Abstract
Long-term potentiation (LTP) in the dentate gyrus was previously shown to be enhanced by nicotine, an effect dependent on both homomeric α7 and heteromeric α2β2 nicotinic acetylcholine receptors (nAChR). In our experiments, bath-applied nicotine produced no significant enhancement of LTP. The α7 nAChR silent agonist NS6740, a weak activator of α7 nAChR ion channels but an effective modulator of the cholinergic anti-in-flammatory pathway, decreased LTP and, additionally, produced a substantial reduction in the baseline synaptic function prior to the high frequency stimulation used to induce LTP. The effects of NS6740 on the various ligand-gated ion channels associated with the generation and modulation of dentate LTP were evaluated with receptors expressed in Xenopus oocytes. A 60 s pre-application of 5μM NS6740 to α7 receptors blocked the response to subsequent applications of acetylcholine (ACh). In contrast, the responses of α2β2 nAChR to control applications of ACh were not significantly affected by NS6740. Likewise, responses of cells expressing GluRl + GluR2 AMPA- type glutamate receptor subunits or GABAA αl, β2, and γ2L subunits to control agonist applications (100 μM kainic acid or 10 μM GABA, respectively), were unaffected by NS6740. The effects of NS6740 on α7 were in-consistent with simple antagonism since, while unresponsive to ACh, the receptors exposed to NS6740 were effectively activated by the positive allosteric modulator PNU-120596. The results support the hypothesis that NS6740 switches the mode of α7 signaling in a channel-independent manner that can reduce synaptic function.
Keywords: Hippocampus, Dentate gyrus, LTP, Nicotinic acetylcholine receptors, α7 Signaling, NS6740, PNU-120596
1. Introduction
The α7 subtype of nicotinic acetylcholine receptor (nAChR) has long been considered a potential therapeutic target for treating cogni-tive disorders, and α7-selective drugs have been shown to be effective at improving baseline or compromised behavior in animal models of learning and memory [5,13,27,45,51,53,55]. Drugs targeting α7 have also been identified as effective at reducing inflammation and asso-ciated pain [43,47]. While α7 channel activation appears to be im-portant for cognitive effects [8], it does not seem to be important for the modulation of inflammation [4,26,49,54]. In fact, NS6740, which is a compound with good activity in models of inflammation and pain, is an extremely weak partial agonist of α7 and is otherwise a strong de-sensitizer of α7 channel activity [32]. NS6740 is, however, able to activate large α7 ion channel currents in the presence of a positive allosteric modulator (PAM), identifying it as a “silent agonist”. The concept that there are two distinct types of α7-mediated signaling is supported by reports that NS6740 is not only ineffective in cognitive tests, it is able to block the effects of cognition-enhancing α7 channel activators, for example, the effects of the α7 agonist BMS-902483 on improving novel object recognition [44]. On its own, BMS-902483 has been also shown to increase hippocampal long-term potentiation (LTP), an effect hypothesized to be related to its positive cognitive effects.
LTP is arguably the most widely accepted neurophysiological cor-relate of learning and memory, involving long-lasting change in sy-naptic efficacy, which can be experimentally induced by high-frequency stimulation (HFS) of presynaptic axons. Mechanisms that have been shown to be important for LTP have also been demonstrated essential for spatial learning [28,41], fear conditioning [48], and passive avoidance learning [60]. LTP in the dentate gyrus of the hippocampus has been reported to be enhanced by acute application of nicotine [59]. This effect was reportedly blocked by methyllycaconitine (MLA) and absent in a7 nAChR knockout mice. However, other nAChR, including heteromeric α2β2 receptors, have also been implicated in the induction and modulation of hippocampal LTP [30,31].
Here we report the effects of NS6740 on synaptic function and plasticity in the dentate gyrus of the rat hippocampus. Using hetero-logously expressed receptors, we investigated the effects of NS6740 on several ion channel receptors associated with synaptic function in the hippocampus, including important subtypes of AMPA-sensitive glutamate receptors, GABAa receptors, and α2β2 subtypes of nAChR.
2. Methods and materials
2.1. Chemicals
NS6740 was supplied by Dr. Ganesh Thakur (Northeastern Univ., Boston MA). PNU-120596 was supplied by Professor Nicole Horenstein (University of Florida, Gainesville FL). All other chemicals and reagents were purchased from Sigma (St. Louis MO).
2.2. Animals
Procedures involving animal subjects have been reviewed and ap-proved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Male Sprague-Dawley (SD) rats (p22-p31) were obtained from colony at Harlan Sprague Dawley Inc. through the University of Florida Animal Care and Service facility. Animals were maintained on a 12:12 h light schedule, and provided ad lib access to water.
2.3. LTP experiments
LTP experiments were carried out on transverse slices of Sprague- Dawley (SD) male rats (p22-p31) as previously described [59]. Rats were anesthetized with isoflurane (Halocarbon Laboratories, River Edge, NJ) and swiftly decapitated. The brains were rapidly removed, and slices were cut at a thickness of 350 μm using a vibratome (Pelco, Redding, CA) and placed in a beaker with oxygenated high Mg2+/low Ca2+ ice-cold artificial cerebral spinal fluid (ACSF) containing (in mM) 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.5 MgSO4, 1 CaCl2, 10 D-glucose, and 25.9 NaHCO3, at room temperature for at least 2 h. The slices were then transferred to a recording chamber for submerged slices and con-tinuously superfused at a rate of 2 ml/min at 30-32 °C. The normal recording ACSF contained (in mM): 126 NaCl, 3 KCl, 1.2 NaH2PO4, 25.9 NaHCO3, 1.5 MgSO4, 2.4 CaCl2, 11 D-glucose, and 0.1 picrotoxin to block GABAA-mediated activity. Pre-synaptic stimulation was applied to the medial perforant pathway of the dentate gyrus using a concentric bipolar electrode, and field excitatory post-synaptic potentials (fEPSPs) were recorded at a frequency of 0.033 Hz from the middle one-third of the molecular layer of the dentate gyrus with a borosilicate glass electrode. The inner blade of the dentate gyrus was used in all studies. To ensure pathway specificity, paired-pulse stimuli were given (40 ms apart), and paired-pulse depression was used as the criterion to confirm correct placement in the medial perforant pathway. For all experiments, the amplitude of the test fEPSP was adjusted to 35% of the maximum. LTP was induced by HFS consisting of eight trains (each of eight stimuli at 200 Hz, intertrain interval 2 s) with the stimulation current increased during the HFS to elicit the maximum fEPSP amplitude. Nicotinic drugs were bath-applied for 10 min prior to HFS.
