Abstract
Background
The developmental competence of an embryo is principally dictated by the oocyte. Usually, oocyte selection is based on morphological properties; however, all morphological criteria that are currently used for the grading and screening of oocytes are not able to eliminate the subjectivity. Despite recent studies of the molecular factors related to oocyte quality, it is technically difficult to develop an index based on these factors, and new indices that reflect intracellular conditions are necessary.
Methods
Morphological and molecular factors influencing developmental competence were comprehensively reviewed, and intracellular temperature was evaluated as a new marker of oocyte quality.
Main findings
The intracellular temperature of mature oocytes was high in fresh oocytes and decreased with time after polar body release. Under the same conditions, the intracellular temperature and its distribution differed among oocytes, suggesting that temperature represents the state of each oocyte.
Conclusion
Intracellular temperature is advantageous as an objective and quantitative indicator of oocyte quality. Further studies should evaluate the link between temperature and cellular phenomena to establish its use as an indicator of quality.
Keywords: assisted reproductive techniques, meiosis, oocyte, temperature
1. INTRODUCTION
The oocyte is known to be a unique and highly specialized cell responsible for creating, activating, and controlling the embryonic genome, as well as supporting basic processes, such as cellular homeostasis, metabolism, and cell cycle progression in the early embryo.1 An oocyte is formed in the ovarian follicle and is the largest single cell. Meiosis in the mammalian oocyte is initiated during fetal development and is arrested at the diplotene stage of the first meiotic prophase. After stimulation by endogenous luteinizing hormone surge, oocyte meiosis resumes and progresses to the second meiotic phase with a dynamic change. Ovulation leads to the release of an oocyte into the oviduct where meiosis stops at metaphase II (MII) until fertilization.2, 3, 4 Ovulated oocytes are presumed to have acquired fertilization and developmental competence, which is related to the ability to undergo meiotic maturation, fertilization, embryonic development, and successful pregnancy. Developmental competence is gradually acquired during oogenesis, and the final stage is important for optimal development prior to ovulation because the synchronization between nuclear and cytoplasmic maturation in the oocyte is completed at this stage.5
Usually, for in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), oocyte selection is based on morphological parameters related to the cumulus cells, polar body, and cytoplasm.6, 7 It has been speculated that some morphological irregularities that are easily assessed under light microscopy may reflect a compromised developmental ability and could therefore be useful for selecting competent oocytes prior to fertilization.8 The first polar body (PB1) is the easiest indicator for judging nuclear maturation. However, studies using polarized light microscopy have shown that oocytes displaying a polar body may still be immature.9 In addition, just after the extrusion of PB1, oocytes do not acquire sufficient developmental competence, despite exhibiting the morphologic features of the MII stage.10 Furthermore, if the structure of the mitotic spindle collapses due to overmaturation, developmental competence decreases.10 These previous findings indicate that developmental competence changes, even in the MII oocyte.
Accurate sorting of mature oocytes that are healthy and have a high developmental competence will improve the pregnancy rate. However, the morphological criteria that are currently used for the grading and screening of oocytes are subjective and controversial, and they may not be related to the intrinsic competence of the oocyte.11, 12 The identification of objective and noninvasive molecular markers that predict oocyte ability is a major research goal. Factors related to the quality of oocytes are being elucidated at the molecular level, but it is technically difficult to develop an index based on the visualization of these factors. Accordingly, new indices that reflect intracellular conditions are necessary. In this review, an overall summary of morphological factors related to oocyte quality is provided, recent studies of molecular markers are reviewed, and intracellular temperature is introduced as a potentially effective marker.
2. FACTORS INFLUENCING OOCYTE QUALITY
Fresh matured oocytes with intact PB1 are enclosed within the zona pellucida, made up of glycoprotein, and consist of meiotic spindle with aligned chromosomes, microtubule‐organizing centers (pericentriolar materials, PCMs) located at the spindle poles, mitochondria, microfilaments, and regularly aligned cortical granules underneath the oocyte cortex in the cytoplasm. The zona pellucida is covered with abundant cumulus cells. Aging or overmaturation of oocytes is associated with numerous morphological and cellular alterations, including changes in the structure of the plasma membrane, zona pellucida, cytoskeleton, and mitochondria. It is also associated with displacement of the spindle, misalignment of chromosomes, and displacement of PB1 and cortical granules.4 The presence of clear PB1 and the attachment level of cumulus cells are major indicators used to determine the quality of oocytes because these are easy to observe using a microscope.
Many studies have reported that changes in oocyte constituents are linked to oocyte quality. We provide an overview of factors contributing to oocyte quality, focusing on the PB1, meiotic spindle, cumulus cells, mitochondria, and oxygen consumption.
