Abstract
Background
Despite the large number of studies on ginseng, pharmacological activities of ginseng seed oil (GSO) have not been established. GSO is rich in unsaturated fatty acids, mostly oleic and linoleic acids. Unsaturated fatty acids are known to exert a therapeutic effect in nonalcoholic fatty liver disease (NAFLD). In this study, we investigated the protective effect and underlying mechanisms of GSO against NAFLD using in vitro and in vivo models.
Methods
In vitro lipid accumulation was induced by free fatty acid mixture in HepG2 cells and by 3 wk of high fat diet (HFD)-feeding in Sprague–Dawley rats prior to hepatocyte isolation. The effects of GSO against diet-induced hepatic steatosis were further examined in C57BL/6J mice fed a HFD for 12 wk.
Results
Oil Red O staining and intracellular triglyceride levels showed marked accumulation of lipid droplets in both HepG2 cells and rat hepatocytes, and these were attenuated by GSO treatment. In HFD-fed mice, GSO improved HFD-induced dyslipidemia and hepatic insulin resistance. Increased hepatic lipid contents were observed in HFD-fed mice and it was lowered in GSO (500 mg/kg)-treated mice by 26.4% which was evident in histological analysis. Pathway analysis of hepatic global gene expression indicated that GSO increased the expression of genes associated with β-oxidation (Ppara, Ppargc1a, Sirt1, and Cpt1a) and decreased the expression of lipogenic genes (Srebf1 and Mlxipl), and these were confirmed with reverse transcription and quantitative polymerase-chain reaction.
Conclusion
These findings suggest that GSO has a beneficial effect on NAFLD through the suppression of lipogenesis and stimulation of fatty acid degradation pathway.
Keywords: dyslipidemia, ginseng seed oil, global gene expression analysis, nonalcoholic fatty liver, triglyceride
1. Introduction
Panax ginseng is a popular traditional herbal medicine used for diseases related to fatigue and inflammation. Various phytochemical and pharmacological investigations have been performed on ginseng, and the root of ginseng is considered to be the most active part of the ginseng plant. In recent years, growing research indicates that other parts of the ginseng plant including berries, leaves, and flower buds also have potential therapeutic activities [1], [2], [3], [4]. However, there is considerably less experimental evidence regarding the medicinal properties of ginseng seeds. Compositional analyses of ginseng seeds revealed that ginseng seeds contain many unsaturated fatty acids (UFA), phytosterols, and ginsenosides [5], [6], [7], implying the need for pharmacological studies on ginseng seeds. Although the levels of fatty acids and phytosterols of ginseng seed oil (GSO) vary with extraction methods, it is consistent that the fatty acids of GSO are mostly oleic and linoleic acids, and also GSO has high levels of squalene and stigmasterol [5]. Both UFA and phytosterols have been reported for their therapeutic values in metabolic syndrome by reducing cholesterol and improving insulin sensitivity [8], [9], [10].
Nonalcoholic fatty liver disease (NAFLD) is a common form of chronic liver disease manifested with obesity, insulin resistance, and abnormal lipid profiles, and thus NAFLD is considered to be the hepatic expression of metabolic syndrome [11]. The pathological signature of NAFLD is the excessive intracellular accumulation of lipids in hepatocytes mainly due to the disruption of hepatic lipid metabolism. Current treatment options for NAFLD are insulin sensitizers such as metformin, vitamin E, ursodeoxycholic acid, and polyunsaturated fatty acids with the mainstay being lifestyle modification [12]. With the potential aid of UFA and phytosterols, we hypothesized that GSO could exert antisteatotic activity and alter the expression of genes related to lipid metabolism. Therefore, in this study, we examined the therapeutic value of GSO in vitro and in diet-induced steatotic mice, and elucidated the underlying mechanisms using global gene expression analysis.
2. Materials and methods
2.1. Materials
GSO was provided by Chungbuk Ginseng Farming Association (Chungcheongbuk-do, Korea). Cell culture reagents including Dulbecco's modified Eagle medium (DMEM) and penicillin–streptomycin were purchased from Gibco (Grand Island, NY, USA). All reagents were from Sigma (St Louis, MO, USA) unless otherwise stated.
2.2. Fatty acid analysis
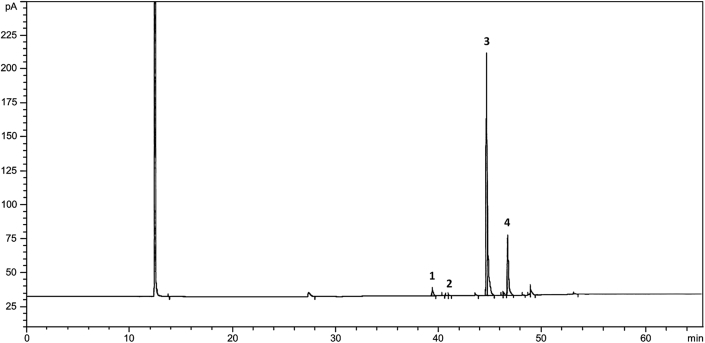
Fatty acid composition of GSO was determined using GC. The standard material for fatty acid identification was the Sulpeco 37-Component FAME mix. The capillary GC column (SP-2560; 100 m × 0.25 mm internal diameter, 0.20 μm film) was connected to a GC (Agilent 7890A; Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector. Helium was used as the carrier gas with velocity 20 cm/s. The oven temperature was held initially at 140°C for 5 min and was then increased to 240°C at a rate of 4°C/min. The chromatogram profile was detected with flame ionization at 285°C.
GSO used in this experiment was mainly composed of palmitic acid (2.01%), palmitoleic acid (0.25%), oleic acid (76.12%) and linoleic acid (17.33%; Fig. 1).
Fig. 1.
Chromatographic profile of fatty acids detected from ginseng seed oil extracted by pressing method using 5-year-old ginseng seeds. 1: palmitic acid (2.01%), 2: palmitoleic acid (0.25%), 3: oleic acid (76.12%), 4: linoleic acid (17.33%).
