Abstract
Successful episodic recollection can vary in the precision of the information recalled. The hypothesis that recollection precision requires functional neuroanatomical contributions distinct from those required for recollection success remains controversial. Some findings in individuals with hippocampal lesions have indicated that precision is dependent on the hippocampus. However, other neuroimaging and lesion studies have implicated regions outside of the mesial temporal lobe (MTL) in precision, such as parietal cortex. To further elucidate distinctions of recollection precision versus success, we examined whether they were differentially sensitive to aging and to unilateral MTL lesions. Precision and success were measured using a novel task that required memory for item-location associations across different spatial contexts. We found impairments in recollection precision, but not success, in older adults (59–80 years) relative to younger adults (18–33 years). Recollection precision was also selectively impaired in individuals with unilateral MTL resections made to treat refractory epilepsy. Moreover, recollection precision was significantly worse when resections included the hippocampus compared to when only nonhippocampal MTL tissue was resected. These findings suggest that the MTL is critically involved in the high-resolution binding required to support spatial recollection precision, and thus provide evidence for functional neuroanatomical differences between recollection success and precision.
Keywords: graded recollection, familiarity, context, memory impairment
1. Introduction
Episodic recollection is the retrieval of an event comprised of arbitrary and complex associations among individual features (Yonelinas, 2002). Recollection has typically been conceptualized as an all-or-none experience, such that individuals can either be successful or unsuccessful at recollecting an event. This is often contrasted with familiarity-based recognition, in which memory for single items can vary in strength without specific recall of event associative information (Eichenbaum, Yonelinas, & Ranganath, 2007; Yonelinas, Aly, Wang, & Koen, 2010). However, even when recollection is successful, the quality of the information that is retrieved can vary (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007; Harlow & Donaldson, 2013; Harlow & Yonelinas, 2016; Jeye, Karanian, & Slotnick, 2016; Parks, Murray, Elfman, & Yonelinas, 2011; Wilding, 2000), with highly precise and detailed memory in some cases (e.g., “the bus stop was on the left side of the street, four blocks ahead of the first stop sign”) and more general memory in others (e.g., “the bus stop was on the left side of the street”). Most studies have used paired-associative memory tests, source memory tests, or remember-know paradigms to measure recollection success, but have not objectively assessed varying levels of recollection precision. That is, typical tests of recollection cannot determine if recollection precision is functionally and/or neuroanatomically distinct from general recollection success.
Damage to the hippocampus impairs recollection (Eichenbaum, et al., 2007; Giovanello, Verfaellie, & Keane, 2003; Konkel, Warren, Duff, Tranel, & Cohen, 2008; Yonelinas, et al.,2002). Numerous studies have also demonstrated that recollection declines with age (Dulas & Duarte, 2012; McIntyre & Craik, 1987; Schacter, Kaszniak, Kihlstrom, & Valdiserri, 1991) (Craik & Rose, 2012; Koen & Yonelinas, 2016; Spencer & Raz, 1995). Age-related recollection impairments correspond to reductions in hippocampal integrity (Wolk, Dunfee, Dickerson, Aizenstein, & DeKosky, 2011) and hippocampal-cortical network connectivity (Hampstead, Khoshnoodi, Yan, Deshpande, & Sathian, 2016; Poppenk & Moscovitch, 2011). However, the tests utilized in these studies predominantly measure recollection success, without corresponding measures of precision.
Experiments involving human MTL lesions have provided some evidence that damage to the hippocampus has a greater impact on recollection precision than success. Kolarik et al. (2016) used a virtual-reality analog of the Morris water maze task (Morris, Garrud, Rawlins, & O’Keefe, 1982) in which participants were asked to explore a virtual-reality room and were trained to find and later retrieve a target location. A young adult with bilateral hippocampal damage was able to use coarse allocentric search strategies to find the target, but demonstrated significant deficits in spatial precision relative to healthy controls (Kolarik, et al., 2016). In a similar virtual-reality experiment, five amnestic patients with MTL damage demonstrated precision impairments without deficits of overall recollection success. They spent less time close to the target location relative to age-matched controls, but equal time in the correct general area (Kolarik, Baer, Shahlaie, Yonelinas, & Ekstrom, 2017). In both studies, precision, but not success, was impaired, thereby suggesting a role for the MTL and especially the hippocampus in spatial recollection precision.
