Abstract
BACKGROUND:
In the current study, the authors attempted to describe the incidence, most common sites, and mortality of second primary malignancies among survivors of common cancers.
METHODS:
The authors identified patients aged ≥18 years who were diagnosed with a primary malignancy from the 10 most common cancer sites (prostate, breast, lung, colon, rectum, bladder, uterus, kidney, melanoma, and non-Hodgkin lymphoma) between 1992 and 2008 from Surveillance, Epidemiology, and End Results data. Factors associated with the incidence of second primary malignancies were explored using bivariable and multivariable models, and mortality attributable to first and second primary malignancies was examined.
RESULTS:
A cohort of 2,116,163 patients was identified, 170,865 of whom (8.1%) developed a second primary malignancy. Survivors of bladder cancer had the highest risk of developing a second cancer. In a multivariable model controlling for age, race, tumor grade, stage of disease, marital status, educational level, and income, a history of non-Hodgkin lymphoma (hazard ratios of 2.70 and 2.88, respectively, for men and women) and bladder cancer (hazard ratios of 1.88 and 1.66, respectively, for men and women) predicted the highest risk of developing a second cancer. For patients with 2 incident cancers, 13% died of their initial cancer, but greater than one-half (55%) died of their second primary malignancy. Lung cancer was the cause of death in 12% of patients with 2 incident cancers.
CONCLUSIONS:
Nearly 1 in 12 patients diagnosed with a common cancer developed a second malignancy, the most common of which was lung cancer. Greater than one-half of patients with 2 incident cancers died of their secondary malignancy. The findings from the current study may inform care strategies among cancer survivors.
Keywords: epidemiology, screening, second primary neoplasms, Surveillance, Epidemiology, and End Results (SEER) program, survivors
INTRODUCTION
It is estimated that 1 in 4 deaths in the United States is related to cancer; however, death rates are reported to have fallen by 22% between 1991 and 2011.1 These improved outcomes have resulted in a growing population of cancer survivors in the United States. In fact, 14 million cancer survivors were alive in the United States in 2012, and that number is expected to increase to nearly 20 million by 2024.2,3 With such a large population, many of these cancer survivors are at risk of developing a second primary malignancy. Multiple primary cancers now account for approximately 17% of all incident cancers reported each year to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.4 Furthermore, among select subgroups of cancer survivors, the lifetime risk of developing second primary malignancies may be as high as 33%, with many of these being lethal.5–8 Cancer survivors may be especially susceptible to developing second primary malignancies due to a variety of unique factors, including genetic syndromes,9 common etiologic exposures,10,11 and the late effects of chemotherapy and radiotherapy.12–14
Given the longer duration of cancer survivorship and the substantial percentage of survivors at risk of developing second primary malignancies, the incidence and mortality from second primary malignancies are likely to increase. There is ample literature describing the risk of second primary malignancies in certain site-specific survivor groups, such as patients with testicular cancer,8 head and neck cancer,15 and thyroid cancer.12 However, to the best of our knowledge, less isknown regarding the risk of second primary malignancies across the spectrum of cancer survivors diagnosed with other, more common malignancies. In addition, it also is unclear from which sites these secondary malignancies most commonly arise or the cancer-specific survival rates for each second primary malignancy. Given that screening practices have been widely adopted for several commonly diagnosed cancers (breast, prostate, and lung), we believed a better understanding of the epidemiology of second primary malignancies could help to inform long-term outcomes for cancer survivors, among whom screening recommendations aimed at a broader population may not apply.
As such, the objective of the current study was to more clearly understand the risk of developing and dying of a second primary malignancy for survivors of the most commonly diagnosed cancers. We sought to describe the sites from which these second primary malignancies arise and estimate the mortality attributable to first and second primary malignancies in a large, population-based cohort. A clearer comprehension of the risk of developing and dying of a second primary malignancy could help to provide a better understanding of the appropriate long-term surveillance strategies.
MATERIALS AND METHODS
Data Set
We obtained data from the April 2013 release of the publicly available SEER database. This release from the SEER program compiled information regarding cancer incidence and survival from 18 population-based cancer registries throughout the United States and covers approximately 28% of the general population.16
Patient Cohort
We identified all patients diagnosed with a primary malignancy among the 10 cancer sites with the highest incidence in both sexes (ie, prostate, breast, lung, colon, rectum, bladder, uterus, kidney, melanoma, and non-Hodgkin lymphoma [NHL]).1 We included cases of carcinoma in situ and clinically localized, regionally advanced, and metastatic disease. Although data were available through 2011, we limited the current study cohort to patients diagnosed between 1992 and 2008 to ensure 3 years of follow-up after a cancer diagnosis. We began by identifying all individuals in the data set who met the above criteria. We subsequently excluded cases in which the second malignancy had the same histology as the first malignancy (68,786 cases) and cases in which the second primary malignancy was diagnosed within 1 year of the first malignancy (557,346 cases) to prevent misclassification of metastatic primary malignancies as second primary malignancies. Finally, patients aged <18 years (6415 patients) were excluded. From this cohort, individuals with >1 pathologic diagnosis of cancer were identified and denoted as the second primary malignancy group. Cancer cases were categorized by site using codes from the ninth revision of the International Statistical Classification of Diseases and Related Health Problems maintained by the World Health Organization.
Primary Outcome and Covariates of Interest
The primary outcome was the diagnosis of a second primary malignancy, based on a diagnosis of 1 of the 10 most common incident cancer types after a prior diagnosis of another common malignancy. The secondary outcome of interest was death from a primary or secondary malignancy. Patient demographics included age in years (1835, 36–50, 51–65, 66–80, and ≥80), marital status (single, married/domestic partner, divorced/widowed/separated, or unknown), race (white, black, unknown, and other [included American Indian, Alaska Native, and Asian/Pacific Islander]), sex (male or female), and year of diagnosis (1992–1996, 1997–2000, 2001–2004, and 2005–2008). SEER merged ZIP code-level data for educational level and annual household income from the 2008 US Census data. Individual-level data were imputed from the percentage of patients holding a Bachelor’s degree and the median annual household income in each patient’s ZIP code, which was then stratified into quartiles. Tumor characteristics included grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated, and unknown/not applicable) and stage (in situ and/ or localized, regional, distant, unstaged, and unknown) based on the SEER Summary Staging Manual – 2000.
