Abstract
Background
Acute hepatic failure is a life-threatening critical condition associated with rapid deterioration of liver function and liver transplantation. Several studies have shown that Panax ginseng Mayer has antidiabetic and hepatoprotective effects. However, the hepatoprotective effect of ginseng berry is still unveiled. In this study, we evaluated the hepatoprotective effects of ultrasonication-processed ginseng berry extract (UGBE) on acute hepatic failure model in rats.
Methods
Ginseng berry extract (GBE) was ultrasonically processed. The GBE, silymarin, and UGBE were orally administered to male Sprague-Dawley rats for 4 wk. Twenty-four h after the last administration, rats were challenged with D-galactosamine (D-GalN)/lipopolysaccharide (LPS).
Results
After ultrasonication, the component ratio of ginsenosides Rg2, Rg3, Rh1, Rh4, Rk1, Rk3, and F4 in GBE had been elevated. Administration of UGBE significantly increased the survival rate of D-GalN/LPS-challenged rats. Pretreatment with UGBE significantly decreased serum alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels in D-GalN/LPS-challenged rats in a dose-dependent manner. The levels of enzymatic markers for oxidative stress (superoxide dismutase, glutathione peroxidase, catalase, and glutathione) were increased by UGBE treatment in a dose-dependent manner. Tumor necrosis factor alphalevel, inducible nitric oxide synthase activities, and nitric oxide productions were reduced by UGBE treatment. In addition, hemeoxygenase-1 levels in liver were also significantly increased in the UGBE-treated group. The protein expression of toll-like receptor 4 was decreased by UGBE administration. Hematoxylin and eosin staining results also supported the results of this study showing normal appearance of liver histopathology in the UGBE-treated group.
Conclusion
UGBE showed a great hepatoprotective effect on D-GalN/LPS-challenged rats via the toll-like receptor 4 signaling pathway.
Keywords: acute liver failure, ginseng berry, hepatotoxicity, toll-like receptor 4, ultrasonication
1. Introduction
Acute liver failure (ALF) is an uncommon but life-threatening critical condition in which rapid degeneration of liver function results from catastrophic injury to the liver [1]. ALF usually occurs with no pre-existing liver disease, within a relatively short period of time [2]. There are many causes of ALF, such as viruses, drugs, toxins, alcohol, metabolic disease, or chronic autoimmune hepatitis [3], [4]. Liver transplant is known to be the only effective therapy; however, there are critical drawbacks such as a shortage of liver donors or the rapidity of the progression of ALF [5]. Therefore, prevention of ALF may prove to be more important than curative therapy.
D-galactosamine (D-GalN) is a hexosamine derived from galactose that causes necrosis of liver cells [6]. D-GalN induces liver damage through a decrease in the cellular uridine-5'-triphosphate (UTP) concentration and a reduction in the synthesis of hepatocyte RNA [7]. Metabolism of D-GalN by D-glucosamine pathways results in transcriptional blockade in the liver [8]. Lipopolysaccharide (LPS) is a representative endotoxic compound from the outer leaflet of Gram-negative bacteria, which can induce an inflammatory reaction [9]. Treatment of D-GalN in combination with LPS makes the liver more sensitive to LPS [10]. LPS induces intrahepatic inflammation by the activation of Kupffer cells and chemical mediators such as superoxide, nitric oxide (NO), and tumor necrosis factor alpha (TNF-α) [11]. Throughout oxidative stress, treatment of D-GalN/LPS (G/L) induces severe hepatic damage [12]. Considering these facts, the G/L-induced ALF experimental model is useful for ALF studies [13].
Panax ginseng Mayer is one of the most widely used medicinal herb, which has a long medicinal history in East Asia [14]. It is famous for having beneficial effects on diseases associated with the livers, brain, and heart [15]. Ginsenosides, which are representative compounds of P. ginseng, have been proved to contribute to these diverse biological effects [16]. Ginsenosides are triterpene saponins that belong to the steroidal family, which are dispersed in the roots, leaves, and berries of P. ginseng [17], [18]. Among the ginsenosides identified (approximately 40), ginsenosides Rg1, Rb1, Re, and Rd are the most commonones [19].
P. ginseng berry extract (GBE) is known to have antiobesity and antidiabetic effects, which may be due to ginsenoside Re, which is the major compound of P. ginseng [20]. In our experiments, however, ultrasonication-processed P. ginseng berry extracts (UGBEs) were used. After the ultrasonication process, the composition of GBE was changed. Ginsenosides Rh1, Rh4,Rg2, Rg3, Rk1, Rk3, and F4 have rapidly been increased, while ginsenoside Re has been decreased. Several studies have examined the physiological effects of these compounds on the liver [21].
In the present study, we investigate the hepatoprotective effect of UGBE on the G/L-induced ALF model in rats, from an antioxidative perspective. Silymarin and GBE were used as positive controls. To identify the hepatoprotective effect of UGBE, the survival rate, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (T. bilirubin) levels were measured. Furthermore, the activity and expression of several enzymes related to oxidative stress and cytokines were evaluated.
2. Materials and methods
2.1. Materials
D-GalN, LPS (Escherichia coli 0111:B4), and silymarin were purchased from Sigma-Aldrich Co., LLC (St. Louis, MO, USA). The glutathione peroxidase (GPx), catalase (CAT) activity, and NO assay kits were purchased from Biovision, Inc. (San Francisco, CA, USA). The TNF-α enzyme-linked immunosorbent assay (ELISA), glutathione (GSH), bilirubin, and albumin assay kits were also obtained from Biovision, Inc. The hemeoxygenase-1 (HO-1) ELISA kit was purchased from Enzo Life Sciences, Inc. (New York, NY, USA). The inducible nitric oxide synthase (iNOS) ELISA kit was purchased from CUSABIO (Wuhan, China). The toll-like receptor 4 (TLR4) ELISA kit was purchased from MyBioSource (San Diego, CA, USA). The anti-TLR4 antibody was purchased from Abcam (Cambridge, UK). The secondary antibody (Rabbit/Mouse) was purchased from Dako Real Envision Detection System (Agilent, Santa Clara, CA, USA) GBE and UGBE were kindly supplied by Professor Sung Kwon Ko, Semyung University (Jecheon, Korea). Ginsenoside standards were purchased from Chromadex (Irvine, CA, USA). Other essential materials and reagents were purchased from Sigma-Aldrich Co., Limited Liability Company.
