Abstract
CRISPR-Cas technologies have greatly reshaped the biology field. In this review, we discuss the CRISPR-Cas with a particular focus on the associated technologies and applications of CRISPR-Cas9 and CRISPR-Cas12a, which have been most widely studied and used. We discuss the biological mechanisms of CRISPR-Cas as immune defense systems, recently-discovered anti-CRISPR-Cas systems, and the emerging Cas variants (such as xCas9 and Cas13) with unique characteristics. Then, we highlight various CRISPR-Cas biotechnologies, including nuclease-dependent genome editing, CRISPR gene regulation (including CRISPR interference/activation), DNA/RNA base editing, and nucleic acid detection. Last, we summarize up-to-date applications of the biotechnologies for synthetic biology and metabolic engineering in various bacterial species.
Keywords: Cas9, Cas12a (Cpf1), Cas13, Base editing, DNA/RNA detection
1. Introduction
CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated) technologies have greatly advanced our genetic engineering capabilities in the past few years. Widely found in bacteria and archaea, CRISPR-Cas systems constitute the adaptive immune systems that act against invading foreign nucleic acids [1]. In general, CRISPR-Cas systems are composed of a CRISPR RNA (crRNA) and Cas proteins. The crRNA is complementary to the target sequence and thus guides the Cas proteins for the sequence-specific recognition and cleavage. The genetic modification can then be introduced by either the error-prone non-homologous end joining (NHEJ) or homology-directed repair (HDR) that creates precise genomic modifications. While eukaryotes use both mechanisms to respond to DNA breakages [2,3], most prokaryotes employ HDR [[4], [5], [6]]. These mechanisms can be exploited for various CRISPR-Cas based biotechnologies.
A number of promising CRISPR-Cas technologies have been developed, revolutionizing research and application in biology. Compared to traditional DNA engineering strategies [7,8] such as λ-Red recombineering, CRISPR-Cas genome editing is a marker-free, versatile and efficiency method, and requires less screening to identify the positive clones. Furthermore, engineering the Cas proteins to nuclease-deficient Cas (dCas) further expands the power of CRISPR-Cas based systems to easy, efficient, and multi-target transcriptional repression and activation, enabling expression level control of potentially any genes of interest without manipulating the genomic sequence. Further, new technologies based on CRISPR-Cas are constantly being developed. For example, by fusion of deaminases to dCas, CRISPR-Cas systems can be adapted to enable base editing on DNA and RNA, without requirement of DNA cleavage or any donor templates. Additionally, based on the collateral effect of Cas proteins, CRISPR-Cas systems have been exploited to detect specific nucleic acids in attomole level [[9], [10], [11]].
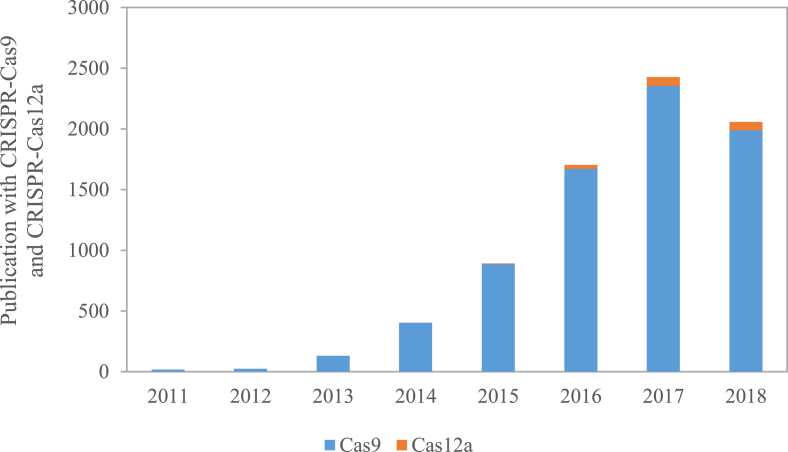
CRISPR-Cas systems are classified as Class 1 and Class 2, which are based on multi-protein effector complex and one single Cas protein, respectively. Depending on their complexity and signature proteins, CRISPR-Cas systems are further divided into six types (Type I-VI). Among them, the type II-A CRISPR-Cas9 and type V-A CRISPR-Cas12a (previously referred as Cpf1) have been most widely studied and developed as genetic tools in bacteria. Notably, based on the PubMed results using terms “Cas9” and “Cas12a (or Cpf1)”, ∼5000 articles have been published in the past two years (Fig. 1), indicating the emergence of a hot research topic. There are many high-profile reviews on CRISPR-Cas applications in eukaryotic organisms, such as yeast, filamentous fungi, plant, and mammalian cells [[12], [13], [14], [15]]. Here, we will discuss the CRISPR-Cas with a particular focus on the associated technologies and applications of CRISPR-Cas9 and CRISPR-Cas12a in various bacterial species.
Fig. 1.
Numbers of NCBI PubMed publications containing Cas9 and Cas12a (Cpf1). The 2018 data are collected at the end of August.
2. The biology of CRISPR-Cas
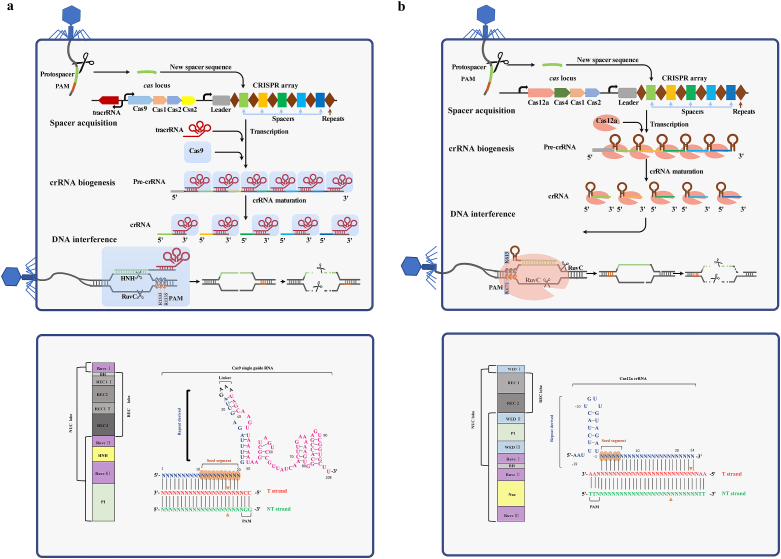
Type II CRISPR-Cas9 and type V CRISPR-Cas12a systems naturally evolve to defend against invading foreign DNAs [16]. The defense process includes three phases: spacer acquisition, crRNA biogenesis, and target interference (Fig. 2). Interestingly, anti-CRISPR-Cas systems evolved by phages are discovered recently, revealing an evolutionary arms race between CRISPR-Cas systems and foreign DNA invaders. Furthermore, the native systems, especially the Cas9 and Cas12a effectors, can be engineered for broader applications and higher specificities.
Fig. 2.
The biological mechanisms of type II CRISPR-Cas9 (a) and type V CRISPR-Cas12a (b). The immune defense presented on the upper contains three steps: spacer acquisition, crRNA biogenesis, and target interference. The Cas domain organizations are presented at the bottom. spCas9 has two nuclease domains HNH and RuvC which cleaves complementary and non-complementary DNA strands respectively, while fnCas12a uses the single nuclease domain RuvC for both DNA cleavage. Orange triangles, the cleavage site; PAM, protospacer adjacent motif; NUC, nuclease lobe; REC, recognition lobe; PI, PAM interaction domain; WED, wedge domain; BH, bridge helix; tracrRNA, trans-activating CRISPR RNA.
2.1. CRISPR defense
2.1.1. Spacer acquisition
Bacterial immunity memory is located at CRISPR arrays containing unique DNA spacers (known as protospacers) flanked by short repeats (Fig. 2). Detection and integration of exogenous DNA into the CRISPR array is the first step of CRISPR-mediated immunity, allowing host organisms to memorize invaders [17]. The spacer acquisition machinery varies across different CRISPR-Cas types. In the type II-A system, four Cas proteins (Cas9, Cas1, Cas2, and Csn2) and a trans-activating CRISPR RNA (tracrRNA) are required [18,19] (Fig. 2). Similarly, type V-A system adopts Cas12a, Cas1, Cas2, and Cas4 for spacer acquisition [20] (Fig. 2).
The selection of new protospacers is often non-random. Within the invading DNA, a 2-5 nucleotide protospacer adjacent motif (PAM) next to the protospacer is critical for acquisition [[21], [22], [23], [24], [25]]. Cas9 first selects a protospacer adjacent to a PAM, and then recruits the acquisition proteins (Cas1, Cas2 and Csn2) for integration of the new spacer into the CRISPR array [19]. To yield a new spacer, a distinct sequence of the invading DNAs is inserted into the leader end of the CRISPR array by the Cas1-Cas2 complex (Fig. 2) and the first repeat of the array is duplicated to maintain the repeat-spacer-repeat architecture [26]. The PAM site only exists in targets but not the CRISPR assay, thus avoiding self-targeting [27,28].
2.1.2. crRNA biogenesis
Successful protection from DNA invading requires the CRISPR array to be transcribed into a long precursor CRISPR RNA (pre-crRNA) and further processed into mature CRISPR RNAs (crRNA) [29,30]. In the type II-A Cas9 system, the pre-crRNA expression is controlled by a promoter embedded within the AT-rich leader sequence preceding the CRISPR array. A tracrRNA with complementary sequence to the pre-crRNA (Fig. 2) is required for the processing of pre-crRNA, and forms a mature dual RNA (crRNA:tracrRNA). The duplex is specifically recognized and stabilized by Cas9, and further cleaved by an endogenous RNase III [31]. Then, an unknown nuclease trims the 5′ end of the crRNA, leading to the mature crRNA [31,32]. The crRNA and tracrRNA can also be artificially engineered and fused into a chimeric single guide RNA (sgRNA) for genome editing [33]. Unlike type II-A system, the type V-A Cas12a system capable of processing the crRNA maturation by itself, doesn't require tracrRNA or RNase III [24,25].
2.1.3. Interference
In the third stage of the defense, the effector complex guided by the crRNA recognizes and cleaves the invading DNAs [25,32,34]. Cas9 and Cas12a act as nucleases and play important roles during the interference process. The crystal structure of Cas9 reveals two lobes, an α-helical recognition (REC) lobe and a nuclease (NUC) lobe [28,35] (Fig. 2). The REC lobe, consisting bridge helix (BH), REC1, REC2, and REC3 domains, is indispensable for binding to sgRNA and DNA. The NUC lobe is composed of a PAM-interacting (PI) domain and two nuclease domains, HNH and RuvC, which cleaves complementary and non-complementary strands of the target DNA respectively. Similar to Cas9, Cas12a also adopts a bi-lobed structure comprising the REC and NUC lobes (Fig. 2b). The REC lobe consists of REC1 and REC2 domains, and the NUC lobe is comprised of Wedge (WED), PI, BH, Nuc, and RuvC domains. Unlike Cas9, Cas12a uses the single nuclease domain RuvC for cleavage of both DNA strands [36] and use WED III domain as the RNase for process its own crRNA [36,37].
In the type II-A system, Cas9 identifies target DNAs using its PI domain through the recognition of a cognate PAM sequence (such as S. pyogenes spCas9 PAM, 5′-NGG-3′) located directly downstream of the protospacer [27,28]. spCas9 recognizes the conserved PAM dG-2 and dG-3 nucleotides via major-groove interactions with two arginine residues (R1333 and R1335). Interference also depends on a PAM-proximal 10–12 nt seed segment at the 3′-end of the 20-nt target RNA [38] (Fig. 2a). The guide RNA loading regulates spCas9 activity by converting its apo-state to a target-recognition mode, where a central cleft is generated between the two lobes to accommodate the RNA-DNA heteroduplex [28,34]. Upon recognition of the PAM and preorganization of seed sequence in an A-form helical conformation, spCas9 triggers target DNA unwinding and R-loop formation. The target-DNA duplex adjacent to the PAM is destabilized through a phosphate lock loop (K1107-S1109) mechanism [27]. The configuration change stabilizes the structural distortion in the targeted strand and plays an important role for base pairing between the guide RNA and DNA [27]. Then, interacting with the pre-ordered seed region, a RNA-DNA heteroduplex is formed along the REC and NUC lobes, while the non-targeted strand is displaced [39]. The RNA-DNA heteroduplex along with the non-targeted strand forms a R-loop structure (Fig. 2). The R-loop triggers the cleavage at a specific site 3-bp from the NGG PAM, yielding predominantly blunt ends [33,40]. Fluorescence experiments show that the HNH domain is mobile, and whether it is in place relies on the PAM-distal-end complementarity [38]. The HNH domain allosterically controls the RuvC domain to guarantee high-fidelity of target DNA cleavage [38,40].
In the type V-A system, unlike Cas9, Cas12a does not require any tracrRNA and only needs crRNA for cleavage. Francisella novicida fnCas12a recognizes a 5′-TTN-3′ PAM located directly upstream (not downstream as in Cas9's case) of the protospacer by the base and shape readout mechanism [24,25]. Two invariant residuals (K613 and K671) in the WED domain are inserted in the minor and major grooves of the T-rich region [36,37]. The seed region which is approximately 5–6 nt at the 5′-end of the spacer-derived segment of crRNA, preorders in an A-form helical conformation by Cas12a (Fig. 2b). The residues of Lys823 and Gly826 interact with the phosphate group between dT-1 and dT0 of the targeted strand that initiates base pairing [41]. Similar to Cas9, Cas12a also undergoes large structural rearrangements, forming a cleft to accommodate the RNA-DNA heteroduplex [20,42]. fnCas12a, via RuvC catalytic domain, generates a staggered double-stranded break (DSB) with a 5-nt overhang distal to the PAM (19-bp from the PAM) [25]. However, the details of cleavage in Cas12a remain unknown and open for further investigations.
2.2. Anti-CRISPR-Cas systems
To survive in the arm race between phages and the CRISPR-Cas system, some “smart” phages have evolved anti-CRISPR-Cas systems that can inhibit CRISPR-Cas systems [[43], [44], [45]]. For a while, acquisition of point mutations within the protospacer or the PAM was thought to be the only way to escape the CRISPR-Cas systems. It is only until recently the anti-CRISPR proteins (Acr) [46] was discovered. The first Acr is found in pro-phages from the host Pseudomonas aeruginosa and allows pro-phages to escape destruction from CRISPR-Cas systems [46]. Later, plenty of Acrs are discovered from various bacterial hosts, such as Pseudomonas aeruginosa [[46], [47], [48]], Neisseria meningitides [48], Listeria monocytogenes [49], and Shewanella xiamenensis [48], and even from plasmids and conjugative DNA islands [46,47].
The Acrs discovered from ∼20 unique families are all small proteins with 50–150 amino acids, and have no sequence similarity to any known proteins [43,44]. Up to date, several mechanisms of the anti-CRISPR-Cas have been characterized, including interfering with DNA binding activity [[50], [51], [52], [53]], inhibiting Cas3 recruitment [54,55], and preventing DNA cleavage [56]. For anti-type II CRISPR-Cas9 [49], some Acrs act as DNA-mimicking inhibitors and occupy the PAM-binding site, thus preventing DNA target binding [[57], [58], [59]]. Alternatively, some Acrs disable Cas9 function by binding to the HNH domain via critical catalytic residues [56]. The anti-CRISPR-Cas can be potentially adapted to enhance the CRISPR-Cas editing by reducing its off-targeting effects and allowing for spatial, temporal, and conditional controls [58,60].