Statistical Analysis
We generated descriptive statistics for the current study cohort and evaluated the association between covariates of interest and the development of a second primary malignancy using chi-square and 2-sided Student t tests. Next, we displayed the survival distribution estimate of a second primary malignancy using the Kaplan-Meier method. We then estimated hazard ratios (HRs), stratified by sex, using multivariable Cox proportional hazards models that incorporated our covariates of interest (first cancer site, age, race, marital status, tumor grade, stage, percentage of patients holding a Bachelor’s degree, and median annual household income). We used the highest incident cancer as the referent group, which was prostate cancer for men and breast cancer for women. Finally, we calculated distributions of the cause of death in all patients and in patients with multiple malignancies stratified by first cancer site, and whether cause of death was due to a primary or secondary malignancy. All statistical tests were performed at the 5% significance level and conducted using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC). The current study was deemed to be exempt by the Institutional Review Board of the University of California at Los Angeles.
RESULTS
We identified 2,116,163 patients with first incident cancers who were diagnosed between 1992 and 2008, among whom 170,865 (8.1%) developed a second primary malignancy (Table 1). The mean follow-up of the entire cohort was 7.09 years (standard deviation, 4.26 years), and when stratified by primary cancer type, the mean follow-up was >6 years in all primary cancer types except for patients with primary lung cancer, in whom the mean follow-up was 4.18 years (standard deviation, 3.52 years) (Table 2). The majority of patients (60%) with a second primary malignancy were aged >65 years, and were older than patients without a second primary malignancy (aged 66 years vs aged 63 years; P<.001). Of those patients with a second primary malignancy, 85% were white; 63% were male; and 73% were survivors of either prostate, breast, colorectal, or bladder cancer. The majority of patients with a second primary malignancy (55%) had a welldifferentiated or moderately differentiated first cancer.
Table 1.
Patient Demographics of the Analytic Cohort
| Covariate | All Patients n = 2,116,163 |
Patients With 2 Malignancies n = 170,865 |
Patients With 1 Malignancy n = 1,945,298 |
P |
|---|---|---|---|---|
| Age at diagnosis of first malignancy, y | <.0001 | |||
| 18–35 | 65,765 (3.11) | 1097 (0.6) | 64,670 (3.3) | |
| 36–50 | 315,219 (14.9) | 11,621 (6.8) | 303,598 (15.6) | |
| 51–65 | 743,755 (35.2) | 56,270 (32.9) | 687,485 (35.3) | |
| 66–80 | 783,457 (37.0) | 87,586 (51.3) | 695,871 (35.8) | |
| ≥80 | 207,965 (9.8) | 14,291 (8.4) | 193,674 (10.0) | |
| Marital status | <.0001 | |||
| Single | 237,754 (11.2) | 14,897 (8.7) | 222,857 (11.5) | |
| Married/domestic partner | 1,282,229 (60.6) | 109,923 (64.3) | 1,172,306 (60.3) | |
| Divorced/widowed/separated | 445,631 (21.1) | 34,732 (20.3) | 410,899 (21.1) | |
| Unknown | 150,549 (7.1) | 11,313 (6.6) | 139,236 (7.2) | |
| Race/ethnicity | <.0001 | |||
| White | 1,749,143 (82.7) | 146,323 (85.6) | 1,602,820 (82.4) | |
| Black | 203,106 (9.6) | 15,727 (9.2) | 187,379 (9.6) | |
| Other | 132,734 (6.3) | 8695 (5.1) | 124,039 (6.4) | |
| Unknown | 31,180 (1.5) | 120 (0.1) | 31,060 (1.6) | |
| Sex | <.0001 | |||
| Male | 1,096,043 (51.8) | 107,097 (62.7) | 988,946 (50.8) | |
| Female | 1,020,120 (48.2) | 63,768 (37.3) | 956,352 (49.2) | |
| % Bachelor’s degree, quartile | <.0001 | |||
| 1st | 535,710 (25.3) | 42,926 (25.1) | 492,784 (25.3) | |
| 2nd | 532,701 (25.2) | 43,455 (25.4) | 489,246 (25.2) | |
| 3rd | 506,193 (23.9) | 39,001 (22.8) | 467,192 (24.0) | |
| 4th | 541,559 (25.6) | 45,483 (26.6) | 496,076 (25.5) | |
