Despite the widespread success of transition metal-catalyzed cross-coupling methodologies, significant limitations still exist in reactions at sp3-hybridized carbon atoms, with most approaches relying on prefunctionalized alkylmetal or bromide coupling partners1,2. While the use of native functional groups (e.g., carboxylic acids, alkenes, and alcohols) has improved the overall efficiency of such transformations by expanding the range of potential feedstocks3–5, the direct functionalization of carbon-hydrogen (C–H) bonds—the most abundant moiety in organic molecules—represents a more ideal approach to molecular construction. In recent years, an impressive range of C(sp3)-heteroatom bond forming reactions of strong C–H bonds have been reported6,7. Additionally, valuable technologies have been developed for the formation of carbon-carbon bonds from the corresponding C(sp3)–H bonds via substrate-directed transition metal C–H insertion8, undirected C–H insertion by captodative rhodium carbenoid complexes9, or hydrogen atom transfer (HAT) from weak, hydridic C–H bonds by electrophilic open-shell species10–14. Despite these advancements, a mild and general platform for the coupling of strong, neutral C(sp3)-H bonds with aryl electrophiles has not been realized. Here we describe a protocol for the direct C(sp3) arylation of a diverse set of aliphatic, C–H bond-containing organic frameworks via the combination of light-driven, polyoxometalate-facilitated hydrogen atom transfer (HAT) and nickel catalysis. This dual-catalytic manifold enables the generation of carbon-centered radicals from strong, neutral C–H bonds, which thereafter act as nucleophiles in nickel-mediated cross-coupling with aryl bromides to afford sp3-sp2 cross-coupled products. This technology enables unprecedented, single-step access to a broad array of complex, medicinally relevant molecules directly from natural products and chemical feedstocks via functionalization at sites unreactive under traditional methods.
Metallaphotoredox catalysis has recently emerged as an effective strategy for C(sp3)–H functionalization15. Specifically, the merger of photoredox-mediated hydrogen atom transfer (HAT) and transition metal catalysis has delivered several methods for the selective functionalization of activated C–H bonds based on low bond dissociation energies (BDEs) and/or polarity effects (α-heteroatom, benzylic, and formyl)10–14. Inspired by these studies and a strong oxidant-mediated protocol for C(sp3)–H arylation16, we proposed that combining (i) an HAT catalyst capable of generating high-energy carbon-centered radicals from strong, inert C–H bonds with (ii) the elementary steps of nickel catalysis (aryl oxidative addition, reductive elimination) would allow, for the first time, the coupling of aliphatic carbon frameworks with a range of aryl bromide coupling partners.
We postulated that polyoxometalates (POMs), many of which possess high-energy excited states able to perform the desired C–H abstraction, would be ideal cocatalysts for the proposed transformation17. Of particular interest was the decatungstate anion ([W10O32]4–), a POM which has been broadly utilized as an efficient HAT photocatalyst in a variety of oxygenations, dehydrogenations, conjugate additions, and, more recently, fluorinations of strong, unactivated, aliphatic C–H bonds18–23 with BDEs up to 100 kcal mol–1[24]. To our knowledge, the decatungstate anion has not previously been merged with transition metal cross-couplings, and we hoped that such a combination of catalytic processes would allow access to a significant breadth of carbon-centered radicals and aryl-functionalized products from abundant feedstocks (Figure 1). Furthermore, the observed selectivity of decatungstate for abstraction of electron-rich, sterically accessible C–H bonds25 combined with the steric preference of nickel catalyzed cross-couplings suggested that our proposed dual-catalytic system could provide site-specific arylation of complex organic frameworks.
Figure 1 |. Undirected Aliphatic C–H Arylation.
