Abstract
Background:
No pharmacological treatments exist for active suicidal ideation (SI), but the glutamatergic modulator ketamine elicits rapid changes in SI. We developed data-driven subgroups of SI trajectories after ketamine administration, then evaluated clinical, demographic, and neurobiological factors that might predict SI response to ketamine.
Methods:
Data were pooled from five clinical ketamine trials. Treatment-resistant inpatients (n = 128) with DSM-IV-TR-diagnosed major depressive disorder (MDD) or bipolar depression received one subanesthetic (0.5 mg/kg) ketamine infusion over 40 min. Composite SI variable scores were analyzed using growth mixture modeling to generate SI response classes, and class membership predictors were evaluated using multinomial logistic regressions. Putative predictors included demographic variables and various peripheral plasma markers.
Results:
The best-fitting growth mixture model comprised three classes: Non-Responders (29%), Responders (44%), and Remitters (27%). For Responders and Remitters, maximal improvements were achieved by Day 1. Improvements in SI occurred independently of improvements in a composite Depressed Mood variable for Responders, and partially independently for Remitters. Indicators of chronic SI and self-injury were associated with belonging to the Non-Responder group. Higher levels of baseline plasma interleukin-5 (IL-5) were linked to Remitters rather than Responders.
Limitations:
Subjects were not selected for active suicidal thoughts; findings only extend to Day 3; and plasma, rather than CSF, markers were used.
Conclusion:
The results underscore the heterogeneity of SI response to ketamine and its potential independence from changes in Depressed Mood. Individuals reporting symptoms suggesting a longstanding history of chronic SI were less likely to respond or remit post-ketamine.
Keywords: Growth mixture modeling, Suicidal ideation, Ketamine, Depression, Suicide
1. Introduction
Suicide poses a serious threat to public health. Worldwide, suicide accounts for approximately 1 million deaths, and 10 million suicide attempts are reported annually (World Health Organization, 2014). In the United States, the national suicide rate has increased by approximately 28% over the last 15 years (Curtin et al., 2016). At the same time, relatively few interventions for suicide risk exist. While treatments such as clozapine and lithium have demonstrated effects on suicidal behavior over weeks to months, these effects may be limited to specific diagnoses (Cipriani et al., 2005; Griffiths et al., 2014). Currently, no FDA-approved medications exist to treat suicidal ideation (SI), leaving those who experience a suicidal crisis with limited options for a reprieve of symptoms. Consequently, a critical need exists for rapid-acting treatments that can be used in emergency settings.
A promising off-label agent for this purpose is the rapid-acting antidepressant ketamine, which past studies have suggested reduces suicidal thoughts (Diazgranados et al., 2010a; Murrough et al., 2015; Price et al., 2009). A recent meta-analysis of 167 patients with a range of mood disorder diagnoses found that ketamine reduced suicidal thoughts compared to placebo as rapidly as within a few hours, with effects lasting as long as seven days (Wilkinson et al., 2017). These results are reinforced by newer findings of reduced active suicidal ideation post-ketamine compared to a midazolam control (Grunebaum et al., 2018). As the efficacy literature develops in the era of personalized medicine, two important issues must be addressed. First, little is known about the acute course of SI following ketamine. The speed with which antidepressant response occurs, and how much improvement can be expected on average, has been documented for single administrations of ketamine (Mathew et al., 2012; Sanacora et al., 2017); in the limited available literature, researchers have emulated previous studies examining antidepressant effect, where a cutoff of 50% improvement demarcated response (Nierenberg and DeCecco, 2001). Nevertheless, it remains unknown whether this categorization accurately reflects the phenomenon of suicidal thoughts. Empirically-derived approaches to the description of SI trajectory after ketamine may be useful in operationalizing “response” in future clinical trials.
Second, identifying demographic, clinical, or biological predictors of SI response to ketamine would allow researchers and clinicians to determine who is most likely to exhibit an SI response to ketamine. A broad literature describes clinical and demographic predictors for suicide risk (Franklin et al., 2017), and a smaller literature connects suicidal thoughts and behaviors to plasma markers such as brain-derived neurotrophic factor (BDNF) and cytokines (Bay-Richter et al., 2015; Falcone et al., 2010; Isung et al., 2012; Serafini et al., 2017; Serafini et al., 2013). However, no biomarkers have been shown to predict SI/ behavior response to intervention, a finding reinforced by the National Action Alliance for Suicide Prevention’s Research Prioritization Task Force’s Portfolio Analysis (National Action Alliance for Suicide Prevention: Research Prioritization Task Force, 2015). Notably, predictor analyses have the potential to reveal insights into personalized treatments for suicidal individuals, as well as the neurobiology of SI response. With respect to antidepressant response, for example, this approach yielded the observation that individuals with a family history of alcohol dependence may be more likely to exhibit an antidepressant response to ketamine (Krystal et al., 2003; Niciu et al., 2014; PermodaOsip et al., 2014).
