Abstract
Cisplatin is a widely used chemotherapy drug that can damage auditory and vestibular tissue and cause hearing and balance loss through the intracellular release of reactive oxygen species (ROS). Curcumin has anticancer efficacy and can also counteract cisplatin’s damaging effect against sensory tissue by scavenging intracellular ROS, but curcumin’s applicability is limited due to its low bioavailability. EF-24 is a synthetic curcumin analog that is more bioavailable than curcumin and can target cancer, but its effects against cisplatin-mediated ROS in auditory and vestibular tissue is currently unknown. In this study, we employed a novel zebrafish inner ear tissue culture system to determine if EF-24 counteracted cisplatin-mediated ROS release in two sensory endorgans, the saccule and the utricle. The zebrafish saccule is associated with auditory function and the utricle with vestibular function. Trimmed endorgans were placed in tissue culture media with a fluorescent reactive oxygen species indicator dye, and intracellular ROS release was measured using a spectrophotometer. We found that cisplatin treatment significantly increased ROS compared to controls, but that EF-24 treatment did not alter or even decreased ROS. Importantly, when equimolar cisplatin and EF-24 treatments are combined, ROS did not increase compared to controls. This suggests that EF-24 may be able to prevent intracellular ROS caused by cisplatin treatment in inner ear tissue.
Keywords: tissue culture, zebrafish, reactive oxygen species, cisplatin, curcuminoid, inner ear
1. Introduction
The platinum-based chemotherapy compound, cis-diamminedichloridoplatinum(II) (cisplatin) (Fig. 1), is a widely-prescribed FDA-approved drug that can produce several negative side-effects including damage to auditory and vestibular tissue resulting in hearing and balance loss [1–9]. Curcumin, a naturally occurring plant compound, can act to prevent cisplatin-mediated damage to auditory tissue, although, its effects in vestibular tissue have not yet been characterized [4, 10]. However, curcumin exhibits poor solubility and bioavailability in mammals [11–12]. Therefore, there is considerable interest in discovering whether synthetic curcumin analogs (curcuminoids) with improved solubility and bioavailability can act like curcumin to prevent cisplatin-mediated damage to sensory tissue.
Figure 1.
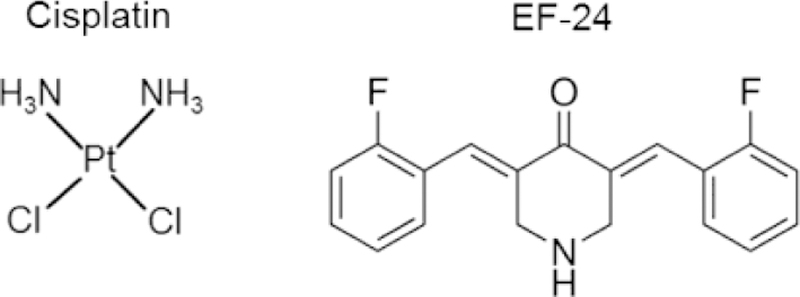
The chemical structures of the platinum-based chemotherapy compound, cisplatin, and the curcuminoid, EF-24. Cisplatin treatment can cause intracellular ROS release that damages auditory and vestibular tissue. EF-24 can affect ROS release in cancer and non-cancer cells, but its effects on ROS in auditory and vestibular tissue is not known.
Cisplatin can induce ROS release in auditory and vestibular hair cells causing these sensory cells to undergo apoptosis [5–7, 13–16]. Unlike cisplatin, curcumin can scavenge ROS, but curcumin’s efficacy is limited due to its low bioavailability [10–11, 17–20]. However, some synthetic curcuminoid compounds do not exhibit limited bioavailability [14, 21–24] and could potentially counteract intracellular ROS release. (3E,5E)-3,5-bis[(2-fluorophenyl) methylene]-4-piperidinone (EF-24) (Fig. 1) is a curcuminoid with anti-cancer efficacy that prevents reactive oxygen species damage in some tissues [25–26] and can counteract chemically-induced ROS in cancer cells [14]. These results suggested to us that EF-24 might be able to reduce ROS release in auditory and vestibular tissue treated with cisplatin.
Most auditory studies conducted in zebrafish focus on the lateral line system due to its ease of access and manipulation [27–31]. ROS release has been successfully studied in lateral line hair cells [32]. However, lateral line hair cells exhibit some differences from their mammalian counterparts including requiring mechanotransduction for cisplatin uptake [33], and functionally the lateral line is restricted to low frequency sound detection [30]. Although the zebrafish inner ear model can be used in drug-related studies [34], and it is sensitive to a broader spectrum of sound frequencies than the lateral line system [30], its use until now has been hampered by its inaccessibility.