2.3. Cas variants
Cas9 and Cas12a as important effectors in CRISPR-Cas, have been engineered to generate variants for different purposes. Cas9 has two cleaving domains RuvC and HNH. The mutants RuvC (D10A) and/or HNH (H841A) are introduced into spCas9 to form the nuclease-deactivated Cas9 (dCas9, intact DNA binding activity but no cleavage activity) and the Cas9-nickase (nCas9, introducing a nick after single strand cleavage). As only bearing the RuvC domain for DNA cut, Cas12a can be modified to a nuclease-deactivated Cas12a (dCas12a, such as Acidaminococcus sp. dCas12a (E993A)), and cannot be engineered to form Cas12a-nickase [36,42,61]. The nCas9, dCas9, and dCas12a have been broadly applied for transcription regulation, base editing, etc. [3,62]. Recently, a new class of Cas9 variants with broad PAM compatibility and high DNA specificity, termed as xCas9, have been created [63]. These xCas9 proteins potentially increase the flexibility for choosing genomic loci.
The requirement of PAM site at target sequences limits Cas9 applications. For example, the PAM NGG recognized by canonical spCas9 only occurs once every 8–16 base pairs among selected genomes [63], and thus limits the applications that need precise positions. Although harnessing either previously engineered CRISPR nucleases [64,65] or other naturally-occurring CRISPR nucleases recognizing different PAMs [65,66] has enabled a wider selection of the targeting sequence, many genomic loci still remain inaccessible. Liu group used a “phage-assisted continuous evolution (PACE)” method [[67], [68], [69]] to generate the xCas9 variants [63], and observed notable recurring mutations (E480K, E543D, E1219V, A262T, K294R, S409I, M694I, K294R, Q1256K, and R324L) within the xCas9 variants [63]. Based on the crystal structure of spCas9, E1219 is close to the two residues, R1333 and R1335, which are involved in PAM recognition [27]. The R324, S409, and M694 residues are predicted to be close to the DNA-sgRNA interface, and possibly mediates target recognition and Cas9 conformation change. DNA cleavage test showed that a variant xCas9-3.7 was able to recognize GAA, GAT, CAA, NG, and NNG PAM sites [63], greatly expanding the PAM recognition sites. The xCas9 have proved to be effective in human cells for genome editing, transcriptional activation, and base editing. Interestingly, the xCas9 presented higher DNA specificity and lower off-target activity than spCas9, presumably because the xCas9 mutation residues are close to PAM or the DNA-sgRNA interface refines the DNA–RNA-contact region [63]. Except for the engineered xCas9, Nishimasu et al. recently reported that a rationally engineered spCas9 variant was able to recognize relaxed NG PAMs [70]. These results present potentials for further optimizing Cas variants.
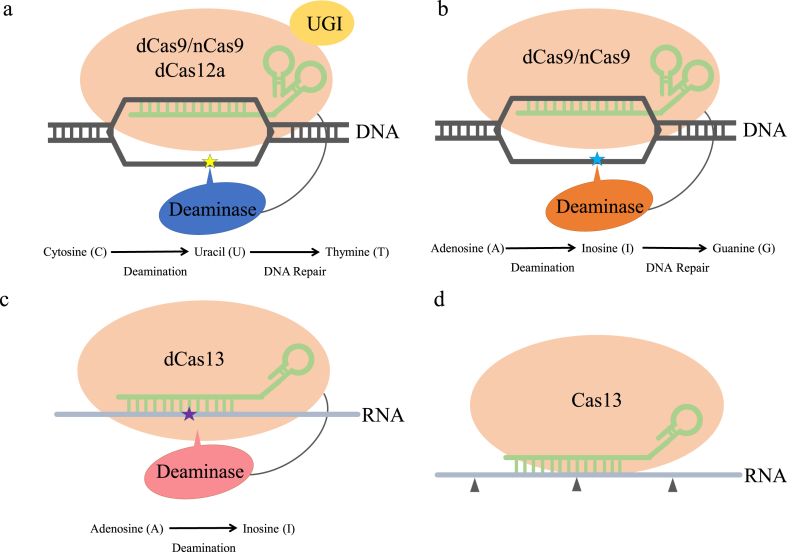
Besides Cas9 and Cas12a, another CRISPR nuclease Cas13a (formerly known as C2c2) is attracting great attentions [71,72]. Cas13a is the effector protein of the type VI CRISPR-Cas system. Unlike Cas9 and Cas12a, Cas13a is an RNA-guided RNase that cleaves both pre-crRNA and its single-stranded RNA (ssRNA) target (Fig. 4d). The structural study reveals that the RNase active pockets for cutting pre-crRNA and target RNA are located on the Helical-1 and HEPN domains, respectively [73]. The protospacer flanking sequence (PFS, equivalent to the PAM site) of Cas13a from Leptotrichia shahii is located at the 3′ end of the interval region, and is composed of A, U or C bases (not G). It is shown in vitro that after cleavage of crRNA-targeted RNA, Cas13a cleaves collateral RNA that has no complementarity to the designed crRNA [72]. Such promiscuous RNA cleavage is used for detecting specific nucleic acid (described in section 3.4). In E. coli, the activated nonspecific RNase activity cause cellular toxicity, retarding cell growth. Interestingly, when expressed in the human cells, Cas13a from Leptotrichia wadei only targets the RNA designated by crRNA, while all the other RNA sequences in the cells remain intact [71]. Furthermore, a new type VI CRISPR RNase Cas13b from Prevotella sp. was found to be more efficient compared to Cas13a and doesn't require any PFS [74], thus was further developed as an RNA base editor [74] (described in 3.3). Because Cas13 does not cleave DNAs, it is believed to be a better alternative for gene therapy.
Fig. 4.
The emerging CRISPR biotechnologies for in vivo manipulations. a) DNA base editing to switch C to T. the system contains a nuclease-defective Cas (dCas9/nCas9/dCas12a), a fused cytidine deaminase, and a fused uracil glycosylase inhibitor (UGI). b) DNA base editing to switch A to G. A nuclease-defective Cas (dCas9/nCas9) is fused to an evolved tRNA adenosine deaminase that converts A to G via I. c) RNA base editing to switch A to I. A catalytically-inactive dCas13 tethered to an adenosine deaminase, acts on RNA to convert A to I. d) RNA-guided RNase Cas13 mediates RNA cleavages. Asterisk, nucleotide change; triangle, cleavage.
3. CRISPR-Cas9/Cas12a-based biotechnology
The natural CRISPR-Cas systems were initially utilized for genome engineering and were later developed for gene regulation. These two technologies have extensive applications in prokaryotic and eukaryotic cells. Other CRISPR-related biotechnologies, such as DNA imaging, bacterial vaccination, virome tracking, and DNA cloning have also been created and well summarized in other reviews [3,13,[75], [76], [77]]. dCas-based DNA optical probes fused to fluorescent proteins have been developed for in vivo imaging of specific genomic loci, and this technology propels our understanding of chromosomal organizations and dynamics. Analysis of the CRISPR array sequences also enables precise genotyping of bacterial strains and provides a record of past virome infections that hints on the interactions between bacterial species and bacteriophages. Such information is also valuable to develop CRISPR-Cas vaccination to prevent bacteriophage infections, which helps minimize the risk of failure in industrial fermentations. Additionally, CRISPR DNA cloning can facilitate the in vitro DNA assembly in a simple way [[78], [79], [80], [81]]. Here, we discuss the widely-used CRISPR-based genome engineering and CRISPR gene regulation, and the emerging CRISPR-biotechnologies including base editing and nucleic acid detection.
3.1. Nuclease-dependent CRISPR genome editing
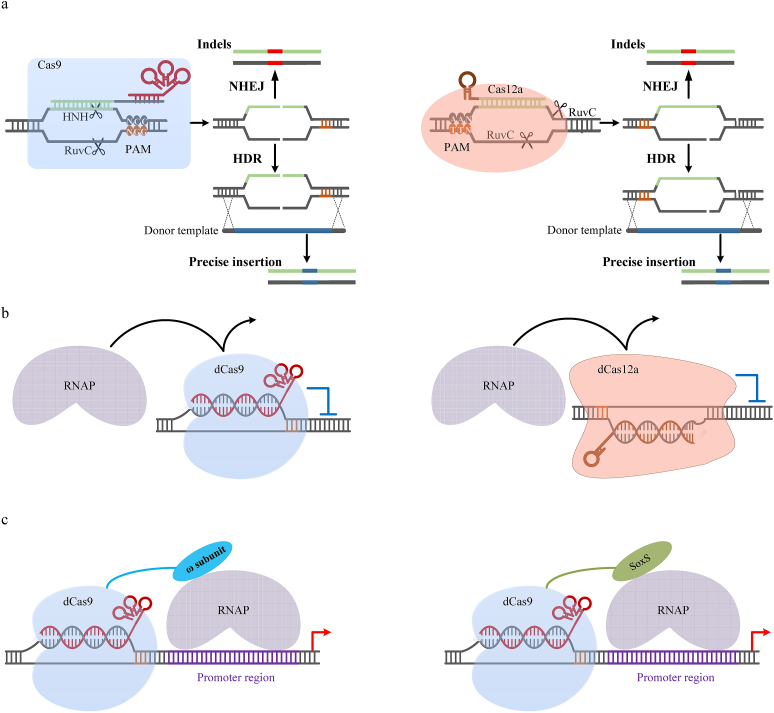
Before CRISPR-Cas, zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) [82] are used for programmable sequence-specific genome editing, aided by fusion of FokI nuclease. ZFNs and TALENs use repeat domains to recognize triple and single base pairs respectively, and modular domain repeats need to be linked in a sequential manner to recognize contiguous sequences. Therefore, huge difficulties arise for custom domain design. In contrast to ZFNs and TALENs, CRISPR-Cas only requires a simple design of guide RNAs to lead to specific DNA targets. The CRISPR genome editing initiates from the introduction of DSB (described in 2.1.3 section), and followed by DNA repair through NHEJ or HDR (Fig. 3a). In bacteria, the NHEJ system depends on a dedicated DNA ligase (LigD) and the DNA-end-binding proteins, and leads to error-prone repair by introducing imprecise indels [83]. Most of bacteria lack NHEJ, and need donor DNA templates for precise HDR repair.
Fig. 3.
CRISPR genome editing (a) and CRISPR gene regulation including CRISPR interference (b, CRISPRi) and CRISPR activation (c, CRISPRa). The genome editing starts from the introduction of DSBs (double-stranded breaks) followed by NHEJ and HDR DNA repair. CRISPRi uses dCas (dCas9 or dCas12a) to sterically block RNA polymerase (RNAP) to repress gene expression. CRISPRa is achieved by fusing the ω-subunit of the RNAP or the bacterial RNAP activator SoxS to dCas9, and activates transcription by recruitment of the RNAP assembly.
In the first reported bacterial genome editing [84], a dual-RNA:Cas9 was constructed for the cleavage at targeted genomic sites in Streptococcus pneumoniae and in E. coli. Together with a donor template, the CRISPR-induced HDR generated marker-less mutations. The editing efficiency was significantly improved by introducing the lambda–Red [84] or the RecET [85] recombineering systems derived from phagenic repair systems. Later, replacement of the dual-RNA (tracrRNA:crRNA) by the sgRNA further simplified system [33]. Moreover, Cas12a further facilitated the genome editing by targeting to multiple genomic loci through a single transcript [72].
3.2. CRISPR gene regulation: CRISPR interference and CRISPR activation
The ability to precisely regulate gene expression is important to understand desired genes' functions. ZFNs and TALENs have also been developed for gene regulation [82]. However, their applications are often limited by difficulties in designing the effective domains. By contrast, dCas provides a simple and robust technology for gene repression and activation, and can target almost any DNA sequence aided by the sgRNA. The nuclease-defective S. pyogenes dCas9 (D10A and H841A) is the widely used for various applications. Working like bacterial transcriptional repressors that are limited by recognitions of specific-DNA-sequences [86,87], the CRISPR interference (CRISPRi) inhibits transcription by sterically blocking the RNA polymerase (RNAP) (Fig. 3b). The first CRISPRi in bacteria was reported by Qi et al., demonstrating an RNA-guided dCas9 system for fluorescent-protein gene repression [88]. Interestingly, the dCas-sgRNA complex alone was enough for strong gene repression in bacteria [88], whereas auxiliary inhibitors were required to fuse to dCas for the strong repression in eukaryotic cells [89]. For multi-gene repression, the dCas9 CRISPRi needs independent expression of multiple sgRNAs [88], while dCas12a CRISPRi only needs the expression of a single CRISPR array [61].
CRISPR-based gene activation, termed CRISPRa, is also achieved in E. coli by fusing the ω-subunit of the RNAP to the dCas9 complex [90] (Fig. 3c). The dCas9 complex guided by crRNA binds to the upstream promoter regions and recruits the RNAP, and further activates transcription. However, the fold of activation in the initial CRISPRa system was not significant, limiting further applications. Recently, Hu et al. adopted a mutated ω subunit ω-(I12N) tethered to dCas9 to boost transcriptional activation up to over 100-fold [63]. This system was then used in “phage-assisted non-continuous evolution” to evolve the xCas9 variants [63], demonstrating great potential for other applications. Besides the application in E. coli, the dCas9-ω CRISPRa was also used in Myxococcus xanthus for improvement of epothilone production by gene-cluster activation [91]. Furthermore, a new CRISPRa system [92] was recently set up in E. coli by fusing a bacterial RNAP activator SoxS, a member of the AraC family of transcription factors (Fig. 3c). The CRISPRa system obviously increased the activation by over 100-fold compared to the control and successfully applied for ethanol biosynthesis.
In conclusion, owing to the simpler design, better performance and less sequence constraint, the capacity of CRISPRa/i to activate and repress gene expression is more powerful compared to the widely adopted transcription factor/DNA pairs.
3.3. Base editing
Currently, DNA and RNA base editing approaches are available in both prokaryotic and eukaryotic cells, greatly advancing DNA manipulations in a safer manner. The classic nuclease-dependent CRISPR genome editing (described in section 3.1) generates DSBs that introduce toxicity and massive random indels derived from the NHEJ and other known/unknown mechanisms [93,94], thus resulting in inefficient genome-editing and potential risks [[95], [96], [97]]. To overcome such problems, a novel CRISPR technology “DNA base editing” has been first developed to change single DNA nucleotides without introducing DSBs or requiring any homology-directed repair [[96], [97], [98]]. The first developed base editor is cytosine deaminase-based DNA base editor [[97], [98], [99]], which converts C to T within target sequences. The system consists of a) an inactive CRISPR-Cas along with a sgRNA for specific binding, b) a cytidine deaminase that converts C to U which is later converted to T via endogenous DNA repair systems, within a narrow nucleotide window on a single-stranded DNA, and c) an uracil glycosylase inhibitor (UGI) that blocks uracil excision (Fig. 4a). The inactive Cas dCas9, dCas12a, and nCas9 were tested, among which nCas9 (D10A) nicking the non-edited DNA strand showed higher efficacy. The cytidine deaminase (such as apolipoprotein B mRNA-editing enzyme, catalytic polypeptide–like (APOBEC) and activation-induced deaminase (AID) cytidine deaminase) and the UGI were fused to dCas to form the base editing system. Moreover, adenine base editors (ABEs) were later developed, converting A to G using an evolved tRNA adenosine deaminase [96] (Fig. 4b), exhibiting high editing efficacy and purity.