2.3. Cell culture and lipid accumulation
Human hepatoma cell line HepG2 was purchased from the Korean Cell Line Bank (Seoul, Korea). HepG2 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin in a 5% CO2 incubator at 37°C. To induce intracellular lipid accumulation, HepG2 cells were incubated for 24 h in DMEM containing 1mM of a mixture of free fatty acids (FFA; oleic acid:palmitic acid = 2:1) and 1% FFAs–free bovine serum albumin. For GSO experiments, HepG2 cells were treated with different concentrations of GSO as indicated 2 h prior to the addition of FFA mixture.
2.4. Isolation of primary hepatocytes
To induce hepatic lipid overload, 3-wk-old male Sprague–Dawley rats were fed a high fat diet (HFD; 60% Kcal from fat) for 3 wk. Primary hepatocytes were isolated by two-step collagenase perfusion. Rats were anesthetized with ether inhalation, then livers were perfused with ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N'-tetraacetic acid solution [10mM HEPES pH 7.8, 142mM NaCl, 6.7mM KCl, and 0.5mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N'-tetraacetic acid pH 8.0] into the hepatic portal vein at a flow rate of 20 mL/min for 20 min. Following the perfusion, hepatocytes were dissociated from the liver tissues using sterile filtered collagenase solution (100mM HEPES, 66.7mM NaCl, 6.7mM KCl, 4.8mM CaCl2, and 1 mg/mL of collagenase H; Roche, Mannheim, Germany) at a flow rate of 20 mL/min for 15 min. The hepatocytes were harvested and washed three times in serum-free DMEM and centrifuged at 50g for 3 min. Isolated hepatocytes were gently resuspended in DMEM supplemented with 10% FBS, 1% penicillin–streptomycin, 10nM insulin, and 500nM dexamethasone, and seeded at a density of 5 × 105 cells/well in collagen-coated six-well plate. The viability of the hepatocytes was examined by trypan blue exclusion. After 6-h incubation in a humidified atmosphere of 5% CO2 at 37°C, the culture media were replaced with DMEM containing 10% FBS and 1% penicillin–streptomycin. Cells were incubated for 24 h before experimentation.
2.5. Oil red O staining and triglyceride assay
HepG2 cells or rat hepatocytes were washed with phosphate-buffered saline (PBS) twice, fixed with 10% neutral-buffered formalin for 1 h, and then stained with oil red O solution for 1 h at room temperature. The stained lipid droplets in cells were examined under the microscope (Olympus CKX41, Tokyo, Japan) and photographed using an Olympus DP70 digital camera (Tokyo, Japan). Cellular oil red O was eluted with 100% isopropanol and quantified spectrophotometrically at 540 nm. For determination of intracellular triglyceride (TG) content, GSO-treated HepG2 cells or rat hepatocytes were washed twice with PBS. Cells were then scraped and centrifuged at 300g for 5 min. Cell pellets were lysed in 1% triton X-100/PBS. The concentration of intracellular TG was determined using Triglyceride Reagents (Asan Pharmaceutical Co., Seoul, Korea) according to the manufacturer's instruction, and normalized with protein concentration.
2.6. Animal experiment and serum analysis
The animal experiment protocol was approved by the Institutional Animal Care and Use Committee in Kyung Hee University [Institutional Animal Care and Use Committee approval number: KHUASP(SE)-15-069)]. Five-wk-old C57BL/6J mice (Envigo, Indianapolis, IN, USA) were housed in a temperature- (22 ± 2°C) and humidity-controlled (50 ± 5%) room with a 12/12 h light/dark cycle. Regular diet (RD; 10% kcal% fat; #D12450B) and HFD (60% kcal% fat; #D12492) experimental diets were purchased from Research Diets (New Brunswick, NJ, USA) and food and drinking water were provided to mice ad libitum. After 1 wk of acclimation, mice were randomly divided into following groups: a RD-fed group (n = 7), a HFD-fed group (n = 7), and three treatment groups (n = 8 for each group) fed a HFD plus GSO 250 mg/kg (GSO250) group, a HFD plus GSO 500 mg/kg (GSO500) group, and a HFD plus metformin 300 mg/kg group as a positive control group. Mice were orally administrated with vehicle or corresponding agents at the indicated dose once per d for 12 wk, and the body weight was measured every week. After 12 wk of treatment, the mice were fasted overnight and sacrificed for tissue and blood collections. After collection of the blood sample, the blood was allowed to clot at room temperature, and then serum was prepared by centrifugation at 2,000g for 10 min at 4°C. Serum was stored at –75°C for later use. Serum levels of total cholesterol, TG, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), alanine aminotransferase, and aspartate aminotransferase were determined using commercial kits (Stanbio Laboratory, Boerne, TX, USA) with an automated chemistry analyzer (SMARTLAB, Mannheim, Germany). The liver tissue was removed, weighed, and immediately frozen in liquid nitrogen, then stored at –75°C until future analysis.
2.7. Histological analysis and determination of lipid contents in the liver
Liver tissues were fixed in 10% neutral-buffered formalin for 48 h, embedded in paraffin wax and sectioned at 5 μm. For histological examination, sections were stained with hematoxylin and eosin and visualized using light microscope Olympus BX51 (Tokyo, Japan) equipped with Olympus DP22 digital camera (Tokyo, Japan). For the determination of hepatic lipid and TG levels, liver tissue was homogenized in extraction solution (chloroform: methanol = 2:1). Homogenates were mixed with 50mM NaCl and incubated at 4°C overnight, then centrifuged at 1,300g for 10 min at room temperature. The lower phase of extraction solution containing lipids was dried with nitrogen gas and weighed to determine the total lipid contents. The dried lipid pellet was dissolved in 1% triton X-100/PBS and used for TG content measurement using Triglyceride Reagents (Asan Pharmaceutical Co.).
2.8. RNA preparation and gene expression profiling
Total RNA was extracted from the livers of RD, HFD, and GSO500 groups using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. RNA purity and integrity were evaluated by the ratio of absorbance at 260 and 280 nm, and using Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), respectively. Two independent RNA samples of each group were used for analysis of global gene expression using Affymetrix GeneChip Mouse Gene 2.0 ST arrays (Affymetrix, Santa Clara, CA, USA). Expression data were normalized by the robust multiarray average method using oligo R package [13]. Differentially expressed genes (DEGs) were identified using Rank Product algorithm [14] based on the percentage of false prediction < 0.1. All data were analyzed using R 3.2.5 (www.r-project.org), and Kyoto Encyclopedia of Genes and Genomes pathway was visualized using log-fold change (logFC) gene expression level in Cytoscape [15]. Gene set enrichment analysis (GSEA) [16], [17] was conducted using gene sets of up- and down-DEGs between HFD and RD microarrays and the gene expression level logFC (GSO500 vs. HFD) was considered as a preranked list.