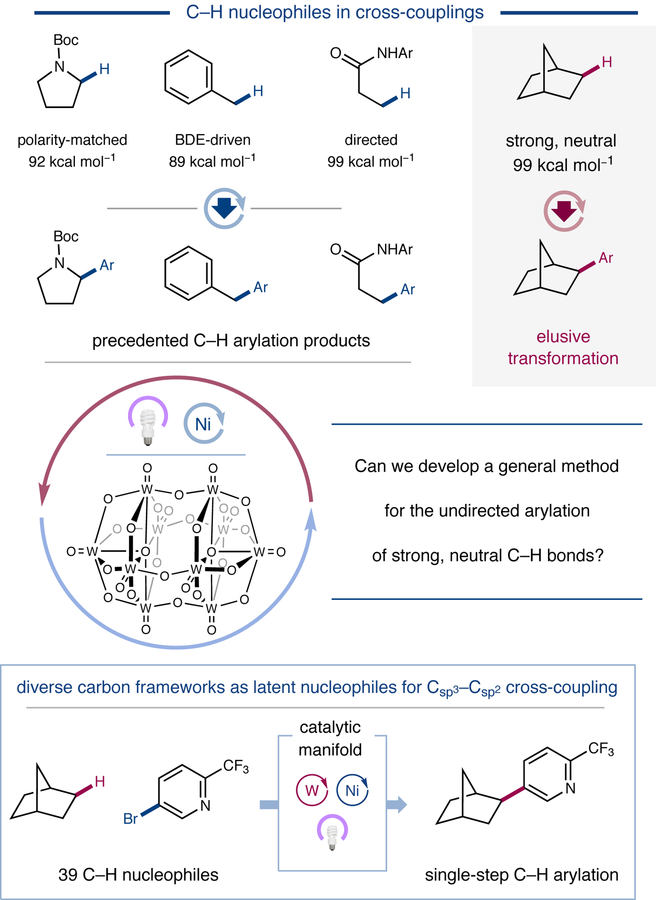
Traditional transition metal-catalyzed Csp3–H arylation methods rely on adjacent or distal functionality to facilitate C–H bond activation (top). The use of a highly oxidizing polyoxometalate photocatalyst in combination with nickel catalysis enables direct arylation of strong, unactivated C–H bonds (bottom). Boc, tert-butoxycarbonyl; Ar, aryl; BDE, bond dissociation energy.
A detailed description of our proposed mechanism is illustrated in Figure 2. Photoexcitation of tetrabutylammonium decatungstate (TBADT, 1) followed by intersystem crossing would produce the triplet excited state (2) (τ = 55 ns)26. Subsequent hydrogen atom abstraction from an alkyl nucleophile such as norbornane (3) by excited-state decatungstate (2) would readily afford singly-reduced decatungstate (4) and carbon-centered radical 5. Disproportionation of singly-reduced decatungstate (4) would regenerate the active HAT photocatalyst 1 and concurrently form doubly reduced decatungstate (6)26. Two successive single-electron reductions of precatalyst NiBr2•dtbbpy (dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) [Ep (NiII/Ni0) = –1.47 V versus Ag/Ag+ in MeCN, see Supplementary Information] by doubly-reduced decatungstate (6) [E1/2red ([W10O32]5–/[W10O32]6–) = –1.52 V versus Ag/Ag+ in MeCN, see Supplementary Information] could initially afford Ni0 species 7, which after capture of alkyl radical 5 would furnish NiI-alkyl species 8. Subsequent oxidative addition into aryl halide 9 by NiI-alkyl species 8 would afford NiIII(aryl)(alkyl) species 10. Reductive elimination would provide desired cross-coupled product 11 as well as NiI species 12. A final single-electron transfer step between this NiI species and the doubly-reduced polyoxometalate 6 would regenerate active Ni0 catalyst 7, as well as singly-reduced TBADT (4), closing both catalytic cycles. An alternative mechanism involving oxidative addition of Ni0 catalyst 7 to aryl halide 9 could also be operative27.
Figure 2 |. Reaction scheme and proposed mechanism for sp3 C–H arylation via a dual polyoxometalate HAT and nickel catalytic manifold.