Since creating the DNA base editing systems, many groups have focused on optimization of the systems, and applications for disease treatment in vertebrate [96,100] and crop improvement in plant [101]. A bacteriophage Mu protein that can bind to DSBs was fused to nCas9, further reducing the frequency of indels during base editing [102]. The narrow window (caused by the R-loop) around suitably positioned PAM sites are confirmed to be important for high-efficiency base editing [[95], [96], [97]]. The derivatives of Cas9 and Cas12a with broad PAM compatibilities could further extend the capacity of base-editing [3,96,99]. Owing to the substitutions of C to T and A to G in most of known human genetic diseases, the base editing systems are promising in clinical applications [96,100].
Besides DNA base editing, RNA base editing is created using Cas13b by Zhang group [74]. An adenosine deaminase that acts on RNA converting adenosine (A) to inosine (I), was tethered into the catalytically-inactive Cas13b (dCas13b), forming a RNA Editing for Programmable A to I Replacement (REPAIR) base editing system (Fig. 4c). The REPAIR system only edited full-length RNA transcripts and did not alter the DNA sequence, thus presenting a promising and safe base editing platform for treating diseases that need short-term changes in transcription level [74].
3.4. Nucleic acid detection by Cas13 and Cas12a
Based on the promiscuous RNase ability to cleave collateral RNAs, Cas13a has been developed as a highly sensitive diagnostic tool for nucleic acid detection in vitro [103]. Once Cas13a-crRNA complex recognizes the target RNA and performs the specific cleavage, the activated Cas13a will cut the nearby non-targeted RNAs. A RNA reporter-quenched fluorescent RNA, which does not emit any fluorescence until the RNA gets cleaved, was used in the system. The system was further optimized through the technology “isothermal amplification” with recombinase polymerase amplification (for amplifying signals) and T7 transcription (for detecting DNA signals) [104,105], generating a “Specific High-Sensitivity Enzymatic Reporter UnLOCKing” (SHERLOCK) system. The system can detect DNAs and RNAs with single-base mismatch specificity and attomolar detection sensitivity. Later, indiscriminate single-stranded DNA (ssDNA) cleavage activity by the activated Cas12a was disclosed [9,11]. Results showed that the RNA-guided DNA binding activated LbCas12a for both cleavages on site-specific dsDNA and collateral ssDNA. Interestingly, turnover values for cleavage of site-specific dsDNAs were much lower than those for the collateral ssDNA. The cleavage of collateral dsDNA after the specific cleavage was arguable based on current results from two groups [9,11]. Also, the Cas12a was explored to generate a DNA endonuclease-targeted CRISPR trans reporter (DETECTR) system for DNA detection with the attomolar sensitivity [9]. Further, using Cas13 or Cas12a along with an auxiliary CRISPR-associated enzyme (Csm6), SHERLOCK was optimized to generate SHERLOCK v2 and successfully applied in detection of Dengue and Zika virus [10]. In sum, these Cas13/Cas12a-based nucleic acid detection technologies provide a simple, fast, portable, and quantitative detection platform for molecular diagnostics.
4. Applications of CRISPR-Cas9/Cas12a biotechnology in bacteria
CRISPR-Cas9/Cas12a are well-studied CRISPR nucleases, and thoroughly engineered and optimized in bacteria for broad applications, especially in metabolic engineering and synthetic biology. Bacteria as cell factories can take up simple and cheap feedstock, like renewable biomass and even wastes, for basic cell metabolism and biosynthesis of value-added chemicals. To enhance performance of the cell factory, genetic manipulation on them are often required. Although the traditional methods based on recombination systems are available, they are time consuming and labor intensive. Nowadays, CRISPR-Cas9/Cas12a-based biotechnologies have greatly facilitated the genetic manipulation on model and non-model bacteria for higher editing efficiency and specificity. Here, we summarize the CRISPR biotechnology applications on a few characterized bacteria, including E. coli, Streptomyces, Clostridium, Corynebacterium, Bacillus, lactic acid bacteria, cyanobacteria, and pathogenic bacteria (Table 1).
Table 1.
Applications of CRISPR-Cas9/Cas12a biotechnologies in various bacteria.
| Bacteria | Biotechnologies | Applications |
|---|---|---|
| Escherichia coli | ||
| E. coli [84,115,116,122,123] | Cas9-mediated genome editing | Production of uridine, adipic acid, β-carotene, and isopropanol |
| E. coli [72,243] | Cas12a-mediated genome editing | Biotechnology demo |
| E. coli [63,90,92] | dCas9-mediated CRISPRa | Biotechnology demo |
| E. coli [[117], [118], [119], [120], [121],[124], [125], [126], [127]] | dCas9-mediated CRISPRi | Production of lycopene, isoprene, 4-hydroxybutyrate, malate, butanol, naringenin, malonyl-CoA, and mevalonate |
| E. coli [61] | dCas12a-mediated CRISPRi | Biotechnology demo |
| E. coli [[96], [97], [98]] | DNA base editing | Biotechnology demo |
| E. coli [74] | RNA base editing | Biotechnology demo |
| E. coli [71,72] | RNA cleavage | Biotechnology demo |
| Cyanobacteria | ||
| Synechococcus [134] | Cas12a-mediated genome editing | Biotechnology demo |
| Synechocystis [134] | Cas12a-mediated genome editing | Biotechnology demo |
| Anabaena [134] | Cas12a-mediated genome editing | Biotechnology demo |
| S. elongatus [150,153] | Cas9-mediated genome editing | Biotechnology demo |
| Synechocystis [151] | Cas9-mediated genome editing | Biotechnology demo |
| Streptomyces | ||
| S. coelicolor [171,173,174,176] | Cas9-mediated genome editing | Production of secondary metabolites |
| S. ablus [172,177] | Cas9-mediated genome editing | Activation of silent BGCs |
| S. viridochromogenes [172,177] | Cas9-mediated genome editing | Activation of silent BGCs |
| S. lividans [172,177] | Cas9-mediated genome editing | Activation of silent BGCs |
| S. coelicolor [174] | dCas12a-mediated CRISPRi | Production of secondary metabolites |
| S. hygroscopicus [174] | Cas12a-mediated genome editing | Production of 5-oxomilbemycin |
| S. rimosus [175] | Cas9-mediated genome editing | Production of oxytetracycline |
| S. coelicolor [176] | dCas9-mediated CRISPRi | Production of secondary metabolites |
| S. venezuelae [177] | Cas9-mediated genome editing | Activation of silent BGCs |
| Lactic acid bacteria | ||
| L. reuteri [85] | Cas9-mediated genome editing | Biotechnology demo |
| L. casei [186] | Cas9-mediated genome editing | Biotechnology demo |
| Clostridium | ||
| C. beijerinckii [193,194] | Cas9-mediated genome editing | Biotechnology demo |
| C. saccharoperbutylacetonicum [195] | Cas9-mediated genome editing | Production of butanol |
| C. acetobutylicum [196] | dCas9-mediated CRISPRi | Biotechnology demo |
| C. beijerinckii [196] | nCas9-mediated genome editing, dCas9-mediated CRISPRi | Biotechnology demo |
| C. ljungdahlii [198] | Cas9-mediated genome editing | Production of ethanol from synthetic gas |
| C. tyrobutyricum [199] | Cas9-mediated genome editing | Production of butanol |
| C. pasteurianum [200] | Cas9-mediated genome editing | Production of butanol from waste glycerol |
| C. difficile [201] | Cas12a-mediated genome editing | Biotechnology demo |
| C. cellulolyticum [202,203] | nCas9-mediated genome editing | Production of biofuels and chemicals from lignocellulosic biomass |
| C. acetobutylicum [204] | dCas9-mediated CRISPRi | Relief of catabolite repression |
| C. beijerinckii [205] | dCas9-mediated CRISPRi | Biotechnology demo |
| C. cellulovorans [206] | dCas9-mediated CRISPRi | Production of solvents (acetone, butanol and ethanol) |
| Corynebacterium | ||
| C. glutamicum [209] | Cas12a-mediated genome editing | Biotechnology demo |
| C. glutamicum [[210], [211], [212], [213]] | Cas9-mediated genome editing | Production of γ–aminobutyric acid, 1,2-propanediol |
| C. glutamicum [214] | Cas9 and nCas9-mediated genome editing, base editing | Production of glutamate |
| C. glutamicum [[215], [216], [217]] | dCas9-mediated CRISPRi | Production of l-lysine, l-glutamate and homo-butyrate |
| Bacillus | ||
| B. subtilis [219,220,223,224] | Cas9-mediated genome editing | Production of l-valine and β-cyclodextrin glycosyltransferase |
| B. subtilis [219,221,222] | dCas9-mediated CRISPRi | Production of hyaluronic acid and N-acetylglucosamine |
| B. smithii [225,226] | Cas9-mediated genome editing | Biotechnology demo on a moderate thermophile |
| Pathogenic bacteria | ||
| S. aureus [229] | DNA base editing | Biotechnology demo |
| S. aureus [230] | Cas9-mediated CRISPR | Biotechnology demo |
| S. aureus [231] | dCas9-mediated CRISPRi | Biotechnology demo |
| M. tuberculosis [[232], [233], [234]] | dCas9-mediated CRISPRi | Biotechnology demo |
| P. aeruginosa [240] | dCas9-mediated CRISPRi | Biotechnology demo |
| Klebsiella [241] | Cas9-mediated genome editing | Biotechnology demo |
| K. pneumoniae [242] | dCas9-mediated CRISPRi | Biotechnology demo |
| Y. pestis [243] | Cas12a-mediated genome editing | Biotechnology demo |
4.1. E. coli
E. coli is one of the most widely used host organisms for microbial cell factories and synthetic biology applications [86,87,[106], [107], [108]]. In the past, a large number of tools have been established to edit genomic sequences to knock-in or knock-out genes, which are primarily based on homologous recombination-based systems (e.g., λ-Red, Cre-lox, and FLP-FRT). To expand the capacity of such systems to large-scale genomic editing that can efficiently modify multiple genomic loci, multiplex automated genome engineering (MAGE) and conjugative assembly genome engineering (CAGE) have been developed [109,110]. While these tools greatly improved our capability to edit E. coli genome, they often require the expression of selection markers in the genome or need either screening or genome sequencing to identify the desired clone. In addition, a number of tools to control endogenous gene expression levels without modifying the genome are also available in E. coli. For example, zinc finger proteins (ZFPs) and antisense RNAs can be designed to mostly turn down expression [111,112]. However, these tools often require the introduction of heterologous sequence to the genome and thus leave scars, or have limited design consensus [113].
Although tools in E. coli is relatively abundant compared to many other host organisms, CRISPR-Cas still greatly propels our capability to engineer E. coli. Such capabilities have largely facilitated metabolic engineering and proved to improve the titers, productivities and yields for various chemicals. Compared to the previously available tools, CRISPR-Cas-based systems have a few advantages that have been exploited in various applications. First, CRISPR-Cas system is highly efficient in cleaving the target sequence and thus can be combined with λ-Red recombineering to achieve precise, efficient, and marker-free editing. For example, Jiang et al. developed a two-plasmid system that allowed iterative genome editing of multiple targets and curing of the plasmids after completion [114]. These systems saved the efforts to recycle selection markers and enable multiple modifications to be introduced simultaneously, thus greatly improved the engineering throughput. Li et al. exploited this capacity to integrate a β-carotene synthetic pathway into the genome and performed combinatorial modulations to test 33 genomic modifications to search for clones with improved β-carotene production, with the best producer yielding 2.0 g/L β-carotene in fed-batch fermentation [115]. In another study, genome libraries of isopropanol pathway with close to 1000 variants were rapidly constructed with CRISPR, and thus allowed more genetic space to be explored to identify the superior performer. The best performer reached a titer of 7.1 g/L isopropanol within 24 h [116]. Second, CRISPR-Cas systems are versatile and can be used to transcriptionally repress or activate gene expression. CRISPRi and genome editing have been successfully applied in metabolic engineering to optimize gene expression levels for improved production of various molecules, including lycopene [117], isoprene [117], 4,4′-dihydroxybiphenyl (4HB) [118], malate [119], n-butanol [120], naringenin 7-sulfate [121], uridine [122], adipic acid [123], etc. Wu et al. constructed the CRISPRi system to perturb the expression of multiple genes in the central metabolic pathway and achieved improved malonyl-CoA level by 223% [124]. Li et al. targeted the DNA replication machinery with CRISPRi to decouple cell growth from production of biochemicals, and led to an increase in mevalonate yield by 41% [125]. Further, CRISPRi and CRISPRa also expand the toolkit of genetic parts to build gene circuits, as orthogonal control can be achieved by just using different sgRNAs. For example, orthogonal sgRNA/promoter pairs were constructed by Nielsen et al. and used to build multi-input logic gates with high on-target repression [126]. The circuit was further connected to the native E. coli metabolism by targeting the output sgRNA to a transcription factor. Linking CRISPR-Cas to cellular metabolism in gene circuits also provides promising potentials for metabolic engineering applications, and the prospects of engineering dynamic CRISPR-Cas circuits to regulate metabolic pathways has been discussed in another recent review [127]. In addition, emerging technologies such as programmable DNA/RNA base editing further opens up opportunities to expedite metabolic engineering processes by allowing for point mutations without DSBs [128] (discussed in section 3.3, Table 1). Moreover, CRSPRa with SoxS has been recently expanded to allow for simultaneous multiplex genome editing of up to six different genes and genes at least 41 loci [129]. Overall, CRISPR-Cas has greatly advanced our capability to achieve multi-target genome editing and gene expression control, and expanded tools for synthetic biology applications.
4.2. Cyanobacteria
Cyanobacteria is a group of photosynthetic bacteria, habiting in various conditions. Unlike the broadly-used chassis E. coli that requires food-based carbon feedstock [[130], [131], [132], [133], [134]], cyanobacteria is able to utilize CO2 and solar energy for growth and biosynthesis [[135], [136], [137], [138], [139], [140], [141], [142]]. Numerous products have been successfully produced by cyanobacteria, ranging from biofuels, pharmaceuticals, and nutrients [130,133,[143], [144], [145], [146], [147], [148], [149]]. Also, integration of CO2 into biomass by cyanobacteria can restore the energy balance [136]. Thus, it is believed that cyanobacteria are next-generation cell factories for synthetic biology and metabolic engineering.
However, the time to successfully engineer cyanobacteria is much longer than other established cell hosts [134,144,145,150,151]. This is because cyanobacteria are ploidy, i.e. cyanobacteria have multiple copies of chromosomes, varying from 3 to up to 218 copies [152]. For example, there are more than 12 chromosome copies per cell in Synechocystis PCC 6803, and ∼3 copies per cell in Synechococcus PCC 7942. For conventional homologous recombination-based editing method, multiple rounds of segregations are required to get a fully-segregated mutant in which all chromosomes are modified. Thus, there is a great need for the development of a fast one-step gene editing system. So far, CRISPR-Cas9 and CRISPR-Cas12a systems have been developed to facilitate genome editing in cyanobacteria by several groups [134,150,151,153]. Cas9 has been successfully applied for genome engineering in Synechococcus elongatus UTEX 2973 [150], S. elongatus PCC 7942 [153], and Synechocystis sp. PCC 6803 [151]. Due to the toxicity caused by high expression of Cas9, the transient or low-level expression of Cas9 are adopted and allows for efficient genome editing. Using the Cas9-assisted genome editing, Li et al. performed multiple gene knock-in and knock-out to generate a succinate-producing Synechococcus with high performance (succinate titer 0.44 mg/L, an 11-fold increase compared to the starter) [153]. Moreover, Xiao et al. developed an inducible Cas9 system to cure the endogenous plasmids in Synechocystis sp. PCC 6803 and created a E. coli- Synechocystis shuttle vector for stable expression of heterologous genes [151]. Moreover, Cas12a is reported to present less toxicity compared to Cas9, and the Cas12a-assisted genome editing has been applied in Synechocystis sp. PCC 6803, S. elongatus 7942, and Anabaena sp. PCC 7120 [134]. These results showed that Cas12a worked effectively in Synechococcus (efficiency 90%), whereas its efficiency was modest in Anabaena (efficiency 63%) and Synechocystis (efficiency 44%).
