Topical gel reduces pesticide-induced systemic acetylcholinesterase inhibition, thus preventing neuronal dysfunction and mortality.
Abstract
Organophosphate-based pesticides inhibit acetylcholinesterase (AChE), which plays a pivotal role in neuromuscular function. While spraying in the field, farmworkers get exposed to pesticides through the dermal route. Internalized pesticide inhibits AChE, which leads to neurotoxicity, cardiotoxicity, cognitive dysfunction, loss of endurance, and death in severe cases. Here, we present a nucleophilic pyridine-2-aldoxime–functionalized chitosan-based topical gel (poly-Oxime gel) that rapidly deactivates organophosphates, methyl parathion (MPT), on the skin of rats, which leads to reduced AChE inhibition in the blood and tissues. Testing the robustness of poly-Oxime gel, we report reduction in AChE inhibition following repeated dermal administration of MPT in the presence of poly-Oxime gel. Furthermore, poly-Oxime gel prevented MPT-induced neuromuscular dysfunction, loss of endurance, and locomotor coordination. We observe a 100% survival in rats following topical MPT administration in the presence of poly-Oxime gel. This prophylactic gel may therefore help farmworkers by limiting pesticide-induced toxicity and mortality.
INTRODUCTION
Acetylcholinesterase (AChE) is abundant in both central and peripheral nervous system (1, 2). In addition to chemical warfare agents (CWAs), organophosphate pesticides [such as methyl parathion (MPT), malathion, paraoxon, and chlorpyrifos] are AChE inhibitors. Inhibition of AChE leads to the accumulation of the neurotransmitter acetylcholine, resulting in neurological disorders, suffocation, paralysis, and death in severe cases (3, 4). India is one of the prime countries in pesticide usage in the world, with a vast majority of agricultural workers being repeatedly exposed to pesticides in the field (5, 6). In addition to neurotoxic effects, AChE inhibition leads to cardiotoxicity, reduced immunity, infertility, and delayed sexual maturation (7). Cohort studies reveal that pre- and postnatal exposures to pesticides impede neurodevelopment in children living in agricultural communities and increase susceptibility to neuropsychiatric disorders (8, 9).
According to the World Health Organization (WHO) Recommended Classification of Pesticides by Hazard, organophosphate pesticides oxydemeton-methyl and methamedophos belong to class I (extremely/highly hazardous); dimethoate, fenthion, quinalphos, chlorpyrifos, prothiofos, and diazinon belong to class II (moderately hazardous); and malathion and pirimifos-methyl belong to class III (slightly hazardous) (10, 11). It is clear that systemic exposure to pesticides through the dermal route is a health hazard. Preventive strategies to eliminate or minimize dermal exposure to reduce pesticide-induced toxicity are a less attended clinical need. Although the personal protective equipment (PPE) such as suits, gloves, face masks, headgear, and boots are available, they are scarcely used, mainly because of high cost and discomfort under tropical conditions (12–14). The most effective way to prevent pesticide exposure is chemical deactivation of pesticides, and existing PPE lack this ability. There have been attempts to formulate physical barrier creams in the past to attenuate exposure to pesticides or CWAs (15–17). This design comes with an inherent limitation of exposure, as the pesticide that is arrested on the surface can still enter the system through the oral or ocular route via hand contact. In addition, each pesticide brand has different additive formulations, and these formulations have variable penetrance through physical barrier creams. The noncatalytic nature of this approach also limits the amount of pesticide the barrier creams can arrest. Therefore, physical barrier creams are not effective in preventing exposure. The metal nanoparticles, like CeO2, are studied as potential chemical deactivators of pesticides/CWAs (18, 19). However, CeO2 nanoparticles are known to cause DNA damage in dermal fibroblasts, an effect that necessarily limits their use in dermal protectants (20, 21).
To understand the awareness of agricultural workers about the pesticide-induced toxicity, we interacted with several farmers and their families in the field. All of them were aware of pesticide-induced toxicity, as they experience pain right after they spray pesticides in the field. However, because of the lack of any protective technologies, they suffer, and they expressed their willingness to adopt any topical technologies that can prevent pesticide exposure. Because of the low purchasing power of this sector, the affordable cost would be a key differentiating factor to have compliance to adopt these technologies by the end user—the farmer. This unmet need inspired us to develop prophylactic technologies to prevent pesticide exposure during spraying in the field. An ideal user-compliant solution would be a low-cost, easy-to-use, nonobstructive biocompatible material, which could inactivate pesticides on the skin and prevent their penetration. A topical gel with catalytic activity to cleave/hydrolyze pesticides before entering into the skin may serve as a prophylactic strategy to reduce chronic exposure (Fig. 1A). The aim/objective of this study was to determine whether a nucleophilic pyridine-2-aldoxime–functionalized chitosan-based topical gel (poly-Oxime gel) could be used as a prophylactic gel to deactivate pesticides on the skin before they enter the body. However, multiple technical hurdles exist in designing this catalytically robust prophylactic topical gel. The first is the identification of a reactive nucleophile that can act by rapidly hydrolyzing organophosphate ester on the skin before it enters. Also, it should catalytically, not stoichiometrically, cleave organophosphates. Second, the topical gel should be chemically active in different climate conditions to be effective in tropical and cold countries.
Fig. 1. Nucleophilic poly-Oxime gel to deactivate pesticides to limit toxicity.
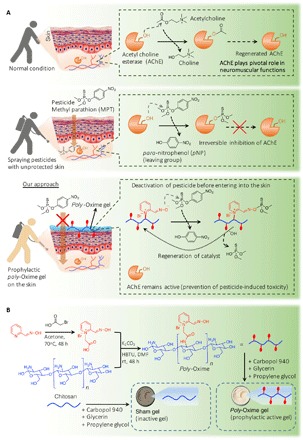
(A) Dermal penetration of pesticide (MPT) leads to the inhibition of AChE, which plays a pivotal role in biological functions including neuronal signaling and neuromuscular coordination (NMC). MPT-mediated inhibition of AChE leads to severe toxicity including neuromuscular dysfunction, loss of endurance, and locomotor function. In our approach, the presence of α-nucleophile (an oxime) attached polymer (poly-Oxime) formulated as a topical gel could deactivate organophosphorus ester, MPT, through hydrolysis. This limits MPT penetration into the skin, which leads to the reduction of pesticide-induced toxicity. (B) Synthesis of poly-Oxime using pyridine-2-aldoxime connected to chitosan through acetamide linker. With humectants like glycerin, propylene glycol, and carbopol 940, topical gel was prepared. Two types of topical gels, poly-Oxime–containing active gel and, as a control, unfunctionalized chitosan–containing inactive sham gel, were prepared. rt, room temperature; DMF, N,N′-dimethylformamide.
Here, we present a nucleophilic topical gel (poly-Oxime) that is capable of catalytically deactivating organophosphates efficiently on the skin, thereby reducing inhibition of AChE quantitatively and limiting pesticide-induced toxicity and mortality. The N-hydroxy α-nucleophile–containing poly-Oxime was converted into a gel by addition of excipients like carbopol 940, glycerin, and propylene glycol (Fig. 1B). This α-nucleophile–based topical gel solves the aforementioned challenges. A direct dermal exposure of a widely used pesticide, MPT, in rats significantly inhibited the activity of AChE in blood, brain, lung, liver, and heart. A single-dose dermal exposure of MPT (150 mg/kg) in rats dampened the locomotor coordination function, altered neuromuscular signaling, and led to death with a median survival time of 4 days. The same dose of MPT in the presence of poly-Oxime topical gel did not induce any adverse effects, and a 100% survival was observed.