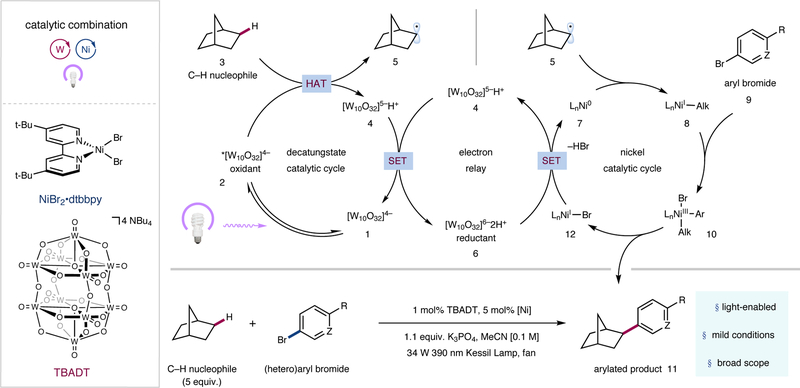
The catalytic cycle begins with photoexcitation of the decatungstate anion 1 to provide triplet excited state 2. Hydrogen atom transfer (HAT) from nucleophile 3 affords reduced photocatalyst 4 and open-shell species 5. Disproportionation of the reduced decatungstate species regenerates the active photocatalyst and affords the reducing hexa-anion 6. Ni0 species 7 subsequently captures alkyl radical 5, furnishing NiI-alkyl species 8. Oxidative addition into aryl electrophile 9 provides NiIII species 10, which undergoes reductive elimination to afford the product (11) and NiI–Br species 12. Single electron transfer (SET) between 6 and 12 regenerates 7 and 4, closing both catalytic cycles. dtbbpy, 4,4′-di-tert-butyl-2,2′-dipyridyl; TBADT, tetrabutylammonium decatungstate; Me, methyl; t-Bu, tert-butyl.
We began our investigation into the proposed transformation by exposing 5-bromo-2-trifluoromethylpyridine and cyclohexane to near-UV light [Kessil 34 W 390 nm light-emitting diodes (LEDs)] in the presence of the commercially available HAT photocatalyst TBADT, NiBr2•dtbbpy, and potassium phosphate in acetonitrile. To our delight we observed a 70% analytical yield of the desired cyclohexyl C–H arylation product. Critical to the success of the reaction was the exclusion of both oxygen and water (see Supplementary Information); however, the use of standard benchtop techniques was sufficient in this regard. Moreover, while five equivalents of the C–H nucleophile affords optimal yields, lower substrate loadings can be used albeit with diminished efficiency (see Supplementary Information).
With optimized conditions in hand, we next sought to examine the scope of the transformation with respect to the C–H bearing partner. As shown in Figure 3, a diverse array of organic frameworks proved to be competent coupling partners for the C–H arylation protocol. Cycloalkanes with various ring sizes ranging from five to eight carbons were arylated in good yields (13 to 16, 57 to 70% yield). Linear aliphatic systems were likewise successful in the protocol (17 to 20, 41 to 56% yield), with a greater-than-statistical preference observed for arylation of the less sterically demanding 2-position for all substrates, including n-hexane (SI-1, 48% yield, 60% selectivity). Electron-withdrawing substituents further improved this regiocontrol, highlighting the selectivity of decatungstate for more hydridic C–H bonds imparted by the electrophilic nature of its excited state26. Accordingly, we found ketones to be particularly effective in modulating regioselectivity, affording products functionalized distal to the electron-withdrawing carbonyl moiety (21, 22, 31–35, 31 to 65% yield).
Figure 3 |. Scope of the alkyl nucleophile coupling partner.
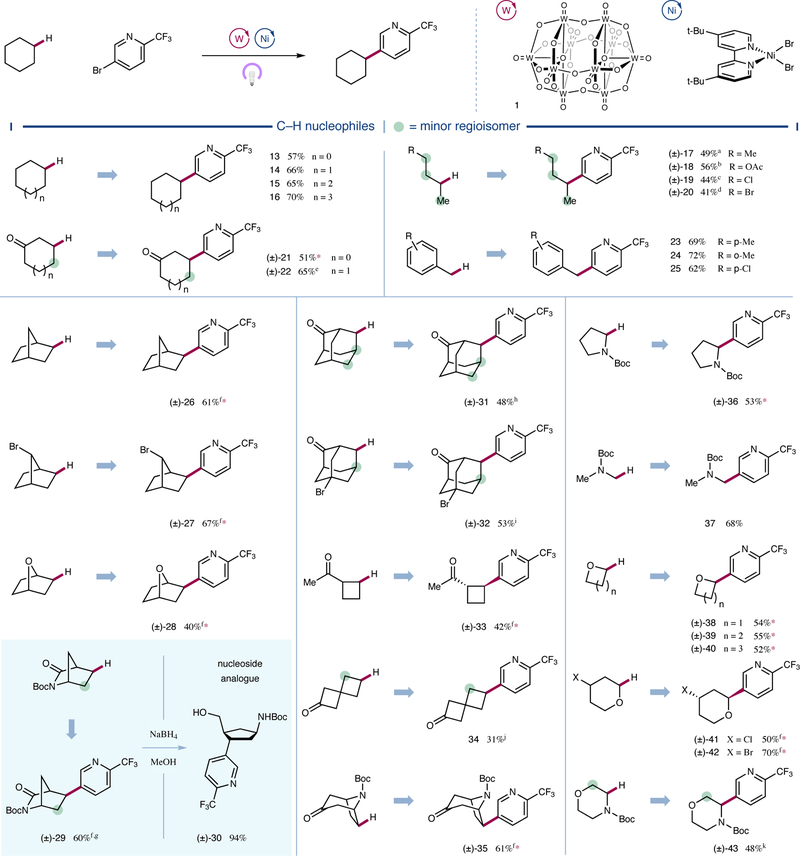
A broad range of C–H nucleophiles are selectively functionalized by this arylation protocol. Cyclic, acyclic, and bicyclic aliphatic systems are amenable substrates. Heteroatom and carbonyl substituents electronically influence regioselectivity, and alkyl halides remain intact. All yields are isolated yields. Conditions as in Figure 2. Green circles denote sites where significant amounts of other regioisomers are observed. See Supplementary Information for experimental details. Ac, acetyl; d.r., diastereomeric ratio; r.r., regioisomeric ratio. *>20:1 r.r.; a70% selectivity; b53% selectivity; c79% selectivity; d93% selectivity; e1.4:1 r.r.; f>20:1 d.r; g2.5:1 r.r.; h79% selectivity, 3:1 d.r. (major); i1.8:1 r.r., 5.4:1 d.r. (major); j8.8:1 r.r.; k3.4:1 r.r.