2.9. Reverse transcription and quantitative polymerase-chain reaction
The expression level of genes selected from microarray analysis was confirmed by reverse transcription–quantitative polymerase-chain reaction (RT-qPCR). Total RNA was isolated from the livers of RD, HFD, and GSO500 groups as described above. Reverse transcription was performed with 2 μg of total RNA, oligo(dT) primers (Thermo Scientific, Rockford, IL, USA), and M-MLV reverse transcriptase (Promega, Madison, WI, USA) in a total volume of 50 μL. Equal amount of complementary DNA was used for the amplification of specific target genes by qPCR using SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) in a 20 μL reaction volume with StepOnePlus Real-time PCR (Applied Biosystems, Foster City, CA, USA). The temperature profile of the reaction was 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min. The relative gene expression levels of target genes were normalized to expression of β-actin and fold change of expression was calculated using 2(–ΔΔCt) method. Oligonucleotide primer sequences used in qPCR were as follows: peroxisome proliferator activated receptor α (Ppara), sense, 5′-AACATCGAGTGTCGAATATGTGG-3′, antisense, 5′-CCGAATAG TTCGCCGAAAGAA-3′; peroxisome proliferator activated receptor γ coactivator 1α (Ppargc1a), sense, 5′-TATGGAGTGACATAGAGTGTGCT-3′, antisense, 5′-CCACTTCAATCCACCCAGAAAG-3′; sirtuin 1 (Sirt1), sense, 5′-TGATTGGCACCGATCCTCG-3′, antisense, 5′-CCACAGCGTCATATCATCCAG-3′; carnitine palmitoyltransferase 1a (Cpt1a), sense, 5′-AGATCAATCGGACCCTAGACAC-3′, antisense, 5′-CAGCGAGTAGCGCATAGTCA-3′; carnitine palmitoyltransferase (Cpt2), sense, 5′-CAAAAGACTCATCCGCTTTGTTC-3′, antisense, 5′-CATCACGACTGGGTTTGGGTA-3′; acyl-coenzyme A dehydrogenase medium chain (Acadm), sense, 5′-AACACAACACTCGAAAGCGG-3′, antisense, 5′-TTCTGCTGTTCCGTCAACTCA-3′; insulin receptor substrate 2 (Irs2), sense, 5′-ACCGACTTGGTCAGCGAAG-3′, antisense, 5′-CACGAGCCCGTAGTTGTCAT-3′; hepatic nuclear factor 4 α (Hnf4a), sense, 5′-AAGGTGCCAACCTCAATTCATC-3′, antisense, 5′-CACATTGTCGGCTAAACCTGC-3′; sterol regulatory element binding transcription factor 1 (Srebf1), sense, 5′-GATGTGCGAACTGGACACAG-3′, antisense, 5′-CATAGGGGGCGTCAAACAG-3′; MLX interacting protein-like (Mlxipl), sense, 5′-AGATGGAGAACCGACGTATCA-3′, antisense, 5′-ACTGAGCGTGCTGACAAGTC-3′; fatty acid binding protein 5 (Fabp5), sense, 5′- TGAAAGAGCTAGGAGTAGGACTG-3′, antisense, 5′-CTCTCGGTTTTGACCGTGATG-3′; tumor necrosis factor (Tnf), sense, 5′-CCCTCACACTCAGATCATCTTCT-3′, antisense, 5′-GCTACGACGTGGGCTACAG-3′; chemokine ligand 2 (Ccl2), sense, 5′-TTAAAAACCTGGATCGGAACCAA-3′, antisense, 5′-GCATTAGCTTCAGATTTACGGGT-3′, antisense, 5′-GCATTAGCTTCAGATTTACGGGT-3′; collagen type 1 α 1 (Col1a1), sense, 5′-GCTCCTCTTAGGGGCCACT-3′, antisense, 5′-CCACGTCTCACCATTGGGG-3′.
2.10. Statistical analysis
All data are expressed as mean ± standard deviation. Statistical comparison between groups was calculated by Graphpad Prism 5.01 software using one-way analysis of variance followed by Tukey's post-test. Differences with p < 0.05 were considered to be statistically significant.
3. Results
3.1. GSO suppresses intracellular lipid accumulation in HepG2 cells and rat primary hepatocytes
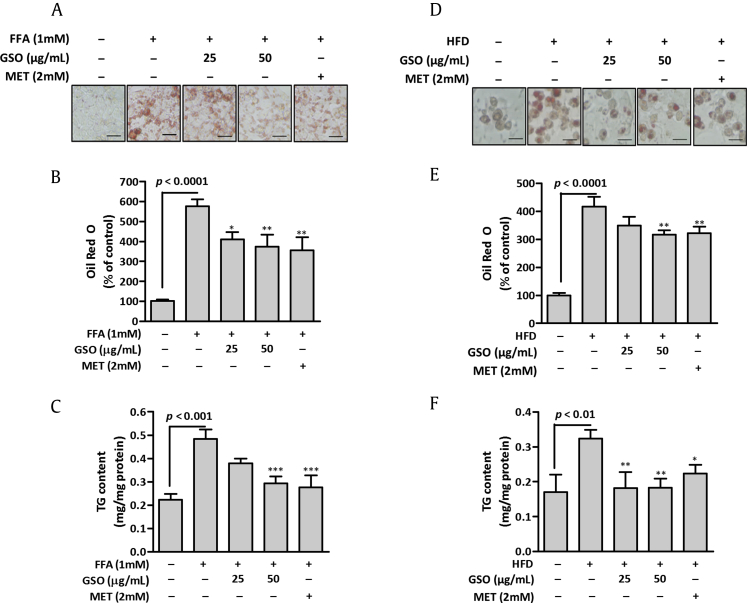
To explore the effect of GSO on hepatic lipid accumulation, cellular lipid deposits were induced by FFA supplementation and HFD-feeding in HepG2 cells and rat hepatocytes, respectively. The cellular lipids were markedly increased in the FFA-induced HepG2 cells > 5-fold compared with those in control cells. Treatment with GSO 2 h prior to FFA induction in HepG2 cells resulted in a concentration-dependent reduction in lipid accumulation examined by oil red O staining (Figs. 2A and 2B). The results were consistent when cellular TG content was quantified. TG content of GSO- (50 μg/mL) treated cells was 1.7-fold decreased compared with that of FFA-induced cells (Fig. 2C). Primary cultured rat hepatocytes after 3 wk of HFD feeding showed a marked accumulation of intracellular lipids compared to the hepatocytes from RD-fed rats. The hepatocytes were treated with different concentrations of GSO for 24 h. Oil red O staining (Fig. 2D) and spectrophotometric measurement of stained lipids (Fig. 2E) showed that 50 μg/mL of GSO treatment effectively reduced HFD-induced intracellular lipid contents. Subsequent measurement of TG contents proved concentration-dependent reduction of cellular TG upon GSO treatment in HFD-fed hepatocytes (Fig. 2F). These results demonstrate that GSO effectively decreases cellular lipid accumulation in vitro.
