Abstract
Background
The physiological and biochemical demands of intense exercise elicit both muscle-based and systemic responses. The main adaptations to endurance exercise include the correction of electrolyte imbalance, a decrease in glycogen storage and the increase of oxidative stress, intestinal permeability, muscle damage, and systemic inflammatory response. Adaptations to exercise might be influenced by the gut microbiota, which plays an important role in the production, storage, and expenditure of energy obtained from the diet as well as in inflammation, redox reactions, and hydration status.
Methods
A systematic and comprehensive search of electronic databases, including MEDLINE, Scopus, ClinicalTrials.gov, ScienceDirect, Springer Link, and EMBASE was done. The search process was completed using the keywords: “endurance”, “exercise”, “immune response”, “microbiota”, “nutrition”, and “probiotics”.
Results
Reviewed literature supports the hypothesis that intestinal microbiota might be able to provide a measureable, effective marker of an athlete's immune function and that microbial composition analysis might also be sensitive enough to detect exercise-induced stress and metabolic disorders. The review also supports the hypothesis that modifying the microbiota through the use of probiotics could be an important therapeutic tool to improve athletes' overall general health, performance, and energy availability while controlling inflammation and redox levels.
Conclusion
The present review provides a comprehensive overview of how gut microbiota may have a key role in controlling the oxidative stress and inflammatory responses as well as improving metabolism and energy expenditure during intense exercise.
Keywords: Endurance, Exercise, Immune response, Microbiota, Nutrition, Probiotics
1. Introduction
Endurance exercise can be defined as cardiovascular exercise—such as running, cross-country skiing, cycling, aerobic exercise, or swimming—that is performed for an extended period of time.1 The physiological and biochemical demands of endurance exercise elicit both muscle-based and systemic responses. Endurance athletes expose their bodies to extreme physiological circumstances that disrupt inner body's homeostasis, overwhelming organs and the system's normal function. Physical exertion at a very high level for a prolonged time means that the whole body initiates a defense response through the synthesis of acute phase proteins, hormone release, and shifts in fluid and metabolic balance. The main adaptations to endurance exercise include an improvement of mechanical, metabolic, neuromuscular, and contractile functions in muscle,2 a rebalance of electrolytes,3 a decrease in glycogen storage,4 and an increase in mitochondrial biogenesis in muscle tissue.5 Furthermore, endurance exercise has a profound impact on oxidative stress,5 intestinal permeability, muscle damage, systemic inflammation, and immune responses.5 An increase in body temperature changes blood flow and increases dehydration, which causes the release of adrenaline and glucocorticoids as a way to reestablish homeostatic equilibrium.6
The human gut harbors a vast array of microorganisms that significantly affect host nutrition, metabolic function, gut development, and maturation of the immune system and epithelial cells.7 Overall, the gut microbiota comprises 5 phyla and approximately 160 species in the large intestine.8 Although very few of these species are shared between unrelated individuals, the functions carried out by these species appear to be similar in everybody's gastrointestinal tract.9 The gut microbiota promotes digestion and food absorption for host energy production,10 whereas in the colon, complex carbohydrates are digested and subsequently fermented into short chain fatty acids (SCFAs) such as n-butyrate, acetate, and propionate. Propionate and acetate are carried in the bloodstream to a variety of different organs where they are used as substrates for energy metabolism, particularly by the hepatocyte cells, which use propionate for gluconeogenesis.11 The gut microbiota also plays a fundamental role in the induction and function of the host immune system,12 protection from pathogens, and stimulation and maturation of epithelial cell.13 Endurance athletes present a high prevalence of upper respiratory tract infections and gastrointestinal troubles, including increased permeability of the gastrointestinal epithelial wall, also called “leaky gut”, disruption of mucous thickness and higher rates of bacterial translocation.14 Understanding the effect of exercise on gut microbiota composition and structure is still in its infancy and the function of microbiota on exercise adaptation remains unknown, but a few studies have shown the impact exercise has on the gut microbiota composition.
A recent observational study comparing the fecal bacterial profile of male elite rugby players with non-athlete healthy subjects15 showed that athletes had lower levels of bacteroidetes and greater amounts of firmicutes than controls. After analyzing the gut microbiota composition of the participants of the American Gut Project,16 it was concluded that increasing moderate exercise frequency from never to daily causes greater diversity among the firmicutes phylum (including Faecalibacterium prausnitzii, and species from the genus Oscillospira, Lachnospira, and Coprococcus) which contribute to a healthier gut environment. In the limited studies available in animal models, exercise in rats was associated with higher bacteroidetes and lower firmicutes in fecal matter,17, 18 whereas the cecal microbiota following 6 weeks of exercise activity presented a greater abundance of selected firmicutes species and lower Bacteroides/Prevotella genera.19 Similarly, at the phyla level, exercise reduced bacteroidetes, while it increased firmicutes, proteobacteria, and actinobacteria in mice.20, 21 Additionally, gut microbiota can improve some of the other exercise-induced disturbances in the gastrointestinal tract like oxidative stress19, 22 and hydration levels.23
Given the gut microbiota's fundamental role in the regulation of energy metabolism, hydration, inflammatory response and oxidative stress, the aim of this systematic review is to review papers published from 2007 that allow greater understanding of how the gut microbiota may exert beneficial effects on elite athletes. With a specific focus on endurance, the proposed systematic review will first answer whether the microbiota–host relationships specifically could influence the energy metabolism, hydration, oxidative stress, and inflammation in the gastrointestinal tract. Second, it will identify probiotics, prebiotics, or other functional foods that could modify the microbiota composition and improve both overall health (i.e., improving the conditions of the intestinal epithelium and the immune system response) and performance (i.e., improving energy availability from diet and controlling the inflammation levels in athletes).
2. Methods
This study was executed according to the requirements established in the preferred reporting items for systematic review and meta-analysis protocols (PRISMA).24
The articles selected were divided into the following categories: (i) generic articles about gut microbiota and exercise; (ii) articles about the relationship between probiotics consumption and exercise response. With regard to the generic articles about gut microbiota and exercise, publications of any type were included if they reported data that linked intestinal microbiota and exercise in humans and animal models. The search was not restricted to the type of exercise, exercise intensity, gender, clinical condition, sample size, specie, year of publication, publication status, or length of follow-up. No study design limit was imposed on the search, although only studies published in English were included. We included randomized controlled trials (RCTs) that compared athletes' gut microbiota from sedentary individuals, case–control studies, and prospective cohort studies. Given that the studies in humans and animals analyzing the gut microbiota's involvement in various responses to exercise are still in its infancy, we did not restrict the criteria papers selected for this review. The search process was completed using the keywords “exercise”, “endurance”, and “microbiota”. For each study, the following information was retrieved: species, number of individuals, individual characteristics, level and frequency of exercise, experimental design, and duration of follow-up. The primary outcome was the gut microbiota profile, the indicators of immune response, oxidative stress, dehydration, or other clinical outcome.
To understand the relationship between probiotics consumption, gut microbiota, and exercise response, we selected observational studies in humans, including case–control, prospective cohort studies, randomized, blinded and counterbalanced cross-over designs, and pre–post controlled trial with control (but no placebo treatment). The search process was completed using the keywords “athletes”, “exercise”, “endurance”, “microbiota”, “nutrition”, “probiotics”, and “prebiotics”. For each study, the following information was retrieved: number of individuals, individual characteristics, the experimental intervention, the type of control used, dosage, frequency, and duration of treatment, patient characteristics, duration of follow-up, and the primary outcomes.
This review was not registered a priori and nor was a protocol published prior to the start of the study. An addendum of the original article by Clarke et al.15 has been recently published under the title of Exercise and the Microbiota.25 Based on data from the Irish international rugby football team, they discuss the relationship between exercise, associated dietary habits, and gut microbiota composition.25 They also describe the potential mechanisms by which exercise may exert a direct or indirect effect on gut microbiota, but they do not explain how the gut microbiota may contribute to the individual's exercise performance and health. Similarly, the recently published review entitled Exercise, Fitness, and the Gut26 explains the benefits of regular exercise in the treatment and prevention of gastrointestinal conditions, but it does not explain how the gut microbiota may contribute to individual's exercise performance.
Due to the nature of our review, no request was performed for the ethics committee's approval. We searched MEDLINE, Scopus, ClinicalTrials.gov, ScienceDirect, Springer Link, and EMBASE for publications in English documenting the role of microbiota in exercise. Bibliographies of the identified reviews and original research publications were hand-selected for additional studies that may have been missed by the database searches. We also searched Web of Science for conference proceedings and abstracts that may not have been indexed in the databases mentioned before.
Records were imported into a bibliographic database. The 2 authors independently assessed titles and abstracts for eligible publications. If eligibility could not be determined, the full article was retrieved.
A search conducted in October 2015 yielded the following list of key term combinations (microbiota and exercise = 46; probiotic and athlete = 30). Clearly the focus on the research community investigating how exercise and healthy gut microbiota help maintain good health and sport performance is in its early states. Finally, a total of 33 experimental studies met the inclusion criteria and were included in the review. All reports were journal articles, except for 1 PhD thesis. Most of the studies were randomized controlled trials. Periods of data collection spanned from 2007 to 2015, including data from human and animal models (i.e., mice and rats).
3. Results and discussion
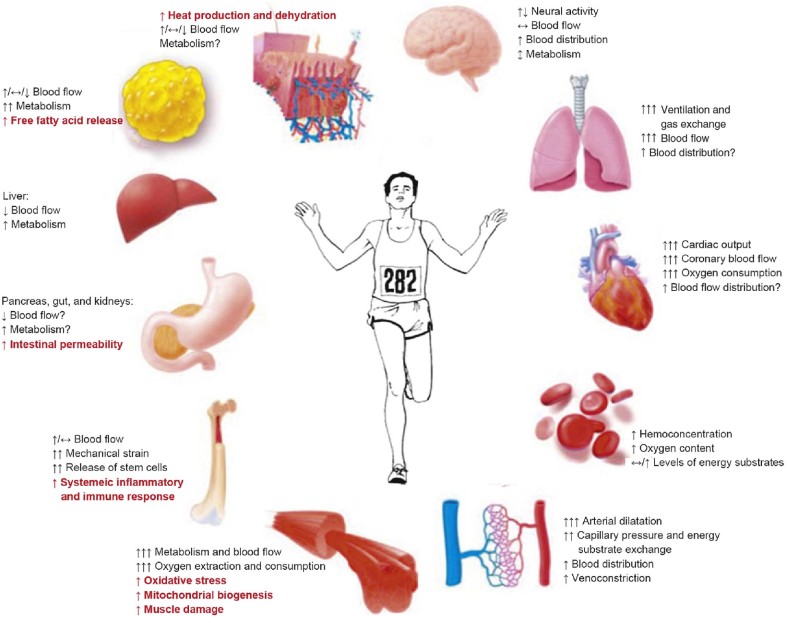
Endurance exercise has a profound impact on metabolism in tissues other than skeletal muscle, including the heart, brain, adipose tissue, and liver (Fig. 1).27 By reviewing the respective role microbiota has on exercise regulation, we were able to identify a large number of biological functions that fit neatly into the well-characterized context of adaptive regulation in response to endurance exercise, including energy metabolism, inflammatory response, stress resistance, and oxidative stress. These changes may help supply the working muscles with energy or control excessive inflammatory reactions. They might be involved in “staleness” and the transient immunosuppression that can occur during and/or after endurance exercise. Additionally, we discovered to what extent probiotics, in conjunction with diet composition and type of exercise and intensity, affect health and exercise performance in athletes.
Fig. 1.