However, not all results are consistent with this conclusion. One recent fMRI experiment segregated memory precision from success in younger adults. During recall, recollection success was related to hippocampal activity whereas precision was related to parietal cortex activity (Richter, Cooper, Bays, & Simons, 2016). Furthermore, two patients with bilateral parietal lobe lesions had successful autobiographical memory for general events but showed impairments when probed for specific details (Berryhill, et al., 2007). It is therefore possible that precision is supported by regions outside of the MTL, such as the parietal cortex.
Because only few studies using diverse methods have attempted to distinguish the functional neuroanatomy of recollection success from precision, it remains unclear if and how these memory processes are distinct. Furthermore, previous studies have tested spatial recollection within the same visuospatial context in which it was originally encoded. Such tests do not account for the possibility that precision and success could also be supported in part by perceptual recognition processes (Graf & Schacter, 1989; Quamme, Yonelinas, & Norman, 2007; Staresina & Davachi, 2010) rather than by relational/associative memory processes. To limit the possible contributions of perceptual memory to success and precision, we tested younger adults, older adults, and adults with unilateral MTL lesions using a memory task in which objects were studied at locations within a background context, and then later tested within a different background context. Importantly, the change in context ensured that recognition of the object-in-scene perceptual information alone could not support accurate performance. Instead, recollection precision and success were necessarily based on the arbitrary link between the object and its associated location. We hypothesized that if recollection precision and success were distinct processes, functional neuroanatomical changes associated with age would differently affect precision versus success in older adults relative to younger adults. Further, we hypothesized that lesions of the MTL, specifically those that included hippocampus, would particularly disrupt precision relative to success.
2. Methods
2.1. Participants
20 younger and 20 older right-handed adults with no history of neurological or psychiatric conditions participated in the experiment. Data from one older adult and one younger adult were excluded for poor memory performance (at least two standard deviations below overall mean performance for each group) and data from one additional younger adult participant was excluded due to computer malfunction. Thus, data from 18 younger adults (mean age=25.0, range=18–33 years, 11 females) and 19 older adults (mean age=70.57, range=59–80 years) were included in the final analyses. Adults with unilateral MTL resection, performed as a treatment for refractory epilepsy, also participated (N=8; mean age=39.63, range=22–50 years, described in Table 1). MTL patients participated approximately 3 years after resection surgery (mean=2.82, SE=0.26 years). Before surgery, after surgery, and on the day of the experiment, the Wechsler Abbreviated Scale of Intelligence (WASI-II,(Wechsler, 2008)) was administered to characterize verbal comprehension, perceptual reasoning and IQ (Table 1). All participants gave written informed consent and were monetarily compensated for their time, as approved by the Institutional Review Board at Northwestern University.
Table 1.
Unilateral MTL resection participant demographics.
| WASI-II |
|||||||
|---|---|---|---|---|---|---|---|
| ID | Age | Hemisphere | Damage | Resection | FSIQ | VCI | PRI |
| 1 | 31 | L | H- | 18.1 | 104 | 107 | 106 |
| 2 | 50 | L | H- | 2.6 | 99.6 | 103.6 | 99.6 |
| 3 | 40 | R | H- | 23.8 | 83 | 85.6 | 86.6 |
| 4 | 36 | L | H- | 3.5 | 108.3 | 107.3 | 120.3 |
| 5* | 39 | R | H- | 38.6 | 81 | 82.5 | 84.5 |
| 6* | 22 | L | H+ | 1.7 | 118.5 | 116 | 118 |
| 7 | 49 | R | H+ | 23.5 | 90.67 | 94 | 84.3 |
| 8 | 50 | L | H+ | 1.3 | 113.3 | 108.6 | 121.3 |
Each resection participant is characterized based on age, hemisphere of resection (L=Left, R=Right), whether the hippocampus was intact (H+) or removed as part of the MTL resection (H-), and resection volume in milliliters (mL) in standardized space. Mean scores from the Wechsler Abbreviated Scale of Intelligence – Second Edition (WASI-II,(Wechsler, 2008)) including the Full-Scale Intelligence Quotient (FSIQ), Verbal Comprehension Index (VCI), and Perceptual Reasoning Index (PRI).
Participants are missing post-surgery WASI-II assessment.