This C–H arylation protocol was also found to effectively functionalize a range of electronically diverse primary and secondary benzylic C–H bonds, which were arylated in moderate to good yields (23 to 25, 62 to 71% yield, see Supplementary Information for three additional examples). Bridged bicyclic alkanes afforded arylated products with complete exo-selectivity (26 to 29 and 35, 40 to 67% yield), likely due to selective radical capture by nickel catalyst 7 on the less-hindered face. Functionalization of norbornane occurred selectively on the ethylene bridge (26, 61% yield). Notably, a bromide substitutent on the bridging methylene of norbornane was tolerated and, moreover, strongly influenced site-selectivity, giving only the anti product (27, 67% yield). Notably, heteroatom-containing bicycles afforded the desired products in moderate to good yields (28 and 29, 40 and 60% yield, respectively). Arylated lactam 29 was subsequently subjected to ring-opening reductive conditions to afford the carbocyclic nucleoside analogue 30 (94% yield), highlighting the utility of the C–H arylation protocol. Significantly, adamantane derivatives underwent arylation predominantly at the methylene position (31 and 32, 48 and 53% yield, respectively), an unexpected chemoselectivity given decatungstate-catalyzed adamantane functionalization affords 5:1 selectivity for methine positions when corrected for equivalent hydrogen atoms28. This result further highlights the role of the nickel catalyst in determining the regioselectivity of C–C bond formation, presumably via reversible radical capture and selectivity-determining reductive elimination27. Four-membered rings were also competent substrates for this arylation protocol, with both an exocyclic ketone and a spirocyclic ketone affording the desired product in moderate yields (33 and 34, 42 and 31% yield, respectively). Tropinone, a common scaffold among natural products and pharmaceuticals29, was also effectively subjected to this dual-catalysis protocol (35, 61% yield).
It is important to note that this transformation is not restricted to electronically neutral, unactivated C–H systems. Indeed, a variety of α-heteroatom C–H nucleophiles were readily modified with excellent regioselectivity. As follows from decatungstate’s preference for the most hydridic and sterically accessible C–H bond, Boc-protected pyrrolidine was functionalized selectively at the α-amino position (36, 53% yield). Primary α-amino C–H nucleophile N-Boc dimethylamine also found to be an effective substrate for the transformation (37, 68% yield). In addition to nitrogen-containing nucleophiles, a variety of cyclic ethers were regioselectively functionalized in moderate to good yield at the α-oxy position (38 to 43, 48 to 70% yield). Notable among these substrates, alkyl halides were well-tolerated (41 and 42, 50 and 70% yield, respectively), opening avenues for subsequent synthetic manipulations. N-Boc-morpholine underwent C–H arylation predominantly at the α-amino C–H bond (43, 48% yield, 3.4:1 r.r.). Notably, useful amounts of the α-oxy product are generated in this case, in contrast to the quinuclidine-mediated triple catalytic arylation reported previously by our laboratory10 (see Supplementary Information) as well as benzophenone-mediated cyanation30, wherein exclusive α-amino functionalization is observed.