The physiological and biochemical demands of endurance exercise elicit both muscle-based and systemic responses. The main adaptations to endurance exercise include an improvement of mechanical, metabolic, neuromuscular and contractile functions in muscle, a rebalance of electrolytes, a decrease in glycogen storage and an increase in mitochondrial biogenesis in muscle tissue. Moreover, endurance exercise has a profound impact on oxidative stress, intestinal permeability, muscle damage, systemic inflammation and immune responses. Additionally, there is increased ventilation and pumping function of the heart associated with substantially decreased peripheral vascular resistance in the muscles. This facilitates the delivery of oxygen and nutrients to working muscles, which consume high amounts of oxygen and nutrients, especially when exercise intensity increases. ↑: increases; ↔: no change in response; ↓: decreases; ↕: may increase or decrease.
Adapted with permission.109
3.1. The link between exercise and alterations in the gut microbiota
The impact gastrointestinal tract microbiota has on health and performance, including metabolism of nutrients, growth and maturation of the immune response, protection from pathogens, and stimulation of epithelial cell proliferation, is becoming increasingly apparent.28, 29 In humans, there is growing evidence that perturbations of the gut microbiota composition and functions may play an important role in the development of the host metabolism and diseases.28 The current perception is that microbiota composition and structure are regulated by the metabolic niche (mainly diet, antimicrobials, and lifestyle behaviors), host genetics, microbe–microbe interactions, inflammation status, and host–microorganism crosstalk.30, 31, 32 Generally, the gut microbiota composition is estimated by analyzing fresh fecal samples because they are relatively easy to obtain. The strength of the associations between fecal samples and species richness, enterotypes, which are classifications of bacteriological ecosystems, and the bacterial community composition in gut emphasizes the importance of fecal sample assessment in gut metagenome-wide association studies.33
Although little is known about how the gut microbiome may contribute to an individual's exercise performance, accumulating literature shows that exercise alone induces modifications in the gut microbiota composition (Table 1). After analyzing a total of 1493 human fecal samples from the participants of the American Gut Project, McFadzean16 concluded that exercise leads to an increase in α-diversity, which is the number and distribution of kinds of taxa or lineages in 1 sample within an individual, especially in certain members of the firmicutes phylum. Similarly, a recent study in elite rugby players suggested that athletes have a greater gut microbial diversity compared to sedentary individuals.15 They found significantly higher proportions of the genus Akkermansia in the rugby players as well as in low body mass index control group,15 which is generally associated with a healthier metabolic profile.34 Although the authors were careful in their interpretation and did not associate correlation into causation, they suggested that increased microbial diversity is one of the beneficial effects of exercise.25 Similar positive effects on the gut microbiota have also been observed in laboratory animals. Matsumoto et al.35 showed that rats that participated in voluntary running exercise had increased colonic butyrate concentrations compared to sedentary rats due to higher levels of butyrate-producing bacterium from the firmicutes phylum (SM7/11 and T2-87) in their cecum. Most of the published works on murine models examine the combined effects of exercise, dietary interventions, and diseases. For example, according to Evans et al.,17 exercise increases the bacteroidetes phylum while it decreases firmicutes in a manner that is proportional to the distance ran by mice who were fed a high-fat diet. In a case–control study, Queipo-Ortuno et al.18 described that moderate exercise in rats may affect the α-diversity of the gut microbiota by increasing Lactobacillus, Bifidobacterium, and Blautia coccoides–Eubacterium rectale species while decreasing Clostridium and Enterococcus genera compared to sedentary male rats. Similarly, Lambert et al.19 showed that 6 weeks of exercise activity in diabetic and control mice resulted in a greater abundance of some firmicutes species and lower Bacteroides/Prevotella genera compared to sedentary counterparts. Accordingly, it has been reported20 that exercise alone causes modifications in mice gut microbiota at nearly the same magnitude as high-fat diet. Exercise reduced the Streptococcus genus and bacteroidetes and tenericutes phyla, while increasing the firmicutes phylum. The results from Petriz et al.36 in obese, non-obese, and hypertensive male rats showed that moderate exercise altered the composition and a-diversity of gut microbiota. A recent human study elucidated how intense exercise (4-day cross-country ski-march) modifies gut microbiota composition. Compared with controls, individuals following an intense training showed an increased level of microbial diversity, an increased abundance of members of the commensal microbiota that may become pathogenic under certain circumstances and a decreased abundance of the dominant beneficial species, such as members of the Bacteroidaceae, and Lachnospiraceae families.37 Exercise also enhanced the relative abundance of Lactobacillus, while Streptococcus, Aggregatibacter, and Sutterella were shown to be more abundant before exercise training. They also made a significant correlation between the bacterial families clostridiaceae and bacteroidaceae and the Oscillospira and Ruminococcus genera and blood lactate accumulation. Results from Choi et al.21 showed that oral exposure to polychlorinated biphenyls (PCB, 150 µmol/kg) significantly changed the mice gut microbiota mainly by reducing the amount of proteobacteria. They also discovered that exercise decreased the PBC-induced alterations in the gut ecology, which may protect them against dangerous xenobiotic effects.
Table 1.
Relationship between gut microbiota and exercise performance.
| Author | Year | Species | Number of individuals | Level and frequency of exercise | Experimental design | Duration of experiment | Results/conclusions |
|---|---|---|---|---|---|---|---|
| Choi et al.21 | 2013 | Mice | 12 (n = 6/group) | Mice were randomly distributed into 2 groups: (i) exercised and (ii) sedentary. For the exercised mice, activity on a running wheel was monitored 24 h/day, 7 day/week. Wheels were locked in the cages of sedentary mice. A total PCB dose of 150 µmol/kg was administered to mice, resulting in a PCB plasma level of 5 µmol/L. | Randomized controlled trial | 5 weeks, 1 sampling point | Exercise attenuates changes in microbiota induced by oral exposure to PCBs. Exercise increased phylum firmicutes, class bacilli, and most of these were in the order lactobacillales. The taxa that were decreased in the exercised group belonged to phyla tenericutes and bacteroidetes. Exercised mice had a decrease in Erysipelotrichaceae bacterium C11_K211 from phylum tenericutes compared to sedentary mice. |
| Clarke et al.15 | 2014 | Human | 86 | Male professional rugby players (n = 40) and healthy male controls (n = 46) were included in the study. Controls were divided into 2 groups based on their physical size (BMI) relative to the athletes, gender, and age. Each participant completed a detailed food frequency questionnaire. | Case–control study | 1 sampling point | Athletes had a higher diversity of gut microorganisms, representing 22 distinct phyla. Bacteroidetes was significantly less abundant in athletes. The top changes in relative abundance were in the firmicutes, Ruminococcaceae, S24-7, Succinivibrionaceae, RC9 gut, and Succinivibrio groups. Notably, there were significantly higher proportions of Akkermansiaceae and Akkermansia in elite athletes compared to the high BMI controls. |
| Evans et al.17 | 2014 | Mice | 48 (n = 12/group) | Male littermates (5 weeks old) were randomly distributed into 4 groups: (i) LF/Sed, (ii) LF/Ex, (iii) HF/Sed, and (iv) HF/Ex. Mice were individually housed and LF/Ex and HF/Ex cages were equipped with a wheel and odometer to record exercise. | Randomized controlled trial | 12 weeks, 3 sampling points: baseline, 6 weeks, and 12 weeks | Exercise induces a unique shift in the gut microbiota that was different from dietary effects. The bacteroidetes phylum increased while it decreased firmicutes in a manner that was proportional to the distance run in mice fed with HFD. |
| Hsu et al.10 | 2015 | Mice | 24 (n = 8/group) | Male (12 weeks old) were (i) SPF, (ii) GF, or (iii) BF gnotobiotic. Swimming was performed in plastic containers. Mice were considered exhausted when they failed to rise to the surface of the water to breathe after 7 s. | Prospective cohort study | 1 sampling point | Endurance swimming time was longer for SPF and BF than GF mice, and the weight of liver, muscle, brown adipose, and epididymal fat pads was higher for SPF and BF than GF mice. The serum levels of GPx and catalase were greater in SPF than GF mice. SOD activity was lower in BF than SPF and GF mice. In addition, hepatic GPx level was higher in SPF than GF and BF mice. Gut microbial status could be crucial for exercise performance and its potential action linked with the antioxidant enzyme system in athletes. |
| Kang et al.20 | 2014 | Mice | 40 (n = 10/group) | Male (8 weeks old) were randomly distributed into 4 groups: (i) ND, (ii) ND + exercise, (iii) HFD, and (iv) HFD + exercise. Exercised groups were placed in running wheels for 1 h at 7 m/min every morning for 5 day/week. Control “sedentary” groups were placed in adjacent running wheels that rotated at a speed that just prevented them from sleeping (~1 m/min) to control for environmental enrichment and handling. | Randomized controlled trial | 1 sample point | Exercise alone caused massive shifts in the gut microbiome at nearly the same magnitude as diet but shifts were unrelated (orthogonal). At the phyla level, exercise reduced bacteroidetes, while increased firmicutes, proteobacteria and actinobacteria. Additionally, exercise reduced the abundance of Porphyromonadaceae, Streptococcaceae, Peptococcaceae_2 family, while increased the peptostreptococcacea, the cryomorphaceae, the rhizobiaceae, and the Incertae Sedis IV. |
| Lambert et al.19 | 2015 | Mice | 38 (n = 9–10/group) | Male (6 weeks old) with (i) type 2 diabetic db/db and (ii) db/+ (heterozygote; control) were randomly distributed into 2 groups: sedentary or exercise group for 6 weeks. Exercise consisted of low-intensity treadmill running for 5 day/week. db/+ mice exercised for 60 min/session at 4.79 m/min (287 m/session) and db/db mice for 66 min/session at 2.87 m/min (189 m/session). | Randomized controlled trial | 6 weeks, 1 sampling point | The interaction between diabetic state and exercise training affected the cecal abundance of total bacteria; enterobacteriaceae and Bifidobacterium spp. Specifically, total bacteria and enterobacteriaceae were similar in db/+ mice regardless of exercise, but lower with exercise in db/db mice. Bifidobacterium spp. was greater in exercised non-diabetic mice. Exercise was independently associated with lower abundance of Bacteroides/Prevotella spp. and Methanobrevibacter spp., and greater Lactobacillus spp. and Clostridium leptum. |
| Matsumoto et al.35 | 2008 | Rats | 14 (n = 7/group) | Male (6 weeks old) were randomly distributed by matched weight into 2 groups: (i) sedentary (control) group or (ii) a voluntary wheel-running exercise group. The rats in the exercise group were moved to cages equipped with running wheels. | Randomized block design | 5 weeks, 1 sampling point | Exercised rats presented increased colonic butyrate concentrations compared with sedentary rats. The temperature gradient gel electrophoresis analysis suggested that the appearance of the butyrate-producing bacteria associated with the alteration in the cecal microbiota was the reason for the n-butyrate increase in the cecum. |
| McFadzean16 | 2014 | Human | 1493 | Each participant was categorized according to his or her exercise frequency into: (i) never, (ii) rarely, (iii) occasionally, (iv) regularly, and (v) daily. | Prospective cohort study | 1 sampling point | Faecalibacterium prausnitzii was the only species to be significantly different among exercise. They showed an increase in Faecalibacterium prausnitzii as exercise increases. There was a significant increase in α-diversity among individuals who exercised more frequently. |
| Petriz et al.36 | 2014 | Rats | 15 (n = 5/group) | Three different strains from 2 different genotypes were included in the study: (i) an obese genotype, homozygous (fa/fa) obese (Obese rats), (ii) hypertensive genotype (Hypertensive rats), and (iii) a strain obtained by the selective breeding of Wistar-Kyoto rats with high blood pressure. Duration and speed on treadmill were increased progressively (up to 12.5 m/min for obese rats; 20 m/min for hypertensive and Wistar rats). All animals were trained for 30 min/day, 5 day/week for 4 weeks. | Prospective cohort study | 4 weeks | Exercise altered the composition and diversity of gut bacteria at genus level in all rat lineages. In obese rats, Pseudomonas and Lactobacillus were both significantly altered after exercise training. Minimal variation in Pseudomonas relative abundance was observed between samples, while Lactobacillus presented the higher relative abundance after exercise from all identified genera. Another 3 genera were shown to be more abundant before exercise training (Streptococcus, Aggregatibacter, and Sutterella). A significant correlation was seen in the clostridiaceae and bacteroidaceae families and Oscillospira and Ruminococcus genera with blood lactate accumulation. |
| Queipo-Ortuno et al.18 | 2013 | Rats | 40 (n = 10/group) | Weight-matched rats (5 weeks old) were randomly assigned to 1 of 4 experimental groups: (i) ABA group; (ii) control ABA group: rats submitted to the same food restriction schedule as ABA with no wheel access exercise, (iii) exercise group: rats feed ad libitum with free access to the activity wheel, and (iv) ad libitum group: rats feed ad libitum but without access to the activity wheel. | Case–control study | 6 days | Nutritional status and exercise affected the diversity and similarity of the gut microbiota. The number of Lactobacillus, Bifidobacterium, and Blautia coccoides–Eubacterium rectale group was greater in the exercise group with respect to the ABA, control ABA, and ad libitum groups. Both Bifidobacteria and Lactobacillus had the capacity to produce the organic acid lactate, which is converted into butyrate by butyrate-producing bacteria in the gut. Clostridium and Enterococcus appeared decreased in the exercise group. |
Abbreviations: ABA = activity based anorexia; BF = bacteroides fragilis; BMI = body mass index; Ex = exercise; GF = germ free; GPx = glutathione peroxidase; HFD = high fat diet; HF/Ex = high fat exercise; HF/Sed = high fat sedentary; LF/Ex = low fat exercise; LF/Sed = low fat sedentary; ND = normal diet; PCB = polychlorinated biphenyl; Sed = sedentary; SOD = superoxide dismutase; SPF = specific pathogen-free.