2.2. Memory paradigm
Participants completed two study-test blocks of an object location memory task adapted from (Bridge & Voss, 2014a, 2014b). During the study phase, participants viewed 24 objects presented at randomized locations on a specific background scene (Yue, Vessel, & Biederman, 2007) on a screen (52.0×29.25 cm), viewed with an eye-to-screen distance of ~60 cm. Objects (3.25×4.06 cm, (Moreno-Martinez & Montoro, 2012)) were presented one at a time for 3000ms each. A red dot was centered on top of each object to identify its exact location. Participants were instructed to remember the object locations as accurately as possible. After each study phase, participants played a visuo-spatial distractor task (“Tetris”) for 90 seconds. Following this filled delay, a cued recall test was administered. 24 studied objects were randomly presented one at a time in the center of the screen and participants were required to use a mouse to recall associated locations (for up to 5000ms) on a different background scene than was presented with the item during study. Distance error (the distance between the location the object was originally studied and the location the object was recalled) was our main dependent variable. The change in background scene between study and test is an important manipulation because it encourages the hippocampal-dependent process of binding independent features (object and location) into an associative event and discourages other strategies involving the perceptual unitization of the object superimposed on the entire scene (Graf & Schacter, 1989; Quamme, et al., 2007; Staresina & Davachi, 2010).
Participants completed these study-test blocks as part of a larger experimental design that also included two additional study-test blocks with a “passive” manipulation, where participants were prompted to move each object from the center of the screen to a pre-selected box, and a final recognition test. These data were not analyzed for this report as they did not contribute to our assessment of recollection success versus precision.
2.3. Behavioral analysis
Statistical analyses were done in R (Team, 2013). Trials were scored based on distance error (difference between recalled and studied locations). The threshold for recollection success was determined using two separate approaches. First, we used the geometry of the screen, and defined successful recollection as the trials recalled within the same quadrant as studied. A similar approach has also been used in other spatial memory tests of precision (Kolarik, et al., 2017; Kolarik, et al., 2016), as quadrant based success is similar to rodent spatial memory tests (Kesner & Goodrich-Hunsaker, 2010; Morris, et al., 1982). Second, we used growth-mixture modeling (as described in (Harlow & Yonelinas, 2016)) to fit distance error to a Cauchy distribution (for successful recollection) and a uniform distribution (representing random guessing). The modeling results in a mixture parameter (λ) denoting the proportion of success relative to guess. For all participants (excluding those with unilateral temporal lobe resection, N=37), the mixture-modeling approach indicated that 65.5% of trials fit the Cauchy distributions with a good fit (p=0.15). Distributions for each group demonstrate a slightly sloped guess distribution (rather than uniform flat) due to the relatively low probability that items were either studied or recalled near the corners of the rectangular screen. Although the estimation in mixture modeling is limited by relatively low trial counts (48 trials per participant in this experiment), the fit value that was obtained is consistent with that identified in other studies using similar paradigms (Harlow & Donaldson, 2013; Harlow & Yonelinas, 2016; Nilakantan, Bridge, Gagnon, VanHaerents, & Voss, 2017). Using this modeling approach, the threshold for successful recollection corresponded to 7.66 cm. Recollection precision was then measured as the mean distance error (i.e., the distance between the studied object-location and the recalled location) for trials successfully recollected. Two-sample t-tests were used to compare recollection success and recollection precision among groups. For the targeted hippocampal analysis in n=5 of left hemisphere resection participants, a Welch-two sample t-test was used, where variance is not assumed to be equal among groups of small sample sizes.
2.4. Magnetic resonance imaging
To provide anatomical characterization of the unilateral MTL lesions, MRI structural data were collected from these participants using a Siemens 3T TIM Trio whole-body magnet with a 32-channel head coil. An MPRAGE T1-weighted scans structural image (TR=2400ms, TE=3.16ms, FOV=256×256, flip angle=8°, with 1.0×1.0×1.0mm voxel resolution over 176 sagittal volumes) was acquired from each participant. Structural images were preprocessed using AFNI (Cox, 1996). Each structural image was AC-PC aligned and transformed to Talaraich-Tournoux (stereotaxic) space. Each resection was then manually drawn as a mask using the contralateral hemisphere as reference. Whole brain-volume was estimated using a manually inspected AFNI brain segmentation from the structural scan, plus the estimated volume of resected tissue.
3. Results
3.1. Is recollection precision and/or success affected by healthy aging?
Overall memory performance (mean distance error for all trials, irrespective of any success or precision distinction) was not significantly different for younger (mean=6.83, SE=0.50 cm) compared to older adults (mean=7.94, SE=0.54 cm) (T(35)=1.50, p=0.14).