The goals of this study were to elucidate trajectories of SI response and identify predictors of that response, with the ultimate goal of adding to the growing literature surrounding ketamine’s specific effects on SI. In particular, we sought to determine whether the heterogeneous patterns of change in SI after ketamine administration were better explained by a model with two or more latent groups of trajectories rather than a single average trajectory, using secondary analyses from previously published clinical trials. These classes were then used to evaluate potential clinical, demographic, and plasma biomarker predictors of SI response to ketamine in order to generate hypotheses.
2. Methods
Data from five independent studies of ketamine in treatment-resistant major depressive disorder (MDD) and bipolar I or II depression without psychotic features were combined; details regarding the patient population have been published previously (Diazgranados et al., 2010b; Ibrahim et al., 2012; Nugent et al., 2018; Zarate et al., 2012; Zarate et al., 2006). A total of 128 patients (ages 19 to 66 (M = 43.83, SD = 12.12); 58 males and 70 females; see Table 1) were admitted for study to the Mood and Anxiety Disorders research unit at the National Institutes of Health (NIH), Bethesda, MD, USA. Participants were screened and deemed eligible for further evaluation upon meeting research criteria, which included meeting criteria for a major depressive episode of at least moderate severity (≥ 18 on the Hamilton Depression Rating Scale (HAM-D) (Zarate et al., 2006) or ≥ 20 (Diazgranados et al., 2010b; Nugent et al., 2018; Zarate et al., 2012) on the Montgomery-Asberg Depression Rating Scale (MADRS). Once at the NIH, patient diagnosis was established using the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (First et al., 2002) and corroborated by a team of clinicians using all available information. All subjects were in good physical health, as determined by medical history, physical examination, and laboratory tests. Exclusion criteria included pregnancy, breastfeeding, or illicit comorbid substance abuse with the previous three months. Written informed consent was obtained from all participants, and the NIH combined Neuroscience Institute Review Board approved the studies.
Table 1.
Demographics of sample used in growth mixture model and predictor analysis.
| Growth mixture model Sample (N = 128) | Predictor analysis sample(N = 107) | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age (years) | 43.83 | 12.12 | 43.07 | 12.18 |
| Body mass index | 29.55 | 6.79 | 29.04 | 6.35 |
| Length of Illness (years) | 25.01 | 12.49 | 24.78 | 12.67 |
| Clinical ratingsMADRS | 33.47 | 4.68 | 33.47 | 4.68 |
| Suicidal ideation score | 0.35 | 0.18 | 0.36 | 0.18 |
| Depressed mood score | 0.59 | 0.10 | 0.60 | 0.10 |
| n | % | n | % | |
| Sex (Male) | 58 | 45 | 50 | 47 |
| Race (White) | 105 | 83 | 93 | 88 |
| Diagnosis Major depressive disorder | 86 | 67 | 65 | 61 |
| Bipolar disorder | 42 | 33 | 42 | 39 |
| History of suicide attempt | 48 | 38 | 42 | 39 |
| History of psychiatric hospitalization | 80 | 63 | 70 | 65 |
Note: Suicidal Ideation and Depressed Mood scores are composite scores on a scale of 0 (no symptoms) to 1 (most severe symptoms). The Depressed Mood factor comprises items from the Beck Depression Inventory (BDI; Item 1), the Montgomery–Asberg Depression Rating Scale (MADRS; Items 1, 2, 6, 8), and the Hamilton Depression Rating Scale (HAMD; Items 1, 2, 16). The Suicidal Thoughts factor includes items from the MADRS (Item 10) and BDI (Items 2 and 9).
2.1. Design
As noted above, subject data were obtained from one of five studies; one of these examined ketamine’s antidepressant effects in MDD (Zarate et al., 2006), two examined the use of ketamine to treat bipolar depression (Diazgranados et al., 2010b; Zarate et al., 2012), one examined the use of riluzole to extend ketamine’s antidepressant effects (Ibrahim et al., 2012), and one explored ketamine’s mechanism of action (Nugent et al., 2018) (Clinical Trials Identifier: NCT0088699; NIH Protocol 04-M-0222, substudies 1, 2, 3, and 4, respectively). All patients received a single subanesthetic (0.5 mg/kg) intravenous infusion of ketamine hydrochloride over 40 min. Patients enrolled in the MDD ketamine crossover and ketamine-riluzole studies were free of all psychotropic medications for at least two weeks (five weeks for fluoxetine) prior to the first infusion. The two bipolar studies were both randomized, double-blind, placebo-controlled crossover studies where subjects were maintained on therapeutic levels of either lithium or valproate for at least two weeks prior to the first infusion. The current analyses included the ketamine-only condition (all bipolar patients and all MDD patients with the exception of those randomized to add-on riluzole), at baseline, Day 1, Day 2, and Day 3. It should be noted that many participants were taking numerous medications at the time of their admission to the NIH; participants subsequently completed a screening phase, medication taper, and medication washout period, which could range from weeks to months before entry into the present study and ketamine administration. Therefore, we make a distinction between variables that were collected upon admission to the NIH (which were included as potential clinical and sociodemographic predictors of SI response) and baseline (which were ratings administered just before ketamine infusion, as part of these randomized control trials).