Only 1 study in mice has evaluated how the gut microbiota affects exercise performance. While most of the reviewed literature focuses on the effect that exercise produces in gut microbiota, Hsu et al.10 investigated the influence that intestinal microbiota has on endurance swimming time in specific pathogen-free (SPF), germ-free (GF), and Bacteroides fragilis (BF) gnotobiotic mice. Additionally, they found that the serum levels of glutathione peroxidase (GPx) and catalase (CAT) were greater in SPF than GF mice, while serum superoxide dismutase (SOD) activity was lower in BF than SPF and GF mice. In addition, hepatic GPx level was higher in SPF than GF and BF mice. The authors found that endurance swimming time was longer for SPF and BF than GF mice, suggesting that gut microbiota composition is crucial for exercise performance and could also potentially be linked to antioxidant enzyme systems in athletes (see Section 3.4 for further details).
Additionally, it must be taken into consideration that all the published articles to date are very bacteria-centric when looking at the gut microbiota after exercise; there are no papers that have looked at the viral component (or virome) and other eukaryotes such as protozoa and fungi. Despite this, there is no evidence to date that the depletion or enrichment of a single species (bacterial, fungal, viral, or other eukaryotes) is associated with better performance or health in athletes. As pinpointed by Marchesi et al.,9 we know that the gut microbiota is essential for the proper function and development of the host (e.g., energy metabolism, the inflammatory response, stress resistance, and oxidative stress), but we are unsure which are the key species and whether the microbiota's function as a whole is more important than any individual member of the ecological community for regulating the exercise response.
3.2. The role of gut microbiota in energy metabolism during endurance exercise
As previously mentioned, energy availability is an important limiting factor in the final performance during endurance exercise. After several minutes of muscle contractions, the concentration of phosphocreatine (PCr) declines, resulting in a need to use other fuels. This stimulus is rapidly sensed and transduced through signaling pathways into a coordinated transcriptional, post-transcriptional and allosteric response leading to the synthesis of specific molecules needed to restore the cellular energy homeostasis. First, the transcription of genes involved in glycogenolysis is induced to ensure the production of ATP meets the demand of the cross-bridge cycle (increased myosin ATPase activity) and muscle ion pumps. Unfortunately, mitochondria are unable to oxidize all the pyruvate produced during intense exercise, which leads to its conversion into lactate in the myoplasm.38 Intracellular acidosis may cause fatigue such as inhibiting energy metabolism.39 At the same time, lipolysis of adipose tissue provides an increase in fatty acids as well as increased plasma free fatty acid uptake and fatty acid oxidation.
Given the energy requirements during endurance exercise40 and the recently described complex and reciprocal relationship between the gut microbiota and whole body energy metabolism,19 it is not surprising that efforts to identify the mechanisms via which gut microbiota exerts positive performance effects in elite athletes are increasing. Carbohydrate fermentation is a core activity of the human gut microbiota, driving the energy and carbon metabolism of the colon, although the range of end products generated by protein digestion is broader than that of carbohydrates.41 In the colon, complex plant-derived polysaccharides (such as cellulose, β-glucan, xylan, mannan, and pectin) are digested and subsequently fermented by gut microorganisms into short-chain fatty acids (SCFAs) and gases (Fig. 2), which are also used as carbon and energy sources by other more specialized bacteria such as reductive acetogens, sulfate-reducing bacteria, and methanogens.9 The SCFAs affect a range of host processes including energy utilization, host–microbe signaling and control of colonic pH, with consequent effects on microbiota composition, intestinal gut motility, gut permeability, and epithelial cell proliferation.42 Gut motility, a gross measure of forces (resistive and propulsive) in the small intestine transit flow,43 is increasingly gaining interest since it is highly correlated with transit time and stool consistency. In turn, it has been recently published that stool consistency is a good biomarker for species richness and community composition.33
Fig. 2.
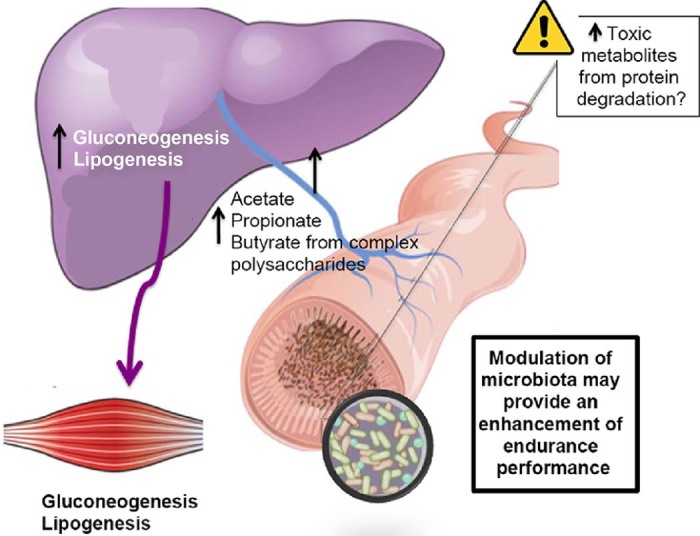
Complex polysaccharides are metabolized by the colonic microbiota to oligosaccharides and monosaccharides and then fermented to short-chain fatty acid (SCFA) end products, mainly acetate, propionate, and butyrate. The SCFAs are absorbed in the colon, where butyrate provides energy for colonic epithelial cells, and acetate and propionate reach the liver and peripheral organs, where they are substrates for gluconeogenesis and lipogenesis. The types and amount of SCFAs produced by gut microorganisms are determined by the composition of the gut microbiota and the metabolic interactions between specie.45 In addition to being energy sources, SCFAs control colonic gene expression involved in the immune response. It must be borne in mind that endurance diets are rich in protein (1.2–1.6 g/kg/day), which besides liberating beneficial SCFAs, produces a range of potentially harmful compounds in the intestine.
In addition to being a local nutrient source for colonocytes11 and a minor nutrient source for microorganisms such as Desulfotomaculum genus in the gut,44 n-butyrate has also been shown in cell-culture models and mice to regulate energy homeostasis by stimulating leptin production in adipocytes as well as provoking intestinal enteroendocrine L cells to secrete glucagon-like peptide-1 (GLP-1).11 The main genera that produce n-butyrate are Clostridia, Eubacteria, and Roseburia. Additionally, n-butyrate produced by gut bacteria regulates neutrophil function and migration, inhibits inflammatory cytokine-induced expression of vascular cell adhesion molecule-1, increases expression of tight junction proteins in colon epithelia and exhibits anti-inflammatory effects.29 Other SCFAs such as propionate and acetate are substrates for gluconeogenesis and lipogenesis in the liver and peripheral organs (e.g., muscle and adipose tissue11, 45). The types and amount of SCFAs produced by gut microorganisms are determined by the composition of the gut microbiota and the metabolic interactions between microbial species,46 but also by the amount, type, and balance of the main dietary macro- and micronutrients.41, 47, 48 The dietary regime of endurance athletes is based on high protein and carbohydrate consumption and very low fat intake together with the consumption of certain key micronutrients such as iron, calcium, and essential fatty acids.49 Overall, the dietary protein intake necessary for endurance athletes ranges from 1.2 to 1.6 g/kg/day in the top sport elite athletes50, 51 so that amino acids are spared for protein synthesis and are not oxidized to assist in meeting energy needs.51 Carbohydrate intake ranges from 7 to 12 g/kg/day and fat < 1 g/kg/day (<20% of total calories consumed).49 Although the fermentation of amino acids can produce beneficial by-products such as SCFAs, a range of potentially harmful compounds can also be produced.9 Studies in animal models and in vitro show that compounds like ammonia, phenols, p-cresol, certain amines and hydrogen sulfide play important roles in the initiation or progression of increased intestinal permeability or “leaky gut” and inflammation.52 With this in mind, the modulation of the microbiota and its fermentation capacity may provide the scientific basis for designing diets aimed at improving performance by enhancing carbohydrate fermentation during exercise and limiting those that produce toxic metabolites from protein degradation. Modifying athletes' diets in a way in which they positively impact the activities of their gut microbiota through newly recognized inter-kingdom axes of communication such as the gut–liver axis9 may also benefit sport performance.
It is clear then that the interaction between diet and exercise needs to be further studied to better assess the contributions of diet and microbial activities in athletic performance. In fact, an important confounding effect of the study of Clarke et al.15 that documented that gut microbial diversity increases with exercise is that the professional rugby players' diet differed from that of the controls. The athletes ate more calories, fat, carbohydrates, sugars, protein, protein supplements, and saturated fat per day than the controls. Therefore it is difficult to draw conclusions from the study of Clarke et al.15 and assess the impact diet has on gut microbial diversity and exercise performance.53 As such, further prospective studies are now planned to determine whether dietary changes or exercise affect elite athletes' gut microbiota profile.54 In the same line, Liu et al.55 have published a protocol to study the effect of exercise and dietary intervention on the microbiota profile during 6 months in 200 postmenopausal women and middle-age pre-diabetic men along with follow-ups 6 and 12 months later. If their program proves to be effective in reducing serum glucose levels and fatty liver content while improving gut microbiota composition in the participants, it will open new strategies to combat chronic diseases through exercise and modifications of gut microbiota composition.
