Abstract
Concussion, or mild traumatic brain injury, incidence rates have reached epidemic levels and impaired postural control is a cardinal symptom. The purpose of this review is to provide an overview of the linear and non-linear assessments of post-concussion postural control. The current acute evaluation for concussion utilizes the subjective balance error scoring system (BESS) to assess postural control. While the sensitivity of the overall test battery is high, the sensitivity of the BESS is unacceptably low and, with repeat administration, is unable to accurately identify recovery. Sophisticated measures of postural control, utilizing traditional linear assessments, have identified impairments in postural control well beyond BESS recovery. Both assessments of quiet stance and gait have identified lingering impairments for at least 1 month post-concussion. Recently, the application of non-linear metrics to concussion recovery have begun to receive limited attention with the most commonly utilized metric being approximate entropy (ApEn). ApEn, most commonly in the medial-lateral plane, has successfully identified impaired postural control in the acute post-concussion timeframe even when linear assessments of instrumented measures are equivalent to healthy pre-injury values; unfortunately these studies have not gone beyond the acute phase of recovery. One study has identified lingering deficits in postural control, utilizing Shannon and Renyi entropy metrics, which persist at least through clinical recovery and return to participation. Finally, limited evidence from two studies suggest that individuals with a previous history of a single concussion, even months or years prior, may display altered ApEn metrics. Overall, non-linear metrics provide a fertile area for future study to further the understanding of postural control impairments acutely post-concussion and address the current challenge of sensitive identification of recovery.
Keywords: Balance, Concussion, Mild traumatic brain injury, Postural control, Variability
1. Concussion overview
The National Institute of Health has deemed concussion, a mild traumatic brain injury (mTBI), a major public health disorder.1 Concussions have reached epidemic levels both epidemiologically and in the popular media.2, 3 Indeed, the discussion of concussion permeates major sporting events from the Super Bowl to the World Cup to the Olympic Games.4, 5 There are estimated 1.6–3.8 million concussions which occur annually in the US; however, between 50% and 80% of concussions may go underreported either through intentional non-disclosure or lack of injury recognition.6, 7, 8 However, sports and recreation related concussions comprise only a small minority (4%–11%) of all head injuries in children in the United States.9 Beyond sports and recreation, TBI has been referred to as the signature injury of the current military engagements in Iraq and Afghanistan with up to 23% of active deployment military personal suffering an mTBI.10 The costs associated with all TBI exceed US$60 billion annually in direct and indirect costs with mTBI comprising 75%–90% of all TBIs with an annual economic burden of ~US$22 billion alone.11, 12
While many neurological pathologies are associated with specific supraspinal structures or pathways, the pathophysiology of concussion is less well understood.13 Frequently described as a functional injury, recent advances in imaging have identified microstructural anatomical damage post-concussion.13 The acute pathophysiology, often termed the neurometabolic cascade, is associated with elevated glutamate, potassium, and calcium levels resulting in an energy crisis due to impaired mitochondrial dysfunction.13 This process does not affect a specific structure or pathway, rather it is termed a “spreading depression” in which diffuse areas of the brain are affected.13 Although some recent human studies have suggested prolonged recovery, animal studies indicate that reduced cerebral blood flow and glucose metabolism persist for up to 7–10 days post-concussion which correlates well with symptom resolution.13, 14, 15, 16 Thus, it is not surprising that the 4th International Consensus Statement on Concussion in Sports suggests that 80%–90% of concussions recover, based on standard clinical markers, within this same 7- to 10-day window.2 Herein, clinical recovery refers to achieving baseline (pre-morbid) values on the assessment battery utilized by the clinicians which typically includes assessment of concussion related symptoms, cognition, balance, and computerized neuropsychological tests.2
The consequences of concussion include elevated risks acutely, the potential for recurrent concussions, and later life neuropathological consequences. Specifically, in the acute post-concussion period there is a risk of the rare, but potentially fatal and debated, second impact syndrome which is believed to occur when a subsequent head impact occurs prior to resolution of the neurometabolic cascade.17, 18 Furthermore, there is an elevated risk of recurrent concussion which will likely present worse and have prolonged recovery as well as the recent suggestion of increased risk of non-concussion sports injuries.19, 20, 21 The well-publicized long-term complications of repeated concussions include elevated risk of clinically diagnosed depression, mild cognitive impairment, earlier onset of Alzheimer's disease and the much discussed, albeit debated, chronic traumatic encephalopathy.22, 23, 24 Finally, there is even emerging evidence for elevated rate of future traumatic death amongst individuals in the general population who suffered mTBI.25
There are currently no well accepted methods to predict or prevent concussions, thus the key to reduce the associated risks are proper acute evaluation and identification of recovery for safe return to participation. Encouragingly, a multifaceted concussion assessment battery is highly sensitive, 0.89–0.96, in the acute concussion evaluation and most athletic trainers are utilizing this type of battery.26, 27, 28 However, the sensitivity of this battery drops to unacceptably low levels (0.14–0.30) within a week likely due to a practice effect from repeat administration of the assessments.29 Currently, computerized neuropsychological testing is the standard of care to identify post-concussion cognitive recovery; whereas, assessment of postural control remains substantially limited with the balance error scoring system (BESS) is the current clinical recommendation despite extremely low sensitivity (0.07) at 1 week post-injury.2, 27, 28, 29 However, recovery of cognitive processing capabilities occurs independent of postural control and therefore neurocognitive testing alone is insufficient to identify recovery.30, 31, 32, 33, 34 Thus, a multifaceted approach, including postural control assessment, is critical in both the acute recognition of concussion as well as the determination of recovery and the safe return to participation.35 The purpose of this review is to provide an overview of the linear and non-linear assessments of post-concussion postural control.
