Abstract
Metal induced hypersensitivity is driven by dendritic cells (DCs) that migrate from the site of exposure to the lymph nodes, upregulate costimulatory molecules and initiate metal specific CD4+ T cell responses. Chronic beryllium disease (CBD), a life-threatening metal induced hypersensitivity, is driven by beryllium specific CD4+ Th1 cells that expand in the lung-draining lymph nodes (LDLNs) after beryllium exposure (sensitization phase) and are recruited back to the lung where they orchestrate granulomatous lung disease (elicitation phase). To understand more about how beryllium exposures impact DC function during sensitization, we examined the early events in the lung and LDLNs after pulmonary exposure to different physiochemical forms of beryllium. Exposure to soluble or crystalline forms of beryllium induced alveolar macrophage death/release of IL-1α and DNA, enhanced migration of CD80hi DCs to the LDLNs and sensitized HLA-DP2 transgenic mice after single low dose exposures, while exposures to insoluble particulate forms beryllium did not. IL-1α and DNA released by alveolar macrophages upregulated CD80 on immature BMDCs via IL-1R1 and TLR9, respectively. Intrapulmonary exposure of mice to IL-1R and TLR9 agonists without beryllium was sufficient to drive accumulation of CD80hi DCs in the LDLNs, while blocking both pathways prevented accumulation of CD80hi DCs in the LDLNs of beryllium-exposed mice. Thus, in contrast to particulate forms of beryllium, which are poor sensitizers, soluble or crystalline forms of beryllium promote death of alveolar macrophages and their release of IL-1α and DNA, which act as DAMPs to enhance DC function during beryllium sensitization.
Introduction
Metal hypersensitivity reactions are generated against a variety of metals. Clinical manifestations of metal sensitization vary depending on the metal and site of exposure, ranging from skin rashes to the development of pulmonary granulomatous lung disease, as occurs in chronic beryllium disease (CBD). CBD occurs in genetically susceptible workers who are exposed to beryllium by inhaling fumes or airborne particulates. Sensitization occurs when beryllium reactive CD4+ T cells expand, circulate to the lung and secrete IFNγ. Τhese cells orchestrate pulmonary granulomas in chronic stages of disease (1–3). In HLA-DP2+ patients, beryllium ions coordinate changes in the HLA-DP2/self-peptide complex. This effectively results in the generation of a neo-antigen that is presented to naïve CD4+ T cells (4–6).
During sensitization, expansion of CD4+ Th1 cells that recognize the beryllium-altered HLA-DP2/peptide complex (beryllium-specific CD4+ T cells) occurs before clinical development of granulomas that characterizes CBD. Beryllium sensitization is required but does not always lead to chronic lung inflammation/CBD (7–9). Different physiochemical forms of beryllium may impact rates of sensitization (10–12). Development of a mouse model for CBD has provided the opportunity to study CBD pathogenesis, and we used it here to study how different forms of beryllium impact sensitization. This model utilizes mice expressing the human HLA-DP2 gene under control of the MHC II promoter (HLA-DP2 transgenic (Tg) mice) (13). These mice express HLA-DP2 in DCs, B cells and macrophages (13). After pulmonary beryllium exposure, HLA-DP2 Tg mice become sensitized as detected by CD4+ T cells in the spleen, lung-draining LNs (LDLNs) and lung that secrete IFNγ and IL-2 upon beryllium restimulation in vitro (13). In addition, a subset of CD4+ T cells in the lungs of beryllium-exposed HLA-DP2 Tg mice recognize the same HLA-DP2/peptide/Be2+ epitopes as beryllium specific CD4+ T cells derived from the lungs of humans with CBD (6, 13–15). After long term exposure to beryllium, HLA-DP2 Tg mice develop features of CBD, including pulmonary mononuclear infiltrates and granulomatous inflammation (13). Thus, this animal model recapitulates the major features of the human disease.
Acute inflammation, as opposed to chronic granulomatous inflammation in CBD, can occur immediately after Be(OH)2 exposure, is not dependent upon HLA-DP2 or CD4+ T cells, and involves signaling through innate pattern recognition receptors (16). Thus, analysis of acute inflammation provides insights into innate receptor pathways that could be involved in early phases of beryllium sensitization. The effects of innate receptor mediated activation and mobilization of DCs plays a central role in the sensitization phase of metal hypersensitivity (17, 18). Metal exposures that engage innate pattern recognition receptors or induce cellular death and the release of DAMPs are associated with higher rates of sensitization and disease after low dose exposures (19, 20). Under steady state conditions, presentation of foreign antigens by DCs to naïve T cells is tolerogenic (21); however, activated DCs presenting neo-antigens together with strong costimulatory signals, such as CD80, drive expansion of effector memory T cells with the capacity to migrate back to the site of exposure (22). Innate receptors upregulate expression of costimulatory molecules on the plasma membranes of DCs and accelerate migration of DCs to the draining LNs where naïve T cells scan for foreign antigen (22, 23). Naïve CD4+ T cells licensed by DCs, expand and differentiate into effector cells that can home to inflamed tissues and orchestrate chronic inflammation (24). Thus, determining the roles that innate receptors play during beryllium sensitization will define early immunological mechanisms in CBD pathogenesis.
We previously showed that exposure of mice to Be(OH)2 drives release of IL-1α and DNA into the airways and IL-1R/MyD88 dependent neutrophil recruitment. Be(OH)2 exposure drives upregulated expression of CD80 on migratory DCs in the lung and LDLNs, which is reduced in MyD88KO mice but is not affected in TLR9KO or IL-1RKO mice (16). The presence of CD80hi DCs in the LDLN is associated with enhanced CD4+ T cell responses, but the receptors driving MyD88-dependent upregulation of CD80 on DCs following beryllium exposure are not known.
In this study we expand these findings to determine if these mechanisms are initiated by exposures to different physiochemical forms of beryllium. We first set out to determine whether beryllium exposure promoted death of alveolar macrophages as a potential early source of IL-1α and DNA. We show that soluble and partially soluble crystalline forms of beryllium (BeSO4 and Be(OH)2) were toxic to alveolar macrophages, leading to release of DNA and IL-1α, while insoluble particulate forms of beryllium (BeO or Be metal powder) did not. Then we examined how different forms of beryllium impacted cell death and the release of DNA and IL-1α in vivo. Pulmonary exposure of mice to BeSO4 or Be(OH)2 resulted in release of IL-1α and DNA into the airways, accumulation of CD80hi DCs in the LDLNs, and sensitization of HLA-DP2 Tg mice after a single exposure, while exposure to insoluble particulate forms of beryllium (BeO and Be metal powder) mediated none of these effects. We then assessed how IL-1α and DNA from alveolar macrophages impacted DCs. We found that the DNA and IL-1 released from dying alveolar macrophages engage TLR9 and IL1RI, respectively, to drive upregulation of CD80 expression on DCs in vitro. We show that intrapulmonary exposure of mice to IL-1RI and TLR9 agonists in the absence of beryllium is sufficient to promote accumulation of CD80hi DCs in the LDLNs and exposure to either agonist alone upregulates CD80 on DCs. Furthermore, blocking both the TLR9 and IL1RI pathways impairs accumulation of CD80hi DCs in the LDLNs of Be(OH)2 exposed mice. Together, these data show that exposure to certain physiochemical forms of beryllium may drive hypersensitivity at low doses. The data suggest that this effect is related to their toxic effects on alveolar macrophages and the release of IL-1α and DNA, which act as DAMPs by engaging IL-1RI and TLR9 and driving upregulation of CD80 and migration of pulmonary DCs to the LDLNs.
Materials and Methods
Mice and treatments
WT and MyD88KO C57BL6/J (B6) mice were purchased from Jackson Laboratories and housed in the Office of Laboratory Animal Resources (OLAR) vivarium at the University of Colorado Anschutz Medical Campus. TLR9KO B6 mice were kindly provided by Jillian Poole with permission from Dr. Shizuo Akira and bred at the vivarium. HLA-DP2 Tg congenic B6 mice were provided by Andrew Fontenot (13) and housed/bred at the vivarium. Gnotobiotic B6 mice were bred and maintained within the Gnotobiotic Facility at the University of Colorado. Mice were housed, bred and treated in accordance with IACUC protocol standards at the CU Anschutz Medical Campus. Male and female mice (8–12 weeks of age) were used in our experiments. For intratracheal (i.t.) instillations, mice were removed from isoflurane anesthesia and supported on a table at 45 degrees. While depressing the tongue, 50μl of DPBS +/− beryllium, IL-1α or CpG ODN were administered to the tracheal opening and observed for inhalation during breathing. In some experiments, mice were treated with 200 μg mouse anti-mouse IL-1α antibody in DPBS (XBiotech, Austin, TX) or 100mg/kg sIL-1R antagonist by i.p. injection 30–45 minutes prior to DPBS or beryllium instillations. In some experiments mice were treated with 10ng rIL-1α or 10ug CpG in PBS by i.t. instillation.