RESULTS
Synthesis of poly-Oxime gel and deactivation of pesticides in vitro and ex vivo
Most pesticides are organophosphate esters. Thus, a nucleophile-mediated ester hydrolysis is an efficient way to deactivate pesticides. α-Nucleophiles are known as efficient groups for hydrolytic cleavage of organophosphates (22–24). Oxime (N-hydroxy compound) is a potent α-effect O-nucleophile (25, 26). Therefore, we reasoned that pyridine-2-aldoxime would be a powerful α-nucleophile, which can be functionalized on a biopolymer, chitosan (Fig. 1B). Chitosan, a biocompatible polymer, has been extensively used to develop a wide range of biomaterials (27, 28). Conjugation of pyridine-2-aldoxime to chitosan through an acetamide linker in solution phase generated poly-Oxime (Fig. 1B and Supplementary Methods). The degree of functionalization was 40%. A detailed characterization of poly-Oxime is given in the Supplementary Materials. The kinetics of MPT hydrolysis was obtained by monitoring the appearance of p-nitrophenoxide spectrophotometrically at 400 nm (Fig. 1A and see Materials and Methods). The soluble poly-Oxime polymer was used to study the catalytic activity toward decontamination of different pesticides. The observed pseudo–first-order (kobs) values for the cleavage of MPT, paraoxon, and chlorpyrifos were 4.59 × 10−4, 2.28 × 10−4, and 5.55 × 10−4 s−1, respectively. Data in table S1 show that poly-Oxime is an effective catalyst for the hydrolytical deactivation of multiple organophosphates, in that they afford more than two orders of magnitude rate enhancement over the background reaction. Poly-Oxime also showed activity at different temperatures (at 20°C, kobs = 3.37 × 10−4 s−1; at 30°C, kobs = 7.18 × 10−4 s−1; at 40°C, kobs = 17.57 × 10−4 s−1).
To evaluate the ability of poly-Oxime gel to prevent pesticide penetration into the skin, we formulated the poly-Oxime (2%, w/w) into a topical gel using the following additives: glycerin (6.8%, w/w), propylene glycol (3.74%, w/w), and carbopol 940 (1.8%, w/w; see Materials and Methods). Sham gel, as a control, was prepared using the same composition, with unmodified chitosan replacing the poly-Oxime. Both gels have shown standard gel-like behavior in rheology studies (fig. S1) and could be applied to the skin. A temperature-dependent rheology studies at 25°, 35°, and 45°C with poly-Oxime gel revealed that at higher temperatures, it maintains its gel-like behavior but becomes softer as seen in the reduction in G′ values (fig. S1, C to E). The fact that, in linear viscoelastic range, all the gels showed G′ > G″ suggests that they behave like viscoelastic solids or gels. Carbopol 940 is a hydrophilic colloidal gum that offers a high thickening gel property due to water absorption, leading to the formation of macroscopic swollen particles. This formation is attributed to hydrogen bonding between carboxylic groups of the polymer and water molecules. The observed softening of gel upon addition of the poly-Oxime polymer could be explained because of charges in quaternary N and deprotonated oxime groups that promote electrostatic interaction with solvent, rather than hydrogen bonding. This might result in reduced solvent interaction in terms of hydrogen bonding and thus formation of softer gels. Franz diffusion apparatus was used to mimic the transdermal penetration (Fig. 2A). The uncoated dialysis membrane [molecular weight cutoff (MWCO), 3.5 kDa] or the membrane coated with a thin layer of sham gel or poly-Oxime gel was placed between donor and acceptor chambers. Subsequently, MPT was added into the donor chamber and the acceptor chamber was filled with phosphate-buffered saline (PBS). The chamber’s temperature was maintained at 37°C. At different time points (0, 0.25, 0.5, 1, 2, 3, 4, and 5 hours), the concentration of MPT and its hydrolytically degraded product p-nitrophenol (pNP) was quantified in both donor and acceptor chambers using ultrafast liquid chromatography (UFLC; Fig. 2, B to E). In the presence of the uncoated membrane or sham gel–coated membrane, MPT was quantitatively penetrated into the acceptor chamber within 3 hours, whereas poly-Oxime gel prevented MPT penetration (Fig. 2, B and C). It suggests that sham gel does not act as a physical barrier and MPT can easily pass through the gel. Data in Fig. 2 (D and E) show that poly-Oxime gel was able to degrade MPT into pNP, which was quantified in both donor and acceptor chambers. After 3 hours, the deactivation was nearly complete and the pNP that formed in the donor compartment started to diffuse into the acceptor compartment. pNP was not generated in any of those chambers in the absence of poly-Oxime gel. These results suggest that poly-Oxime gel prevents the penetration of MPT, not as a physical barrier but through chemically hydrolyzing MPT.
Fig. 2. Poly-Oxime gel deactivates MPT to limit MPT-mediated AChE inhibition ex vivo.
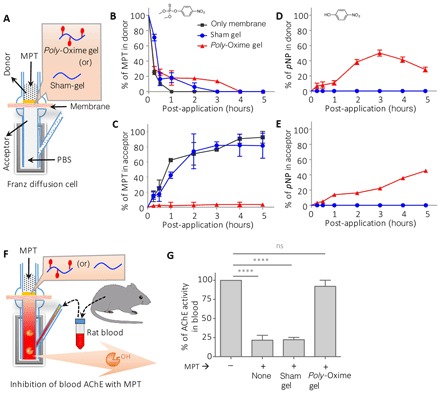
(A) Schematic of Franz diffusion cell: A thin layer of either poly-Oxime gel or sham gel was applied on a dialysis membrane, which was placed between donor and acceptor chambers. (B to E) Concentration of MPT and pNP (hydrolytic degradation product of MPT) in donor and acceptor chambers was measured using UFLC. The presence of sham gel did not prevent the diffusion of MPT into the acceptor chamber and could not hydrolyze MPT to generate pNP, whereas poly-Oxime actively hydrolyzed to limit the penetration of toxic MPT into the acceptor chamber. (F and G) An ex vivo assay to demonstrate the ability of poly-Oxime to limit MPT-induced assay AChE inhibition using rat blood. AChE containing rat blood was placed in the acceptor chamber, and MPT was added in the donor chamber in the presence of either poly-Oxime or sham gel. Active AChE was measured in the blood before and 3 hours after addition of MPT. In the absence of poly-Oxime gel, MPT diffused into an acceptor chamber and significantly inhibited AChE activity. However, poly-Oxime gel could hydrolyze MPT before diffusion, therefore limiting the MPT-induced inhibition of AChE. Data are means ± SD (n = 3, performed at least twice); P values were determined by one-way analysis of variance (ANOVA). ****P < 0.0001. ns, not significant.