4.3. Streptomyces
Streptomyces is the largest genus of Actinobacteria with an extremely high GC content in their genomes. Streptomyces have evolved to produce a wide variety of bioactive secondary metabolites, including antibiotics, anticancer agents, herbicides, and immunosuppressants [[154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166]]. However, the potential for metabolite biosynthesis is not fully explored. Whole genome sequencing analysis reveals that Streptomyces harbor a large number of silent biosynthetic gene clusters (BGCs), providing useful clues for discovery of unique secondary metabolite pathways [[167], [168], [169], [170]]. The lack of efficient tools for exploiting Streptomyces genomes, impedes the discovery process, and thus advanced genetic tools are urgently needed.
Recently, the CRISPR-Cas technique provides a powerful tool for in-frame gene deletion [52,[171], [172], [173], [174]], single/double-site mutations [175], reversible gene expression control [176], and activation of silent BGCs in multiple Streptomyces strains [177]. Several studies utilize a codon-optimized spCas9 for genome editing [[171], [172], [173],176,178]. The efficiency of genome editing in three independent research achieved 66–100% efficiency in S. ablus and S. viridochromogenes [172], and 70–100% efficiency in S. coelicolor [171,176]. Cobb et al. [172] compared a dual tracr/crRNA expression cassette with a sgRNA expression cassette. The results showed that the usage of sgRNA in the CRISPR system for multiplex engineering presented better efficiency, leading to efficient genome deletion ranging from 20 bp to 30 kb. To introduce spCas9 into industrial Streptomyces, Li et al. [174] developed a fnCas12a-assisted genome editing system in S. coelicolor, and expanded the system into seven other Streptomyces strains including important industrial strains S. pristinaespiralis HCCB10218 and S. hygroscopicus SIPI-KF. The efficiency of gene deletion in S. coelicolor reached 0–50% by NHEJ and 75–95% by HDR, respectively. In the same study, a dCas12a-based integrative CRISPRi system for transcriptional repression in Streptomyces has been created, achieving up to 95% repression [174]. Furthermore, Lei et al. [78] and Zhang et al. [177] reported an efficient CRISPR-Cas9-mediated and CRISPR-Cas12a-mediated promoter knock-in strategy to activate silent BGCs within different species, including S. albus, S. lividans, S. roseosporus, S. venezuelae, and S. viridochromogenes. In Zhang's work [177], the native promoters were replaced by a strong and constitutive promoter kasO*p using CRISPR-Cas9 system. Consequently, the biosynthesis of related compounds was enhanced, and some new products (such as a novel type II polyketide) were identified. This strategy presents an improved technique for activation of silent BGCs and contributes to discovery of new uncharacterized compounds.
4.4. Lactic acid bacteria (LAB)
LAB constitute a wide group of low-GC Gram-positive bacteria that are non-sporulating, non-motile, facultative anaerobic, and acid-tolerant [179]. The main applications of LAB are food starters and health-promoting probiotics [180,181]. LAB possess specific characteristics, including small genome sizes, high sugar uptake, high tolerance to environmental stress, and uncoupled growth and energy metabolism. Such features render them natural cell factories for industrial production of metabolites and enzymes [[182], [183], [184], [185]].
Exogenous CRISPR-Cas9 systems have been broadly adapted for genome editing in LAB [85,186]. Moreover, Stout et al. [187] reported a mechanism of native CRISPR targeting escape in L. gasseri JV-V03 and NCK1342, which contributes to a better understanding of the occasional target failure of type II systems. On the other hand, targeted mutagenesis with high efficiencies (90–100%) in Lactobacillus reuteri was achieved through spCas9-mediated single-strand DNA recombineering [85]. Song et al. [186] developed a highly efficient nCas9 system for in-frame gene deletions and chromosomal insertion of exogenous genes in L. casei, with efficiencies ranging from 25 to 62%.
4.5. Clostridium
The Gram-positive, anaerobic, spore-forming Clostridium has drawn tremendous attention, because Clostridium includes diverse species with vital importance for human disease and industrial biotechnology [188]. In particular, they have great potentials for the production of biochemical and biofuels from renewable carbon sources [189,190]. Because Clostridium is difficult to be genetically engineered, the development and implementation of efficient genetic engineering tools is a prerequisite of constructing cell factory [191,192].
The CRISPR-Cas tools have been extensively employed as a counter-selection tool for selecting rare homologous recombination events in the Clostridium [193,194]. Wang et al. [193] reported efficient and marker-less chromosomal gene deletion in Clostridium beijerinckii NCIMB 8052 using spCas9. The system was then optimized and expanded in the same strain for large DNA fragment deletion, gene integration and single nucleotide modification by combining Cas9 expression with an inducible promoter and plasmid-borne editing templates [194]. The strategy of inducible Cas9 expression significantly improved HDR efficacy, which has been recognized by many researchers [[195], [196], [197], [198]]. For example, Wang et al. [195] applied this customized genome editing tool along with optimized gRNA expression for a hyper-butanol-producing strain C. saccharoperbutylacetonicum N1-4, resulting in a double deletion mutant strain capable of producing 19.0 g/L butanol. Huang et al. [198] developed a similar system in C. ljungdahlii. They tested several available promoters for expressions of Cas9 and sgRNA, and selected strong promoters Pthl (thiolase) and ParaE (phosphotransbutyrylase) to control the expression. All genetic elements were constructed in a single plasmid vector and transferred into C. ljungdahlii by electroporation. The precise single gene deletions were achieved with efficiencies ranged from 50% to 100%. Besides spCas9, the exploitation of the native type I-B CRISPR-Cas within C. pasteurianum and C. tyrobutyricum was also reported [199,200]. The native CRISPR-Cas can mitigate Cas9 toxicity and improve transformation efficiency up to 100%. In addition, exogenous CRISPR-Cas12a was exploited for deletion of large DNA fragment (49.2 kb) and multiplex genome editing with high efficiencies in C. difficile [201].
As nCas9-based genome editing and gene repression can circumvent lethal effects of Cas9-induced DSBs, this strategy has been widely used in C. acetobutylicum, C. beijerinckii, and C. cellulolyticum [196,202,203] with up to 100% efficiency. Xu et al. [202] reported applications of nCas9 in precise gene deletions and insertions in C. cellulolyticum. The nCas9 editing was further explored to facilitate antisense RNA-mediated repression [203]. First, the authors used a synthetic promoter P4 and a ferredoxin promoter from C. cellulolyticum to drive the expression of nCas9 and sgRNA respectively, generating a single-nick-triggered homologous recombination within one step. Then, plasmid-borne homologous arms with 0.1-kb, 0.2-kb, 0.5-kb or 1-kb DNA length, were tested as donor templates, demonstrating longer regions of homology on the donor had higher efficiencies (more than 95%). Lastly, such Cas9-nickase genome editing facilitated antisense RNA-mediated repression targeting pta encoding phosphotransacetylase, leading to reduce acetate titer in both wild-type and lactate deficient mutant [203]. Also, the dCas9-CRISPRi technology has been developed as an efficient tool for gene repression in Clostridium species [196,[204], [205], [206]]. In particular, Wen et al. [206] adopted CRISPRi strategy to down-regulate the expression of a putative hydrogenase and resulted in decreased hydrogen production.
4.6. Corynebacterium
Gram-positive Corynebacterium is a major workhorse for production of amino acids and a variety of related compounds which are used as polymer subunits, biofuels, feed additives, nutritional supplements, cosmetics, and pharmaceutical intermediates [207,208]. The CRISPR-Cas genome editing methods have been developed in several Corynebacterium species [[209], [210], [211], [212]]. The first publication on genome editing in C. glutamicum was reported by Yang group, who adapted fnCas12a along with single-stranded DNA recombineering for genetic alterations [209]. Since Cas12a is capable of processing its own crRNA, multiplex genome editing using a customized CRISPR array is easily to be attained. The genes for fnCas12a and crRNA were combined with homologous arms in one single vector for large gene deletions and insertions. Aided by the system, the condon saturation mutagenesis of proB encoding γ-glutamyl kinase successfully enabled to remove a l-proline feedback inhibition. Unlike Cas12a, the CRISPR-Cas9 genome editing needs fine-tuning of Cas9 expression to relieve the Cas9 toxicity. Several attempts utilized a two-plasmid system, one expressing Cas9 under an inducible promoter, and the other carrying sgRNA and a repair template [210,211]. Furthermore, Cho et al. [212] developed another two-plasmid system, one vector was used to express Cas9-sgRNA, and the other vector was used to express RecT from a Ptac promoter. RecT expression played an important role to obtain positive transformants using ssDNA as an editing template. After genome editing, plasmids could be cured to obtain plasmid-free strains. This tool was then applied to perform multiple gene knockout for enhanced γ–aminobutyric acid production. Wang et al. [213] optimized the single-plasmid method by utilizing a chromosome-borne Cas9-RceET and eliminated the instability of Cas9 on the plasmid. Aided by this system, 1,2-propanediol production reached as high as 6.75 g/L in a small-scale fermentation. In addition to nuclease-dependent genome editing, nuclease-free DNA base editing technologies have also been developed in C. glutamicum based on the experience from animals and plants systems [214]. Integrated in robotic systems, a multiplex automated C. glutamicum based editing method (MACBETH) was created to increase glutamate production. To improve glutamate production, MACBETH was applied to construct a multiple gene inactivation library, in which pyk&ldhA double inactivation phenotype showed the best performance. The ease and automation of MACBETH could significantly speed up the generation of rationally engineered strains.
In addition, CRISPRi was established earlier than the CRISPR-based genome editing in C. glutamicum. Cleto et al. [215] applied CRISPRi to repress glycolytic pathway genes, pgi, pck, and pyk, and enhanced l-lysine production and l-glutamate productivity within as short as three days. Park et al. [216] expanded CRISPRi to repress two genes simultaneously and increased l-lysine yield by 1.3-fold in C. glutamicum DM1919. In another study, Yoon and Woo [217] adopted CRISPRi to repress acn encoding aconitase for higher homo-butyrate production.
4.7. Bacillus
Bacillus is gram-positive and rod-shaped, including free-living and parasitic pathogenic species. Many Bacillus species are generally recognized as safe (GRAS) microorganisms and perform well as cell factories. Thus, Bacillus have been commonly applied for the industrial value-added production, especially recombinant proteins [218]. Recently, the CRISPR tools for Bacillus have been developed to facilitate genetic modification. Chou group [219] developed CRISPR-Cas9 genome editing based on chromosomal expression system and CRISPRi in Bacillus subtilis, which can effectively perform continuous genome editing, multiplexing of gene mutations, and gene repression. This system is comprised of two essential elements, PxylA.SphI+1-gRNA transcription cassette and counter-selectable gRNA delivery vectors, thus avoiding instability and metabolic burden caused by multiple plasmids. Using this strategy, this group successfully improved the production of l-valine [220] and hyaluronic acid [221]. In detail, the l-valine titer was increased to 4.61 g/L in shake flask cultures using the following strategies: (i) releasing l-valine feedback inhibition; (ii) redirecting more carbon towards l-valine biosynthetic pathway; (iii) blocking l-valine degradation pathway and competing pathways; (iv) increasing precursor pyruvate concentrations. Other industrially relevant examples for Bacillus genome editing include disruption of essential genes to construct N-acetylglucosamine (GlcNAc) producing strains [222] and β-cyclodextrin glycosyltransferase producing strains [223], and knocking out protease genes to construct protease-deficient strains [224]. Wu et al. [222] developed a xylose-induced CRISPRi system, which efficiently down-regulated GlcNAc-competing pathway genes: zwf in the pentose phosphate pathway, pfkA in the glycolytic pathway, and glmM in the peptidoglycan pathway. The engineered strain was able to produce 17.4 g/L of GlcNAc at a yield of 0.42 (g/g) from glucose and xylose. After combination of sgRNA and optimization of temporal control system, the final strain could synthesize 103.1 g/L of GlcNAc in a 3-L fermenter via fed-batch culture. In a thermophilic B. smithii study, to overcome obstacles of the regular spCas9's working temperature (<42 °C), Mougiakos et al. [225,226] developed a thermos-tolerant Cas9 (ThermoCas9) from a thermophilic bacterium G. thermodenitrificans T12. Finally, the CRISPR-mediated gene deletion can be achieved at 55 °C in vivo. Besides Cas9, Li et al. [227] developed a nCas9 genome editing method for single, double and large-fragment gene deletions and gene integration, reaching efficiencies of 100%, 11.6%, 79%, and 76.5%, respectively.
4.8. Pathogenic bacteria
Pathogenic bacteria is an important class of bacteria, because they cause various human diseases. The antibiotic-resistance and health problems caused by the pathogenic bacteria are still global challenges [228], while CRISPR-based tools have greatly facilitated their research. Besides the above-mentioned C. difficile, other classic pathogens are discussed here, including Mycobacterium tuberculosis, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoni, and Yersinia pestis. To genetically modify S. aureus, Ji group has developed efficient and fast tools including nCas9-APOBEC1 base editing and CRISPR-Cas9 genome editing [229,230]. The dCas9 system is also available in S. aureus [231]. In M. tuberculosis that is notoriously difficult to be genetically manipulated, Cas9-CRISPRi techniques facilitated functional gene analysis [[232], [233], [234]]. A recently developed Cas12a-mediated genome editing system in M. smegmatis [235] may pave a way for efficient genome modification in M. tuberculosis, owing a lower toxicity of Cas12a in Mycobacterium. Pseudomonas is a chassis broadly used for biodegradation and biosynthesis [236,237] and some species such as P. aeruginosa are lethal pathogens. The efficient CRISPR-Cas9/Cas12a genome editing tools have been developed for P. putida [238,239] and the CRISPRi systems have also been applied for gene repression in P. aeruginosa, P. fluorescens and P. putida [240]. Also, the CRISPR-Cas9 based genome editing [241] and interference [242] have been developed in Klebsiella pneumoni, and a CRISPR-Cas12a assisted recombineering system is available in Yersinia pestis [243].
5. Conclusions and perspectives
Novel CRISPR-Cas mechanisms are continually being discovered nowadays, refreshing our knowledge and allowing us to further optimize the CRISPR-Cas biotechnologies. The discovery of anti-CRISPR-Cas reveals an evolutionary arms race between virus and CRISPR systems [48,50,53,55]. Interesting questions arise as how microorganisms harboring CRISPR-Cas fight back against virus to keep the race balanced. We believe more novel CRISPR-Cas proteins and mechanisms will be discovered in the future.