Reagents and antibodies
BeO 1877 was purchased and characterized by National Institute of Standards and Technology (NIST). Be metal powder 99+% −325 mesh was purchased from Alfa Aesar. BeSO4 solution was provided kindly by Dr. Lisa Maier at National Jewish Health. Be(OH)2 was precipitated from BeSO4 solution and characterized as previously described (16). All stocks were prepared using EndoGrade water (Hyglos) or DPBS (Hyclone) and tested for endotoxin activity <0.1EU/ml with the Endpoint Chromogenic Limulus amebocyte assay (Lonza). Prior to use, Be(OH)2, BeO and Be stocks were placed in a sonicating water bath for 15 minutes to disperse particles. Mouse anti-mouse IL-1α was a kind gift of XBiotech, Austin, TX. Soluble IL-1R antagonist was kindly provided by Dr. Charles Dinarello. Functional grade LE rIL-1α was purchased from eBioscience, functional anti-IL1RI (JAMA-1) was purchased from BioXCel, mouse genomic DNA was purchased from Promega, and VacciGrade LE CpG type C ODN (5’-tcgtcgttttcggcgc:gcgccg-3’ (22 mer)) was purchased from InVivogen. The following monoclonal antibodies (mAbs) against mouse targets were used for flow cytometry: anti-CD11c (N418), anti-CD11b (M1/70), anti-F4/80 (BM8), anti-IAb (AF6.120.1), anti-CD4 (GK1.5), anti-Ly6G (1A8-Ly6G), anti-CD103 (2E7), anti-CD80 (16.10A1), anti-CD86 (GL-1), anti-CD8, (all from eBioscience); anti-CD16/CD32 (2.4G2), anti-CD44 (IM7), and anti-Siglec-F (E250–4440) (from BD Biosciences). Fixable Viability Dye (FVD) eFluor 506 (eBioscience) was used to assess viability. Collagenase D and DNase I (both from Roche) were used for LN digestion, and collagenase from Clostridium histolyticum (Sigma) was used for lung digestion. ELISA kits for mouse IL-1β and IL-1α and ELISPOT kits for IL-2 and IFNγ were purchased from eBioscience and used according to manufacturer instructions. The AEC Substrate set (BD Biosciences) and ELISPOT plates (Millipore) were used for ELISPOT assays. GM-CSF was purchased from PeproTech. Lipofectamine 2000 and OptiMEM media were purchased from ThermoFisher. Mitochondrial DNA extraction kits were purchased from BioVision and used per manufacturer instructions. DNA stocks (extracted and purchased) were brought to final concentration of 0.9% NaCl using 9% sterile saline in endograde water (DNA vehicle).
Tissue collection and processing
BAL was harvested with 3 washes of 1 ml DPBS and harvested into 15ml conical tubes containing 1ml cRPMI. The upper right mediastinal LNs (LDLNs) were dissected into cRPMI and teased apart with 18 gauge needles. Lungs were flushed by placing an incision in the left atrium of the heart followed by infusion of 10 ml DPBS through the lung vasculature via insertion of a 25-gauge needle into the right ventricle of the heart. Flushed lungs were dissected into cRPMI, chopped into 3mm slices using razor blades and transferred into 15ml tubes containing 3ml cRPMI with 1mg/ml collagenase and incubated 30 min at 37°C. Remaining tissue fragments were disrupted by serial passage through 16- and 18-gauge needles (5–6 times each). Lung digests were pelleted, and red cells were lysed for 2 min in ammonium chloride red cell lysis buffer. Each digest was brought up to 15ml with DPBS and the cells were strained through a 70 μm filter (Falcon). Cells were pelleted and resuspended in cRPMI media. LNs were teased apart and digested with 1 mg/ml collagenase D and 100 μg/ml DNAse in cRPMI (RPMI 1640 (Invitrogen) containing 0.2 U/ml penicillin, 0.2 μg/ml streptomycin, 0.6 μg/ml L-glutamine, 10mM HEPES, 1 mM sodium pyruvate (Invitrogen) and 10% FCS) for 30 min at 37°C, 5% CO2. An equal volume 100 mM EDTA in DPBS pH7.4 was added to each digest, and cells were washed with DPBS. As a source of APCs in ELISPOT assays, spleens from naïve B6 HLA-DP2 Tg mice were harvested into DPBS and pressed through 70 μm cell strainers. Red blood cells were lysed and cells were resuspended in cRPMI. Viable cells were counted on a hemocytometer using trypan blue.
ELISPOTs
Lung CD4+ T cells were purified using the mouse CD4+ T cell positive selection kit and LS columns (Miltenyi) according to manufacturer instructions. B cells from 3–5 naive HLA-DP2 Tg spleens were purified using CD19 positive selection kit (Miltenyi). 105 CD4+ T cells were co-cultured with 105 B cells in cRPMI media +/− 100 μM BeSO4 on coated and blocked IL-2 or IFNγ ELISPOT plates overnight and the number of spots was determined as previously described (13) using ImmunoSpot Reader and software (CTL).
Ex vivo analysis of DNA and cytokines in the BALF
BAL cells were pelleted and BALF was separated from the cells. For cytokine analysis BALF was concentrated 10-fold using 3K Amicon concentrators. Concentrated BALF was tested for IL-1α and IL-1β by ELISA. DNA in unconcentrated BALF was quantified using the HS DS DNA Qubit Assay (Invitrogen). Cell free media (for in vitro experiments) and PBS used to harvest BAL (for in vivo experiments) were used as blanks in determining DNA concentration in all experimental samples.
In vitro assays for alveolar macrophage death
For alveolar macrophage assays, BAL cells were harvested and pooled from naïve B6 mice and purified by plastic adherence. Alveolar macrophages were stimulated in cDMEM media (DMEM high glucose with L-glutamine and sodium pyruvate, supplemented with 0.2 U/ml penicillin, 0.2 μg/ml streptomycin, 0.6 μg/ml L-glutamine, 10mM HEPES (All from Invitrogen) and 10% FCS (Heat inactivated, Hyclone)) +/− indicated concentrations of BeSO4, Be(OH)2, BeO, or Be metal powder in a final volume of 200 μl/well. After culture, cells were observed by microscopy, plates were spun and supernatants were harvested for DNA and IL-1α analysis and cells were analyzed by flow cytometry.
Generation of BMDC and BMDC activation assays
BMDC were generated as previously described (25) by culture of bone marrow cells from WT, MyD88KO or TLR9KO in GM-CSF/cRPMI media. After culture, BMDCs were counted and resuspended to 5 × 106/ml in media with 5ng/ml GM-CSF and 100 μl was added to the wells of a 96 well plate. In some wells 10 μg/ml anti-IL1RI was added to the cells. For assessing the DAMP activity in BALF from Be-exposed mice, cohorts of 10 mice were exposed i.t. to either PBS or PBS containing Be(OH)2. 18hrs later mice were sacrificed and BAL were obtained as described above in PBS and cells were removed by centrifugation at 400 x g and concentrated 10-fold using Amicon 3K concentrators. For assessing the DAMP activity of factors released from alveolar macrophages, supernatants were collected from alveolar macrophages cultured 18hr in media +/− 50 μg/ml Be(OH)2 as described above. BALF and SN were spun at 10,000 rpm in the microfuge to remove beryllium crystals and debris. The soluble fractions were retained and tested for the presence of DNA. To assess whether the soluble fractions were toxic to macrophages (and thus contained residual soluble beryllium), 1 × 10^5 alveolar macrophages/well were stimulated for 18hr with 50 μl of each fluid and viability was assessed by flow cytometry. To assess DAMP activity in the soluble fractions WT, TLR9KO and MyD88KO BMDC plated as described above in cRPMI media +/− 50 μl of BALF from PBS or Be treated mice or with 50 μl SN from media or Be(OH)2 treated alveolar macrophages. In some experiments DNA was extracted from the BALF or SN using the mitochondrial DNA extraction kit from BioVision per manufacturer instructions. DNA was brought to a 0.9% saline concentration, quantified and normalized so that 35ng would be delivered to BMDC in lipofectamine at a 1:2 ratio of ng DNA/nl lipofectamine 2000. In these assays BMDC were plated in 100 μl OptiMEM media containing 5ng/ml GM-CSF without antibiotics or FCS. The liposomal DNA from the test groups, CpG DNA, mouse genomic DNA, or DNA vehicle controls were added to each well and the BMDC were cultured for 6hr, after which FCS (final concentration 10%) was added to the cells for the remaining 18hr of culture. BMDCs were stimulated in triplicate in each experiment and were incubated for a total of 24hr at 37 °C, 5% CO2. After culture, BMDCs were stained and analyzed by flow cytometry.
Flow Cytometry
To assess viability, alveolar macrophages or BAL cells were stained with eFluor 506 Fixable Viability Dye. BAL cells were stained with the following monoclonal antibodies (mAB): FcBlock, PE anti-Siglec, FITC anti-Ly6G, PerCP-Cy5.5 anti-CD11b, eFluor anti-F4/80, and PE-Cy7 anti-CD11c in flow wash (DPBS containing 10mg/ml BSA, 0.1mg/ml NaN3). LDLN were stained with the following mAbs: FcBlock, PE-Cy7 anti-CD11c, PE anti-CD80, PerCP-Cy5.5 anti-CD11b, eFluor 450 anti-IAb, and APC anti-CD103. LN cells from HLA-DP2 sensitized mice were labeled with 20 μM PE-labeled plexinA4/HLA-DP2/Be2+ tetramers (6) in a volume of 25 μl cRPMI media containing FcBlock and normal mouse serum for 2.5 hours at 37°C, 5% CO2. Cells were then stained with the following antibodies on ice for 30 min: AlexaFluor700 anti-CD4, PerCP-Cy5.5 anti-CD44, eFluor 450 Dump (anti-IAb, -F4/80, -B220, -CD8α). BMDC were stained with the following antibodies for 30 min: FcBlock, PE-Cy7 anti-CD11c, PE anti-CD80, PerCP-Cy5.5 anti-CD11b, eFluor 450 anti-IAb and APC CD86. Cells were washed with flow wash and analyzed on a Canto II flow cytometer using FlowJo software (Treestar).