3.3. Effects of gut microbiota on immune response during intense exercise
Immune response activation appears to play a key role in endurance performance. More specifically, there is evidence that several immune responses are suppressed during prolonged periods of intense exercise training. These include total leukocyte count, granulocyte, monocyte, lymphocyte and natural killer cell counts, total T cell counts, cell proliferation in response to mitogens, and serum immunoglobulin levels among others.56 As intense exercise continues, plasma cortisol levels rise, inducing an influx of neutrophils from bone marrow and an efflux of other leukocyte subsets.57, 58 In addition to cellular immune alterations, several studies have reported that intense exercise causes an acute-phase inflammatory response, which has some similarities to those seen in sepsis and trauma.59, 60 In contrast to habitual light exercise and fitness,15 strenuous exercise causes an increase in the number of pro-inflammatory cytokines, such as Tumor necrosis factor alpha (TNF-α), Interleukin 1(IL-1), IL-6, IL-1 receptor antagonist, TNF receptors, as well as anti-inflammatory modulators like IL-10, IL-8, and macrophage inflammatory protein-1,61 indicating a dose–response effect between biological responses to exercise and host immunity25 (Fig. 3). It has been reported that strenuous aerobic exercise in mice leads to an increase in TNF-α62 and IL-1063 in intestinal lymphocytes. In addition, it has also been demonstrated that intense exercise increases immunoglobulin A (IgA) expression, which coats the bacteria helping maintain a tolerant, non-inflammatory host–microbial relationship64 and may thus strengthen the resistance of exercised mice to infections by intestinal pathogens and the colonization of commensal microbiota in mice.65 Conversely, when studying 38 elite America's Cup yacht racing athletes over 50 weeks of training, a clear correlation was found between increased training and competition load and decreased levels of salivary IgA.66 These findings have led to a theory that an “open window” of impaired immunity exists in which viruses and bacteria are more likely to take over and increase the risk of subclinical and clinical infections in endurance athletes.67 In fact, it is known that strenuous exercise increases the prevalence of upper respiratory tract infections (URTI) and digestive troubles in athletes.68 For instance, 2311 runners had a higher incidence of URTI during the week after they had taken part in the 1988 Olympic marathon competition compared to a control group.69 Athletes are more prone to URTI because of the physical and psychological stress of exercise combined with imbalanced diet, foreign travel across time zones, disturbed sleep and exposure to environmental extremes.70 Their exposure to pathogens may also be increased because of elevated lung ventilation during exercise, skin abrasions, and exposure to large crowds.70 As mentioned previously, some athletes are also susceptible to the development of gastrointestinal symptoms such as abdominal discomfort and diarrhea which often occur during long-distance runs or competitions.71
Fig. 3.
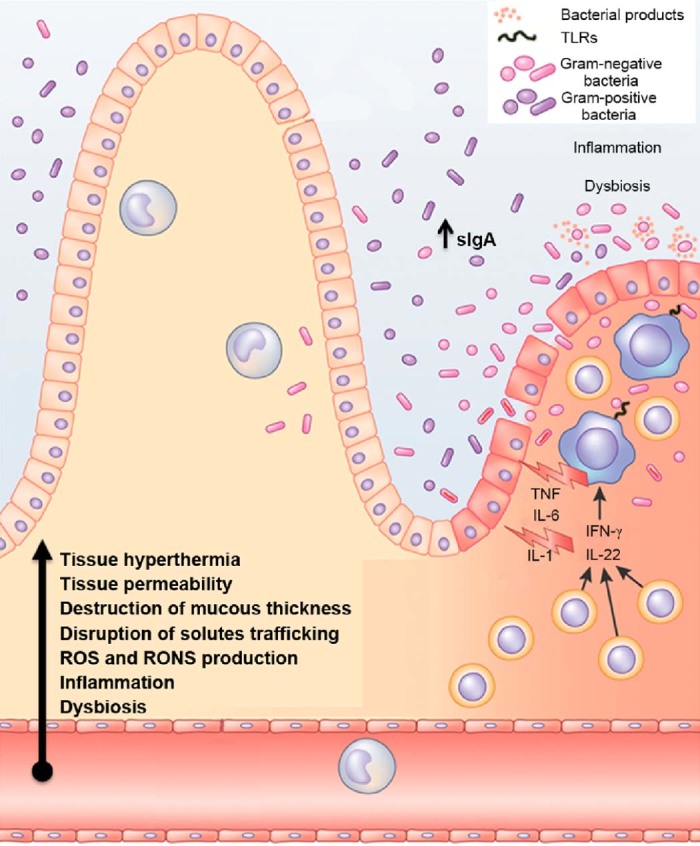
Endurance: crosstalk between intestinal microbiota, immune responses and redox status. Endurance exercise may cause an increase in the number of pro-inflammatory cytokines, such as TNF-α, IL-1, IL-6, IL-1 receptor antagonist, TNF receptors, but also anti-inflammatory modulators (e.g., IL-10, IL-8), sIgA and intestinal lymphocytes. In turn, this inflammatory response may induce disbiosis and modifications of intestinal microbiome composition and their secreted products. Additionally there is an increase of tissue hyperthermia, gastrointestinal permeability and destruction of gut mucous thickness. Moreover, the activity of antioxidant enzymes may become weaker, which modify the mesenteric redox environment. In parallel, the epithelial barrier disruption enhances the TLRs-mediated recognition of gut commensal bacteria by effector cell types, which potentiate the immune response. IgA = immunoglobulin A; IL = interleukin; RONS = reactive oxygen and nitrogen species; ROS = reactive oxygen species; sIgA = secretory IgA; TLRs = toll-like receptors; TNF = tumor necrosis factor.
Adapted with permission.111
Another way endurance training can cause changes in immune response is by reducing the gastrointestinal blood flow, oxygen, and nutrients while increasing tissue hyperthermia, permeability of the gastrointestinal epithelial wall and the destruction of gut mucous thickness,53 which stimulates an inflammatory immune response. This so-called “leaky gut” might lead to endotoxemia14 in which pathogens or endotoxins are able to cross the intestinal barrier into the bloodstream, causing a disruption of the immune system–microbiota homeostasis. Thus, in stressful situations, loosening of the tight junctions and adherens junction which strictly control paracellular trafficking of solutes and fluids between gut epithelial cells may occur, causing larger substances to escape the intestines provoking an immune response.14, 29 The loosening of the tight junctions as a result of exercise occurs through a dynamic interaction with specific toll-like receptors (TLRs) which are able to recognize motifs expressed by bacterial cells. On the one hand, the activation of the TLRs starts signaling cascades that involve the activation of proteins and transcription factors, inducing the secretion of proinflammatory and cytokines in the gastrointestinal tract.72 In fact, it has recently been shown that in healthy individuals, commensal bacteria cannot access the liver through the portal vein and reach the spleen because of the existence of a gut–vascular barrier which controls the type of antigens that are translocated across blood endothelial cells to the portal vein.73 Whether the gut–vascular barrier is compromised under intense exercise is not known yet.
Other studies have shown how increased intestinal permeability caused by exercise can increase serum endotoxicity as well as stimulate an immune response. Jeukendrup et al.74 analyzed lipopolysaccharide (LPS) levels, which are endotoxins found in the outer membrane of gram-negative bacteria, in blood samples from 29 athletes before, immediately after, and 1 h, 2 h, and 16 h after a long-distance triathlon. There was an increase in LPS immediately after exercise and was even higher 1 h after the race (meaning there was an increase in intestinal permeability after intense exercise). The highest measured value was 15.0 pg/mL. If it is assumed that endotoxemia is present when LPS concentrations are >5.0 pg/mL, then at 1 h after the race, 68% of the athletes had endotoxemia. Marycz et al.75 have documented that prolonged strenuous exercise expands the population of developmentally early stem cells in bone marrow and transports them into peripheral blood which may be in part mediated by the derived bacterial LPS. Therefore, Marycz et al.75 proposed to measure the levels of serum endotoxin in future studies investigating the effect of exercise on gut microbiota.
To counteract these inflammatory responses brought on by intense exercise, the gut microbiota and their resulting SCFA metabolites may reduce colonic mucosa permeability and inhibit inflammatory cytokines. These anti-inflammatory effects of gut microbiota may help delay the fatigue symptoms in endurance performances. In line with this, the study with elite rugby players suggested that the observed microbial modifications were accompanied by lower inflammatory status compared to controls (higher IL-10 and IL-8; lower IL-6, TNF-α, and IL-1B).15 Whole metagenome sequencing for functional genomic analysis along with blood immune parameters profiling is needed to ascertain which, if any, immune response functional pathways are altered during exercise training. Additionally, it is also plausible to imagine that the gut microbiota composition and structure could be measured in fecal samples from patients partaking in endurance exercise and serve as useful biomarkers to trace metabolic and systemic stress during and after exercise. Since the alterations of the microbial diversity have been linked to changes in the prevalence of gastrointestinal and respiratory tract alterations among professional athletes, it is important to address this important issue and find, for example, dietary habits that help ameliorate immune responses via the modulation of gut microbiota (see probiotics section below).
3.4. The role of gut microbiota in oxidative stress during endurance exercise
One of the main physiological adaptations to endurance exercise is the modulation of oxidative and nitrosative stress as a way to avoid tissue damage, intestinal permeability, and bacterial translocation.14, 76 The gastrointestinal tract is a key source of reactive oxygen species (ROS) and nitrogen oxide species (RONS) substances, which are by-products of normal cellular metabolism. The homeostatic control of the intestinal epithelia redox environment, which is the balance between antioxidant defense and/or oxidative stress, is central to the functions of the gut in nutrient digestion and absorption, stem cell proliferation, apical enterocyte apoptosis, and immune response.77 The control and removal of ROS and RONS substances are accomplished by (i) an enzymatic system (e.g., SOD, CAT, and GPx) and (ii) a non-enzymatic system (e.g., urate, glutathione, ubiquinone, thioredoxin, ferritin, and lactoferri).78 During exercise, the activity of antioxidant enzymes such as SOD, CAT, and GPx becomes weaker during chronic fatigue and intense exercise. Additionally, there is an increased production of catecholamines that subsequently undergo autoxidation which may increase the oxidative stress79 and thus limit the final performance.
Investigation of the effect microbiota has in controlling the gastrointestinal redox environment is still in its infancy. However, some initial datasets have opened the way toward identifying microbiota–redox status relationships that are specifically regulated in the gastrointestinal tract. Xu et al.22 have demonstrated that the host redox status is related to a balanced gut microbiota composition. They observed that oxidative status was negatively correlated with Lactobacillus and Bifidobacterium and positively correlated with gut Escherichia coli. In mice, data reveal that the colonic microbiota where there are higher levels of bacteroidetes play a critical role in protecting against intestinal infection by inducing pro-inflammatory and pro-oxidant responses that control pathogen load as well as ion transporter gene expression which may prevent fatal dehydration.80 In mice, it has also been reported that gut microbiota affects the host's amino acid metabolism which thus regulates glutathione metabolism.81 Given the modulatory effects of gut microbiota on antioxidant enzyme activity and the ability of antioxidant enzymes to augment recovery following extreme exercise, Hsu et al.10 examined the antioxidant enzyme activity and endurance exercise time in SPF, GF, and BF gnotobiotic mice following an exhaustive exercise challenge. As mentioned above, the absence of microbiota decreased antioxidant enzyme activities and the overall exercise performance. Therefore, the authors concluded that different microbiota composition and structure might affect exercise performance by modifying the activity of the antioxidant enzymes, such as CAT and GPx. The higher levels of CAT in SPF than GF and BF gnotobiotic mice led them to conclude that gut microbiota may promote increased CAT activity and thus reduce exercise-induced fatigue. It was also observed that GF animals presented lower serum and hepatic GPx activity as well as lower epididymal fat pad weight, which may limit the exercise performance. No effects were observed when analyzing the SOD activity which is responsible for the breakdown of superoxide into hydrogen peroxide and oxygen.82 While the role the microbiota has on controlling the redox homeostasis during exercise is not well-defined,77 we believe that understanding the signaling events initiated by free radicals as well as the role of microbiota in such processes is key to furthering our understanding of ROS and RONS-mediated response in the gastrointestinal tract during exercise.