Owing to merits such as fast growth in low-cost cultivation and ease of genetic manipulation and scale-up fermentation, the bacteria are often used for both fundamental research and practical application. The CRISPR-Cas technologies are boosting research like synthetic biology and metabolic engineering for fine and bulk bio-products [106,[244], [245], [246]]. The classic CRISPR-Cas9/Cas12a-based biotechnologies, such as gene repression and nuclease-dependent genome editing, have been broadly applied in various bacteria (Table 1) and eukaryotic cell, whereas the emerging CRISPR biotechnologies DNA/RNA base editing and RNA cleavage are available only in eukaryotic cells [3,101,103,247,248] and E. coli (Table 1). Thus, it leaves a huge space to extend these biotechnologies in non-conventional bacterial hosts. In the future, the CRISPR technologies and the novel Cas9 variants (such as xCas9) with unique characters [63] are expected to be further developed as versatile tools and broadly applied in bacteria. Additionally, the collateral effects of Cas13 and Cas12a [[9], [10], [11]] have been exploited for in vitro detection of specific nucleic acids. This new technology is expected for medical use within a foreseeable future. These applications present a huge potential of CRISPR biotechnology in industrial and medical fields.
The challenges of the CRISPR technology [5], including the off-target effects and Cas toxicity, are recognized to limit its applications. Off-target effects cause unexpected modifications on the genomes, resulting in concerns in biosafety and efficiency. Owing to small-size genome, the off-target effect is less common in bacteria and can be further reduced by rational designs of sgRNA. Various bioinformatics tools have been developed to assist the design of these guide RNA sequences with high specificity and efficacy [93,249], including WU-CRISPR [250], CRISPR. mit [251], GuideScan [252], CCTop [253], sgRNA Scorer2.0 [254], CHOPCHOP [255], CRISPRscan [256], CLD [257], and E-CRISP [258]. Additionally, toxicity caused by heterologous expression of Cas9 proteins has been widely reported [94,134,151,259]. However the toxicity mechanisms [134,151] are not fully characterized. Such toxicity can be alleviated by replacing Cas9 with Cas12a in some cases [134,209]. On the other hand, DSBs caused by Cas proteins is lethal to host cells [84,150,259] and can induce DNA damage response mediated by other known/unknown host factors (such as p53 proteins that can conserve the stability by preventing genome mutation) [94,259]. To overcome this problem, the nuclease-free DNA base editing without introduction of DSBs, is created for safer genome modification [3,74,96]. In conclusion, the CRISPR-Cas9/Cas12a technologies have revolutionized the research on bacteria. We anticipate that new CRISPR discoveries and technologies will further enhance our understanding of life and capacity to genetically modify organisms.
Acknowledgement
This work was sponsored by Natural Science Foundation of Shanghai (Grant No. 18ZR1420500) and the Science and Technology Commission of Shanghai Municipality (Grant No. 18JC1413600). Y.X. acknowledges National 1000 Youth Talents Program. W.L. and Y.Z. are recipients of China Postdoctoral Science Foundation (2018M632119 and 2018M632098).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Hille F., Richter H., Wong S.P., Bratovic M., Ressel S., Charpentier E. The biology of CRISPR-Cas: backward and forward. Cell. 2018;172:1239–1259. doi: 10.1016/j.cell.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Koonin E.V., Makarova K.S., Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78. doi: 10.1016/j.mib.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitsunobu H., Teramoto J., Nishida K., Kondo A. Beyond native Cas9: manipulating genomic information and function. Trends Biotechnol. 2017;35:983–996. doi: 10.1016/j.tibtech.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Bowater R., Doherty A.J. Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2006;2:e8. doi: 10.1371/journal.pgen.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian P., Wang J., Shen X., Rey J.F., Yuan Q., Yan Y. Fundamental CRISPR-Cas9 tools and current applications in microbial systems. Synth Syst Biotechnol. 2017;2:219–225. doi: 10.1016/j.synbio.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selle K., Barrangou R. Harnessing CRISPR-Cas systems for bacterial genome editing. Trends Microbiol. 2015;23:225–232. doi: 10.1016/j.tim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 7.David F., Siewers V. Advances in yeast genome engineering. FEMS Yeast Res. 2015;15:1–14. doi: 10.1111/1567-1364.12200. [DOI] [PubMed] [Google Scholar]
- 8.Esvelt K.M., Wang H.H. Genome-scale engineering for systems and synthetic biology. Mol Syst Biol. 2013;9:641. doi: 10.1038/msb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J.S., Ma E., Harrington L.B., Da Costa M., Tian X., Palefsky J.M., Doudna J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gootenberg J.S., Abudayyeh O.O., Kellner M.J., Joung J., Collins J.J., Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S.Y., Cheng Q.X., Liu J.K., Nie X.Q., Zhao G.P., Wang J. CRISPR-Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res. 2018;28:491–493. doi: 10.1038/s41422-018-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian J., HamediRad M., Zhao H. Advancing Metabolic Engineering of Saccharomyces cerevisiae Using the CRISPR/Cas System. Biotechnol J. 2018 doi: 10.1002/biot.201700601. [DOI] [PubMed] [Google Scholar]
- 13.Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9:1911. doi: 10.1038/s41467-018-04252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H., Gao R., Liao X., Cai Y. CRISPR system in filamentous fungi: current achievements and future directions. Gene. 2017;627:212–221. doi: 10.1016/j.gene.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Soda N., Verma L., Giri J. CRISPR-Cas9 based plant genome editing: significance, opportunities and recent advances. Plant Physiol Biochem. 2018;131:2–11. doi: 10.1016/j.plaphy.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Swarts D.C., Jinek M. Wiley Interdiscip Rev RNA; 2018. Cas9 versus Cas12a/Cpf1: structure-function comparisons and implications for genome editing; p. e1481. [DOI] [PubMed] [Google Scholar]
- 17.Jackson S.A., McKenzie R.E., Fagerlund R.D., Kieper S.N., Fineran P.C., Brouns S.J. CRISPR-Cas: adapting to change. Science. 2017;356 doi: 10.1126/science.aal5056. [DOI] [PubMed] [Google Scholar]
- 18.Heler R., Samai P., Modell J.W., Weiner C., Goldberg G.W., Bikard D., Marraffini L.A. Cas9 specifies functional viral targets during CRISPR-Cas adaptation. Nature. 2015;519:199–202. doi: 10.1038/nature14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y., Terns R.M., Terns M.P. Cas9 function and host genome sampling in Type II-A CRISPR-Cas adaptation. Genes Dev. 2015;29:356–361. doi: 10.1101/gad.257550.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stella S., Alcon P., Montoya G. Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature. 2017;546:559–563. doi: 10.1038/nature22398. [DOI] [PubMed] [Google Scholar]
- 21.Deveau H., Barrangou R., Garneau J.E., Labonte J., Fremaux C., Boyaval P., Romero D.A., Horvath P., Moineau S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol. 2008;190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mojica F.J., Diez-Villasenor C., Garcia-Martinez J., Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 23.Swarts D.C., Mosterd C., van Passel M.W., Brouns S.J. CRISPR interference directs strand specific spacer acquisition. PloS One. 2012;7 doi: 10.1371/journal.pone.0035888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonfara I., Richter H., Bratovic M., Le Rhun A., Charpentier E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 2016;532:517–521. doi: 10.1038/nature17945. [DOI] [PubMed] [Google Scholar]
- 25.Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 27.Anders C., Niewoehner O., Duerst A., Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513:569–573. doi: 10.1038/nature13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang F., Zhou K., Ma L., Gressel S., Doudna J.A. STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015;348:1477–1481. doi: 10.1126/science.aab1452. [DOI] [PubMed] [Google Scholar]
- 29.Brouns S.J., Jore M.M., Lundgren M., Westra E.R., Slijkhuis R.J., Snijders A.P., Dickman M.J., Makarova K.S., Koonin E.V., van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haurwitz R.E., Jinek M., Wiedenheft B., Zhou K., Doudna J.A. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–1358. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deltcheva E., Chylinski K., Sharma C.M., Gonzales K., Chao Y., Pirzada Z.A., Eckert M.R., Vogel J., Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasiunas G., Barrangou R., Horvath P., Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jinek M., Jiang F., Taylor D.W., Sternberg S.H., Kaya E., Ma E., Anders C., Hauer M., Zhou K., Lin S. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimasu H., Ran F.A., Hsu P.D., Konermann S., Shehata S.I., Dohmae N., Ishitani R., Zhang F., Nureki O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156:935–949. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamano T., Nishimasu H., Zetsche B., Hirano H., Slaymaker I.M., Li Y., Fedorova I., Nakane T., Makarova K.S., Koonin E.V. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell. 2016;165:949–962. doi: 10.1016/j.cell.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao P., Yang H., Rajashankar K.R., Huang Z., Patel D.J. Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 2016;26:901–913. doi: 10.1038/cr.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternberg S.H., LaFrance B., Kaplan M., Doudna J.A. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature. 2015;527:110–113. doi: 10.1038/nature15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczelkun M.D., Tikhomirova M.S., Sinkunas T., Gasiunas G., Karvelis T., Pschera P., Siksnys V., Seidel R. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc Natl Acad Sci U S A. 2014;111:9798–9803. doi: 10.1073/pnas.1402597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang F., Taylor D.W., Chen J.S., Kornfeld J.E., Zhou K., Thompson A.J., Nogales E., Doudna J.A. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016;351:867–871. doi: 10.1126/science.aad8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swarts D.C., van der Oost J., Jinek M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR-Cas12a. Mol Cell. 2017;66:221–233 e4. doi: 10.1016/j.molcel.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stella S., Alcon P., Montoya G. Class 2 CRISPR-Cas RNA-guided endonucleases: swiss Army knives of genome editing. Nat Struct Mol Biol. 2017;24:882–892. doi: 10.1038/nsmb.3486. [DOI] [PubMed] [Google Scholar]
- 43.Borges A.L., Davidson A.R., Bondy-Denomy J. The discovery, mechanisms, and evolutionary impact of anti-CRISPRs. Annu Rev Virol. 2017;4:37–59. doi: 10.1146/annurev-virology-101416-041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxwell K.L. The anti-CRISPR story: a battle for survival. Mol Cell. 2017;68:8–14. doi: 10.1016/j.molcel.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Pawluk A., Davidson A.R., Maxwell K.L. Anti-CRISPR: discovery, mechanism and function. Nat Rev Microbiol. 2018;16:12–17. doi: 10.1038/nrmicro.2017.120. [DOI] [PubMed] [Google Scholar]
- 46.Bondy-Denomy J., Pawluk A., Maxwell K.L., Davidson A.R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature. 2013;493:429–432. doi: 10.1038/nature11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawluk A., Bondy-Denomy J., Cheung V.H., Maxwell K.L., Davidson A.R. A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa. mBio. 2014;5 doi: 10.1128/mBio.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawluk A., Staals R.H., Taylor C., Watson B.N., Saha S., Fineran P.C., Maxwell K.L., Davidson A.R. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat Microbiol. 2016;1:16085. doi: 10.1038/nmicrobiol.2016.85. [DOI] [PubMed] [Google Scholar]
- 49.Rauch B.J., Silvis M.R., Hultquist J.F., Waters C.S., McGregor M.J., Krogan N.J., Bondy-Denomy J. Inhibition of CRISPR-Cas9 with bacteriophage proteins. Cell. 2017;168:150–158. doi: 10.1016/j.cell.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bondy-Denomy J., Garcia B., Strum S., Du M., Rollins M.F., Hidalgo-Reyes Y., Wiedenheft B., Maxwell K.L., Davidson A.R. Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature. 2015;526:136–139. doi: 10.1038/nature15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chowdhury S., Carter J., Rollins M.F., Golden S.M., Jackson R.N., Hoffmann C., Nosaka L., Bondy-Denomy J., Maxwell K.L., Davidson A.R. Structure reveals mechanisms of viral suppressors that intercept a CRISPR RNA-guided surveillance complex. Cell. 2017;169:47–57. doi: 10.1016/j.cell.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong D., Guo M., Wang S., Zhu Y., Wang S., Xiong Z., Yang J., Xu Z., Huang Z. Structural basis of CRISPR-SpyCas9 inhibition by an anti-CRISPR protein. Nature. 2017;546:436–439. doi: 10.1038/nature22377. [DOI] [PubMed] [Google Scholar]
- 53.Maxwell K.L., Garcia B., Bondy-Denomy J., Bona D., Hidalgo-Reyes Y., Davidson A.R. The solution structure of an anti-CRISPR protein. Nat Commun. 2016;7:13134. doi: 10.1038/ncomms13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X., Yao D., Xu J.G., Li A.R., Xu J., Fu P., Zhou Y., Zhu Y. Structural basis of Cas3 inhibition by the bacteriophage protein AcrF3. Nat Struct Mol Biol. 2016;23:868–870. doi: 10.1038/nsmb.3269. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Ma J., Cheng Z., Meng X., You L., Wang M., Zhang X., Wang Y. A CRISPR evolutionary arms race: structural insights into viral anti-CRISPR/Cas responses. Cell Res. 2016;26:1165–1168. doi: 10.1038/cr.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrington L.B., Doxzen K.W., Ma E., Liu J.J., Knott G.J., Edraki A., Garcia B., Amrani N., Chen J.S., Cofsky J.C. A broad-spectrum inhibitor of CRISPR-Cas9. Cell. 2017;170:1224–1233. doi: 10.1016/j.cell.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang H., Patel D.J. Inhibition mechanism of an anti-CRISPR suppressor AcrIIA4 targeting SpyCas9. Mol Cell. 2017;67:117–127. doi: 10.1016/j.molcel.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin J., Jiang F., Liu J.J., Bray N.L., Rauch B.J., Baik S.H., Nogales E., Bondy-Denomy J., Corn J.E., Doudna J.A. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv. 2017;3 doi: 10.1126/sciadv.1701620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong Guo M., Wang S., Zhu Y., Wang S., Xiong Z., Yang J., Xu Z., Huang Z. Structural basis of CRISPR-SpyCas9 inhibition by an anti-CRISPR protein. Nature. 2017;546:436–439. doi: 10.1038/nature22377. [DOI] [PubMed] [Google Scholar]