2.1. First polar body (PB1)
The removal of cumulus cells from oocytes allows a detailed observation of the morphological characteristics. Extrusion of PB1 is a cellular landmark of meiotic maturation. Recent studies have investigated the correlation between PB1 morphology and oocyte competence; although PB1 does not participate in the developmental process, studies of mouse oocytes have supported this connection.7 PB1 morphology is frequently used to evaluate oocyte quality. Oocytes with an intact PB1 have high fertilization rates and a high oocyte quality, whereas those displaying a PB1 characterized by large size, irregular shape, rough surface, or fragmentation are developmentally less competent after IVF, yielding low pregnancy rates after embryo transfer.13, 14 PB1 degeneration occurs within a few hours after extrusion, and it is associated with oocyte aging.15, 16, 17 Some PB1 shows displacement from the MII spindle at nuclear maturation, and the distance between PB1 and MII spindle increases over time during oocyte aging. Moreover, the perivitelline space increases over time and facilitates the lateral displacement of the degenerating PB1.17
2.2. Meiotic spindle
The meiotic spindle is a chromosome distribution cytoskeletal structure, critically important for the accurate distribution of chromosomes to the dividing blastomeres, thereby ensuring accurate embryonic development.4 In fresh oocytes, spindles display a vertical orientation with respect to the oolemma and spindle poles associate with PCMs to create a compact bipolar spindle. This morphology changes during aging at the MII stage; the spindle becomes elongated and/or loses tension in its microtubules and becomes weak.18, 19, 20, 21 The shape of the spindle is determined by the position of the spindle pole and changes over time after spindle formation. Oocytes exhibiting a reduction in the distance between the PCMs at the spindle pole have a higher developmental potential than those exhibiting an increase in the distance between the PCMs.10 When the distance between PCMs is short, a small rhomboid spindle body is formed, and as the distance increases, a large spindle is formed. The morphology of the mitotic spindle is an important indicator of oocyte condition. Conventionally, spindles were mainly visualized by confocal microscopy, which requires cell fixation and hence cannot be applied to live cells. Alternatively, meiotic spindles can be observed directly using a polarization microscope.22
The molecular mechanisms underlying meiotic spindle in fresh oocytes have shown the importance of meiotic spindles in fertilization and embryonic development.23, 24 The meiotic spindle is essential for the accurate separation of homologous chromosomes or two sets of chromatids during germ cell division.25 Oocyte aging results in significant increases in premature chromosome separation, which is strongly associated with aneuploidy.26, 27 Aneuploidy is involved in inheriting too many or too few of any of the chromosomes. Most aneuploid embryos that inherit only one copy of an autosome develop severe abnormalities and die before pregnancy. In contrast, inheriting an extra copy of an autosome is also associated with severe developmental abnormalities and miscarriages. Chromosome 21 trisomy, the cause of Down's syndrome, is by far the most frequent aneuploidy affecting live births.28, 29, 30 Chromosomes in 2‐day‐old oocytes are no longer aligned at the spindle equator but are scattered within the degenerating spindle. In oocytes aged 3‐4 days, chromosomes become more decondensed and display nuclear alterations. Chromosome loss, fragmentation, or the clumping of chromosomes and chromatid separation have been observed in aged oocytes.18, 31, 32
2.3. Cumulus cells
Cumulus cells are critical for oocyte maturation, ovulation, and fertilization,33 and are a determinant of oocyte quality.34 Cumulus cells support energy production in the cumulus‐oocyte complex.35, 36 Additionally, cumulus cells that surround oocytes may protect against the damaging effects of reactive oxygen species (ROS).37 Recent studies suggest that the mitochondrial function of cumulus cells can directly influence the ability to achieve a successful pregnancy.38, 39 The identification of surrogate markers of oocyte competence and favorable reproductive outcomes in assisted reproductive technology is a goal of many transcriptome, proteome, and metabolome studies. The analysis of granulosa and cumulus cells is considered one of the best noninvasive strategies available today.40
2.4. Mitochondria