2.2. Preparation and analysis of UGBE
UGBE and GBE water solvent extracts were kindly provided by Professor Sung Kwon Ko of Semyung University. We analyzed the constituents of UGBE and GBE bythe Waters 1525 binary HPLC system (Waters, Milford, MA, USA). Separation of UGBE was performed on an analytical column (Eurospher, 100-5 C18, 250 mm × 3.0 mm, 5μm; Knauer, Berlin, Germany) by gradient elution at room temperature. The eluent was a mixture of acetonitrile for HPLC (A) and distilled water for HPLC (B). The elution process was performed according to the following conditions: 0 min, 17% of A; 25 min, 25% of A; 50 min, 40% of A; 105 min, 60% of A; 110 min, 100% of A. The flow rate was 0.8 mL/min, injection volume was 20μL, and chromatograms were acquired by a UV/VIS Waters 2478 Dual λ Absorbance Detector (Waters) at 203 nm. After 10 h of ultrasonification, ginsenosides Rb1, Rb2, Rd, Re, Rf, Rg1, Rg2, Rg3, Rg6, Rh1, Rh4, Rk1, Rk3, F1, and F4 were identified. UGBE solution was orally administered to the rats once/d at doses of 100 mg/kg body weight (b.w.), 250 mg/kg b.w., and 500mg/kg b.w.
2.3. Animal models
Specific pathogen-free male Sprague-Dawley rats (200–250g) were purchased from Samtako Bio (Osan, Korea). The rats were group housed in pathogen-free cages in a room controlled for temperature (24–25°C) and humidity (70–75%), on a 12-h dark/light cycle, and were provided with filtered tap water and fed a normal laboratory diet from Samtako Bio. Animals were fasted for 24 h prior to being sacrificed, but were allowed free access to tap water throughout. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Chung-Ang University, in accordance with the guide for the Korean Association for Laboratory Animals, Korea (CAUIACUC-20140031).
The 54 specific pathogen-free male Sprague-Dawley rats were randomly divided into six groups—a control group, an ALF group, a silymarin group, and three experimental groups. The rats in the control and ALF groups were given 2mL/kg b.w. distilled water once/d. The silymarin group was given 150 mg/kg b.w. silymarin once/d. The experimental groups were given 100 mg/kg b.w., 250 mg/kg b.w., and 500 mg/kg b.w. UGBE once/d. Each solution was administered for 28 d. During the administration period, food and water consumption and body weight were checked daily. One h following the last administration, D-GalN (300mg/kg, intraperitoneal injection; i.p.) and LPS (30μg/kg, i.p.intraperitoneal injection; i.p.) were injected simultaneously to all rats except those in the control group (normal saline, 5 mL/kg, i.p.intraperitoneal injection; i.p.). Twenty-four h after G/L injection, all rats were sacrificed by cervical dislocation. Blood and liver samples were removed immediately after sacrificing. This procedure is summarized in Supplementary Fig. 1.
Another experiment was carried out in the same manner for lethality analysis (previously described). Following G/L injection, the survival rate was assessed over 10 d.
2.4. Measurements of serum biochemical parameters
Serum samples were diluted for optimal reactions before being measured. Serum ALT and AST activities were measured by the International Federation of Clinical Chemistry methods using Beckman-Coulter reagents (Beckman-Coulter, Brea, CA, USA) [22]. The T. bilirubin level was measured by a bilirubin kit. Serum TNF-αprotein expression was measured by ELISA. All assay procedures were progressed according to the manufacturer's instructions.
2.5. Measurements of hepatic biochemical parameters
Activities of superoxide dismutase (SOD), GPx, CAT and GSH levels were measured by colorimetric assays. The activity of hepatic iNOS was measured by an ELISA kit (CUSABIOS, Wuhan, China). NO production was measured by an NO colorimetric assay kit. Hepatic HO-1 protein expression was measured by ELISA. All assay procedures were progressed according to the manufacturer's instructions.
2.6. Measurement of TLR4 protein expression
The hepatic protein expression of TLR4 was measured by ELISA. In brief, liver tissues were stored overnight at −20°C, followed by washing with phosphate-buffered saline (PBS) and homogenization. Homogenates were centrifuged for 5 min at 5,000g at 2–8°C. The supernatant was collected and assayed immediately. All other assay procedures were processed in accordance with the manufacturer's instructions.
For immunohistochemistry assay, liver samples were perfused with 10% of formaldehyde solution. After perfusion, liver samples were collected and immersed for 2 wk at room temperature. After immersion 2 wk, liver samples were embedded in liquid paraffin and cut into 5-μm-thick sections. The TLR4 antibody (1:100) was used for staining of the expression of TLR4 with the Dako Real EnVision Detection System Rabbit/Mouse (1:200).
Stained tissues were observed with a Leica DMR 6000 microscope and images were photographed with a Leica DM 480 camera (Wetzlar, Hesse, Germany) at 40 × 10 magnifications.
2.7. Hematoxylin and eosin staining
Liver samples for hematoxylin and eosin (H&E) staining were perfused with 10% formalin solution and harvested in same solution. Two wk later, the liver tissues were embedded in liquid paraffin, cut into 4-μm-thick sections, and stained with H&E. For pathological observation under a light microscope, 20–30 views were randomly selected from each side at a magnification of 200× and different optical fields were measured.
2.8. Statistical analysis
Data are expressed as the mean ± standard error of the mean. Significant differences between means were analyzed by a Student t test or Tukey's multiple comparisons after analysis of variance using SPSS Statistics 24 (IBM, Armonk, NY, USA). Differences were considered to be significant at p < 0.05.