2.2. Plasma biomarkers
Whole blood samples were obtained using the vacutainer system at 60 min before ketamine infusion and at one day post-infusion. Plasma markers were evaluated due to prior evidence of a link between suicidal thoughts and behaviors (Bay-Richter et al., 2015; Falcone et al., 2010; Isung et al., 2012; Serafini et al., 2017; Serafini et al., 2013). Peripheral plasma assays were evaluated for neurotrophic factors (BDNF, vascular endothelial growth factor (VEGF), and S100 calcium-binding protein B (S100B)), kynurenine pathway analytes (indoleamine 2,3-dioxygenase (IDO), kynurenine (Kyn), kynurenine acid (KynA), and quinolinic acid (QA)), pro- and anti-inflammatory cytokines (tumor necrosis factor alpha (TNF-α), soluble tumor necrosis factor receptor 1 (sTNFR1), interferon gamma (IFN-γ), interleukin 1 (IL-1), interleukin 2 (IL-2), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 8 (IL-8), and interleukin 10 (IL-10)), as well as cortisol. Further details can be found in the Supplementary Materials.
2.3. Measures
The variable of interest in this study was a composite SI score comprising two items from the Beck Depression Inventory (BDI; Hopelessness and Suicidal Thoughts) and one item from the HAM-D (Suicidal Thoughts). A second composite variable, Depressed Mood, comprised items from the BDI (Sadness), the HAM-D (Depressed Mood, Inability to Work, Psychomotor Retardation), and the MADRS (Reported Sadness, Concentration Difficulties, Inability to Feel, Apparent Sadness). These subscales were empirically derived from an exploratory factor analysis of the BDI, HAM-D, MADRS, and the Snaith–Hamilton Pleasure Scale (SHAPS); in that analysis, the scales were shown to have differential response patterns to ketamine and placebo, suggesting potential utility in measuring unidimensional constructs, rather than the broad heterogeneity of symptoms commonly assessed by depression rating scales (Ballard et al., 2018). Baseline ratings were obtained 60 min prior to infusion, and possible scores on these composites ranged from 0 to 1, reflecting the average proportion of available points per item. It should be noted that these composites were developed to be dimensional measures across a range of symptoms rather than dichotomizing patients as suicide ideators/non-ideators. Thus, in this analysis, only three patients scored a “0″ on this composite measure. Given our desire to study individuals across a spectrum of suicidal thoughts, all participants were included in this analysis. Sociodemographic and clinical variables—obtained at admission to the hospital during the screening phase—were evaluated as potential predictors of SI response. These variables were selected based on prior evidence of their association with antidepressant response to ketamine (including body-mass index (BMI) and family history of an alcohol use disorder) (Niciu et al., 2014). We also included variables that have not specifically been associated with antidepressant response to ketamine, but are nevertheless linked with suicide risk; these included personal history of physical or sexual abuse, personal history of suicide attempt, SI on admission to the NIH, and family history of suicidal behavior (Franklin et al., 2017). This information was not collected for one study (Zarate et al., 2006), which was excluded from these analyses. All relevant analyses appear in Supplementary Table 1.
2.4. Statistical analysis
Growth mixture modeling is a person-centered latent variable method to parse heterogeneity in a sample. It is an extension of conventional latent growth modeling, which treats heterogeneity in the parameters as explained by an unobserved (latent) class variable. We used the latent class growth curve analysis—the simplest type of growth mixture model—because more complex models did not converge reliably. In this model, the mean values of the intercept, slope, and quadratic term are estimated and allowed to vary between classes.Models with up to five classes were enumerated and compared using the following relative fit indices: the loglikelihood, the Bayesian information criterion, the adjusted Bayesian information criterion, Aikake’s information criterion, and the consistent Aikake’s information criterion (Masyn, 2013). Bayes’ factor and the approximate-weight-of-evidence criterion were used to help interpret information criteria. Finally, the Vuong-Lo-Mendell-Rubin likelihood ratio test was used to assess the degree of improvement in model fits with additional classes (Masyn, 2013). All analyses were performed with a censored distribution assumed for the dependent variable. Study was initially entered as a covariate but was subsequently excluded from the models because it was not statistically significant (p > .10).
General linear mixed models were used to help disentangle improvements between SI and Depressed Mood; these models included fixed effects of class and time, as well as a repeated effect (with compound symmetry covariance structure) of time. Assumptions were evaluated with visual inspection of residual plots. Cohen’s d effect sizes were calculated using least squares mean difference estimates and associated degrees of freedom.
Predictor analyses were performed in a subset of the sample (N = 107, excluding 21 subjects with incomplete data); given the high degree of entropy (the measure of classification uncertainty ranging from 0 to 1, in which higher is better), most-likely class assignment was entered as the dependent variable in multinomial logistic regressions. To conserve space, only a subset of the putative predictors is presented in the text (those with the most existing evidence for inclusion), but the results of all analyses are available in Supplementary Table 1. Given the exploratory, hypothesis-generating nature of these analyses, alpha was set to 0.05. We did not adjust for multiple comparisons. All growth mixture model analyses were completed in MPlus version 7.4 (Muthen and Muthen, 2012); other analyses were performed in IBM SPSS Statistics 24 (2016).