Fig. 2.
Effects of ginseng seed oil (GSO) on lipid accumulation in HepG2 cells and primary rat hepatocytes. (A) Oil Red O staining of HepG2 cells after free fatty acids (FFA)-induced accumulation of lipid droplets. HepG2 cells were pre-treated with GSO for 2 h and incubated with 1mM FFA for 24 h. Scale = 100 μm. (B) Relative intracellular lipid level was determined in HepG2 cells by spectrophotometric quantification of oil red O dye at 540 nm. (C) Intracellular triglyceride (TG) content was measured in HepG2 cells. (D) Oil red O staining of isolated rat hepatocytes after 3 weeks of high-fat diet (HFD) feeding. The hepatocytes were treated with GSO for 24 h as indicated. (E) Relative intracellular lipid level was determined in rat hepatocytes by spectrophotometric quantification of oil red O dye at 540 nm. (F) Intracellular TG content was measured in hepatocytes using commercial kit. Each bar represents the mean ± standard deviation of three independent experiments in triplicate.*p < 0.05; **p < 0.01 versus FFA-treated cells; ***p < 0.001 versus hepatocytes of HFD-fed rat. MET, metformin.
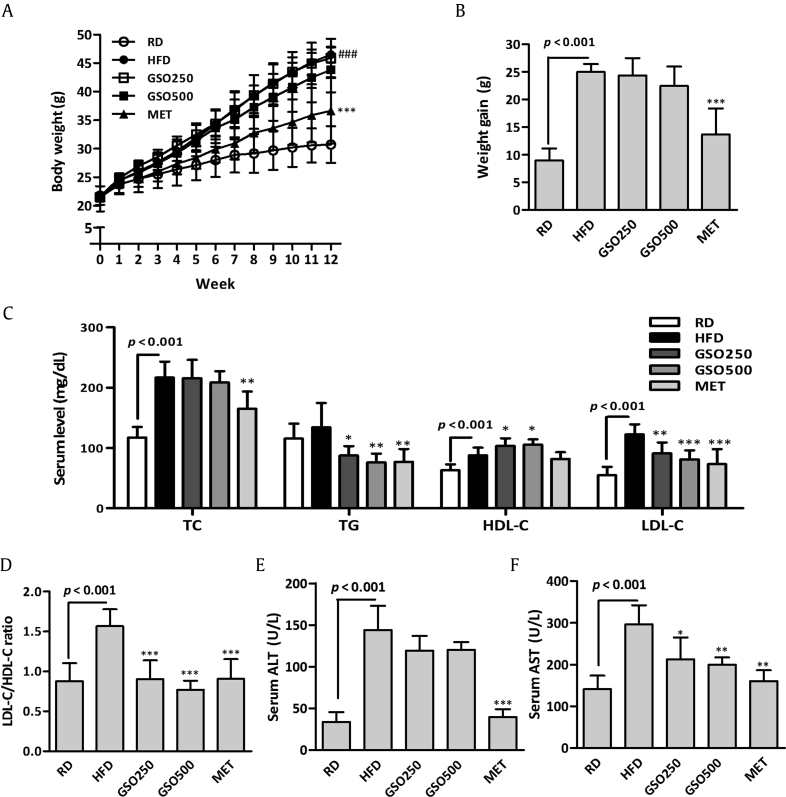
3.2. GSO ameliorates HFD-induced metabolic changes in mice
The in vivo effects of GSO were assessed in the HFD-fed C57BL/6J mice. Six-wk-old C57BL/6J mice were fed a HFD and treated with GSO at dose of 250 mg/kg or 500 mg/kg for 12 wk. No significant differences in weight gain were observed in GSO-treated mice compared to that of HFD-fed mice (Figs. 3A and 3B). Serum lipid profiles of each group are shown in Fig. 3C. Serum total cholersterol levels were significantly increased in HFD-fed mice compared with that of RD-fed mice; however, the cholesterol levels were not different between HFD and GSO groups. Although there were no significant differences between serum TG levels of RD and HFD groups, GSO-treated groups had significantly lowered TG levels compared with that of HFD group. GSO significantly increased HDL-C level compared with that of HFD group, while preventing elevation of LDL-C due to HFD feeding. The ratio of LDL-C to HDL-C was significantly reduced by 42.3% and 50.9% in GSO250 and GSO500 groups, respectively, when compared to that of HFD group (Fig. 3D). Serum aspartate aminotransferase level was decreased in mice administered with GSO in a dose dependent fashion, but serum alanine aminotransferase level was not affected by GSO administration although decreasing tendency was seen compared to those fed an HFD (Figs. 3E and 3F).
Fig. 3.
Effects of ginseng seed oil (GSO) on high-fat diet (HFD)-induced changes in C57BL/6J mice. (A, B) Weekly body weight change was measured, and the body weight gain was calculated. (C) Serum lipid profiles including total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured. (D) The ratio of LDL-C over HDL-C was calculated. (E, F) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured as liver toxicity parameters. *p < 0.05; **p < 0.01; ***p < 0.001 versus HFD. MET, metformin; RD, regular diet.