Recordings were performed using a MultiClamp 700A amplifier (Molecular Devices, Union City CA), digitized using a Digidata 1322A (Molecular Devices), and analyzed using Clampfit 10.3 (Molecular Devices). Values are the means ± SEM for n slices. All data were normalized to the averaged fEPSP slope for 5 min prior to HFS. Recordings were only performed in slices with the maximum fEPSP amplitude larger than 1 mV. An LTP (+) slice is defined if the fEPSP slope was > 120% of the baseline at 60 min after HFS. All data were normalized to the averaged fEPSP slope between 30 min and 10 min prior to HFS. Values are the means ± SEM for n slices. Two-tailed unpaired student’s t-tests were done using Excel (Microsoft). P < 0.05 is considered statistically significant. Comparisons were made based on the fEPSP ratios between the average of the last 5 response before the HFS and either the first 5 responses after the HFS (early LTP) or the last 5 responses of the recordings (55-60 min after the HFS, late LTP).
2.4. Heterologous expression of ligand-gated channels in Xenopus laevis oocytes
The human α7 nAChR clone was obtained from Dr. J. Lindstrom (University of Pennsylvania, Philadelphia PA), and the human α2 and β2 clones and concatamer were a gift from Dr. Ed Johnson (Astra Zeneca, Boston MA). Rat GluR1 and GluR2 clones were provided by Michael Hollmann and Steve Heinemann (Salk Institute, La Jolla CA), and rat GABAA α1, β2, and γ1L subunit clones were provided by Dr. David Weiss (Univ. Texas, San Antonio TX). The human resistance-to- cholinesterase 3 (RIC-3) clone was provided by Millet Treinin (Hebrew University, Jerusalem, Israel) and co-injected with α7 to improve the level and speed of α7 receptor expression without affecting the phar-macological properties of the receptors [19]. Subsequent to lineariza-tion and purification of the plasmid cDNAs, cRNAs were prepared using the mMessage mMachine in vitro RNA transcription kit (Ambion, Austin TX).
Oocytes were surgically removed from mature Xenopus laevis frogs (Nasco, Ft. Atkinson WI) and injected with appropriate nAChR subunit cRNAs as described previously [37]. Frogs were maintained in the Animal Care Service facility of the University of Florida, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee. In brief, the frog was first anesthetized for 15-20 min in 1.51 frog tank water containing 1 g of 3-aminobenzoate methanesulfonate buffered with sodium bicarbonate. The harvested oocytes were treated with 1.4 mg/ml collagenase (Worthington Bio-chemicals, Freehold, NJ) for 3 h at room temperature in calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, and 12 mg/l tetracycline, pH 7.6) to remove the follicular layer. Stage V oocytes were subsequently isolated and injected with 50 nl of 5-20 ng nAChR subunit cRNA. Oocytes were maintained in Barth’s solution with calcium and recordings were carried out 2-11 days after injection.
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City, CA) [37]. Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage-clamped at — 60 mV. The oocytes were bath-perfused with Ringer’s solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 μM atropine, pH 7.2) at 2 ml/min for α7 nAChR and at 4 ml/min for the other receptors tested. To evaluate the effects of NS6740 on the various receptor sub-types expressed in oocytes, control responses were defined as the average of two initial applications of a reference agonist made before NS6740 applications. The solutions were applied from a 96-well plate via disposable tips. 400 μl drug applications were 12 s in duration fol-lowed by a 181s washout period for α7 nAChR and 6 s in duration followed by a 241 s washout period for the heteromeric receptors. The reference agonist for the nAChR was acetylcholine (ACh). The ACh concentration was 60 μM for the α7 receptors. Note that α2β2 receptors can configure into two different stoichiometries, with two α subunits and three β subunits or the reverse. The use of an α — β concatamer expressed with either β or α subunit monomers allows for the con-trolled expression of either α2(2)β2(3) or α2(3)β2(2) configurations, referred to as high sensitivity (HS) or low sensitivity (LS) forms, re-spectively [39]. The α2β2 experiment was run on four cells of each configuration. The ACh control was 10 μM for the HS-expressing cells (n = 4) and 100 μM for the LS-expressing cells (n = 4). Since the nor-malized responses were equivalent, the data for the two α2β2 types were pooled. The reference agonist for the cells expressing GluR1 and GluR2 was 100 μM kanic acid, and 10 μM GABA was used for the cells expressing the α1, β2, and γ1L subunits. The oocyte responses were calculated as both peak current amplitudes and net charge, as previously described [36], and the average of the two initial controls were used for normalization purposes. Net charge responses were used for α7 [36] and peak currents for the other receptors studied.
Data were collected at 50 Hz, filtered at 20 Hz (α7) or 5 Hz (het- eromeric), and analyzed by Clampfit 9.2 or 10.0 (Molecular Devices) and Excel (Microsoft, Redmond, WA). Data were expressed as means ± SEM from at least seven oocytes for each experiment and plotted by Kaleidagraph 4.5.2 (Abelbeck Software, Reading, PA). Multi-cell averages were calculated for comparisons of complex responses. Averages of the normalized data were calculated for each of the 10,322 points in each of the 206.44 s traces (acquired at 50 Hz), as well as the standard errors for those averages.
3. Results
3.1. Nicotine effects on LTP
We hypothesized that the nicotinic modulation of LTP in the dentate gyrus would be most effectively facilitated by α7 nAChR-selective li-gands that are efficacious for ion channel activation. We first estab-lished the control LTP data in standard ACSF containing 100 μM pi-crotoxin to suppress the GABAA-mediated inhibition in the dentate gyrus. The HFS-induced control LTP attained a peak amplitude of 180 ± 4% over baseline immediately following the stimulation and declined to stable potentiation of 143 ± 6% at 60 min following HFS (n = 6, Fig. 1A & C). The effect of nicotine on LTP was then investigated by bath perfusion of 5 or 10 μM nicotine prior to HFS for 10 min. Bath application of nicotine had no effect on the baseline synaptic response. Although neither 5 μM nor 10 μM nicotine significantly increased LTP over the control values, visual inspection of the data suggest slight in-creases (Fig. 1A and B). Early potentiation was 184 ± 4% and 201 ± 6% in the presence of 5 and 10 μM nicotine, respectively, and late potentiation was 167 ± 13 and 153 ± 8% in the presence of 5 and 10 μM nicotine, respectively.