3. Results
3.1. Phytochemistry of UGBE
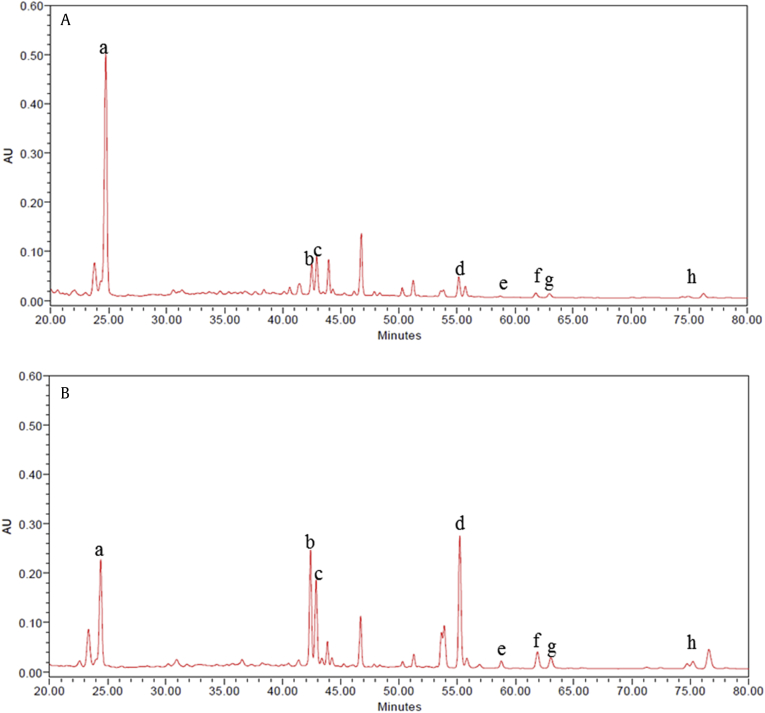
Table 1 shows the composition ratio of GBE and UGBE by the HPLC analysis [23]. Ginsenosides Rh1, Rh4,Rg2, Rg3, Rk1, Rk3, and F4 were rapidly increased after ultrasonication process. Among these ginsenosides, the composition ratio of ginsenosides Rg2, Rh1, and F4 was largely increased, which are produced by the process of manufacture of red ginseng [24]. Furthermore, ginsenosides Rg3, Rh4, and Rk1 were newly produced by ultrasonication process, which were not identified in GBE. HPLC chromatogram data also proved these changes (Figs. 1A and 1B). While other ginsenosides were increased by ultrasonication process, ginsenoside Re was significantly decreased in UGBE.
Table 1.
Composition ration of GBE and UGBE
| Ginsenoside | GBE | UGBE |
|---|---|---|
| Rb1 | 0.758±0.179 | 0.072±0.052# |
| Rb2 | 0.594±0.114 | 0# |
| Rd | 1.534±0.182 | 0.025±0.007# |
| Re | 11.169±0.158 | 0.288±0.037# |
| Rf | 0.330±0.115 | 0# |
| Rg1 | 0.567±0.013 | 0.033±0.004# |
| Rg2 | 0.801±0.215 | 2.278±0.368∗ |
| 20S-Rg3 | 0 | 0.432±0.063∗ |
| 20R-Rg3 | 0 | 0.400±0.059∗ |
| Rg6 | 0.044±0.026 | 0.445±0.063∗ |
| Rh1 | 0.629±0.095 | 1.350±0.208∗ |
| Rh4 | 0 | 0.083±0.011* |
| Rk1 | 0 | 0.2071±0.030* |
| Rk3 | 0 | 0.039±0.005* |
| F1 | 0.193±0.149 | 0.035±0.017# |
| F4 | 0.191±0.026 | 1.210±0.137# |
Data represent mean ± SEM
GBE, P. ginseng berry extract; SEM, standard error of the mean; UGBE, ultrasonication-processed P. ginseng berry extract
* p < 0.05 increased composition ratio of UGBE compared with the same ginsenoside in GBE
# p < 0.05 decreased composition ratio of UGBE compared with the same ginsenosides in GBE [23]
Fig. 1.
HPLC chromatogram of ginsenosides detected from the ginseng berry extracts processed with ultrasonication. Separation of sample was performed on an analytical column by gradient elution at room temperature. Chromatograms of (A) GBE and (B) UGBE were obtained using a UV/VIS Waters 2478 Dual λ Absorbance Detector at 203 nm. The lowercase letters represent the following: a, ginsenoside Re; b, ginsenoside Rg2; c, ginsenoside Rh1: d, ginsenoside F4; e, ginsenoside Rk3: f, ginsenoside Rh4; g, ginsenoside Rg; and h, ginsenoside Rk1. AU, absorbance units; HPLC, high-performance liquid chromatography; GBE, P. ginseng berry extract; UGBE, ultrasonication-processed P. ginseng berry extract.
3.2. Body weight gain, food intake, water consumption, and survival rate
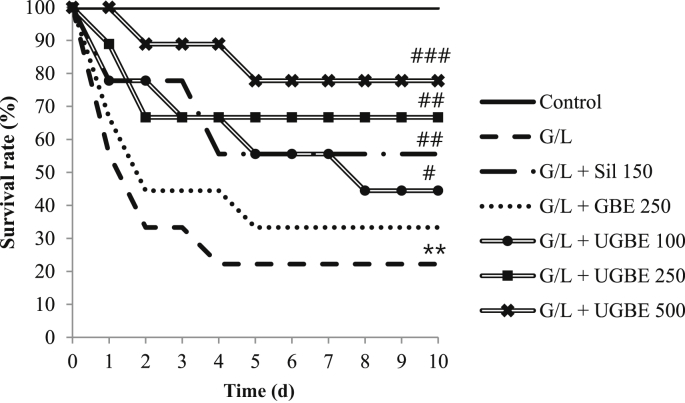
There was no significant difference in body weight gain, food intake, or water consumption between the different treatment groups before G/L challenge (data not shown). However, as shown in Fig. 2, the survival rate of the liver-damaged group was significantly decreased. Ten d following G/L treatment, only 22.2% of the damaged rats had survived. On the contrary, the UGBE-treated (250 mg/kg and 500 mg/kg) group showed a huge increase in survival rate (77.8% and 66.7%, respectively). This increase was higher than that in the silymarin-treated (150 mg/kg) group (55.6%). With respect to the GBE- (250 mg/kg) and UGBE-treated (100 mg/kg) groups, only 33.3% and 44.4% of the nine rats, respectively, survived.