The mitochondrion is directly involved in many essential cellular functions, including energy production, management of ROS levels, and regulation of apoptosis. Mitochondria play an extremely important role in supplying the energy that is consumed during the maturation process.41, 42 The primary function of mitochondria is to synthesize adenosine triphosphate (ATP), the preferred energy source of cells. Synthesis of ATP in adequate amounts is critical for cell survival, and severe ATP deficiency often leads to apoptosis.43 Although several metabolic pathways of ATP production have been identified, most of the ATP generated from glucose is produced via mitochondrial oxidative phosphorylation (OXPHOS).44 All the complex processes that occur in the oocyte prior to ovulation and fertilization require energy, which is derived mainly from ATP production via OXPHOS.45 Moreover, higher ATP content in oocytes and embryos has been correlated with better reproductive results among infertile patients.46 In contrast, mitochondrial dysfunction has been implicated in decreased oocyte quality, and clinical and experimental data have suggested decreased oocyte quality as the main factor in the age‐related deterioration of reproductive capacity. However, the molecular mechanisms underlying this mitochondrion‐related decrease in oocyte quality remain poorly understood.47, 48
The distribution and organization of mitochondria during oocyte maturation are dynamic, and these changes may be related to mitochondrial function. Oocytes with higher concentrations of ATP have significantly higher fertilization and blastocyst rates.42, 49 Lower ATP content in oocytes is at least partially responsible for positive spindle formation in in vitro maturation mammalian oocytes.50, 51 Decreasing the ATP content in mouse oocytes by treatment with carbonyl cyanide p‐trifluoromethoxyphenylhydrazone, an inhibitor of OXPHOS, leads to a reduction in the percentage of oocytes with nuclear maturation, normal spindle formation, and chromosome alignment, evenly distributed mitochondria, and the ability to form blastocysts.52 ATP is extremely important for nuclear and cytoplasmic maturation events. Spindle formation and chromosome movements depend on the expression and activity of motor proteins, which use ATP as their energy source. Due to the critical role of energy metabolism in oocyte maturation, ATP content has been proposed as an indicator of the developmental potential of oocytes.53, 54, 55
Oxidative stress (OS) results from an imbalance between the production of ROS and neutralizing antioxidant molecules.56 In mammalian mature oocytes, OS causes substantial mitochondrial dysfunction, impacting both mitochondrial ATP synthesis and the activation of mitochondrial‐mediated apoptotic mechanisms.57, 58 Enhanced and unbalanced ROS production may be a predominant cause of impaired mitochondrial OXPHOS.59 External factors contribute to the higher OS observed in vitro, including exposure to visible light, non‐ideal pH and temperature, centrifugation, cryopreservation, culture medium composition, oxygen concentrations, and oocyte and embryo manipulation processes.60 mtDNA is particularly susceptible to several elements causing OS, and mtDNA disruption leads to critical loss of function and, ultimately, diminished capacity to generate ATP.58, 59 Oocyte mtDNA content increases until the stage that immediately precedes fertilization. In healthy embryos, the accumulated mtDNA is divided equally among all cells during embryogenesis.61, 62, 63, 64 Recent studies have proposed quantification of mtDNA in cumulus, granulosa, and trophectoderm cells as a promising strategy for predicting embryo quality and viability.62, 65 Since mtDNA content in cumulus cells is correlated with that in oocytes for each cumulus‐oocyte complex, it is suggested that the mitochondrial characteristics of the cells may serve as a marker of the oocyte quality.40 Furthermore, mutations or deletions in mtDNA have been correlated with organelle dysfunction, low ATP levels, and embryonic developmental arrest.66 With aging, mtDNA deficiency of luteinizing granulosa cells and cumulus cells increases, which leads to a decrease in pregnancy rate.66, 67 These findings corroborate the understanding that mitochondrial function of granulosa and cumulus cells directly influences embryonic development, as well as the maturation and fertilization of oocytes.68
Various mitochondrial anomalies have been linked to the age‐related deterioration of oocyte quality, and at least some of these may be reflective of the changes in specific mitochondrial subpopulations.69 The most prominent of these defects are atypical mitochondrial localization and aggregation, reduced mtDNA content, reduced membrane potential (consequently, bioenergetic capacity), increased OS, and increased frequency of mtDNA mutations and deletions.47, 50, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79
3. MOLECULAR MARKERS RELATED TO OOCYTE QUALITY
3.1. Meta‐analysis of microarray studies
Multiple microarray studies have been performed to identify markers associated with oocyte quality and developmental competence in oocytes or both oocytes and cumulus cells.80, 81, 82, 83, 84 Based on a meta‐analysis of previously published microarray data for various models of oocyte and embryo quality, 63 candidate genes associated with oocyte quality across several species were identified. Biological networks and transcription factor regulation associated with oocyte quality were also identified.85 If factors that control oocyte quality and molecular mechanisms are clarified, it might provide a basis for objectively evaluating oocyte quality.