Statistical Analysis
A one-way ANOVA and Bonferroni posttest was used to test for differences for normally distributed data, a Mann-Whitney test was used to test for differences for data without a normal distribution. Data were analyzed using Prism GraphPad Software.
Results
BeSO4 and Be(OH)2 induce death of alveolar macrophages and their release of IL-1α and DNA into the airways, while BeO and Be metal powder do not
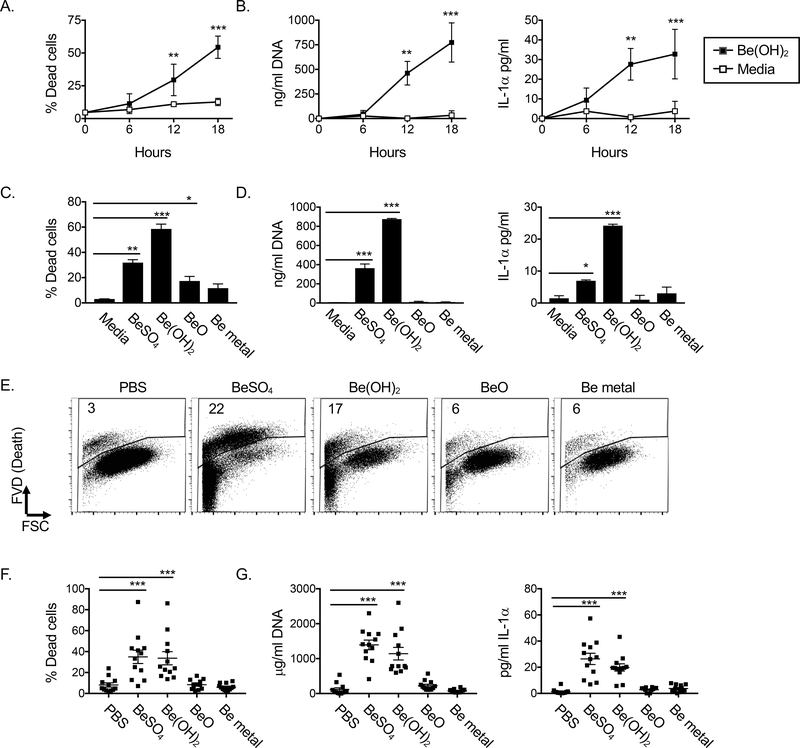
Our previous study showed that pulmonary exposure of mice to Be(OH)2 resulted in a drop in alveolar macrophage numbers in the bronchoalveolar lavage (BAL) after 6hrs, followed by a rapid recovery in alveolar macrophage numbers by 18hrs (16). Due to autofluorescence, the phenotype of the dying cells could not be reliably assessed by flow cytometry. Additionally, during inflammation alveolar macrophages may alter their adhesion molecules and be more difficult to recover with bronchoalveolar lavage. Thus, we could not conclude in this previous study whether alveolar macrophages were dying after Be(OH)2 exposure. To test this, we isolated and cultured alveolar macrophages with media +/− Be(OH)2 for 6, 12 or 18hr and assessed cell death and concentrations of DNA and IL-1α in the culture supernatants. Be(OH)2 enhanced death of alveolar macrophages 12–18hr after exposure compared to alveolar macrophages cultured in media alone (Fig 1A). Similarly, DNA and IL-1α concentrations were elevated in the supernatants 12–18hr after exposure to Be(OH)2. These data indicate that alveolar macrophages die after exposure to Be(OH)2 and subsequently release their cellular contents including DNA and IL-1α (Fig 1B).
Figure 1. BeSO4 and Be(OH)2 induce DNA and IL-1α release from alveolar macrophages upon cell death, while BeO and Be metal do not. A-D.
Analysis of alveolar macrophage death and release of DNA and IL-1α into culture supernatants after culture in media +/− beryllium. A. Percent cell death and B. concentrations of DNA and IL-1α in the supernatants of alveolar macrophages cultured in media alone (open squares) or with 50 μg/ml Be(OH)2 (solid squares). Data were combined from three independent experiments performed on separate days with 3 separate cohorts of alveolar macrophage donors. Dots on graphs represent mean of the 3 experimental values from each treatment group (calculated first within each experiment as an average of triplicate measures for each group). Error bars indicate SD of the experimental means to indicate the variation between the three experiments. C. Percent cell death D. DNA and IL-1α concentrations in the supernatants of alveolar macrophages cultured for 18hr with media +/− 50 μg/ml BeSO4, Be(OH)2, BeO or Be metal powder. Data are combined from two independently performed experiments performed on separate days with 2 separate cohorts of alveolar macrophage donors. Bars on graphs represent means and bars indicate SD as described for A-B. E-G. Impact of intrapulmonary exposure to different forms of beryllium on cell death and the presence of DNA and IL-1α in the BALF 18hr later. E. Flow plots show gating strategy for dead cells in a representative example from each treatment group. F. Percent dead cells and G. DNA and IL-1α in the BALF from mice treated 18hr previously with PBS +/− 80 μg BeSO4, Be(OH)2, BeO or Be metal powder. Data in F-G are combined from 3 independent experiments (n=12 mice per group altogether). Dots on graphs indicate values for individual mice, bars indicate overall mean values and error bars indicate SEM. A one-way ANOVA was performed to determine significant differences between means of each treatment compared to media or PBS treated controls. Asterisks indicate p<0.05 (*), p<0.01 (**), p<0.001 (***).
To extend this analysis to other forms of beryllium, we incubated alveolar macrophages with media alone or media containing soluble BeSO4, partially soluble/crystalline Be(OH)2, or two different forms of insoluble beryllium (BeO or Be metal powder), (Table 1) and assessed death and release of IL-1α or DNA 18hrs later. BeSO4, Be(OH)2 and BeO induced higher rates of alveolar macrophage death compared to media stimulation (Fig 1C). Furthermore, BeSO4 and Be(OH)2 were the only forms of beryllium that induced the release of DNA and IL-1α into the supernatants compared to alveolar macrophages cultured in media alone (Fig 1D).
Table 1: Beryllium forms used in this study.
BeSO4 is soluble while Be(OH)2 is a solid amorphous crystalline particle in water at neutral pH with low solubility. Be(OH)2 is more soluble in lysosomes at pH 4.5 due to its amphoteric chemistry and BeOH- ions are the main form that Be ions take in physiological solutions (41). BeO and Be metal powder are both insoluble with limited solubility at lysosomal pH (soluble dissociation rate ~10−7 mmol/kg/day at pH 4.5).
| Formula | Chemical properties | Potential exposure |
|---|---|---|
| BeSO4 (Be2+)aq | Clear solution 3Solubility 2×10−1 mol/kg |
Processing: fumes, furnace work, chemical purification |
| Be(OH)2 |
1Crystals 0.2–20 μm Amorphous, white gel *3Solubility 5 × 10−7 mol/kg |
Processing: chemical purification Soluble form (BeOH−) is the main form that Be2+ ions take in physiological solutions |
| BeO (NIST standard reference) |
2Particles 0.12 +/− 1.5 μM Light white powder Insoluble |
Processing Machining Ceramics |
| Be |
2Particles <44 μm Grey powder Insoluble |
Mining Processing Machining |
Particle size determined by microscopy.
Particle size determined by manufacturer.
solubility in aqueous solution at pH 7.4, 25°C.
Solubility of amphoteric Be(OH)2 increases in acid solutions
To determine the impact of different forms of beryllium on cell death and release of DNA and IL-1α in vivo, we exposed mice i.t. to PBS alone +/− BeSO4, Be(OH)2, BeO or Be metal powder and assessed cell death in the BAL and the presence of DNA and IL-1α in the BALF 18hr later. Pulmonary exposure of mice to either BeSO4 or Be(OH)2, led to an increased percentage of dead cells in the BAL compared to exposure to PBS, but exposure to BeO or Be metal did not (Fig 1E, F). Similarly, exposure of mice to BeSO4 or Be(OH)2 induced elevated levels of DNA and IL-1α in the BALF compared to PBS controls, while exposure to BeO or Be metal powder did not (Fig 1G). We detected no IL-1β in the BALF of mice exposed to any form of beryllium, even after concentrating the BALF 10-fold (data not shown). These data show that the physiochemical form of beryllium has profound effects on alveolar macrophage viability and the release of IL-1α and DNA in vitro and in vivo.