We next turned our attention to the scope of the aryl halide coupling partner. As shown in Figure 4, a broad range of electron-deficient aryl bromides provided the desired products in good yield (44 to 49, 60 to 70% yield). Furthermore, neutral and electron-rich substrates displayed useful coupling efficiencies (50 to 54, 52 to 62% yield). Chlorine- and fluorine-bearing aryl bromides were alkylated selectively as well (55 and SI-5, 50 and 55% yield, respectively). Free alcohol-containing substrate 56 was also found to be a competent coupling partner (55% yield). Ortho-substituted aryl bromides were likewise alkylated in moderate to good yields (57 and 58, 45 and 71% yield, respectively). With respect to heteroaryl bromides, N-Boc-indole 59 underwent the desired transformation in useful efficiency (38% yield). A range of bromopyridines were alkylated in useful to good yields as well (60 to 69, 25 to 64% yield). Bromopyrimidines were effective substrates (70 and 71, 55 and 51% yield, respectively), and both electron-rich and electron-deficient 2-bromothiazoles afforded the desired product in moderate yields (72 and 73, 54 and 51% yield, respectively). Last, Celebrex precursor 74, a pharmaceutically-relevant aryl halide, was subjected to the reaction conditions with a variety of alkyl C–H nucleophiles. Cyclohexane, cyclohexanone, and 7-bromonorbornane were all coupled in good efficiencies (75a-c, 60 to 67% yield), demonstrating the utility of the protocol in cross-coupling complex aryl fragments with structurally diverse C–H nucleophiles.
Figure 4 |. Scope of the aryl halide coupling partner.
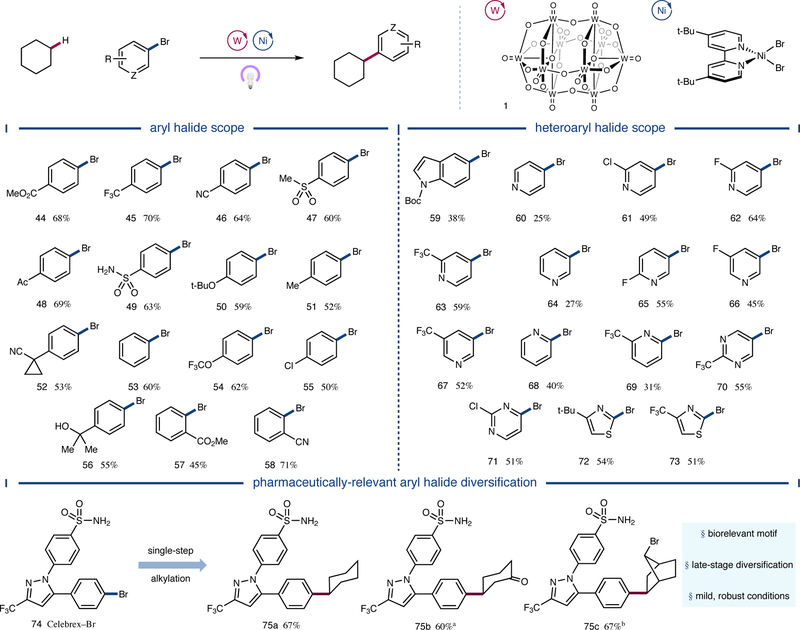
A variety of electronically diverse aryl bromides were functionalized in moderate to good yields, and unprotected polar functionalities such as alcohols and sulfonamides were well tolerated. Moreover, heteroaryl bromides including indoles, pyridines, pyrimidines, and thiazoles were competent coupling partners in the transformation. Finally, the dual catalytic manifold was applied to the synthesis of several Celebrex analogues. All yields are isolated yields. Conditions as in Figure 2. See Supplementary Information for experimental details. a1.4:1 r.r.; b>20:1 d.r.
