs and alcohol (72.1 vs 56.8; f1,73 = 3.61; P = .06).
DISCUSSION
In this randomized clinical trial of a culturally adapted, a Web-based version of CBT for primarily Spanish-speaking individuals, the primary a priori hypothesis was confirmed in that those assigned to CBT4CBT-Spanish had a significantly greater reduction in days of their primary substance use over time than did those who received standard treatment only. These effects were detectable through a 6-month follow-up. Results from a pre–post treatment quiz indicated significantly greater learning of CBT concepts for those assigned to the program. As with previous randomized trials evaluating the CBT4CBT program,10,11,13,28 no adverse events occurred that were attributable to the program. Finally, exploratory analyses indicated that the efficacy of adding CBT4CBT-Spanish on substance use outcomes extended even to participants with current psychotic or bipolar disorder, suggesting the program’s efficacy may extend to a wide range of individuals.
Strengths and Limitations
Strengths of this trial included a diverse sample of adult Latinos with long histories of substance use and psychiatric disorders seeking treatment in different settings and multiple methodologic features associated with high rigor in studies of Web-based therapies,29,30 including computerized randomization, a clinical population meeting DSM-IV criteria for current substance abuse or dependence, close monitoring of treatment delivery across conditions,31 high and consistent rates of data availability extending through a 6-month follow-up, and biologic verification of self-report. In terms of weaknesses, it should be noted that this trial evaluated CBT4CBT-Spanish as an add-on to standard treatment, rather than as a stand-alone intervention. However, the English version from which the Spanish cultural adaptation was developed has been shown to be effective as a stand-alone treatment, with minimal clinical monitoring, in 2 independent trials.10,28 The power to evaluate outcomes by specific substance or psychiatric disorder types, as well as gender and severity, was limited. Finally, the US Latino population is heterogeneous; studies in other geographic areas are ongoing and necessary to determine generalizability to other samples.
Public Health Implications
To our knowledge, this is the first randomized trial to evaluate a culturally adapted, Web-based intervention specifically for Spanish-speaking individuals with substance use disorders. Its efficacy across sites suggests good generalizability; the high rates of completion are notable for Web-based interventions32 for which rates of attrition are typically high, demonstrating strong acceptability. Moreover, that this is the first trial to indicate that the beneficial effects of a Web-based intervention carried over to those with severe mental illness is unprecedented and suggests that this approach can be used in a wide range of settings.
As the number of Latinos accessing the Internet grows (from 64% to 81% between 2009 and 2015),6 these findings underscore the fact that technology has the potential to provide easily accessible, inexpensive forms of treatment of this population.33 Considering the multiple barriers facing this community, particularly the lack of culturally adapted and evidence-based interventions coupled with a shortage of adequately trained bilingual providers,34 the public health significance for approaches such as these is compelling.
ACKNOWLEDGMENTS
This research was supported by the National Institute on Drug Abuse (grants R01 DA030369, P50 DA09241, U10 DA015831, and R37/01 DA15969).
K. M. C. is a member in trust of CBT4CBT, LLC, which makes some versions of CBT4CBT available to clinical providers on an affordable basis. The conflict is managed through Yale University.
We are deeply grateful to the staff and patients at the Hispanic Clinic of the Connecticut Mental Health Center, particularly Robert Cole and Esperanza Diaz, MD; the Substance Abuse Treatment Unit of the Connecticut Mental Health Center, particularly Donna LaPaglia, PhD, and Catherine Segura, and Multicultural Ambulatory Addiction Services, particularly Kristen Bonilla and Asher Delerme. We are indebted to Julian Pozzi, who directed the video vignettes, and especially Rick Leone, Craig Tomlin, and Doug Forbush of the Yale Broadcast Center and Lucas Swineford and Thom Stylinski of the Yale Center for Teaching and Learning; we appreciate their creativity, resourcefulness, and dedication. Jose Szapocznik and Viviana Horigian of the University of Miami provided valuable consultation on the cultural adaptation.
HUMAN PARTICIPANT PROTECTION
The Yale University Human Investigation Committee institutional review board approved this study.