3. Results
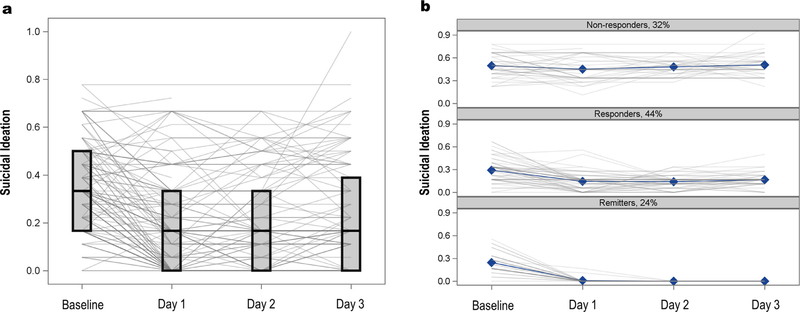
Demographic and clinical information for participants included in the growth mixture model and predictor analyses are presented in Table 1. The average baseline SI subscale score was 0.35 (SD = 0.18) on a scale of 0–1 (0 = no symptoms, 1 = most severe symptoms). While the average score decreased across visits, considerable heterogeneity was apparent (Fig. 1A). The relative fit indices suggested that the three-class solution was best fit to the data (see Table 2). Class 1, referred to hereafter as “Non-Responders”, exhibited no SI response, and was characterized by the highest baseline levels of SI and no change in SI at Days 1, 2, or 3. Class 2, hereafter referred to as “Responders”, was the largest class. Responders had a statistically significant slope and quadratic term (p < .001), reflecting improvement in SI during the study. Finally, Class 3, hereafter referred to as “Remitters”, improved significantly during the course of the study (slope and quadratic terms, p < .001), such that all members had scores of zero by Day 2. Responders and Remitters did not differ in baseline SI (see Fig. 1B). Entropy of the selected model was high (0.93), and the average posterior probability for each class exceeded 0.95; thus, most-likely class membership was used for the remainder of the analyses.
Fig. 1.
Suicidal Ideation (SI) composite scores (Panel A) by SI latent class (Panel B).
Table 2.
Results of growth mixture model.
| 1-class | 2-class | 3-class | 4-class | 5-class | |
|---|---|---|---|---|---|
| Parameters | 7 | 11 | 15 | 19 | 23 |
| LL | −115 | −6.4 | 51 | 71 | 81 |
| AIC | 245 | 34 | −73 | −105 | −117 |
| BIC | 265 | 66 | −30 | −51 | −51 |
| ABIC | 243 | 31 | −77 | −111 | −124 |
| VLMR (p) | .02 | .0006 | .16 | .12 | |
| Bayes’ Factor | 1.6304E + 43 | 7.0167E + 20 | 36,315 | 1.65 | |
| AWE | 319 | 153 | 89 | 99 | 130 |
Note: LL = loglikelihood (larger is better); BIC = Bayesian information criterion (smaller is better); ABIC = adjusted Bayesian information criterion (smaller is better); AIC = Aikake’s information criterion (smaller is better); Bayes’ Factor (larger is better, > 10 is strong evidence); AWE = approximate weight of evidence criterion (smaller is better); VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test (p-value, smaller is better).
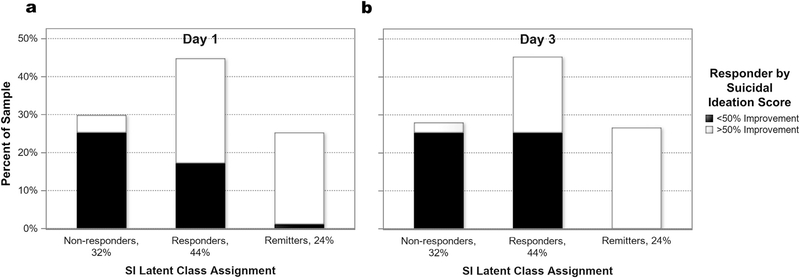
3.1. Relationship of class membership to a standard definition of response
A 50% improvement in depressive symptoms is often considered an antidepressant response in the literature. Fig. 2 depicts the relationship between SI response class membership and 50% reduction in the SI composite score at both Day 1 (panel A) and Day 3 (panel B) after ketamine administration. Those who exhibited a 50% SI response were represented in each SI class at Day 1 and in two of the three classes at Day 3.
Fig. 2.
Suicidal Ideation (SI) latent class assignment by traditional responder groupings at post-ketamine Day 1 (Panel A) and Day 3 (Panel B).