Fig. 2.
Survival rate of rats in different treatment groups. Serum samples were collected from different groups. The sample number in each group is 0 (n = 9). **p < 0.01 compared with control. #p < 0.05 versus G/L. ##p < 0.01 versus G/L. ###p < 0.005 versus G/L. GBE, P. ginseng berry extract; G/L, D-galactosamine/lipopolysaccharide; Sil, silymarin; UGBE, ultrasonication-processed P. ginseng berry extract.
3.3. Serum ALT, AST, and T. bilirubin levels
Serum ALT, AST, and T. bilirubin levels are shown in Table 2. In the G/L group, serum ALT, AST, and T. bilirubin levels were significantly increased compared with those in the control group, and were reduced by the treatment with 250 mg/kg and 500 mg/kg UGBE and 150 mg/kg silymarin. The value for 250 mg/kg UGBE was similar to that for 150 mg/kg silymarin. Therefore, it can be concluded that UGBE ameliorated liver function and attenuated the liver damage induced by G/L treatment.
Table 2.
TaSerum AST, ALT, and T. bilirubin levels in different treatment groups
| AST (IU/L) | ALT (IU/L) | T. Bilirubin (mg/dL) | |
|---|---|---|---|
| Control | 92.43 ± 10.94 | 39.29 ± 7.11 | 0.14 ± 0.04 |
| G/L | 3425.917 ± 766.71∗∗∗ | 2590.3 ± 591.63∗∗∗ | 1.08 ± 0.3∗ |
| G/L +Sil 150 | 1452.1 ± 236.56## | 697.6 ± 196.94### | 0.47 ± 0.1## |
| G/L + GBE 250 | 2478 ± 508.65 | 1872.6 ± 205.36 | 0.99 ± 0.1 |
| G/L + UGBE 100 | 2468.08 ± 912.39# | 1907.33 ± 170.45 | 0.74 ± 0.14# |
| G/L + UGBE 250 | 1706.5 ± 306.76## | 616.17 ± 237.13## | 0.54 ± 0.12### |
| G/L + UGBE 500 | 303.57 ± 178.15### | 203.14 ± 122.79### | 0.30 ± 0.09### |
Data represent mean ± SEM
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GBE, P. ginseng berry extract; G/L, D-galactosamine/lipopolysaccharide; SEM, standard error of the mean; T. bilirubin, total bilirubin; UGBE, ultrasonication-processed P. ginseng berry extract
* p < 0.05, compared with control
*** p < 0.005 compared with control
# p < 0.05 compared with G/L
## p < 0.01 compared with G/L
### p < 0.001 compared with G/L
3.4. Hepatic SOD, GPx, and CAT activities, and GSH level
Hepatic SOD, GPx, and CAT activities, and GSH level were measured in order to evaluate the antioxidant capacity of the liver (Table 3). Treatment with UGBE (100 mg/kg, 250 mg/kg, and 500 mg/kg) or silymarin (150 mg/kg) significantly recovered the antioxidant capacity of the liver damaged by G/L. However, GBE (250 mg/kg) did not recover the decreased antioxidant capacity of the liver.
Table 3.
Hepatic SOD, GPx, and CAT activities and GSH levels in different treatment groups
| SOD (U/mg) | GPx (U/mg) | CAT (U/mg) | GSH (ng/mg) | |
|---|---|---|---|---|
| Control | 83.20 ± 5.99 | 25.59 ± 5.38 | 24.81 ± 9.48 | 10.41 ± 2.84 |
| G/L | 15.26 ± 1.92∗∗∗ | 10.19 ± 2.28∗∗ | 7.85 ± 3.37∗∗∗ | 3.65 ± 1.54∗∗∗ |
| G/L +Sil 150 | 52.72 ± 11.55## | 17.73 ± 0.7### | 17.02 ± 3.89# | 6.44 ± 1.74# |
| G/L + GBE 250 | 26.62 ± 9.11 | 10.94 ± 3.77 | 6.78 ± 1.27 | 3.45 ± 1.23 |
| G/L + UGBE 100 | 37.87 ± 3.63## | 15.87 ± 0.45## | 15.77 ± 3.92# | 6.51 ± 2.33# |
| G/L + UGBE 250 | 54.62 ± 3.51## | 19.66 ± 0.4### | 18.70 ± 3.83## | 9.41 ± 5.86# |
| G/L + UGBE 500 | 71.70 ± 2.08### | 22.05 ± 1.92### | 23.45 ± 3.55### | 9.47 ± 2### |
Data represent mean ± SEM
CAT, catalase; GBE, P. ginseng berry extract; G/L, D-galactosamine/lipopolysaccharide; GPx, glutathione peroxidase; GSH, glutathione; Sil, silymarin; SEM, standard error of the mean; SOD, superoxide dismutase; UGBE, ultrasonication-processed P. ginseng berry extract
* p < 0.05 compared with control
*** p < 0.005 compared with control
# p < 0.05 compared with G/L
## p < 0.01 compared with G/L
### p < 0.001 compared with G/L
3.5. Hepatic iNOS activity and NO levels
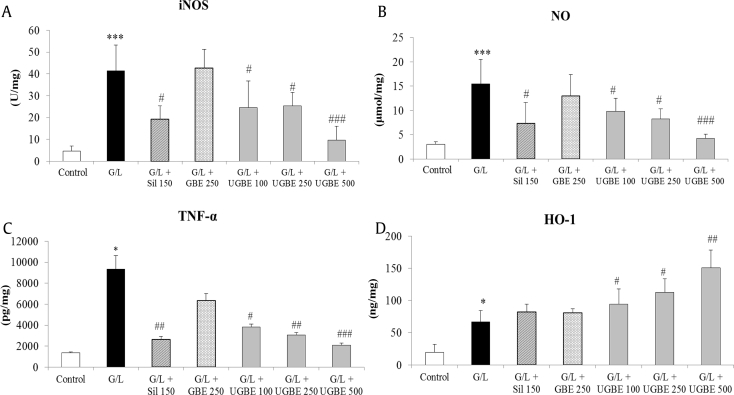
Figs. 3A and 3B show the hepatic iNOS activity and NO levels in the different treatment groups. The iNOS activity and NO levels in the liver were significantly increased after G/L treatment. Treatment with silymarin or UGBE significantly decreased iNOS activity and NO levels to such an extent that UGBE protected oxidative stress from NO production. However, treatment with GBE did not affect the iNOS activity or NO level in the liver.