Fig. 1.
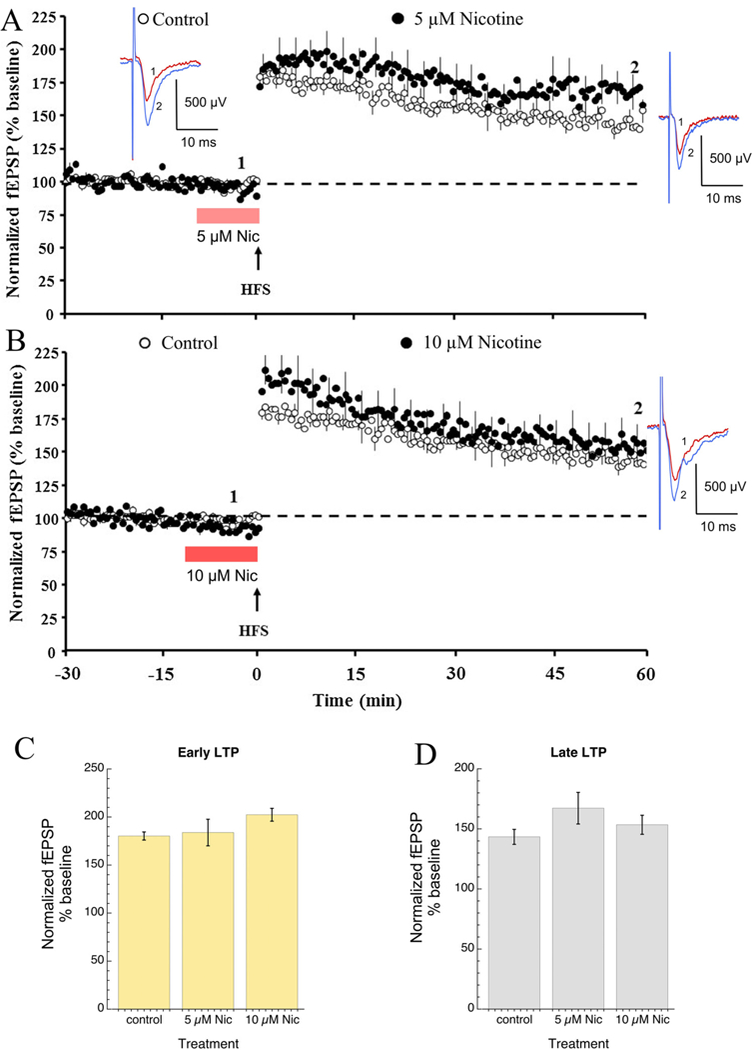
The nonselective nicotinic acetylcholine receptor (nAChR) agonist nicotine showed modest effects on long-term potentiation (LTP) induced by high frequency stimulation (HFS) in the dentate gyrus of hippocampal slices ob-tained from rats. A) The time course of the field EPSP (fEPSP) measurements obtained from dentate gyrus 30min before and 60min after HFS under the control condition (open circle, n = 6) and following the application of 5 μM nicotine (filled circles, n = 7). B) The time course of the field EPSP (fEPSP) measurements obtained from dentate gyrus 30 min before and 60 min after HFS under the control condition (open circle, n = 6) and after the application of 10 μM nicotine (filled circle, n = 7). For both experiments, LTP was induced by HFS consisting of eight trains (each of eight stimuli at 200 Hz, inter-train interval 2 s, indicated by the arrow) with the stimulation current increased during the HFS to elicit the maximum fEPSP amplitude. The periods of nicotine bath appli-cation began 10 min prior to the HFS, as in-dicated by the bars. Bath applications of nico-tine had no effect on the baseline synaptic response. Shown are representative fEPSP traces at the time points (1 and 2) indicated in the time course for baseline (blue) and 60 min following HFS (red). Each trace is an average of 5 consecutive traces before and after HFS under either control conditions or with 5 μM or 10 μM nicotine as indicated. C) Bar charts of the average ( ± SEM) early (first 5 responses after HFS) and late (55-60 min after HFS) potentiation observed under the three conditions shown in panels A & B, left and right graphs, respectively. (For interpretation of the refer-ences to colour in this figure legend, the reader is referred to the web version of this article.)
Previous studies reported the presence of high expression levels of α7 nAChRs in the rat dentate gyrus [16]. In the present study, to ex-plore the role of α7 nAChR on the effects of nicotine on LTP in the dentate gyrus we used the a7 nAChR competitive antagonist, MLA co-applied with 10 μM nicotine (Fig. 2A). MLA at 100 μM, a concentration known to be selective for α7, has previously been reported to eliminate the nicotine enhancement of LTP [59]. In our experiments bath co-application of nicotine and MLA had no effect on the baseline fEPSP. Although a visual inspection of the data (Fig. 2A) suggests a small effect of MLA compared to the results with 10 μM nicotine alone, the results were not statistically significant, probably due to the fact that we could not reproduce the previously reported robust effects of nicotine [59]. With the addition of 100 nM MLA to 10 μM nicotine there was 186 ± 7% early potentiation and 145 ± 7% late potentiation.
Fig. 2.