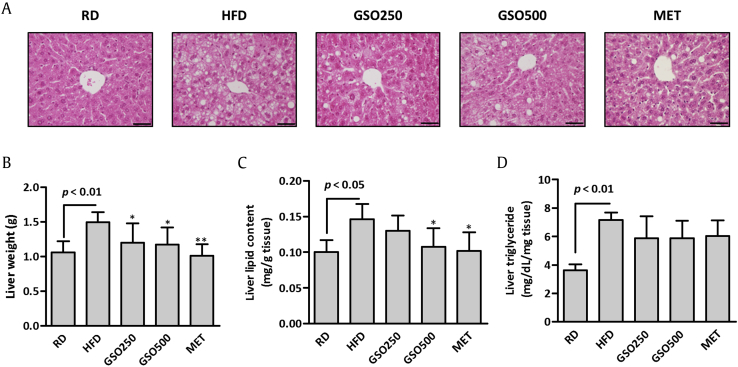
3.3. GSO prevents HFD-induced hepatic steatosis in mice
Histological analysis revealed an increased intracellular lipid deposition in the liver of HFD group (Fig. 4A). The lipid droplets were markedly reduced in the livers of GSO-treated mice. HFD feeding increased the liver weight markedly when compared with RD, and GSO significantly reduced the liver weights by 19.7% and 21.4% in HFD-fed mice (Fig. 4B). We measured lipid and TG contents in the livers of HFD- and GSO-treated mice. Hepatic lipid content was decreased by 11.0% and 26.4% in GSO250 and GSO500 groups, respectively, when compared with that of HFD group (Fig. 4C). Hepatic TG contents of GSO- and metformin-treated groups were also decreased slightly compared to that of HFD control mice, although statistical differences between groups were not observed (Fig. 4D).
Fig. 4.
Effects of ginseng seed oil (GSO) on high-fat diet (HFD)-induced hepatic lipid accumulation in mice. (A) Histological analysis of the liver by hematoxylin and eosin staining. Scale = 50 μm. (B–D) Liver weight, lipid contents and triglyceride levels were measured.∗p < 0.05; ∗∗p < 0.01 versus HFD. MET, metformin; RD, regular diet.
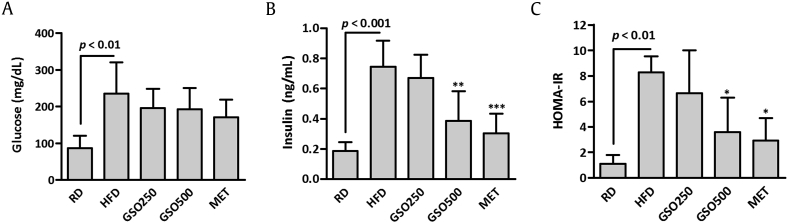
3.4. GSO prevents HFD-induced hyperinsulinemia in mice
Fasting serum glucose level was increased by 2.7-fold in HFD-fed mice in comparison to that of RD-fed mice (Fig. 5A). Elevated fasting glucose level in HFD-fed mice was decreased by 17.0% and 18.2% in GSO250 and GSO500-treated mice, respectively, although statistical significances were not seen. HFD-feeding significantly induced hyperinsulinemia in mice, and it was effectively prevented by GSO500 treatment in mice (Fig. 5B). Homeostatic model assessment of insulin resistance index of GSO500-treated mice was 56.9% lowered than that of HFD-fed mice, suggesting that GSO500 improved insulin resistance in HFD-fed mice (Fig. 5C).
Fig. 5.
Effects of ginseng seed oil on high-fat diet (HFD)-induced insulin resistance in mice. (A) Fasting glucose and (B) insulin levels were measured in the serum of mice. (C) Homeostatic Model Assessment of Insulin Resistance Index (HOMA-IR)was calculated using the following formula: fasting insulin (μU/L) × fasting glucose (nmol/L)/22.5.*p < 0.05; **p < 0.01; ***p < 0.001 versus HFD.GSO, ginseng seed oil; MET, metformin; RD, regular diet.
3.5. GSO altered the expression of lipid metabolic genes in the liver of HFD-fed mice
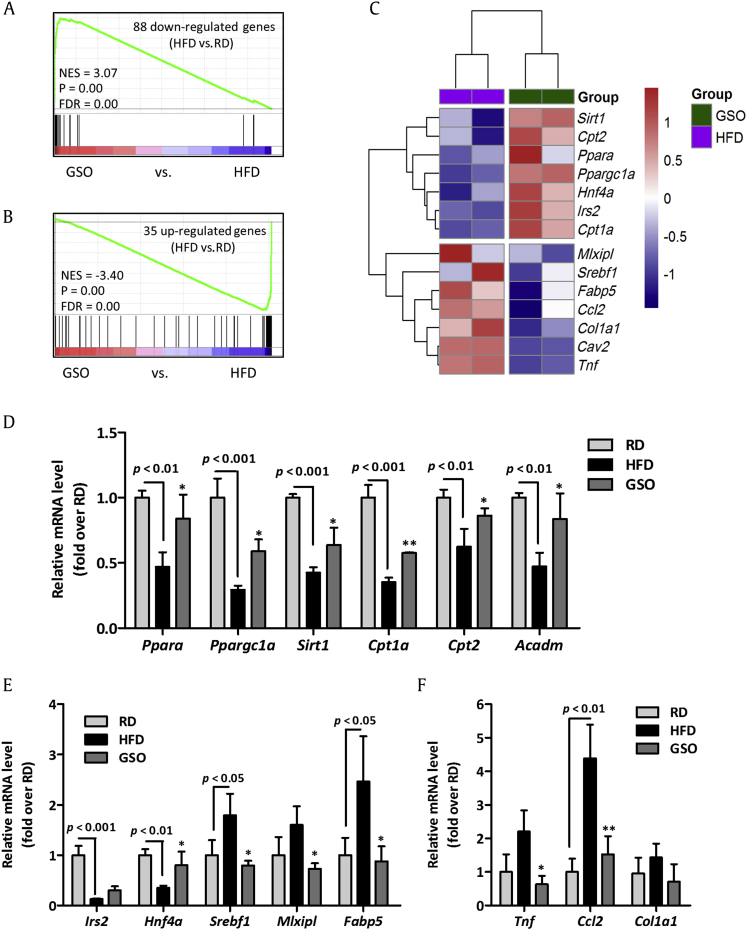
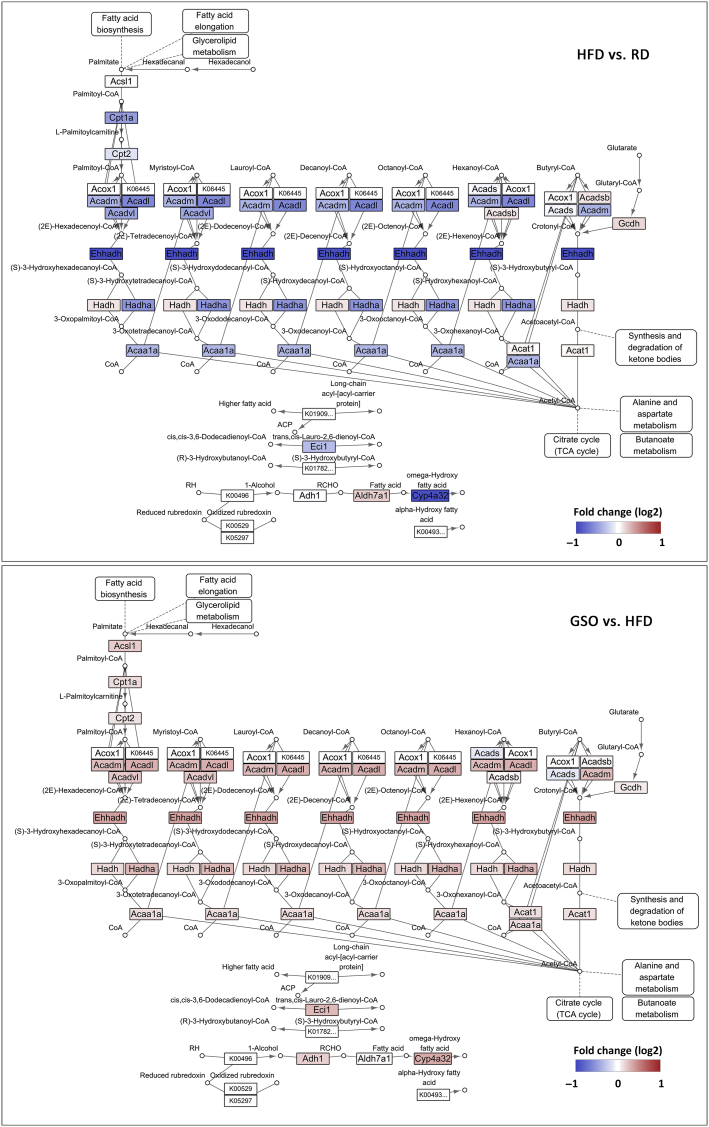