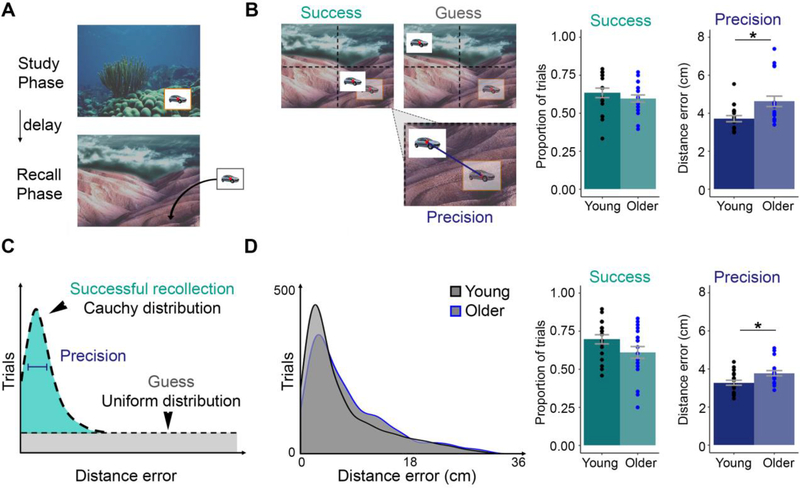
We first used the geometry of the screen to characterize recollection success and precision (Figure 1B). Recollection success was not significantly different for younger adults (mean=63.43%, SE=3.13%) compared to older adults (mean=59.64%, SE=2.39%) (T(35)=0.96, p=0.34). However, recollection precision was impaired for older adults (mean=4.63, SE=0.27 cm) relative to younger adults (mean=3.72, SE=0.16 cm) (T(35)=2.82, p=0.008).
Figure 1. Recollection precision is impaired in older adults.
(A) Participants studied trialunique objects at randomly assigned locations within a background scene. Subsequent memory testing involved object-cued recall of associated locations on a different background scene. Proportion of trials successfully recollected and mean distance error (recollection precision) of those successfully recollected trials are presented for younger and older adults determined by (B) the geometry of the screen and (C) mixture modeling (see methods). (D) Distance error distributions for each group. Individual participant scores are plotted on each bar graph as black or blue circles. Original studied locations are outlined in yellow and quadrant demarcations are for representation in the figure only.
Results were consistent when we used a mixture-modeling approach to define recollection success versus precision (Figure 1C). Recollection success did not significantly differ for younger adults (mean=69.7%, SE=2.99%) compared to older adults (mean=61.1%, SE=3.84%)(T(35)=1.75, p=0.09). Recollection precision was impaired for older adults (mean=3.78 cm, SE=0.14 cm) relative to younger adults (mean=3.28 cm, SE=0.13 cm; T(35)=2.65, p=0.01, Figure 1D).
To further establish the specificity of these age-related effects on precision, we tested the effects of aging on distance error for guess trials. Recollection precision for younger adults (mean=14.64 SE=0.45 cm) was not significantly different compared to older adults (mean=14.35 cm, SE=0.36 cm) (T(35)=0.51, p=0.61). Therefore, we found that age selectively impaired recollection precision, but not recollection success or guess distance errors.
3.2. Is MTL necessary for recollection precision and/or success?
To test the role of the MTL in recollection success and precision, we assessed memory performance in individuals with unilateral MTL resections. The amount of tissue resected varied among these participants, with most resections limited to the anterior third of the MTL (Table 1, Figure 1A). Overall memory performance (mean distance error) was marginally worse for individuals with MTL resection (mean=9.06 cm, SE=1.14 cm) relative to younger adults (mean=6.83 cm, SE=0.50 cm; T(24)=2.1, p=0.045). To use the modeling approach to dissociate recollection precision from success, the distribution of distance error must reliably fit the canonical Cauchy-uniform distribution (described in Figure 1C). However, the distribution of distance error for individuals with MTL resections were highly variable and as a group, they did not demonstrate a consistent mixed cauchy-uniform distribution of distance error. Recollection success was therefore only defined using the quadrant approach, as in other studies involving individuals with MTL lesions (Kolarik, et al., 2017; Kolarik, et al., 2016). Recollection success was not significantly different for individuals with unilateral MTL resection (mean=54.69%, SE=3.13%) than for the younger adults (T(24)=1.56, p=0.13). Recollection precision was significantly impaired for individuals with unilateral MTL resection participants (mean=5.02 cm, SE=0.59 cm) relative to the younger adults (mean=3.72 cm, SE=0.16 cm; T(24)=2.87, p=0.008; Figure 2B). Notably, although participants with unilateral MTL resections were older than younger adult controls (T(24)=5.14, p<0.001), there was a wide range of ages for MTL resection participants. Furthermore, there was no significant correlation between age and recollection precision for the MTL resection participants (r=0.139, p=0.74; Table 1), suggesting that age did not contribute significantly to the precision impairments attributed to MTL lesions.