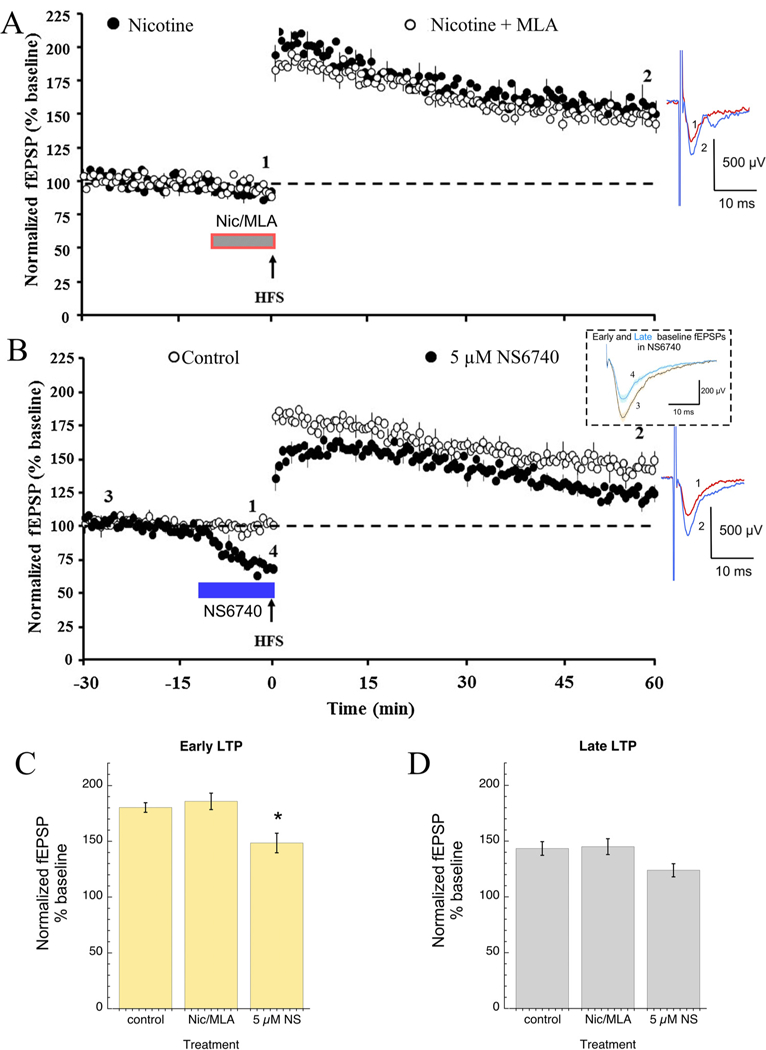
A) Effects of the selective α7 nAChR antagonist MLA co-applied with 10 μM nicotine on LTP in the dentate gyrus of hippocampal slices obtained from rats. Data from 1 B with bath applied 10 μM nicotine are shown for comparison, (open circles, n = 7) along with data when 10 μM nicotine was co-applied with 100nM MLA (filled circle, n = 7). LTP was induced by HFS (indicated by the arrow). Bath co-application of nicotine and MLA had no effect on the baseline synaptic response and LTP after the HFS was slightly reduced to the level seen on control data (Fig. 1). B) The α7 nAChR silent agonist NS6740 significantly attenuated LTP that was induced by HFS in the dentate gyrus of hippocampal slices obtained from rats. The main panel shows the time course of the fEPSP measurements obtained from dentate gyrus 30 min before and 60 min after HFS under control condition (control data from 1A above shown for comparison, open circle, n = 6) and in the presence of 5 μM NS6740 (filled circle, n = 6). The insert shows the averaged fEPSP responses of slices (n = 6) at the beginning (time point 3, black line) and at the end of the baseline period in NS6740 (time point 4, blue line). Shaded areas are the standard errors of the mean calculated for the point-by-point averages. For both experiments, LTP was induced by HFS (indicated by the arrow). The periods of drug application began 10min prior to the HFS, as indicated by the bars. Bath application of NS6740 (n = 6) both reduced the baseline synaptic response (time points 3 and 4) and decreased the LTP produced by HFS. Also shown are representative field EPSP (fEPSP) traces at the time points (1 and 2) indicated in the time course for baseline (blue) and 60 min following HFS (red). Each trace is an average of 5 consecutive traces before and after HFS. C) Bar charts of the average ( ± SEM) early (first 5 responses after HFS) and late (55-60min after HFS) potentiation observed under the three conditions shown in panels A & B, left and right graphs, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The involvement of α7 nAChR in the dentate gyrus LTP was further investigated using the α7 silent agonist NS6740 [32] (Fig. 2B). Un-expectedly, bath application of 5 μM NS6740 for 10 min prior to HFS demonstrated a significant (p < 0.001) inhibitory effect on the base-line fEPSP, reducing response amplitude by 34 ± 6% during the 10 min incubation period (see insert Fig. 2B). The addition of 5 μM NS6740 also significantly reduced early potentiation compared to controls (P < 0.05) (148 ± 8%) and this decayed to 124 ± 6% after 60 min.
3.2. NS6740 effects on specific ligand-gated ion channels expressed in Xenopus oocytes
NS6740 is an α7-selective partial agonist that is a poor channel activator but particularly effective at producing long-lived desensiti-zation [8,32,38] that can be partially reversed with PAMs. In order to confirm that the effects of NS6740 on reducing hippocampal excitatory transmission and LTP were due to α7 nAChR, we tested its effects on α7 and other channel receptors involved in hippocampal transmission ex-pressed in Xenopus oocytes. As shown in Fig. 3A, a 60 s pre-application of 5 μM NS6740 suppressed α7 response to a following ACh application. The receptors remained desensitized and showed minimal response to two follow-up ACh applications at four-minute intervals. We have previously shown that α4β2 nAChR are insensitive to NS6740 activa-tion and desensitization [32]; however, α2β2 nAChR have also been implicated in the nicotinic modulation of hippocampal LTP [30,31], so oocytes expressing high-or low-sensitivity α2β2 receptors [39] (see Methods) were tested. We also tested oocytes expressing AMPA-type glutamate receptors (GluR1 co-expressed with GluR2) [20], and GABAA receptors [10]. Using a protocol like that described for the α7-expres-sing cells shown in Fig. 3A, we determined that NS6740 had no significant effects on the agonist-evoked responses of any of these other receptor subtypes.
Fig. 3.