Organophosphates are known to inhibit AChE in blood (29). An ex vivo assay was carried out using rat blood in Franz diffusion cell to evaluate the ability of poly-Oxime gel to limit MPT-induced AChE inhibition (Fig. 2F). Akin to the previous experiment, three groups were taken (uncoated, sham gel, and poly-Oxime gel–coated membranes between both chambers) along with the no MPT group, 1000× diluted rat blood was taken in the acceptor chamber, and the percentage of the AChE activity was quantified using modified colorimetric Ellman’s assay (see Materials and Methods) (30). In the absence of MPT, no change was observed in the activity of AChE after 3 hours (Fig. 2G). The addition of MPT (1 μM) in the donor chamber led to a significant inhibition of AChE activity in blood. The presence of native membrane or sham gel–coated membrane did not limit MPT-mediated AChE inhibition (Fig. 2G). On the contrary, poly-Oxime gel deactivated MPT before entering into the acceptor chamber, thereby completely reducing MPT-induced inhibition of blood AChE activity. Poly-Oxime gel could hydrolytically cleave a wide range of commercial organophosphate formulations (fig. S2). Under similar reaction conditions, sham gel, which was made with native chitosan without oxime groups, did not deactivate pesticides, suggesting that poly-Oxime α-nucleophile is essential for the reactivity (fig. S2). These results suggest that poly-Oxime gel could limit pesticide-induced AChE inhibition by chemically deactivating organophosphates.
To validate the role of the oxime group in imparting the ability to limit pesticide exposure, we blocked the active oxime group with methyl ether. O-methoxy pyridine-2-aldoxime (methoxyOxime) was covalently attached to chitosan (poly-methoxyOxime) using the same process that was used for the poly-Oxime polymer (a detailed synthesis protocol and characterization is given in Supplementary Methods). Akin to poly-Oxime gel, the poly-methoxyOxime gel was prepared and its ability to reduce MPT-induced AChE inhibition in the blood was evaluated using Franz diffusion cell ex vivo. While poly-Oxime gel completely decreased the MPT-induced AChE inhibition, blocking of oxime nucleophile with methoxy ether (poly-methoxyOxime gel) led to the loss of its activity (fig. S3). The inability of poly-methoxyOxime gel to prevent AChE inhibition validates the importance of oxime nucleophile to deactivate MPT.
Poly-Oxime gel prevents pesticide-induced AChE inhibition in blood and tissue in vivo
To evaluate the ability of poly-Oxime gel to prevent MPT-induced AChE inhibition, 18 Sprague-Dawley rats (10 to 13 weeks) were randomly divided into three groups (n = 6 per group). The dorsal coat was clipped, and the 10-cm2 area was exposed to MPT directly or in the presence of the sham gel or poly-Oxime gel layer (see Materials and Methods). According to the WHO, pesticides that have LD50 (median lethal dose) of 10 to 100 mg/kg of body weights in rats (dermal exposure) are considered as “highly hazardous” (class Ib) (10, 11). To test the robustness of poly-Oxime gel, we dermally exposed all groups to MPT (150 mg/kg) (Fig. 3A). Quantification of active AChE in the blood at pre-exposure (0 hours) and post-exposure (96 hours) revealed that dermal exposure of MPT alone or in the presence of sham gel significantly decreased the active AChE, whereas in the presence of poly-Oxime gel no such decrease was seen (Fig. 3B). Animals that were exposed to MPT alone or in the presence of sham gel lost ~20% of their initial body weight by day 4, while animals that were exposed to MPT in the presence of poly-Oxime gel did not lose their weight and showed normal weight gain (fig. S4). In addition, on day 4, animals were sacrificed, and tissues like brain, heart, liver, and lung were isolated and quantified for active AChE in comparison with the naïve rat tissues. Data in Fig. 3 (C to F) suggest that the MPT exposure leads to a decrease in active AChE levels, while sham gel could not protect from the MPT exposure. On the contrary, the presence of poly-Oxime gel limited the reduction of active AChE. These results demonstrate that poly-Oxime gel does not act like a typical barrier gel, but it hydrolytically deactivates organophosphate, leading to limiting AChE inhibition.
Fig. 3. Poly-Oxime gel limits AChE inhibition after exposure to a lethal dose of MPT in vivo.
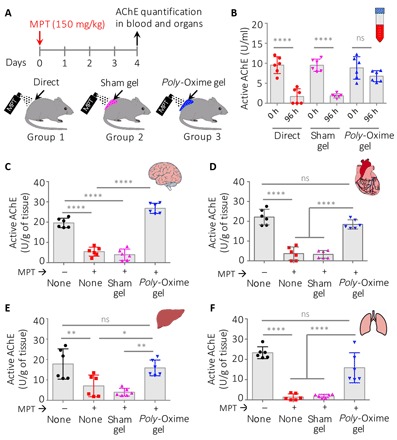
(A) Coat on the dorsal side of rats was clipped 1 day before the exposure. A dose of MPT (150 mg/kg) was applied on the skin either directly or in the presence of the sham gel (220 mg per animal) or poly-Oxime gel (220 mg per animal) layer. (B) Active AChE in the blood was quantified using Ellman’s assay. Direct exposure of MPT significantly reduced the active AChE in the blood. Sham gel could not limit MPT-induced AChE inhibition, while poly-Oxime gel deactivated MPT before entering into the skin, therefore reducing the MPT-induced AChE inhibition. (C to F) On day 5, rats were sacrificed, tissue was collected, and the amount of active AChE was quantified. Dermal exposure of MPT either directly on the skin or in the presence of sham gel led to the decrease in the active AChE in all tissues such as brain, heart, liver, and lung, while poly-Oxime gel significantly reduced this MPT-mediated deactivation of AChE. Data in (B) to (F) are means ± SD (n = 6 rats per group); P values were determined by one-way ANOVA with Tukey post hoc test. *P < 0.05, **P < 0.01, ****P < 0.0001.
Poly-Oxime gel prevents pesticide-induced mortality in vivo
As multiple exposures of MPT could cause mortality, we investigated the ability of poly-Oxime gel to prevent MPT-induced mortality in rats. A single dose of 150 mg/kg leads to 100% mortality in a direct exposure group with a mean survival time (MST) of 4 days. Therefore, for survival experiments, the dose was brought down to 100 mg/kg to understand response to multiple exposures and thus partly mimic the field scenario of multiple exposures. First, 18 Sprague-Dawley rats were randomized into three groups (n = 6), and all groups received MPT (100 mg/kg per day) once a day until >50% mortality was observed in one of the groups. Group 1 animals received MPT without any gel, sham gel was applied daily on group 2 animals, and poly-Oxime gel was applied daily on group 3 animals, 30 min before the MPT exposure (Fig. 4A). Animals in groups 1 and 2 showed the characteristics of organophosphate pesticide poisoning in the rats (31); symptoms include salivation, muscular fibrillation, diarrhea, lacrimation, respiratory distress, gasping, decreased body temperature, and tremors. On the contrary, group 3 animals that received MPT after the application of prophylactic poly-Oxime gel did not show any signs of toxicity. All animals directly exposed to the MPT group died in 5 days, while all the animals in the sham gel group died within 7 days, with an MST of 4 and 6 days, respectively, and all animals that received MPT after the poly-Oxime gel application survived (Fig. 4B). A 100% survival rate was observed in the presence of poly-Oxime gel (n = 6; P = 0.0001, Mantel-Cox test). The blood was collected before exposing to MPT (day 0) and at the terminal stage (on day 3 for groups 1 and 2 and on day 30 for group 3). Animals in group 3 were tested for any delayed signs of toxicity for 30 days, and the study was terminated on day 30. No visible delayed signs of toxicity like muscular fibrillation, diarrhea, lacrimation, and respiratory distress were observed. Quantification of active AChE in the blood revealed that direct exposure of MPT and the sham gel group showed a decrease in active AChE, while poly-Oxime gel limited the inhibition of AChE activity (Fig. 4C). In addition, brain tissue was collected from group 1 and 2 animals immediately after they died. Because not even a single animal died in group 3, they were sacrificed on day 30 and their brain tissue was harvested. The active AChE in tissues was quantified. Exposure of MPT either directly or in the presence of sham gel significantly decreased the active AChE, while poly-Oxime gel prevented reduction of active AChE in the brain (fig. S5A). In addition, while animals in groups 1 and 2 that received MPT lost their body weight, group 3 animals that were exposed to MPT in the presence of poly-Oxime gel continue to gain weight normally until the end of the study (fig. S5B).