REFERENCES
- 1.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR. Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health Aff (Millwood) 2016;35(8):1416–1423. doi: 10.1377/hlthaff.2015.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alegria M, Alvarez K, Falgas-Bague I. Clinical care across cultures: what helps, what hinders, what to do. JAMA Psychiatry. 2017;74(9):865–866. doi: 10.1001/jamapsychiatry.2017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrero EG, Marsh JC, Khachikian T, Amaro H, Vega WA. Disparities in Latino substance use, service use, and treatment: implications for culturally and evidence-based interventions under health care reform. Drug Alcohol Depend. 2013;133(3):805–813. doi: 10.1016/j.drugalcdep.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Department of Health and Human Services; 2016. [PubMed] [Google Scholar]
- 6.Brown A, López G, Lopez MH. Digital Divide Narrows for Latinos as More Spanish Speakers and Immigrants Go Online: Broadband Use Little Changed in Recent Years Among Hispanics. Washington, DC: Pew Research Center; 2016. [Google Scholar]
- 7.Muñoz RF. Using evidence-based Internet interventions to reduce health disparities worldwide. J Med Internet Res. 2010;12(5):e60. doi: 10.2196/jmir.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López L, Green AR, Tan-McGrory A, King R, Betancourt JR. Bridging the digital divide in health care: the role of health information technology in addressing racial and ethnic disparities. Jt Comm J Qual Patient Saf. 2011;37(10):437–445. doi: 10.1016/s1553-7250(11)37055-9. [DOI] [PubMed] [Google Scholar]
- 9.Rogers MA, Lemmen K, Kramer R, Mann J, Chopra V. Internet-delivered health interventions that work: systematic review of meta-analyses and evaluation of website availability. J Med Internet Res. 2017;19(3):e90. doi: 10.2196/jmir.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiluk BD, Devore KA, Buck MB et al. Randomized trial of computerized cognitive behavioral therapy for alcohol use disorders: efficacy as a virtual stand-alone and treatment add-on compared with standard outpatient treatment. Alcohol Clin Exp Res. 2016;40(9):1991–2000. doi: 10.1111/acer.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll KM, Kiluk BD, Nich C et al. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171(4):436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: a 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100(1–2):178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll KM, Ball SA, Martino S et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165(7):881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;(suppl 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 15.Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9(2):183–205. [Google Scholar]
- 16.Carroll KM, Nich C, DeVito EE, Shi JM, Sofuoglu M. Galantamine and computerized cognitive behavioral therapy for cocaine dependence: a randomized clinical trial. J Clin Psychiatry. 2018;79(1):17m11669. doi: 10.4088/JCP.17m11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Añez LM, Paris M, Jr, Bedregal LE, Davidson L, Grilo CM. Application of cultural constructs in the care of first generation Latino clients in a community mental health setting. J Psychiatr Pract. 2005;11(4):221–230. doi: 10.1097/00131746-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Añez LM, Silva MA, Paris MP, Bedregal LE. Engaging Latinos through the integration of cultural values and motivational interviewing. Prof Psychol Res Pr. 2008;39(2):153–159. [Google Scholar]
- 19.Torrens M, Serrano D, Astals M, Pérez-Domínguez G, Martín-Santos R. Diagnosing comorbid psychiatric disorders in substance abusers: validity of the Spanish versions of the psychiatric research interview for substance and mental disorders and the structured clinical interview for DSM-IV. Am J Psychiatry. 2004;161(7):1231–1237. doi: 10.1176/appi.ajp.161.7.1231. [DOI] [PubMed] [Google Scholar]
- 20.Butler SF, Redondo JP, Fernandez KC, Villapiano A. Validation of the Spanish Addiction Severity Index Multimedia Version (S-ASI-MV) Drug Alcohol Depend. 2009;99(1–3):18–27. doi: 10.1016/j.drugalcdep.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll KM, Martino S, Ball SA et al. A multisite randomized effectiveness trial of motivational enhancement therapy for Spanish-speaking substance users. J Consult Clin Psychol. 2009;77(5):993–999. doi: 10.1037/a0016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 23.Hedeker D, Mermelstein RJ. Analysis of longitudinal substance use outcomes using ordinal random-effects regression models. Addiction. 2000;95(suppl 3):S381–S394. doi: 10.1080/09652140020004296. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons RD, Hedeker D, Elkin I et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 25.Singer JD, Willett JB. Applying Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 26.Schwartz RH. Urine testing in the detection of drugs of abuse. Arch Intern Med. 1988;148(11):2407–2412. [PubMed] [Google Scholar]
- 27.Donovan DM, Bigelow GE, Brigham GS et al. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107(4):694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiluk BD, Nich C, Buck MB et al. Randomized clinical trial of stand-alone computerized cognitive behavioral therapy and clinician-delivered CBT in comparison with standard outpatient treatment for substance use disorders: primary within-treatment and follow-up outcomes. Am J Psychiatry. 2018;175(9):853–863. doi: 10.1176/appi.ajp.2018.17090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiluk BD, Sugarman DE, Nich C et al. A methodological analysis of randomized clinical trials of computer-assisted therapies for psychiatric disorders: toward improved standards for an emerging field. Am J Psychiatry. 2011;168(8):790–799. doi: 10.1176/appi.ajp.2011.10101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danaher BG, Seeley JR. Methodological issues in research on web-based behavioral interventions. Ann Behav Med. 2009;38(1):28–39. doi: 10.1007/s12160-009-9129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa C, Campbell AN, Miele GM, Brunner M, Winstanley EL. Using e-technologies in clinical trials. Contemp Clin Trials. 2015;45(pt A):41–54. doi: 10.1016/j.cct.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rooke S, Thorsteinsson E, Karpin A, Copeland J, Allsop D. Computer-delivered interventions for alcohol and tobacco use: a meta-analysis. Addiction. 2010;105(8):1381–1390. doi: 10.1111/j.1360-0443.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 33.Rodriquez EJ, Pérez-Stable EJ. The time is now for eHealth research with Latinos. Am J Public Health. 2017;107(11):1705–1707. doi: 10.2105/AJPH.2017.304055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paris M, Silva MA, Diaz E, Bedregal LE, Cole RA, Añez-Nava LM. The Connecticut Latino Behavioral Health System: a culturally informed community-academic collaboration. Psychol Serv. 2016;13(2):140–147. doi: 10.1037/ser0000065. [DOI] [PubMed] [Google Scholar]