- 60.Pawluk A., Amrani N., Zhang Y., Garcia B., Hidalgo-Reyes Y., Lee J., Edraki A., Shah M., Sontheimer E.J., Maxwell K.L. Naturally occurring off-switches for CRISPR-Cas9. Cell. 2016;167:1829–18238 e9. doi: 10.1016/j.cell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X., Wang J., Cheng Q., Zheng X., Zhao G., Wang J. Multiplex gene regulation by CRISPR-ddCpf1. Cell Discov. 2017;3:17018. doi: 10.1038/celldisc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dominguez A.A., Lim W.A., Qi L.S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol. 2016;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu J.H., Miller S.M., Geurts M.H., Tang W., Chen L., Sun N., Zeina C.M., Gao X., Rees H.A., Lin Z. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. doi: 10.1038/nature26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleinstiver B.P., Prew M.S., Tsai S.Q., Nguyen N.T., Topkar V.V., Zheng Z., Joung J.K. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat Biotechnol. 2015;33:1293–1298. doi: 10.1038/nbt.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., Gonzales A.P., Li Z., Peterson R.T., Yeh J.R. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrangou R., Doudna J.A. Applications of CRISPR technologies in research and beyond. Nat Biotechnol. 2016;34:933–941. doi: 10.1038/nbt.3659. [DOI] [PubMed] [Google Scholar]
- 67.Badran A.H., Liu D.R. Development of potent in vivo mutagenesis plasmids with broad mutational spectra. Nat Commun. 2015;6:8425. doi: 10.1038/ncomms9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hubbard B.P., Badran A.H., Zuris J.A., Guilinger J.P., Davis K.M., Chen L., Tsai S.Q., Sander J.D., Joung J.K., Liu D.R. Continuous directed evolution of DNA-binding proteins to improve TALEN specificity. Nat Methods. 2015;12:939–942. doi: 10.1038/nmeth.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badran A.H., Guzov V.M., Huai Q., Kemp M.M., Vishwanath P., Kain W., Nance A.M., Evdokimov A., Moshiri F., Turner K.H. Continuous evolution of Bacillus thuringiensis toxins overcomes insect resistance. Nature. 2016;533:58–63. doi: 10.1038/nature17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimasu H., Shi X., Ishiguro S., Gao L., Hirano S., Okazaki S., Noda T., Abudayyeh O.O., Gootenberg J.S., Mori H. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259–1262. doi: 10.1126/science.aas9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., Verdine V., Cox D.B.T., Kellner M.J., Regev A. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abudayyeh O.O., Gootenberg J.S., Konermann S., Joung J., Slaymaker I.M., Cox D.B., Shmakov S., Makarova K.S., Semenova E., Minakhin L. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353 doi: 10.1126/science.aaf5573. aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu L., Li X., Wang J., Wang M., Chen P., Yin M., Li J., Sheng G., Wang Y. Two distant catalytic sites are responsible for C2c2 RNase activities. Cell. 2017;168 doi: 10.1016/j.cell.2016.12.031. 121-34 e12. [DOI] [PubMed] [Google Scholar]
- 74.Cox D.B.T., Gootenberg J.S., Abudayyeh O.O., Franklin B., Kellner M.J., Joung J., Zhang F. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–1027. doi: 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho S., Shin J., Cho B.K. Applications of CRISPR/Cas system to bacterial metabolic engineering. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donohoue P.D., Barrangou R., May A.P. Advances in industrial biotechnology using CRISPR-Cas systems. Trends Biotechnol. 2018;36:134–146. doi: 10.1016/j.tibtech.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Choi K.R., Lee S.Y. CRISPR technologies for bacterial systems: current achievements and future directions. Biotechnol Adv. 2016;34:1180–1209. doi: 10.1016/j.biotechadv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Lei C., Li S.Y., Liu J.K., Zheng X., Zhao G.P., Wang J. The CCTL (Cpf1-assisted Cutting and Taq DNA ligase-assisted Ligation) method for efficient editing of large DNA constructs in vitro. Nucleic Acids Res. 2017;45:e74. doi: 10.1093/nar/gkx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li S.Y., Zhao G.P., Wang J., C-Brick A new standard for assembly of biological parts using Cpf1. ACS Synth Biol. 2016;5:1383–1388. doi: 10.1021/acssynbio.6b00114. [DOI] [PubMed] [Google Scholar]
- 80.Li S.Y., Zhao G.P., Wang J. Protocols for C-brick DNA standard assembly using Cpf1. J Vis Exp. 2017;15:124. doi: 10.3791/55775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang W., Zhao X., Gabrieli T., Lou C., Ebenstein Y., Zhu T.F. Cas9-Assisted Targeting of CHromosome segments CATCH enables one-step targeted cloning of large gene clusters. Nat Commun. 2015;6:8101. doi: 10.1038/ncomms9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaj T., Gersbach C.A., Barbas C.F., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shuman S., Glickman M.S. Bacterial DNA repair by non-homologous end joining. Nat Rev Microbiol. 2007;5:852–861. doi: 10.1038/nrmicro1768. [DOI] [PubMed] [Google Scholar]
- 84.Jiang W., Bikard D., Cox D., Zhang F., Marraffini L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oh J.H., van Pijkeren J.P. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014;42:e131. doi: 10.1093/nar/gku623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu D., Xiao Y., Evans B., Zhang F. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor-actuator. ACS Synth Biol. 2014;4:132–140. doi: 10.1021/sb400158w. [DOI] [PubMed] [Google Scholar]
- 87.Xiao Y., Bowen C.H., Liu D., Zhang F. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis. Nat Chem Biol. 2016;12:339–344. doi: 10.1038/nchembio.2046. [DOI] [PubMed] [Google Scholar]
- 88.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P., Lim W.A. Resource repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bikard D., Jiang W., Samai P., Hochschild A., Zhang F., Marraffini L.A. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–7437. doi: 10.1093/nar/gkt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng R., Wang Y., Feng W.W., Yue X.J., Chen J.H., Hu X.Z., Li Z.F., Sheng D.H., Zhang Y.M., Li Y.Z. CRISPR/dCas9-mediated transcriptional improvement of the biosynthetic gene cluster for the epothilone production in Myxococcus xanthus. Microb Cell Fact. 2018;17:15. doi: 10.1186/s12934-018-0867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong C., Fontana J., Patel A., Carothers J.M., Zalatan J.G. Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat Commun. 2018;9:2489. doi: 10.1038/s41467-018-04901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mendoza B.J., Trinh C.T. Enhanced guide-RNA design and targeting analysis for precise CRISPR genome editing of single and consortia of industrially relevant and non-model organisms. Bioinformatics. 2018;34:16–23. doi: 10.1093/bioinformatics/btx564. [DOI] [PubMed] [Google Scholar]
- 94.Haapaniemi E., Botla S., Persson J., Schmierer B., Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24:927–930. doi: 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 95.Cox D.B., Platt R.J., Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaudelli N.M., Komor A.C., Rees H.A., Packer M.S., Badran A.H., Bryson D.I., Liu D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A., Liu D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishida K., Arazoe T., Yachie N., Banno S., Kakimoto M., Tabata M., Mochizuki M., Miyabe A., Araki M., Hara K.Y. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science. 2016;353 doi: 10.1126/science.aaf8729. [DOI] [PubMed] [Google Scholar]
- 99.Li X., Wang Y., Liu Y., Yang B., Wang X., Wei J., Lu Z., Zhang Y., Wu J., Huang X. Base editing with a Cpf1-cytidine deaminase fusion. Nat Biotechnol. 2018;36:324–327. doi: 10.1038/nbt.4102. [DOI] [PubMed] [Google Scholar]
- 100.Liu Z., Lu Z., Yang G., Huang S., Li G., Feng S., Liu Y., Li J., Yu W., Zhang Y. Efficient generation of mouse models of human diseases via ABE- and BE-mediated base editing. Nat Commun. 2018;9:2338. doi: 10.1038/s41467-018-04768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimatani Z., Kashojiya S., Takayama M., Terada R., Arazoe T., Ishii H., Teramura H., Yamamoto T., Komatsu H., Miura K. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat Biotechnol. 2017;35:441–443. doi: 10.1038/nbt.3833. [DOI] [PubMed] [Google Scholar]
- 102.Eid A., Alshareef S., Mahfouz M.M. CRISPR base editors: genome editing without double-stranded breaks. Biochem J. 2018;475:1955–1964. doi: 10.1042/BCJ20170793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gootenberg J.S., Abudayyeh O.O., Lee J.W., Essletzbichler P., Dy A.J., Joung J., Verdine V., Donghia N., Daringer N.M., Freije C.A. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- 105.Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keasling J.D. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 107.Ma Q., Zhang Q., Xu Q., Zhang C., Li Y., Fan X., Xie X., Chen N. Systems metabolic engineering strategies for the production of amino acids. Synth Syst Biotechnol. 2017;2:87–96. doi: 10.1016/j.synbio.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pontrelli S., Chiu T.Y., Lan E.I., Chen F.Y., Chang P., Liao J.C. Escherichia coli as a host for metabolic engineering. Metab Eng. 2018 doi: 10.1016/j.ymben.2018.04.008. pii: S1096-7176(18)30074-0. [DOI] [PubMed] [Google Scholar]
- 109.Wang H.H., Isaacs F.J., Carr P.A., Sun Z.Z., Xu G., Forest C.R., Church G.M. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isaacs F.J., Carr P.A., HH W, MJ L, B S, L K, AC T, TA G, DB G, NB R Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333:348–353. doi: 10.1126/science.1205822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brophy J.A.N., Voigt C.A. Antisense transcription as a tool to tune gene expression. Mol Syst Biol. 2016;12:854–868. doi: 10.15252/msb.20156540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsia J., Holtz W.J., Maharbiz M.M., Arcak M., Keasling J.D. Modular Synthetic Inverters from Zinc Finger Proteins and Small RNAs. PloS One. 2016;11 doi: 10.1371/journal.pone.0149483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Connor AH-o, Moon T.S. Development of design rules for reliable antisense RNA behavior in E. coli. ACS Synth Biol. 2016;5:1441–1454. doi: 10.1021/acssynbio.6b00036. [DOI] [PubMed] [Google Scholar]
- 114.Jiang Y., Chen B., Duan C., Sun B., Yang J. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. 2015;81:2506–2514. doi: 10.1128/AEM.04023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Lin Z., Huang C., Zhang Y., Wang Z. Metabolic engineering of Escherichia coli using CRISPR – Cas9 meditated genome editing. Metab Eng. 2015;31:13–21. doi: 10.1016/j.ymben.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 116.Liang L., Liu R., Garst A.D., Lee T., Sànchez V., Beckham T., Gill R.T. CRISPR enabled trackable genome engineering for isopropanol production in Escherichia coli. Metab Eng. 2017;41:1–10. doi: 10.1016/j.ymben.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Keun S., Hwan G., Seong W., Kim H., Kim S-w, Lee D-h, Lee S-g. CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production. Metab Eng. 2016;38:228–240. doi: 10.1016/j.ymben.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Lv L., Ren Y-l, Chen J-c, Wu Q., Chen G-q. Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes , a case study : Controllable P ( 3HB- co -4HB ) biosynthesis. Metab Eng. 2015;29:160–168. doi: 10.1016/j.ymben.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 119.Gao C., Wang S., Hu G., Guo L., Chen X. Engineering Escherichia coli for malate production by integrating modular pathway characterization with CRISPRi-guided multiplexed metabolic tuning. Biotechnol Bioeng. 2017;115:661–672. doi: 10.1002/bit.26486. [DOI] [PubMed] [Google Scholar]
- 120.Kim S.K., Seong W., Han G.H., Lee D.H., Lee S.G. CRISPR interference - guided multiplex repression of endogenous competing pathway genes for redirecting metabolic flux in Escherichia coli. Microb Cell Fact. 2017:1–15. doi: 10.1186/s12934-017-0802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chu L.L., Dhakal D., Shin H.J., Jung H.J., Yamaguchi T., Sohng J.K. Metabolic engineering of Escherichia coli for enhanced production of naringenin 7-sulfate and its biological activities. Front Microbiol. 2018;9:1671. doi: 10.3389/fmicb.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu H., Li Y., Ma Q., Li Q., Jia Z., Yang B., Xu Q., Fan X., Zhang C., Chen N. Metabolic engineering of Escherichia coli for high-yield uridine production. Metab Eng. 2018;49:248–256. doi: 10.1016/j.ymben.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Zhao M., Huang D., Zhang X., Koffas M.A.G., Zhou J., Deng Y. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway. Metab Eng. 2018;47:254–262. doi: 10.1016/j.ymben.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Wu J., Du G., Chen J., Zhou J. Enhancing flavonoid production by systematically tuning the central metabolic pathways based on a CRISPR interference system in Escherichia coli. Sci Rep. 2015;5:13477. doi: 10.1038/srep13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li S., Bille C., Grünberger A., Ronda C., Ingemann S., Noack S., Toftgaard A., Novo T., Foundation N., Lyngby K. Enhanced protein and biochemical production using CRISPRi-based growth switches. Metab Eng. 2016;38:274–284. doi: 10.1016/j.ymben.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Nielsen A.A.K., Voigt C.A. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Mol Syst Biol. 2014;10:763. doi: 10.15252/msb.20145735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fontana J., Voje W.E., Zalatan J.G., Carothers J.M. Prospects for engineering dynamic CRISPR – Cas transcriptional circuits to improve bioproduction. J Ind Microbiol Biotechnol. 2018;45:481–490. doi: 10.1007/s10295-018-2039-z. [DOI] [PubMed] [Google Scholar]
- 128.Arazoe T., Kondo A., Nishida K. Targeted Nucleotide Editing Technologies for Microbial Metabolic Engineering. Biotechnol J. 2018;13 doi: 10.1002/biot.201700596. [DOI] [PubMed] [Google Scholar]
- 129.Banno S., Nishida K., Arazoe T., Mitsunobu H., Kondo A. Deaminase-mediated multiplex genome editing in Escherichia coli. Nat Microbiol. 2018;3:423–429. doi: 10.1038/s41564-017-0102-6. [DOI] [PubMed] [Google Scholar]
- 130.Huang H.H., Camsund D., Lindblad P., Heidorn T. Design and characterization of molecular tools for a Synthetic Biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010;38:2577–2593. doi: 10.1093/nar/gkq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Armshaw P., Carey D., Sheahan C., Pembroke J.T. Utilising the native plasmid, pCA2.4, from the cyanobacterium Synechocystis sp. strain PCC6803 as a cloning site for enhanced product production. Biotechnol Biofuels. 2015;8:201. doi: 10.1186/s13068-015-0385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ng A.H., Berla B.M., Pakrasi H.B. Fine-tuning of photoautotrophic protein production by combining promoters and neutral sites in the cyanobacterium Synechocystis sp. strain PCC 6803. Appl Environ Microbiol. 2015;81:6857–6863. doi: 10.1128/AEM.01349-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramey C.J., Baron-Sola A., Aucoin H.R., Boyle N.R. Genome engineering in cyanobacteria: where we are and where we need to go. ACS Synth Biol. 2015;4:1186–1196. doi: 10.1021/acssynbio.5b00043. [DOI] [PubMed] [Google Scholar]
- 134.Ungerer J., Pakrasi H.B. Cpf1 is a versatile tool for CRISPR genome editing across diverse species of cyanobacteria. Sci Rep. 2016;6:39681. doi: 10.1038/srep39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matson M.M., Atsumi S. Photomixotrophic chemical production in cyanobacteria. Curr Opin Biotechnol. 2017;50:65–71. doi: 10.1016/j.copbio.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 136.Zhang A., Carroll A.L., Atsumi S. Carbon recycling by cyanobacteria: improving CO2 fixation through chemical production. FEMS Microbiol Lett. 2017:364. doi: 10.1093/femsle/fnx165. [DOI] [PubMed] [Google Scholar]