2. Linear assessment of post-concussion postural control
Postural control requires controlling the body's orientation in space and encompasses both postural stability and postural steadiness.36 Traditionally, linear dynamic postural control modeling stems from a stimulus-response paradigm, during which output is predicted using linear equations (e.g., position, displacement, velocity, acceleration).36 Within this model, postural steadiness is measured by variations in the center of pressure (CoP) as a function of time whereas an increase in the area of CoP measures is associated with greater impairment in postural stability.36 Following a concussive injury, the impairments to the postural control system are thought to result from an impaired interaction between the somatosensory, visual, and vestibular systems.37, 38 However, this approach may be limited by emphasis on the sensory system (e.g., primarily static and single tasks challenges) and resulting limited consideration of the motor and cognitive systems (e.g., dynamic and dual task challenges).38, 39 The Romberg test was one of the first static balance tests to be used in the clinical setting for concussion management which challenges the sensory system by evaluating sway during quiet stance with both the eyes open and closed.40 However, the Romberg has been criticized as being insensitive due to its subjective nature of the interpretation and it has never been validated for clinical management of sports concussion.40, 41 These limitations, in an effort to objectify balance impairments, lead to the development of the BESS.40, 42 While an improvement over the Romberg test, the BESS also suffers from multiple limitations leading to the implementation of instrumented measures of postural control, primarily using force plates and the sensory organization test (SOT).
The most commonly utilized postural control assessment for sports-related concussion is the BESS.27, 28 The BESS was created as a cost effective and clinically feasible test which assessing balance on multiple surfaces (firm and foam) in multiple stances (double limb, single limb, and tandem).42 The BESS has demonstrated strong correlations with force plate sway measures in five static balance positions (single-leg firm surface, tandem firm surface, double leg foam surface, single leg foam surface, and tandem foam surface), has with an intertester reliability coefficient correlations (ICC) ranging from 0.78 to 0.96, and produced results similar to those on the SOT.40, 42, 43 However, the total BESS score, the score utilized clinically, has low to moderate ICC values (0.57–0.74) that have been identified raising reliability concerns. Following a concussion, BESS scores typically increases by 3–6 errors which is less than the minimal detectable change scores (7.3–9.4 errors) which could explain the test's low initial sensitivity (0.34).29, 44 Further, environmental distractions, fatigue, functional ankle instability, and dehydration may all impair performance whereas improved performance may occur secondary to a learning effect associated with repeat administration.45, 46, 47, 48, 49, 50, 51 These limitations may be underlying the low initial sensitivity which decreases substantially within the first week post-injury (0.07).29
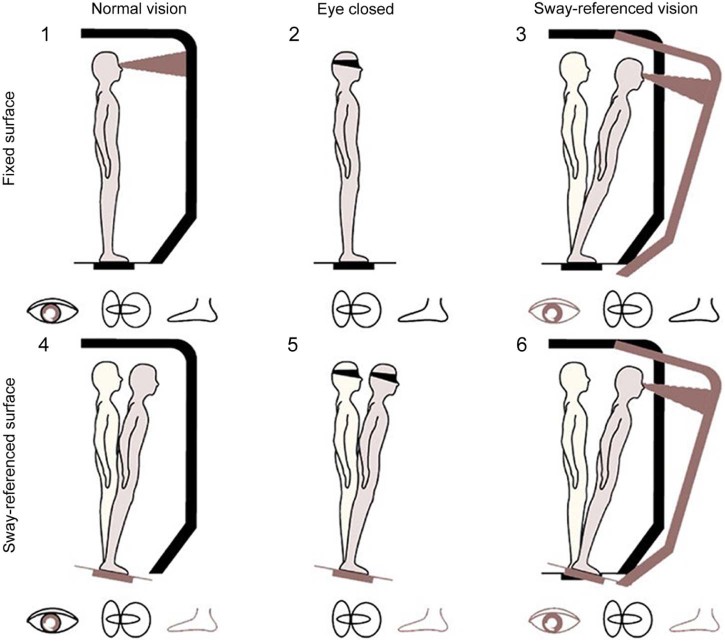
To address these considerations, instrumented measures of postural control, primarily the SOT, has been incorporated into research testing paradigms. The SOT disrupts information available to the somatosensory and visual systems while it measures an individual's ability to maintain a steady equilibrium.52 The SOT consists of three 20-s trials with three different visual conditions (eyes open, eyes closed, and sway referenced) as well as two stance conditions (fixed and sway referenced).38 Within the SOT, the term sway-referencing refers to tilting of the forceplate or the test apparatus (Fig. 1).38 An overall equilibrium score is derived from performance on all six stances while composite scores are calculated for somatosensory, visual, and vestibular.38 While the SOT has the advantage of providing objective, reliable, and valid postural control data, the implementation of the SOT within the sports medicine community is limited by cost, expertise, and lack of portability.27, 53
Fig. 1.
Six testing conditions for the sensory organization test. Vision is removed in Conditions 2 and 5. The sway-referenced AP angular motion of the surrounding wall reduces optic flow stimulation in Conditions 3 and 6, which is useful for the perception of self-motion relative to the visual field. In Conditions 4 and 6, the sway-referenced AP angular motion of the force plates reduces somatosensory stimulation, which is useful for the perception of AP self-motion relative to the surface of support78 (adapted with permission). AP = anterior-posterior.