Figure 2. Recollection precision is impaired in individuals with unilateral mesial temporal lobe (MTL) resection.
(A) Overlap map depicting resected MTL tissue (with brighter colors representing more overlap across participants). (B) Mean recollection success and recollection precision of individuals with unilateral MTL resection relative to younger adults. (C). Recollection precision for left hemisphere resection participants whose hippocampus was removed (H-) as part of the MTL resection relative to participants whose hippocampus remains intact (H+). Individual participant scores are marked in blue for MTL resection participants and in black for young adults.
We next tested whether MTL lesions that included the hippocampus were especially disruptive for precision rather than success, compared to MTL lesions that did not include the hippocampus. The right-hemisphere resection patients overall had greater amount of tissue removed and lower IQ than left-hemisphere resection patients, and so we restricted this analysis to left-lateralized (n=5) resection patients (Figure 2C). Individuals whose left MTL resections included the hippocampus (H-, n=3, mean=5.71, SE=0.27 cm) had worse precision relative to those with no hippocampal resection (H+, n=2, mean=2.64, SE=0.03 cm) (T(2.04)=11.27, p=0.007). However, recollection success did not vary significantly for H-versus H+ participants (H-: mean=45.83%, SE=4.33%; H+: mean=71.8%, SE=7.73%) (T(1.72)=3.07, p=0.11). The amount of tissue resected did not differ for the two groups (H-mean=8.06 SE=5.03 mL; H+ mean=1.53 SE=0.19 mL) (T(2.01)=1.30, p=0.32), even when corrected for estimated whole-brain volume (H-mean= 0.50, SE=0.31%; H+ mean=0.09 SE=0.38%;T(2.00)=1.32, p=0.32).
4. Discussion
We examined recollection precision and success in younger adults, older adults, and individuals with unilateral MTL resections. Our task probed the associative/relational components of precision and success by assessing object-location memory in different background contexts than were studied. The change in background scene prevents perceptual recognition strategies involving encoding the object and background scene as a single unit (Graf & Schacter, 1989; Quamme, et al., 2007; Staresina & Davachi, 2010). Older adults showed a specific impairment for recollection precision but not success, and no overall memory impairment, relative to younger adults. Precision impairment in older adults could be related to altered MTL function and structure, as many memory impairments due to age are associated with atrophy of the hippocampus, diminished structural connectivity, and altered functional connectivity of the MTL (Andrews-Hanna, et al., 2007; Bakkour, Morris, Wolk, & Dickerson, 2013; Leal & Yassa, 2013; Pini, et al., 2016), although such functional neuroanatomical changes were not measured in the present study. The necessary contribution of the MTL was assessed with individuals with unilateral surgical resections of MTL tissue. While overall performance was impaired relative to controls, precision was significantly impaired with no impairment of success. Notably, resections that included hippocampal tissue produced significantly worse precision compared to resections that included only non-hippocampal MTL tissue, with no significant difference in success. Thus, recollection success and precision were distinguished by the functional neuroanatomical changes of healthy aging as well as by MTL lesions, particularly those involving the hippocampus.
Our results are consistent with other studies of spatial episodic memory that did not limit the role of perceptual memory in success and precision. In those studies, MTL and hippocampal damage was related to impairments in recollection precision rather than in general spatial strategy or recollection success (Kolarik, et al., 2017; Kolarik, et al., 2016). There are notable caveats to our findings as well as to these previous studies. Although the change in background scene was designed to prevent perceptual recognition strategies, it could have also increased interference from the new scene background on recall performance, which could affect different memory processes (Sun, et al., 2017) and have harmed MTL-resection and older adult participants more so than younger adult participants (Fidalgo, Changoor, Page-Gould, Lee, & Barense, 2016; H. C. Watson & Lee, 2013). Furthermore, our analysis was limited by our small sample size, which included only two individuals with resections that spared the hippocampus. Evidence demonstrating a role for the hippocampus in recollection precision would be strongest in a larger cohort with comparisons to a control group with brain lesions outside of the MTL. Precision impairments due to hippocampal damage do not rule out the possibility that other regions, such as parietal cortex, make critical contributions to precision. Indeed, there is lesion (Berryhill, et al., 2007) and fMRI (Richter, et al., 2016) evidence for parietal cortex involvement in recollection precision, with the fMRI data indicating that parietal cortex might be particularly involved during memory retrieval (Richter, et al., 2016). Future studies could include additional perceptual controls, compare the effects of MTL lesions to parietal lesions on memory success versus precision, and fMRI studies in particular could determine whether these regions are differentially involved during memory formation versus retrieval.