Here, we have employed a novel zebrafish inner ear tissue culture system to test the effects of cisplatin and EF-24 treatment on intracellular ROS release. Using a spectrophotometric ROS indicator dye assay [35–36], we were able to determine the effects of cisplatin, EF-24 and combined cisplatin-EF-24 treatment on two zebrafish sensory endorgan tissues, the saccule and utricle (Fig. 2). The zebrafish saccule has primarily auditory function, and the utricle has primarily vestibular function [37–38]. We found that low (100 µM) and high (500 µM) cisplatin treatments caused significantly increased ROS release in both endorgan tissues relative to controls, but both low (100 µM) and high (500 µM) molarity EF-24 treatment either did not alter reactive oxygen species release or reduced it compared to controls. Interestingly, we found that administering equimolar concentrations of cisplatin and EF-24 did not change ROS release compared to controls, which indicates that EF-24 may counteract cisplatin-mediated ROS release in these zebrafish sensory tissues. Our results also suggest that the zebrafish inner ear tissue culture system may be a versatile new technique for assessing whether novel oto- and vestibuloprotective candidates, given in conjunction with known ototoxic therapies, modulate ROS release in sensory tissue.
Figure 2.
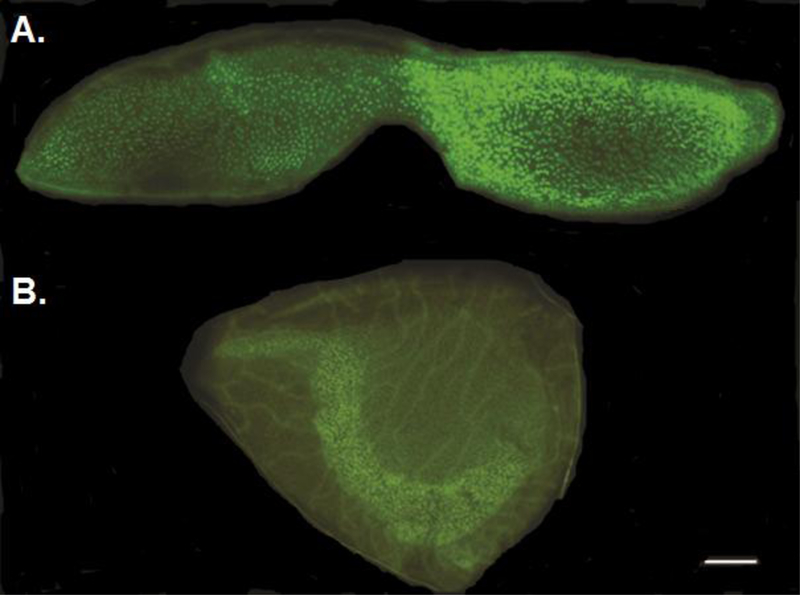
Representative samples of dissected and trimmed zebrafish auditory and vestibular endorgans. A. Saccule (auditory endorgan) stained with the nuclear marker, DAPI, and the f-actin labelling marker, Alexa Fluor 488. B. Utricle (vestibular endorgan) stained with DAPI and Alexa Fluor 488. Bar = 10 µm.
2. Material and methods
2.1. Zebrafish maintenance
Zebrafish (Danio rerio) used in this study were obtained from a commercial supplier (Segrest Farms, Gibsonton, FL) and maintained in the Western Kentucky University animal facility following protocols approved by the Institutional Animal Care and Use Committee. All zebrafish were a mix of male and female adult animals at least 6 months of age and were maintained according to standard methods [39].
2.2. Tissue sample preparation
Tissue samples were prepared according to established procedures [34, 40]. First, zebrafish were euthanized using an overdose of tricaine methanesulfonate (MS-222) (Argent, Redmond, WA) per American Veterinary Medical Association (AVMA) protocol. Then, saccules and utricles were carefully dissected out and trimmed, and set aside in filter-sterilized phosphate buffer solution (LabChem, Pittsburgh, PA) for ROS analysis. A separate set of dissected and trimmed endorgans were photographed to provide illustrative samples of saccules and utricles used in the study (Fig. 2). These samples were first stained with Alexa Fluor 488-conjugated phalloidin (1:100; Life Technologies, Eugene, OR) for 30 minutes to label filamentous actin (F-actin). Tissue samples were then placed on glass slides and nuclei were labelled with Prolong Gold antifade reagent (4’,6-diamidino-2-phenylindole [DAPI], Life Technologies, Carlsbad, CA) and a cover-slip was placed over the samples. Saccules and utricles were subsequently viewed through the FITC and DAPI filters of a Zeiss Axioplan2 epifluorescence microscope (Carl Zeiss, Jena, Germany) at 5X and 10X magnification and photographed using an AxioCam MRm camera.