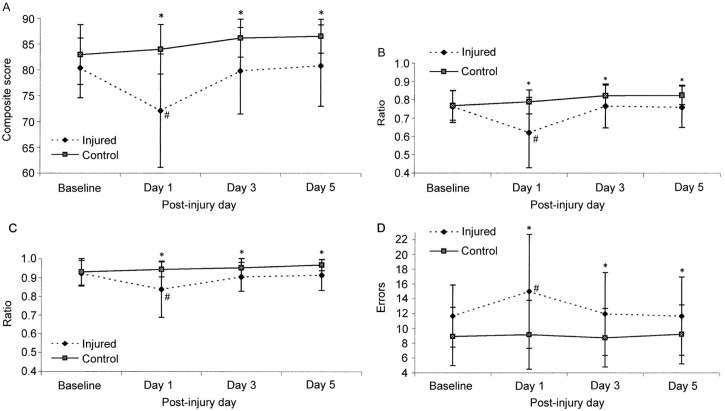
In the immediate aftermath of a concussion, impaired postural control is a cardinal symptom.2 On the BESS assessment, performance typically worsens by 3–6 errors, compared to baseline, when first assessed within 24 h.14, 29, 38, 54 However, the BESS typically returns to baseline values within 2–5 days post-injury and, with repeat administration, will continue to improve with nearly 20% less errors committed after a week of testing.14, 29 This apparent clinical recovery, operationally defined as achieving baseline/premorbid values, on the BESS exists despite persistent cognitive deficits and concussion related symptoms reported by patients.14 Even when utilizing the instrumented SOT, collegiate student-athletes recovery patterns were similar to BESS with recovery occurring within 3–5 days post-injury, with both tests displaying a similar recovery curve suggesting test–retest limitations may also exist (Fig. 2).38, 42, 54, 55 Overall, these results suggest that the BESS is a moderately valid clinical tool for the post-concussion assessment of postural control. However, the aforementioned limitations must be considered when evaluating the conclusion that postural control recovers within several days post-concussion.14, 29
Fig. 2.
Means ± SD on the NeuroCom Smart Balance Master. (A) Composite score, (B) visual ratio, (C) vestibular ratio, and (D) Balance Error Scoring System for 36 injured and 36 control subjects across test sessions (pre-season through day 5 post-injury). *Significant group difference; #significant difference from baseline (p < 0.05). Higher scores represent better performance on A, B, and C, and a lower score is indicative of better performance in D54
(adapted and revised with permission).
These studies, primarily from the early to mid-2000s, were largely viewed as conclusive findings on linear measures of post-concussion postural control with general agreement that balance recovers within a few days. However, in recent years several other approaches have identified conflicting findings suggesting that post-concussion impairments in postural control may persist far beyond a few days. Both traditional (e.g., 95% area, velocity, stability index) and novel biomechanical measures of quiet stance and associated CoP metrics have been utilized to challenge the BESS and SOT findings. Slobounov et al.,30 utilizing virtual time to contact (VTC) (an assessment of instantaneous CoP values including position, velocity, and acceleration), identified residual postural abnormalities at least 30 days post-concussion in collegiate student-athletes despite no apparent differences in traditional CoP metrics. Further, when used in conjunction with electroencephalogram, VTC was a predictor of future postural control impairments.56 Similarly, Powers et al.57 identified increased anterior-posterior (A/P) CoP displacement in collegiate student-athletes, compared to a healthy control group, on the day the individual returned to athletic participation (26 + 14 days post-injury) and suggested this was secondary to poor sensorimotor integration of the lateral vestibulospinal tract. Slobounov et al.31 concurred with deficits noted in CoP area for at least 30 days post-concussion when compared within subject to baseline values, but encouragingly noted apparent recovery at 6 months post-injury. Taken together, these studies suggest that instrumented measures of postural control identify impairments persisting at least 30 days post-concussion and through clinical recovery. However, the feasibility of these tests remains low and thus is unlikely to be incorporated into clinical settings due to cost, time demand, and expertise.
Dynamically, a conservative gait strategy (slower gait velocity, reduced separation of the CoP–center of mass (COM), increased mediolateral range of motion of the COM, and increased time in double support) has been routinely identified post-concussion in collegiate student-athletes.32, 53, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 These noted deficits tend to be exacerbated when the challenge is increased either in the motor task (e.g., obstacle avoidance) or when a cognitive task is incorporated.53, 69 It is important to note that the majority of gait studies reviewed were performed at one laboratory and were largely delimited to grade II concussions, as defined by the American Academy of Neurology (no LOC and symptoms persisting longer than 15 min),70 had fairly homogeneous and small (n = 10–17/group) participant populations for most studies, lacked within-subject pre-injury data, and have involved a variety of gait tasks including single task gait, dual task gait with working memory challenges, and obstacle avoidance tasks.57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 Beyond traditional gait testing, Buckley et al.33 identified an impaired motor strategy during gait termination through altered propulsive and braking forces post-concussion which persisted beyond recovery on the multifaceted clinical concussion assessments and was also independent of gait velocity. Thus, sophisticated measures of postural control and gait have successfully identified lingering impairments in post-concussion postural control which persist far beyond the clinical markers of recovery.