3.2. microRNAs
Gamete maturation requires extensive signaling between germ cells and their surrounding somatic cells. In the ovary, from the theca cells, mural granulosa cells, cumulus cells, and oocyte secrete factors that are critical for ovulation of high‐quality oocyte, throughout follicle growth and oocyte maturation. Recent studies of a variety of species have uncovered the presence of cell‐secreted vesicles in follicular fluid.86 These cell‐secreted vesicles contain small non‐coding regulatory RNAs called microRNAs, which can be shuttled between maturing gametes and surrounding somatic cells.87 In humans, it is known that extracellular microRNAs of follicular fluid are associated with fertilization ability and early embryo quality,85 although little is known about the exact mechanism by which microRNAs are loaded into these cell‐secreted vesicles or are transferred and modulate gene expression and function. However, recent studies suggest that microRNAs in cell‐secreted vesicles are involved in oocyte maturation. These microRNAs involved in gamete maturation are potential therapeutic targets and diagnostic markers associated with fertility.86
4. VISUALIZATION OF OOCYTE QUALITY BY INTRACELLULAR TEMPERATURE IMAGING
The effects of temperature within the cell have drawn recent attention. Temperature affects various physiological functions and is important for maintaining homeostasis. Cellular functions are fundamentally regulated by intracellular temperature.88, 89, 90 The biological reactions responsible for cellular functions occur either exothermically or endothermically at particular locations within a cell, such as inside organelles. Thus, temperature distributions inside a living cell reflect the functions of cellular components.91 Okabe et al developed a novel fluorescent polymer thermometer (FPT) that can diffuse throughout whole cells; using this intracellular temperature imaging approach, they were able to evaluate the thermal profiles of living cells.92 The temperature resolutions of FPT were 0.18‐0.58°C; it can detect differences of approximately 0.2°C in the cell. There appears to be a temperature variation in 1‐2°C within somatic cells. Additionally, the temperatures of the nucleus and centrosome in somatic cells are significantly higher than that of the cytoplasm, and the temperature gap between the nucleus and the cytoplasm differed depending on the cell cycle. The FPT also detects heat production from mitochondria. The heterogeneous temperature distribution is inherently related to basic cellular processes, such as the cell cycle and mitochondrial function.92
In oocytes, during the transition from the germinal vesicle to the MII stage, virtually all major organelles undergo important changes in structure, function, and/or distribution.93, 94, 95, 96, 97, 98, 99 The oocyte during MII arrest is in a highly dynamic state, with spindle microtubules keeping all of the chromosomes perfectly aligned on the metaphase plate via proteins, such as maturation promoting factor (MPF).100 Furthermore, in the mature oocyte at MII, substantial changes, such as spindle formation, chromosome alignment, and mitochondrial activity to acquire developmental competence, occur, with the potential for an elevated temperature. We investigated the intracellular temperature and its relationship to oocyte quality using the FPT developed by Okabe et al. Intracellular temperature in mature oocytes was higher in fresh oocytes immediately after PB1 extrusion, and the temperature decreased with time after polar body release (submitted data). The differences in oocyte intracellular temperature can correlate with developmental competence. Fresh oocytes had high‐temperature regions localized around the cell membrane and around the spindle (Figure 1).
Figure 1.
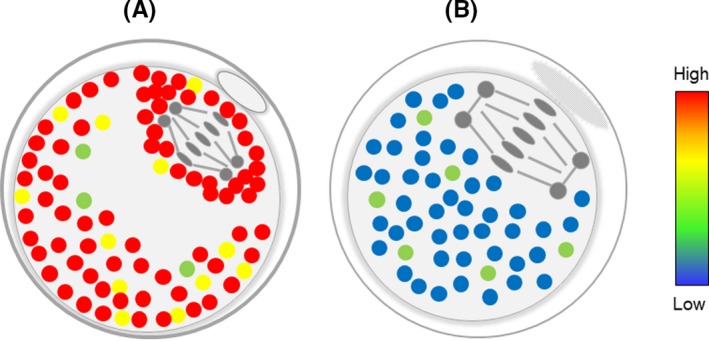
Schematic representation of intracellular temperature in matured oocytes. A, Fresh oocyte, and B, overmatured or aged oocyte. Fresh oocytes had high‐temperature regions localized around the cell membrane and around the spindle. Red and yellow spots indicate high temperature, and blue and green spots indicate low temperature
The dynamic changes in the cytoskeleton are considered to contribute to intracellular thermal variations. The temperature of a centrosome, the main microtubule‐organizing center, is higher than that of the surrounding area in COS7 and HeLa cells.92 However, the thermogenic mechanism at the centrosome remains unclear. It is presumed to be caused by the hydrolysis of tubulin‐GTP, ATP‐driven motion of motor proteins, and phosphorylation/dephosphorylation of centrosomal proteins by kinase/phosphatase.101 In addition, repeated shortening and elongation of microtubules are necessary for the formation and maintenance of the spindle, and this repetition might be responsible for heat generation.