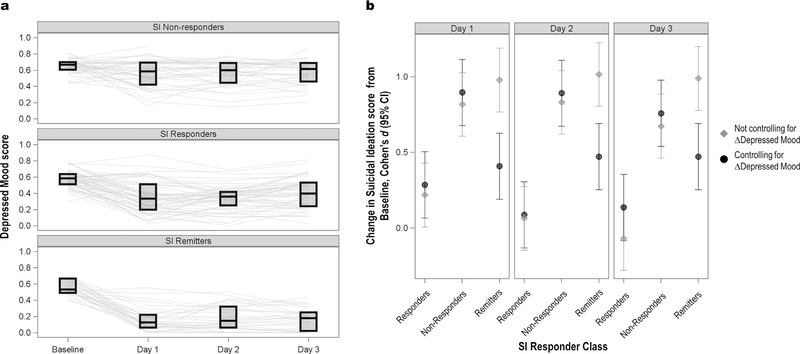
3.2. The role of depressed mood
Because depressed mood and SI are closely related, average antidepressant response of the Non-Responders, Responders, and Remitters was explored in relation to the Depressed Mood composite score (see Fig. 3, panel A). No difference in baseline Depressed Mood was observed among the three SI classes. SI Remitters had the most dramatic antidepressant response (71% improvement), falling into the subclinical range by Day 1. SI Responders also exhibited an antidepressant response, with an average 39% improvement by Day 1. The average improvement in antidepressant symptoms in the Non-Responder group was 12% at Day 1.
Fig. 3.
Independence of change in Suicidal Ideation (SI) from change in Depressed Mood. Panel A illustrates Depressed Mood composite scores by SI latent class. Panel B illustrates the change from baseline post-ketamine (Cohen’s d with 95% CI) in each class, with or without change in Depressed Mood entered as a covariate.
Next, we created a model to assess whether within-class improvements in SI, and between-class differences in SI trajectory, were explained by general improvements in Depressed Mood. In the initial model predicting SI scores, a significant class-by-time interaction was observed [F(6,350) = 11.58, p < .0001]; post-hoc tests indicated that the classes were significantly different from one another at all timepoints. Within the classes, change from baseline was significant for the Responders and Remitters but not the Non-Responders (see Fig. 3, panel B). Because this analysis is an iteration of the growth mixture model, these results were not surprising; the focus was the final model, which included a fixed effect of change in Depressed Mood score (Day 3 – baseline). The three-way interaction between change in Depressed Mood, class, and time was not significant [F(6,324) = 0.86, p = .52], indicating that the class-by-time interaction did not differ with various levels of antidepressant response. The class-by-time interaction did remain statistically significant after controlling for antidepressant response [F(6,324) = 2.32, p = .03], but the nature of the interaction was altered from the original model. As illustrated in Fig. 3 (panel B), change in Depressed Mood appeared to account for about half of the improvement in SI among the Remitters. In contrast, the improvement in SI among the Responders remained unchanged after controlling for change in Depressed Mood, and Non-Responders similarly exhibited no response.
3.3. Predictors of class membership
Multinomial logistic regressions were conducted to assess whether clinical or biological variables predicted SI response group. Among demographic variables, older age (in years) was associated with slightly greater odds of being in the Remitter compared to the Responder group (OR = 1.05, 95% CI: 1.01 – 1.10), as was longer length of the current depressive episode (in months) (OR = 1.01, 95% CI: 1.00 – 1.02).
Analyses of clinical variables suggested that history of sexual abuse was associated with slightly greater odds of being in the Remitter compared to the Non-Responder group (OR = 4.12, 95% CI: 1.17 – 14.49). In contrast, positive history of self-injury was associated with lower odds of being in the Remitter compared to the Non-Responder group (OR = 0.08, 95% CI: 0.01 – 0.70). Participants with SI at admission to the NIH had slightly lower odds of being in the Remitter (OR = 0.22, 95% CI: 1.12 – 17.76) or Responder (OR = 0.19, 95% CI: 0.06 – 0.60) groups than in the Non-Responder group.
3.4. Peripheral plasma measures
Only one statistically significant predictor emerged from among the plasma biomarkers. Specifically, higher baseline levels of IL-5 were associated with slightly greater odds of being in the Remitter than Responder group (OR = 1.79, 95% CI: 1.07 – 2.98). See Table 3 and Supplementary Table 1 for results from all multinomial logistic regression analyses.
Table 3.
Predictors of SI class membership.
| OR (95% CI) |
||||
|---|---|---|---|---|
| Predictor | Overall model fit | Remitter: Non-Responder | Remitter: Responder | Responder: Remitter |
| Age | χ2(1) = 6.14, p = .05 | 1.02 (0.98–1.07) | 1.05 (1.01 –1.10) | 0.97 (0.94–1.01) |
| History of Self-Injury | χ2(1) = 9.50, p = .01 | 0.08 (0.01–0.70) | 0.23 (0.03–2.03) | 0.36 (0.12–1.04) |
| History of Sexual Abuse | χ2(1) = 5.25, p = .07 | 4.12 (1.17–14.49) | 0.57 (0.19–1.64) | 2.33 (0.74–7.35) |
| Length of Current Episode (months) | χ2(1) = 6.66, p = .04 | 1.00 (1.00–1.01) | 1.01 (1.00–1.02) | 0.99 (0.98–1.00) |
| Suicidal Ideation on Hospital Admission | χ2(1) = 10.44, p = .01 | 0.22 (0.06–0.90) | 1.17 (0.25–5.38) | 0.19 (0.06–0.60) |
| IL-5 | χ2(1) = 5.51, p = .06 | 1.40 (0.85–2.31) | 1.79 (1.07–2.98) | 0.78 (0.51–1.19) |
Note: OR = Odds ratio for membership in the first response class versus the second response class listed. Results of multinomial logistic regressions predicting class membership (Remitter, Responder, Non-Responder) from the given predictor variable. The full list of results is found in Supplementary Table 1; only those results discussed in the text are presented here.