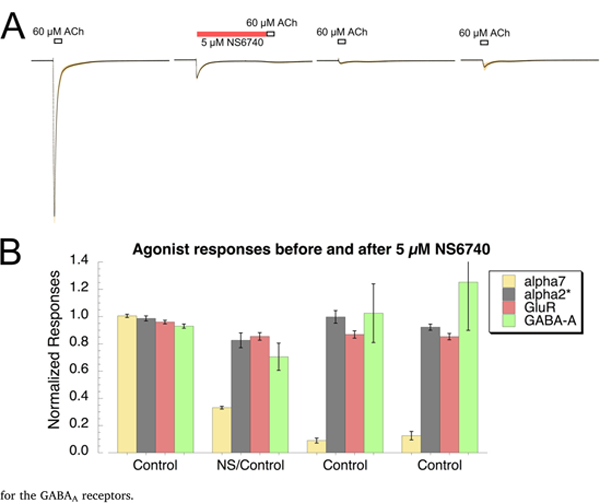
NS6740 selectively modulated α7 nAChR ligand-gated channels. A) Control applications of 60 ACh were delivered to oo-cytes expressing α7 (n = 8, second of two ACh controls shown). Subsequently, 5 μM NS6740 was applied for 60 s prior to another application of ACh. This was followed by two additional control applications of ACh at four- minute intervals. The data shown are the point-by-point averages, solid line) of the data from each cell after they were normalized to the amplitude of the initial ACh responses from each cell. The standard errors of the mean at each point are plotted as the shaded area. B) Responses of cells expressing α7 prior to and following the application of 5 μM NS6740 were compared to those of other receptors important for hippocampal physiology: α2β2 nAChR, AMPA-type glutamate receptors (cells co-expressing GluRl and GluR2), and a representative GABAa receptor (cells co-expressing al, β2, and γlL subunits) using the same application protocol illustrated in A. The α7 responses were calculated as net charge and the others as peak currents. ACh control concentrations used were 10 μM and 100 μM, respectively for the high sensitivity and low sensitivity forms of the α2β2 receptors (see Methods), 100 μM kainic acid was the control for the glutamate receptors, and 10 μM GABA
MLA, the selective α7 antagonist, decreased the effects of nicotine on LTP but did not affect baseline synaptic function, at least not in the presence of nicotine. Therefore, we used the same protocol that de-monstrated persistent desensitization of α7 by NS6740 to determine the magnitude and duration of the effects of 100 nM MLA on α7 expressed in oocytes. While a 60 s pre-application of MLA blocked the response to an initial ACh application, there was a progressive recovery of ACh responses to subsequent applications (Fig. 4A), such that the net-charge response [36] to the third ACh application shown was back up to 75 ± 5% of the original controls. As shown in Fig. 4B, when the re-ceptors were recovering from the MLA inhibition, they were insensitive to the allosteric modulator PNU-120596. This was in great contrast to receptors desensitized by a 60 s exposure to 5 μM NS6740 (Fig. 4C). Under conditions when ACh responses were only 12.6 ± 3% initial controls (Fig. 3A), an application of 10 μM PNU-120596 alone evoked responses that were 710 ± 86% the size of initial ACh controls. Note that the data in Fig. 4 are not “representative traces” but actual calculated multi-cell averages ( ± SEM).
Fig. 4.
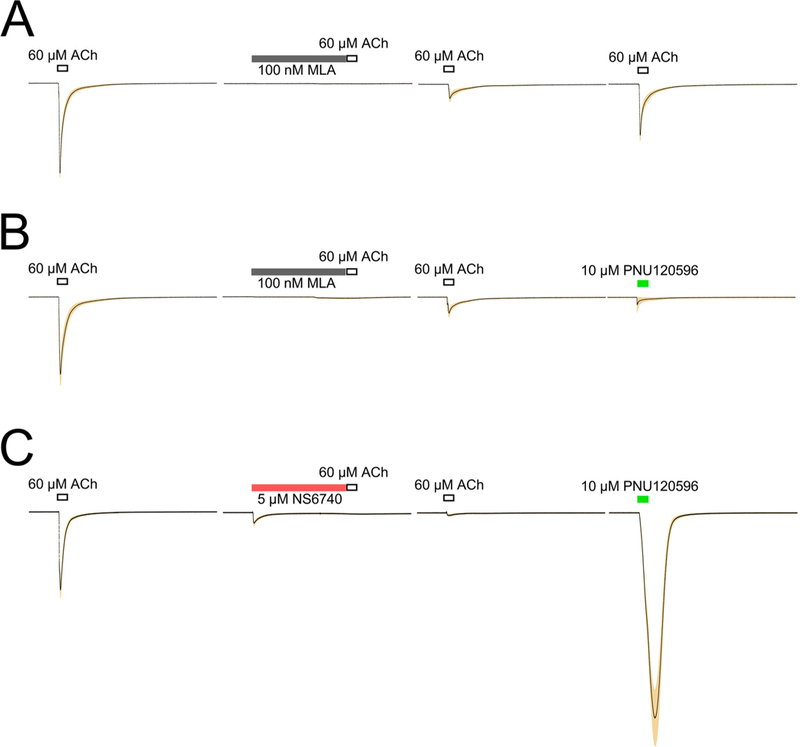
A) Responses of oocytes expressing α7 (n = 8) showing the effect of 100nM MLA on α7 receptor response to 60 μM ACh application. After the acquisition of control responses to ACh (the second of two controls is shown), MLA was applied for 60s prior to a second application of ACh. This was followed by two additional control applications of ACh at four- minute intervals. B) Responses of oocytes expressing α7 (n = 8). After the acquisition of control responses to ACh, nM MLA was applied for 60 s prior to a second application of ACh. This was followed by an application of 10 μM PNU-12096, a PAM of α7 nAChR. C) Responses of oocytes expressing a7 (n = 8) showing that the effect of NS6740 on the α7 receptors was distinct from simple inhibition produced by MLA, as shown by the ability of the PAM PNU-120596 to evoke large responses from the receptors that were unresponsive to ACh. The data in each trace are the point-by-point averages (solid line) of the data from each cell after they were normalized to the amplitude of the initial ACh responses from each cell. The standard errors of the mean at each point are plotted as the shaded area.
In the slice experiments (Fig. 1B), 100 nM MLA was present throughout the entire nicotine treatment and should have effectively antagonized α7 function without inducing desensitization. In contrast, while NS6740 would have reduced α7 channel activation, it would have induced other, potentially important, conformational states [32,38].
4. Discussion
Our experimental design was based the methods described by Welsby et al. [59], who previously reported that 5 μM nicotine pro-duced a robust enhancement of dentate LTP, with an early potentiation of 250% compared to a control of 200%, and increase at 60 min after HFS of 182% compared to 143% in the control slices. Although we saw significant LTP under all conditions (P < 0.05), we observed lower levels of LTP under control conditions and no significant effects of 5 or 10 μM nicotine compared to controls. It should be noted that nicotine is a very non-selective agonist and at concentrations greater than 1 μM would predominantly desensitize all heteromeric nAChR [40]. Like-wise, at such concentrations nicotine would produce only very transient activation of α7 nAChR and virtually no steady-state currents [35,40].