Fig. 4. Either daily or a single application of poly-Oxime gel prevented mortality during repeated exposure of MPT in vivo.
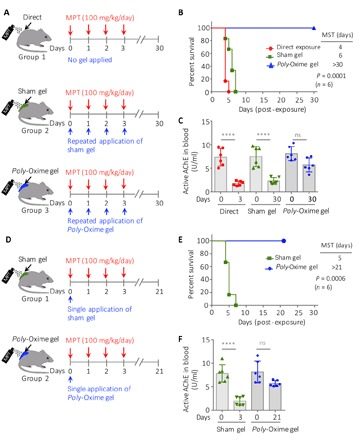
(A) Sprague-Dawley rats (10 weeks, males) were randomized in three groups (n = 6 rats per group): (i) direct exposure of MPT, (ii) sham gel + MPT, and (iii) poly-Oxime gel + MPT. Either sham gel or poly-Oxime gel (220 mg per rat per day) was applied every day before the MPT exposure (100 mg/kg per day for 4 days). Organs were collected either immediately after mortality or on day 30. AChE activity in blood and organs was quantified using modified Ellman’s assay. (B) MST for direct exposure of MPT and MPT received in the presence of sham gel was 4 and 6 days, respectively, while poly-Oxime gel–applied group did not show mortality. (C) Blood AChE activity dropped markedly in direct MPT and sham gel groups, while inhibition was reduced in poly-Oxime gel–applied animals. (D) Rats were divided into two groups (n = 6 rats per group). On day 0, 220 mg of sham gel and poly-Oxime gel was applied dermally on group 1 and 2 animals, respectively. Without further applying any gel, MPT (100 mg/kg per day) was given daily for 4 days. Organs were collected either immediately after mortality or on day 21. (E) MST for sham gel was 5 days, while poly-Oxime gel–applied group did not show mortality. (F) Blood AChE activity dropped markedly in group 1, while the inhibition was reduced in group 2. P values were determined by Mantel-Cox test (B and E) and one-way ANOVA with Tukey post hoc test (C and F). **P < 0.01, ****P < 0.0001.
An oxime could hydrolyze multiple organophosphate molecules in a truly catalytic manner. After cleaving one molecule of organophosphate, an oxime nucleophile could regenerate and cleave more organophosphate molecules (Fig. 1A). To test the robust nature of poly-Oxime gel in vivo, we randomized 12 Sprague-Dawley rats into two groups (n = 6). On day 0, we applied 220 mg of sham gel or poly-Oxime gel on group 1 and 2 animals, respectively. In one-time post-application of the gel, both groups received MPT (100 mg/kg per day) for 4 days (Fig. 4D). All the animals in the sham gel group died within 7 days with an MST of 5 days, whereas a 100% survival was observed in one-time poly-Oxime gel–treated group (n = 6; P = 0.0006, Mantel-Cox test; Fig. 4E). Analysis of active AChE in the blood revealed that sham gel could not reduce MPT-mediated inhibition of AChE in the blood and brain tissue compared to the presence of prophylactic poly-Oxime gel (Fig. 4F and fig. S6A). In addition, animals in the poly-Oxime gel group did not lose their body weight compared to the sham gel group (fig. S6B). When poly-Oxime gel was applied before the MPT exposure, in addition to providing 100% survival, there were no visible signs such as shivering and stress-induced porphyrin discharge, which are hallmarks of pesticide-induced toxicity and stress. Cumulatively, these results suggest that poly-Oxime gel can deactivate organophosphate pesticides in a robust manner to prevent pesticide-induced toxicity and mortality. We propose that this property of poly-Oxime gel could be protective for farmers and workers who are exposed to high doses of pesticides repeatedly.
Poly-Oxime gel prevents pesticide-induced loss of motor coordination and altered neuromuscular signaling
Organophosphate pesticides bind and subsequently inhibit the AChE in synaptic clefts of the neuromuscular junction (32–34). Lethal effects are cardiovascular collapse and respiratory failure. Signaling at motor end plate gets impaired because of AChE inhibition. Therefore, pesticide exposure leads to the reduction in motor coordination and altered neuromuscular signaling (35–37). Pesticide-induced stress reduces the endurance (38, 39). The endurance and motor coordination of rats exposed to MPT with and without poly-Oxime gel were tested using the rotarod experiment. Three groups of male Sprague-Dawley rats (n = 4 in each group) were trained on a rotarod for 4 days (Fig. 5A), and while training, animals in all the groups showed similar latency to fall (Lf; time to fall from rotating rod with 2 to 20 rpm). Lf on day 4 was considered as 100%. After recording Lf on day 4, group 1 animals were left unexposed, group 2 animals were directly exposed to MPT, and group 3 animals received MPT in the presence of poly-Oxime gel. After the MPT exposure, group 2 animals showed a significant drop in the latency to fall, thus indicating an MPT-induced reduction in endurance (Fig. 5B). On the contrary, group 3 animals that received MPT in the presence of prophylactic poly-Oxime gel did not show a reduction in latency to fall and continued improvement in the performance like unexposed group 1, which suggests that poly-Oxime gel prevents the pesticide-induced reduction in endurance. We used acceleration in rotation speed from 2 to 60 rpm, with a step size of 4 rpm to study the neuromuscular coordination (NMC) of rats under constant need to readjust the muscle recruitment. After training for 6 days, animals were similarly divided into three groups (n = 5 per group) and tested for NMC. Animals in group 2 showed a significant drop in NMC, while poly-Oxime gel could prevent this drop of NMC and showed performance similar to that of unexposed animals (Fig. 5C).
Fig. 5. Poly-Oxime gel prevented loss of endurance, NMC, nerve function impairment, and uncontrolled muscle activity in vivo.
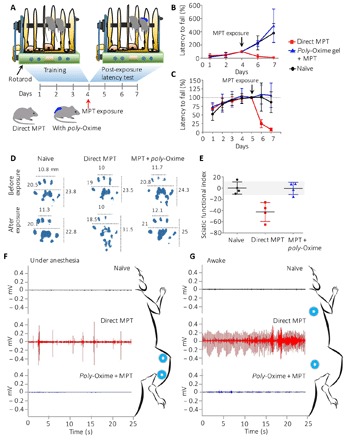
(A) Rotarod was used to study endurance and NMC in MPT animals either directly or in the presence of poly-Oxime gel. (B) Latency to fall was measured by measuring the time the animal stayed on rotarod at a constant speed of 20 rpm, and it was normalized to the day of exposure. Directly exposed MPT animals showed significant reduction endurance that was prevented by poly-Oxime cream. (C) When animals were subjected to increasing speed from 2 to 60 rpm, the rpm reached before falling was taken as a measurement to assign NMC score, and data were normalized to the day of exposure. Poly-Oxime cream showed complete rescue of loss of NMC, which was observed with direct exposure MPT animals. (D and E) Paw prints of animals were exposed either directly or in the presence of poly-Oxime gel before and after exposure. Prints were used to calculate SFI. Direct exposure animals showed greatly reduced SFI that represents impairment of sciatic nerve function, which was again rescued by poly-Oxime cream. (F) EMGs were recorded under 2.5% isoflurane anesthesia and (G) when animals were awake. Poly-Oxime cream showed complete prevention of muscle spasm.