Furthermore, heat production by mitochondria is considered a factor influencing intracellular temperature. Substantial heat generation in the mitochondria was observed when HeLa cells expressing tsGFP1‐mito, a genetically encoded thermosensor specifically targeting the mitochondria, were treated with carbonyl cyanide 3‐chloro‐phenylhydrazone.102 Simultaneous visualization of tsGFP1‐mito in HeLa cells using JC‐1, a dye that visualizes high mitochondrial membrane potential, and ATeam, a genetically encoded ATP sensor, revealed high temperature in mitochondria with high membrane potential and that there was a positive correlation between ATP levels and membrane potential.103, 104 This result demonstrates that constitutive thermogenesis occurs via the respiratory chain or OXPHOS in a subpopulation of mitochondria in HeLa cells.101 Interestingly, even in oocytes collected under the same conditions, the intracellular temperature and temperature distribution differed among oocytes, suggesting that the temperature represents the state of each oocyte well. Taken together, these reports indicate that the intracellular temperature may be affected by the function of organelles such as microtubules and mitochondria. Thus, the intracellular temperature of oocytes can be a strong predictor of oocyte quality and developmental competence.
5. CONCLUSION
Individuals with identical morphological features can differ with respect to developmental competence. It is well known that oocyte quality determines the developmental potential of embryos after fertilization.6 Oocytes arrested at the MII stage are normally fertilized within a few hours after ovulation or PB1 emission. If fertilization does not occur within the proper time, the unfertilized oocyte undergoes a time‐dependent deterioration in quality, resulting in oocyte aging, a cause of fertilization failure. Sakai et al reported that the optimal period of fertilization can be specified based on the distance between the PCMs of the meiotic spindle.10 It is important to accurately determine the generation capacity of individual oocytes, but it is also necessary to perform IVF/ICSI according to the fertilization period.
Factors related to oocyte quality are becoming evident at the molecular level. However, it is not easy to accurately evaluate the developmental competence of mature oocytes based on morphology or molecular activity without damage. Typically, oocyte selection is based on microscopically determined morphological properties; however, all morphological criteria that are currently used for the grading and screening of oocytes are sometimes subjective and may not reflect the intrinsic competence of the oocyte. Moreover, morphological evaluation depends greatly on the experience and subjectivity of the observer and lacks quantitativeness. A major advantage of using intracellular temperature as a predictor is that it can be evaluated objectively and quantitatively using a temperature imaging system. If we can definitively prove that the temperature accurately reflects phenomena in the cell, it could be an indicator of oocyte quality. We are working toward elucidating the mechanism by which temperature influences cellular processes. In this study, FPT has been injected into oocytes to measure intracellular temperature, but in the future, noninvasive methods for temperature measurement should be developed for visualization.
DISCLOSURES
Conflict of interest: The author declares no conflict of interest. Human rights statement and informed consent: This article does not contain any experiment performed with human subjects. Animal studies: All institutional and national guidelines for the care and use of laboratory animals were followed. All the experiments were approved and conducted in accordance with the guidelines of the Committee of Animal Experiments of Hiroshima University, Hiroshima, Japan.
Hoshino Y. Updating the markers for oocyte quality evaluation: intracellular temperature as a new index. Reprod Med Biol. 2018;17:434–441. 10.1002/rmb2.12245
REFERENCES
- 1. Mtango NR, Potireddy S, Latham KE. Oocyte quality and maternal control of development. Int Rev Cell Mol Biol. 2008;268:223‐290. [DOI] [PubMed] [Google Scholar]
- 2. Edwards RG. Maturation in vitro of human ovarian oocytes. Lancet. 1965;2(7419):926‐929. [DOI] [PubMed] [Google Scholar]
- 3. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200‐214. [DOI] [PubMed] [Google Scholar]
- 4. Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15(5):573‐585. [DOI] [PubMed] [Google Scholar]
- 5. Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 6. Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 7. Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte quality? Ann N Y Acad Sci. 2004;1034:132‐144. [DOI] [PubMed] [Google Scholar]
- 8. Van Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation. Hum Reprod. 1992;7(3):379‐390. [DOI] [PubMed] [Google Scholar]