Intrapulmonary exposure to BeSO4 or Be(OH)2 leads to accumulation of neutrophils in the airways
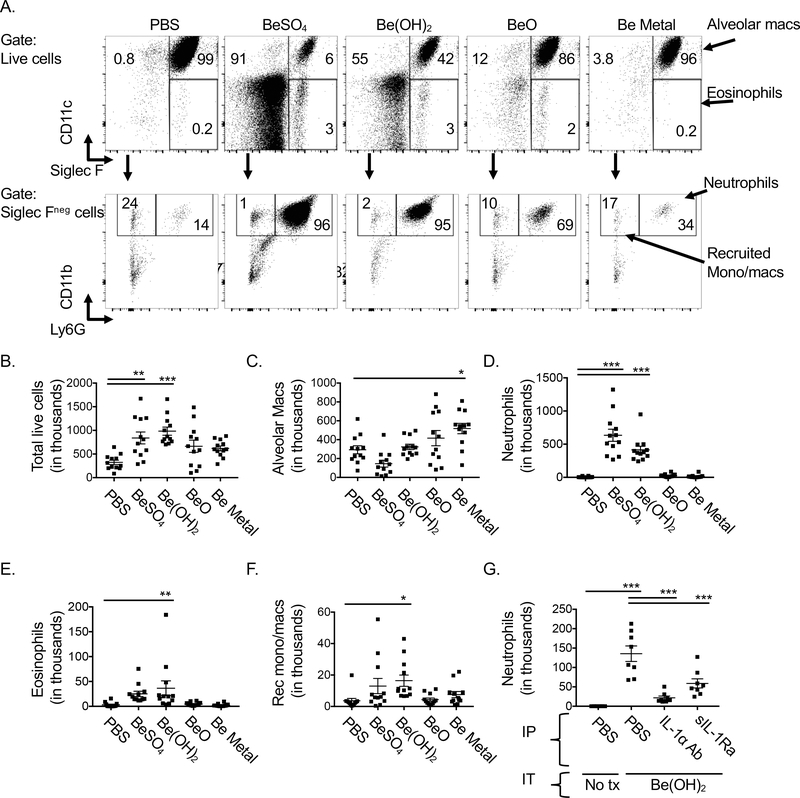
We previously showed that neutrophils accumulated in the airways 18hr after pulmonary exposure of mice to Be(OH)2 and that this acute inflammation was IL-1R dependent (16). To determine whether the different levels in IL-1α released after exposure to different forms of beryllium resulted in higher numbers of neutrophils, we exposed mice i.t. to PBS, BeSO4, Be(OH)2, BeO or Be metal powder and analyzed the cells in the BAL 18hr later (See gating strategy in Fig 2A). Total numbers of cells in the BAL were increased in mice exposed to BeSO4 or Be(OH)2 compared to PBS treated control mice, but not in those exposed to BeO or Be metal powder (Fig 2B). We previously showed that exposure to Be(OH)2 results in a drop in alveolar macrophages 6–12hr after pulmonary instillation, but that total numbers of these cells recover by 18hrs (16), consistent with the data in Fig 2C for Be(OH)2. Total alveolar macrophage numbers were similar in the BAL 18hrs after i.t. exposure of mice to PBS, BeSO4, Be(OH)2 or BeO (Fig 2C). In contrast, the total number of alveolar macrophages in the BAL of Be metal exposed mice was increased compared to PBS treated controls (Fig 2C). Neutrophil numbers were increased in both BeSO4 and Be(OH)2 exposed mice compared to PBS controls, but not in mice exposed to BeO or Be metal powder (Fig 2D). Eosinophils and recruited monocyte/macrophage populations were only increased in mice exposed to Be(OH)2 (Fig 2E, F).
Figure 2. Exposure of mice to BeSO4 or Be(OH)2 induces release of IL-1α and DNA and acute inflammation, while exposure to BeO or Be metal particles do not.
Flow cytometry analysis of BAL 18hr after i.t. exposure to PBS +/− 80 μg BeSO4, Be(OH)2, BeO, or Be powder. A. Gating strategy is shown on viable BAL cells for analysis of alveolar macrophages (SiglecFhi CD11chi), eosinophils (SiglecFhi CD11clo) upper row). Siglec Fneg cells (second row) were then analyzed for the presence of neutrophils (Ly6Ghi CD11bhi) and recruited monocyte/macrophages (Ly6GloCD11bhi). Numbers on graphs indicate percent of parent population in indicated gates for each representative sample in each treatment group. B. The total number of viable BAL cells in PBS or beryllium treated mice is shown as determined by cell counts. Total alveolar macrophages (C), neutrophils (D), eosinophils (E) and inflammatory monocyte/macrophages (F) were determined using total cell counts and percentages from flow cytometric analysis. Data in B-F are combined data from 3 independent experiments (n=12 mice per treatment group. data in G are combined from 2 independent experiments (n=8 mice per treatment group). Points on graphs indicate values for individual mice, bars indicate means, and error bars indicate SEM. A one-way ANOVA was used to test for differences between treatment groups, asterisks indicate p<0.05 (*), p<0.01 (**), p<0.001 (***).
Treatment of Be(OH)2-exposed mice with an anti-IL-1α antibody prevented neutrophil recruitment (Fig 2G). The effect of anti-IL1α antibody treatment on neutrophil accumulation was comparable to the effects of treatment with soluble human IL-1 receptor antagonist (sIL-1Ra, anakinra), which impairs both IL-1α and IL-1β activity through the IL-1R1 (Fig 2G). Together these data show that pulmonary exposure to BeSO4 and Be(OH)2 induce release of IL-1α, which drives neutrophil accumulation. In contrast, BeO or Be metal do not induce release of DNA or IL-1α nor do they induce an acute inflammatory response.
Be(SO)4 and Be(OH)2 sensitize B6 HLA-DP2 Tg mice after single low dose exposures, while BeO and Be metal do not
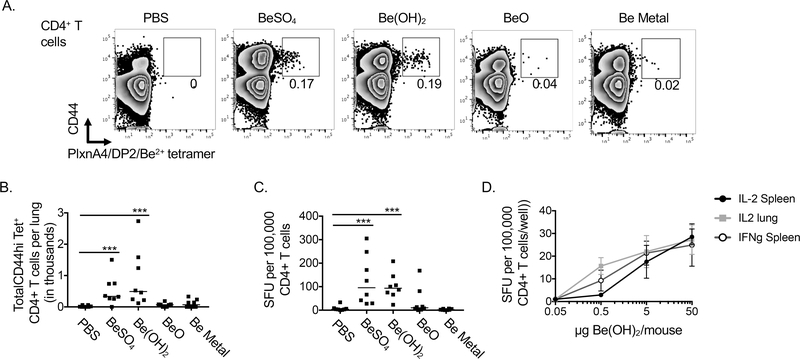
HLA-DP2 Tg mice mount a CD4+ T cell response against plexinA4 peptide presented in beryllium-loaded HLA-DP2 molecules after 7 exposures of BeO over 21 days (6). Due to workplace controls, beryllium workers are no longer exposed to high doses of beryllium. Yet beryllium sensitization can still occur at very low doses. To determine whether different forms of beryllium drive expansion of beryllium-specific CD4+ T cells after a single exposure, we exposed B6 HLA-DP2 Tg to a single dose of PBS with or without BeSO4, Be(OH)2, BeO or Be metal and analyzed the presence of Be-specific CD4+ T cells in the lungs 14 days later using HLA-DP2/plexinA4/Be2+ tetramers (Fig 3A.). Total cell numbers and total number of CD4+ T cells were similar in the lungs of mice in each treatment group (data not shown). However, total numbers of HLA-DP2/plexnA4/Be tetramer+ CD44hi CD4+ T cells were elevated in lungs of mice exposed to BeSO4 or Be(OH)2 but not in lungs of mice exposed to BeO or Be metal (Fig 3B). In accordance with this, lung CD4+ T cells from BeSO4 and Be(OH)2 exposed mice secreted IFNγ in response to beryllium restimulation, while CD4+ T cells from BeO or Be metal exposed mice did not (Fig 3C). Thus, analysis of lung cells from mice using the similar assays used to assess beryllium sensitization in BAL samples from beryllium workers, showed that BeSO4 and Be(OH)2 were potent beryllium sensitizers as compared to BeO or Be metal powder. Furthermore, a single exposure to 500ng Be(OH)2 per mouse was sufficient to detect beryllium sensitization in HLA-DP2 Tg mice as detected by ELISPOT, the most sensitive measure of beryllium sensitization (Fig 3D).
Figure 3. Exposure of HLA-DP2 Tg mice to a single dose of BeSO4 or Be(OH)2 induces accumulation of beryllium specific CD4+ Th1 cells in the lung (sensitization).
. B6 HLA-DP2 Tg mice were treated i.t. with PBS +/− BeSO4, Be(OH)2, BeO or Be metal powder and lungs were harvested 14 days later to assess beryllium sensitization by tetramer staining and ELISPOT. A. CD4+ T cells (CD3+ F480/MHCII/CD11b/B220neg) from lungs of mice in each treatment group were analyzed for CD44 expression and positive staining with HLA-DP2/plexinA4/Be2+ tetramers as shown for a representative mouse in each treatment group. Numbers on graphs are the percent of CD4+ T cells that are CD44hi HLA-DP2/plexinA4/Be2+ tetramer+ for each sample shown. B. Total CD4+ CD44hiTet+ cells/lung are shown for each treatment group based on total lung counts for each mouse, CD4+ T cell percentages and the percentage of CD4+ T cells that were CD44hiTet+. C. Be-specific CD4+ T cell IFNγ production in the lung as detected by ELISPOT. Each dot represents the mean number of IFNγ spot forming units (SFUs) per 200,000 CD4+ T cells in the presence of Be2+ stimulation normalized to media stimulated controls for an individual mouse. D. B6 HLA-DP2 Tg mice were exposed to indicated doses of Be(OH)2 and whole lung or spleen cells were analyzed for IL-2 or IFNγ producing cells. For B-C, points on scatter plots indicate values of individual mice. Data are combined from two independent experiments (n=8 mice per group) and lines indicate median values. In D points on graphs indicate means (n=8 mice per dose) are bars indicate SD. A Mann-Whitney test was used to test for differences between groups in B-C. Asterisks indicate p<0.05 (*), p<0.01 (**), p<0.001 (***) between indicated groups.