Gait analysis is a conventionally used noninvasive method to study sciatic nerve injury and recovery. The pattern of walking is decided by the posture of the foot, angle formed with the ground, and the force exerted. These things combined will establish a particular print length, toe spread, and intertoe spread of an animal, and it gets affected if there is any impairment of nerve function (Fig. 5D) (40, 41). When analyzed for sciatic functional index (SFI), which is an empirically derived formula to evaluate nerve function (see Materials and Methods), the direct exposure group showed values decreasing to −30 to −60, which indicates partial impairment of sciatic nerve function. However, the poly-Oxime gel–protected animals showed no significant reduction in SFI, suggesting complete prevention of nerve function impairment (Fig. 5E).
Visible muscular spasms were observed in the animals directly exposed to MPT. We recorded electromyogram (EMG) for biceps femoris of the left hind limb under 2.5% isoflurane anesthesia and between spinotrapezius and gluteus maximus when the animal was awake. In either case, EMG showed frequent muscle activity. This involuntary muscle activity was completely absent in the animals protected with poly-Oxime gel before the application of MPT (Fig. 5, F and G).
DISCUSSION
Increasing resistance in pest is forcing agriculture farmers to use a cocktail of potent organophosphates in the field. However, the lack of any protective measure during spraying in the field is causing a direct exposure to pesticides. High dermal penetration of pesticides leads to acute toxicity, including neural dysfunction, muscle dysfunction, and respiratory arrest. Currently, adverse toxic effects of pesticides are a major concern for farmworkers. Although the use of PPE could be an alternative route, the lack of compliance due to uncomforted use in developing countries is a major hurdle. In developing countries, the manual spray of pesticides is still the primary practice, as compared to developed countries where the machine spray or aerial spray is being practiced. A manual spray of pesticides leads to direct dermal exposure. Human studies reveal that pre- and postnatal exposure to pesticides delays neuronal development in children who are living in agricultural communities. In addition, it increases susceptibility to neuropsychiatric disorders (8, 9). Therefore, a few attempts were made to develop physical barrier creams (15, 16). Because of the high skin penetration of pesticides, commercial physical barrier creams were not sufficient to prevent pesticide entry into the body (fig. S7). This prompted us to devise a new strategy, which is a chemical deactivation of pesticides on the skin to prevent their entry into the body. We have designed a polymeric super-nucleophile (α-nucleophile)–mediated hydrolysis of pesticides on the skin.
Poly-Oxime could be formulated into the dermal gel using excipients. Our data suggest that a thin layer of poly-Oxime gel can hydrolyze organophosphates on the skin; therefore, it can prevent AChE inhibition quantitatively in blood and in all internal organs such as brain, lung, liver, and heart. Previously, there were no examples that demonstrated prevention of AChE inhibition in vivo. Our data demonstrate that poly-Oxime gel does not act as a physical barrier. Instead, it hydrolyzes organophosphate ester, MPT, into nonharmful hydrolytic products such as dimethyl phosphate and pNP, which cannot inhibit AChE. Poly-Oxime gel can hydrolytically cleave a wide range of pesticides, including commercial organophosphate formulations such as chlorpyriphos, prophenophos, and monocrotophos, and therefore can prevent pesticide-induced AChE inhibition. The activity of poly-Oxime gel against a broader range of pesticides shows its robustness. As farmers spray pesticides in all seasons including cold winters and hot summers, the prophylactic gel should be active at a wide range of temperature. The fact that the poly-Oxime showed an efficient catalytic activity at temperatures ranging from 20° to 40°C and retained its activity even after prolonged exposure to ultraviolet (UV) light shows stability and ability to function in varying weather conditions (fig. S8).
Farming in developing countries is labor-intensive and demands strenuous physical activity from agriculture workers. As pesticide exposure is known to affect endurance and NMC, farmers lose their ability to work with full capacity. In rat models, we recapitulated the loss of endurance and NMC due to pesticide exposure. Our data demonstrate that the presence of poly-Oxime gel completely prevented pesticide-induced loss of endurance and NMC. In addition, pesticide exposure can severely damage nerves such as the sciatic nerve. Using Gait analysis, we demonstrated that direct exposure to pesticide significantly damaged the sciatic nerve, while the presence of poly-Oxime gel prevented this damage.
To demonstrate the efficiency of poly-Oxime gel to prevent pesticide-induced mortality, we used two sets of experiments. In the first set, rats were repeatedly exposed to a pesticide, while every day a thin layer of poly-Oxime gel was applied before pesticide exposure. While rats died in the absence of poly-Oxime gel (MST, 4 days), the presence of poly-Oxime gel prevented mortality and a 100% survival was observed. In the second set, poly-Oxime gel was applied only once on day 0 and repeatedly exposed to pesticide for 4 days. Even a single application of poly-Oxime gel completely prevented mortality with a 100% survival. A daily or single application of sham gel led to 100% mortality with an MST of 7 and 6 days, respectively. This demonstrates the stability, robustness, and true catalytic nature of poly-Oxime gel.
In conclusion, we have developed a prophylactic topical gel (poly-Oxime) that can limit pesticide-induced systemic AChE inhibition and therefore can prevent loss of motor coordination, loss of endurance, altered neuromuscular function, and mortality. Thus, in addition to bringing awareness to agriculture workers, this technology may offer a great promise for farmers and workers who use pesticides. The ability of poly-Oxime gel to deactivate organophosphate in a catalytic manner also has a potential to be used for protection against organophosphate-based CWAs.
MATERIALS AND METHODS
Study design
The goal of this study was to design a nucleophilic topical gel to chemically deactivate pesticides on the skin to prevent pesticide-induced neuronal dysfunction and mortality. We hypothesized that nucleophile-mediated hydrolysis of pesticides into nonharmful products could prevent systemic AChE inhibition.
In vitro
We initially examined the hydrolytic cleavage of pesticides using a nucleophilic topical gel, thereby preventing inhibition of AChE using Franz diffusion cells.
In vivo
A series of experiments were designed to recapitulate pesticide-induced systemic AChE inhibition, loss of endurance, loss of NMC, sciatic nerve damage, and lethality in rats upon dermal exposure of pesticide, MPT. We tested the hypothesis of the presence of nucleophilic poly-Oxime gel that can deactivate pesticides on the skin and prevent pesticide-induced toxicity and mortality.
Rats
For all experiments, 10- to 14-week-old Sprague-Dawley albino rats were used for experiments. Animals were provided by the animal house at the National Centre for Biological Sciences, Bengaluru. Animals were caged (maximum, four per cage) before the experiment and individually after starting the experiment. Food and water were offered ad libitum. All rat studies were performed according to institutional and national guidelines for humane animal use. Experimental protocols were approved by the Institute Animal Ethical Committee at the Institute for Stem Cell Biology and Regenerative Medicine (INS-IAE-2016/09).
Materials
Pyridine-2-aldoxime, bromoacetic acid, chitosan (medium molecular weight), dimethyl formamide, potassium carbonate, O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU), parathion methyl (PESTANAL), methyl paraoxon (PESTANAL), chlorpyrifos (PESTANAL), carbopol 940, glycerin, propylene glycol, triethanolamine (TEA; S. D. Fine-Chem Limited, Bangalore), snake skin dialysis membrane (MWCO, 3.5 kDa; Thermo Fisher Scientific), trifluoroacetic acid, pNP (Alfa Aesar), isoflurane (Isotroy), Triton X-100, EDTA (Thermo Fisher Scientific), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ASChI), and deionized water were used. Unless mentioned otherwise, all chemicals were procured from Sigma-Aldrich.