3. Non-linear assessments of post-concussion postural control
A dynamic system, the human body, may appear to be in a static state during upright standing, but fluctuations in movement provide evidence that the body is in slight disequilibrium with the environment and thus the term “quiet” is more appropriate than “static” stance.71 Dynamic systems theory is an area of applied mathematics used to describe a dynamic system or a system which evolves over time.71 Non-linear measures represent alternative instruments to explore movement variability and are based on dynamic systems principles.72, 73 Variability, it should be noted, is not inherently “good” or “bad”, but rather provides a range of coordinated patterns that can be utilized to complete a motor task.72, 74 However, the dynamic systems theory argues that increased variability in a movement pattern generally indicates a loss of stability whereas decreased variability generally indicates a more stable pattern.75
Despite the growth of dynamic systems analysis and the related subfield of non-linear entropy measures, these assessment protocols have received limited attention in the sports-concussion literature.74 The most commonly utilized metric in post-concussion postural control measures, approximate entropy (ApEn), takes into account the sequential order of successive data points and is based on how likely a given pattern is to reappear within the time series.76 ApEn is a non-linear measure which characterizes orderliness in the temporal output of a complex system.36 Mathematically, ApEn is a measure of the logarithmic likelihood that a series of data points of length m + 1 will be close, given that observations of length m are close.76 In this calculation, the length of data points (m) is defined by the investigator, often m = 2 per convention, and closeness (r) is defined as a percentage of the standard deviation (SD) of the time series of interest. Conventionally, m = 2, r = 0.2 × CoP SD.76 The ApEn is a unitless quantity that ranges from 0, perfect repeatability, such as a sine wave, to 2, complete randomness, such as Gaussian noise.76 As it relates to postural control, high sway regularity would have a low ApEn (approaching 0), and high sway irregularity would have a high ApEn (approaching 2). Currently, it is unknown what specific pathophysiology or compensatory strategies are represented by high or low sway regularity and suggested mechanisms are presented in subsequent paragraphs. Importantly, ApEn does not necessarily correlate well with traditional linear analysis of a data series and thus may elucidate impairments in postural control not currently identified by traditional linear metrics.36 The test–retest reliability of ApEn values calculated from raw data obtained using the SOT for the anterior-posterior (AP) time series is moderate to good (ICC(2,1) = 0.79–0.90); however, the ML time series were less consistent between trials (ICC(2,1) = 0.53–0.77).77
Only five studies have utilized non-linear analysis to identify potential changes in postural control strategies amongst acutely concussed collegiate student-athletes.78, 79, 80, 81 These studies described alterations in postural control that were not otherwise recognized using traditional linear CoP measures including area, displacement, and velocity. Further, in two studies this also included achieving baseline values on the SOT overall equilibrium score as well as the vestibular, visual, and somatosensory ratios.78, 82 Acutely post-concussion, operationally defined as within 48 h of injury, multiple studies have identified altered postural control utilizing ApEn measures in both the A/P and medial-lateral (M/L) directions.78, 80, 82 Specifically, Cavanaugh et al.82 identified increased regularity (lower ApEn values) with the largest changes occurring in SOT Condition 1 (eyes open, surface fixed) and 2 (eyes closed, surface fixed) which were almost three times as large as the standard error of the mean, exceeded all changes observed in the other conditions for the concussed and all conditions in the healthy participants. This finding suggested that despite the lack of differences noted in SOT, significant alterations in CoP regularity were identified utilizing ApEn during the acute phase of concussion. Further, these differences, the magnitude of decline, were greater in the M/L plane which the authors suggested was due to a higher level of irregularity during the baseline test and therefore had a greater potential to identify alterations.82 Higher irregularity in the M/L plane may be a result of lower boundary area compared to the A/P plane or may be a result of greater muscle co-activation (e.g., tibialis anterior and gastrocnemius/soleus) in the A/P plane compared to gluteus medius activation in the M/L plane. However, the authors do not speculate why there were no differences in SOT measures which have routinely identified acute postural control impairments post-concussion.26, 38, 42, 55
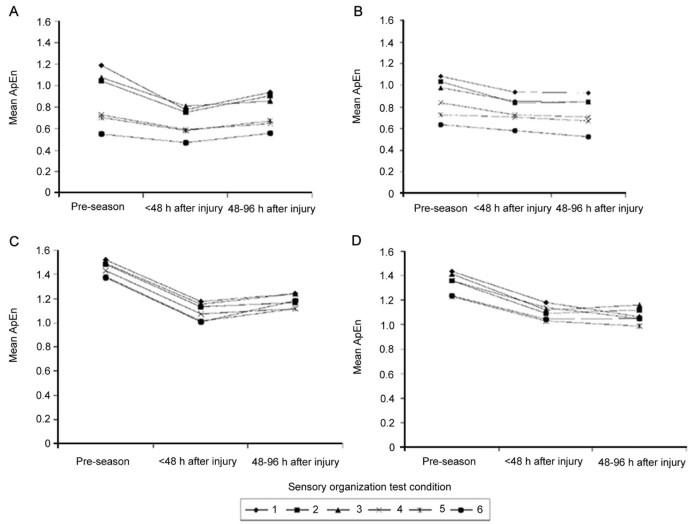
Once immediate changes in CoP regularity were identified, the next logical step was to assess the duration of these alterations. The same group followed up with a study which extended the assessment to 2–4 days post-concussion, placing the testing timeframe within the normal recovery period when utilizing the BESS test (Fig. 3).78 During the SOT, the A/P ApEn began to return to baseline values; however, changes in M/L ApEn persisted, especially in Conditions 1 and 2 of the SOT, through Day 4 with noted reductions in randomness (lower ApEn values). Once again, the noted differences between Day 4 and baseline were substantial with the differences three to four times larger than the standard error of the mean and these changes were largely independent of patient self-reported symptoms. This finding suggests that altered postural control may persist for a longer period of time than indicated based on BESS or SOT testing. The decline in ApEn values were again suggested to be associated with increased cortical regularity, but the authors herein also speculated increased co-contraction of the lower extremity musculature to gain control over postural sway may have reduced the CoP oscillations; however, electromyography data were not presented to support or refute this hypothesis.78 From these two findings, the authors suggest that postural control is achieved through unconstrained and irregular patterns of motor output.78, 80 Unfortunately, this study, nor subsequent published studies by this group, tracked these changes for longer periods of time, thus the time to full resolution, based on ApEn values, remains unknown.
Fig. 3.
Mean approximate entropy (ApEn) values for center of pressure (CoP) anterior-posterior (A, B) and medial-lateral (C, D) time series in athletes (A, C) with (n = 13) and (B, D) without (n = 16) postural instability after concussion. ApEn values are displayed for the six Sensory Organization Test conditions. Athletes were tested at pre-season, within 48 h after injury, and between 48 and 96 h after injury. Lower scores reflect greater regularity of CoP oscillations78
(adapted with permission).