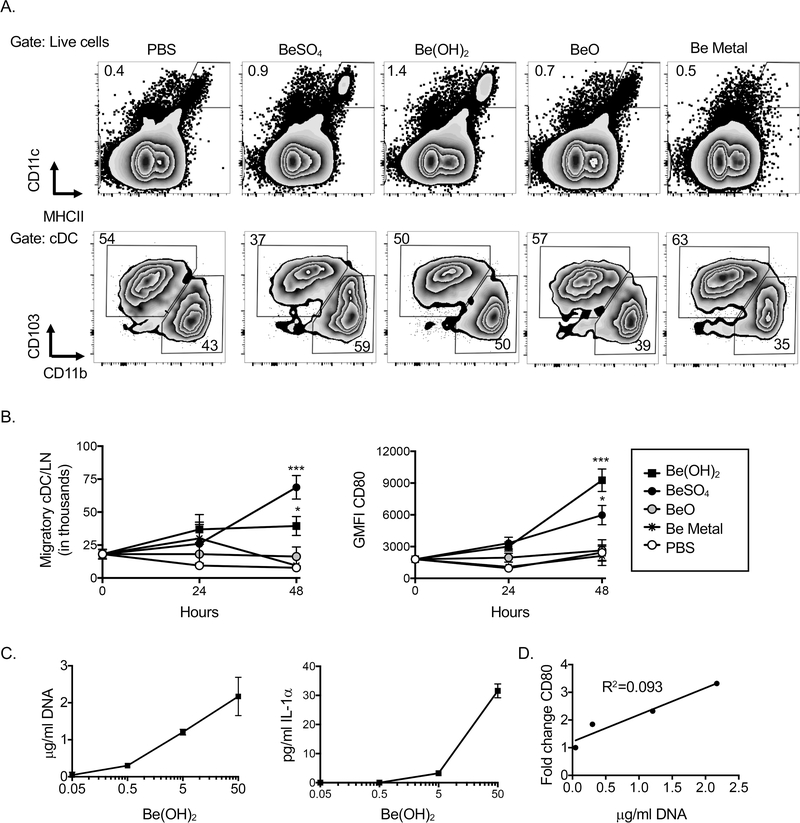
Pulmonary DCs upregulate CD80 and are mobilized to the LDLNs after exposure to BeSO4 or Be(OH)2
To test whether different forms of beryllium impact DC function, we exposed mice i.t. to PBS +/− BeSO4, Be(OH)2, BeO or Be metal powder and assessed the accumulation of migratory CD11bhi and CD103hi DCs in the LDLNs by flow cytometry (Fig 4A, gating strategy). The percentage of migratory DCs in the LDLNs was enhanced after exposure to either BeSO4 or Be(OH)2, and there was an increased percentage of CD11bhi DCs in these mice compared to PBS controls (Fig 4A). These effects were not observed in the LDLNs of mice exposed to BeO or Be metal powder. The total number of migratory DCs was enhanced 48hr after exposure to either BeSO4 or Be(OH)2 compared to PBS controls, but not in mice exposed to BeO or Be metal (Fig 4B). Upregulation of CD80 on DCs is associated with enhanced CD4+ responses in Be(OH)2 exposed mice (16). Exposure to either BeSO4 or Be(OH)2 led to upregulation of this costimulatory molecule on DCs in the LDLN, while exposure to BeO or Be metal did not (Fig 4B). To determine if exposure to Be(OH)2 at low doses had similar effects, we exposed mice to 500ng, 5 μg or 50 μg Be(OH)2 and assessed concentrations of DNA and IL-1α in the BALF and DC expression of CD80 in the LDLN 48hrs later. Pulmonary exposure of mice to doses of Be(OH)2 as low as 500ng/mouse resulted in elevated levels of DNA and IL-1α in the BALF (Fig 4D). There was a positive correlation between the concentration of DNA released in the BALF and upregulation of CD80 on DCs in the LDLNs (Fig 4D).
Figure 4. Accumulation of CD80hi DCs in the LDLN after exposure to physiochemical forms of beryllium that induce DAMP release.
Flow cytometry analysis of cDCs in the LDLN after i.t. exposure of mice to PBS +/− BeSO4, Be(OH)2, BeO or Be metal powder. A. Gating strategy for LN cells analyzed for the presence of migratory (CD11chiMHCIIhi) DCs, and CD11bhi and CD103hi subsets for a representative example in each treatment group B. Total number of migratory cDCs and geometric mean fluorescence intensity of staining for CD80 on migratory DCs 24–48hr after exposure to different forms of beryllium C. Mice were treated i.t. with indicated doses of Be(OH)2. C. concentrations of DNA and IL-1α in the BALF. D. Association between concentration of DNA in the BALF and change in CD80 expression on DCs was assessed. Data in B are combined data from 2 independent experiments (n=8 per treatment group). Data in C are combined from 3 independent experiments (n=9 per treatment group). Points on line graphs indicate mean values and error bars indicate SEM. A one-way ANOVA was used to test for differences between treatment groups. Asterisks indicate p<0.05 (*), p<0.01 (**), p<0.001 (***) between indicated groups or between the indicated group and PBS control on line graphs.
Be(OH)2 exposure promotes migration of activated DCs to the LDLN in gnotobiotic mice
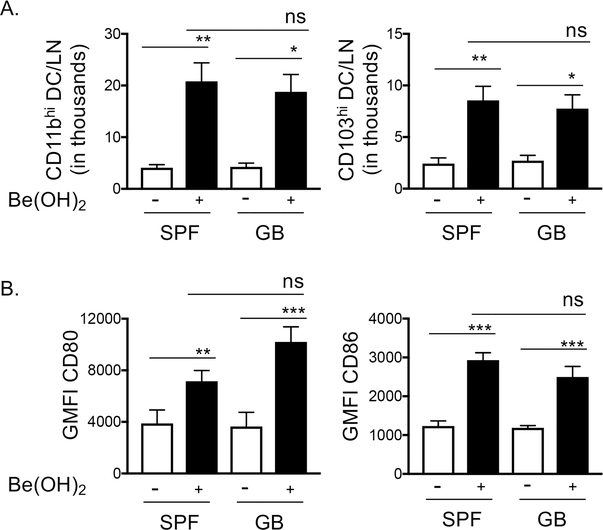
Exposure to Be(OH)2 enhances the mobilization of CD80hi pulmonary DCs to the LDLNs, which peaks 48hr after exposure and is MyD88KO dependent (16). We hypothesized that this was due to the release of DAMPs that engaged TLRs or the receptors of the IL-1 family that drive MyD88-dependent activation. However, it is also possible that exposure to Be(OH)2 may promote entry of bacteria and the engagement of TLRs by bacterial products. To test this, we treated regularly housed mice (specific pathogen free – SPF) or gnotobiotic mice (GB) with PBS or Be(OH)2 and examined the presence of CD80hi cDCs in the LDLN 48hrs later. SPF and GB mice exposed to Be(OH)2 exhibited similar accumulations of CD11bhi and CD103hi DCs in the LDLNs and increased expression of CD80 (Fig 5), indicating that bacterial PAMPs are not responsible for these effects.
Figure 5. Accumulation migratory DC in the LDLNs and their upregulation of CD80 and CD86 is similar in Be(OH)2 exposed SPF housed and gnotobiotic mice.
CD11bhi and CD103hi DCs in the LDLNs were analyzed by flow cytometry as described in Fig 4A. WT B6 mice housed in specific pathogen free (SPF) room or bred and housed in the gnotobiotic (GB) core facility were treated with PBS (open bars) or PBS + 80 μg Be(OH)2 (solid, black bars). A. Total DC/LN in thousands and B. GMFI of staining for CD80 and CD86 on migratory DC. Data are combined from two separate experiments (n=8 mice per treatment group), bars indicate mean values and error bars indicate SEM. A one-way ANOVA was used to test for differences between treatment groups. Asterisks indicate p<0.05 (*), p<0.01 (**), p<0.001 (***) between indicated groups.