To further investigate the transcriptional changes by which GSO affects hepatic steatosis, we performed microarray analysis using complementary DNA extracted from livers of RD, HFD, and GSO500 groups. DEG analysis identified 35 genes were upregulated and 88 genes were downregulated by HFD as compared to the gene expression levels of RD. These DEGs were used for GSEA analysis within DEGs between GSO and HFD. Genes that were downregulated by HFD feeding were highly enriched among genes that were upregulated by GSO treatment (Fig. 6A). Also, the upregulated genes in HFD-fed mice compared with RD-fed mice showed significant enrichment among the downregulated genes in response to GSO feeding (Fig. 6B). The microarray analysis showed that GSO enhanced hepatic expression of genes responsible for lipid metabolism (Sirt1, Hnf4a, Irs2, Ppara, Ppargc1a, Cpt1a, and Cpt2) while suppressing the expression of lipogenic genes (Srebf1, Mlxipl, Fabp5, and Cav2) and inflammation-associated genes (Tnf, Ccl2, and Col1a1) when compared with their expression in the HFD group (Fig. 6C). The target genes of GSO selected from microarray analysis were validated by RT-qPCR. In line with the microarray data, the genes involved in β-oxidation, lipogenesis, and hepatic inflammation were regulated by GSO500 treatment (Figs. 6D–6F). Kyoto Encyclopedia of Genes and Genomes pathway analysis revealed that most of the genes encoding enzymes involved in fatty acid degradation were downregulated in the liver of HFD-fed mice compared to that of RD-fed mice (Fig. 7). The downregulated fatty acid degradation pathway in HFD mice was reversed in GSO-treated mice with increased expression of genes including Acadl, Acadm, Acadvl, Ehhadh, Hadh, Hadha, and Acaa1a.
Fig. 6.
Effects of ginseng seed oil (GSO) on high-fat diet (HFD)-induced hepatic gene expression in mice. (A) Gene set enrichment analysis plot of 88 genes significantly down-regulated by HFD feeding within genes ranked log2-fold change in GSO versus HFD. (B) Gene set enrichment analysis of 35 upregulated genes in HFD versus regular diet (RD) as compared to genes ranked log2-fold change in GSO versus HFD. (C) Heatmap displaying expression level of genes involved in lipid metabolism and hepatic inflammation. Genes up- and down-regulated are depicted in red and blue, respectively. Reverse transcription–quantitative polymerase-chain reaction validation was performed on the target genes of GSO involved in (D) β-oxidation, (F) lipogenesis, and (F) hepatic inflammation.*p < 0.05; **p < 0.01 versus HFD. FDR, false discovery rate; mRNA, messenger RNA; NES, normalized enrichment score.
Fig. 7.
Expression pattern of genes related to fatty acid degradation pathway (Kyoto Encyclopedia of Genes and Genomes entry: mmu00071) in the liver from ginseng seed oil-treated mice. Genes up- and downregulated are depicted in red and blue, respectively. HFD, high fat diet; RD, regular diet; TCA, tricarboxylic acid.