While ApEn provides valuable insights into non-linear analysis of postural control via CoP oscillations, others have suggested alternative forms of entropy assessment including Shannon, Renyi, Kolmogorov, sample, and multiscale entropies with each approach having their own unique strengths and limitations (Table 1).80, 83, 84 Gao et al.80 suggested the short data sets utilized in the SOT were an inherent limitation to the analysis and therefore utilized a 120-s bipedal stance data capture while utilizing alternative entropy measures: Shannon and Renyi entropies. Shannon entropy can be calculated by mapping a CoP trace on to a grid and then determining the probability of the CoP occupying a given box during the CoP trace. Renyi entropy is a mathematical generalization of Shannon entropy, which does not assume the additivity of independent events. Interestingly, Gao et al.80 identified a substantial influence of samples on the outcome measures. Specifically, during bipedal stance when only the first 20 s of data were analyzed, there were no differences between Day 1 and Day 12 post-concussion. Indeed Day 1 had slightly lower Shannon entropy which would lead one to conclude that either there were no differences in Shannon entropy or that the entropy actually decreased. However, when the entire 120 s trial is analyzed, a greater than linear increase is observed on Day 1 whereas on Day 12 the entropy value plateau's around 40 s (Fig. 4). These findings suggest that in order to identify altered postural control post-concussion, a 20-s assessment may be insufficient and only longer data collections may reveal the impairments. Clinically, this could be relevant as the BESS test stances are only held for 20 s and longer assessment points could better discriminate balance impairments. Further when applying this approach, apparent alterations in postural control were present for at least 1–2 weeks post-concussion and well beyond clinically defined recovery. Unfortunately, this study lacked baseline data such that within-subject comparison was not available and therefore determining an individual's recovery was not plausible; however, this result provides a proof of concept that may provide for future approaches and investigations.80
Table 1.
Description of entropy measures used in concussion populations and their limitations.
| Entropy measure | Description | Limitation |
|---|---|---|
| Approximate entropy | Calculated by determining the probability that if two series data points are similar for a length of m points, then they will remain similar at the next point | Biased toward regularity in that it includes self-matches and lacks relative consistency in that it is more sensitive to changes in input parameters, m and r |
| Sample entropy | A modification of approximate entropy that does not include self-matches and is independent of data length | Does not discriminate between groups with a shorter data as well as approximate entropy |
| Shannon entropy | Calculated by mapping a CoP trace onto a grid and then determining the probability of the CoP occupying a given box during the CoP trace | Substantial influence of samples on Shannon entropy |
| Renyi entropy | A mathematical generalization of Shannon entropy, which does not assume the additivity of independent events | Substantial influence of samples on Renyi entropy |
Abbreviation: CoP = center of pressure.
Fig. 4.

Variation of Shannon entropy with data length. Shannon entropy vs. data length for a subject on Day 1 and Day 12 after concussion79 (open access, permission not required).
The primary focus of this review has been the assessment of postural control in the acute aftermath of a concussion; however, several studies have investigated the chronic effects of concussion on postural control utilizing both linear and non-linear measures.79, 81, 85 Despite only limited differences when evaluated with traditional linear metrics, both Sosnoff et al.79 and De Beaumont et al.81 identified altered non-linear measures during quiet stance and SOT testing. Specifically, Sosnoff et al.79 observed that as the task difficulty increased, individuals with a previous history of concussions (>1) decreased M/L irregularity while increasing their A/P irregularity. Further, the SOT test failed to identify between group differences in three of the four functional balance scores and the only difference was noted in the visual ratio score, but the authors suggested this was not a meaningful clinical change.79 Similarly, De Beaumont et al.81 identified both lower ApEn and CoP displacement amplitude in the A/P, but not M/L, direction during a 30-s quiet stance task amongst athletes with a history of >1 concussion (2.65 ± 1.45) at least 9 months prior (19.03 ± 13.77 months). The primary focus of investigations related to the long-term consequences of concussion are cognitive, emotional, and quality of life related; however, these limited studies suggest the potential of postural impairments in individuals with even a single concussion. How these potential impairments will affect quality of life and fall risk as these participants age are currently unknown but worthy of investigation given the personal and societal implications associated with falls.86
Another potential area for future consideration is the application of a dual task (DT) methodology whereby an individual performs a motor task while also addressing a cognitive challenge.87 During gait trials, DT has successfully identified lingering impairments in postural control despite apparent resolution on motor only tasks.58, 59, 60, 64, 65, 66, 68 During quiet stance with a cognitive challenge, digit recall, healthy participants increased the A/P ApEn, but not M/L ApEn, independent of changes in the amplitude of CoP oscillations.88 This approach provides an additional potential avenue for future investigations to utilize DT methodologies while applying non-linear metrics. However, as described herein, this topic remains largely unexplored within this population.
4. Conclusion
Taken together, these studies suggest that ApEn outcome measures, as well as other entropy measures, can successfully identify impairments in postural control not detected by traditional linear outcome measures arguing in favor of their inclusion in an instrumented assessment battery. The successful identification of impaired postural control during the eyes open and closed with fixed surface conditions of the SOT (Conditions 1 and 2) suggested these may be the only conditions required for testing, thus increasing the clinical plausibility of the approach as new instruments could incorporate fixed surfaces.75 Unfortunately, access to the SOT and/or forceplates is extremely limited within most sports medicine clinics and therefore currently this approach may have limited applicability.27, 28 Future approaches to post-concussion postural control assessments should focus toward the development of clinician friendly protocols potentially utilizing cost-efficient commercially available products (e.g., Wii Fit balance board) with custom written software that could provide easy to interpret outcomes measures for clinicians. Currently, of course this will require extensive further experimentation to identify the measure(s) which are both highly sensitive and specific to changes post-concussion. Unfortunately, no gold standard currently exists on which to compare these assessments protocols. Moving forward, improved and clinically applicable measures of postural control, utilizing both linear and non-linear approaches, are warranted to further the neuropathological and clinical challenges associated with concussion.