- 137.Carroll A.L., Case A.E., Zhang A., Atsumi S. Metabolic engineering tools in model cyanobacteria. Metab Eng. 2018 doi: 10.1016/j.ymben.2018.03.014. pii: S1096-7176(18)30038-7. [DOI] [PubMed] [Google Scholar]
- 138.Gao F., Zhao J., Chen L., Battchikova N., Ran Z., Aro E.M., Ogawa T., Ma W. The NDH-1L-PSI supercomplex is important for efficient cyclic electron transport in cyanobacteria. Plant Physiol. 2016;172:1451–1464. doi: 10.1104/pp.16.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y., Gao Y., Li C., Gao H., Zhang C.C., Xu X. Three substrains of the cyanobacterium Anabaena sp. strain PCC 7120 display divergence in genomic sequences and hetC function. J Bacteriol. 2018;200 doi: 10.1128/JB.00076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luan G., Lu X. Tailoring cyanobacterial cell factory for improved industrial properties. Biotechnol Adv. 2018;36:430–442. doi: 10.1016/j.biotechadv.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 141.Xie M., Wang W., Zhang W., Chen L., Lu X. Versatility of hydrocarbon production in cyanobacteria. Appl Microbiol Biotechnol. 2017;101:905–919. doi: 10.1007/s00253-016-8064-9. [DOI] [PubMed] [Google Scholar]
- 142.Huang X., Liang Y., Yang Y., Lu X. Single-step production of the simvastatin precursor monacolin J by engineering of an industrial strain of Aspergillus terreus. Metab Eng. 2017;42:109–114. doi: 10.1016/j.ymben.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 143.Taton A., Unglaub F., Wright N.E., Zeng W.Y., Paz-Yepes J., Brahamsha B., Palenik B., Peterson T.C., Haerizadeh F., Golden S.S. Broad-host-range vector system for synthetic biology and biotechnology in cyanobacteria. Nucleic Acids Res. 2014;42:e136. doi: 10.1093/nar/gku673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song X., Wang Y., Diao J., Li S., Chen L., Zhang W. Direct photosynthetic production of plastic building block chemicals from CO2. Adv Exp Med Biol. 2018;1080:215–238. doi: 10.1007/978-981-13-0854-3_9. [DOI] [PubMed] [Google Scholar]
- 145.Li S., Sun T., Xu C., Chen L., Zhang W. Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metab Eng. 2018;48:163–174. doi: 10.1016/j.ymben.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Zhou J., Meng H., Zhang W., Li Y. Production of industrial chemicals from CO2 by engineering cyanobacteria. Adv Exp Med Biol. 2018;1080:97–116. doi: 10.1007/978-981-13-0854-3_5. [DOI] [PubMed] [Google Scholar]
- 147.Zhou J., Zhang F., Meng H., Zhang Y., Li Y. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria. Metab Eng. 2016;38:217–227. doi: 10.1016/j.ymben.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 148.Zhou J., Zhu T., Cai Z., Li Y. From cyanochemicals to cyanofactories: a review and perspective. Microb Cell Fact. 2016;15:2. doi: 10.1186/s12934-015-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ni J., Tao F., Xu P., Yang C. Engineering cyanobacteria for photosynthetic production of C3 platform chemicals and terpenoids from CO2. Adv Exp Med Biol. 2018;1080:239–259. doi: 10.1007/978-981-13-0854-3_10. [DOI] [PubMed] [Google Scholar]
- 150.Wendt K.E., Ungerer J., Cobb R.E., Zhao H., Pakrasi H.B. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microb Cell Fact. 2016;15:115. doi: 10.1186/s12934-016-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xiao Y., Wang S., Rommelfanger S., Balassy A., Barba-Ostria C., Gu P., Galazka J.M., Zhang F. Developing a Cas9-based tool to engineer native plasmids in Synechocystis sp. PCC 6803. Biotechnol Bioeng. 2018;115:2305–2314. doi: 10.1002/bit.26747. [DOI] [PubMed] [Google Scholar]
- 152.Griese M., Lange C., Soppa J. Ploidy in cyanobacteria. FEMS Microbiol Lett. 2011;323:124–131. doi: 10.1111/j.1574-6968.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- 153.Li H., Shen C.R., Huang C.H., Sung L.Y., Wu M.Y., Hu Y.C. CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab Eng. 2016;38:293–302. doi: 10.1016/j.ymben.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 154.Robertsen H.L., Weber T., Kim H.U., Lee S.Y. Toward systems metabolic engineering of streptomycetes for secondary metabolites production. Biotechnol J. 2018;13 doi: 10.1002/biot.201700465. [DOI] [PubMed] [Google Scholar]
- 155.Xu D.B., Ye W.W., Han Y., Deng Z.X., Hong K. Natural products from mangrove actinomycetes. Mar Drugs. 2014;12:2590–2613. doi: 10.3390/md12052590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lv M., Ji X., Zhao J., Li Y., Zhang C., Su L., Ding W., Deng Z., Yu Y., Zhang Q. Characterization of a C3 deoxygenation pathway reveals a key branch point in aminoglycoside biosynthesis. J Am Chem Soc. 2016;138:6427–6435. doi: 10.1021/jacs.6b02221. [DOI] [PubMed] [Google Scholar]
- 157.Tan G.Y., Deng K., Liu X., Tao H., Chang Y., Chen J., Chen K., Sheng Z., Deng Z., Liu T. Heterologous biosynthesis of spinosad: an omics-guided large polyketide synthase gene cluster reconstitution in Streptomyces. ACS Synth Biol. 2017;6:995–1005. doi: 10.1021/acssynbio.6b00330. [DOI] [PubMed] [Google Scholar]
- 158.Tan G.Y., Liu T. Rational synthetic pathway refactoring of natural products biosynthesis in actinobacteria. Metab Eng. 2017;39:228–236. doi: 10.1016/j.ymben.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 159.Song L.Q., Zhang Y.Y., Pu J.Y., Tang M.C., Peng C., Tang G.L. Catalysis of extracellular deamination by a FAD-linked oxidoreductase after prodrug maturation in the biosynthesis of saframycin A. Angew Chem Int Ed Engl. 2017;56:9116–9120. doi: 10.1002/anie.201704726. [DOI] [PubMed] [Google Scholar]
- 160.Lin Z., He Q., Liu W. Bio-inspired engineering of thiopeptide antibiotics advances the expansion of molecular diversity and utility. Curr Opin Biotechnol. 2017;48:210–219. doi: 10.1016/j.copbio.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 161.Gao Q., Tan G.Y., Xia X., Zhang L. Learn from microbial intelligence for avermectins overproduction. Curr Opin Biotechnol. 2017;48:251–257. doi: 10.1016/j.copbio.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 162.Gui C., Liu Y., Zhou Z., Zhang S., Hu Y., Gu Y.C., Huang H., Ju J. Angucycline glycosides from mangrove-derived streptomycesdiastaticus subsp. SCSIO GJ056. Mar Drugs. 2018:16. doi: 10.3390/md16060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pokhrel A.R., Nguyen H.T., Dhakal D., Chaudhary A.K., Sohng J.K. Implication of orphan histidine kinase (OhkAsp) in biosynthesis of doxorubicin and daunorubicin in Streptomyces peucetius ATCC 27952. Microbiol Res. 2018;214:37–46. doi: 10.1016/j.micres.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 164.Hahn D.R., Graupner P.R., Chapin E., Gray J., Heim D., Gilbert J.R., Gerwick B.C. Albucidin: a novel bleaching herbicide from Streptomyces albus subsp. chlorinus NRRL B-24108. J Antibiot (Tokyo) 2009;62:191–194. doi: 10.1038/ja.2009.11. [DOI] [PubMed] [Google Scholar]
- 165.Kim M., Sang Yi J., Kim J., Kim J.N., Kim M.W., Kim B.G. Reconstruction of a high-quality metabolic model enables the identification of gene overexpression targets for enhanced antibiotic production in Streptomyces coelicolor A3(2) Biotechnol J. 2014;9:1185–1194. doi: 10.1002/biot.201300539. [DOI] [PubMed] [Google Scholar]
- 166.Andexer J.N., Kendrew S.G., Nur-e-Alam M., Lazos O., Foster T.A., Zimmermann A.S., Warneck T.D., Suthar D., Coates N.J., Koehn F.E. Biosynthesis of the immunosuppressants FK506, FK520, and rapamycin involves a previously undescribed family of enzymes acting on chorismate. Proc Natl Acad Sci U S A. 2011;108:4776–4781. doi: 10.1073/pnas.1015773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baltz R.H. Gifted microbes for genome mining and natural product discovery. J Ind Microbiol Biotechnol. 2017;44:573–588. doi: 10.1007/s10295-016-1815-x. [DOI] [PubMed] [Google Scholar]
- 168.Saha S., Zhang W., Zhang G., Zhu Y., Chen Y., Liu W., Yuan C., Zhang Q., Zhang H., Zhang L. Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem Sci. 2017;8:1607–1612. doi: 10.1039/c6sc03875a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yan Y., Liu Q., Zang X., Yuan S., Bat-Erdene U., Nguyen C., Gan J., Zhou J., Jacobsen S.E., Tang Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature. 2018;559:415–418. doi: 10.1038/s41586-018-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Doroghazi J.R., Albright J.C., Goering A.W., Ju K.S., Haines R.R., Tchalukov K.A., Labeda D.P., Kelleher N.L., Metcalf W.W. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat Chem Biol. 2014;10:963–968. doi: 10.1038/nchembio.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang H., Zheng G., Jiang W., Hu H., Lu Y. One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim Biophys Sin (Shanghai) 2015;47:231–243. doi: 10.1093/abbs/gmv007. [DOI] [PubMed] [Google Scholar]
- 172.Cobb R.E., Wang Y., Zhao H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth Biol. 2015;4:723–728. doi: 10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zeng H., Wen S., Xu W., He Z., Zhai G., Liu Y., Deng Z., Sun Y. Highly efficient editing of the actinorhodin polyketide chain length factor gene in Streptomyces coelicolor M145 using CRISPR/Cas9-CodA(sm) combined system. Appl Microbiol Biotechnol. 2015;99:10575–10585. doi: 10.1007/s00253-015-6931-4. [DOI] [PubMed] [Google Scholar]
- 174.Li L., Wei K., Zheng G., Liu X., Chen S., Jiang W., Lu Y. CRISPR-Cpf1 assisted multiplex genome editing and transcriptional repression in Streptomyces. Appl Environ Microbiol. 2018 doi: 10.1128/AEM.00827-18. pii: e00827-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jia H., Zhang L., Wang T., Han J., Tang H. Development of a CRISPR/Cas9-mediated gene-editing tool in Streptomyces rimosus. Microbiology. 2017;163:1148–1155. doi: 10.1099/mic.0.000501. [DOI] [PubMed] [Google Scholar]
- 176.Tong Y., Charusanti P., Zhang L., Weber T., Lee S.Y. CRISPR-Cas9 based engineering of actinomycetal genomes. ACS Synth Biol. 2015;4:1020–1029. doi: 10.1021/acssynbio.5b00038. [DOI] [PubMed] [Google Scholar]
- 177.Zhang M.M., Wong F.T., Wang Y., Luo S., Lim Y.H., Heng E., Yeo W.L., Cobb R.E., Enghiad B., Ang E.L. CRISPR-Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat Chem Biol. 2017;13:607–611. doi: 10.1038/nchembio.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bao Z., Xiao H., Liang J., Zhang L., Xiong X., Sun N., Si T., Zhao H. Homology-integrated CRISPR-Cas (HI-CRISPR) system for one-step multigene disruption in Saccharomyces cerevisiae. ACS Synth Biol. 2015;4:585–594. doi: 10.1021/sb500255k. [DOI] [PubMed] [Google Scholar]
- 179.Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1983;49:209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- 180.Sauer M., Russmayer H., Grabherr R., Peterbauer C.K., Marx H. The efficient clade: lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017;35:756–769. doi: 10.1016/j.tibtech.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 181.Vlasova A.N., Kandasamy S., Chattha K.S., Rajashekara G., Saif L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol. 2016;172:72–84. doi: 10.1016/j.vetimm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lindlbauer K.A., Marx H., Sauer M. Effect of carbon pulsing on the redox household of Lactobacillus diolivorans in order to enhance 1,3-propanediol production. N Biotechnol. 2017;34:32–39. doi: 10.1016/j.nbt.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 183.Liu F., Yu B. Efficient production of reuterin from glycerol by magnetically immobilized Lactobacillus reuteri. Appl Microbiol Biotechnol. 2015;99:4659–4666. doi: 10.1007/s00253-015-6530-4. [DOI] [PubMed] [Google Scholar]
- 184.Dishisha T., Pyo S.H., Hatti-Kaul R. Bio-based 3-hydroxypropionic- and acrylic acid production from biodiesel glycerol via integrated microbial and chemical catalysis. Microb Cell Fact. 2015;14:200. doi: 10.1186/s12934-015-0388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang Y., Kang Z., Zhou J., Chen J., Du G. High-level expression and characterization of recombinant acid urease for enzymatic degradation of urea in rice wine. Appl Microbiol Biotechnol. 2015;99:301–308. doi: 10.1007/s00253-014-5916-z. [DOI] [PubMed] [Google Scholar]
- 186.Song X., Huang H., Xiong Z., Ai L., Yang S. CRISPR-Cas9(D10A) nickase-assisted genome editing in Lactobacillus casei. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.01259-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stout E.A., Sanozky-Dawes R., Goh Y.J., Crawley A.B., Klaenhammer T.R., Barrangou R. Deletion-based escape of CRISPR-Cas9 targeting in Lactobacillus gasseri. Microbiology. 2018;164:1098–1111. doi: 10.1099/mic.0.000689. [DOI] [PubMed] [Google Scholar]
- 188.He M., Miyajima F., Roberts P., Ellison L., Pickard D.J., Martin M.J., Connor T.R., Harris S.R., Fairley D., Bamford K.B. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45:109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patakova P., Kolek J., Sedlar K., Koscova P., Branska B., Kupkova K., Paulova L., Provaznik I. Comparative analysis of high butanol tolerance and production in clostridia. Biotechnol Adv. 2018;36:721–738. doi: 10.1016/j.biotechadv.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 190.Ren C., Wen Z., Xu Y., Jiang W., Gu Y. Clostridia: a flexible microbial platform for the production of alcohols. Curr Opin Chem Biol. 2016;35:65–72. doi: 10.1016/j.cbpa.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 191.Joseph R.C., Kim N.M., Sandoval N.R. Recent developments of the synthetic biology toolkit for Clostridium. Front Microbiol. 2018;9:154. doi: 10.3389/fmicb.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xue C., Zhao J., Chen L., Yang S.T., Bai F. Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnol Adv. 2017;35:310–322. doi: 10.1016/j.biotechadv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 193.Wang Y., Zhang Z.T., Seo S.O., Choi K., Lu T., Jin Y.S., Blaschek H.P. Markerless chromosomal gene deletion in Clostridium beijerinckii using CRISPR/Cas9 system. J Biotechnol. 2015;200:1–5. doi: 10.1016/j.jbiotec.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 194.Wang Y., Zhang Z.T., Seo S.O., Lynn P., Lu T., Jin Y.S., Blaschek H.P. Bacterial genome editing with CRISPR-Cas9: deletion, integration, single nucleotide modification, and desirable “clean” mutant selection in Clostridium beijerinckii as an example. ACS Synth Biol. 2016;5:721–732. doi: 10.1021/acssynbio.6b00060. [DOI] [PubMed] [Google Scholar]
- 195.Wang S., Dong S., Wang P., Tao Y., Wang Y. Genome editing in Clostridium saccharoperbutylacetonicum N1-4 with the CRISPR-Cas9 system. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00233-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Q., Chen J., Minton N.P., Zhang Y., Wen Z., Liu J., Yang H., Zeng Z., Ren X., Yang J. CRISPR-based genome editing and expression control systems in Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnol J. 2016;11:961–972. doi: 10.1002/biot.201600053. [DOI] [PubMed] [Google Scholar]