2.3. ROS measurement
Sets of 3–4 wells in black plastic 96-well plates (Nunclon, Roskilde, Denmark) were prepared for either experimental, control (tissue-media-dye; media-dye; DMSO-media-dye) or blank treatments. Experimentals consisted of either 100 µM cisplatin, 500 µM cisplatin, 100 µM EF-24 or 500 µM EF-24 in L-15 media supplemented with 10 mM HEPES, 0.5% w/v bovine serum albumin fraction V, 10 nM retinoic acid, 0.4 mg/L amphotericine B (Fungizone), 200 U/ml penicillin and 200 µg/ml streptomycin, as well as 5 µM H2DCFDA indicator dye in phosphate-buffered saline (PBS). Tissue-media-dye and media-dye control treatments consisted of only L-15 media with supplementation and ROS indicator dye. DMSO-media-dye controls were prepared the same as the other controls except that DMSO concentrations equivalent to those introduced during the experiments were added. Blanks consisted of L-15 media prepared as before without dye. Stock solutions of EF-24 and H2DCFDA were initially solubilized in DMSO and then diluted 1:100 in media (EF-24) or PBS (H2DCFDA). Stock solutions of cisplatin were prepared in supplemented media. Trimmed saccule or utricle endorgans were then placed in experimental and tissue-media-dye control wells and incubated at 28 °C in a Quincy Lab (Chicago, IL) model 12–140E incubator for 45 minutes. Next, the 96-well plates were placed in a BioTek Synergy (Winooski, VT) microplate reader and read using the fluorescent mode set at 495 nm (excitation) and 527 nm (emission) wavelengths (t = 0 hours). Plates were then immediately placed back into the incubator and read again using the same spectrophotometer settings at t = 18 hours. For experiments where cisplatin and EF-24 treatments were combined, either 100 or 500 µM cisplatin was initially placed in the wells and then tissue samples were introduced. This was followed by incubation for 45 minutes and then an initial (t = 0 hours) spectrophotometer reading as before. After 3 hours, equimolar to cisplatin concentrations of EF-24 (100 or 500 µM) were introduced. Plates were read a final time at t = 18 hours using the same excitation and emission wavelengths. ROS background measured in the media-dye control samples was subtracted from the experimental and tissue-media-control values to obtain final adjusted values which were then converted to a percent of control. Unless stated otherwise, reagents were purchased from Gibco (Gaithersburg, MD), Thermo Fisher (Waltham, MA) or Sigma-Aldrich (St. Louis, MO).
2.4. Statistical analysis
GraphPad Prism v6 (La Jolla, CA) was used for all statistical analysis. Data sets were analyzed using unpaired two-tailed t tests (p ≤ 0.05).
3. Results
A fluorescent spectrophotometric assay was used to measure ROS release within zebrafish saccules and utricles following cisplatin, EF-24 and cisplatin-EF-24 combination treatments. Our control results showed that DMSO and tissue only preparations did not exhibit altered ROS release compared to media-dye only controls (data not shown). However, samples treated with 100 µM of cisplatin exhibited a statistically significant higher ROS release in both saccules (169% of control; p < 0.001) and utricles (148% of control; p < 0.05) (Fig. 3A). Tissue samples treated with a higher (500 µM) concentration of cisplatin had significantly higher ROS release than the 100 µM samples for both saccules (298% of control; p < 0.0001) and utricles (263% of control; p < 0.001) (Fig. 3B).
Figure 3.
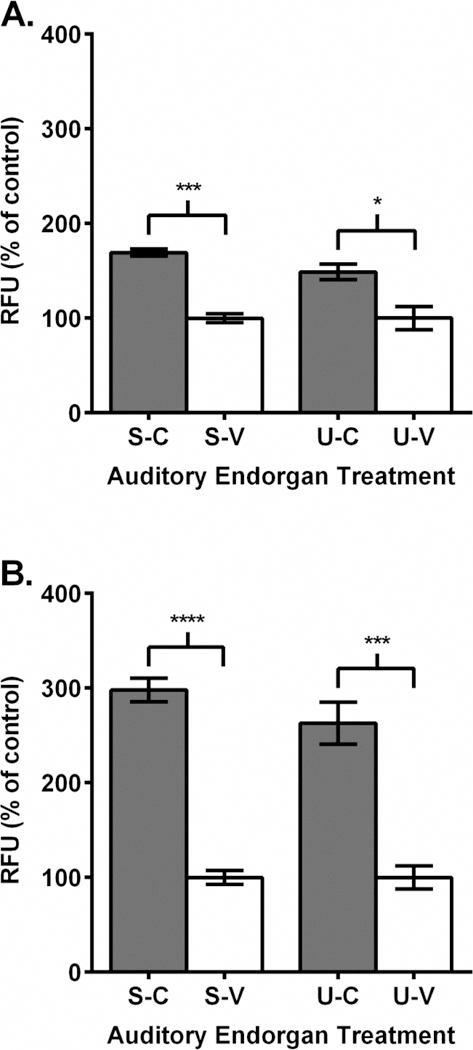
Cisplatin treatment increased reactive oxygen species in zebrafish sensory endorgans. A.-B. Endorgan treatment labeling: saccule-cisplatin (S-C), saccule-vehicle (S-V), utricle-cisplatin (U-C), utricle-vehicle (U-V). A. 100 µM cisplatin (dark gray) treatment increased ROS in tissue samples compared to vehicle (white) treatment. B. 500 µM cisplatin (dark gray) treatment increased ROS in tissue samples compared to vehicle (white) treatment. RFU = relative fluorescence units. N = 3–6; “*”, p < 0.05; “***”, p < .001; “****”, p < 0.0001.