Authors' contributions
All authors were involved in the development of this review article. JRO performed the review and writing of the linear analysis section of the paper. JBC performed the review and writing of the non-linear analysis section of the paper. TAB was responsible concussion overview section and for the overall paper. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
Acknowledgment
This project was funded, in part, by an NIH/NINDS grant (No. 1R15NS070744-01A1). The funding agency had no role in the development of the manuscript, the interpretation of the results, or the decision where to submit the manuscript.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.National Institute of Neurological Disorders and Stroke Traumatic Brain Injury. 2012. http://www.ninds.nih.gov/disorders/tbi/detail_tbi.htm Available at. accessed 12.12.2012.
- 2.McCrory P., Meeuwisse W.H., Aubry M., Cantu R.C., Dvořák J., Echemendia R.J. Consensus statement on concussion in sport. The 4th International Conference on Concussion in Sport. Zurich, Switzerland. November 2012. J Athl Train. 2013;48:554–575. doi: 10.4085/1062-6050-48.4.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cusimano M.D., Sharma B., Lawrence D.W., Ilie G., Silverberg S., Jones R. Trends in North American newspaper reporting of brain injury in ice hockey. PLoS One. 2013;8:e1865. doi: 10.1371/journal.pone.0061865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams J.M., Langdon J.L., McMillan J., Buckley T. English professional footballers concussion knowledge and attitudes. J Sport Health Sci. 2015 doi: 10.1016/j.jshs.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuominen M., Stuart M.J., Aubry M., Kannus P., Parkkari J. Injuries in men's international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49:30–36. doi: 10.1136/bjsports-2014-093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langlois J.A., Rutland-Brown W., Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 7.McCrea M., Hammeke T., Olsen G., Leo P., Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Llewellyn T., Burdette G.T., Joyner A.B., Buckley T.A. Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clin J Sport Med. 2014;24:76–79. doi: 10.1097/01.jsm.0000432853.77520.3d. [DOI] [PubMed] [Google Scholar]
- 9.Quayle K.S., Powell E.C., Mahajan P., Hoyle J.D., Jr, Nadel F.M., Badawy M.K. Epidemiology of blunt head trauma in children in U.S. emergency departments. N Engl J Med. 2014;371:1945–1947. doi: 10.1056/NEJMc1407902. [DOI] [PubMed] [Google Scholar]
- 10.Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 11.Gerberding J.L., Binder S. Report to congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. 2003. http://www.cdc.gov/traumaticbraininjury/pdf/mtbireport-a.pdf Available at. accessed 16.02.2015.
- 12.Finkelstein E.A., Corso P.S., Miller T.R. Incidence and economic burden of injuries in the United States. J Epidemiol Community Health. 2007;61:926. [Google Scholar]
- 13.Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 15.Meier T.B., Bellgowan P.S., Singh R., Kuplicki R., Polanski D.W., Mayer A.R. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 2015;72:530–538. doi: 10.1001/jamaneurol.2014.4778. [DOI] [PubMed] [Google Scholar]
- 16.Maugans T.A., Farley C., Altaye M., Leach J., Cecil K.M. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129:28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantu R.C. Second-impact syndrome. Clin Sports Med. 1998;17:37–44. doi: 10.1016/s0278-5919(05)70059-4. [DOI] [PubMed] [Google Scholar]
- 18.McCrory P., Davis G., Makdissi M. Second Impact syndrome or cerebral swelling after sporting head injury. Curr Sports Med Rep. 2012;11:21–23. doi: 10.1249/JSR.0b013e3182423bfd. [DOI] [PubMed] [Google Scholar]
- 19.Zemper E.D. Two-year prospective study of relative risk of a second cerebral concussion. Am J Phys Med Rehabil. 2003;82:653–659. doi: 10.1097/01.PHM.0000083666.74494.BA. [DOI] [PubMed] [Google Scholar]
- 20.Collins M.W., Lovell M.R., Iverson G.L., Cantu R.C., Maroon J.C., Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–1181. doi: 10.1097/00006123-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Nordstrom A., Nordstrom P., Ekstrand J. Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. Br J Sports Med. 2014;48:1447–1450. doi: 10.1136/bjsports-2013-093406. [DOI] [PubMed] [Google Scholar]
- 22.Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Cantu R.C., Randolph C. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 23.Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Cantu R.C., Mihalik J.R. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 24.McKee A.C., Stein T.D., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaaramo K., Puljula J., Tetri S., Juvela S., Hillbom M. Head trauma with or without mild brain injury increases the risk of future traumatic death: a controlled prospective 15-year follow-up study. J Neurotrauma. 2015;32:1579–1583. doi: 10.1089/neu.2014.3757. [DOI] [PubMed] [Google Scholar]
- 26.Broglio S.P., Macciocchi S.N., Ferrara M.S. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050–1057. doi: 10.1227/01.NEU.0000255479.90999.C0. [DOI] [PubMed] [Google Scholar]
- 27.Kelly K.C., Jordan E.M., Burdette G.T., Buckley T.A. NCAA Division I athletic trainers concussion management practice patterns. J Athl Train. 2014;49:665–673. doi: 10.4085/1062-6050-49.3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley T.A., Burdette G.T., Kelly K. NCAA Division II and III athletic trainers concussion management practice patterns: how the other half lives. J Athl Train. 2015;50:879–888. doi: 10.4085/1062-6050-50.7.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrea M., Barr W.B., Guskiewicz K., Randolph C., Marshall S.W., Cantu R. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 30.Slobounov S., Cao C., Sebastianelli W., Slobounov E., Newell K. Residual deficits from concussion as revealed by virtual time-to-contact measures of postural stability. Clin Neurophysiol. 2008;119:281–289. doi: 10.1016/j.clinph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Slobounov S., Sebastianelli W., Hallett M. Residual brain dysfunction observed one year post-mild traumatic brain injury: combined EEG and balance study. Clin Neurophysiol. 2012;123:1755–1761. doi: 10.1016/j.clinph.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckley T.A. Acute and lingering impairments in post-concussion postural control. In: Slobounov S.M., Sebastianelli W., editors. Concussions in athletics: from brain to behavior. 1st ed. Springer Scientific Publishing; New York, NY: 2014. pp. 139–165. [Google Scholar]