- 9. Rienzi L, Ubaldi F, Iacobelli M, Minasi MG, Romano S, Greco E. Meiotic spindle visualization in living human oocytes. Reprod Biomed Online. 2005;10(2):192‐198. [DOI] [PubMed] [Google Scholar]
- 10. Sakai C, Hoshino Y, Sato Y, Sato E. Evaluation of maturation competence of metaphase II oocytes in mice based on the distance between pericentriolar materials of meiotic spindle: distance of PCM during oocyte maturation. J Assist Reprod Genet. 2011;28(2):157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12(6):1267‐1270. [DOI] [PubMed] [Google Scholar]
- 12. Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13(12):3431‐3433. [DOI] [PubMed] [Google Scholar]
- 13. Ebner T, Moser M, Yaman C, Feichtinger O, Hartl J, Tews G. Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy. Fertil Steril. 1999;72(4):599‐603. [DOI] [PubMed] [Google Scholar]
- 14. Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, Tews G. First polar body morphology and blastocyst formation rate in ICSI patients. Hum Reprod. 2002;17(9):2415‐2418. [DOI] [PubMed] [Google Scholar]
- 15. Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15(2):427‐430. [DOI] [PubMed] [Google Scholar]
- 16. Hardarson T, Lundin K, Hamberger L. The position of the metaphase II spindle cannot be predicted by the location of the first polar body in the human oocyte. Hum Reprod. 2000;15(6):1372‐1376. [DOI] [PubMed] [Google Scholar]
- 17. Miao Y, Ma S, Liu X, et al. Fate of the first polar bodies in mouse oocytes. Mol Reprod Dev. 2004;69(1):66‐76. [DOI] [PubMed] [Google Scholar]
- 18. Eichenlaub‐Ritter U, Stahl A, Luciani JM. The microtubular cytoskeleton and chromosomes of unfertilized human oocytes aged in vitro. Hum Genet. 1988;80(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 19. Goud AP, Goud PT, Van Oostveldt P, Diamond MP, Dhont M. Dynamic changes in microtubular cytoskeleton of human postmature oocytes revert after ooplasm transfer. Fertil Steril. 2004;81(2):323‐331. [DOI] [PubMed] [Google Scholar]
- 20. Segers I, Adriaenssens T, Coucke W, Cortvrindt R, Smitz J. Timing of nuclear maturation and postovulatory aging in oocytes of in vitro‐grown mouse follicles with or without oil overlay. Biol Reprod. 2008;78(5):859‐868. [DOI] [PubMed] [Google Scholar]
- 21. Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001;75(2):348‐353. [DOI] [PubMed] [Google Scholar]
- 22. Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001;16(7):1464‐1468. [DOI] [PubMed] [Google Scholar]
- 23. Sun QY, Schatten H. Centrosome inheritance after fertilization and nuclear transfer in mammals. Adv Exp Med Biol. 2007;591:58‐71. [DOI] [PubMed] [Google Scholar]
- 24. Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol. 2008;129(6):667‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang WH, Sun QY. Meiotic spindle, spindle checkpoint and embryonic aneuploidy. Front Biosci. 2006;11:620‐636. [DOI] [PubMed] [Google Scholar]
- 26. Mailhes JB, Young D, London SN. Postovulatory ageing of mouse oocytes in vivo and premature centromere separation and aneuploidy. Biol Reprod. 1998;58(5):1206‐1210. [DOI] [PubMed] [Google Scholar]
- 27. Steuerwald NM, Steuerwald MD, Mailhes JB. Post‐ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod. 2005;11(9):623‐630. [DOI] [PubMed] [Google Scholar]
- 28. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280‐291. [DOI] [PubMed] [Google Scholar]
- 29. Herbert M, Kalleas D, Cooney D, Lamb M, Lister L. Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births. Cold Spring Harb Perspect Biol. 2015;7(4):a017970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age‐old problem. Nat Rev Genet. 2012;13(7):493‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodman TC. Chromatid disjunction in unfertilized ageing oocytes. Nature. 1971;233(5316):191‐193. [DOI] [PubMed] [Google Scholar]
- 32. Van Wissen B, Bomsel‐Helmreich O, Debey P, Eisenberg C, Vautier D, Pennehouat G. Fertilization and ageing processes in non‐divided human oocytes after GnRHa treatment: an analysis of individual oocytes. Hum Reprod. 1991;6(6):879‐884. [DOI] [PubMed] [Google Scholar]
- 33. Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev. 2002;61(3):414‐424. [DOI] [PubMed] [Google Scholar]
- 34. Da Broi MG, Giorgi V, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. 2018;35(5):735–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte‐cumulus cell complexes. Biol Reprod. 1999;60(6):1446‐1452. [DOI] [PubMed] [Google Scholar]
- 36. Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta‐oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod. 2013;88(5):111. [DOI] [PubMed] [Google Scholar]
- 37. Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakada T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71(4):1150‐1157. [DOI] [PubMed] [Google Scholar]
- 38. Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229(3):353‐361. [DOI] [PubMed] [Google Scholar]
- 39. Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715‐725. [DOI] [PubMed] [Google Scholar]
- 40. Boucret L, Chao de la Barca JM, Moriniere C, et al. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30(7):1653‐1664. [DOI] [PubMed] [Google Scholar]