Having demonstrated the applicability of the C–H arylation protocol to a broad array of C–H nucleophiles and aryl halide electrophiles, we next investigated its efficacy on naturally occurring aliphatic systems. As illustrated in Figure 5a, a variety of inexpensive, abundant natural products were successfully functionalized under our standard conditions, enabling rapid arylation of complex stereo-defined frameworks at carbon sites that lack adaptive functional handles. Observed regioselectivities were in accordance with the expected preferences of this dual-catalytic manifold as described above. A moderate yield of heteroarene coupled eucalyptol was observed (76, 55% yield), with a strong preference for arylation at the most hydridic and sterically accessible C–H bond. Useful efficiencies were observed for the terpene fenchone, which was also found to be a suitable substrate on 5.0 mmol scale (77, 38 and 41% yield, respectively. See Supplementary Information for experimental details). A free alcohol derivative of fenchone was also readily exploited in this protocol (78, 52% yield). Camphene, a terminal olefin-containing natural product, was also an effective substrate for arylation (79, 70% yield). Lastly, we sought to illustrate the generality of this method by derivatizing the lactone sclareolide with a range of aryl and heteroaryl bromides (80a-c, 35% to 43% yield). Notably, an alkyl acid chloride provided the sclareolide-derived ketone in useful efficiency (80d, 33% yield), illustrating the capability of our transformation to install a range of functionality onto complex aliphatic substrates without the need for directing groups.
Figure 5 |. Functionalization, synthesis, and derivatization of natural products.

(a) A series of abundantly available terpenoids serve as competent coupling partners in this dual catalytic protocol, affording complex arylated scaffolds in good yields. Major and minor isomers are denoted. (b) The naturally occurring alkaloid (±)-N-Boc-epibatidine was synthesized in two steps from commercially available materials, and subsequently a small library of analogous compounds was constructed. *>20:1 r.r., >20:1 d.r.; acyclopropane carbonyl chloride employed as electrophilic coupling partner.
Finally, we demonstrated the application of this C–H arylation protocol toward the rapid generation of complex pharmaceutically-relevant molecules. We set our sights on the natural product epibatidine, a potent non-opioid analgesic. Due to its high toxicity, epibatidine has seen limited potential as a commercial pharmaceutical31. However, a range of epibatidine analogues have been investigated in a clinical setting32. We first targeted the synthesis of (±)-N-Boc-epibatidine from commercially available 7-azabicyclo[2.2.1]heptane. When N-Boc-protected amine substrate 81 and 2-chloro-5-bromopyridine were subjected to the reaction conditions, we observed an unoptimized 28% yield of protected epibatidine (82a) in two steps from the commercially available unprotected amine (Figure 5b, see Supplementary Information for experimental details). To our knowledge, this is the shortest formal synthesis of (±)-epibatidine in the multitude of reported procedures to date33. Subsequently, we sought to demonstrate that diversification was possible by variation of both the alkyl fragment and the aryl bromide fragment. A representative sampling of heteroaryl bromides was coupled with bridged bicyclic amines to afford a small set of analogues in synthetically useful yields (82b to 83c, 17 to 44% yield).
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the NIH National Institute of General Medical Sciences (R01 GM103558–03) and gifts from MSD, Bristol-Myers Squibb, Eli Lilly, Genentech, Pfizer and Johnson & Johnson. The authors thank C. Kraml, N. Byrne and L. Wilson (Lotus Separations) for compound purification, and I. Pelczer for assistance in structure determination.
Footnotes
The authors declare no competing financial interests.
Author Information Reprints and permissions information is available at www.nature.com/reprints.
Readers are welcome to comment on the online version of the paper.