4. Discussion
This analysis used a data-driven approach to characterize SI response to ketamine. The data were best explained by three trajectory classes: one with severe average baseline SI and little to no response to ketamine (Non-Responders), one with moderate average baseline levels of SI and significant response to ketamine (Responders), and a third with moderate average baseline levels of SI and complete remission of SI by two days post-ketamine (Remitters). These findings suggest a diversity of post-ketamine changes in SI that may not be captured under traditional methods of categorizing response to treatment. Furthermore, we found evidence that SI response and antidepressant response could be distinguished from each other; one subset of participants experienced improvement in SI that was partially explained by improvements in Depressed Mood, while the other group’s improvements in SI occurred independently of antidepressant response. With regard to predictors of SI response trajectory, preliminary results suggest the individuals least likely to experience improvement in SI post-ketamine were those with the most severe SI and a history of self-injury. Few plasma markers emerged as predictors of SI response in this study, highlighting the limitations of connecting SI ratings of response with biological markers.
The growth mixture modeling approach used here underscored the heterogeneity of SI response to ketamine, which would not have been captured by simply calculating the average trajectory. The class assignment from this approach also differed from the definition of response (50% reduction in symptoms) traditionally used in the antidepressant literature, which often focuses on a specific timepoint rather than the entire symptom trajectory. In comparing classification using a 50% response at Day 1 and Day 3 with the latent trajectory classes, we found representation of almost every SI class across each responder group, highlighting the potential limitations of the 50% response approach. Further study is needed to determine which of these approaches will prove more fruitful. Complete remission of SI has previously been used as an outcome measure in clinical trials and in a meta-analysis of ketamine’s efficacy (Grunebaum et al., 2017; Grunebaum et al., 2018; Wilkinson et al., 2017), as well as in a study examining the relationship between SI response to ketamine and changes in nocturnal wakefulness (Vande Voort et al., 2017). One strength of the present study is that this data-driven approach provides classifications that directly reflect the phenomena under study as they are, as opposed to what they should be. Especially when used in larger samples than the current study, this approach is particularly promising in its ability to provide a more nuanced understanding of the nature of SI response to ketamine.
Our results also support the idea that SI response in particular can be distinguished from depressed mood and can be treated as a distinct target. First, it should be noted here that SI classes were not distinguishable by baseline Depressed Mood scores; patients with the most severe SI did not differ meaningfully in Depressed Mood scores from those with the mildest SI. Second, while previous analyses of these data documented that BMI and family history of alcohol dependence predicted antidepressant response (Niciu et al., 2014), SI response was not associated with these variables in the current analysis. Third, the antidepressant response profiles of the SI classes suggest that SI response and antidepressant response are not wholly redundant. This aligns with previous clinical trials and meta-analytic reviews of the literature suggesting that SI response to ketamine occurs partially independently of antidepressant response (Grunebaum et al., 2018; Wilkinson et al., 2017). Nevertheless, this independence did not hold true across both SI response groups. Specifically, antidepressant and SI response were clearly linked in Remitters, with depression accounting for half of the changes in SI; however, in Responders, improvements in SI occurred independently from improvements in Depressed Mood. These discrepancies could be related to ketamine’s complex neurobiological mechanisms or to the potentially low levels of clinical severity observed in the Remitters.
Interestingly, the current analyses found no baseline demographic variables that reliably distinguished Responders from Remitters. Some phenotypic characteristics were uniquely associated with belonging to the Non-Responder group, suggesting that a long-standing history of self-injury or SI may indicate resistance to rapid changes in SI. Relatedly, a recent, randomized clinical trial of repeat-dose ketamine compared to placebo found that ketamine had no effect on SI in a sample of patients selected for their longstanding, chronic history of SI (Ionescu, 2017). These results highlight the importance of patient selection, particularly for suicide risk. It should be stressed, however, that SI does not necessarily translate to suicidal attempts or deaths; to our knowledge, no study has yet linked ketamine with reduced risk of suicidal behavior. Indeed, in the present study the SI Non-Responders experienced limited antidepressant effects in response to ketamine, but may nevertheless have improved on other, unmeasured symptoms that could provide important benefit and relief. As the ketamine literature develops, it will be important to identify which clinical symptom profiles are most likely to have a robust anti-SI and anti-suicidal behavior response to ketamine and which ones may benefit from other interventions.