DNA and IL-1α released by alveolar macrophages upregulate CD80 on DCs
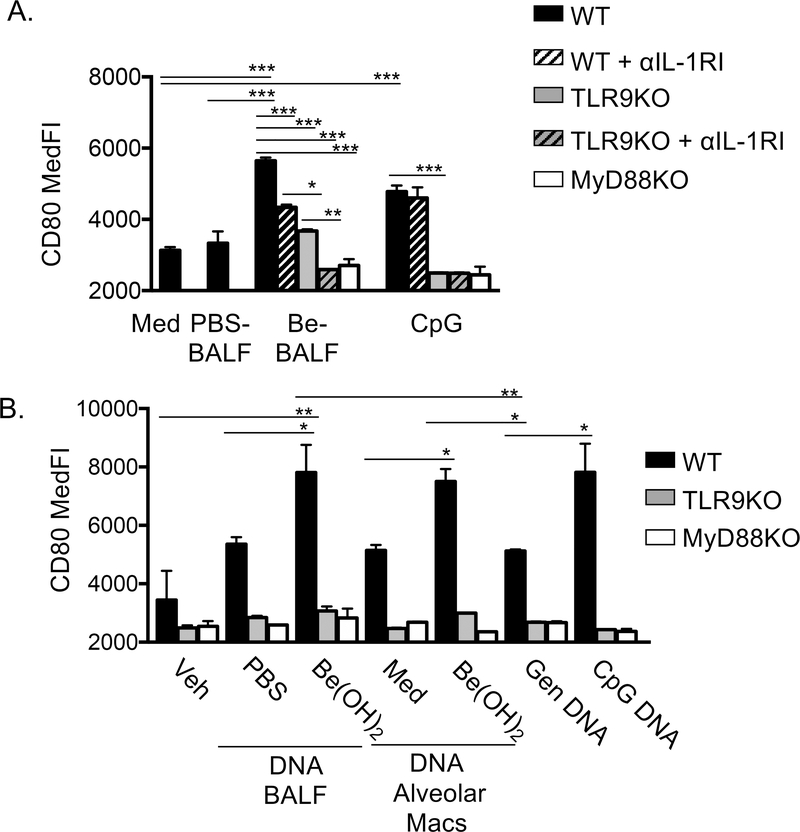
To assess impact of IL-1α and DNA released into the airways following beryllium exposure, we developed an in vitro assay to test their effects on the upregulation of CD80 on DCs. We first purified DCs from spleens and lungs of naïve WT B6 mice, however processing of the organs led to spontaneous activation of the DCs after in vitro culture in media alone, masking any effects of TLR9 mediated activation (Fig S1). Therefore, we generated bone marrow derived DCs (BMDCs) (25), as a source of large numbers of immature DCs. BMDCs upregulated MHCII, CD80 and CD86 in response to CpG (Fig S2). We first wanted to test whether IL-1α and/or DNA in the BALF of Be(OH)2 exposed mice (Be-BALF) could directly activate BMDCs. Cell free soluble BALF from PBS (PBS-BALF) or Be(OH)2 exposed mice (Be-BALF) were not toxic to alveolar macrophages (Fig S3). Be-BALF upregulated CD80 on WT BMDCs, compared to BMDCs cultured with PBS-BALF or media alone (Fig 6A, black bars). The DAMP activity in the BALF from Be-exposed mice was reduced in WT BMDCs in the presence of an IL-1R1 neutralizing antibody (black hatched bars) and in TLR9KO BMDCs (solid grey bars) suggesting both receptors induce upregulation of CD80 in response to Be-BALF. Accordingly, Be-BALF did not induce any upregulation of CD80 in TLR9KO DCs treated with the IL-1R1 antibody or in MyD88KO DC (Fig 6A grey hatched bars or white bars). In contrast, anti-IL1RI had no effect on the upregulation of CD80 on WT BMDCs stimulated with CpG ODN, which as expected, was entirely TLR9 dependent (Fig 6A).
Figure 6. DNA and IL-1α released by dying alveolar macrophages upregulate CD80 on BMDC.
A. WT, TLR9KO or MyD88KO BMDC were incubated with media, 50 μl soluble BALF from PBS exposed mice (PBS-BALF), 50 μl soluble BALF from Be(OH)2 exposed mice (Be-BALF), or 10 μg/ml CpG ODN for 24hr. In some cultures a blocking antibody against the IL-1RI was used to block IL-1 signaling. BMDC were gated as described in supplemental figure 2 and CD80 median fluorescence intensity (MedFI). B. WT, TLR9KO and MyD88KO BMDC were stimulated with 35ng/well DNA extracted from PBS-BALF or Be-BALF, 35ng DNA extracted from supernatants from media or Be(OH)2 stimulated alveolar macrophages, 35ng genomic DNA from untreated mice, or 35ng CpG ODN. DNA was delivered to DC internally using lipofectamine as described in methods. After 24hr, DC CD80 expression was analyzed as described in A. Data are combined from 3 independent experiments for A and B. We used 3 separate cohorts of PBS- or Be-BALF for A and for the DNA sources in B and three separate alveolar macrophage cultures for B. BMDC in each independent experiment were grown from separate donor mice. Bars on graphs indicate mean experimental values (n=3 values), which were determined in each experiment as the average value between triplicate stimulations. Error bars indicate SD of the experimental means to show interexperimental variability. One way ANOVA was performed to test for experimental variation. Asterisks indicate p<0.05 (*), p<0.01 (**) and p<0.001 (***) between select groups. In B p<0.001 between WT and TLR9KO DCs and WT and MyD88KO DCs for all treatment groups except vehicle controls.
To confirm that DNA in Be-BALF could activate TLR9, we extracted DNA from the BALF samples. Vertebrate DNA can activate TLR9 when delivered to endosomes, a process that can be influenced by factors in the tissue (26). To determine if isolated DNA could activate TLR9, DNA (35ng/well) was delivered to BMDC by liposomes (27). DNA extracted from Be-BALF induced a higher degree of TLR9 dependent upregulation of CD80 on BMDC compared to equivalent amounts of DNA extracted from PBS-BALF or of genomic DNA from unexposed mice (Fig 6B). The effects of Be-BALF DNA was comparable to equivalent amounts of CpG ODN (Fig 6B). DNA released into the BALF fluid may include DNA from other cells, including neutrophils (16), therefore we tested whether DNA released from Be-exposed alveolar macrophages could promote TLR9 dependent upregulation of CD80 on BMDCs. DNA released from Be-exposed alveolar macrophages upregulated CD80 via TLR9 on BMDCs compared to vehicle controls, equivalent amounts of DNA extracted from media stimulated alveolar macrophages or equivalent amounts of genomic DNA from naïve mice (Fig 6B). Together these data indicate that DNA and IL-1α released by alveolar macrophages after exposure to toxic forms of beryllium, act as DAMPs to promote upregulation of CD80 on DCs via TLR9 and IL1RI, respectively.
Engagement of IL-1R1 and TLR9 in the lung is sufficient to promote accumulation of CD80hi DCs in the LDLNs
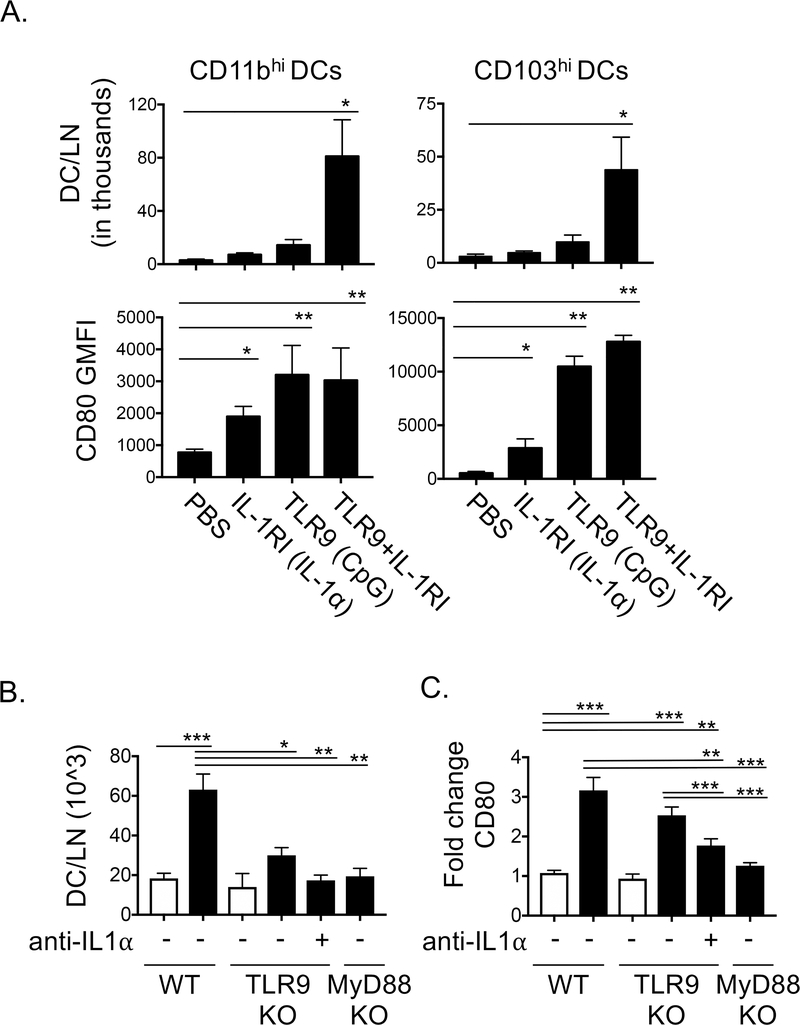
To test whether engagement of IL-1R1, TLR9 or both receptors promotes effects on DCs in vivo, we treated mice i.t. to agonists of either receptor (rIL-1α or CpG) or a combination of both rIL-1α and CpG and analyzed DCs in the LDLN 48hr later. Treatment of mice with either rIL-1α or DNA alone was not sufficient to enhance the numbers of CD11bhi or CD103hi DCs in the LDLNs 48hrs later (Fig 7A). Treatment with both agonists, however, promoted accumulation of CD11bhi and CD103hi DC in the LDLNs (Fig 7B). In contrast, rIL-1α or CpG DNA treatment alone were each sufficient to induce upregulation of CD80 on CD11bhi and CD103hi DCs (Fig 5B). These data suggest that the TLR9 and IL-1RI receptor pathways can synergize to drive migration of DC from the lung to the LDLN to enhance priming of naïve T cells, but that engagement of either receptor alone is sufficient to promote upregulation of CD80 on LN DCs. These effects on CD80 mirror those of BMDCs exposed to IL-1α and DNA released from alveolar macrophages in vitro (Fig 6).