| Median household income, quartile | <.0001 | |||
| 1st | 512,048 (24.2) | 40,572 (23.8) | 471,476 (24.2) | |
| 2nd | 530,670 (25.1) | 42,938 (25.1) | 487,732 (25.1) | |
| 3rd | 536,631 (25.4) | 44,570 (26.1) | 492,061 (25.3) | |
| 4th | 536,814 (25.4) | 42,785 (24.0) | 494,029 (25.4) | |
| Y of diagnosis | <.0001 | |||
| 1992–1996 | 344,153 (16.3) | 45,783 (26.8) | 298,370 (15.3) | |
| 1997–2000 | 385,229 (18.2) | 41,503 (24.3) | 343,726 (17.7) | |
| 2001–2004 | 672,300 (31.8) | 53,467 (31.3) | 618,833 (31.8) | |
| 2005–2008 | 714,481 (33.8) | 30,112 (17.6) | 684,369 (35.2) | |
| Primary site | <.0001 | |||
| Prostate | 595,385 (28.1) | 57,946 (33.9) | 537,439 (27.6) | |
| Breast | 491,223 (23.2) | 29,194 (17.1) | 462,029 (23.8) | |
| Lung | 174,431 (8.2) | 8296 (4.9) | 166,135 (8.5) | |
| Colorectal | 275,715 (13.0) | 22,679 (13.3) | 253,036 (13.0) | |
| Non-Hodgkin lymphoma | 108,266 (5.1) | 9499 (5.6) | 98,767 (5.1) | |
| Bladder | 112,207 (5.3) | 15,571 (9.1) | 96,636 (5.0) | |
| Melanoma | 124,587 (5.9) | 9563 (5.6) | 115,024 (5.9) | |
| Endometrial | 90,242 (4.3) | 7298 (4.3) | 82,944 (4.3) | |
| Kidney | 70,945 (3.4) | 6839 (4.0) | 64,106 (3.3) | |
| Thyroid | 73,162 (3.5) | 3980 (2.3) | 69,182 (3.6) | |
| Grade | <.0001 | |||
| Well differentiated | 224,008 (10.6) | 19,677 (11.5) | 204,331 (10.5) | |
| Moderately differentiated | 823,121 (38.9) | 74,927 (43.9) | 748,194 (38.5) | |
| Poorly differentiated | 485,378 (22.9) | 34,769 (20.4) | 450,609 (23.2) | |
| Undifferentiated | 47,044 (2.2) | 3708 (2.2) | 43,336 (2.2) | |
| Unknown/not applicable | 536,612 (25.4) | 37,784 (22.1) | 498,828 (25.6) | |
| Stage | <.0001 | |||
| In situ or localizeda | 1,330,915 (62.9) | 116,668 (68.3) | 1,214,247 (62.4) | |
| Regional | 396,089 (18.7) | 25,650 (15.0) | 370,439 (19.0) | |
| Distant | 127,696 (6.0) | 3852 (2.3) | 123,844 (6.4) | |
| Unstaged | 195,826 (9.3) | 13,824 (8.1) | 182,002 (9.4) | |
| Unknown | 65,637 (3.1) | 10,871 (6.4) | 54,766 (2.8) |
Includes cases of prostate cancer coded as “localized/regional.”
Table 2.
Follow-Up For Analytic Cohort
| Disease Site | Mean Follow-Up (SD)a |
|---|---|
| All sites | 7.09 (4.26) |
| Lung | 4.18 (3.52) |
| Prostate | 7.54 (4.07) |
| Breast | 7.72 (4.29) |
| Colorectal | 6.44 (4.17) |
| Melanoma | 7.76 (4.36) |
| Bladder | 6.77 (4.18) |
| Non-Hodgkin lymphoma | 6.72 (4.10) |
| Thyroid | 8.16 (4.24) |
| Kidney | 6.45 (3.90) |
| Endometrial | 7.79 (4.48) |
Abbreviation: SD, standard deviation.
Follow-up time shown in years.
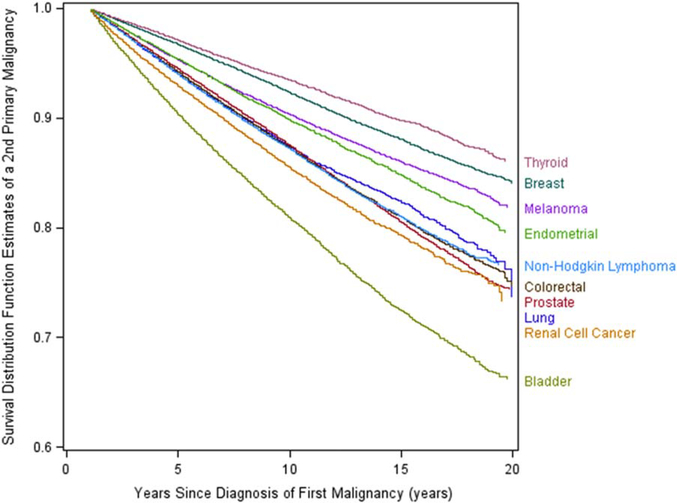
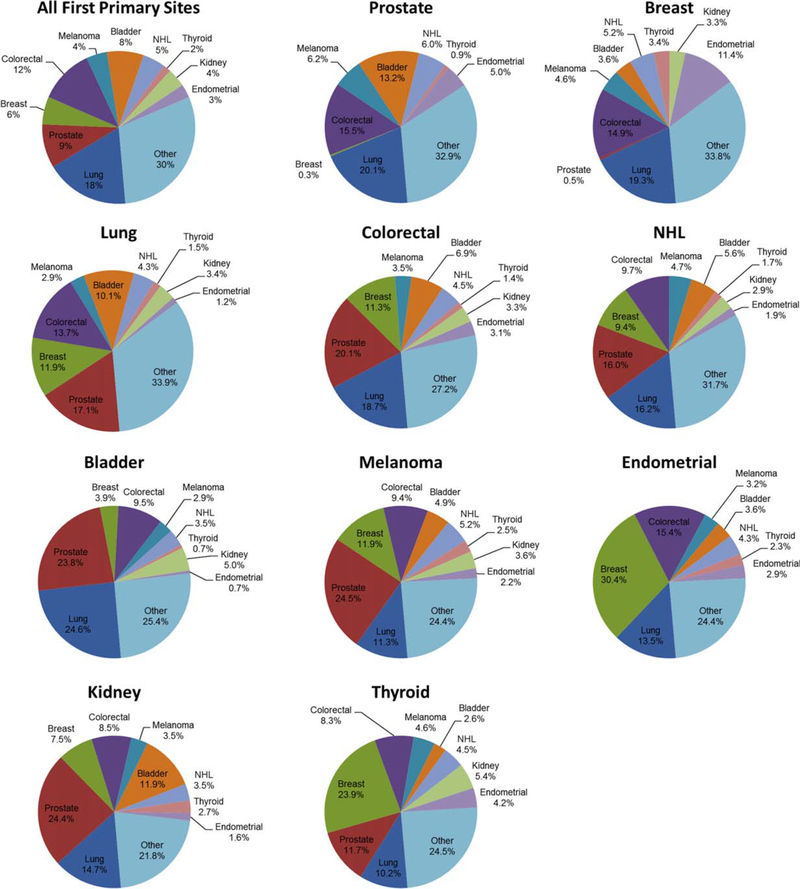
Figure 1 shows the estimates of survival distribution functions of second primary malignancies by primary cancer site. Survivors of bladder cancer demonstrated the worst second cancer-free survival of all survivors of primary cancer, with 19% and 34% of survivors, respectively, diagnosed with a second primary cancer at 10 and 20 years. After bladder cancer, second primary malignancies were most commonly diagnosed among survivors of lung, prostate, colorectal, and kidney cancers. Figure 2 displays the distribution of second primary malignancy sites for the 10 primary cancers evaluated. The most commonly diagnosed second primary malignancy was lung cancer, representing 18% of all second primary malignancies, followed by colorectal cancer (12%), prostate cancer (9%), and bladder cancer (8%). Lung cancer was particularly common among survivors of bladder cancer, representing 25% of all second cancer diagnoses in this group. Among survivors of endometrial and thyroid cancer, breast cancer was the most frequently diagnosed second primary malignancy at 30.4% and 23.9%, respectively. In survivors of lung cancer, kidney cancer, colorectal cancer, and melanoma, prostate cancer was the most frequently diagnosed second primary malignancy.