3.5. The role of gut microbiota on dehydration status during endurance exercise
Endurance athletes are at particular risk for dehydration, primarily because of increased fluid losses from sweating as a result of prolonged and intensive periods of exercise.49 Exercise performance is impaired when an individual is dehydrated and loses as little as 2% of one's body weight. An excess loss of 5% body weight can decrease athletic performance by about 30%.83 Therefore, an adequate hydration status is essential for endurance performance. A primary physiologic function of mucosal epithelial cells is electrolyte transport.84 Water transport and mucosal hydration function are thought to be necessary components of a normal functioning and protective intestinal barrier.84 The study by Musch et al.85 reported that activation of electrogenic Cl− secretion in the intestinal mucosa altered the composition of mucus and intestinal microbiota by increasing the abundance of Lactobacillus (firmicutes phylum) and Alistipes genera. In addition, another study has reported that active electrogenic Cl− secretion functions as a primitive innate defense mechanism, substantially shifting the colonic microbiota with notable changes (increasing the number of bacteria of the firmicutes and bacteroidetes phyla).86 Very recently the gut microbiota has been related to the maintenance of proper hydration during exercise and the prevention of an inflammatory response. Redondo et al.23 demonstrated that the bacteroidetes phylum reduced plasma sodium levels, whereas the Clostridium genus reduced the plasma osmolality levels in 23 healthy young individuals. In plasma, the sodium substance concentration, together with potassium, bicarbonate, urea, and glucose, constitutes 95% of total osmolarity.87 These results suggested that microbiota influence the cellular transport of solutes through the gut mucosa and contribute to the hydration state, while reducing the plasma osmolality. In the same experiment, an abundance of Bifidobacterium influenced T lymphocyte levels, reflecting an interaction with the immune response of the host. Because a good hydration state and a well-functioning protective intestinal barrier are essential for sports performance, and since ultra-endurance athletes typically do not meet their fluid needs during exercise, it is important to understand the role that microbiota has on water transport and the associated changes to the mucus intestinal layer.
3.6. Diet modulation of gut microbiota profiles and its fermentation capacity to improve endurance performance
The overall aim of the studies of the gut microbiota in health and disease is to find associations between lifestyle changes, primarily diet, and functional consequences of alterations in the gut microbiota. Currently, it is known that the ingestion of probiotics, prebiotics, polyphenols, and antibiotics modify the gut microbiota,9 but their effects in athletes are in the early stages of investigation.
There is now a reasonable body of evidence that shows consuming probiotics regularly may positively modify the gut microbiota's population and structure and may influence immune function as well as intestinal epithelium cell proliferation, function, and protection in individuals who follow exercise programs (Table 2). Probiotics are food supplements that contain live microorganisms, especially lactic acid bacteria, which when administered in adequate amounts confer a health benefit for the host.88 They are available commercially in tablets, capsule form, as a powder (added to drinks), probiotic-enriched chews or in selected dairy products such as fermented milk or yogurt.40 For further details on probiotic supplementation in athletes, see the recently published review by Pyne et al.40
Table 2.
Effect of probiotics and prebiotics in trained individuals. Studies were selected from 2006 to 2016. Updated from Ref. 40.
| Author | Year | Number of individuals | Functional food treatment | Experimental design | Duration of experiment | Results/conclusions |
|---|---|---|---|---|---|---|
| Clancy et al.91 | 2006 | 27 | A total of 18 healthy athletes and 9 fatigued athletes were included in the study. Fatigued athletes were self-referred to a medical sports clinic complaining of fatigue, recurrent sore throats, and impaired performance. All individuals were supplemented with Lactobacillus acidophilus, 2 × 1010 cell/day. | Prospective single group intervention | 4 weeks, 1 sampling point | Athletes complaining of fatigue had significantly less secretion of IFN γ from blood CD4+ T cells than healthy control athletes. After treatment with L. acidophilus there was a significant increase in secretion of whole-blood IFN γ to levels similar to those found in the control athlete group. No effect on whole blood culture secretion of IL-4, IL-12 or S-IgA concentration was observed. |
| Cox et al.90 | 2010 | 20 (n = 10/group) | Distance runners were randomly distributed into 1 of the 2 groups: (i) supplementation with Lactobacillus fermentum daily at the dose of 1.26 × 1010 cells or (ii) placebo capsules contained an inert excipient. | Randomized, blinded, placebo-controlled, cross-over trial | 4 weeks of winter training | L. fermentum VRI-003 treatment elicited greater change in the whole-blood culture of IFN γ compared to placebo, and significantly reduced (50%) the number of days of respiratory illness and its severity. No substantial changes in running performance measures were seen over the study. There were no significant differences in the mean change in S-IgA and IgA1 levels, or in IL-4 and IL-12 levels between treatments. |
| Gill et al.102 | 2016 | 8 | Endurance trained males were randomly assigned to 1 of the 2 groups: (i) supplementation with Lactobacillus casei (1 × 1011 cell/day) or (ii) placebo. After treatment, individuals were exposed to EHS, which comprised of 2 h running exercise at 60%VO2max in hot ambient conditions (34.0°C and 32%RH). | Randomized, blinded, and counterbalanced cross-over trial | 1 week, 7 sampling points: baseline, pre-EHS, post-EHS (1 h, 2 h, 4 h, and 24 h). | L. casei supplementation did not show significant changes in resting circulatory endotoxin concentration or plasma cytokine profile compared to placebo. A main effect of time was observed for IL-6, TNF-α, IL-10, and IL-8; whereby levels increased in response to EHS. Relative to pre-EHS concentrations, higher plasma concentrations of endotoxin, and plasma TNF-α concentration was observed after probiotic supplementation compared to placebo group. |
| Gill et al.103 | 2016 | 8 | Endurance trained males were randomly assigned to 1 of 2 groups: (i) supplementation of Lactobacillus casei (1 × 1011 cell/day) or (ii) placebo. After treatment, individuals were exposed to EHS, which comprised of 2 h running exercise at 60%VO2max in hot ambient conditions (34.0°C and 32%RH). | Randomized, blinded, and counterbalanced cross-over trial | 1 week 7 sampling points: baseline, pre-EHS, post-EHS (1 h, 2 h, 4 h, and 24 h). | Probiotic supplementation did not induce significant changes in resting S-AMP responses compared with placebo. Increases in S-IgA, S-α-amylase, and S-cortisol responses, but not S-lysozyme responses, were observed after EHS. No main effects of trial or time × trial interaction were observed for S-AMP and S-cortisol responses. |
| Gleeson et al.104 | 2012 | 66 (n = 33/group) | Highly active individuals were randomly distributed into 1 of the 2 groups: (i) probiotic supplementation (Lactobacillus salivarius; 2.0 × 1010 live cell/day) or (ii) placebo. | Randomized, blinded, placebo-controlled trial | 16 weeks, 3 sampling points: baseline, 8 and 16 weeks | The proportion of subjects on placebo group who experienced 1 or more weeks with URTI symptoms was not different from individuals supplemented with probiotics. The number of URTI episodes was similar between groups. Severity and duration of symptoms were not significantly different between treatments. Blood leukocyte, neutrophil, monocyte, and lymphocyte counts; S-IgA; and lysozyme concentrations did not change over the course of the study and were not different between groups. |
| Gleeson et al.88 | 2011 | 84 (n = 42/group) | Endurance runners were randomly distributed into 1 of the 2 groups: (i) probiotic supplementation with Lactobacillus casei Shirota (6.5 × 109 live cell/day) or (ii) placebo. | Randomized, blinded, placebo-controlled trial | 16 weeks, 3 sampling points: baseline, 8 and 16 weeks | The proportion of subjects on placebo group who experienced 1 or more weeks with URTI symptoms was 36% higher than those on probiotic supplementation. The number of URTI episodes was significantly higher in the placebo group than in the probiotic group. Severity and duration of symptoms were not significantly different between treatments. S-IgA concentration was higher on probiotic group than placebo. |
| Haywood et al.96 | 2014 | 30 | Rugby union players were randomly distributed into 1 of the 2 groups: (i) probiotics multi-species (Lactobacillus gasseri: 2.6 × 1012 cell/day, Bifidobacterium bifidum: 0.2 × 1012 cell/day, and Bifidobacterium longum: 0.2 × 1012 cell/day) or (ii) placebo. | Randomized controlled, single cross-over design with 28-day washout period | 4 weeks | During the probiotic treatment 14/30 participants never experienced a single URTI or gastrointestinal episode, compared to 6/30 on the placebo supplementation. The number of days of illness tended to be higher for the placebo than probiotic. There was no significant difference in the severity of the symptoms between the 2 treatment groups. |
| Kekkonen et al.67 | 2007 | 141 | Marathon runners were randomly assigned to 1 of the 2 groups: (i) Lactobacillus rhamnosus GG (4.0 × 1010 cell/day) or (ii) placebo. | Randomized, double-blinded intervention study | 12 weeks | The number of healthy days was 79.0 in the probiotic group and 73.4 in the placebo group. There were no differences in the number of respiratory infections or gastrointestinal-symptom episodes. The duration of gastrointestinal-symptom episodes in the probiotic group was 2.9 days vs. 4.3 days in the placebo group during the training. Hematological parameters within reference range for both groups throughout study. |
| Lamprecht et al.79 | 2012 | 23 | Trained men were randomly distributed into 1 of the 2 groups: (i) multi-species probiotic group (1 × 1010 cell/day, Ecologic®Performance or OMNi-BiOTiC®POWER, n = 11) or (ii) placebo group (n = 12). Individuals were submitted to a triple-step test cycle ergometry. | Randomized, double-blinded, placebo-controlled trial | 14 weeks, 2 sampling points: baseline and 16 weeks | Zonulin, a marker indicating improved intestinal barrier integrity, decreased in feces (~25%) after probiotic supplementation. Probiotic supplementation reduced TNF concentration by ~25% at rest and post-exercise, and exercise-induced protein oxidation by ~8% and IL-6 production. |
| Martarelli et al.98 | 2011 | 24 (n = 12/group) | Active individuals were randomly distributed in 1 of the 2 groups: (i) mixture of the 2 probiotic strains (1:1 Lactobacillus rhamnosus IMC 501 and Lactobacillus paracasei IMC 502; ~10 × 109 cell/day) or (ii) control group. They did not consume any supplements during the 4 weeks. | Pre–post controlled trial with control (but no placebo treatment) group | 4 weeks | Probiotic supplementation increased plasma antioxidant levels (~9%), thus neutralizing reactive oxygen species. The 2 strains, L. rhamnosus and L. paracasei exerted strong antioxidant activity. |
| Nieman et al.105 | 2014 | 19 | Cyclists were engaged in two 75 km time trials after 2 weeks pistachio or no pistachio supplementation (480 kcal per 3 of serving) with a 2-week washout period. Pistachios were used because they are nutrient-dense nuts that contain a unique nutrient profile of proteins and carbohydrates (∼30% of energy), fats (∼70% of energy), minerals (in particular, copper, iron, magnesium), potassium, vitamins B6 and thiamin, carotenoids, phytosterols, and phenolic acids. | Randomized, cross-over design | Sampling at 5 min pre-exercise, and immediately post-, 1.5 h post-, and 21 h post-exercise | Two weeks pistachio nut supplementation was associated with reduced 75 km cycling time trial performance and increased post-exercise plasma levels of raffinose, sucrose, and metabolites related to leukotoxic effects and oxidative stress. |
| O'Brien et al.106 | 2015 | 67 | Active individuals were randomly assigned to 1 of the 4 groups: (i) endurance training + control beverage, (ii) endurance training + kefir beverage, (iii) active control + control beverage, or (iv) active control + kefir beverage. The exercise groups completed 15 weeks of structured endurance training while the active control groups maintained their usual exercise routine. Additionally, each group was assigned to either a kefir or a calorie/macronutrient matched placebo beverage that was consumed twice per week. | Prospective group intervention | 15 weeks | The endurance training was effective in improving 1.5 mile (2.41 km) times and kefir supplementation may have been a factor in attenuating the increase in CRP that was observed over the course of the intervention period. |
| Salarkia et al.89 | 2013 | 46 (n = 23/group) | Endurance swimmers girls were randomly assigned into 1 of the 2 groups: (i) consumption 400 mL of probiotic yogurt or (ii) ordinary yogurt daily. | Randomized controlled design | 8 weeks, 2 sampling points: beginning and at the end of the study | Consumption of probiotic yogurt resulted in a reduction in the number of episodes of respiratory infections and in duration of some of their symptoms. Intake of probiotic yogurt also resulted in a significant improved in VO2max possibly due to the reduction of upper respiratory tract infections. |