- 41. Krisher RL, Bavister BD. Responses of oocytes and embryos to the culture environment. Theriogenology. 1998;49(1):103‐114. [DOI] [PubMed] [Google Scholar]
- 42. Stojkovic M, Machado SA, Stojkovic P, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64(3):904‐909. [DOI] [PubMed] [Google Scholar]
- 43. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond). 2010;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ben‐Meir A, Burstein E, Borrego‐Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao J, Li Y. Adenosine triphosphate content in human unfertilized oocytes, undivided zygotes and embryos unsuitable for transfer or cryopreservation. J Int Med Res. 2012;40(2):734‐739. [DOI] [PubMed] [Google Scholar]
- 47. Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28(9):773‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilding M, Di Matteo L, Dale B. The maternal age effect: a hypothesis based on oxidative phosphorylation. Zygote. 2005;13(4):317‐323. [DOI] [PubMed] [Google Scholar]
- 49. Nagano M, Katagiri S, Takahashi Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote. 2006;14(4):299‐304. [DOI] [PubMed] [Google Scholar]
- 50. Eichenlaub‐Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8(1):45‐58. [DOI] [PubMed] [Google Scholar]
- 51. Zeng HT, Ren Z, Yeung WS, et al. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod. 2007;22(6):1681‐1686. [DOI] [PubMed] [Google Scholar]
- 52. Ge H, Tollner TL, Hu Z, et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev. 2012;79(6):392‐401. [DOI] [PubMed] [Google Scholar]
- 53. Slotte H, Gustafson O, Nylund L, Pousette A. ATP and ADP in human pre‐embryos. Hum Reprod. 1990;5(3):319‐322. [DOI] [PubMed] [Google Scholar]
- 54. Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in‐vitro fertilization and embryo transfer. Hum Reprod. 1995;10(2):415‐424. [DOI] [PubMed] [Google Scholar]
- 55. Leese HJ, Biggers JD, Mroz EA, Lechene C. Nucleotides in a single mammalian ovum or preimplantation embryo. Anal Biochem. 1984;140(2):443‐448. [DOI] [PubMed] [Google Scholar]
- 56. Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86(3):503‐512. [DOI] [PubMed] [Google Scholar]
- 57. Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress‐induced cell death in mouse zygotes. Biol Reprod. 2000;62(6):1745‐1753. [DOI] [PubMed] [Google Scholar]
- 58. Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria‐derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16(10):841‐850. [DOI] [PubMed] [Google Scholar]
- 59. Lord T, Aitken RJ. Oxidative stress and ageing of the post‐ovulatory oocyte. Reproduction. 2013;146(6):R217–R227. [DOI] [PubMed] [Google Scholar]
- 60. Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol. 2014;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fragouli E, Spath K, Alfarawati S, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11(6):e1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. St John JC, Facucho‐Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16(5):488‐509. [DOI] [PubMed] [Google Scholar]
- 64. Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83(1):52‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Desquiret‐Dumas V, Clement A, Seegers V, et al. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017;32(3):607‐614. [DOI] [PubMed] [Google Scholar]
- 66. Tsai HD, Hsieh YY, Hsieh JN, et al. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J Reprod Med. 2010;55(11–12):491‐497. [PubMed] [Google Scholar]
- 67. Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78(5):1046‐1048. [DOI] [PubMed] [Google Scholar]
- 68. Cecchino GN, Seli E, Alves da Motta EL. Garcia‐Velasco JA. The role of mitochondrial activity in female fertility and assisted reproductive technologies: overview and current insights. Reprod Biomed Online. 2018;36(6):686‐697. [DOI] [PubMed] [Google Scholar]
- 69. Woods DC, Khrapko K, Tilly JL. Influence of maternal aging on mitochondrial heterogeneity, inheritance, and function in oocytes and preimplantation embryos. Genes (Basel). 2018;9(5):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jansen RP, Burton GJ. Mitochondrial dysfunction in reproduction. Mitochondrion. 2004;4(5–6):577‐600. [DOI] [PubMed] [Google Scholar]
- 71. Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17(6):838‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging‐associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108(30):12319‐12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tarin JJ, Perez‐Albala S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002;61(3):385‐397. [DOI] [PubMed] [Google Scholar]
- 74. Hsieh RH, Tsai NM, Au HK, Chang SJ, Wei YH, Tzeng CR. Multiple rearrangements of mitochondrial DNA in unfertilized human oocytes. Fertil Steril. 2002;77(5):1012‐1017. [DOI] [PubMed] [Google Scholar]
- 75. Reynier P, May‐Panloup P, Chretien MF, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425‐429. [DOI] [PubMed] [Google Scholar]