We then investigated the effect of EF-24 treatment on ROS release in the dissected sensory endorgans. When tissue samples were treated with 100 µM of EF-24, ROS release was essentially unchanged in saccular tissue (95% of control) (Fig. 4A). In utricles, application of a 100 µM concentration caused a statistically significant decrease in ROS release (71% of control; p < 0.01) (Fig. 4A). Our 500 µM EF-24 treatments decreased ROS release by non-significant amounts in both saccules (71% of control) and utricles (87% of control) (Fig. 4B).
Figure 4.
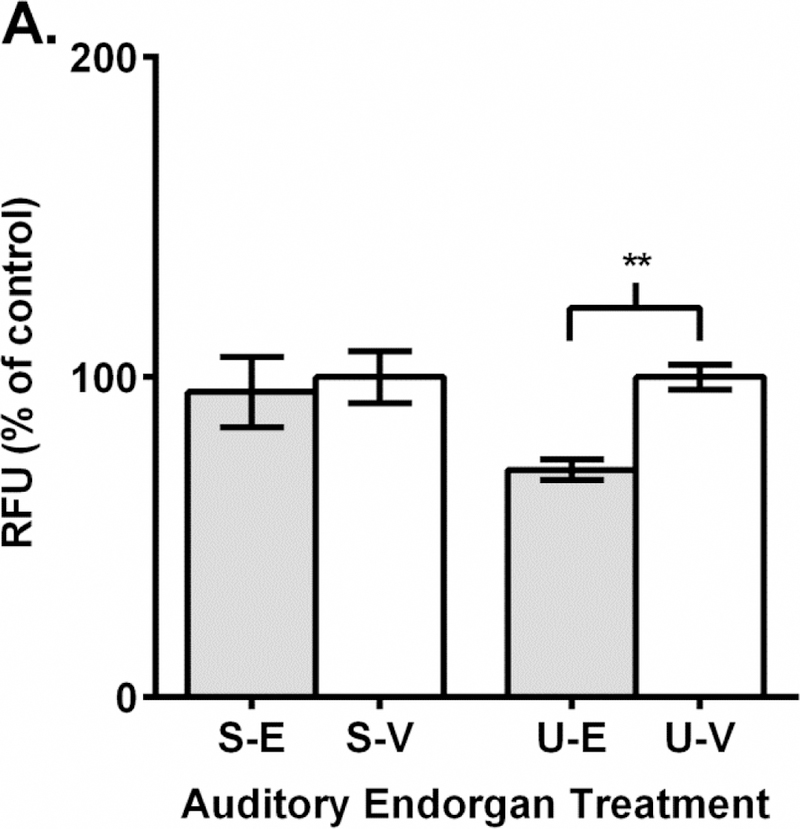
Reactive oxygen species in zebrafish sensory endorgans is unchanged or reduced after EF-24 treatment. A.-B. Endorgan treatment labelling: saccule-EF-24 (S-E), saccule-vehicle (S-V), utricle-EF-24 (U-E), utricle-vehicle (U-V). A. 100 µM EF-24 (light gray) treatment does not alter ROS in saccular tissue but decreases ROS in utricular tissue compared to vehicle (white) treatment. B. 500 µM EF-24 (light gray) treatment does not alter ROS in tissue samples compared to vehicle (white) control. RFU = relative fluorescence units. N = 3–4; p > 0.05; “**”, p < 0.01.
As a final experiment, we treated auditory endorgan samples initially with cisplatin and then an equimolar concentration of EF-24 after 3 hours had elapsed. When equimolar 100 µM treatments were introduced, ROS release in tissues increased slightly, but this was not statistically different from controls in both saccules (112% of control) and utricles (114% of control) (Fig. 5A). Similarly, ROS release in tissues exposed to combined 500 µM equimolar treatments of cisplatin and EF-24 were not significantly different from controls with saccules being equivalent (100% of control) and utricles exhibiting a slight decline (87% of control) (Fig. 5B). Overall, these results suggest that equimolar EF-24 treatment can counteract cisplatin-induced ROS release in these two zebrafish sensory endorgans.