Fig. 3.
Activities of iNOS, NO levels, TNF-α levels, and HO-1 expression. (A)Activity of iNOS in the livers. (B) NO levels in the livers. (C) Serum TNF-α protein expression. (D) Hepatic HO-1 protein expression. Liver samples were collected from different groups. The sample number in each group is 9 (n = 9). Data represent mean ± SEM. *p < 0.05 compared with control. ***p < 0.005 compared with control. #p < 0.05 compared with G/L. ##p < 0.01 compared with G/L. ###p < 0.001 compared with G/L. GBE, P. ginseng berry extract; G/L, D-galactosamine/lipopolysaccharide; HO-1, hemeoxygenase-1; iNOS, inducible nitric oxide synthase; NO, nitric oxide; SEM, standard error of the mean; Sil, silymarin; TNF-α, tumor necrosis factor alpha; UGBE, ultrasonication-processed P. ginseng berry extract.
3.6. Protein expression of serum TNF-α and hepatic HO-1
As shown in Fig. 3C, G/L challenge caused a great increase in serum TNF-α protein expression compared with that of the control group, which was reduced with silymarin or UGBE treatment. The suppression of TNF-α caused by the treatment with 250 mg/kg UGBE was similar to that of 500 mg/kg UGBE, which were more potent than 150 mg/kg silymarin. UGBE also increased hepatic HO-1 protein expression in a dose-dependent manner (Fig. 3D). However, there was no significant increase in HO-1 protein expression, with only slight upregulation in the silymarin-treated group. These results suggest that UGBE effectively prevents the inflammatory response to G/L treatment and enhances HO-1 protein expression, contributing to the antioxidant capacity of the liver.
3.7. Protein expression of TLR4
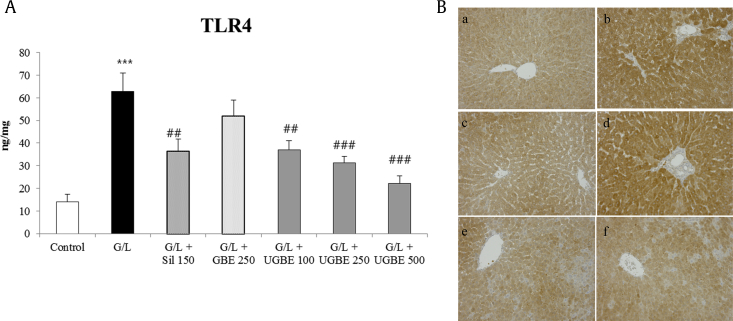
A G/L challenge caused a great increase in TLR4 protein expression compared with that of the control group, which was reduced with silymarin or UGBE treatment (Fig. 4A). Suppression of TLR4 caused by the treatment with 250 mg/kg UGBE was similar to that of 500 mg/kg UGBE, which were more potent than 150 mg/kg silymarin. Fig. 4B shows the representative immunohistochemistry result of TLR4 in each rat.
Fig. 4.
Effect of UGBE on TLR4 expression in G/L-challenged liver injury model. (A) Protein expression of TLR4 in the liver. (B) Photomicrographs of TLR4 immunohistochemistry assay: a, control group; b, G/L group; c, G/L + silymarin (150 mg/kg) group; d, G/L + GBE (200 mg/kg) group; e, G/L + UGBE (250 mg/kg) group; and f, G/L + UGBE (500 mg/kg) group. The sample number in each group is 9 (n = 9). Data represent mean ± SEM. ***p < 0.005 compared with control. ##p< 0.01 compared with G/L. ###p < 0.005 compared with G/L. GBE, P. ginseng berry extract; G/L, D-galactosamine/lipopolysaccharide; SEM, standard error of the mean; Sil, silymarin; TLR4, toll-like receptor 4; UGBE, ultrasonication-processed P. ginseng berry extract.
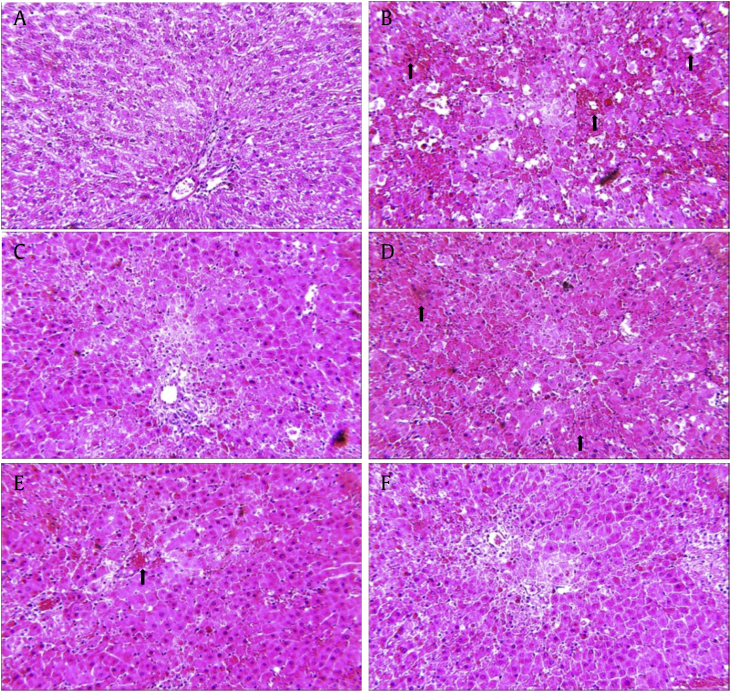
3.8. Histopathological study
Fig. 5 shows the H&E staining result in the different treatment groups. Compared with the control group, G/L challenge group showed severe abnormal changes in the hepatic lobules. In addition, inflammatory cell infiltration, centritubuler necrosis, and inflammatory foci were frequently detected (black arrows in Fig. 5B). These histological changes reduced in the silymarin- or UGBE-treated groups. Especially, UGBE 500 mg/kg-treated group showed normal appearance in liver morphology so that the appearance of histopathology is similar to that of the control group. This grading of histological lesions in the liver confirmed that UGBE significantly reduced hepatic injury induced by G/L challenge.