It is important to note that tissue damage can impact large-scale network function (Gratton, Nomura, Perez, & D’Esposito, 2012), including lesions of the hippocampus (Henson, et al., 2016; Voets, et al., 2014). Likewise, although aging disproportionately impacts MTL-network function (Jagust, 2013), normal aging can involve a variety of neurological changes, including abnormal protein aggregation and distributed neurodegeneration (Jack, et al., 2013). It is therefore possible that memory precision and success are supported by different patterns of hippocampal-cortical connectivity. Support for this hypothesis comes from our previous studies in which a repetitive TMS protocol that increases functional connectivity among the hippocampus and regions of the posterior-medial parietal and occipital cortex (Wang, et al., 2014) resulted in a selective increase in recollection precision without affecting success (Nilakantan, et al., 2017). Nonetheless, the current results that hippocampal damage was particularly detrimental for precision relative to other non-hippocampal MTL tissue suggests that the hippocampus is critical for high-resolution memory (Yonelinas, 2013), although other regions are likely also involved.
The present results are also consistent with studies of visual working memory (Zhang & Luck, 2008), which demonstrate impaired high-resolution but not low-resolution memories or general memory capacity in aging (Peich, Husain, & Bays, 2013; Pertzov, Heider, Liang, & Husain, 2015) and in individuals with bilateral hippocampal damage (Koen, Borders, Petzold, & Yonelinas, 2017; P. D. Watson, Voss, Warren, Tranel, & Cohen, 2013). One short-term memory study demonstrated seemingly contradictory results, suggesting that the hippocampus is not necessarily involved in memory precision (Warren, Duff, Cohen, & Tranel, 2014). In this study, participants studied boxes shown in specific associated colors, and trials included one, three, or six boxes at a time. After a brief delay, cued with a box’s location, participants had to select the associated color using a continuous color wheel scale. A modeling approach was then used to segregate the probability that the item was remembered relative to the quality (color precision) of the item. Amnestic patients were less likely to remember items at test overall, but showed no impairment for the quality of the associated color (Warren, Duff, Cohen, & Tranel, 2014) for all trials. However, when load was matched to the other studies of precision (only one item-color association was studied at a time), four of the five amnesic patients showed no impairment of general recollection yet demonstrated reduced recollection precision relative to controls. Thus, the lack of relative precision impairment only emerged with greater loads, suggesting that precision is impaired in both amnesics and controls when high-resolution information about multiple items must be maintained (see also (Jeneson, Mauldin, & Squire, 2010; Jeneson, Wixted, Hopkins, & Squire, 2012). Although the current results are agnostic to whether short versus long retention intervals are required to observe recollection precision impairments following MTL damage, they further support the conclusion that the hippocampus is necessary to bind complex and high-resolution information (Yonelinas, 2013), and that this remains the case even when perceptual qualities of the stimulus could not alone govern precision performance.
It is also important to note that recollection is usually tested using tasks that do not explicitly measure precision versus success, and such recollection tasks are consistently impaired by hippocampal damage (Aggleton, et al., 2005; Scoville & Milner, 1957). This raises the question of why performance is affected in such tasks if hippocampal impairments are relatively specific to precision. It is possible that many of these tests involve recollection of varying degrees of qualitative information, and that precision is therefore relevant to performance even though it is not specifically measured. Furthermore, although recollection precision and success are orthogonal in theory (Bays, Catalao, & Husain, 2009) and could therefore potentially be dissociated, recollection precision depends on success in our experiment and in others that have attempted to distinguish them. That is, memory for high-resolution details are not assessed (i.e., “the bus stop is four blocks ahead of the first stop sign”) without successful recollection (i.e., “the bus stop was on the left”). Experiments that systematically address these questions are necessary to fully understand neural mechanisms for memory precision and how they might relate to those of other memory processes. Nonetheless, our results provide evidence that memory precision and success are supported by distinct functional neuroanatomy, with the MTL and the hippocampus particularly involved in recollection precision.