4. Discussion
The present study has demonstrated that GSO significantly ameliorated NAFLD in HFD-fed mice. Moreover, GSO effectively improved dyslipidemia and insulin resistance. The majority of NAFLD patients demonstrate the features of metabolic syndrome including central obesity, insulin resistance, hypertension, and dyslipidemia, and thus NAFLD is considered to be a hepatic manifestation of metabolic syndrome [18]. In our study, 12 wk of HFD feeding was sufficient to develop features of NAFLD in mice parallel to a previous report [19]. Compared with RD-fed mice, HFD-fed mice showed increased body weight (51.3%), LDL/HDL ratio (78.6%), liver weight (38.8%), hepatic lipid content (46%), hepatic TG content (98.8%), fasting glucose level (172%), and fasting insulin level (302%). These metabolic parameters were ameliorated by GSO administration in HFD-fed mice, in that GSO500-treated mice showed a 42.3% lower LDL/HDL ratio, a 21.4% lower liver weight, a 26.4% lower hepatic lipid content, a 17.9% lower hepatic TG content, a 18.2% lower fasting glucose level and a 48.3% lower fasting insulin level, compared with those of HFD-fed mice. These results indicate that GSO has preventive effect on hepatic steatosis and conditions associated with NAFLD in diet-induced obese mice.
The high content of monounsaturated fatty acid, specifically oleic acid, in GSO could be responsible for the beneficial effects of GSO against HFD-induced disruption in hepatic lipid metabolism. GSO is composed of 76.12% of oleic acid (C18:1, n-9) and 17.33% of linoleic acid (C18:2, n-6; Fig. 1), similar to olive oil which contains 73.7% of oleic acid and 7.9% of linoleic acid [20]. It is well documented that dietary oils enriched in oleic acid have beneficial effects in cardiovascular disease by reducing plasma lipid levels, especially LDL-C level [8], [21], [22], [23]. Likewise, treatment with oleic acid-rich GSO significantly improved dyslipidemia as demonstrated by reduced serum levels of TG and LDL-C and increased level of HDL-C comparable to HFD (Fig. 3C). Dyslipidemia is commonly present in up to 80% of patient with NAFLD and it is critical as the patient show increased incidence of cardiovascular disease [24].
It is widely known that dietary fatty acids influence hepatic lipid metabolism through transcriptional regulation of gene encoding enzymes involved in lipid metabolic pathways. Thus, in the present study, we performed global gene expression analysis to elucidate possible transcriptional modulation action of GSO. We found that hepatic messenger RNA expression of GSO-treated mice was rather similar to the expression profile of RD-fed mice. This was demonstrated by GSEA analysis, where downregulated genes of HFD-fed mice showed enrichment in the upranked expression in response to GSO treatment, and upregulated genes in steatotic liver were enriched in downregulated genes of GSO-treated mice (Figs. 6A and 6B). The results suggest that GSO could modulate global hepatic gene expression profile to regulate hepatic lipid metabolism. Pathway analysis discovered that expression of enzymes involved in fatty acid degradation are reduced in HFD-induced steatotic liver and this was reversed by GSO treatment (Fig. 7). Unsaturated C18 fatty acids including oleic acid and linoleic acid have been recognized to activate peroxisome proliferation activated receptor-α (PPARα) by binding directly to this receptor, which in turn upregulate apoA-I, apoA-II, lipoprotein lipase, and the genes involved in fatty acid oxidation [25]. As expected from the previous report, GSO increased expression of PPARα and decreased sterol-regulatory element binding protein 1 (SREBP1). Transcriptional modulation of β-oxidation-regulatory genes in response to GSO was associated to elevation of Sirt1 and Ppargc1a. Hepatic sirtuin 1 has been studied for its role in regulation of hepatic fatty acid metabolism via activation of PPARα signaling pathway [26]. Upregulation of Sirt1 by GSO was presented with increased expression of Ppara and Ppargc1a, and subsequently increased expression of genes that are associated to fatty acid β-oxidation including Cpt1a and Cpt2 (Fig. 6C). The similar results have been observed previously by Lim et al. [27] as they reported that oleic acid increases the expression of genes involved in fatty acid oxidation pathway via sirtuin 1–peroxisome proliferator-activated receptor γ coactivator 1-α pathway.
The pathogenesis of NAFLD is accompanied by peripheral insulin resistance and it has been suggested that insulin resistance is a prerequisite condition for the development of hepatic steatosis [28], [29]. In the state of hyperinsulinemia, hepatic lipid metabolism is altered via transcriptional modulation of genes, which in turn increases de novo lipogenesis, adipocyte lipolysis, and impairs hepatic β-oxidation. It is evident as reported in previous study that hepatic lipid synthesis is elevated in NAFLD patients with hyperinsulinemia, compared with healthy individuals [30]. Hepatic lipid metabolism shift towards de novo lipogenesis due to insulin resistance is known to be influenced by transcriptional activity of carbohydrate response element-binding protein, and SREBP1 [31], [32]. Our microarray and RT-qPCR data revealed that GSO enhanced HFD-induced aberration of insulin signaling as evident by increased hepatic messenger RNA expression of insulin receptor substrate (Irs2) and decreased expression of downstream target genes encoding SREBP1 (Srebf1) and carbohydrate response element-binding protein (Mlxipl). In parallel, GSO significantly lowered fasting insulin level with a minimal effect on fasting glucose level in HFD-fed mice (Fig. 5). Amelioration of hyperinsulinemia resulted in improved homeostatic model assessment of insulin resistance index demonstrating GSO inhibits HFD-induced insulin resistance. Furthermore, hepatic inflammation has been described as the most plausible link between insulin resistance and pathogenesis of NAFLD [33]. Elevation in hepatic lipid availability due to impaired fatty acid oxidation and enhanced lipogenesis is closely linked to hepatic lipotoxicity as hepatic lipid overload initiates hepatic inflammatory responses. It thereby progresses to steatohepatitis by activation of tumor necrosis factor-α orchestrated with various cytokines and chemokines [34]. It is worth noting that the proinflammatory cytokines and fibrogenic markers, namely, Tnf, Ccl2, and Col1a1 were downregulated in GSO-treated liver. Such alterations in hepatic gene expression profile by GSO support the antisteatotic effect of GSO and suggest that GSO may protect from disease progression to steatohepatitis.