Fig. 5.
Effect of UGBE on liver histopathological changes. Representative hematoxylin and eosin-stained high magnification (original magnification 200×) of liver tissue from different groups. (A) Control group. (B) G/L group. (C) G/L + silymarin (150 mg/kg) group. (D) G/L + GBE (200 mg/kg) group. (E) G/L + UGBE (250 mg/kg) group. (F) G/L + UGBE (500 mg/kg) group. GBE, P. ginseng berry extract; G/L, D-galactosamine/lipopolysaccharide; UGBE, ultrasonication-processed P. ginseng berry extract.
4. Discussion
Owing to its various physiological effects, P. ginseng gained significant interest for use in a variety of medicinal applications [25]. Several studies have shown the effect of ginseng berry on diabetes and obesity, which is attributed to ginsenoside Re [20]. However, other protective effects of ginsenosides on liver failure have yet to be unveiled. In the present study, we aimed to produce a novel GBE containing a high concentration of active ginsenosides and to investigate the effect of UGBE on ALF.
We examined the composition ratio of UGBE by HPLC. Among several trials with time variations, we selected 10 h ultrasonication processing for the highest content of ginsenosides Rg2, Rh1, and F4, and total saponin (data not shown). After 10 h of ultrasonication processing, the composition ratio of ginsenosides Rg2, Rg3, Rh1, Rh4, Rk1, Rk3, and F4 was markedly increased, while ginsenoside Re was decreased (Table 1). Formation of ginsenosides in UGBE has been reported to be related to enzymatic production, fermentation, or bacterial metabolisms. In the present study, ultrasonication processing in a specific condition facilitated this kind of reactions instead of physiological function. However, the other transformation mechanisms involved in ginsenoside Rg3, Rk1, and F4 still remain to be unveiled and should be researched further. Ginsenosides Rg2, Rg3, Rh1, Rk1, and F4 are most common ginsenosides in red ginseng, which are known to have an protective effect against liver injuries [24], [26], [27]. With the newly developed ultrasonication processing, we may have produced more effective ginsenosides than with typical red ginseng manufacturing. Ginsenoside Rk1 has been reported to have an anticancer effect against hepatocellular carcinoma cells [28], [29]. Ginsenoside Rg3 is known to have antioxidative and hepatoprotective effects by the HO-1 enzyme activity [30]. G Rh1 has shown an anti-inflammatory function via inhibition of iNOS and cyclooxygenase-2 function [31]. Ginsenoside F4 has an apoptotic effect, which contributes to the protection of damaged organs [32].
In the present study, we significantly increased the levels of the representative indices of liver injury (ALT, AST, and T. bilirubin), oxidative stress (SOD, GPx, GSH, CAT, iNOS, and HO-1), and inflammation (TNF-α) by G/L injection, for building an ALF rat model. However, administration of UGBE recovered the liver function and increased survival time, dose dependently. H&E staining results also supports these results (Fig. 5). The combined administration of D-GalN and LPS caused liver failure and rapid death within 1–2 d [5]. In the G/L-treated group, more than 60% of rats died within 2 d. Oral administration of UGBE significantly increased the survival rate. These survival effects at a higher concentration of UGBE are greater than that in the silymarin-treated group. Meanwhile, GBE did not affect the survival rate of the G/L-treated group. The D-GalN-induced ALF model is characterized by increased serum levels of ALT and AST [33]. In our experiment, serum ALT, AST, and T. bilirubin levels were reduced in the high-concentration-UGBE-treated group (Table 2). The differences between GBE and UGBE on liver protection may be due to the difference in composition ratio of ginsenosides, especially ginsenosides Rk1, Rg2, Rg3, Rh1, and F4.
The enzyme-dependent antioxidant system plays a key role in the ALF condition [34], [35], [36], [37]. The treatment of G/L causedan imbalance of reactive oxygen species (ROS) production and a reduction of the antioxidant capacity of the liver [38]. Overproduction of ROS impaired antioxidant enzymes such as SOD, CAT, and GPx in the ALF condition in rats [39], [40], [41], [42]. TNF-α is an important proinflammatory cytokine that triggers liver damage in the G/L-induced ALF mode [43], [44]. The findings of the present study revealed that administration of UGBE protects antioxidative enzymes from oxidative damage (Table 3). Hepatic TNF-α levels were also decreased by UGBE treatment in a dose-dependent manner. Although it has not been investigated that the oral administration of UGBE directly scavenges ROS, there is a strong likelihood that UGBE increased the capacity of antioxidant, and ameliorated hepatotoxicity and oxidative stress induced by G/L challenge.
HO-1 plays a key role in cytoprotection by inhibiting the leukocytic response and ameliorating hepatic microvascular perfusion from LPS-induced oxidative damage [45]. Bilirubin is one of the key mediators of the HO-1-mediated cytoprotection process for intracellular homeostatic balance and inflammation against oxidative stress [46]. In accordance with many other findings, an increased HO-1 expression in the G/L-treated group was significantly increased. Furthermore, the protein expression of HO-1 increased dose dependently according to UGBE treatment (Fig. 3D). This result indicates that UGBE enhanced the hepatoprotective effect by upregulating the protein expression of HO-1 and is likely mediated by ginsenoside Rg3 [30]. However, the role of iNOS in liver injury still remains controversial [47]. NO, which is produced by iNOS, rapidly reacts with ROS to form a peroxynitrite (ONOOK)that is very harmful to the liver [48]. However, during this process, NO acts as a scavenger of ROS [49]. In this study, UGBE ameliorated G/L-induced hepatotoxicity in view of the former action. It is assumed that this action was due to ginsenoside Rh1 [31].