While we evaluated a range of potential plasma markers previously linked to suicidal ideation and behavior, in the present analysis only IL-5 was associated with the SI Responder subgroup. Ketamine is known to have anti-inflammatory effects (Zunszain et al., 2013), but the relationship between antidepressant response and change in cytokine levels remains unclear (Park et al., 2017). Cytokines have been linked to suicidal behavior in the past; a recent meta-analysis found that lower levels of IL-2 and IL-4, and higher levels of TGFbeta, were associated with suicidal thoughts and behaviors (Serafini et al., 2013); however, to our knowledge IL-5 has not previously been linked to SI. Given the large number of comparisons and lack of precedent in the literature, this result may have been spurious and should be interpreted with caution. A number of other results may reflect meaningful relationships, but the high degree of variability—and the associated wide confidence intervals—suggests that larger sample sizes are needed to better elucidate the nature of any such relationships (e.g. baseline VEGF: χ2 = 6.13, p = .05, but OR (95% CI) 13.33 (0.93–200.00)). Somewhat surprisingly, plasma BDNF levels were not associated with responder class. Previous studies of bipolar, but not MDD, samples found that plasma BDNF levels were associated with SI response after ketamine (Grunebaum, 2017; Grunebaum et al., 2017), suggesting that the mixed diagnostic composition of this study may explain differences from previous work. Studies exploring the relationship between BDNF and antidepressant response to ketamine have also yielded mixed findings (Haile et al., 2014; Machado-Vieira et al., 2009). Other data-driven approaches have considered both biological and behavioral variables in characterizing depression (Drysdale et al., 2017); a similar approach might prove useful for predicting SI response.
The present study is associated with several strengths as well as limitations. Strengths include the relatively large sample size of participants who received ketamine, the use of composite SI scores from previous exploratory factor analyses as opposed to individual items, and the combination of clinical and biological markers as potential predictors of class membership. Limitations include patient selection methods, as these patients were part of an antidepressant trial and were not selected for active suicidal thoughts, as well as the exploratory nature of the analysis. As stated above, suicidal thoughts do not necessarily equate to suicidal behavior, and class membership would thus not necessarily correspond with an overall reduction in suicide risk. Another limitation is that results were collapsed across several clinical trials with slight variations in study design, and findings were thus only extended to Day 3 rather than a week after ketamine administration. As a result, only a subset of the sample could be used for predictive analyses. In addition, plasma—rather than CSF—markers were used, and the latter might better indicate underlying biology due to proximity to the brain, though certain markers such as plasma BDNF may be related to platelet storage, rather than the brain (Chacón-Fernández et al., 2016). Comparison of results to trajectories of suicide-specific measures, such as the Scale for Suicide Ideation (Beck et al., 1979), may also give further insight into specific SI content. Finally, many clinical predictors were collected upon hospital admission; future analyses could use formal assessments, such as the Childhood Traumatic Questionnaire (Bernstein et al., 1994), assessment of personality disorders, or diagnoses such as post-traumatic stress disorder (PTSD) as potential indicators of response.
Despite these limitations, the study demonstrates the utility of a data-driven approach for characterizing the heterogeneity of SI response to a rapid-acting intervention. This allows for a more fine-grained analysis of symptoms than would be permitted by traditional approaches, such as overall average response or dichotomization at 50% reduction in symptoms. This study identified several findings of note. These included distinguishing at least three patterns of SI response to ketamine and finding that subjects who exhibited more severe SI at baseline were not likely to experience an SI response to ketamine. Furthermore, for those participants who did exhibit an SI response to ketamine, a distinction could be made regarding whether the SI response was explained by general improvements in depressive symptoms or occurred independently of antidepressant response. Further prospective work is needed to replicate these classes among suicide-specific samples, as this analysis was meant to be hypothesis-generating. Due to the growing literature on personalized medicine, and its concomitant impact on rapid-acting treatments for acutely suicidal subjects, the need for such empirically-derived subgrouping of SI response will only increase.
Supplementary Material
Acknowledgements
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Role of funding source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH) (NCT00088699; ZIA-MH002857), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. These organizations had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Declaration of interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
All other authors have no conflict of interest to disclose, financial or otherwise.
Author disclosures
None
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2018.07.077.