- 197.Nagaraju S., Davies N.K., Walker D.J., Kopke M., Simpson S.D. Genome editing of Clostridium autoethanogenum using CRISPR/Cas9. Biotechnol Biofuels. 2016;9:219. doi: 10.1186/s13068-016-0638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang H., Chai C., Li N., Rowe P., Minton N.P., Yang S., Jiang W., Gu Y. CRISPR/Cas9-Based efficient genome editing in Clostridium ljungdahlii, an autotrophic gas-fermenting bacterium. ACS Synth Biol. 2016;5:1355–1361. doi: 10.1021/acssynbio.6b00044. [DOI] [PubMed] [Google Scholar]
- 199.Zhang J., Zong W., Hong W., Zhang Z.T., Wang Y. Exploiting endogenous CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab Eng. 2018;47:49–59. doi: 10.1016/j.ymben.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 200.Pyne M.E., Bruder M.R., Moo-Young M., Chung D.A., Chou C.P. Harnessing heterologous and endogenous CRISPR-Cas machineries for efficient markerless genome editing in Clostridium. Sci Rep. 2016;6:25666. doi: 10.1038/srep25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hong W., Zhang J., Cui G., Wang L., Wang Y. Multiplexed CRISPR-Cpf1-mediated genome editing in Clostridium difficile toward the understanding of pathogenesis of C. difficile infection. ACS Synth Biol. 2018;7:1588–1600. doi: 10.1021/acssynbio.8b00087. [DOI] [PubMed] [Google Scholar]
- 202.Xu T., Li Y., Shi Z., Hemme C.L., Zhu Y., Van Nostrand J.D., He Z., Zhou J. Efficient genome editing in Clostridium cellulolyticum via CRISPR-Cas9 nickase. Appl Environ Microbiol. 2015;81:4423–4431. doi: 10.1128/AEM.00873-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu T., Li Y., He Z., Van Nostrand J.D., Zhou J. Cas9 nickase-assisted RNA repression enables stable and efficient manipulation of essential metabolic genes in Clostridium cellulolyticum. Front Microbiol. 2017;8:1744. doi: 10.3389/fmicb.2017.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bruder M.R., Pyne M.E., Moo-Young M., Chung D.A., Chou C.P. Extending CRISPR-Cas9 technology from genome editing to transcriptional engineering in the genus Clostridium. Appl Environ Microbiol. 2016;82:6109–6119. doi: 10.1128/AEM.02128-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Y., Zhang Z.T., Seo S.O., Lynn P., Lu T., Jin Y.S., Blaschek H.P. Gene transcription repression in Clostridium beijerinckii using CRISPR-dCas9. Biotechnol Bioeng. 2016;113:2739–2743. doi: 10.1002/bit.26020. [DOI] [PubMed] [Google Scholar]
- 206.Wen Z., Minton N.P., Zhang Y., Li Q., Liu J., Jiang Y., Yang S. Enhanced solvent production by metabolic engineering of a twin-clostridial consortium. Metab Eng. 2017;39:38–48. doi: 10.1016/j.ymben.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 207.Baumgart M., Unthan S., Kloss R., Radek A., Polen T., Tenhaef N., Muller M.F., Kuberl A., Siebert D., Bruhl N. Corynebacterium glutamicum chassis C1*: building and testing a novel platform host for synthetic biology and industrial biotechnology. ACS Synth Biol. 2018;7:132–144. doi: 10.1021/acssynbio.7b00261. [DOI] [PubMed] [Google Scholar]
- 208.Becker J., Rohles C.M., Wittmann C. Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab Eng. 2018 doi: 10.1016/j.ymben.2018.07.008. pii: S1096-7176(18)30152-6. [DOI] [PubMed] [Google Scholar]
- 209.Jiang Y., Qian F., Yang J., Liu Y., Dong F., Xu C., Sun B., Chen B., Xu X., Li Y. CRISPR-Cpf1 assisted genome editing of Corynebacterium glutamicum. Nat Commun. 2017;8:15179. doi: 10.1038/ncomms15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu J., Wang Y., Lu Y., Zheng P., Sun J., Ma Y. Development of a CRISPR/Cas9 genome editing toolbox for Corynebacterium glutamicum. Microb Cell Fact. 2017;16:205. doi: 10.1186/s12934-017-0815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peng F., Wang X., Sun Y., Dong G., Yang Y., Liu X., Bai Z. Efficient gene editing in Corynebacterium glutamicum using the CRISPR/Cas9 system. Microb Cell Fact. 2017;16:201. doi: 10.1186/s12934-017-0814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cho J.S., Choi K.R., Prabowo C.P.S., Shin J.H., Yang D., Jang J., Lee S.Y. CRISPR/Cas9-coupled recombineering for metabolic engineering of Corynebacterium glutamicum. Metab Eng. 2017;42:157–167. doi: 10.1016/j.ymben.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 213.Wang B., Hu Q., Zhang Y., Shi R., Chai X., Liu Z., Shang X., Wen T. A RecET-assisted CRISPR-Cas9 genome editing in Corynebacterium glutamicum. Microb Cell Fact. 2018;17:63. doi: 10.1186/s12934-018-0910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang Y., Liu Y., Liu J., Guo Y., Fan L., Ni X., Zheng X., Wang M., Zheng P., Sun J. MACBETH: multiplex automated Corynebacterium glutamicum base editing method. Metab Eng. 2018;47:200–210. doi: 10.1016/j.ymben.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 215.Cleto S., Jensen J.V., Wendisch V.F., Lu T.K. Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi) ACS Synth Biol. 2016;5:375–385. doi: 10.1021/acssynbio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Park J., Shin H., Lee S.M., Um Y., Woo H.M. RNA-guided single/double gene repressions in Corynebacterium glutamicum using an efficient CRISPR interference and its application to industrial strain. Microb Cell Fact. 2018;17:4. doi: 10.1186/s12934-017-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yoon J., Woo H.M. CRISPR interference-mediated metabolic engineering of Corynebacterium glutamicum for homo-butyrate production. Biotechnol Bioeng. 2018;115:2067–2074. doi: 10.1002/bit.26720. [DOI] [PubMed] [Google Scholar]
- 218.Gu Y., Xu X., Wu Y., Niu T., Liu Y., Li J., Du G., Liu L. Advances and prospects of Bacillus subtilis cellular factories: from rational design to industrial applications. Metab Eng. 2018 doi: 10.1016/j.ymben.2018.05.006. pii: S1096-7176(17)30482-2. [DOI] [PubMed] [Google Scholar]
- 219.Westbrook A.W., Moo-Young M., Chou C.P. Development of a CRISPR-Cas9 tool kit for Comprehensive engineering of Bacillus subtilis. Appl Environ Microbiol. 2016;82:4876–4895. doi: 10.1128/AEM.01159-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Westbrook A.W., Ren X., Moo-Young M., Chou C.P. Metabolic engineering of Bacillus subtilis for L-valine overproduction. Biotechnol Bioeng. 2018 doi: 10.1002/bit.26789. [DOI] [PubMed] [Google Scholar]
- 221.Westbrook A.W., Ren X., Oh J., Moo-Young M., Chou C.P. Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab Eng. 2018;47:401–413. doi: 10.1016/j.ymben.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 222.Wu Y., Chen T., Liu Y., Lv X., Li J., Du G., Ledesma-Amaro R., Liu L. CRISPRi allows optimal temporal control of N-acetylglucosamine bioproduction by a dynamic coordination of glucose and xylose metabolism in Bacillus subtilis. Metab Eng. 2018;49:232–241. doi: 10.1016/j.ymben.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 223.Zhang K., Duan X., Wu J. Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Sci Rep. 2016;6:27943. doi: 10.1038/srep27943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang K., Su L., Wu J. Enhanced extracellular pullulanase production in Bacillus subtilis using protease-deficient strains and optimal feeding. Appl Microbiol Biotechnol. 2018;102:5089–5103. doi: 10.1007/s00253-018-8965-x. [DOI] [PubMed] [Google Scholar]
- 225.Mougiakos I., Bosma E.F., Weenink K., Vossen E., Goijvaerts K., van der Oost J., van Kranenburg R. Efficient genome editing of a facultative thermophile using mesophilic spCas9. ACS Synth Biol. 2017;6:849–861. doi: 10.1021/acssynbio.6b00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mougiakos I., Mohanraju P., Bosma E.F., Vrouwe V., Finger Bou M., Naduthodi M.I.S., Gussak A., Brinkman R.B.L., van Kranenburg R., van der Oost J. Characterizing a thermostable Cas9 for bacterial genome editing and silencing. Nat Commun. 2017;8:1647. doi: 10.1038/s41467-017-01591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li K., Cai D., Wang Z., He Z., Chen S. Development of an efficient genome editing tool in Bacillus licheniformis using CRISPR-Cas9 nickase. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.02608-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Petchiappan A., Chatterji D. Antibiotic resistance: current perspectives. ACS Omega. 2017;2:7400–7409. doi: 10.1021/acsomega.7b01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gu T., Zhao S., Pi Y., Chen W., Chen C., Liu Q., Li M., Han D., Ji Q. Highly efficient base editing in Staphylococcus aureus using an engineered CRISPR RNA-guided cytidine deaminase. Chem Sci. 2018;9:3248–3253. doi: 10.1039/c8sc00637g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen W., Zhang Y., Yeo W.S., Bae T., Ji Q. Rapid and efficient genome editing in Staphylococcus aureus by using an engineered CRISPR/Cas9 system. J Am Chem Soc. 2017;139:3790–3795. doi: 10.1021/jacs.6b13317. [DOI] [PubMed] [Google Scholar]
- 231.Dong X., Jin Y., Ming D., Li B., Dong H., Wang L., Wang T., Wang D. CRISPR/dCas9-mediated inhibition of gene expression in Staphylococcus aureus. J Microbiol Methods. 2017;139:79–86. doi: 10.1016/j.mimet.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 232.Choudhary E., Thakur P., Pareek M., Agarwal N. Gene silencing by CRISPR interference in mycobacteria. Nat Commun. 2015;6:6267. doi: 10.1038/ncomms7267. [DOI] [PubMed] [Google Scholar]
- 233.Rock J.M., Hopkins F.F., Chavez A., Diallo M., Chase M.R., Gerrick E.R., Pritchard J.R., Church G.M., Rubin E.J., Sassetti C.M. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol. 2017;2:16274. doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Singh A.K., Carette X., Potluri L.P., Sharp J.D., Xu R., Prisic S., Husson R.N. Investigating essential gene function in Mycobacterium tuberculosis using an efficient CRISPR interference system. Nucleic Acids Res. 2016;44:e143. doi: 10.1093/nar/gkw625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sun B., Yang J., Yang S., Ye R.D., Chen D., Jiang Y. A CRISPR-Cpf1-Assisted Non-Homologous End Joining Genome Editing System of Mycobacterium smegmatis. Biotechnol J. 2018 doi: 10.1002/biot.201700588. [DOI] [PubMed] [Google Scholar]
- 236.Gao Y.Z., Liu H., Chao H.J., Zhou N.Y. Constitutive expression of a nag-like dioxygenase gene through an internal promoter in the 2-Chloronitrobenzene Catabolism gene cluster of Pseudomonas stutzeri ZWLR2-1. Appl Environ Microbiol. 2016;82:3461–3470. doi: 10.1128/AEM.00197-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yu H., Hausinger R.P., Tang H.Z., Xu P. Mechanism of the 6-hydroxy-3-succinoyl-pyridine 3-monooxygenase flavoprotein from Pseudomonas putida S16. J Biol Chem. 2014;289:29158–29170. doi: 10.1074/jbc.M114.558049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Aparicio T., de Lorenzo V., Martinez-Garcia E. CRISPR/Cas9-Based Counterselection Boosts Recombineering Efficiency in Pseudomonas putida. Biotechnol J. 2018;13 doi: 10.1002/biot.201700161. [DOI] [PubMed] [Google Scholar]
- 239.Sun J., Wang Q., Jiang Y., Wen Z., Yang L., Wu J., Yang S. Genome editing and transcriptional repression in Pseudomonas putida KT2440 via the type II CRISPR system. Microb Cell Fact. 2018;17:41. doi: 10.1186/s12934-018-0887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tan S.Z., Reisch C.R., Prather K.L.J. A robust CRISPR interference gene repression system in Pseudomonas. J Bacteriol. 2018;200 doi: 10.1128/JB.00575-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shen J., Zhou J., Chen G.Q., Xiu Z.L. Efficient genome engineering of a virulent Klebsiella bacteriophage using CRISPR-Cas9. J Virol. 2018;92 doi: 10.1128/JVI.00534-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang J., Zhao P., Li Y., Xu L., Tian P. Engineering CRISPR interference system in Klebsiella pneumoniae for attenuating lactic acid synthesis. Microb Cell Fact. 2018;17:56. doi: 10.1186/s12934-018-0903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yan M.Y., Yan H.Q., Ren G.X., Zhao J.P., Guo X.P., Sun Y.C. CRISPR-Cas12a-Assisted recombineering in bacteria. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00947-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang Y.H., Wei K.Y., Smolke C.D. Synthetic biology: advancing the design of diverse genetic systems. Annu Rev Chem Biomol Eng. 2012;4:69–102. doi: 10.1146/annurev-chembioeng-061312-103351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gronenberg L.S., Marcheschi R.J., Liao J.C. Next generation biofuel engineering in prokaryotes. Curr Opin Chem Biol. 2013;17:462–471. doi: 10.1016/j.cbpa.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Woolston B.M., Edgar S., Stephanopoulos G. Metabolic engineering: past and future. Annu Rev Chem Biomol Eng. 2013;4:259–288. doi: 10.1146/annurev-chembioeng-061312-103312. [DOI] [PubMed] [Google Scholar]
- 247.Shao J., Wang M., Yu G., Zhu S., Yu Y., Heng B.C., Wu J., Ye H. Synthetic far-red light-mediated CRISPR-dCas9 device for inducing functional neuronal differentiation. Proc Natl Acad Sci U S A. 2018;115:E6722–E6730. doi: 10.1073/pnas.1802448115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sontheimer E.J., Davidson A.R. Inhibition of CRISPR-Cas systems by mobile genetic elements. Curr Opin Microbiol. 2017;37:120–127. doi: 10.1016/j.mib.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cui Y., Xu J., Cheng M., Liao X., Peng S. Review of CRISPR/Cas9 sgRNA design tools. Interdiscip Sci. 2018;10:455–465. doi: 10.1007/s12539-018-0298-z. [DOI] [PubMed] [Google Scholar]
- 250.Wong N., Liu W., Wang X. Wu-CRISPR: characteristics of functional guide RNAs for the CRISPR/Cas9 system. Genome Biol. 2015;16:218. doi: 10.1186/s13059-015-0784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Perez A.R., Pritykin Y., Vidigal J.A., Chhangawala S., Zamparo L., Leslie C.S., Ventura A. GuideScan software for improved single and paired CRISPR guide RNA design. Nat Biotechnol. 2017;35:347–349. doi: 10.1038/nbt.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stemmer M., Thumberger T., Del Sol Keyer M., Wittbrodt J., Mateo J.L. CCTop: an Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PloS One. 2015;10 doi: 10.1371/journal.pone.0124633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chari R., Yeo N.C., Chavez A., Church G.M. sgRNA scorer 2.0: a species-independent model to predict CRISPR/Cas9 activity. ACS Synth Biol. 2017;6:902–904. doi: 10.1021/acssynbio.6b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Montague T.G., Cruz J.M., Gagnon J.A., Church G.M., Valen E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014;42:W401–W407. doi: 10.1093/nar/gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Moreno-Mateos M.A., Vejnar C.E., Beaudoin J.D., Fernandez J.P., Mis E.K., Khokha M.K., Giraldez A.J. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods. 2015;12:982–988. doi: 10.1038/nmeth.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Heigwer F., Zhan T., Breinig M., Winter J., Brugemann D., Leible S., Boutros M. CRISPR library designer (CLD): software for multispecies design of single guide RNA libraries. Genome Biol. 2016;17:55. doi: 10.1186/s13059-016-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Heigwer F., Kerr G., Boutros M. E-CRISP: fast CRISPR target site identification. Nat Methods. 2014;11:122–123. doi: 10.1038/nmeth.2812. [DOI] [PubMed] [Google Scholar]
- 259.Ihry R.J., Worringer K.A., Salick M.R., Frias E., Ho D., Theriault K., Kommineni S., Chen J., Sondey M., Ye C. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med. 2018;24:939–946. doi: 10.1038/s41591-018-0050-6. [DOI] [PubMed] [Google Scholar]