- 33.Buckley T.A., Munkasy B.A., Tapia-Lovler T.G., Wikstrom E.A. Altered gait termination strategies following a concussion. Gait Posture. 2013;38:549–551. doi: 10.1016/j.gaitpost.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guskiewicz K.M., Register-Mihalik J.K. Postconcussive impairment differences across a multifaceted concussion assessment protocol. PM R. 2011;3:S445–51. doi: 10.1016/j.pmrj.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Littleton A., Guskiewicz K. Current concepts in sport concussion management: a multifaceted approach. J Sport Health Sci. 2013;2:227–235. [Google Scholar]
- 36.Cavanaugh J.T., Guskiewicz K.M., Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005;35:935–950. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- 37.Guskiewicz K.M., Perrin D.H., Gansneder B.M. Effect of mild head injury on postural stability in athletes. J Athl Train. 1996;31:300–306. [PMC free article] [PubMed] [Google Scholar]
- 38.Guskiewicz K.M. Balance assessment in the management of sport-related concussion. Clin Sports Med. 2011;30:89–102. doi: 10.1016/j.csm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Shumway-Cook A., Woollacott M.H. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2012. Motor control: translating research into clinical practice. [Google Scholar]
- 40.Riemann B.L., Guskiewicz K.M., Shields E.W. Relationship between clinical and forceplate measures of postural stability. J Sport Rehabil. 1999;8:71–82. [Google Scholar]
- 41.Broglio S.P., Guskiewicz K.M. Concussion in sports: the sideline assessment. Sports Health. 2009;1:361–369. doi: 10.1177/1941738109343158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riemann B.L., Guskiewicz K.M. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- 43.Guskiewicz K.M. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001;11:182–189. doi: 10.1097/00042752-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Finnoff J.T., Peterson V.J., Hollman J.H., Smith J. Intrarater and interrater reliability of the Balance Error Scoring System (BESS) PM R. 2009;1:50–54. doi: 10.1016/j.pmrj.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Rahn C., Munkasy B.A., Barry Joyner A., Buckley T.A. Sideline performance of the balance error scoring system during a live sporting event. Clin J Sport Med. 2015;25:248–253. doi: 10.1097/JSM.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onate J.A., Beck B.C., Van Lunen B. On-field testing environment and balance error scoring system performance during preseason screening of healthy collegiate baseball players. J Athl Train. 2007;42:446–451. [PMC free article] [PubMed] [Google Scholar]
- 47.Fox Z.G., Mihalik J.P., Blackburn J.T., Battaglini C.L., Guskiewicz K.M. Return of postural control to baseline after anaerobic and aerobic exercise protocols. J Athl Train. 2008;43:456–463. doi: 10.4085/1062-6050-43.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Docherty C.L., McLeod T.C.V., Shultz S.J. Postural control deficits in participants with functional ankle instability as measured by the balance error scoring system. Clin J Sport Med. 2006;16:203–208. doi: 10.1097/00042752-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Weber A.F., Mihalik J.P., Register-Mihalik J., Mays S., Prentice W.E., Guskiewicz K.M. Dehydration and performance on clinical concussion measures in collegiate wrestlers. J Athl Train. 2013;48:153–160. doi: 10.4085/1062-6050-48.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burk J.M., Munkasy B.A., Joyner A.B., Buckley T.A. Balance error scoring system performance changes after a competitive athletic season. Clin J Sport Med. 2013;23:312–317. doi: 10.1097/JSM.0b013e318285633f. [DOI] [PubMed] [Google Scholar]
- 51.McLeod T.V., Perrin D.H., Guskiewicz K.M., Shultz S.J., Diamond R., Gansneder B.M. Serial administration of clinical concussion assessments and learning effects in healthy young athletes. Clin J Sport Med. 2004;14:287–295. doi: 10.1097/00042752-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Guskiewicz K.M. Assessment of postural stability following sport-related concussion. Curr Sports Med Rep. 2003;2:24–30. doi: 10.1249/00149619-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Buckley T.A. Concussion and gait. In: Li L., Holmes M., editors. Gait biometrics: basic patterns, role of neurological disorders and effects of physical activity. 1st ed. Nova Science Publishers; Hauppauge, NY: 2014. pp. 141–164. [Google Scholar]
- 54.Guskiewicz K.M., Ross S.E., Marshall S.W. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–273. [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson C.L., Ferrara M.S., Mrazik M., Piland T., Elliott T. Evaluation of neuropsychological stability following cerebral domain scores and postural concussion in sports. Clin J Sport Med. 2003;13:230–237. doi: 10.1097/00042752-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Slobounov S., Cao C., Sebastianelli W. Differential effect of first versus second concussive episodes on wavelet information quality of EEG. Clin Neurophysiol. 2009;120:862–867. doi: 10.1016/j.clinph.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers K.C., Kalmar J.M., Cinelli M.E. Recovery of static stability following a concussion. Gait Posture. 2014;39:611–614. doi: 10.1016/j.gaitpost.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 58.Howell D.R., Osternig L.R., Chou L.S. Return to activity after concussion affects dual-task gait balance control recovery. Med Sci Sports Exerc. 2015;47:673–680. doi: 10.1249/MSS.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 59.Howell D.R., Osternig L.R., Koester M.C., Chou L.S. The effect of cognitive task complexity on gait stability in adolescents following concussion. Exp Brain Res. 2014;232:1773–1782. doi: 10.1007/s00221-014-3869-1. [DOI] [PubMed] [Google Scholar]