Figure 5.
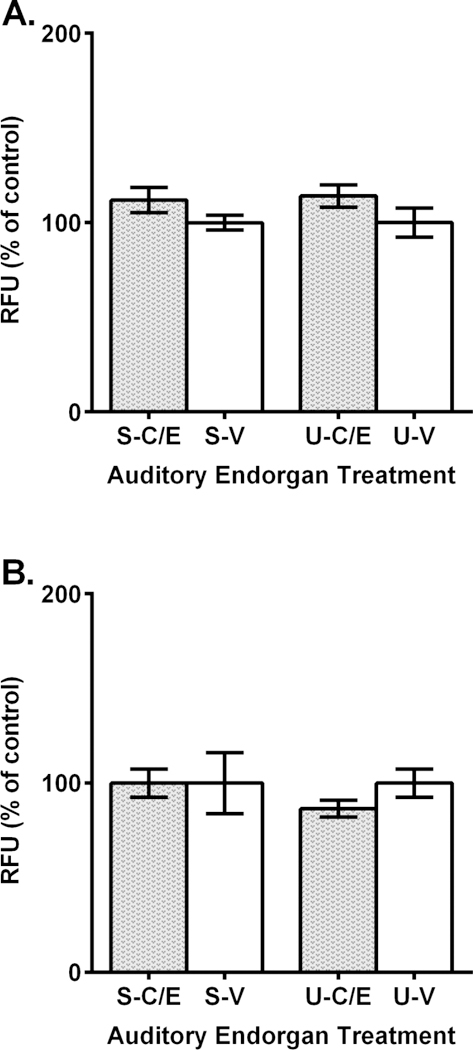
EF-24 prevents reactive oxygen species in zebrafish sensory endorgan tissues treated with cisplatin. A.-B. Endorgan treatment labelling: saccule-cisplatin and EF-24 (S-C/E), saccule-vehicle (S-V), utricle-cisplatin and EF-24 (U-C/E), utricle-vehicle (U-V). A. ROS is not significantly different than control (white) treatment when 100 µM EF-24 treatment follows 100 µM cisplatin treatment (dotted gray). B. ROS is not significantly different than control (white) treatment when 500 µM EF-24 treatment follows 500 µM cisplatin treatment (dotted gray). RFU = relative fluorescence units. N = 3; p > 0.05.
4. Discussion
In this study, we used a novel zebrafish inner ear tissue culture method to determine if the curcuminoid, EF-24, prevents reactive oxygen species release in auditory and vestibular tissue treated with the chemotherapy compound and ototoxin, cisplatin. Once in cells, cisplatin enters the nucleus where it binds to DNA, causing apoptosis and increased ROS release that can damage auditory and vestibular cells [2, 4–9, 41]. As an initial step, we investigated whether cisplatin treatment caused ROS release in zebrafish saccular and utricular tissue samples and if release was concentration dependent. We found that ROS release in zebrafish saccular and utricular tissue is significantly higher than controls when treated with 100 µM cisplatin treatment (Fig. 3A). This concentration (100 µM) is approximately double that of reported cisplatin IC50 values in some cancer cell lines [42–43] and equivalent to or below IC50 values in some cisplatin resistant cell lines, e.g., A2780/C30, MDA/CH [42–44]. Therefore, our tissue culture assay found statistically significant ROS release at cisplatin concentrations encountered in cancer cell culture experiments. Further, we found that ROS release is sharply elevated from the 100 µM result at a cisplatin concentration of 500 µM (Fig. 3B). As the H2DCFDA dye was prepared in DMSO, which can neutralize cisplatin [45], we diluted the dye 1:100 in PBS to prevent this effect. If the DMSO chemically affected cisplatin in these experiments, we would expect no or very minor changes in ROS release compared to control. However, our ROS yields with cisplatin treatment were extremely robust (148 to 169% of control at 100 µM and 263–298% of control at 500 µM), which strongly supports the interpretation that the cisplatin was intact throughout the experiments. Thus, our results suggest that the increased ROS release in the endorgan tissues was due solely from the cisplatin and follows a dosage-dependent profile.