- 76. May‐Panloup P, Chretien MF, Jacques C, Vasseur C, Malthiery Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod. 2005;20(3):593‐597. [DOI] [PubMed] [Google Scholar]
- 77. Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584‐591. [DOI] [PubMed] [Google Scholar]
- 78. Duran HE, Simsek‐Duran F, Oehninger SC, Jones HW Jr, Castora FJ. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril. 2011;96(2):384‐388. [DOI] [PubMed] [Google Scholar]
- 79. Simsek‐Duran F, Li F, Ford W, Swanson RJ, Jones HW Jr, Castora FJ. Age‐associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8(5):e64955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kocabas AM, Crosby J, Ross PJ, et al. The transcriptome of human oocytes. Proc Natl Acad Sci U S A. 2006;103(38):14027‐14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Su YQ, Sugiura K, Woo Y, et al. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol. 2007;302(1):104‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pan H, Ma P, Zhu W, Schultz RM. Age‐associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316(2):397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Montfoort AP, Geraedts JP, Dumoulin JC, Stassen AP, Evers JL, Ayoubi TA. Differential gene expression in cumulus cells as a prognostic indicator of embryo viability: a microarray analysis. Mol Hum Reprod. 2008;14(3):157‐168. [DOI] [PubMed] [Google Scholar]
- 84. Kenigsberg S, Bentov Y, Chalifa‐Caspi V, Potashnik G, Ofir R, Birk OS. Gene expression microarray profiles of cumulus cells in lean and overweight‐obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15(2):89‐103. [DOI] [PubMed] [Google Scholar]
- 85. O'Shea LC, Mehta J, Lonergan P, Hensey C, Fair T. Developmental competence in oocytes and cumulus cells: candidate genes and networks. Syst Biol Reprod Med. 2012;58(2):88‐101. [DOI] [PubMed] [Google Scholar]
- 86. da Silveira JC, de Avila A, Garrett HL, Bruemmer JE, Winger QA, Bouma GJ. Cell‐secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol. 2018;236(1):R15–R27. [DOI] [PubMed] [Google Scholar]
- 87. Lu J, Clark AG. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22(7):1243‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bahat A, Tur‐Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat Med. 2003;9(2):149‐150. [DOI] [PubMed] [Google Scholar]
- 89. Warner DA, Shine R. The adaptive significance of temperature‐dependent sex determination in a reptile. Nature. 2008;451(7178):566‐568. [DOI] [PubMed] [Google Scholar]
- 90. Seymour RS. Biophysics and physiology of temperature regulation in thermogenic flowers. Biosci Rep. 2001;21(2):223‐236. [DOI] [PubMed] [Google Scholar]
- 91. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652‐660. [DOI] [PubMed] [Google Scholar]
- 92. Okabe K, Inada N, Gota C, Harada Y, Funatsu T, Uchiyama S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat Commun. 2012;3:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797‐813. [DOI] [PubMed] [Google Scholar]
- 94. Dalton CM, Carroll J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J Cell Sci. 2013;126(Pt 13):2955‐2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol. 1995;170(2):607‐615. [DOI] [PubMed] [Google Scholar]
- 96. Van Blerkom J, Davis P, Alexander S. Inner mitochondrial membrane potential (DeltaPsim), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum Reprod. 2003;18(11):2429‐2440. [DOI] [PubMed] [Google Scholar]
- 97. Moreno RD, Schatten G, Ramalho‐Santos J. Golgi apparatus dynamics during mouse oocyte in vitro maturation: effect of the membrane trafficking inhibitor brefeldin A. Biol Reprod. 2002;66(5):1259‐1266. [DOI] [PubMed] [Google Scholar]
- 98. Liu M, Sims D, Calarco P, Talbot P. Biochemical heterogeneity, migration, and pre‐fertilization release of mouse oocyte cortical granules. Reprod Biol Endocrinol. 2003;1:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Larson SM, Lee HJ, Hung PH, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin‐II and Ezrin‐Radixin‐Moesin (ERM) proteins. Mol Biol Cell. 2010;21(18):3182‐3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brunet S, Maria AS, Guillaud P, Dujardin D, Kubiak JZ, Maro B. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol. 1999;146(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Okabe K, Sakaguchi R, Shi B, Kiyonaka S. Intracellular thermometry with fluorescent sensors for thermal biology. Pflugers Arch. 2018;470(5):717‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kiyonaka S, Kajimoto T, Sakaguchi R, et al. Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells. Nat Methods. 2013;10(12):1232‐1238. [DOI] [PubMed] [Google Scholar]
- 103. Smiley ST, Reers M, Mottola‐Hartshorn C, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J‐aggregate‐forming lipophilic cation JC‐1. Proc Natl Acad Sci U S A. 1991;88(9):3671‐3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Imamura H, Nhat KP, Togawa H, et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer‐based genetically encoded indicators. Proc Natl Acad Sci U S A. 2009;106(37):15651‐15656. [DOI] [PMC free article] [PubMed] [Google Scholar]