In summary, our results revealed that GSO effectively reduced hepatic steatosis and ameliorated the accompanying metabolic conditions including dyslipidemia and insulin resistance in mice fed a HFD. Antisteatotic activity of GSO are probably due to the transcriptional regulation of enzymes involved in fatty acid degradation by β-oxidation via up-regulation of Ppara, Sirt1, and Ppargc1a. GSO also suppresses genes involved in lipogenesis possibly via sensitizing insulin signaling pathway. Moreover, GSO decreases hepatic expression of inflammatory genes involved in the development of steatohepatitis, indicating that GSO has a capability to prevent progression to steatosis and possibly to steatohepatitis. These results suggest the potential use of GSO as a new functional edible oil for individuals with NAFLD.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgements
This research was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (114023-03-2-HD040) of South Korea.
References
- 1.Seo E., Kim S., Lee S.J., Oh B.C., Jun H.S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients. 2015;7:3038–3053. doi: 10.3390/nu7043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim W.K., Song S.Y., Oh W.K., Kaewsuwan S., Tran T.L., Kim W.S., Sung J.H. Wound-healing effect of ginsenoside Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. Eur J Pharmacol. 2013;702:285–293. doi: 10.1016/j.ejphar.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 3.Yang B.R., Cheung K.K., Zhou X., Xie R.F., Cheng P.P., Wu S., Zhou Z.Y., Tang J.Y., Hoi P.M., Wang Y.H. Amelioration of acute myocardial infarction by saponins from flower buds of Panax notoginseng via pro-angiogenesis and anti-apoptosis. J Ethnopharmacol. 2016;181:50–58. doi: 10.1016/j.jep.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Qian P., Liu P., Wei L., Cao M., Zhou L., Zhou D., Lin Z.X. Effects of Panax notoginseng flower extract on the TGF-beta/Smad signal transduction pathway in heart remodeling of human chymase transgenic mice. Mol Med Rep. 2012;5:1443–1448. doi: 10.3892/mmr.2012.856. [DOI] [PubMed] [Google Scholar]
- 5.Lee M.H., Kim S.S., Cho C.W., Choi S.Y., In G., Kim K.T. Quality and characteristics of ginseng seed oil treated using different extraction methods. J Ginseng Res. 2013;37:468–474. doi: 10.5142/jgr.2013.37.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beveridge T.H., Li T.S., Drover J.C. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 7.Ko S.K., Bae H.M., Cho O.S., Im B.O., Chung S.H., Lee B.Y. Analysis of ginsenoside composition of ginseng berry and seed. Food Sci Biotechnol. 2008;17:1379–1382. [Google Scholar]
- 8.Jones P.J., Senanayake V.K., Pu S., Jenkins D.J., Connelly P.W., Lamarche B., Couture P., Charest A., Baril-Gravel L., West S.G. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014;100:88–97. doi: 10.3945/ajcn.113.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T.C., Ivester P., Hester A.G., Sergeant S., Case L.D., Morgan T., Kouba E.O., Chilton F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014;13:196. doi: 10.1186/1476-511X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y.M., Haastert B., Scherbaum W., Hauner H. A phytosterol-enriched spread improves the lipid profile of subjects with type 2 diabetes mellitus—a randomized controlled trial under free-living conditions. Eur J Nutr. 2003;42:111–117. doi: 10.1007/s00394-003-0401-y. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 12.Than N.N., Newsome P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho B.S., Irizarry R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breitling R., Armengaud P., Amtmann A., Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 15.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurilea E. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 18.Marchesini G., Brizi M., Morselli-Labate A.M., Bianchi G., Bugianesi E., McCullough A.J., Eorlani G., Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z.J., Fan J.G., Ding X.D., Qiao L., Wang G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirex-Tortosa M.C., Grandaos S., Quiles J.L. Chemical composition, types and characteristics of olive oil. In: Quiles J.L., Ramirez-Tortosa C., Yaqoob P., editors. Olive oil and health. CAB International; Wallingford: 2006. pp. 45–62. [Google Scholar]
- 21.Massaro M., De Caterina R. Vasculoprotective effects of oleic acid: epidemiological background and direct vascular antiatherogenic properties. Nutr Metab Cardiovasc Dis. 2002;12:42–51. [PubMed] [Google Scholar]
- 22.Parthasarathy S., Khoo J.C., Miller E., Barnett J., Witztum J.L., Steinberg D. Low density lipoprotein rich in oleic acid is protected against oxidative modification: implications for dietary prevention of atherosclerosis. ProcNatl Acad Sci U S A. 1990;87:3894–3898. doi: 10.1073/pnas.87.10.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.William C.M. Beneficial nutritional properties of olive oil: implications for postprandial lipoproteins and factor VII. Nutr Metab Cardiovasc Dis. 2001;111:246–250. [PubMed] [Google Scholar]
- 24.Souza M.R., Diniz Mde F., Medeiros-Filho J.E., Araujo M.S. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arg Gastroenterol. 2012;49:89–96. doi: 10.1590/s0004-28032012000100015. [DOI] [PubMed] [Google Scholar]
- 25.Lin Q., Ruuska S.E., Shaw N.S., Dong D., Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry. 1999;38:185–190. doi: 10.1021/bi9816094. [DOI] [PubMed] [Google Scholar]
- 26.Purushotham A., Schug T.T., Xu Q., Surapureddi S., Guo X., Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim J.H., Gerhart-Hines Z., Dominy J.E., Lee Y., Kim S., Tabata M., Xiang Y.K., Puigserver P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J Biol Chem. 2013;288:7117–7126. doi: 10.1074/jbc.M112.415729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A.J., Natale S., Forlani G., Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz J.M., Linfoot P., Dare D., Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Shimomura I., Bashmakov Y., Horton J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 32.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J.R., Girard J., Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistnace in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 33.Asrih M., Jornayvaz F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218:R23–R36. doi: 10.1530/JOE-13-0201. [DOI] [PubMed] [Google Scholar]
- 34.Farrell G.C., van Rooyen D., Gan L., Chitturi S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver. 2012;6:149–171. doi: 10.5009/gnl.2012.6.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]