EF-24 can act against cancer cells by increasing intracellular ROS release and enhance cisplatin’s effect against cancer [46–50]. However, EF-24 can also protect non-malignant cells from cisplatin-mediated damage [46]. The curcuminoid, 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid (CLEFMA), causes lung cancer cell death via increased intracellular reactive oxygen species release but doesn’t increase ROS release and cause damage in normal lung cells [51]. This suggested to us that EF-24 might not cause ROS release in non-cancerous auditory and vestibular tissue. We found that both lower (100 µM) and higher (500 µM) concentrations of EF-24 either did not change or even reduced ROS levels in either endorgan compared to controls (Fig. 4). This could mean that in cancer, EF-24 can signal through a mechanism that causes damaging ROS production, but that in normal sensory tissue, EF-24 does not activate a ROS-generating pathway. Additionally, like curcumin in non-cancerous tissue [52–53], our results could suggest that EF-24 functions in our auditory and vestibular tissue culture as a ROS scavenger.
Cisplatin-mediated reactive oxygen species release in auditory and vestibular tissue could be counteracted by EF-24 if the curcuminoid acts through a different pathway, pathway component or as a ROS scavenger. EF-24 and cisplatin can affect distinct antiapoptosis genes in malignant and normal mesothelioma cells [46]. Similarly, EF-24’s ROS signaling in sensory tissue could be separate from cisplatin’s and might function to counteract signals sent through cellular pathways induced by the platinum compound. This interpretation could explain why the equimolar (100 µM and 500 µM) concentrations of EF-24 and cisplatin together did not alter ROS release (Fig. 5). This result could also be explained if EF-24 and cisplatin target different components of the same ROS pathway. EF-24 can form an adduct with the antioxidant glutathione in cancer cells; whereas, cisplatin instead targets glutathione S-transferase, an enzyme that catalyzes the binding of glutathione to ROS [54]. Therefore, in auditory and vestibular tissue, EF-24 could act on a different ROS pathway component than cisplatin, and this might explain how EF-24 can prevent increased ROS generation after cisplatin treatment. The restoration of normal reactive oxygen species levels when EF-24 and cisplatin are combined (Fig. 5) could also be explained by EF-24 neutralizing cisplatin-generated ROS.
In summary, we used a fluorescent spectrophotometric assay and zebrafish sensory tissue culture to show that the curcuminoid, EF-24, can counteract ROS generation caused by the platinum-based chemotherapy compound, cisplatin. Additional work using zebrafish sensory tissue cultures in combination with a variety of fluorescent probes could elucidate if EF-24 and other curcuminoids act as effective otoprotectants against platinum-based chemotherapy compounds that cause destructive reactive oxygen species release in auditory and vestibular hair cells.
Reactive oxygen species release increases in cisplatin-treated zebrafish endorgans
Reactive oxygen species production in cisplatin only samples is dosage dependent
Curcuminoid treatment does not increase endorgan reactive oxygen species
Curcuminoid treatment counteracts cisplatin reactive oxygen species production
5. Acknowledgements
The authors wish to thank the Western Kentucky University Biotechnology Center for providing facilities support and Dr. John Andersland for assistance with microscopy. This research was supported by National Institutes of Health awards T1 R15 CA188890-01A1, 8 P20GM103436-12 KBRIN-IDeA and 2 P20 GM103436-14, a Western Kentucky University (WKU) Research and Creative Activities Program grant to MES and a WKU Faculty-Undergraduate Student Engagement grant to both MHM and BGP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sergent C, Franco N, Chapusot C, Lizard-Nacol S, Isambert N, Correia M, Chauffert B. Human colon cancer cells surviving high doses of cisplatin or oxaliplatin in vitro are not defective in DNA mismatch repair proteins. Cancer Chemother Pharmacol 2002;49:445–52. [DOI] [PubMed] [Google Scholar]
- 2.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 2007;7:3–18. [DOI] [PubMed] [Google Scholar]
- 3.Benard A, Janssen CM, van den Elsen PJ, van Eggermond MC, Hoon DS, van de Velde CJ, Kuppen PJ. Chromatin status of apoptosis genes correlates with sensitivity to chemo-, immune-and radiation therapy in colorectal cancer cell lines. Apoptosis 2014;19:1769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salehi P, Akinpelu OV, Waissbluth S, Peleva E, Meehan B, Rak J, Daniel SJ. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol Neurotol 2014;35:1131–9. [DOI] [PubMed] [Google Scholar]