Synthesis of poly-Oxime and poly-methoxyOxime
A detailed stepwise synthesis procedure and a detailed characterization of intermediate compounds and final polymers are described in Supplementary Methods.
Catalytic efficiency of poly-Oxime
A solution of poly-Oxime (2 mg/ml) in 1% acetic acid was used to study its catalytic ability to cleave pesticides. The reactions were studied spectrophotometrically by monitoring the hydrolysis of pesticides (MPT, methyl paraoxon, or chlorpyrifos) at 25 ± 0.1°C and pH 8.2 by measuring the absorbance at 400 nm as a function of time. Pseudo–first-order rate constants were obtained by using an excess of nucleophile (2.5 × 10−4 M) over the substrate (2.5 × 10−5 M). For all the kinetic runs, the absorbance/time result fits very well to the first-order rate equation. To study temperature stability, the same reactions were carried out at 20°, 30°, and 40°C.
Preparation of poly-Oxime gel
The dermal gel was formulated by using carbopol 940 (1.8%), glycerin (6.81%), propylene glycol (3.74%), and poly-Oxime polymer (2%) mixed in water (85.6%). The pH of the gel was maintained at 8 by TEA. For sham gel, the unfunctionalized chitosan polymer, instead of the poly-Oxime polymer, was used, serving as a control gel with all ingredients but no catalytic activity.
In vitro and ex vivo efficacy of poly-Oxime dermal gel to reduce permeation of pesticide
The dialysis membrane (MWCO, 3.5 kDa) was hydrated overnight in the deionized water at room temperature. It was then placed between the donor and acceptor compartments of the Franz diffusion cells (DBK Diffusion apparatus) and clamped to avoid any leakage. The acceptor compartment was filled with PBS (pH 7.4), making uniform contact with a dialysis membrane. The experiment was performed in three groups (only membrane, sham gel, and poly-Oxime gel) comprising three diffusion cells in each set. Poly-Oxime or sham gel (220 mg) was applied uniformly on 10-cm2 area on the donor compartment side of the dialysis membrane. On the gel, 1 μg of MPT was added in 500 μl of PBS, and in the direct exposure, MPT was added directly on the dialysis membrane. The temperature of the acceptor chamber was maintained at 37 ± 0.5°C using a thermostatic water bath under constant stirring. Samples were withdrawn from acceptor (1 ml) and donor chambers (20 μl) at a regular interval of 1 hour, and an equal amount of phosphate buffer (pH 7.4) was replaced. The amount of MPT that permeated through the membrane and the amount of pNP that formed at each time interval were analyzed using UFLC (photodiode array: SPD-M20A, C18 reverse-phase column: LC-20AD Prominence Chromatograph, Shimadzu). MPT was detected using 60% acetonitrile in double-distilled water (DDW) as mobile phase at 1 ml/min, with a retention time of 5.5 min at 280 nm while maintaining the column at 40°C. For detection of pNP, we used 22% acetonitrile, 0.5% triethylamine, and 1% trifluoroacetic acid in DDW as mobile phase at a flow rate of 0.5 ml/min through a column maintained at 40°C with a retention time of 3.6 min.
Similarly, in another set of experiment, we replaced the solution in the acceptor chamber with rat blood 1000× diluted in phosphate buffer (pH 8.0). The entire diffusion apparatus was maintained at 5°C to maintain the blood AChE activity. Samples were collected at regular intervals and analyzed using Ellman’s method (as mentioned elsewhere) for AChE activity as a proxy for the MPT exposure.
Protection from acute exposure to MPT
Rats were randomized to one of four experimental groups (n = 6 in each group): (i) no exposure (no MPT was given), (ii) direct exposure (MPT, 150 mg/kg), (iii) sham gel (220 mg of gel; MPT, 150 mg/kg), and (iv) poly-Oxime gel (220 mg of gel; MPT, 150 mg/kg). Polymer concentration in the gel was 2% (w/w). The dorsal coat was clipped using a hair clipper under mild anesthesia (2.5% isoflurane) 24 hours before the dermal pesticide application, taking care not to damage the integrity of the skin. Unless specified otherwise, the total area of 10 cm2 was marked and used for dermal exposure experiments. In gel treatment groups, 220 mg of gel was uniformly applied 1 hour before the pesticide exposure (Fig. 3). Before exposure, 200 μl of blood was collected using retro-orbital puncture and evaluated for various parameters as an internal control. After 96 hours of post-exposure, blood was collected using cardiac puncture under anesthesia. After sacrificing the animals, organs such as brain, heart, lungs, and liver were harvested. Organ tissue was homogenized (Polytron PT-MR 2100, 15,000 rpm) in nine volumes of solution D [1 M NaCl, 1% Triton X-100, 0.01 M tris-HCl, 0.01 M EDTA (pH 7.4)] and incubated on ice for 1 hour, followed by centrifugation at 13,300 rpm for 45 min. The supernatant was used to quantitate active AChE. Whole blood diluted 1:1000 in phosphate buffer was used for AChE quantification. The activity of AChE in blood and organs was quantified by modified Ellman’s assay. In this assay, we used DTNB and ASChI, which is specific for AChE. For the colorimetric assay, according to Ellman’s method, reaction mixtures were made up in 0.1 mM phosphate buffer (pH 7.4) containing 0.5 mM DTNB and ASChI at a final concentration of 20 mM. The reaction was performed at 25°C and monitored at 405 nm.
Survival and AChE inhibition study in rats exposed to MPT multiple times
Rats were randomized to three groups (n = 6 rats per group): (i) direct exposure (no gel, MPT given dermally, 100 mg/kg per day for 4 days), (ii) sham gel (220 mg of sham gel applied daily dermally 30 min before the MPT exposure), and (iii) poly-Oxime gel [220 mg of gel applied daily dermally before applying MPT (100 mg/kg per day) daily for 4 days] (Fig. 4A). In all animals before exposure, 200 μl of blood was collected using retro-orbital puncture and evaluated for AChE activity as an internal control. Immediately after mortality or on day 30, animals were sacrificed, and their organs were collected to quantify the AChE level as described above. Similarly, another set of a single application of gel experiments was performed (n = 6 rats per group). In this experiment, 220 mg of sham gel or poly-Oxime gel was applied only once, i.e., on day 0, and on the same gels, these animals received MPT (100 mg/kg per day) for 4 days (Fig. 4D).
Rotarod test
A total of 12 male Sprague-Dawley rats (10 weeks) were used to study the loss of endurance in MPT-exposed animals and the ability of poly-Oxime gel to prevent the same. Animals were placed on a Rotarod treadmill (Rotamex-5 1.4, Columbus Instruments; lane width, 9.3 cm; diameter of rod, 7 cm; fall distance, 48.3 cm), subjected to a uniform increase in acceleration between 0 and 20 rpm, and allowed to run at 20 rpm until the animal got tired and fell off the rod, and the time of fall was recorded. Rats were trained for 3 days before the exposure, where animals were randomly grouped into three groups: (i) direct exposure, (ii) poly-Oxime gel–treated, and (iii) control. On day 4, animals of the direct exposure group were exposed to a dosage of 150 mg/kg, and the animals of treatment group were given poly-Oxime gel and then exposed to the same dosage of MPT. Latency to fall was calculated as the time taken to fall on any day with respect to day 4 (before exposure) and was converted to percentage.