Although our data failed to reproduce the robust enhancement of LTP in the rat dentate with nicotine that was previously reported, our results with NS6740 suggest a more fundamental role for α7 receptor signaling in modulation of synaptic tone in the LTP pathway. The dis-covery of the cholinergic anti-inflammatory pathway (CAP) created an entirely new perspective on how α7 nAChR can function and be tar-geted for therapeutic effects [58]. ACh released by strong electrical stimulation of the vagus nerve was shown to stimulate an anti-inflammatory response mediated by α7 nAChR on macrophages and other cells of the immune system [6,47,57]. Interestingly, GTS-21, a selective but relatively weak partial agonist for α7 nAChR [7] has been shown in many studies to be an effective activator of CAP [9,11,22,25,46,52,62,63]. In contrast to being a weak activator of α7 ion channels, GTS-21 effectively induces prolonged desensitization of receptors that is reversible by the type II PAM PNU-120596 [35]. NS6740, like GTS-21, induces prolonged desensitization that is re-versible by PNU-120596, while stimulating so little channel activation that it has been characterized as a “silent agonist” [12,32,33]. Both GTS-21 and NS6740 were shown to effectively suppress lipopoly- saccharide (LPS) stimulated secretion of Tumor Necrosis Factor-alpha (TNF-a) by microglia cells, an in vitro model of CAP [52]. The effects of PNU-120596 in the oocyte experiments indicate that NS6740 may af-fect other receptor functions such as CAP (Cholinergic Anti-in-flammatory Pathway), consistent with the activity of this agent as modulatory of neuropathic pain [32].
Prior to work of Thomsen and Mikkelsen that demonstrated the CAP activity of NS6740, the drug first appeared in the scientific literature as a putatively inactive α7 agonist that was able to block the cognitive effects of an efficacious α7 agonist [8]. NS6740 was also recently reported to similarly antagonize the effects of BMS-902483 in a novel object recognition test [44]. In the same work, BMS-902483 was shown to enhance hippocampal LTP, an activity presumably related to cogni-tive enhancement. In the present study, we show that NS6740 does not behave as a simple antagonist of α7 but, rather, alters the fundamental behavior of α7 nAChR in the hippocampus without having effects on other types of ion channel receptors. NS6740 then appears as a key to altering the modality of α7-mediated signaling. The activity of GTS-21, NS6740, other α7 silent agonists, and allosteric modulators [1,15,17,32,34,56] in models of inflammatory disease and neuropathic pain appear to be independent of ion channel activation and, rather, depend on activation and modulation of intracellular signal transduction pathways [4,14,26,49,54]. In the case of the α7-mediated control of CAP, the cellular mediators of activity are themselves not even competent for generating nAChR channel currents, and so we must consider the complete receptor protein [50], multiple conformational states, and the complete receptor interactome [29,42] as mediators of the activity.
As we show, NS6740 does not behave like a simple receptor an-tagonist but, rather, is putting receptors into conformational states consistent with the modulation of intracellular signal transduction. There are a number of possible ways that α7-mediated signaling events might be able to alter basal synaptic function, for example, through changes in presynaptic calcium homeostasis or, alternatively, by post-synaptic changes in glutamate receptor trafficking. In any case, the present study challenges the naive assumption that all α7 ligands work by increasing intracellular calcium through ion channel activation. For most therapeutic/in vivo models this mode of action seems hardly even feasible since, in the absence of PAMs, α7 nAChR have extremely low probability of channel opening [61] and are especially ineffective at generating currents when agonist concentrations change slowly, as they would in the brain following a subcutaneous [44] or intraperitoneal injection [8]. It seems likely that the slice experiments NS6740 is down- regulating constitutive α7 ion channel receptor tone reducing tonic pre- synaptic facilitation [18].
The α7 nAChR has been considered an interesting potential target for numerous therapeutic indications over the last two decades, with GTS-21 one of the first candidates proposed for Alzheimer’s disease [2,3,23]. Unfortunately, although many α7-selective agonists have been developed [21], none have made it successfully through clinical trials for cognitive disorders, although small-scale trials continue with GTS-21 and schizophrenia [24]. Appreciation for the potential importance of the cholinergic anti-inflammatory pathway as an avenue for treating diverse indications such as asthma, arthritis, and neuropathic pain has redirected the field to search for different kinds of drugs for these different sorts of diseases, and it may be that attention will shift toward drugs like NS6740 that were once dismissed as an “anti-therapeutic” for cognition.
Acknowledgements
This work was supported by National Institutes of Health Grants GM57481 and AG052258, and the Evelyn F. McKnight Brain Research Foundation. We thank Dr. Michael King for technical assistance in the LTP experiments. We thank Dr. Ganesh Thakur (Northeastern University) for gift of the NS6740.