LPS, a main ligand of TLR4 which is related in innate immune reaction, is thought to play a key role in the progression and pathogenesis of ALF [50], [51]. Impaired gut barrier integrity, by ethanol and its metabolite, induces TLR4-mediated hepatic inflammation and damage by an increase of blood LPS concentration and arrival of LPS to the liver by portal blood [52]. In our findings, the protein expression of TLR4 was upregulated by G/L treatment. However, the treatment of UGBE attenuated TLR4 protein expression in a dose-dependent manner (Fig. 4). Although the exact correlation between effective ginsenosides and the expression of TLR4 is still not clear, UGBE is supposed to have a hepatoprotective effect through TLR4.
Based on these results, it can be summarized that G/L-induced ALF condition was alleviated by oral administration of UGBE. Oral administration of UGBE lengthened the survival time of G/L-treated rats and reduced serum ALT, AST, and T. bilirubin levels. The antioxidant capacity also increased in the UGBE-treated group compared with the damaged liver group. This hepatoprotective effect may be mediated by an increase in HO-1 protein expression and suppression of iNOS levels. These protective effects are related to the inhibition of TLR4. Consequently, these findings strongly support the possibility of P. ginseng berrybeing developed as a preventative remedy for liver injury or as a functional food.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (113021-03).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2017.07.007.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Zhang S., Yang N., Ni S., Li W., Xu L., Dong P., Lu M. Pretreatment of lipopolysaccharide (LPS) ameliorates D-GalN/LPS induced acute liver failure through TLR4 signaling pathway. Int J Clin Exp Pathol. 2014;7:6626–6634. [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal W., Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 3.Schiodt F.V., Lee W.M. Fulminant liver disease. Clin Liver Dis. 2003;7:331–349. doi: 10.1016/s1089-3261(03)00026-6. vi. [DOI] [PubMed] [Google Scholar]
- 4.Bernal W., Auzinger G., Dhawan A., Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Li Y., Xie J., Zhang Y., Wang J., Sun X., Zhang H. Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and D-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol. 2013;15:30–37. doi: 10.1016/j.intimp.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R., Li Z., He C., Li R., Xia H., Li C., Xiao J., Chen Z.Y. Human umbilical cord mesenchymal stem cells and derived hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model. PloS One. 2014;9:e104392. doi: 10.1371/journal.pone.0104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;71:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 8.Galanos C., Freudenberg M.A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rietschel E.T., Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 10.Nowak M., Gaines G.C., Rosenberg J., Minter R., Bahjat F.R., Rectenwald J., MacKay S.L., Edwards C.K., 3rd, Moldawer L.L. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1202–R1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- 11.Su G.L. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 12.El-Agamy D.S., Makled M.N., Gamil N.M. Protective effects of agmatine against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. Inflammopharmacology. 2014;22:187–194. doi: 10.1007/s10787-013-0188-2. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T., Horiai H., Aoki C., Kawamura I., Ota M., Mizuhara H., Tomoi M., Mutoh S. Insulin-like growth factor-I prevents lethal acute liver failure induced by d-galactosamine and lipopolysaccharide in rats. Vivo. 2003;17:293–299. [PubMed] [Google Scholar]
- 14.Yun T.K. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 15.Yang S.O., Park H.R., Sohn E.S., Lee S.W., Kim H.D., Kim Y.C., Kim K.H., Na S.W., Choi H.K., Arasu M.V. Classification of ginseng berry (Panax ginseng C.A. Meyer) extract using 1h NMR spectroscopy and its inhibition of lipid accumulation in 3 T3-L1 cells. BMC Complement Altern Med. 2014;14:455. doi: 10.1186/1472-6882-14-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L., Liu H., Xie Z., Yang S., Xu W., Hou J., Yu B. Ginsenoside rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-kappab pathway: a mouse cardiomyocyte model. PloS One. 2014;9:e103628. doi: 10.1371/journal.pone.0103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C.K., Cho D.H., Lee K.S., Lee D.K., Park C.W., Kim W.G., Lee S.J., Ha K.S., Goo Taeg O., Kwon Y.G. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid Based Complement Altern Med. 2012;2012:490301. doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang H.J., Han I.H., Kim Y.J., Yamabe N., Lee D., Hwang G.S., Oh M., Choi K.C., Kim S.N., Ham J. Anticarcinogenic effects of products of heat-processed ginsenoside re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830–2836. doi: 10.1021/jf5000776. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.K., Yoo D.S., Xu H., Park N.I., Kim H.H., Choi J.E., Park S.U. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat Prod Commun. 2009;4:903–906. [PubMed] [Google Scholar]
- 20.Quan H.Y., Yuan H.D., Jung M.S., Ko S.K., Park Y.G., Chung S.H. Ginsenoside Re lowers blood glucose and lipid levels via activation of AMP-activated protein kinase in HepG2 cells and high-fat diet fed mice. Int J Mol Med. 2012;29:73–80. doi: 10.3892/ijmm.2011.805. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Ma H., Zhang J.W., Deng M.C., Yang L. Influence of ginsenoside Rh1 and F1 on human cytochrome p450 enzymes. Planta Med. 2006;72:126–131. doi: 10.1055/s-2005-873197. [DOI] [PubMed] [Google Scholar]
- 22.Sung K.C., Ryan M.C., Kim B.S., Cho Y.K., Kim B.I., Reaven G.M. Relationships between estimates of adiposity, insulin resistance, and nonalcoholic fatty liver disease in a large group of nondiabetic Korean adults. Diabetes Care. 2007;30:2113–2118. doi: 10.2337/dc07-0512. [DOI] [PubMed] [Google Scholar]