Figure 1.
Second primary malignancy-free survival by primary malignancy site.
Figure 2.
Sites of second primary malignancy by first primary malignancy site. NHL indicates non-Hodgkin lymphoma.
In the Cox regression models, compared with men diagnosed with prostate cancer, men with a history of any other primary cancer type demonstrated an increased risk of developing a second primary malignancy after controlling for age at diagnosis, sex, race, marital status, tumor grade, stage of disease, educational level, and household income (Table 3). Similarly, when compared with those diagnosed with breast cancer, women with a history of any other primary cancer type had an increased risk of developing a second primary cancer after controlling for the aforementioned covariates. In both men and women, the highest risk was observed for patients diagnosed with NHL, who had nearly 3 times the risk of developing a second primary malignancy compared with survivors of prostate cancer (hazard ratio [HR], 2.70; 95% confidence interval [95% CI], 2.58–2.83) and female survivors of breast cancer (HR, 2.88; 95% CI, 2.69–3.07). In addition, both male and female survivors of bladder cancer (men: HR, 1.88 [95% CI, 1.84–1.92] and women: HR, 1.66 [95% CI, 1.60–1.72]) and kidney cancer (men: HR, 1.86 [95% CI, 1.80–1.92] and women: HR, 1.53 [95% CI, 1.46–1.60]) had a considerably higher risk of developing a second primary malignancy compared with survivors of prostate and breast cancer. We observed similar results when assessing the development of an advanced stage second malignancy (ie, stage III or IV). We also noted an increased risk in both sexes of developing a second primary malignancy in all age groups when compared with those aged 18 to 35 years. In women, this risk was greatest among those aged 66 to 80 years (HR, 7.13; 95% CI, 6.63–7.65), whereas in men the risk was highest in those aged >80 years (HR, 15.24; 95% CI, 13.47–17.23).
Table 3.
Risk of Developing a Second Primary Malignancy Estimated From a Cox Proportional Hazards Model
| Females | Males | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Site | ||||
| Prostate | - | Referent | ||
| Bladder | 1.66 (1.60–1.72) | <.0001 | 1.88 (1.84–1.92) | <.0001 |
| Breast | Referent | 1.69 (1.53–1.87) | <.0001 | |
| Colorectal | 1.17 (1.14–1.20) | <.0001 | 1.42 (1.39–1.45) | <.0001 |
| Endometrial | 1.31 (1.27–1.34) | <.0001 | - | |
| Lung | 1.22 (1.17–1.26) | <.0001 | 1.44 (1.39–1.49) | <.0001 |
| Melanoma | 1.37 (1.31–1.43) | <.0001 | 1.69 (1.63–1.75) | <.0001 |
| Non-Hodgkin lymphoma | 2.88 (2.69–3.07) | <.0001 | 2.70 (2.58–2.83) | <.0001 |
| Renal cell cancer | 1.53 (1.46–1.60) | <.0001 | 1.86 (1.80–1.92) | <.0001 |
| Thyroid | 1.29 (1.23–1.34) | <.0001 | 1.73 (1.63–1.84) | <.0001 |
| Age at diagnosis of first malignancy, y | ||||
| 18–35 | Referent | Referent | ||
| 36–50 | 2.34 (2.18–2.52) | <.0001 | 3.28 (2.89–3.71) | <.0001 |
| 51–65 | 4.67 (4.35–5.02) | <.0001 | 8.80 (7.79–9.94) | <.0001 |
| 66–80 | 7.13 (6.63–7.65) | <.0001 | 15.10 (13.37–17.04) | <.0001 |
| ≥80 | 6.20 (5.75–6.69) | <.0001 | 15.24 (13.47–17.23) | <.0001 |
| Race | ||||
| White | Referent | Referent | ||
| Black | 1.04 (1.01–1.07) | .007 | 1.13 (1.11–1.15) | <.0001 |
| Other | 0.83 (0.80–0.86) | <.0001 | 0.79 (0.76–0.81) | <.0001 |
| Unknown | 0.05 (0.04–0.08) | <.0001 | 0.04 (0.04–0.05) | <.0001 |
| Marital status | ||||
| Married/domestic partner | Referent | Referent | ||