Abbreviations:
- ACh
acetylcholine
- ACSF
artificial cerebral spinal fluid
- CAP
cholinergic anti-inflammatory pathway
- fEPSP
field excitatory post-synaptic potentials
- HFS
High-frequency stimulation
- HS
high sensitivity
- MLA
methyllycaconitine
- LTP
long-term potentiation
- LS
low sensitivity
- nAChR
nicotinic acetylcholine receptors
- PAM
positive allosteric modulator
- SD
Sprague-Dawley
- TNF-α
tumor necrosis factor-alpha
References
- [1].Abbas M, Alzarea S, Papke RL, Rahman S, The alpha7 nicotinic acetylcholine receptor positive allosteric modulator attenuates lipopolysaccharide-induced activation of hippocampal IkappaB and CD11b gene expression in mice, Drug Discov. Ther. 11 (2017) 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arendash GW, Sengstock GJ, Sanberg PR, Kem WR, Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21, Brain Res. 674 (1995) 252–259. [DOI] [PubMed] [Google Scholar]
- [3].Azuma R, Komuro M, Korsch BH, Andre JC, Onnagawa O, Black SR, Mathews JM, Metabolism and disposition of GTS-21, a novel drug for Alzheimer’s disease, Xenobiotica 29 (1999) 747–762. [DOI] [PubMed] [Google Scholar]
- [4].Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI, New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on alpha7 nAChRs, Curr. Neuropharmacol. (2017) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G, The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo [2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2- carboxamide improves working and recognition memory in rodents, J. Pharmacol. Exp. Ther. 321 (2007) 716–725. [DOI] [PubMed] [Google Scholar]
- [6].Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin, Nature 405 (2000) 458–462. [DOI] [PubMed] [Google Scholar]
- [7].Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, Decker MW, Donnelly-Roberts D, Elliot RL, Gopalakrishnan M, Holladay MW, Hui Y, Jackson W, Kim DJB, Marsh KC, O’Neill AO, Pendergast MA, Ryther KB, Sullivan JP, Arneric SP, Functional characterization of the novel nicotinic receptor ligand GTS-21 in vitro and in vivo, Pharm. Biochem. Behav. 57 (1997) 231–241. [DOI] [PubMed] [Google Scholar]
- [8].Briggs CA, Gronlien JH, Curzon P, Timmermann DB, Ween H, Thorin- Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M, Role of channel activation in cognitive enhancement mediated by alpha7 nicotinic acetylcholine receptors, Br. J. Pharmacol. 158 (2009) 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bruchfeld A, Goldstein RS, Chavan S, Patel NB, Rosas-Ballina M, Kohn N, Qureshi AR, Tracey KJ, Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis, J. Intern. Med. 268 (2010) 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Campo-Soria C, Chang Y, Weiss DS, Mechanism of action of benzodiazepines on GABAA receptors, Br. J. Pharmacol. 148 (2006) 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chatterjee PK, Al-Abed Y, Sherry B, Metz CN, Cholinergic agonists regulate JAK2/STAT3 signaling to suppress endothelial cell activation, Am. J. Physiol. Cell Physiol. 297 (2009) C1294–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chojnacka K, Papke RL, Horenstein NA, Synthesis and evaluation of a con- ditionally-silent agonist for the alpha7 nicotinic acetylcholine receptor, Bioorg. Med. Chem. Lett. 23 (2013) 4145–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].D’Andrea MR, Nagele RG, Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer’s disease pyramidal neurons, Curr. Pharm. Des. 12 (2006) 677–684. [DOI] [PubMed] [Google Scholar]
- [14].de Jonge WJ, Ulloa L, The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation, Br. J. Pharmacol. 151 (2007) 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Donvito G, Bagdas D, Toma W, Rahimpour E, Jackson A, Meade JA, AlSharari S, Kulkarni AR, Ivy Carroll F, Lichtman AH, Papke RL, Thakur GA, Imad Damaj M, The interaction between alpha 7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-alpha represents a new antinociceptive signaling pathway in mice, Exp. Neurol. 295 (2017) 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A, Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus, J. Neurosci. 21 (2001) 7993–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Freitas K, Carroll FI, Damaj MI, The antinociceptive effects of nicotinic receptors alpha7-positive allosteric modulators in murine acute and tonic pain models, J. Pharmacol. Exp. Ther. 344 (2013) 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Girod R, Barazangi N, McGehee D, Role LW, Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors, Neuropharmacology 39 (2000) 2715–2725. [DOI] [PubMed] [Google Scholar]
- [19].Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M, Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression, J. Biol. Chem. 278 (2003) 34411–34417. [DOI] [PubMed] [Google Scholar]
- [20].Hollmann M, Hartley M, Heinemann S, Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition, Science 252 (1991) 851–853. [DOI] [PubMed] [Google Scholar]
- [21].Horenstein NA, Leonik FM, Papke RL, Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors, Mol. Pharmacol. 74 (2008) 1496–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hu Y, Liu R, Li J, Yue Y, Cheng W, Zhang P, Attenuation of Collagen-Induced Arthritis in rat by nicotinic alpha7 receptor partial agonist GTS-21, BioMed Res. Int. 2014 (2014) 325875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kem WR, The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21), Behav. Brain Res. 113 (2000) 169–181. [DOI] [PubMed] [Google Scholar]
- [24].Kem WR, Olincy A, Johnson L, Harris J, Wagner BD, Buchanan RW, Christians U, Freedman R, Pharmacokinetic limitations on effects of an alpha7 nicotinic receptor agonist in schizophrenia: randomized trial with an extended release formulation, Neuropsychopharmacology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P, GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation, Biochem. Pharmacol. 78 (2009) 863–872. [DOI] [PubMed] [Google Scholar]
- [26].Lee A, Fakler B, Kaczmarek LK, Isom LL, More than a pore: ion channel signaling complexes, J. Neurosci. 34 (2014) 15159–15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Levin ED, McClernon FJ, Rezvani AH, Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization, Psychopharmacology (Berl) 184 (2006) 523–539. [DOI] [PubMed] [Google Scholar]
- [28].Lynch MA, Long-term potentiation and memory, Physiol. Rev. 84 (2004) 87–136. [DOI] [PubMed] [Google Scholar]
- [29].Mulcahy MJ, Blattman SB, Barrantes FJ, Lukas RJ, Hawrot E, Resistance to inhibitors of cholinesterase 3 (Ric-3) expression promotes selective protein associations with the human alpha7-nicotinic acetylcholine receptor interactome, PLoS One 10 (2015) e0134409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakauchi S, Brennan RJ, Boulter J, Sumikawa K, Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors, Eur. J. Neurosci. 25 (2007) 2666–2681. [DOI] [PubMed] [Google Scholar]