| Salehzadeh107 | 2015 | 30 (n = 15/group) | Male students were randomized to (i) probiotic yogurt drink (1 × 105 cell/g) along with physical activities or (ii) ordinary yogurt drink and physical activities. Both groups had 200 mL of yogurt drink daily. | Randomized, double-blinded, placebo-controlled trial | 10 weeks, 2 sampling points: 24 h before the first training session and at the end of the study | Both types of yogurt drink significantly increase HDL and decrease CRP; yet, the decreasing effects of CRP on athletes' records were significantly higher in probiotic group compared to the ordinary drink group. |
| Shing et al.99 | 2014 | 10 (n = 5/group) | Male runners were randomly distributed into 1 of the 2 groups: (i) supplementation with a probiotics capsule (45 billion cell/day of Lactobacillus, Bifidobacterium and Streptococcus strains) or (ii) placebo, separated by a washout period. After each treatment, the runners exercised to fatigue at 80% of their ventilatory threshold at 35°C and 40% humidity. | Randomized, double-blinded, cross-over design | 4 weeks | Four weeks of supplementation with a multi-strain probiotic increased running time to fatigue in high temperatures. There was a small-to-moderate reduction in urine lactulose: rhamnose and a small reduction in symptoms of gastrointestinal discomfort following probiotics supplementation. |
| Valimaki et al.108 | 2012 | 127 | Runners were randomly assigned to 1 of the 2 groups: (i) Lactobacillus rhamnosus GG (probiotic group; 3 × 1010 cell/day) or (ii) placebo drink group. | Randomized, double-blinded intervention | 3 months prior to marathon | Probiotics did not have any effect on oxidized LDL lipids, antioxidants, and serum antioxidant potential during the study. Oxidized LDL lipids increased by 28% and 33% during the preparation period and decreased by 16% and 19% during the marathon run in the placebo and probiotic groups, respectively. No changes were seen in serum antioxidant potential before marathon, but during run serum antioxidant potential raised by 16% in both groups. |
| West et al.95 | 2014 | 465 (241 males, 224 females) | Individuals were randomly distributed to 1 of the 3 groups: (i) Bifidobacterium animalis subsp. lactis Bl-04 (Bl-04), 2.0 × 109 cell/day; (ii) Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. lactis Bi-07 (NCFM & Bi-07) 5 × 109 cell/day; and (iii) placebo mixed in a drink. | Randomized, double-blinded placebo-controlled trial | 150 days, sampling time at baseline, before 6-day preparation, before and immediately after the marathon | The risk of an upper respiratory illness episode was significantly lower in the Bl-04 group compared to placebo. |
| West et al.97 | 2011 | 99 | Cyclists (64 males, 35 females) were randomized distributed into 1 of the 2 groups: (i) probiotic supplementation (1 × 109 cell/day Lactobacillus fermentum or (ii) placebo treatment. | Double-blinded, randomized, controlled trial | 11 weeks | Lactobacillus numbers increased 7.7-fold more in males on the probiotic, while there was an unclear 2.2-fold increase in females taking the probiotic. The number and duration of mild gastrointestinal symptoms were ~2-fold greater in the probiotic group. The load (duration × severity) of lower respiratory illness symptoms was less by a factor of 0.31 in males taking the probiotic compared with placebo but increased by a factor of 2.2 in females. Differences in use of cold and flu medication mirrored these symptoms. There were clear reductions in the magnitude of acute exercise-induced changes in some cytokines. |
Abbreviations: CRP = C-reactive protein; EHS = exertional-heat stress; HDL = high density lipoprotein; IFN γ = interferon gamma; IgA = immunoglobulin A; IL = Interleukin; LDL = low density lipoprotein; RH = relative humidity; S-AMP = salivary antimicrobial protein; S-IgA = salivary immunoglobulin A; TNF = tumor necrosis factor; URTI = upper respiratory tract infections; VO2max = maximum oxygen uptake.
Most recently, interest in the use of probiotics has focused on preventing respiratory illness or persistent common cold and flu-like symptoms in athletes. For example, Salarkia et al.89 studied the effects of probiotic yogurt in 46 female endurance swimmers. The intervention group consumed 400 mL of probiotic yogurt containing Lactobacillus acidophilus spp., Lactobacillus delbrueckii bulgaricus, Bifidobacterium bifidum, and Streptococcus salivarus thermophilus, while the control group received the same amount of ordinary yogurt (produced using a culture of L. delbrueckii subsp. bulgaricus and S. thermophilus). They observed that consuming probiotic yogurt resulted in a reduction in the number of episodes of respiratory infections after endurance swimming competition and the duration of some of the symptoms. Ingesting probiotic yogurt also resulted in a significant improvement in maximum oxygen uptake possibly due to the reduction of URTI. Meanwhile, Cox et al.90 evaluated the ability of the probiotic Lactobacillus fermentum VRI-003 (PCC) to enhance the mucosal immune system in a cohort of 20 highly-trained distance runners. Athletes who were administered the probiotic for 1 month reported less than half the number of days of respiratory symptoms during the 30 days of PCC treatment compared to the control-placebo group. Illness severity was also milder for episodes occurring during the PCC treatment. There were no significant differences in salivary IgA levels or interleukin (IL-4 and IL-12) levels. However, prophylactic probiotic administration treatment elicited a 2-fold greater change in whole-blood culture interferon gamma (IFN γ) compared to the placebo group, suggesting that the maintenance of IFN γ levels may be one of the underlying mechanisms for positive clinical outcomes. A study in endurance athletes (n = 42) who took Lactobacillus casei supplements for 4 months showed a reduced prevalence of upper respiratory illness compared to the control group. The subjects presented improved levels of salivary IgA during a winter period of training and competition.88 Clancy et al.91 also found that fatigued athletes (n = 9) had clinical characteristics similar to those seen in patients who experienced reactivated Epstein–Barr virus infections, including a significantly less secretion of IFN γ from blood CD4+ T cells compared to healthy control athletes (n = 18). After 4 weeks of treatment with capsules containing 2.0 × 1010 cells or colony forming units (CFU) of Lactobacillus acidophilus, the fatigued athletes increased the quantity of IFN γ secretion to levels similar to those of the healthy subjects. The administration of Lactobacillus acidophilus in healthy athletes also resulted in increased concentrations of mucosal IFN γ, suggesting that probiotic therapy may reverse a T cell defect, Treg cells in particular, in fatigued athletes while enhancing mucosal IFN γ concentrations in healthy athletes. A number of research groups continue to explore the role of Tregs in maintaining inflammatory control in various athlete cohorts.92, 93, 94
Other studies have documented beneficial effects of probiotic interventions on improvements in cytokines and immune-marker panels, reductions in oxidative stress as well as respiratory and gastrointestinal symptoms. For example, West et al.95 observed that probiotic supplementation (Bifidobacterium animals subsp. lactis; 2 × 109 CFU/day) for 28 days reduced the risk of respiratory and gastrointestinal illness in a cohort of 465 healthy active men and women compared to a placebo group. In a smaller randomized controlled trial of elite rugby players, the administration of a multi-species probiotic for 4 weeks also reported a reduction in the frequency of upper respiratory tract disorders and gastrointestinal symptoms.96 To address the question if females and males respond differently to probiotics, West et al.97 investigated 99 physically active healthy men and women through a randomized controlled trial. They observed a substantial reduction in respiratory and gastrointestinal symptoms in males, but not females after 88 days of Lactobacillus fermentum supplementation. The extent to which the observed differences between the 2 sexes were biological and/or environmental in nature is unclear. Conversely, a randomized double-blinded intervention study in 141 runners taking either a placebo or probiotic supplement of Lactobacillus rhamnosus for 3 months showed no significant differences in the number of episodes of respiratory or gastrointestinal tract disorders in 2 weeks after the marathon.67 Furthermore, probiotics may counteract exercise-induced oxidative stress. In a randomized double-blinded, placebo-controlled trial, Lamprecht et al.79 reported that trained male athletes who consumed a multispecies probiotic supplement for a 14-week period had normalized fecal zonulin concentrations, which is a marker for intestinal permeability, compared to the placebo group, in which exercise showed no effect on TNF-α serum concentrations. The authors also reported that probiotic supplementation does not enhance antioxidant enzyme level, which subsequently neutralized excessive oxidative stress during intense exercise. However, the oxidative stress in athletes who took Lactobacillus paracasei and Lactobacillus rhamnosus supplements during a 4-week period of intense physical activity was reduced.98 The results demonstrated that intense physical activity induced oxidative stress and that probiotic supplementation with Lactobacillus rhamnosus or Lactobacillus paracasei increased plasma antioxidant levels, thus exerting strong antioxidant activity in athletes. Of particular interest is a small cross-over study of 10 runners who were exercised until they were fatigued at 80% of their ventilatory threshold in 35°C and 40% humidity and supplemented with probiotic capsules containing 45 billion CFU of Lactobacillus, Bifidobacterium, and Streptococcus strains in runners.99 The dominant Lactobacillus and Bifidobacterium strains were selected because they have been shown to increase the expression of tight junction proteins and thus maintain the integrity of the intestinal barrier in response to various physiological stressors. Following probiotic supplementation, serum levels of LPS were reduced, resulting in an improvement in gut mucosa permeability and an increase in the time it took to reach fatigue while exercising in hot temperatures.
Most of the articles reviewed do not specifically identify an ergogenic role of probiotic therapy but suggest that immune function is enhanced, the effects of reactive oxygen species are neutralized and gut mucosa permeability is normalized, which might improve performance in athletes undergoing intense physical training. Thus, probiotic supplementation could act as an indirect ergogenic aid. However, these studies in athletes' performances who take probiotic supplements have design flaws. Dose–response experimental studies of probiotic supplementation should investigate parallel changes in exercise outcome, clinical outcome, immune function as well as dietary and exercise regime over a period of several weeks to a few months. As suggested by Pyne et al.,40 more well-designed studies of probiotic supplementation in various athlete groups are warranted to understand the complex relationship between diet, activity levels, clinical outcome, and gut microbiota modifications caused by probiotic supplementation is essential. Future studies that examine the relationship between probiotic supplementation and exercise-induced disorders should also discuss the most appropriate bacterial strains, the best encapsulation form in which probiotics are manufactured and the concentrations/dosage that could increase its benefits on athletic performance and oxidative stress and immunity.14
Given the small number of studies that have examined the effects of probiotic supplementation in athletes and other highly active individuals, it is somewhat premature to issue definitive clinical and practical guidelines.40 However, it appears that probiotic supplementation in combination with a well-formulated dietary plan could assist athletes with a history of respiratory and gastrointestinal disorders during intense periods of training and competition.40
Unlike probiotics, the effects of prebiotics have not been tested in athletes. Prebiotics represent a specific type of dietary fiber that when fermented mediate measurable changes within the gut microbiota composition such as increasing the levels of bifidobacteria or certain butyrate products. Similarly, no reports exist on the effect polyphenols have on the gut microbiota composition and athletic performance. However, recent studies show that the consumption of polyphenol extracts, such as wine, cocoa, and blueberry, modulates the human gut microbiota toward a more “health-promoting profile” by increasing the relative abundance of bifidobacteria and lactobacilli.100 It is now evident that gut microbiota can play a critical role in transforming dietary polyphenols into bioactive polyphenol-derived metabolites which thus benefit the microbiota composition and host health.100 At the same time, polyphenols may control microbiota sub-populations by changing the intestinal redox state; therefore, the link between microbiota and polyphenol consumption represents an additional marker of oxidative-stress-mediated processes.101 These data again raise the possibility that certain functional foods may tap into the underlying ecological processes that regulate gut microbiome community structure and function, contributing to the athlete's health and performance.9, 100
As we enter the post-metagenomic era and gain a better understanding of the microbiota's role in health and metabolism, we could aim to effectively integrate an athlete's microbiota into some form of personalized training and diet plan. With a more complete understanding of the physiological processes that the microbiota regulate, nutritionists will be able to determine whether or not the gut microbiota is a potential target that we can modulate in order to enhance the performance and health of elite athletes.