Similarly, to study NMC, 15 animals were trained on an increasing rotarod acceleration between 2 and 60 rpm, with an acceleration step of 4 rpm every 8 s. On day 5 of training, animals were divided into three groups: (i) direct exposure, (ii) poly-Oxime gel–treated, and (iii) naïve control. On day 5, animals of the direct exposure group were exposed to a dosage of 150 mg/kg, and the animals of treatment group were given poly-Oxime gel and then exposed to the same dosage of MPT. Animals were given a score of +1 for each step up in the acceleration (increase by 4 rpm) before the fall, and the percentage of NMC was calculated with respect to day 5 (before exposure).
Gait analysis and EMG
Gait analysis was performed on 15 male rats (10 weeks old) to evaluate their walking pattern. To obtain footprints, four paws were colored with different nontoxic water colors and animal was trained to walk through an ally (width, 8 cm; length, 120 cm; height, 10 cm), leading to its home cage. After training, animals were divided randomly into three groups: (i) direct exposure, (ii) poly-Oxime gel–treated, and (iii) naïve control. Footprints were analyzed manually, and SFI was calculated using following formula (40, 41)
where N indicates normal/before exposure, E indicates after exposure, TOF is the distance to the opposite foot, PL is the distance from the heel to the third toe (the print length), TS is the distance from the first toe to the fifth toe (the toe spread), and IT is the distance from the second toe to the fourth toe (the intermediate toe spread).
EMG of animals from these three groups was recorded using Muscle SpikerBox (Backyard Brains). Skin surface electrodes were used with adhesive pads and conductive gel to facilitate the recordings. To track the muscle spasms under anesthesia (2.5% isoflurane in carbogen), EMG from biceps femoris of left hind limb was recorded. To avoid animal from disturbing the electrodes, we recorded EMG between spinotrapezius and gluteus maximus when the animal was awake.
Statistical analysis
The two-tailed Student’s t test was used to compare differences between two experimental groups. In experiments with multiple groups, one-way ANOVA with Tukey post hoc test was used. In survival experiments, Mantel-Cox test was used. P < 0.05 was considered as a statistically significant difference. Statistical analysis and graphing were performed with Prism 6 (GraphPad Software).
Supplementary Material
Acknowledgments
We thank R. Mohamed for helping with animal experiments and P. P. Theja for helping with synthesis. We thank animal house facility/members and Central Imaging & Flow Cytometry facility (CIFF) at inStem and NCBS. Funding: This work was supported by Biotechnology Industry Research Assistance Council (BIRAC) and Centre for Cellular and Molecular Platforms (C-CAMP) through Biotechnology Ignition Grant (C-CAMP/BIG/90H) to P.K.V. and core funds from the Institute for Stem Cell Biology and Regenerative Medicine (inStem). P.K.V. was supported by Ramalingaswami Re-entry Fellowship, Department of Biotechnology (DBT), India. S.C. was supported by the Department of Science and Technology (DST) under the Scheme for Young Scientists and Technologies program (SP/YO/078/2017). S.P. was supported by Junior Research Fellowship by the Lady Tata Memorial Trust. A.A.H. was supported by Senior Research Fellowship by the Council for Scientific and Industrial Research (CSIR). Animal work in the inStem/NCBS Animal Care and Resource Centre was partially supported by the National Mouse Research Resource (NaMoR) grant (BT/PR5981/MED/31/181/2012;2013-2016;2018) from the Department of Biotechnology. Author contributions: P.K.V. conceived and designed the experiments. K.T. and S.P. designed and performed the experiments and analyzed the data. S.C. synthesized the molecules/polymers. K.T., S.P., S.C., N.V., A.A.H., P.S., S.S., S.U., T.P., M.M., and O.S. performed in vitro and in vivo experiments. K.K.M.-N. performed tissue analysis. K.T. and P.K.V. wrote the paper. All authors discussed the results. P.K.V. supervised the project. Competing interests: P.K.V., K.T., S.C., and S.P. are inventors on a provisional patent application related to this work, filed with the Indian Patent Office (IPP application no: 201841006678; filed on 21 February 2018). The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/10/eaau1780/DC1
Fig. S1. Rheological analysis of poly-Oxime and sham gels.
Fig. S2. Ex vivo Franz diffusion assay with commercial organophosphates.
Fig. S3. Limiting MPT-induced AChE inhibition in blood using poly-Oxime and poly-methoxyOxime.
Fig. S4. Change in body weight and body temperature following acute exposure.
Fig. S5. Brain AChE and body weight decrease in repeated exposure of MPT with daily application of poly-Oxime gel.
Fig. S6. Brain AChE and body weight decrease in repeated exposure of MPT with single application of gel.
Fig. S7. Ex vivo Franz diffusion assay with various barrier creams to measure AChE inhibition.
Fig. S8. Ex vivo Franz diffusion assay with poly-Oxime gel before and after exposure to UV light.
Table S1. Pseudo–first-order rate constants for hydrolysis of organophosphates by poly-Oxime polymer.
Supplementary Methods
REFERENCES AND NOTES
- 1.Brightman M. W., Albers R. W., Species differences in the distribution of extraneuronal cholinesterases within the vertebrate central nervous system. J. Neurochem. 4, 244–250 (1959). [DOI] [PubMed] [Google Scholar]
- 2.Koelle G. B., The histochemical localization of cholinesterases in the central nervous system of the rat. J. Comp. Neurol. 100, 211–235 (1954). [DOI] [PubMed] [Google Scholar]
- 3.Speed H. E., Blaiss C. A., Kim A., Haws M. E., Melvin N. R., Jennings M., Eisch A. J., Powell C. M., Delayed reduction of hippocampal synaptic transmission and spines following exposure to repeated subclinical doses of organophosphorus pesticide in adult mice. Toxicol. Sci. 125, 196–208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A. Watson, D. Opresko, R. Young, V. Hauschild, J. King, K. Bakshi, Organophosphate nerve agents, in Handbook of Toxicology of Chemical Warfare Agents, R. C. Gupta, Ed. (Academic Press, 2009), pp. 43–67. [Google Scholar]
- 5.Abhilash P. C., Singh N., Pesticide use and application: An Indian scenario. J. Hazard. Mater. 165, 1–12 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Narayan S., Sinsheimer J. S., Paul K. C., Liew Z., Cockburn M., Bronstein J. M., Ritz B., Genetic variability in ABCB1, occupational pesticide exposure, and Parkinson’s disease. Environ. Res. 143, 98–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajgar J., Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 38, 151–216 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Bouchard M. F., Chevrier J., Harley K. G., Kogut K., Vedar M., Calderon N., Trujillo C., Johnson C., Bradman A., Barr D. B., Eskenazi B., Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 119, 1189–1195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-Alzaga B., Hernández A. F., Rodríguez-Barranco M., Gómez I., Aguilar-Garduño C., López-Flores I., Parrón T., Lacasaña M., Pre- and postnatal exposures to pesticides and neurodevelopmental effects in children living in agricultural communities from South-Eastern Spain. Environ. Int. 85, 229–237 (2015). [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization, The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009 (World Health Organization, 2010), pp. 1–60. [Google Scholar]