Figure 7. Engagement of TLR9 and IL1RI in vivo promotes accumulation of CD80hi DCs in the LDLNs. A-B.
CD11bhi and CD103hi DCs in the LDLNs were analyzed by flow cytometry as described in Fig 4A. A. WT B6 mice were treated i.t. with PBS +/− 10ng rIL-1α (IL-1R1 agonist), 10ug CpG type C DNA (DNA, TLR9 agonist) or 10ng rIL-1α + 10ug DNA. Total DC/LN in thousands and GMFI of staining for CD80 are shown for each DC subset. B-C. WT and TLR9KO B6 mice were treated with nothing or 200 μg anti-IL-1α i.p. and 30 minutes later the mice were treated i.t. with PBS +/− 80 μg Be(OH)2. Migratory DCs in the LDLNs were analyzed by flow cytometry as described in Fig 4A. B. Migratory DCs that accumulated in the LDLN and C. fold change in CD80 expression vs PBS treated WT mice are shown for WT, TLR9KO, TLR9KO anti-IL-1α treated and MyD88KO mice exposed to PBS (open bars) or 80 μg Be(OH)2 (solid, black bars). Data in A-B are combined from 3 independent experiments (n=15 mice per treatment group). Bars on graphs indicate mean values and error bars indicate SEM. Data in B-C are combined from 4 independent experiments (n=16 per treatment group), bars on graphs indicate means and error bars indicate SEM. A one-way ANOVA was used to test for differences between treatment groups, asterisks indicate p<0.05 (*), p<0.01 (**), p<0.001 (***) for select comparisons.
Neutralization of both the DNA/TLR9 and IL-1α/IL-1R pathways impaired accumulation of CD80hi immunogenic DCs in the LDLN of Be(OH)2 exposed mice
We previously showed that upregulation of CD80 on DCs in beryllium exposed mice was MyD88 dependent but was not impaired in Be(OH)2 exposed IL-1RI KO mice or TLR9KO mice (16). However, our in vitro and in vivo experiments (Fig 6, 7A-B), suggest that these signals are redundant in upregulating CD80 on DCs. Thus, we decided to test the effects of neutralizing both TLR9 and IL-1R pathways on DC migration and CD80 expression in Be(OH)2 exposed mice. We treated TLR9KO mice with an anti-IL-1α and analyzed the presence of CD80hi DCs in the LDLNs 48hrs after i.t. exposure to Be(OH)2.
The accumulation of DCs in the LDLNs following Be(OH)2 exposure was partially reduced in TLR9KO mice but was further reduced to background levels in TLR9KO mice treated with anti-IL-1α (Fig 7C). In addition, Be(OH)2-induced upregulation of CD80 was reduced in TLR9KO mice treated with anti-IL-1α compared to WT mice, albeit not to background levels (Fig 7D). These data suggest that IL-1α and DNA are redundant in driving MyD88-dependent upregulation of CD80 on CD11bhi and CD103hi DCs.
Together, these data show that soluble and crystalline forms of beryllium induce death of alveolar macrophages, while highly insoluble particulates (BeO and Be metal) do not. This death is associated with the release of IL-1α and DNA, which promote acute inflammation in the lung and drive migration of CD80hi DCs to the LDLN via TLR9 and IL1R mediated engagement of MyD88. Thus, in contrast to particulate forms of beryllium, which are poor sensitizers, soluble or crystalline forms of beryllium promote death of alveolar macrophages and their release of IL-1α and DNA, which act as DAMPs to enhance DC function during beryllium sensitization.
Discussion
Here we show that soluble and crystalline forms of beryllium are highly toxic to alveolar macrophages. Furthermore, the death of these cells has a profound impact on the mobilization of immunogenic DCs in vivo. We show that pulmonary exposures of mice to BeSO4 and Be(OH)2 drive release of DNA and IL-1α into the lung and neutrophil influx while exposures to BeO and Be metal powder promote limited infiltration of inflammatory cells. The massive neutrophil influx that followed exposure to BeSO4 and Be(OH)2 was driven by IL-1α, suggesting the low levels of IL-1α have potent biological effects in vivo, as has been reported elsewhere (28). Our previous study showed that neutrophils themselves contribute to the presence of DNA and IL-1α in the airways 18hr following Be(OH)2 exposure (16). While both neutrophils and alveolar macrophages are implicated in DAMP release after exposure to Be(OH)2, neutrophils require IL-1α to reach the airways and thus, alveolar macrophages are the likely candidate as the early source of IL-1α. Prevention of neutrophil accumulation in Be(OH)2 exposed mice has no effect on Be(OH)2 induced mobilization of activated DCs to the LDLNs (16), suggesting that the release of DAMPs by alveolar macrophages and other cells is sufficient to impact DCs.
In vitro, alveolar macrophages exposed to Be(OH)2 release higher levels of DAMPs than those exposed to BeSO4. Conversely, we observed similar levels of DAMPs in BeSO4 and Be(OH)2 exposed mice. Be2+ ions can interact with hydroxide ions, phosphate ions and organic molecules in different solutions and biological fluids (29, 30). Therefore, we cannot rule out the possibility that BeSO4 is in a different physiochemical form before entry into alveolar macrophages in our in vitro vs in vivo experiments. In addition, unlike in vitro experiments, delivery of BeSO4 and Be(OH)2 to the deeper small airways of the lung may not be equivalent. In vivo, the depletion of alveolar macrophages is not apparent in the BAL after 18hrs. Previous work showed that after exposure to Be(OH)2, macrophages depletion occurred after 6hrs but not at 18hrs (16). Alveolar macrophages can self-renew (resident alveolar macrophages) or be recruited to the lung during inflammation (recruited alveolar macrophages) (31–33). The differing results in the total number of alveolar macrophages between in vitro and in vivo experiments (Fig 1C vs Fig 2C) are likely due to renewal of the alveolar macrophage pool in vivo by inflammatory monocytes, and lack of renewal of alveolar macrophages in vitro. The in vitro experiments confirm that alveolar macrophages died and released DAMPs after exposure to BeSO4 and Be(OH)2.
Studies of other amphoteric metal salts such as Al(OH)3 (a common vaccine adjuvant) and crystalline particles suggest that phagocytosis of such substances causes cellular stress in macrophages. Phagocytosis of Al(OH)3 by peritoneal macrophages are unable to maintain the integrity of their lysosomal membranes, which leads to cellular stress, assembly of the NLRP3 inflammasome, activation of caspase-1 and release of IL-1β (34). However, phagocytosis of Al(OH)3 particles by alveolar macrophages induced death and release of IL-1α and DNA (35). In contrast to Be(OH)2 IL-1α (but not DNA) appears to play a role in the adjuvant effects of Al(OH)3, after pulmonary exposure (35), while IL-1R and MyD88 are not required for the adjuvant effects of Al(OH)3 via other routes of administration (36, 37). However, the effect of IL-1α release in the lung is limited to driving BALT formation and enhanced antibody production and may not occur at other tissue sites (35) and redundancy between IL-1R1 and TLR9 has not been examined in this model. Interestingly aluminum and beryllium salts both drive DAMP release and both act as adjuvants for adaptive immunity but drive qualitatively different CD4+ T cell responses. Be(OH)2 drives expansion of Th1 cells, while Al(OH)3 drives expansion of Th2 cells, enhanced IgE production and eosinophil dominated inflammation. TLR9 activation in the lung increases IL-12 release (38), a cytokine which is required to drive Th1 responses and likely involved in the adjuvant effects of beryllium, while Al(OH)3 appears to inhibit IL-12 release (39). Perhaps the manner in which these two insoluble metal salts impact innate receptor engagement and cytokine release determines their impact on CD4+ T cells. In addition to IL-1α and DNA, other DAMPs may contribute to the effects on DCs after beryllium exposure, particularly because the role of IL-1α in DC activation was not elucidated until we examined its role in TLR9KO mice. Thus, it is possible that other DAMPs not detected in this study contributed to the MyD88 dependent effects of beryllium exposure on pulmonary DCs.
Comparison of sensitization in B6 HLA-DP2 Tg mice to different forms of beryllium showed that BeSO4 and Be(OH)2 could rapidly sensitize mice with equal genetic predisposition compared to mice exposed to BeO or Be metal. Treatment of HLA-DP2 Tg mice with multiple doses of BeO sensitizes HLA-DP2 Tg mice and induces the expansion of beryllium-specific CD4+ T cells (13). Whether these high-dose exposures to insoluble particles create overload of macrophages or activation of innate pathways in the lung over time are currently under study. We found that DCs in mice exposed to BeSO4 and Be(OH)2 migrate at a higher rate to the LDLN than in mice exposed to BeO or Be metal and that these DCs express high levels of CD80. Upregulation of CD80 on DCs in the LNs in Be(OH)2 exposed mice is MyD88 dependent but not altered in IL-1RKO or TLR9KO mice and associated with enhanced priming of CD4+ T cells to bystander antigen (16).