- 23.Jung H., Bae J., Ko S.K., Sohn U.D. Ultrasonication processed Panax ginseng berry extract induces apoptosis through an intrinsic apoptosis pathway in HepG2 cells. Arch Pharm Res. 2016;39:855–862. doi: 10.1007/s12272-016-0760-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee S.A., Jo H.K., Im B.O., Kim S., Whang W.K., Ko S.K. Changes in the contents of prosapogenin in the red ginseng (Panax ginseng) depending on steaming batches. J Ginseng Res. 2012;36:102–106. doi: 10.5142/jgr.2012.36.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul S., Shin H.S., Kang S.C. Inhibition of inflammations and macrophage activation by ginsenoside-Re isolated from Korean ginseng (Panax ginseng C.A. Meyer) Food Chem Toxicol. 2012;50:1354–1361. doi: 10.1016/j.fct.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.H., Lee Y.C., Choi S.Y., Cho C.W., Rho J., Lee K.W. The changes of ginsenoside patterns in red ginseng processed by organic acid impregnation pretreatment. J Ginseng Res. 2011;35:497–503. doi: 10.5142/jgr.2011.35.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H.M., Kim S.J., Kim J.S., Kang H.S. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem Toxicol. 2012;50:2736–2741. doi: 10.1016/j.fct.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y.J., Kwon H.C., Ko H., Park J.H., Kim H.Y., Yoo J.H., Yang H.O. Anti-tumor activity of the ginsenoside rk1 in human hepatocellular carcinoma cells through inhibition of telomerase activity and induction of apoptosis. Biol Pharm Bull. 2008;31:826–830. doi: 10.1248/bpb.31.826. [DOI] [PubMed] [Google Scholar]
- 29.Ko H., Kim Y.J., Park J.S., Park J.H., Yang H.O. Autophagy inhibition enhances apoptosis induced by ginsenoside Rk1 in hepatocellular carcinoma cells. Biosci Biotechnol Biochem. 2009;73:2183–2189. doi: 10.1271/bbb.90250. [DOI] [PubMed] [Google Scholar]
- 30.Lee C.K., Park K.K., Chung A.S., Chung W.Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/nad(p)h quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem Toxicol. 2012;50:2565–2574. doi: 10.1016/j.fct.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 32.Chen B., Shen Y.P., Zhang D.F., Cheng J., Jia X.B. The apoptosis-inducing effect of ginsenoside F4 from steamed notoginseng on human lymphocytoma JK cells. Nat Prod Res. 2013;27:2351–2354. doi: 10.1080/14786419.2013.828290. [DOI] [PubMed] [Google Scholar]
- 33.Maezona K., Mawatari K., Kajiwara K., Shinkai A., Maki T. Effect of alanine on D-galactosamine induced acute liver failure in rats. J Hepatol. 1996;24:1211–1216. doi: 10.1053/jhep.1996.v24.pm0008903400. [DOI] [PubMed] [Google Scholar]
- 34.Lu J., Chen Y.P., Wan R., Guo C.Y., Wang X.P. Protective effects of ulinastatin on acute liver failure induced by lipopolysaccharide/D-galactosamine. Digest Dis Sci. 2012;57:399–404. doi: 10.1007/s10620-011-1927-0. [DOI] [PubMed] [Google Scholar]
- 35.Singal A.K., Jampana S.C., Weinman S.A. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011;31:1432–1448. doi: 10.1111/j.1478-3231.2011.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esrefoglu M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon. 2012;12:160–167. doi: 10.5812/hepatmon.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ai G., Liu Q., Hua W., Huang Z., Wang D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: in vitro and in vivo studies. J Ethnopharmacol. 2013;146:794–802. doi: 10.1016/j.jep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Xia X., Su C., Fu J., Zhang P., Jiang X., Xu D., Hu L., Song E., Song Y. Role of alpha-lipoic acid in LPS/D-GalN induced fulminant hepatic failure in mice: studies on oxidative stress, inflammation and apoptosis. Int Immunopharmacol. 2014;22:293–302. doi: 10.1016/j.intimp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Bray R.C., Cockle S.A., Fielden E.M., Roberts P.B., Rotilio G., Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J. 1974;139:43–48. doi: 10.1042/bj1390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- 41.Tabatabaie T., Floyd R.A. Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch Biochem Biophys. 1994;314:112–119. doi: 10.1006/abbi.1994.1418. [DOI] [PubMed] [Google Scholar]
- 42.Neihorster M., Inoue M., Wendel A. A link between extracellular reactive oxygen and endotoxin-induced release of tumour necrosis factor alpha in vivo. Biochem Pharmacol. 1992;43:1151–1154. doi: 10.1016/0006-2952(92)90626-t. [DOI] [PubMed] [Google Scholar]
- 43.Liu T.Z., Lee K.T., Chern C.L., Cheng J.T., Stern A., Tsai L.Y. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappab. Ann Clin Lab Sci. 2001;31:383–390. [PubMed] [Google Scholar]
- 44.El-Beshbishy H.A. Aqueous garlic extract attenuates hepatitis and oxidative stress induced by galactosamine/lipopolysaccharide in rats. Phytother Res. 2008;22:1372–1379. doi: 10.1002/ptr.2505. [DOI] [PubMed] [Google Scholar]
- 45.Roller J., Laschke M.W., Scheuer C., Menger M.D. Heme oxygenase (HO)-1 protects from lipopolysaccharide (LPS)-mediated liver injury by inhibition of hepatic leukocyte accumulation and improvement of microvascular perfusion. Langenbeck Arch Surgery. 2010;395:387–394. doi: 10.1007/s00423-010-0603-8. [DOI] [PubMed] [Google Scholar]
- 46.Gomes A.S., Gadelha G.G., Lima S.J., Garcia J.A., Medeiros J.V., Havt A., Lima A.A., Ribeiro R.A., Brito G.A., Cunha F.Q. Gastroprotective effect of heme-oxygenase 1/biliverdin/CO pathway in ethanol-induced gastric damage in mice. Eur J Pharmacol. 2010;642:140–145. doi: 10.1016/j.ejphar.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Lekic N., Canova N.K., Horinek A., Farghali H. The involvement of heme oxygenase 1 but not nitric oxide synthase 2 in a hepatoprotective action of quercetin in lipopolysaccharide-induced hepatotoxicity of D-galactosamine sensitized rats. Fitoterapia. 2013;87:20–26. doi: 10.1016/j.fitote.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Stadler K., Bonini M.G., Dallas S., Jiang J., Radi R., Mason R.P., Kadiiska M.B. Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic Biol Med. 2008;45:866–874. doi: 10.1016/j.freeradbiomed.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loguercio C., Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 50.Mandrekar P., Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inokuchi S., Tsukamoto H., Park E., Liu Z.X., Brenner D.A., Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szabo G., Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.