Highlights:
Recollection precision but not recollection success was impaired in older adults
Precision was also selectively impaired by unilateral mesial temporal resections
Precision was more impaired when resections included the hippocampus
Findings suggest functional neuroanatomical distinction of success and precision
Acknowledgements:
We thank Elise P. Gagnon, Kelly L. Polnaszek, and Irena I. Bellinski for assistance with data collection. Neuroimaging was performed at the Northwestern University Center for Translational Imaging, supported by Northwestern University Department of Radiology. This research was supported by the National Institutes of Health, National Institute on Aging [T32-AG20506 and F31-AG057109], and from the National institute of Neurological Disorders and Stroke [F32NS087885 and R00-NS069788]. The content is solely the responsibilities of the authors and does not necessarily represent the official view of the National Institute of Health.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, & Yonelinas AP (2005). Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia, 43, 1810–1823. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56, 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, & Dickerson BC (2013). The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage, 76, 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Catalao RF, & Husain M (2009). The precision of visual working memory is set by allocation of a shared resource. J Vis, 9, 7 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, & Olson IR (2007). Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J Neurosci, 27, 14415–14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge DJ, & Voss JL (2014a). Active retrieval facilitates across-episode binding by modulating the content of memory. Neuropsychologia, 63, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge DJ, & Voss JL (2014b). Hippocampal binding of novel information with dominant memory traces can support both memory stability and change. J Neurosci, 34, 22032213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Craik FI, & Rose NS (2012). Memory encoding and aging: a neurocognitive perspective. Neurosci Biobehav Rev, 36, 1729–1739. [DOI] [PubMed] [Google Scholar]
- Dulas MR, & Duarte A (2012). The effects of aging on material-independent and materialdependent neural correlates of source memory retrieval. Cereb Cortex, 22, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annu Rev Neurosci, 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo CO, Changoor AT, Page-Gould E, Lee AC, & Barense MD (2016). Early cognitive decline in older adults better predicts object than scene recognition performance. Hippocampus, 26, 1579–1592. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, & Keane MM (2003). Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cogn Affect Behav Neurosci, 3, 186–194. [DOI] [PubMed] [Google Scholar]
- Graf P, & Schacter DL (1989). Unitization and Grouping Mediate Dissociations in Memory for New Associations. Journal of Experimental Psychology-Learning Memory and Cognition, 15, 930–940. [DOI] [PubMed] [Google Scholar]
- Gratton C, Nomura EM, Perez F, & D’Esposito M (2012). Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J Cogn Neurosci, 24, 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Khoshnoodi M, Yan W, Deshpande G, & Sathian K (2016). Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. Neuroimage, 124, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow IM, & Donaldson DI (2013). Source accuracy data reveal the thresholded nature of human episodic memory. Psychon Bull Rev, 20, 318–325. [DOI] [PubMed] [Google Scholar]
- Harlow IM, & Yonelinas AP (2016). Distinguishing between the success and precision of recollection. Memory, 24, 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Greve A, Cooper E, Gregori M, Simons JS, Geerligs L, Erzinclioglu S, Kapur N, & Browne G (2016). The effects of hippocampal lesions on MRI measures of structural and functional connectivity. Hippocampus, 26, 1447–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, & Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol, 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W (2013). Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron, 77, 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, & Squire LR (2010). Intact working memory for relational information after medial temporal lobe damage. J Neurosci, 30, 13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Wixted JT, Hopkins RO, & Squire LR (2012). Visual working memory capacity and the medial temporal lobe. J Neurosci, 32, 3584–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeye BM, Karanian JM, & Slotnick SD (2016). Spatial Memory Activity Distributions Indicate the Hippocampus Operates in a Continuous Manner. Brain Sci, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, & Goodrich-Hunsaker NJ (2010). Developing an animal model of human amnesia: the role of the hippocampus. Neuropsychologia, 48, 2290–2302. [DOI] [PubMed] [Google Scholar]