- 11.Dawson A. H., Eddleston M., Senarathna L., Mohamed F., Gawarammana I., Bowe S. J., Manuweera G., Buckley N. A., Acute human lethal toxicity of agricultural pesticides: A prospective cohort study. PLOS Med. 7, e1000357 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh B., Gupta M. K., Pattern of use of personal protective equipments and measures during application of pesticides by agricultural workers in a rural area of Ahmednagar district, India. Indian J. Occup. Environ. Med. 13, 127–130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacFarlane E., Carey R., Keegel T., El-Zaemay S., Fritschi L., Dermal exposure associated with occupational end use of pesticides and the role of protective measures. Saf. Health Work 4, 136–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang F. K., Chen M. L., Cheng S. F., Shih T. S., Mao I. F., Field protection effectiveness of chemical protective suits and gloves evaluated by biomonitoring. Occup. Environ. Med. 64, 759–762 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bignon C., Amigoni S., Devers T., Guittard F., Barrier cream based on CeO2 nanoparticles grafted polymer as an active compound against the penetration of organophosphates. Chem. Biol. Interact. 267, 17–24 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Chilcott R. P., Dalton C. H., Hill I., Davison C. M., Blohm K. L., Clarkson E. D., Hamilton M. G., Evaluation of a barrier cream against the chemical warfare agent VX using the domestic white pig. Basic Clin. Pharmacol. Toxicol. 97, 35–38 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Millerioux J., Cruz C., Bazire A., Lallement G., Lefeuvre L., Josse D., In vitro selection and efficacy of topical skin protectants against the nerve agent VX. Toxicol. In Vitro 23, 539–545 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Zenerino A., Boutard T., Bignon C., Amigoni S., Josse D., Devers T., Guittard F., New CeO2 nanoparticles-based topical formulations for the skin protection against organophosphates. Toxicol. Rep. 2, 1007–1013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salerno A., Devers T., Bolzinger M.-A., Pelletier J., Josse D., Briançon S., In vitro skin decontamination of the organophosphorus pesticide paraoxon with nanometric cerium oxide CeO2. Chem. Biol. Interact. 267, 57–66 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Benameur L., Auffan M., Cassien M., Liu W., Culcasi M., Rahmouni H., Stocker P., Tassistro V., Bottero J.-Y., Rose J., Botta A., Pietri S., DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology 9, 696–705 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Auffan M., Rose J., Orsiere T., De Meo M., Thill A., Zeyons O., Proux O., Masion A., Chaurand P., Spalla O., Botta A., Wiesner M. R., Bottero J.-Y., CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology 3, 161–171 (2009). [Google Scholar]
- 22.Bhattacharya S., Kumar V. P., Evidence of enhanced reactivity of DAAP nucleophiles toward dephosphorylation and deacylation reactions in cationic Gemini micellar media. J. Org. Chem. 69, 559–562 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Toullec J., Moukawim M., Cetyltrimethylammonium hydroperoxide: An efficient reagent for promoting phosphate ester hydrolysis. Chem. Commun. 0, 221–222 (1996). [Google Scholar]
- 24.Yang Y. C., Szafraniec L. L., Beaudry W. T., Bunton C. A., Perhydrolysis of nerve agent VX. J. Org. Chem. 58, 6964–6965 (1993). [Google Scholar]
- 25.Kumar V. P., Ganguly B., Bhattacharya S., Computational study on hydroxybenzotriazoles as reagents for ester hydrolysis. J. Org. Chem. 69, 8634–8642 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya S., Kumar V. P., Ester cleavage properties of synthetic hydroxybenzotriazoles in cationic monovalent and gemini surfactant micelles. Langmuir 21, 71–78 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Shin M., Park S.-G., Oh B.-C., Kim K., Jo S., Lee M. S., Oh S. S., Hong S.-H., Shin E.-C., Kim K.-S., Kang S.-W., Lee H., Complete prevention of blood loss with self-sealing haemostatic needles. Nat. Mater. 16, 147–152 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Dutta P. K., Dutta J., Tripathi V. S., Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 63, 20–31 (2004). [Google Scholar]
- 29.Nam D. C., Ha Y. M., Park M. K., Cho S. K., The rs662 polymorphism of paraoxonase 1 affects the difference in the inhibition of butyrylcholinesterase activity by organophosphorus pesticides in human blood. Int. J. Clin. Pharmacol. Ther. 54, 622–627 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Worek F., Eyer P., Thiermann H., Determination of acetylcholinesterase activity by the Ellman assay: A versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test. Anal. 4, 282–291 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Edson E. F., Noakes D. N., The comparative toxicity of six organophosphorus insecticides in the rat. Toxicol. Appl. Pharmacol. 2, 523–539 (1960). [DOI] [PubMed] [Google Scholar]
- 32.B. Ballantyne, T. C. Marrs, Overview of the biological and clinical aspects of organophosphates and carbamates, in Clinical and Experimental Toxicology of Organophosphates and Carbamates (Butterworth-Heinemann, 1992), pp. 3–14. [Google Scholar]
- 33.Masuda Y., Cardiac effect of cholinesterase inhibitors used in Alzheimer’s disease—From basic research to bedside. Curr. Alzheimer Res. 1, 315–321 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Saadeh A. M., Farsakh N. A., al-Ali M. K., Cardiac manifestations of acute carbamate and organophosphate poisoning. Heart 77, 461–464 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karalliedde L., Henry J. A., Effects of organophosphates on skeletal muscle. Hum. Exp. Toxicol. 12, 289–296 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Stamper C. R., Balduini W., Murphy S. D., Costa L. G., Behavioral and biochemical effects of postnatal parathion exposure in the rat. Neurotoxicol. Teratol. 10, 261–266 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Spyker J. M., Avery D. L., Neurobehavioral effects of prenatal exposure to the organophosphate Diazinon in mice. J. Toxicol. Environ. Health 3, 989–1002 (1977). [DOI] [PubMed] [Google Scholar]
- 38.Zhu H., Rockhold R. W., Baker R. C., Kramer R. E., Ho I. K., Effects of single or repeated dermal exposure to methyl parathion on behavior and blood cholinesterase activity in rats. J. Biomed. Sci. 8, 467–474 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Buttemer W. A., Story P. G., Fildes K. J., Baudinette R. V., Astheimer L. B., Fenitrothion, an organophosphate, affects running endurance but not aerobic capacity in fat-tailed dunnarts (Sminthopsis crassicaudata). Chemosphere 72, 1315–1320 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Sarikcioglu L., Demirel B. M., Utuk A., Walking track analysis: An assessment method for functional recovery after sciatic nerve injury in the rat. Folia Morphol. 68, 1–7 (2009). [PubMed] [Google Scholar]
- 41.de Medinaceli L., Freed W. J., Wyatt R. J., An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 77, 634–643 (1982). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/10/eaau1780/DC1
Fig. S1. Rheological analysis of poly-Oxime and sham gels.
Fig. S2. Ex vivo Franz diffusion assay with commercial organophosphates.
Fig. S3. Limiting MPT-induced AChE inhibition in blood using poly-Oxime and poly-methoxyOxime.
Fig. S4. Change in body weight and body temperature following acute exposure.
Fig. S5. Brain AChE and body weight decrease in repeated exposure of MPT with daily application of poly-Oxime gel.
Fig. S6. Brain AChE and body weight decrease in repeated exposure of MPT with single application of gel.
Fig. S7. Ex vivo Franz diffusion assay with various barrier creams to measure AChE inhibition.
Fig. S8. Ex vivo Franz diffusion assay with poly-Oxime gel before and after exposure to UV light.
Table S1. Pseudo–first-order rate constants for hydrolysis of organophosphates by poly-Oxime polymer.
Supplementary Methods