4. Conclusion
During endurance exercise, transient immunosuppression and inflammatory alterations are observed as well as the regulation of lipid and carbohydrate metabolism, mitochondrial biogenesis, oxidative stress, and dehydration. In the past decade, interest in human microbiome has increased considerably. Gut microbiota ferment complex dietary polysaccharides into SCFs, which may be used as sources of energy in liver and muscle cells and improve endurance performance by maintaining glycemia over time. In addition, the resulting SCFs seem to regulate neutrophil function and migration, reduce colonic mucosal permeability, inhibit inflammatory cytokines and control the redox environment in the cell, which might help delay fatigue symptoms in endurance athlete. However, the fermentation of amino acids produces a range of potentially harmful compounds. Given that many endurance dietary plans are based on high protein and carbohydrate levels, a key challenge is to design diets that limit the microbial profiles that produce toxic metabolites from protein degradation while increasing the number of microorganisms that improve energy metabolism, reduce oxidative stress and regulate systemic inflammation. Currently, the main dietary strategy to modulate gut microbiota includes probiotics. In athletes, the administration of different Lactobacillus and Bifidobacterium strains might help maintain a state of general health, enhance immune function, improve gut mucosal permeability, reduce oxidative stress and obtain energy from plant-carbohydrate sources. By better understanding the mechanisms by which microbiota respond to exercise, we hope to develop novel therapeutic and nutritional strategies to modulate the microbiota and enhance the athlete's overall performance and health. It is therefore important for future research to tease apart the respective influences that high intensity exercise and the intestinal microbiota have on the immune, redox system, and energy metabolism and to track the impact that ongoing functional foods have on the intestinal microbiota. In the future, it may be possible to use the gut microbiota profile as a tool to predict performance and detect potential disorders before conventional diagnostic tools can.
Authors' contributions
NM is the guarantor; DFB wrote the main manuscript text and prepared all tables; NM wrote the main manuscript text, designed, coordinated, and provided critical revision of the article. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Acknowledgment
We thank Allison Clark from Open University of Catalonia for the English editing.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Joyner M.J., Coyle E.F. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586:35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell A.P., Lamon S., Boon H., Wada S., Güller I., Brown E.L. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J Physiol. 2013;591:4637–4653. doi: 10.1113/jphysiol.2013.255695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz A., Riber C., Trigo P., Castejon-Riber C., Castejon F.M. Dehydration, electrolyte imbalances and renin-angiotensin-aldosterone-vasopressin axis in successful and unsuccessful endurance horses. Equine Vet J. 2010;42:83–90. doi: 10.1111/j.2042-3306.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 4.Snow D.H., Baxter P., Rose R.J. Muscle fibre composition and glycogen depletion in horses competing in an endurance ride. Vet Rec. 1981;108:374–378. doi: 10.1136/vr.108.17.374. [DOI] [PubMed] [Google Scholar]
- 5.Davies K.J., Packer L., Brooks G.A. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- 6.Devenport L., Doughty D., Heiberger B., Burton D., Brown R., Stith R. Exercise endurance in rats: roles of type I and II corticosteroid receptors. Physiol Behav. 1993;53:1171–1175. doi: 10.1016/0031-9384(93)90375-p. [DOI] [PubMed] [Google Scholar]
- 7.Hooper L.V., Gordon J.I. Commensal host–bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 8.Rajilic-Stojanovic M., de Vos W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu Y.J., Chiu C.C., Li Y.P., Huang W.C., Huang Y.T., Huang C.C. Effect of intestinal microbiota on exercise performance in mice. J Strength Cond Res. 2015;29:552–558. doi: 10.1519/JSC.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 11.Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neish A.S. Mucosal immunity and the microbiome. Ann Am Thorac Soc. 2014;11(Suppl. 1):S28–32. doi: 10.1513/AnnalsATS.201306-161MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamprecht M., Frauwallner A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Med Sport Sci. 2012;59:47–56. doi: 10.1159/000342169. [DOI] [PubMed] [Google Scholar]
- 15.Clarke S.F., Murphy E.F., O'Sullivan O., Lucey A.J., Humphreys M., Hogan A. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 16.McFadzean R. 2014. Exercise can help modulate human gut microbiota. Boulder, Co: University of Colorado. Dissertation. [Google Scholar]
- 17.Evans C.C., LePard K.J., Kwak J.W., Stancukas M.C., Laskowski S., Dougherty J. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092193. e92193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Queipo-Ortuno M.I., Seoane L.M., Murri M., Pardo M., Gomez-Zumaquero J.M., Cardona F. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065465. e65465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert J.E., Myslicki J.P., Bomhof M.R., Belke D.D., Shearer J., Reimer R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab. 2015;40:749–752. doi: 10.1139/apnm-2014-0452. [DOI] [PubMed] [Google Scholar]
- 20.Kang S.S., Jeraldo P.R., Kurti A., Miller M.E., Cook M.D., Whitlock K. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J.J., Eum S.Y., Rampersaud E., Daunert S., Abreu M.T., Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121:725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Redondo N., Gheorghe A., Serrano R., Nova E., Marcos A. HYDRAGUT study: influence of HYDRAtion status on the GUT microbiota and their impact on the immune system. The FASEB J. 2015;29(Suppl. 1):593. [Google Scholar]
- 24.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan O., Cronin O., Clarke S.F., Murphy E.F., Molloy M.G., Shanahan F. Exercise and the microbiota. Gut Microbes. 2015;6:131–136. doi: 10.1080/19490976.2015.1011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cronin O., Molloy M.G., Shanahan F. Exercise, fitness, and the gut. Curr Opin Gastroenterol. 2016;32:67–73. doi: 10.1097/MOG.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 27.Boveris A., Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Clavel T., Desmarchelier C., Haller D., Gérard P., Rohn S., Lepage P. Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes. 2014;5:544–551. doi: 10.4161/gmic.29331. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W. Host–gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 30.Schloss P.D., Schubert A.M., Zackular J.P., Iverson K.D., Young V.B., Petrosino J.F. Stabilization of the murine gut microbiome following weaning. Gut Microbes. 2012;3:383–393. doi: 10.4161/gmic.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spor A., Koren O., Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 32.Bearson S.M., Allen H.K., Bearson B.L., Looft T., Brunelle B.W., Kich J.D. Profiling the gastrointestinal microbiota in response to Salmonella: low versus high Salmonella shedding in the natural porcine host. Infect Genet Evol. 2013;16:330–340. doi: 10.1016/j.meegid.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Vandeputte D., Falony G., Vieira-Silva S., Tito R.Y., Joossens M., Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto M., Inoue R., Tsukahara T., Ushida K., Chiji H., Matsubara N. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. 2008;72:572–576. doi: 10.1271/bbb.70474. [DOI] [PubMed] [Google Scholar]
- 36.Petriz B.A., Castro A.P., Almeida J.A., Gomes C.P., Fernandes G.R., Kruger R.H. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15:511. doi: 10.1186/1471-2164-15-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karl J.P., Margolis L.M., Madslien E.H., Murphy N.E., Castellani J.W., Gundersen Y. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. 2017;312:G559–71. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- 38.Cairns S.P. Lactic acid and exercise performance: culprit or friend? Sports Med. 2006;36:279–291. doi: 10.2165/00007256-200636040-00001. [DOI] [PubMed] [Google Scholar]
- 39.Opitz D., Lenzen E., Opiolka A., Redmann M., Hellmich M., Bloch W. Endurance training alters basal erythrocyte MCT-1 contents and affects the lactate distribution between plasma and red blood cells in T2DM men following maximal exercise. Can J Physiol Pharmacol. 2015;93:413–419. doi: 10.1139/cjpp-2014-0467. [DOI] [PubMed] [Google Scholar]
- 40.Pyne D.B., West N.P., Cox A.J., Cripps A.W. Probiotics supplementation for athletes—clinical and physiological effects. Eur J Sport Sci. 2015;15:63–72. doi: 10.1080/17461391.2014.971879. [DOI] [PubMed] [Google Scholar]
- 41.Hold G.L. The gut microbiota, dietary extremes and exercise. Gut. 2014;63:1838–1839. doi: 10.1136/gutjnl-2014-307305. [DOI] [PubMed] [Google Scholar]
- 42.Musso G., Gambino R., Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–380. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 43.Pimentel M., Lin H.C., Enayati P., van den Burg B., Lee H.R., Chen J.H. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–95. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 44.Gibson G.R., Cummings J.H., Macfarlane G.T. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J Appl Bacteriol. 1988;65:241–247. doi: 10.1111/j.1365-2672.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 45.Wong J.M., de Souza R., Kendall C.W., Emam A., Jenkins D.J. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mach N., Berri M., Estellé J., Levenez F., Lemonnier G., Denis C. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ Microbiol Rep. 2015;7:554–569. doi: 10.1111/1758-2229.12285. [DOI] [PubMed] [Google Scholar]
- 49.Antonio J., Kalman D., Stout J.R., Greenwood M., Willoughby D.S., Haff G.G., editors. Essentials of sports nutrition and supplements. Humana Press; Totowa, NJ: 2008. [Google Scholar]
- 50.Tipton K.D., Witard O.C. Protein requirements and recommendations for athletes: relevance of ivory tower arguments for practical recommendations. Clin Sports Med. 2007;26:17–36. doi: 10.1016/j.csm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez N.R., Vislocky L.M., Gaine P.C. Dietary protein, endurance exercise, and human skeletal-muscle protein turnover. Curr Opin Clin Nutr Metab Care. 2007;10:40–45. doi: 10.1097/MCO.0b013e3280115e3b. [DOI] [PubMed] [Google Scholar]
- 52.Windey K., De Preter V., Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 53.Marlicz W., Loniewski I. The effect of exercise and diet on gut microbial diversity. Gut. 2015;64:519–520. doi: 10.1136/gutjnl-2014-307909. [DOI] [PubMed] [Google Scholar]
- 54.Ray K. Gut microbiota. Tackling the effects of diet and exercise on the gut microbiota. Nat Rev Gastroenterol Hepatol. 2014;11:456. doi: 10.1038/nrgastro.2014.109. [DOI] [PubMed] [Google Scholar]
- 55.Liu W.Y., da Lu J., Du X.M., Sun J.Q., Ge J., Wang R.W. Effect of aerobic exercise and low carbohydrate diet on pre-diabetic non-alcoholic fatty liver disease in postmenopausal women and middle aged men—the role of gut microbiota composition: study protocol for the AELC randomized controlled trial. BMC Public Health. 2014;14:48. doi: 10.1186/1471-2458-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shephard R.J., Shek P.N. Potential impact of physical activity and sport on the immune system—a brief review. Br J Sports Med. 1994;28:247–255. doi: 10.1136/bjsm.28.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brenner I., Shek P.N., Zamecnik J., Shephard R.J. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med. 1998;19:130–143. doi: 10.1055/s-2007-971895. [DOI] [PubMed] [Google Scholar]