Here we show that the effects of beryllium exposure on DCs is not due to microbial products. Furthermore, we show that BALF from Be exposed mice and SN from Be exposed alveolar macrophages promoted upregulation of CD80 on BMDC via IL-1R and TLR9. DNA extracted from the airways of Be(OH)2 exposed mice or Be(OH)2 exposed alveolar macrophages can induce upregulation of CD80 in DCs via TLR9 after liposomal and is more potent at activating TLR9 than mouse genomic DNA, similar to CpG ODN. It is unclear if the structure of the DNA or presence of associated histones or other factors such as HMGB1 explains this difference, however these questions are currently being studied.
Our data show that IL-1α and DNA together enhanced migration of DCs to the LDLNs in the absence of beryllium, and each were sufficient to upregulate CD80, suggesting redundancy. We showed that inhibiting both TLR9 and IL-1α/IL-1R1 activity in the same mice reduced upregulation of CD80 and that migration of DCs was reduced to background levels. Thus, cooperation of these pathways promotes optimal migration of immunogenic DCs to the LDLNs.
Together, the data presented here suggest that alveolar macrophages that phagocytose toxic substances like BeSO4 and Be(OH)2 and release DNA and IL-1α can have potent effects on pulmonary DCs via the cooperative effects of IL1R1 and TLR9. In this way, soluble and crystalline forms of beryllium may be particularly dangerous as they not only act as a source of antigenic signals to expand beryllium specific CD4+ T cells, but also promote DAMP release that enhances the adaptive immune response via DCs.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the OLAR Vivarium. Gnotobiotic Core Facility and ClinImmune Flow Core Facility at the University of Colorado Anschutz Medical Campus. The authors would like to acknowledge Abi Shotland for assistance on manuscript revisions.
This study was funded by National Institutes of Health grant numbers HL126736 to AM. AM is also funded by NIH grants ES025534 and HL135872–01.
Footnotes
The authors have no conflicts of interest
References
- 1.Fontenot AP, Falta MT, Kappler JW, Dai S, McKee AS. 2016. Beryllium-Induced Hypersensitivity: Genetic Susceptibility and Neoantigen Generation. Journal of immunology 196:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saltini C, Winestock K, Kirby M, Pinkston P, Crystal RG. 1989. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N Engl J Med 320:1103–1109. [DOI] [PubMed] [Google Scholar]
- 3.Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, Voronov E, Martin MU, Dinarello CA, Apte RN. 2010. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A 107:2574–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowerman NA, Falta MT, Mack DG, Kappler JW, Fontenot AP. 2011. Mutagenesis of beryllium-specific TCRs suggests an unusual binding topology for antigen recognition. J Immunol 187:3694–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton GM, Wang Y, Crawford F, Novikov A, Wimberly BT, Kieft JS, Falta MT, Bowerman NA, Marrack P, Fontenot AP, Dai S, Kappler JW. 2014. Structural basis of chronic beryllium disease: linking allergic hypersensitivity and autoimmunity. Cell 158:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falta MT, Pinilla C, Mack DG, Tinega AN, Crawford F, Giulianotti M, Santos R, Clayton GM, Wang Y, Zhang X, Maier LA, Marrack P, Kappler JW, Fontenot AP. 2013. Identification of beryllium-dependent peptides recognized by CD4+ T cells in chronic beryllium disease. J Exp Med 210:1403–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richeldi L, Sorrentino R, Saltini C. 1993. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science 262:242–244. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot AP, Torres M, Marshall WH, Newman LS, Kotzin BL. 2000. Beryllium presentation to CD4+ T cells underlies disease susceptibility HLA-DP alleles in chronic beryllium disease. Proc Natl Acad Sci U S A 97:12717–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bill JR, Mack DG, Falta MT, Maier LA, Sullivan AK, Joslin FG, Martin AK, Freed BM, Kotzin BL, Fontenot AP. 2005. Beryllium presentation to CD4+ T cells is dependent on a single amino acid residue of the MHC class II beta-chain. J Immunol 175:7029–7037. [DOI] [PubMed] [Google Scholar]
- 10.Cummings KJ, Stefaniak AB, Virji MA, Kreiss K. 2009. A reconsideration of acute Beryllium disease. Environ Health Perspect 117:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefaniak AB, Hoover MD, Day GA, Dickerson RM, Peterson EJ, Kent MS, Schuler CR, Breysse PN, Scripsick RC. 2004. Characterization of physicochemical properties of beryllium aerosols associated with prevalence of chronic beryllium disease. J Environ Monit 6:523–532. [DOI] [PubMed] [Google Scholar]
- 12.Kreiss K, Mroz MM, Zhen B, Wiedemann H, Barna B. 1997. Risks of beryllium disease related to work processes at a metal, alloy, and oxide production plant. Occup Environ Med 54:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack DG, Falta MT, McKee AS, Martin AK, Simonian PL, Crawford F, Gordon T, Mercer RR, Hoover MD, Marrack P, Kappler JW, Tuder RM, Fontenot AP. 2014. Regulatory T cells modulate granulomatous inflammation in an HLA-DP2 transgenic murine model of beryllium-induced disease. Proceedings of the National Academy of Sciences of the United States of America 111:8553–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falta MT, Bowerman NA, Dai S, Kappler JW, Fontenot AP. 2010. Linking genetic susceptibility and T cell activation in beryllium-induced disease. Proc Am Thorac Soc 7:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falta MT, Tinega AN, Mack DG, Bowerman NA, Crawford F, Kappler JW, Pinilla C, Fontenot AP. 2016. Metal-specific CD4+ T-cell responses induced by beryllium exposure in HLA-DP2 transgenic mice. Mucosal Immunol 9:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKee AS, Mack DG, Crawford F, Fontenot AP. 2015. MyD88 dependence of beryllium-induced dendritic cell trafficking and CD4(+) T-cell priming. Mucosal Immunol 8:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, Roth J, Skerra A, Martin SF, Freudenberg MA, Goebeler M. 2010. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol 11:814–819. [DOI] [PubMed] [Google Scholar]
- 18.Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BM, Scheper RJ, van Hoogstraten IM. 2013. Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermatitis 68:331–338. [DOI] [PubMed] [Google Scholar]
- 19.McKee AS, Fontenot AP. 2016. Interplay of innate and adaptive immunity in metal-induced hypersensitivity. Curr Opin Immunol 42:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Huang Z, Gillespie M, Mroz PM, Maier LA. 2014. p38 Mitogen-Activated Protein Kinase in beryllium-induced dendritic cell activation. Hum Immunol 75:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. 1999. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol 163:2592–2600. [PubMed] [Google Scholar]
- 22.Medzhitov R, Janeway CA, Jr., 1997. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 9:4–9. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi H, Akira S. 2005. TLR signalling and the function of dendritic cells. Chem Immunol Allergy 86:120–135. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot AP, Gharavi L, Bennett SR, Canavera SJ, Newman LS, Kotzin BL. 2003. CD28 costimulation independence of target organ versus circulating memory antigen-specific CD4+ T cells. J Clin Invest 112:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. 2006. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci 1082:31–43. [DOI] [PubMed] [Google Scholar]
- 27.Yasuda K, Yu P, Kirschning CJ, Schlatter B, Schmitz F, Heit A, Bauer S, Hochrein H, Wagner H. 2005. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol 174:6129–6136. [DOI] [PubMed] [Google Scholar]
- 28.Manzer R, Dinarello CA, McConville G, Mason RJ. 2008. Ozone exposure of macrophages induces an alveolar epithelial chemokine response through IL-1alpha. Am J Respir Cell Mol Biol 38:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton M, Burastero SR. 2003. Beryllium chemical speciation in elemental human biological fluids. Chem Res Toxicol 16:1145–1154. [DOI] [PubMed] [Google Scholar]
- 30.Skilleter DN, Paine AJ. 1979. Relative toxicities of particulate and soluble forms of beryllium to a rat liver parenchymal cell line in culture and possible mechanisms of uptake. Chem Biol Interact 24:19–33. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. 2011. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda E, Ozasa K, Temizoz B, Ohata K, Koo CX, Kanuma T, Kusakabe T, Kobari S, Horie M, Morimoto Y, Nakajima S, Kabashima K, Ziegler SF, Iwakura Y, Ise W, Kurosaki T, Nagatake T, Kunisawa J, Takemura N, Uematsu S, Hayashi M, Aoshi T, Kobiyama K, Coban C, Ishii KJ. 2016. Inhaled Fine Particles Induce Alveolar Macrophage Death and Interleukin-1alpha Release to Promote Inducible Bronchus-Associated Lymphoid Tissue Formation. Immunity 45:1299–1310. [DOI] [PubMed] [Google Scholar]
- 36.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. 2001. Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2:947–950. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz N, Kurrer M, Kopf M. 2003. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur J Immunol 33:991–1000. [DOI] [PubMed] [Google Scholar]
- 38.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med 202:1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori A, Oleszycka E, Sharp FA, Coleman M, Ozasa Y, Singh M, O’Hagan DT, Tajber L, Corrigan OI, McNeela EA, Lavelle EC. 2012. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur J Immunol 42:2709–2719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.