- 5.Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett 2015;237:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill GW, Morest DK, Parham K. Cisplatin-Induced Ototoxicity: Effect of Intratympanic Dexamethasone Injections. Otol Neurotol 2008;29:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian CJ, Kim YJ, Kim SW, Lim HJ, Kim YS, Choung YH. A combination of cilostazol and Ginkgo biloba extract protects against cisplatin-induced Cochleo-vestibular dysfunction by inhibiting the mitochondrial apoptotic and ERK pathways. Cell Death Dis 2013;4:e509 10.1038/cddis.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Choi J, Kim N, Han GC. Behavioral changes of zebrafish according to cisplatin-induced toxicity of the balance system. Hum Exp Toxicol 2014;33:1167–75. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Cai J, Li X, Li H, Li J, Bai X, Liu W, Han Y, Xu L, Zhang D, Wang H, Fan Z. Allicin protects against cisplatin-induced vestibular dysfunction by inhibiting the apoptotic pathway. Eur J Pharmacol 2017;805:108–117. [DOI] [PubMed] [Google Scholar]
- 10.Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015;20:2728–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teiten MH, Dicato M, Diederich M. Hybrid curcumin compounds: a new strategy for cancer treatment. Molecules 2014;19:20839–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridlender M, Kapulnik Y, Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Front Plant Sci 2015;6:799 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirtes F, Albu S. Prevention and restoration of hearing loss associated with the use of cisplatin. Biomed Res Int 2014;925485 10.1155/2014/925485. [DOI] [PMC free article] [PubMed]
- 14.Tan X, Sidell N, Mancini A, Huang RP, Wang S, Horowitz IR, Liotta DC, Taylor RN,Wieser F. Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells. Reprod Sci 2010;17:931–40. [DOI] [PubMed] [Google Scholar]
- 15.Casares C, Ramírez-Camacho R, Trinidad A, Roldán A, Jorge E, García-Berrocal JR. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur Arch Otorhinolaryngol 2012;269:2455–9. [DOI] [PubMed] [Google Scholar]
- 16.Chang CW, Chen YS, Chou SH, Han CL, Chen YJ, Yang CC, Huang CY, Lo JF. Distinct subpopulations of head and neck cancer cells with different levels of intracellular reactive oxygen species exhibit diverse stemness, proliferation, and chemosensitivity. Cancer Res 2014;74:6291–305. [DOI] [PubMed] [Google Scholar]
- 17.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibellini L, Bianchini E, De Biasi S, Nasi M, Cossarizza A, Pinti M. Natural Compounds Modulating Mitochondrial Functions. Evid Based Complement Alternat Med 2015;527209 10.1155/2015/527209. [DOI] [PMC free article] [PubMed]
- 19.Mahmood K, Zia KM, Zuber M, Salman M, Anjum MN. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int J Biol Macromol 2015;81:877–90. [DOI] [PubMed] [Google Scholar]
- 20.Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res 2015;35:645–51. [PubMed] [Google Scholar]
- 21.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem 2004;12:3871–83. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TP, Hubbard RB 4th, Ehlers TJ, Arbiser JL, Goldsmith DJ, Bowen JP. Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg Med Chem 2005;13:4007–13. [DOI] [PubMed] [Google Scholar]
- 23.Modzelewska A, Pettit C, Achanta G, Davidson NE, Huang P, Khan SR. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg Med Chem 2006;14:3491–5. [DOI] [PubMed] [Google Scholar]
- 24.Lagisetty P, Vilekar P, Sahoo K, Anant S, Awasthi V. CLEFMA-an anti-proliferative curcuminoid from structure-activity relationship studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg Med Chem 2010;18:6109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav VR, Sahoo K, Roberts PR, Awasthi V. Pharmacologic suppression of inflammation by a diphenyldifluoroketone, EF24, in a rat model of fixed-volume hemorrhage improves survival. Pharmacol Exp Ther 2013;347:346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy D, Kabiraj P, Pal R. EF24 prevents rotenone-induced estrogenic status alteration in breast cancer. Cell Biol Int 2014;38:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, Raible DW. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 2010;7:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou HC, Santos F, Raible DW, Simon JA, Rubel EW. Drug screening for hearing loss: using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov Today 2010;15:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou H, Simon JA, Rubel EW, Raible DW. Screening for chemicals that affect hair cell death and survival in the zebrafish lateral line. Hear Res 2012;288:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monroe JD, Rajadinakaran G, Smith ME. Sensory hair cell death and regeneration in fishes. Front Cell Neurosci 2015;9:131 10.3389/fncel.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stawicki TM, Esterberg R, Hailey DW, Raible DW, Rubel EW. Using the zebrafish lateral line to uncover novel mechanisms of action and prevention in drug-induced hair cell death. Front Cell Neurosci 2015;9:46 10.3389/fncel.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esterberg R, Linbo T, Pickett SB, Wu P, Ou HC, Rubel EW, Raible DW. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J Clin Invest 2016;126:3556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci 2013;33:4405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uribe PM, Sun H, Wang K, Asuncion JD, Wang Q, Chen CW, Steyger PS, Smith ME, Matsui JI. Aminoglycoside-induced hair cell death of inner ear organs causes functional deficits in adult zebrafish (Danio rerio). PLoS One 2013;8(3):e58755 https://doi.org 10.1371/journal.pone.0058755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermann AC, Millard PJ, Blake SL, Kim CH. Development of a respiratory burst assay using zebrafish kidneys and embryos. J Immunol Methods 2004;292:119–29. [DOI] [PubMed] [Google Scholar]