- 60.Catena R.D., van Donkelaar P., Chou L.S. The effects of attention capacity on dynamic balance control following concussion. J Neuroeng Rehabil. 2011;8:8. doi: 10.1186/1743-0003-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catena R.D., van Donkelaar P., Chou L.S. Different gait tasks distinguish immediate vs. long-term effects of concussion on balance control. J Neuroeng Rehabil. 2009;6:25. doi: 10.1186/1743-0003-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catena R.D., van Donkelaar P., Halterman C.I., Chou L.S. Spatial orientation of attention and obstacle avoidance following concussion. Exp Brain Res. 2009;194:67–77. doi: 10.1007/s00221-008-1669-1. [DOI] [PubMed] [Google Scholar]
- 63.Parker T.M., Osternig L.R., van Donkelaar P., Chou L.S. Balance control during gait in athletes and non-athletes following concussion. Med Eng Phys. 2008;30:959–967. doi: 10.1016/j.medengphy.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Catena R.D., van Donkelaar P., Chou L.S. Altered balance control following concussion is better detected with an attention test during gait. Gait Posture. 2007;25:406–411. doi: 10.1016/j.gaitpost.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Catena R.D., van Donkelaar P., Chou L.S. Cognitive task effects on gait stability following concussion. Exp Brain Res. 2007;176:23–31. doi: 10.1007/s00221-006-0596-2. [DOI] [PubMed] [Google Scholar]
- 66.Parker T.M., Osternig L.R., van Donkelaar P., Chou L.S. Recovery of cognitive and dynamic motor function following concussion. Br J Sports Med. 2007;41:868–873. doi: 10.1136/bjsm.2006.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parker T.M., Osternig L.R., Van Donkelaar P., Chou L.S. Gait stability following concussion. Med Sci Sports Exerc. 2006;38:1032–1040. doi: 10.1249/01.mss.0000222828.56982.a4. [DOI] [PubMed] [Google Scholar]
- 68.Parker T.M., Osternig L.R., Lee H.J., van Donkelaar P.V., Chou L.S. The effect of divided attention on gait stability following concussion. Clin Biomech (Bristol, Avon) 2005;20:389–395. doi: 10.1016/j.clinbiomech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Lee H., Sullivan S.J., Schneiders A.G. The use of the dual-task paradigm in detecting gait performance deficits following a sports-related concussion: a systematic review and meta-analysis. J Sci Med Sport. 2013;16:2–7. doi: 10.1016/j.jsams.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Kelly J.P., Rosenberg J.H. Diagnosis and management of concussion in sports. Neurology. 1997;48:575–580. doi: 10.1212/wnl.48.3.575. [DOI] [PubMed] [Google Scholar]
- 71.Harbourne R.T., Stergiou N. Movement variability and the use of nonlinear tools: principles to guide physical therapist practice. Phys Ther. 2009;89:267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Preatoni E., Hamill J., Harrison A.J., Hayes K., Van Emmerik R.E., Wilson C. Movement variability and skills monitoring in sports. Sports Biomech. 2013;12:69–92. doi: 10.1080/14763141.2012.738700. [DOI] [PubMed] [Google Scholar]
- 73.Vaillancourt D.E., Sosnoff J.J., Newell K.M. Age-related changes in complexity depend on task dynamics. J Appl Physiol. 2004;97:454–455. doi: 10.1152/japplphysiol.00244.2004. [DOI] [PubMed] [Google Scholar]
- 74.Latash M.L. The bliss (not the problem) of motor abundance (not redundancy) Exp Brain Res. 2012;217:1–5. doi: 10.1007/s00221-012-3000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stergiou N., Decker L.M. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum Mov Sci. 2011;30:869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pincus S.M., Gladstone I.M., Ehrenkranz R.A. A regularity statistic for medical data-analysis. J Clin Monit. 1991;7:335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- 77.Cavanaugh J.T., Mercer V.S., Guskiewicz K. Response stability estimates for the sensory organization test: approximate entropy values and equilibrium scores in healthy young adults. Gait Posture. 2004;20:S55–6. [Google Scholar]
- 78.Cavanaugh J.T., Guskiewicz K.M., Giuliani C., Marshall S., Mercer V.S., Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–313. [PMC free article] [PubMed] [Google Scholar]
- 79.Sosnoff J.J., Broglio S.P., Shin S., Ferrara M.S. Previous mild traumatic brain injury and postural-control dynamics. J Athl Train. 2011;46:85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao J., Hu J., Buckley T., White K., Hass C. Shannon and Renyi entropies to classify effects of mild traumatic brain injury on postural sway. PLoS One. 2011;6:e24446. doi: 10.1371/journal.pone.0024446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Beaumont L., Mongeon D., Tremblay S., Messier J., Prince F., Leclerc S. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46:234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavanaugh J., Guskiewicz K., Giuliani C., Marshall S., Mercer V., Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39:805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yentes J.M., Hunt N., Schmid K.K., Kaipust J.P., McGrath D., Stergiou N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann Biomed Eng. 2013;41:349–365. doi: 10.1007/s10439-012-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao J., Hu J., Tung W. Entropy measures for biological signal analyses. Nonlinear Dynam. 2012;68:431–444. [Google Scholar]
- 85.Martini D.N., Sabin M.J., DePesa S.A., Leal E.W., Negrete T.N., Sosnoff J.J. The chronic effects of concussion on gait. Arch Phys Med Rehabil. 2011;92:585–589. doi: 10.1016/j.apmr.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 86.Gillespie L.D., Robertson M.C., Gillespie W.J., Lamb S.E., Gates S., Cumming R.G. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD007146.pub3. CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woollacott M., Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 88.Cavanaugh J.T., Mercer V.S., Stergiou N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: a methodological report. J Neuroeng Rehabil. 2007;4:42. doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]