| Divorced/widowed/separated | 1.09 (1.07–1.11) | <.0001 | 1.05 (1.03–1.07) | <.0001 |
| Single | 1.06 (1.03–1.09) | <.0001 | 0.97 (0.94–0.99) | .0032 |
| Unknown | 1.04 (1.00–1.08) | .0509 | 1.04 (1.01–1.06) | .0029 |
| Grade | ||||
| Well differentiated | Referent | Referent | ||
| Moderately differentiated | 1.05 (1.03–1.08) | <.0001 | 1.01 (0.99–1.04) | .3413 |
| Poorly differentiated | 1.12 (1.09–1.15) | <.0001 | 1.06 (1.04–1.09) | <.0001 |
| Undifferentiated | 1.20 (1.13–1.27) | <.0001 | 1.11 (1.06–1.16) | <.0001 |
| Unknown/not applicable | 1.01 (0.98–1.04) | .4398 | 0.93 (0.90–0.96) | <.0001 |
| Stage | ||||
| Localized and in situ | Referent | Referent | ||
| Distant | 0.96 (0.92–1.01) | .1312 | 0.84 (0.80–0.88) | <.0001 |
| Regional | 1.01 (0.99–1.03) | .1687 | 0.97 (0.95–0.99) | .0075 |
| Unknown | 0.01 (0.00–0.02) | .9196 | 1.05 (1.03–1.08) | <.0001 |
| Unstaged | 0.52 (0.49–0.55) | <.0001 | 0.69 (0.66–0.72) | <.0001 |
| % Bachelor’s degree, quartile | ||||
| 1st | 1.14 (1.10–1.18) | <.0001 | 1.12 (1.09–1.15) | <.0001 |
| 2nd | 1.08 (1.05–1.12) | <.0001 | 1.07 (1.04–1.09) | <.0001 |
| 3rd | 1.04 (1.01–1.07) | .0029 | 1.01 (0.99–1.03) | .1811 |
| 4th | Referent | Referent | ||
| Median income, quartile | ||||
| 1st | 0.90 (0.87–0.93) | <.0001 | 0.96 (0.93–0.98) | .0012 |
| 2nd | 0.91 (0.88–0.94) | <.0001 | 0.93 (0.91–0.95) | <.0001 |
| 3rd | 0.97 (0.95–0.99) | .0265 | 0.99 (0.98–1.01) | .5978 |
| 4th | Referent | Referent |
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio.
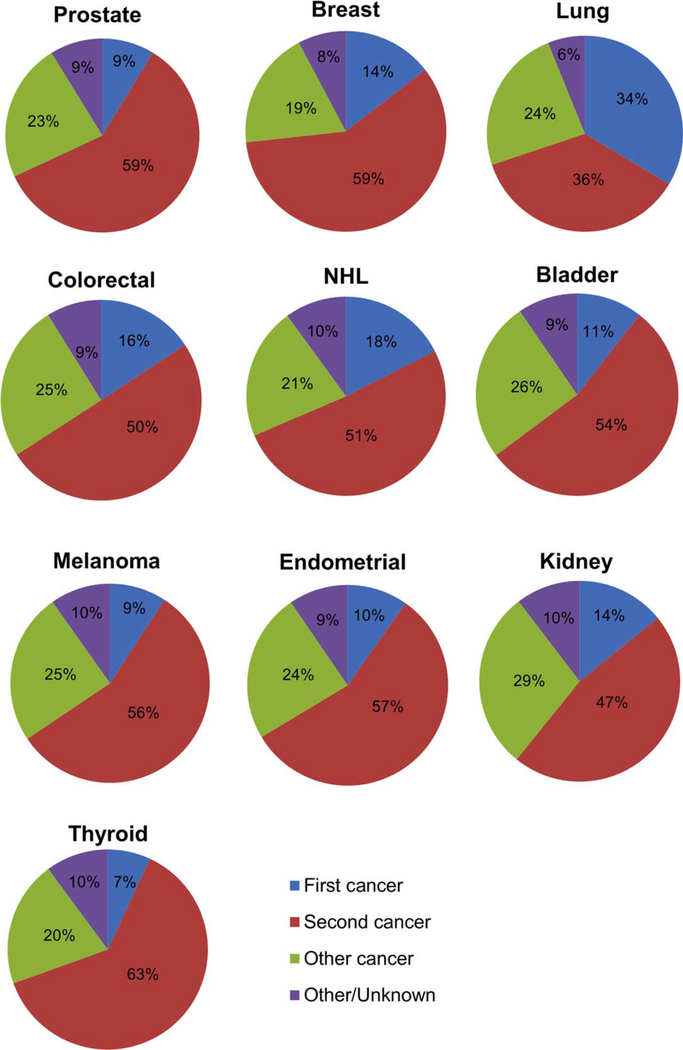
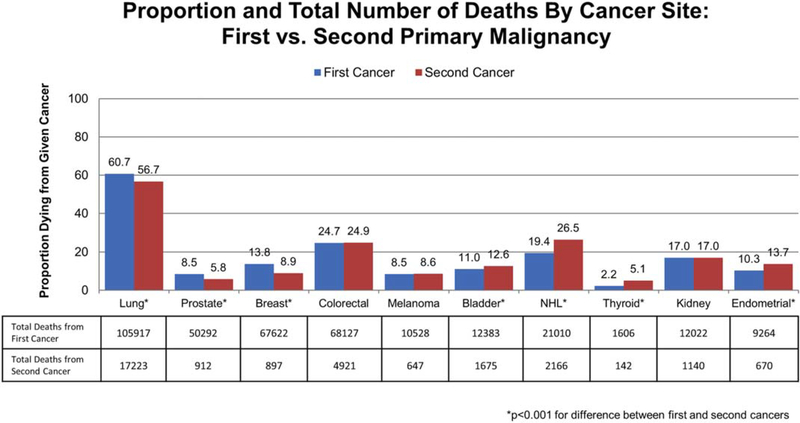
Table 4 describes the causes of death for patients in the current study cohort. In our cohort of 2,116,163 patients, 771,150 (36%) died. Cancer from either a first or second malignancy was responsible for 54% of all deaths. Among patients with a single malignancy, 52% died of their first and only cancer. For patients with 2 incident cancers, 13% died of their initial cancer whereas greater than one-half (55%) died of their second primary malignancy. This was greater than twice the percentage of patients who died of noncancer causes (23%). It is interesting to note that lung cancer was the most lethal malignancy; overall, 6% of the entire cohort and 12% of patients with a second primary malignancy died of lung cancer. Figure 3 shows the cause of death (first malignancy, second malignancy, or other) for patients with 2 primary malignancies, stratified by the site of the malignancy. In this analysis, patients with thyroid cancer were found to have the highest rate of death from their second malignancy, with 63% of patients dying of a second cancer, whereas patients with lung cancer had the lowest rate of death from their second malignancy, with 36% of patients dying of a second malignancy. A second primary cancer was responsible for at least 50% of the deaths noted in survivors of all the malignancies we examined, except for survivors of lung and kidney cancer. Figure 4 demonstrates the percentage and total number of patients dying of a given cancer stratified by first cancer versus second primary malignancy. A greater percentage of patients (56.7%) died of second primary lung cancer than cancer of any other given site. A greater overall number of patients (17,223 patients) died of second primary lung cancer than from all (primary and secondary) cases of melanoma, bladder cancer, thyroid cancer, kidney cancer, or endometrial cancer.