- 36.Ng CH, Kong SM, Tiong YL, Maah MJ, Sukram N, Ahmad M, Khoo AS. Selective anticancer copper(II)-mixed ligand complexes: targeting of ROS and proteasomes. Metallomics 2014;6:892–906. [DOI] [PubMed] [Google Scholar]
- 37.Popper AN, Fay RR. Sound detection and processing by fish: critical review and major research questions. Brain Behav Evol 1993;41:14–38. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Song Q, Yu D, Yang G, Xia L, Su K, Shi H, Wang J, Yin S. Ontogenetic development of the auditory sensory organ in zebrafish (Danio rerio): changes in hearing sensitivity and related morphology. Sci Rep 2015;5:15943 10.1038/srep15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerfield M The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish 4th ed. Eugene: Institute of Neuroscience, University of Oregon; 1994. [Google Scholar]
- 40.Sun H, Lin CH, Smith ME. Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma. PLoS One 2011;6:e28372 10.1371/journal.pone.0028372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem Rev 1999;99:2467–98. [DOI] [PubMed] [Google Scholar]
- 42.Fuller TL, Canada RG. Enhancement of cisplatin cytotoxicity by terbium in cisplatin-resistant MDA/CH human breast cancer cells. Cancer Chemother Pharmacol 1999;44:249–52. [DOI] [PubMed] [Google Scholar]
- 43.Lucantoni F, Lindner AU, O‘Donovan N, Düssmann H, Prehn JHM. Systems modeling accurately predicts responses to genotoxic agents and their synergism with BCL-2 inhibitors in triple negative breast cancer cells. Cell Death and Disease 2018;9:42 10.1038/s41419-017-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bose RN, Maurmann L, Mishur RJ, Yasui L, Gupta S, Grayburn WS, Hofstetter H, Salley T. Non-DNA-binding platinum anticancer agents: Cytotoxic activities of platinum–phosphato complexes towards human ovarian cancer cells. Proc Natl Acad Sci U S A 2008;105:18314 – 18319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall MD, Telma KA, Chang KE, Lee TD, Madigan JP, Lloyd JR, Goldlust IS, Hoeschele JD, Gottesman MM. Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res 2014;74:3913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onen HI, Yilmaz A, Alp E, Celik A, Demiroz SM, Konac E, Kurul IC, Menevse ES. EF24 and RAD001 potentiates the anticancer effect of platinum-based agents in human malignant pleural mesothelioma (MSTO-211H) cells and protects nonmalignant mesothelial (MET-5A) cells. Hum Exp Toxicol 2015;34:117–26. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Zou P, Zhao Z, Chen X, Fan X, Vinothkumar R, Cui R, Wu F, Zhang Q, Liang G, Ji J. Synergistic antitumor activity of rapamycin and EF24 via increasing ROS for the treatment of gastric cancer. Redox Biol 2016;10:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou P, Xia Y, Chen W, Chen X, Ying S, Feng Z, Chen T, Ye Q, Wang Z, Qiu C, Yang S, Liang G. EF24 induces ROS-mediated apoptosis via targeting thioredoxin reductase 1 in gastric cancer cells. Oncotarget 2016;7:18050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He G, Feng C, Vinothkumar R, Chen W, Dai X, Chen X, Ye Q, Qiu C, Zhou H, Wang Y, Liang G, Xie Y, Wu W. Curcumin analog EF24 induces apoptosis via ROS-dependentmitochondrial dysfunction in human colorectal cancer cells. Cancer Chemother Pharmacol 2016;78:1151–1161. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Dai X, Zou P, Chen W, Rajamanickam V, Feng C, Zhuge W, Qiu C, Ye Q, Zhang X, Liang G. Curcuminoid EF24 enhances the anti-tumour activity of Akt inhibitor MK-2206 through ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in gastric cancer. Br J Pharmacol 2017;174:1131–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahoo K, Dozmorov MG, Anant S, Awasthi V. The curcuminoid CLEFMA selectively induces cell death in H441 lung adenocarcinoma cells via oxidative stress. Invest New Drugs 2012;30:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujisawa S, Atsumi T, Ishihara M, Kadoma Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res 2004;24:563–9. [PubMed] [Google Scholar]
- 53.Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One 2011;6:e26012 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjea D, Rybak LP. Pharmacogenomics of cisplatin-induced ototoxicity. Pharmacogenomics 2011;12:1039–50. 10.2217/pgs.11.48. [DOI] [PMC free article] [PubMed] [Google Scholar]