- [31].Nakauchi S, Sumikawa K, Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice, Eur. J. Neurosci. 35 (2012) 1381–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari S, Thakur GA, Damaj GM, The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with nonconducting conformations of the receptor, NeuroPharm 91 (2015) 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Papke RL, Chojnacka K, Horenstein NA, The minimal pharmacophore for silent agonism of alpha7 nAChR, J. Pharmacol. Exp. Ther. 350 (2014) 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Papke RL, Horenstein NA, Kulkarni AR, Stokes C, Corrie LW, Maeng CY,Thakur A, The activity of GAT107 an allosteric activator and positive modulator of alpha7 nicotinic acetylcholine receptors (nAChR), is regulated by aromatic amino acids that span the subunit interface, J. Biol. Chem. 289 (2014) 4515–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA, Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine, J. Pharmacol. Exp. Ther. 329 (2009) 791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Papke RL, Papke JKP, Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis, Br. J. Pharm. 137 (2002) 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Papke RL, Stokes C, Working with OpusXpress: methods for high volume oocyte experiments, Methods 51 (2010) 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Papke RL, Stokes C, Damaj MI, Thakur GA, Manther K, Treinin M, Bagdas D, Kulkarni AR, Horenstein NA, Persistent activation of alpha7 nicotinic ACh receptors associated with stable induction of different desensitized states, Br. J. Pharmacol. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Papke RL, Stokes C, Muldoon P, Imad Damaj M, Similar activity of mecamyla-mine stereoisomers in vitro and in vivo, Eur. J. Pharmacol. 720 (2013) 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Papke RL, Trocme-Thibierge C, Guendisch D, Abbas Al SA, Rubaiy SA, Bloom, Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists, J. Pharmacol. Exp. Ther. 337 (2011) 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC, Storage of spatial information by the maintenance mechanism of LTP, Science 313 (2006) 1141–1144. [DOI] [PubMed] [Google Scholar]
- [42].Paulo J, Brucker W, Hawrot E, Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome, J. Proteome Res. 8 (2009) 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y, Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis, Crit. Care Med. 35 (2007) 1139–1144. [DOI] [PubMed] [Google Scholar]
- [44].Pieschl RL, Miller R, Jones KM, Post-Munson DJ, Chen P, Newberry K, Benitex Y, Molski T, Morgan D, McDonald IM, Macor JE, Olson RE, Asaka Y, Digavalli S, Easton A, Herrington J, Westphal RS, Lodge NJ, Zaczek R, Bristow LJ, Li YW, Effects of BMS-902483 an alpha7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents, Eur. J. Pharmacol. 807 (2017) 1–11. [DOI] [PubMed] [Google Scholar]
- [45].Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel OA Reneerkens, D.G. Flood, D. Hilt, M. Gawryl, S. Bertrand, Bertrand, G. Konig, EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors, Neuropharmacology 62 (2012) 1099–1110. [DOI] [PubMed] [Google Scholar]
- [46].Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ, The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE, Mol. Med. 15 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rosas-Ballina M, Tracey KJ, Cholinergic control of inflammation, J. Intern. Med. 265 (2009) 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sigurdsson T, Doyere V, Cain CK, LeDoux JE, Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory, Neuropharmacology 52 (2007) 215–227. [DOI] [PubMed] [Google Scholar]
- [49].Skok MV, To channel or not to channel? Functioning of nicotinic acetylcholine receptors in leukocytes, J. Leukoc. Biol. 86 (2009) 1–3. [DOI] [PubMed] [Google Scholar]
- [50].Stokes C, Treinin M, Papke RL, Looking below the surface of nicotinic acetylcholine receptors, Trends Pharmacol. Sci. 36 (2015) 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tatsumi R, Fujio M, Satoh H, Katayama J, Takanashi S, Hashimoto K, Tanaka, Discovery of the alpha7 nicotinic acetylcholine receptor agonists. (R)-3’- (5-Chlorothiophen-2-yl)spiro-1-azabicyclo[2.2.2]octane-3,5’-[1’,3’] oxazolidin-2’- one as a novel, potent, selective, and orally bioavailable ligand, J. Med. Chem. 48 (2005) 2678–2686. [DOI] [PubMed] [Google Scholar]
- [52].Thomsen MS, Mikkelsen JD, The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF- alpha release from microglia, J. Neuroimmunol. 251 (2012) 65–72. [DOI] [PubMed] [Google Scholar]
- [53].Tietje KR, Anderson DJ, Bitner RS, Blomme EA, Brackemeyer PJ, Briggs CA, Browman KE, Bury D, Curzon P, Drescher KU, Frost JM, Fryer RM, Fox GB, Gronlien JH, Hakerud M, Gubbins EJ, Halm S, Harris R, Helfrich RJ, Kohlhaas KL, Law D, Malysz J, Marsh KC, Martin RL, Meyer MD, Molesky AL, Nikkel AL, Otte S, Pan L, Puttfarcken PS, Radek RJ, Robb HM, Spies K Thorin-Hagene JF Waring H Ween H Xu M Gopalakrishnan W.H. Bunnelle, Preclinical characterization of A-582941: a novel alpha7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties, CNS Neurosci. Ther. 14 (2008) 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Valbuena S, Lerma J, Non-canonical signaling, the hidden life of ligand-gated ion channels, Neuron 92 (2016) 316–329. [DOI] [PubMed] [Google Scholar]
- [55].Van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R, AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors, Psychopharmacology (Berl) 172 (2004) 375–383. [DOI] [PubMed] [Google Scholar]
- [56].van Maanen MA, Papke RL, Koopman FA, Koepke J, Bevaart L, Clark R, Lamppu D Elbaum GJ LaRosa PP Tak M.J. Vervoordeldonk, Two novel alpha7 nicotinic acetylcholine receptor ligands: in vitro properties and their efficacy in collagen-induced arthritis in mice, PLoS One 10 (2015) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T, The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice, Gastroenterology 130 (2006) 1822–1830. [DOI] [PubMed] [Google Scholar]
- [58].Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ, Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation, Nature 421 (2003) 384–388. [DOI] [PubMed] [Google Scholar]
- [59].Welsby P, Rowan M, Anwyl R, Nicotinic receptor-mediated enhancement of longterm potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus, Eur. J. Neurosci. 24 (2006) 3109–3118. [DOI] [PubMed] [Google Scholar]
- [60].Whitlock JR, Heynen AJ, Shuler MG, Bear MF, Learning induces long-term potentiation in the hippocampus, Science 313 (2006) 1093–1097. [DOI] [PubMed] [Google Scholar]
- [61].Williams DK, Peng C, Kimbrell MR, Papke RL, The intrinsically low open probability of alpha7 nAChR can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures, Mol. Pharmacol. 82 (2012) 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wu S, Zhao H, Luo H, Xiao X, Zhang H, Li T, Zuo X, GTS-21, an alpha7-nicotinic acetylcholine receptor agonist, modulates Th1 differentiation in CD4T cells from patients with rheumatoid arthritis, Exp. Ther. Med. 8 (2014) 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yue Y, Liu R, Cheng W, Hu Y, Li J, Pan X, Peng J, Zhang P, GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-kappaB signaling pathway through the alpha7 nicotinic acetylcholine receptor, Int. Immunopharmacol. 15 (2015) 1–6. [DOI] [PubMed] [Google Scholar]