Table 4.
Causes of Death by Site and by Sequence (First Versus Second Primary Malignancy)
| Total Deaths No. (%) N = 771,150 |
Deaths in Patients With 2 Cancers No. (%) N = 98,279 |
Deaths in Patients With 1 Cancer No. (%) N = 672,871 |
P | |
|---|---|---|---|---|
| Cause of death (by site) | <.0001 | |||
| Lung | 124,965 (16.2) | 20,884 (21.3) | 104,081 (15.5) | |
| Prostate | 51,294 (6.7) | 4198 (4.3) | 47,096 (7.0) | |
| Breast | 68,563 (8.9) | 3275 (3.3) | 65,288 (9.7) | |
| Colorectal | 73,606 (9.5) | 7502 (7.6) | 66,104 (9.8) | |
| Melanoma | 11,294 (1.5) | 1145 (1.2) | 10,149 (1.5) | |
| Bladder | 14,251 (1.9) | 2891 (3.0) | 11,360 (1.7) | |
| Non-Hodgkin lymphoma | 23,434 (3) | 3341 (3.4) | 20,093 (3.0) | |
| Thyroid | 1760 (0.2) | 242 (0.3) | 1518 (0.2) | |
| Renal cell cancer | 13,329 (1.7) | 1761 (1.8) | 11,568 (1.7) | |
| Endometrial | 10,004 (1.3) | 1092 (1.1) | 8912 (1.3) | |
| Other cancer | 28,291 (3.7) | 28,291 (28.8) | NA | |
| Other noncancer | 336,303 (43.6) | 22,803 (23.2) | 313,500 (46.6) | |
| Unknown | 14,056 (1.8) | 854 (0.9) | 13,202 (2.0) | |
| Cause of death (by sequence) | <.0001 | |||
| First cancer | 358,771 (46.5) | 12,602 (12.8) | 346,169 (51.5) | |
| Second cancer | 54,265 (7.0) | 54,265 (55.2) | NA | |
| Other cancer/unknown | 21,811 (2.8) | 8609 (8.8) | 13,202 (2.0) | |
| Other noncancer | 336,303 (43.6) | 22,803 (23.2) | 313,500 (46.6) |
Abbreviation: NA, not applicable.
Figure 3.
Cause of death (first malignancy vs second malignancy vs other) among those with >1 cancer by first primary malignancy site. NHL indicates non-Hodgkin lymphoma.
Figure 4.
Percentage and total number of deaths by cancer site: first versus second primary malignancy. NHL indicates nonHodgkin lymphoma.
DISCUSSION
In this large population-based study, there were 4 notable findings. First, 1 in 12 survivors of common cancers developed a second primary malignancy, with lung cancer being the most commonly diagnosed second primary malignancy. Second, among this group, greater than onehalf (55%) died of their second primary malignancy, exceeding the percentage of patients with only a single cancer who died of that cancer. Third, patients with bladder cancer had the highest risk of being diagnosed with a second malignancy, with lung cancer being the most commonly diagnosed second primary malignancy. Finally, among patients with 2 malignancies, a lung cancer diagnosis was the most lethal, representing 12% of all deaths in this group, and representing a greater number of overall deaths than from melanoma, bladder cancer, thyroid cancer, kidney cancer, or endometrial cancer.