References
- IBM SPSS Statistics for Windows, 2016. Version 21.0. IBM Corporation, Armonk NY Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, Williams D, Machado-Vieira R, Niciu MJ, Park L, Zarate CA Jr., 2018. Parsing the heterogeneity of depression: an exploratory factor analysis across commonly used depression rating scales. J. Affect. Disord 231, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L, 2015. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav. Immun 43, 110–117. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the scale for suicide ideation. J. Consult. Clin. Psychol 47, 343–352. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Chacón-Fernández P, Säuberli K, Conzani M, Moreau T, Gevaert C, Barde YA, 2016. Brain-derived neurotrophic factor in megakaryocytes. J. Biol. Chem 291, 9872–9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Pretty H, Hawton K, Geddes JR, 2005. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am. J. Psychiatry 162, 1805–1819. [DOI] [PubMed] [Google Scholar]
- Curtin SC, Warner M, Hedegaard H, 2016. Increase in Suicide in the United States, 1999–2014. NCHS Data Brief No. 241. National Center for Health Statistics, Hyattsville, MD. [Google Scholar]
- DiazGranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr., 2010a. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 71, 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr., 2010b. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C, 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med 23, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone T, Fazio V, Lee C, Simon B, Franco K, Marchi N, Janigro D, 2010. Serum S100B: a potential biomarker for suicidality in adolescents? PLoS One 5, e11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2002. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCID-I/NP). Biom. Res New York State Psychiatric Institute New York. [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, Musacchio KM, Jaroszewski AC, Chang BP, Nock MK, 2017. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol. Bull 143, 187–232. [DOI] [PubMed] [Google Scholar]
- Griffiths JJ, Zarate CA Jr., Rasimas JJ, 2014. Existing and novel biological therapeutics in suicide prevention. Am. J. Prev. Med 47, S195–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum M, 2017. Ketamine for rapid relief of suicidal thoughts in depression: cognition and biomarkers in a midazolam-controlled trial. In: Proceedings of the ACNP Fifty Sixth Annual Meeting Palm Springs, CA. [Google Scholar]
- Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ, 2017. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 19, 176–183. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ, 2018. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am. J. Psychiatry 175, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ 3rd, De La Garza R 2nd, Charney DS, Newton TF, Mathew SJ, 2014. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int. J. Neuropsychopharmacol 17, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr., 2012. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs. add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37, 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu D, 2017. A randomized, double blind, placebo controlled trial of repeat-dose ketamine augmentation for chronic suicidal thinking. In: Proceedings of the ACNP Fifty Sixth Annual Meeting Palm Springs, California. [Google Scholar]
- Isung J, Mobarrez F, Nordstrom P, Asberg M, Jokinen J, 2012. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J. Biol. Psychiatry 13, 468–473. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, Boutros NN, Trevisan L, Charney DS, 2003. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology 28, 2020–2028. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA Jr., 2009. Brain-derived neurotrophic factor and initial anti-depressant response to an N-methyl-D-aspartate antagonist. J. Clin. Psychiatry 70, 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyn KE, 2013. Latent class analysis and finite mixture modeling. In: Little T (Ed.), The Oxford Handbook of Quantitative Methods in Psychology 2 Oxford University Press Oxford, U.K. [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW, 2012. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 26, 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS, 2015. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol. Med 45, 3571–3580. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO, 2012. Mplus Version 7 User’s Guide. Muthen & Muthen, Los Angeles, CA. [Google Scholar]
- National Action Alliance for Suicide Prevention: Research Prioritization Task Force, 2015. U.S. National Suicide Prevention Research Efforts: 2008–2013 Portfolio Analyses. National Institute of Mental Health and the Research Prioritization Task Force, Rockville, MD. [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, Nolan NM, Zarate CA Jr., 2014. Clinical predictors of ketamine response in treatment-resistant major depression. J. Clin. Psychiatry 75, e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, DeCecco LM, 2001. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J. Clin. Psychiatry 62 (Suppl 16), 5–9. [PubMed] [Google Scholar]
- Nugent AC, Ballard E, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA, 2018. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry 10.1038/s41380-018-0028-2. February 27 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Newman LE, Gold PW, Luckenbaugh DA, Yuan P, Machado-Vieira R, Zarate CA Jr., 2017. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J. Psychiatr. Res 84, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permoda-Osip A, Skibinska M, Bartkowska-Sniatkowska A, Kliwicki S, Chlopocka-Wozniak M, Rybakowski JK, 2014. Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr. Pol 48, 35–47. [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ, 2009. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66, 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, 2017. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74, 399–405. [DOI] [PubMed] [Google Scholar]
- Serafini G, Adavastro G, Canepa G, Capobianco L, Conigliaro C, Pittaluga F, Murri MB, Valchera A, De Berardis D, Pompili M, Lindqvist D, Brundin L, Amore M, 2017. Abnormalities in kynurenine pathway metabolism in treatment-resistant depression and suicidality: a systematic review. CNS Neurol. Disord. Drug. Targets 16, 440–453. [DOI] [PubMed] [Google Scholar]
- Serafini G, Pompili M, Elena Seretti M, Stefani H, Palermo M, Coryell W, Girardi P, 2013. The role of inflammatory cytokines in suicidal behavior: a systematic review. Eur. Neuropsychopharmacol 23, 1672–1686. [DOI] [PubMed] [Google Scholar]
- Vande Voort JL, Ballard ED, Luckenbaugh DA, Bernert RA, Richards EM, Niciu MJ, Park LT, Machado-Vieira R, Duncan WC Jr., Zarate CA Jr., 2017. Antisuicidal response following ketamine infusion is associated with decreased nighttime wakefulness in major depressive disorder and bipolar disorder. J. Clin. Psychiatry 78, 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr., Sanacora G, 2017. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2014. Preventing Suicide: A Global Imperative. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA, 2012. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 71, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM, 2013. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry 18, 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.