- Koen JD, Borders AA, Petzold MT, & Yonelinas AP (2017). Visual short-term memory for high resolution associations is impaired in patients with medial temporal lobe damage. Hippocampus, 27, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, & Yonelinas AP (2016). Recollection, not familiarity, decreases in healthy ageing: Converging evidence from four estimation methods. Memory, 24, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik BS, Baer T, Shahlaie K, Yonelinas AP, & Ekstrom AD (2017). Close but no cigar: Spatial precision deficits following medial temporal lobe lesions provide novel insight into theoretical models of navigation and memory. Hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik BS, Shahlaie K, Hassan A, Borders AA, Kaufman KC, Gurkoff G, Yonelinas AP, & Ekstrom AD (2016). Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia, 80, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, & Cohen NJ (2008). Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci, 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, & Yassa MA (2013). Perturbations of neural circuitry in aging, mild cognitive impairment, and Alzheimer’s disease. Ageing Res Rev, 12, 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JS, & Craik FI (1987). Age differences in memory for item and source information. Can J Psychol, 41, 175–192. [DOI] [PubMed] [Google Scholar]
- Moreno-Martinez FJ, & Montoro PR (2012). An ecological alternative to Snodgrass & Vanderwart: 360 high quality colour images with norms for seven psycholinguistic variables. PLoS One, 7, e37527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, & O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Nilakantan AS, Bridge DJ, Gagnon EP, VanHaerents SA, & Voss JL (2017). Stimulation of the Posterior Cortical-Hippocampal Network Enhances Precision of Memory Recollection. Curr Biol, 27, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CM, Murray LJ, Elfman K, & Yonelinas AP (2011). Variations in recollection:the effects of complexity on source recognition. J Exp Psychol Learn Mem Cogn, 37, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peich MC, Husain M, & Bays PM (2013). Age-related decline of precision and binding in visual working memory. Psychol Aging, 28, 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertzov Y, Heider M, Liang Y, & Husain M (2015). Effects of healthy ageing on precision and binding of object location in visual short term memory. Psychol Aging, 30, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, & Frisoni GB (2016). Brain atrophy in Alzheimer’s Disease and aging. Ageing Res Rev, 30, 25–48. [DOI] [PubMed] [Google Scholar]
- Poppenk J, & Moscovitch M (2011). A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron, 72, 931–937. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, & Norman KA (2007). Effect of unitization on associative recognition in amnesia. Hippocampus, 17, 192–200. [DOI] [PubMed] [Google Scholar]
- Richter FR, Cooper RA, Bays PM, & Simons JS (2016). Distinct neural mechanisms underlie the success, precision, and vividness of episodic memory. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Kaszniak AW, Kihlstrom JF, & Valdiserri M (1991). The relation between source memory and aging. Psychol Aging, 6, 559–568. [DOI] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WD, & Raz N (1995). Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging, 10, 527–539. [DOI] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2010). Object Unitization and Associative Memory Formation Are Supported by Distinct Brain Regions. Journal of Neuroscience, 30, 9890–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SZ, Fidalgo C, Barense MD, Lee ACH, Cant JS, & Ferber S (2017). Erasing and blurring memories: The differential impact of interference on separate aspects of forgetting. J Exp Psychol Gen, 146, 1606–1630. [DOI] [PubMed] [Google Scholar]
- Team RC (2013). R: A Language and Environment for Statistical Computing. In. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Voets NL, Zamboni G, Stokes MG, Carpenter K, Stacey R, & Adcock JE (2014). Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. J Neurosci, 34, 4920–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Hermiller MS, & Voss JL (2014). Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science, 345, 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Cohen NJ, & Tranel D (2014). Hippocampus contributes to the maintenance but not the quality of visual information over time. Learn Mem, 22, 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HC, & Lee AC (2013). The perirhinal cortex and recognition memory interference. J Neurosci, 33, 4192–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, & Cohen NJ (2013). Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus, 23, 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler Abbreviated Scale of Intelligence - Second Edition (WASI-II). In. San Antonio, TX: NCS Pearson. [Google Scholar]
- Wilding EL (2000). In what way does the parietal ERP old/new effect index recollection? Int J Psychophysiol, 35, 81–87. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, & DeKosky ST (2011). A medial temporal lobe division of labor: insights from memory in aging and early Alzheimer disease. Hippocampus, 21, 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2002). The Nature of Recollection and Familiarity: A Review of 30 Years of Research. Journal of Memory and Language, 46, 441–517. [Google Scholar]
- Yonelinas AP (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res, 254, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, & Koen JD (2010). Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus, 20, 1178–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, & Knight RT (2002). Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci, 5, 1236–1241. [DOI] [PubMed] [Google Scholar]
- Yue X, Vessel EA, & Biederman I (2007). The neural basis of scene preferences. Neuroreport, 18, 525–529. [DOI] [PubMed] [Google Scholar]
- Zhang W, & Luck SJ (2008). Discrete fixed-resolution representations in visual working memory. Nature, 453, 233–235 [DOI] [PMC free article] [PubMed] [Google Scholar]