That second primary malignancies are common after all common primary cancers of highest incidence parallels prior reports that 8% of patients diagnosed with cancer between 1975 and 2001 were affected by a second primary malignancy.17 Our reported percentage of second primary malignancies among adult cancer survivors exceeds the 3% to 4% of pediatric cancer survivors who develop a second cancer.18,19 This may reflect a common etiologic exposure (eg, tobacco smoke) contributing to the development of both primary malignancies. Another explanation may be that patients who develop cancer may, by virtue of their exposure to the health care system during treatment of their first cancer, undergo routine screening interventions, in turn leading to the diagnosis of a second primary malignancy. This is consistent with the findings of the current study, in which we noted an especially high rate of secondary prostate, breast, and colorectal cancers, all of which are commonly diagnosed through screening.20
It is important to note that we observed a very high mortality rate associated with second primary malignancies, even exceeding that of patients diagnosed with a single cancer. Several factors may be contributing to this finding. First, lung cancer, which is known to be particularly lethal, represented the largest percentage of secondary cancers among the current study cohort (18%), and was likely a driving factor in the lethality of second primary malignancies in this study. The high incidence of secondary lung cancers in the cancer survivor population likely stems from several factors; these include exposure to tobacco smoke (a known etiologic agent for virtually all malignancies) as well prior treatment with chemotherapeutic agents and radiotherapy, which have been shown to be associated with modest risk increases for lung cancer.21 In addition, patients with a second malignancy who received prior chemotherapy for their first tumor may not respond as well to a second round of systemic treatment. Finally, patients with second primary tumors were older than patients in the single-cancer group, and may have been relatively intolerant of aggressive treatment modalities. It is interesting to note that we found that survivors of bladder cancer demonstrated the highest incidence of second primary malignancies, with 10-year and 20-year incidence rates of 19% and 34%, respectively, and that lung cancer represented the largest percentage of second primary malignancies among survivors of bladder cancer, at 25%. These findings are consistent with prior studies reporting an increased risk of lung cancer in the population of survivors of bladder cancer.22–25 Both bladder cancer and lung cancer harbor some of the highest rates of somatic mutations of any malignancy, and both are strongly linked to exposure to tobacco smoke. The current study finding that survivors of NHL are at an increased risk of developing secondary lung cancers also is consistent with prior reports,26,27 most likely related to the therapeutic alkylating agents and radiotherapy commonly used in the treatment of lymphoma.21
Before considering the implications of the current study findings, several methodological limitations deserve mention. First, the rate of second primary malignancies reported herein may have been overestimated given that these second malignancies could represent misclassified metastases from the primary tumor. However, by excluding all patients in whom the second malignancy was diagnosed within 1 year after the first malignancy (>500,000 patients) and by excluding those cases with identical histology (>68,000 patients), we believe that there is a low likelihood that metastatic recurrences from the first cancer were misclassified as second primary malignancies. In addition, the most common secondary malignancies (eg, prostate cancer, breast cancer, colorectal cancer, bladder cancer, and NHL) are unlikely sites for metastases. Second, SEER is a population-based data set that only captures cases in 18 registries in the United States. However, the comprehensive nature of the registry data, backed by an intense quality assurance protocol, makes generalizability of these results probable. Finally, due to the nature of the SEER data, we were not able to control for several well-known cancer risk factors such as smoking status or obesity, nor we were able to control for treatment-related factors such as radiotherapy or chemotherapy, which may alter findings related to second cancer incidence and survival. These unmeasured covariates are likely related to both the primary cancer as well as the development of a second primary malignancy and, as such, residual confounding cannot be ruled out entirely.
Despite these limitations, the findings of the current study have implications for cancer survivors. There is an increasing recognition of the long-term effects experienced by cancer survivors as a result of their cancer diagnosis or treatment, and site-specific cancer care survivorship guidelines exist for survivors of some adult cancers.28,29 Although these guidelines do address the risk of second primary malignancies, for the most part they recommend screening according to the guidelines developed for the general public, such as those published by the US Preventive Services Task Force. Although these guidelines may be appropriate for survivors of some cancers, the findings of the current study demonstrate that survivors of certain adult cancers are at a higher than average risk, and survivorship guidelines for these patients should incorporate this elevated risk into their recommendations. Survivors of adult malignancies may be screened according to guidelines for high-risk groups of individuals, as determined by certain well-described genetic or treatment-related risk factors. For example, those with BRCA1 or BRCA2 mutations are at risk of developing multiple neoplasms and are candidates for screening with annual breast magnetic resonance imaging,30 as well as for ovarian cancer screening.31 Individuals with Lynch syndrome are advised to undergo early and frequent colonoscopy.32
Our observation that a significant percentage of cancer survivors will develop and die of lung cancer is consistent with the known epidemiology of lung cancer, which is the leading cause of cancer-related death in the United States, representing >25% of all cancer deaths.1 Screening for lung cancer has been evaluated in at least 7 randomized clinical trials; in what to our knowledge was the largest of these studies, a 20% reduction in lung cancer mortality and a 7% reduction in all-cause mortality were observed.33,34 In 2014, the US Preventive Services Task Force released recommendations for lung cancer screening, which recommended annual low-dose chest computed tomography in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or have quit within the last 15 years.35 However, these trials generally excluded patients with a recent diagnosis of malignancy34 and, as such, the applicability of these screening guidelines to survivors of a first malignancy are unproven. It is likely that a significant number of cancer survivors may meet the criteria for lung cancer screening based on age and smoking history alone. However, it may be that a history of prior cancer places an individual at a higher risk of developing a second cancer, independent of a history of exposure to carcinogenic agents, thereby potentially justifying screening for lung cancer, irrespective of age and smoking history. In the case of bladder cancer, for example, the majority of incident tumors are of low grade and stage, and although recurrences are common, they rarely are lethal. As such, the 5-year relative survival from bladder cancer is 80%, and the prevalence of patients with bladder cancer is large: it is estimated that in 2012 there were 577,403 individuals living with bladder cancer in the United States.16 If we apply our finding of a 10-year cumulative risk of second primary malignancy of 19% to this population of survivors of bladder cancer, we estimate that these patients will develop >100,000 new cases of cancer in the subsequent decade, >25,000 of which will be primary lung cancers. Any potential benefits of screening would need to be weighed against potential risks. These include, but are not limited to: 1) falsepositive results triggering unnecessary interventions; 2) false-negative results with subsequent delayed diagnosis; 3) futile detection of small aggressive tumors that have already metastasized and for which early detection does not provide a survival benefit; 4) overdiagnosis of indolent disease for which unnecessary interventions are subsequently undertaken; 5) radiation exposure; 6) psychological and physical stress associated with diagnostic workup and intervention; and 7) increased health care costs. Further study would be required to determine whether screening for second primary lung cancer in cancer survivors has a favorable risk-benefit ratio, given that prior lung cancer screening trials excluded survivors of recent cancers. In addition, the question of whether there are certain high-risk subgroups, such as survivors of bladder cancer, in whom this strategy can improve overall survival will require further research.