- 58.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 59.Cannon J.G., Evans W.J., Hughes V.A., Meredith C.N., Dinarello C.A. Physiological mechanisms contributing to increased interleukin-1 secretion. J Appl Physiol. 1986;61:1869–1874. doi: 10.1152/jappl.1986.61.5.1869. [DOI] [PubMed] [Google Scholar]
- 60.Evans W.J., Meredith C.N., Cannon J.G., Dinarello C.A., Frontera W.R., Hughes V.A. Metabolic changes following eccentric exercise in trained and untrained men. J Appl Physiol. 1986;61:1864–1868. doi: 10.1152/jappl.1986.61.5.1864. [DOI] [PubMed] [Google Scholar]
- 61.Pedersen B.K., Toft A.D. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–251. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pervaiz N., Hoffman-Goetz L. Immune cell inflammatory cytokine responses differ between central and systemic compartments in response to acute exercise in mice. Exerc Immunol Rev. 2012;18:142–157. [PubMed] [Google Scholar]
- 63.Hoffman-Goetz L., Spagnuolo P.A., Guan J. Repeated exercise in mice alters expression of IL-10 and TNF-α in intestinal lymphocytes. Brain Behav Immun. 2008;22:195–199. doi: 10.1016/j.bbi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Palm N.W., de Zoete M.R., Cullen T.W., Barry N.A., Stefanowski J., Hao L. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viloria M., Lara-Padilla E., Campos-Rodríguez R., Jarillo-Luna A., Reyna-Garfias H., López-Sánchez P. Effect of moderate exercise on IgA levels and lymphocyte count in mouse intestine. Immunol Invest. 2011;40:640–656. doi: 10.3109/08820139.2011.575425. [DOI] [PubMed] [Google Scholar]
- 66.Neville V., Gleeson M., Follan J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc. 2008;40:1228–1236. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- 67.Kekkonen R.A., Vasankari T.J., Vuorimaa T., Haahtela T., Julkunen I., Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exerc Metab. 2007;17:352–363. doi: 10.1123/ijsnem.17.4.352. [DOI] [PubMed] [Google Scholar]
- 68.Gleeson M. Immune function in sport and exercise. Appl Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 69.Nieman D.C., Johanssen L.M., Lee J.W., Arabatzis K. Infectious episodes in runners before and after the Los-Angeles marathon. J Sports Med Phys Fitness. 1990;30:316–328. [PubMed] [Google Scholar]
- 70.Thomas L.V., Ockhuizen T. New insights into the impact of the intestinal microbiota on health and disease: a symposium report. Br J Nutr. 2012;107(Suppl. 1):S1–13. doi: 10.1017/S0007114511006970. [DOI] [PubMed] [Google Scholar]
- 71.Simons S.M., Kennedy R.G. Gastrointestinal problems in runners. Curr Sports Med Rep. 2004;3:112–116. doi: 10.1249/00149619-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Trevisi P., De Filippi S., Minieri L., Mazzoni M., Modesto M., Biavati B. Effect of fructo-oligosaccharides and different doses of Bifidobacterium animalis in a weaning diet on bacterial translocation and Toll-like receptor gene expression in pigs. Nutrition. 2008;24:1023–1029. doi: 10.1016/j.nut.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Spadoni I., Zagato E., Bertocchi A., Paolinelli R., Hot E., Di Sabatino A. A gut–vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 74.Jeukendrup A.E., Vet-Joop K., Sturk A., Stegen J.H., Senden J., Saris W.H. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci. 2000;98:47–55. [PubMed] [Google Scholar]
- 75.Marycz K., Mierzejewska K., Śmieszek A., Suszynska E., Malicka I., Kucia M. Endurance exercise mobilizes developmentally early stem cells into peripheral blood and increases their number in bone marrow: implications for tissue regeneration. Stem Cells Int. 2016;2016:5756901. doi: 10.1155/2016/5756901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bessa A., Oliveira V.N., Agostini G., Oliveira R.J., Oliveira A.C., White G.E. Exercise intensity and recovery: biomarkers of injury, inflammation and oxidative stress. J Strength Cond Res. 2016;30:311–319. doi: 10.1519/JSC.0b013e31828f1ee9. [DOI] [PubMed] [Google Scholar]
- 77.Circu M.L., Aw T.Y. Intestinal redox biology and oxidative stress. Semin Cell Dev Biol. 2012;23:729–737. doi: 10.1016/j.semcdb.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamprecht M., Bogner S., Schippinger G., Steinbauer K., Fankhauser F., Hallstroem S. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9:45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosh S., Dai C., Brown K., Rajendiran E., Makarenko S., Baker J. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011;301:G39–49. doi: 10.1152/ajpgi.00509.2010. [DOI] [PubMed] [Google Scholar]
- 81.Mardinoglu A., Shoaie S., Bergentall M., Ghaffari P., Zhang C., Larsson E. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11:834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobashi Y., Yoshimura H., Atarashi E., Takahashi K., Tohei A., Amao H. Upregulation of superoxide dismutase activity in the intestinal tract mucosa of germ-free mice. J Vet Med Sci. 2013;75:49–54. doi: 10.1292/jvms.12-0248. [DOI] [PubMed] [Google Scholar]
- 83.Armstrong L.E., Costill D.L., Fink W.J. Influence of diuretic-induced dehydration on competitive running performance. Med Sci Sports Exerc. 1985;17:456–461. doi: 10.1249/00005768-198508000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Colgan S.P. Swimming through the gut: implications of fluid transport on the microbiome. Dig Dis Sci. 2013;58:602–603. doi: 10.1007/s10620-013-2575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Musch M.W., Wang Y., Claud E.C., Chang E.B. Lubiprostone decreases mouse colonic inner mucus layer thickness and alters intestinal microbiota. Dig Dis Sci. 2013;58:668–677. doi: 10.1007/s10620-012-2509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keely S., Kelly C.J., Weissmueller T., Burgess A., Wagner B.D., Robertson C.E. Activated fluid transport regulates bacterial–epithelial interactions and significantly shifts the murine colonic microbiome. Gut Microbes. 2012;3:250–260. doi: 10.4161/gmic.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sollanek K.J., Kenefick R.W., Cheuvront S.N., Axtell R.S. Potential impact of a 500-mL water bolus and body mass on plasma osmolality dilution. Eur J Appl Physiol. 2011;111:1999–2004. doi: 10.1007/s00421-011-1833-3. [DOI] [PubMed] [Google Scholar]
- 88.Gleeson M., Bishop N.C., Oliveira M., Tauler P. Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab. 2011;21:55–64. doi: 10.1123/ijsnem.21.1.55. [DOI] [PubMed] [Google Scholar]
- 89.Salarkia N., Ghadamli L., Zaeri F., Sabaghian Rad L. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: a randomized controlled trial. Med J Islam Repub Iran. 2013;27:141–146. [PMC free article] [PubMed] [Google Scholar]
- 90.Cox A.J., Pyne D.B., Saunders P.U., Fricker P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med. 2010;44:222–226. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- 91.Clancy R.L., Gleeson M., Cox A., Callister R., Dorrington M., D'Este C. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40:351–354. doi: 10.1136/bjsm.2005.024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Handzlik M.K., Shaw A.J., Dungey M., Bishop N.C., Gleeson M. The influence of exercise training status on antigen-stimulated IL-10 production in whole blood culture and numbers of circulating regulatory T cells. Eur J Appl Physiol. 2013;113:1839–1848. doi: 10.1007/s00421-013-2614-y. [DOI] [PubMed] [Google Scholar]
- 93.Cosgrove C., Galloway S.D., Neal C., Hunter A.M., McFarlin B.K., Spielmann G. The impact of 6-month training preparation for an Ironman triathlon on the proportions of naive, memory and senescent T cells in resting blood. Eur J Appl Physiol. 2012;112:2989–2998. doi: 10.1007/s00421-011-2273-9. [DOI] [PubMed] [Google Scholar]
- 94.Perry C., Pick M., Bdolach N., Hazan-Halevi I., Kay S., Berr I. Endurance exercise diverts the balance between Th17 cells and regulatory T cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074722. e74722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.West N.P., Horn P.L., Pyne D.B., Gebski V.J., Lahtinen S.J., Fricker P.A. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014;33:581–587. doi: 10.1016/j.clnu.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Haywood B.A., Black K.E., Baker D., McGarvey J., Healey P., Brown R.C. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J Sci Med Sport. 2014;17:356–360. doi: 10.1016/j.jsams.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 97.West N.P., Pyne D.B., Cripps A.W., Hopkins W.G., Eskesen D.C., Jairath A. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martarelli D., Verdenelli M.C., Scuri S., Cocchioni M., Silvi S., Cecchini C. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol. 2011;62:1689–1696. doi: 10.1007/s00284-011-9915-3. [DOI] [PubMed] [Google Scholar]
- 99.Shing C.M., Peake J.M., Lim C.L., Briskey D., Walsh N.P., Fortes M.B. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur J Appl Physiol. 2014;114:93–103. doi: 10.1007/s00421-013-2748-y. [DOI] [PubMed] [Google Scholar]
- 100.Queipo-Ortuno M.I., Boto-Ordóñez M., Murri M., Gomez-Zumaquero J.M., Clemente-Postigo M., Estruch R. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012;95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 101.Bolca S., Van de Wiele T., Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24:220–225. doi: 10.1016/j.copbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Gill S.K., Allerton D.M., Ansley-Robson P., Hemmings K., Cox M., Costa R.J. Does short-term high dose probiotic supplementation containing lactobacillus casei attenuate exertional-heat stress induced endotoxaemia and cytokinaemia? Int J Sport Nutr Exerc Metab. 2016;26:268–275. doi: 10.1123/ijsnem.2015-0186. [DOI] [PubMed] [Google Scholar]
- 103.Gill S.K., Teixeira A.M., Rosado F., Cox M., da Costa R.J. High dose probiotic supplementation containing lactobacillus casei for seven days does not enhance salivary antimicrobial protein responses to exertional-heat stress compared with placebo. Int J Sport Nutr Exerc Metab. 2016;26:150–160. doi: 10.1123/ijsnem.2015-0171. [DOI] [PubMed] [Google Scholar]
- 104.Gleeson M., Bishop N.C., Oliveira M., McCauley T., Tauler P., Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exerc Metab. 2012;22:235–242. doi: 10.1123/ijsnem.22.4.235. [DOI] [PubMed] [Google Scholar]
- 105.Nieman D.C., Scherr J., Luo B., Meaney M.P., Dréau D., Sha W. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113725. e113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O'Brien K.V., Stewart L.K., Forney L.A., Aryana K.J., Prinyawiwatkul W., Boeneke C.A. The effects of postexercise consumption of a kefir beverage on performance and recovery during intensive endurance training. J Dairy Sci. 2015;98:7446–7449. doi: 10.3168/jds.2015-9392. [DOI] [PubMed] [Google Scholar]
- 107.Salehzadeh S. The effects of probiotic yogurt drink on lipid profile, CRP and record changes in aerobic athletes. Life Sci. 2015;4:32–37. [Google Scholar]
- 108.Valimaki I.A., Vuorimaa T., Ahotupa M., Kekkonen R., Korpela R., Vasankari T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int J Sports Med. 2012;33:291–296. doi: 10.1055/s-0031-1291223. [DOI] [PubMed] [Google Scholar]
- 109.Heinonen I., Kalliokoski K.K., Hannukainen J.C., Duncker D.J., Nuutila P., Knuuti J. Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology. 2014;29:421–436. doi: 10.1152/physiol.00067.2013. [DOI] [PubMed] [Google Scholar]
- 110.Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 111.Goldszmid R.S., Trinchieri G. The price of immunity. Nat Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]