Data Availability The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Jana R Pathak TP & Sigman MS Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev 111, 1417–1492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tasker SZ, Standley EA & Jamison TF Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo Z & MacMillan DWC Decarboxylative arylation of α-amino acids via photoredox catalysis: a one-step conversion of biomass to drug pharmacophore. J. Am. Chem. Soc 136, 5257–5260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo JC, Gui J, Yabe Y, Pan C-M & Baran PS Functionalized olefin cross-coupling to construct carbon–carbon bonds. Nature 516, 343–348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X & MacMillan DWC Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3–sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc 138, 13862–13865 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margrey KA, Czaplyski WL, Nicewicz DA & Alexanian EJ A general strategy for aliphatic C–H functionalization enabled by organic photoredox catalysis. J. Am. Chem. Soc 140, 4213–4217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimov RR & Hartwig JF Transition-metal-catalyzed selective functionalization of C(sp3)–H bonds in natural products. Angew. Chem. Int. Ed 57, 4234–4241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Wasa M, Chan KSL, Shao Q & Yu J-Q Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev 117, 8754–8786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao K, Negretti S, Musaev DG, Bacsa J & Davies HML Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD & MacMillan DWC Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 352, 1304–1308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le C, Liang Y, Evans RW, Li X & MacMillan DWC Selective sp3 C–H alkylation via polarity-match-based cross-coupling. Nature 547, 79–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X & MacMillan DWC Direct aldehyde C–H arylation and alkylation via the combination of nickel, hydrogen atom transfer, and photoredox catalysis. J. Am. Chem. Soc 139, 11353–11356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitz DR, Tellis JC & Molander GA Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc 138, 12715–12718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields BJ & Doyle AG Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc 138, 12719–12722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twilton J et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem 1, 0052 (2017). [Google Scholar]
- 16.Liu D et al. Nickel-catalyzed selective oxidative radical cross-coupling: an effective strategy for inert Csp3–H functionalization. Org. Lett 17, 998–1001 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Hill CL & Prosser-McCartha CM Photocatalytic and Photoredox Properties of Polyoxometalate Systems. In Photosensitization and Photocatalysis Using Inorganic and Organometallic Compounds (eds Kalyanasundaram K & Grätzel M) 307–330 (Springer, 1993). [Google Scholar]
- 18.Renneke RF & Hill CL Homogeneous catalytic photochemical functionalization of alkanes by polyoxometalates. J. Am. Chem. Soc 108, 3528–3529 (1986). [Google Scholar]
- 19.Renneke RF & Hill CL Selective photochemical dehydrogenation of saturated hydrocarbons with quantum yields approaching unity. Angew. Chem. Int. Ed 27, 1526–1527 (1988). [Google Scholar]
- 20.Ravelli D, Protti S & Fagnoni M Decatungstate anion for photocatalyzed “window ledge” reactions. Acc. Chem. Res 49, 2232–2242 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Schultz DM et al. Oxyfunctionalization of the remote C–H bonds of aliphatic amines by decatungstate photocatalysis. Angew. Chem. Int. Ed 56, 15274–15278 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Halperin SD et al. Development of a direct photocatalytic C–H fluorination for the preparative synthesis of odanacatib. Org. Lett 17, 5200–5203 (2015). [DOI] [PubMed] [Google Scholar]
- 23.West JG, Huang D & Sorensen EJ Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun 6, 10093 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BDE given for cyclohexane from: Luo Y-R. Handbook of Bond Dissociation Energies in Organic Compounds (CRC Press: Boca Raton, FL, 2003). [Google Scholar]
- 25.Ravelli D, Fagnoni M, Fukuyama T, Nishikawa. T & Ryu I Site-selective C–H functionalization by decatungstate anion photocatalysis: synergistic control by polar and steric effects expands the reaction scope. ACS Catal 8, 701–713 (2018). [Google Scholar]
- 26.De Waele V, Poizat O, Fagnoni M, Bagno A & Ravelli D Unraveling the key features of the reactive state of decatungstate anion in hydrogen atom transfer (HAT) photocatalysis. ACS Catal 6, 7174–7182 (2016). [Google Scholar]
- 27.Gutierrez O, Tellis JC, Primer DN, Molander GA & Kozlowski MC Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc 137, 4896–4899 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ermolenko LP & Gianotti C Aerobic photocatalysed oxidations of alkanes in the presence of decatungstates: products and effects of solvent and counter-ion of the catalyst. J. Chem. Soc. Perkin Trans 2, 0, 1205–1210 (1996). [Google Scholar]
- 29.Grynkiewicz G & Gadzikowska M Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep 60, 439–463 (2008). [PubMed] [Google Scholar]
- 30.Hoshikawa T, Yoshioka S, Kamijo S & Inoue M Photoinduced direct cyanation of C(sp3)–H bonds. Synthesis 45, 874–887 (2013). [Google Scholar]
- 31.Bannon AW et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science 279, 77–80 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Badio B, Garraffo HM, Plummer CV, Padgett WL & Daly JW Synthesis and nicotinic activity of epiboxidine: an isoxazole analogue of epibatidine. Eur. J. Pharmacol 321, 189–194 (1997). [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira Filho RE & Omori AT Recent syntheses of frog alkaloid epibatidine. J. Braz. Chem. Soc 26, 837–850 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