Conclusions
Greater than 8% of patients diagnosed with 1 of the top 10 incident cancers were found to develop a second malignancy, with patients with bladder cancer having the highest cumulative incidence, reaching 19% at 10 years and 34% at 20 years. In the cohort of patients with 2 primary malignancies, >50% of patients died of their second cancer, and 12% of patients with 2 cancers died of lung cancer. The growing number of cancer survivors in the United States, coupled with the observed lethality of second primary cancers in the current study, suggests that investigation into effective detection and treatment strategies in this population is warranted.
Acknowledgments
FUNDING SUPPORT
Supported in part by the Urology Care Foundation Research Scholars Program (Christopher P. Filson), the American Cancer Society Postdoctoral Fellowship (CPHPS-PF-14–161-01 to Christopher P. Filson and CPHPS-126217-PF-14–028-01 to Hung-Jui Tan), the National Institutes of Health Loan Repayment Program (Hung-Jui Tan and Karim Chamie), and the STOP Cancer Foundation (Karim Chamie).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Presented as a poster presentation at the American Urological Association (AUA) 2015 Annual Meeting; May 15–19, 2015; New Orleans, LA.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Treatment & Survivorship Facts& Figures: 2014–2015. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 3.de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Council on Radiation Protection and Measurements. Second Cancers and Cardiovascular Disease After Radiation Therapy. Bethesda, MD: National Council on Radiation Protection and Measurements; 2011. NCRP Report No. 170. [Google Scholar]
- 5.Zagars GK, Ballo MT, Lee AK, Strom SS. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22:640–647. [DOI] [PubMed] [Google Scholar]
- 6.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanks GE, Peters T, Owen J. Seminoma of the testis: long-term beneficial and deleterious results of radiation. Int J Radiat Oncol Biol Phys. 1992;24:913–919. [DOI] [PubMed] [Google Scholar]
- 8.Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. [DOI] [PubMed] [Google Scholar]
- 9.Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: clinical perspectives. QJM. 2016;109:151–158. [DOI] [PubMed] [Google Scholar]
- 10.Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86:289–294. [DOI] [PubMed] [Google Scholar]
- 11.Preston-Martin S Evaluation of the evidence that tobacco-specific nitrosamines (TSNA) cause cancer in humans. Crit Rev Toxicol. 1991;21:295–298. [DOI] [PubMed] [Google Scholar]
- 12.Lu CH, Lee KD, Chen PT, et al. Second primary malignancies following thyroid cancer: a population-based study in Taiwan. Eur J Endocrinol. 2013;169:577–585. [DOI] [PubMed] [Google Scholar]
- 13.Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011; 12:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schairer C, Hisada M, Chen BE, et al. Comparative mortality for 621 second cancers in 29356 testicular cancer survivors and 12420 matched first cancers. J Natl Cancer Inst. 2007;99:1248–1256. [DOI] [PubMed] [Google Scholar]
- 15.Montero-Miranda PH, Ganly I. Survivorship–competing mortalities, morbidities, and second malignancies. Otolaryngol Clin North Am. 2013;46:681–710. [DOI] [PubMed] [Google Scholar]
- 16.Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 17.Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–571. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia S, Sklar C. Second cancers in survivors of childhood cancer. Nat Rev Cancer. 2002;2:124–132. [DOI] [PubMed] [Google Scholar]
- 19.Olsen JH, Garwicz S, Hertz H, et al. Second malignant neoplasms after cancer in childhood or adolescence. Nordic Society of Paediatric Haematology and Oncology Association of the Nordic Cancer Registries. BMJ. 1993;307:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corkum M, Hayden JA, Kephart G, Urquhart R, Schlievert C, Porter G. Screening for new primary cancers in cancer survivors compared to non-cancer controls: a systematic review and meta-analysis. J Cancer Surviv. 2013;7:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182–192. [DOI] [PubMed] [Google Scholar]
- 22.Kantor AF, McLaughlin JK. Second cancer following cancer of the urinary system in Connecticut, 1935–82. Natl Cancer Inst Monogr. 1985;68:149–159. [PubMed] [Google Scholar]
- 23.Jensen OM, Knudsen JB, Sorensen BL. Second cancer following cancer of the urinary system in Denmark, 1943–80. Natl Cancer Inst Monogr. 1985;68:349–360. [PubMed] [Google Scholar]
- 24.Greven KM, Spera JA, Solin LJ, Whittington R, Wein AJ, Hanks GE. Secondary malignant neoplasms in patients with bladder carcinoma. Urology. 1992;39:204–206. [DOI] [PubMed] [Google Scholar]
- 25.Salminen E, Pukkala E, Teppo L. Bladder cancer and the risk of smoking-related cancers during followup. J Urol. 1994;152(5 pt 1): 1420–1423. [DOI] [PubMed] [Google Scholar]
- 26.Travis LB, Curtis RE, Boice JD Jr, Hankey BF, Fraumeni JF Jr. Second cancers following non-Hodgkin’s lymphoma. Cancer. 1991; 67:2002–2009. [DOI] [PubMed] [Google Scholar]
- 27.Curtis RE, Freedman DM, Ron E, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. NIH Pub. No. 05–5302. [Google Scholar]
- 28.Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014; 64:225–249. [DOI] [PubMed] [Google Scholar]
- 29.El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin. 2015;65:427–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997–1003. [PubMed] [Google Scholar]
- 31.Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. [DOI] [PubMed] [Google Scholar]
- 32.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Dis Colon Rectum. 2014;57:1025–1048. [DOI] [PubMed] [Google Scholar]
- 33.Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013;159:411–420. [DOI] [PubMed] [Google Scholar]
- 34.National Lung Screening Trial Research Team; Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. [DOI] [PubMed] [Google Scholar]