Summary
Ubiquitin is highly conserved across eukaryotes and is essential for normal eukaryotic cell function. The bacterium Bacteroides fragilis is a member of the normal human gut microbiota, and the only bacterium known to encode a homologue of eukaryotic ubiquitin. The B. fragilis gene sequence indicates a past horizontal gene transfer event from a eukaryotic source. It encodes a protein (BfUbb) with 63% identity to human ubiquitin which is exported from the bacterial cell. The aim of this study was (i) to determine if there was antigenic cross‐reactivity between B. fragilis ubiquitin and human ubiquitin and (ii) to determine if humans produced antibodies to BfUbb. Molecular model comparisons of BfUbb and human ubiquitin predicted a high level (99·8% confidence) of structural similarity. Linear epitope mapping identified epitopes in BfUbb and human ubiquitin that cross‐react. BfUbb also has epitope(s) that do not cross‐react with human ubiquitin. The reaction of human serum (n = 474) to BfUbb and human ubiquitin from the following four groups of subjects was compared by enzyme‐linked immunosorbent assay (ELISA): (1) newly autoantibody‐positive patients, (2) allergen‐specific immunoglobulin (Ig)E‐negative patients, (3) ulcerative colitis patients and (4) healthy volunteers. We show that the immune system of some individuals has been exposed to BfUbb which has resulted in the generation of IgG antibodies. Serum from patients referred for first‐time testing to an immunology laboratory for autoimmune disease are more likely to have a high level of antibodies to BfUbb than healthy volunteers. Molecular mimicry of human ubiquitin by BfUbb could be a trigger for autoimmune disease.
Keywords: autoimmune disease, Bacteroides fragilis, Coeliac, microbiome, multiple sclerosis, rheumatoid arthritis, ubiquitin, ulcerative colitis
Introduction
The resident microbiota of the human gastrointestinal (GI) tract is comprised of ~1500 species, with the predominant phyla being the Bacteroidetes and the Firmicutes. The Gram‐negative strictly anaerobic Bacteroides genus dominates, as evidenced by its prevalence in faeces. B. fragilis, which is well known as an opportunistic pathogen 1, plays an important role in immune cell balancing in the GI tract. Some B. fragilis strains produce a capsular polysaccharide (PSA) that stimulates dendritic cells to alter the ratio of T helper cells and produce interleukin (IL)‐10, which reduces production of the proinflammatory cytokine IL‐17 2. There is evidence that B. fragilis may not only be associated intimately with the colonic mucosa, but also may be present intracellularly 3.
During our annotation of the genome sequence of B. fragilis we discovered a gene (ubb) encoding a protein designated BfUbb, with 63% identity to human ubiquitin (e.g. ubc52), located within an 11 kb region of DNA with a lower GC content than the rest of the genome 4. The closest nucleotide homology for ubb [216 base pairs (bp)] is with the ubiquitin gene of a Migratory Grasshopper Entomopoxvirus (103 of 122 bp), which supports the notion of interkingdom horizontal gene transfer from a eukaryotic source 5. Ubiquitin is a highly conserved protein which, until this discovery, has been found only in eukaryotes and eukaryotic viruses 6, 7. The protein sequence is conserved among mammals. Ubiquitin tagging (ubiquitylation) of proteins governs nearly every eukaryotic cell function, from intracellular proteolysis, membrane–protein endocytosis and intracellular trafficking and chromatin‐mediated regulation of transcription to DNA repair. Ubiquitin is also a major factor involved in development and function of the immune system. To date, B. fragilis is unique in being the only bacterium to encode an identified ubiquitin homologue. The gene has evolved at least two novel features that differentiate the encoded protein from eukaryotic ubiquitin: first, B. fragilis ubiquitin (BfUbb) contains a signal sequence that directs it to the periplasm, and we have found it associated with outer membrane vesicles (OMV) 8; secondly, BfUbb has lost the C‐terminal glycine residues required for thioester bond formation with the catalytic cysteine residue in the eukaryotic E1 activating enzyme. In place of the glycine residues, there is a cysteine that may allow disulphide bond formation with the catalytic residues of E1‐activating and E2‐conjugating enzymes of the ubiquitylation pathway. Consistent with this, we have shown that BfUbb can bind covalently to human E1 under non‐reducing conditions in vitro and can inhibit ubiquitylation if added to a reaction before eukaryotic ubiquitin 4.
Given the intimate association of B. fragilis with the human GI tract and the release of ubiquitin from B. fragilis in OMV, we hypothesized that an immune reaction to BfUbb might be detectable in humans and, potentially, be related to autoimmune disease due to molecular mimicry. Here we show that BfUbb and human ubiquitin (Hubb) are predicted to be structurally highly similar, and we demonstrate that this can be related to cross‐reactive epitopes. We demonstrate that BfUbb also has unique epitope(s). We investigated the reactivity of human serum samples related to various conditions, including systemic lupus erythematosus (SLE), ulcerative colitis, rheumatoid arthritis, coeliac disease and multiple sclerosis, in a pilot study. We now report that BfUbb can generate an immunoglobulin (Ig)G response in humans and that patients referred for autoimmune disease testing are more likely to have high levels of IgG reactive with BfUbb than healthy volunteers.
Methods
Protein structural comparison and epitope mapping
Anti‐BfUbb polyclonal antiserum was produced by inoculation of a New Zealand White rabbit with recombinant (r)BfUbb conjugated to keyhole limpet haemocyanin carrier protein under UK Government Home Office Personal and Project Licences and with local ethical approval, as described previously 4. Serum from a pre‐immune bleed was reserved as a control. Recombinant human ubiquitin (Hubb; > 95% purity) and rabbit polyclonal anti‐Hubb antiserum were obtained from BostonBiochem (Cambridge, MA, USA). Peptides 1,2 and 3 (> 80% purity) and synthetic (s)BfUbb (> 90% purity) were custom synthesized by Genosphere Biotechnologies (Paris, France). Structural protein comparisons were carried out using the Protein Homology/Analogy Recognition Engine (Phyre) 2 web portal for protein modelling, prediction and analysis 9 and molecular graphics generated using the University of California San Francisco (UCSF) Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco, supported by NIGMS P41‐GM103311 10. Linear epitope mapping was carried out by PepScan Presto BV (Lelystad, the Netherlands) on linear arrays of overlapping 15‐mer and 24‐mer peptides generated from the BfUbb and human ubiquitin sequences as well as two chimaeric sequences generated by swapping alternating divergent amino acids from the BfUbb and Hubb sequences. Peptide‐scanning enzyme‐linked immunosorbent assays (ELISAs) were carried out with the rabbit anti‐BfUbb and rabbit anti‐Hubb polyclonal antisera detailed above and substrate colour development was quantified with a charge‐coupled device camera with values recorded in the range of 0 to 3000 mAU 11, 12.
ELISA
Standard ELISAs were carried out in 96‐well Nunc MaxiSorp microtitre plates which had been coated overnight at 4°C with either BfUbb (5 μg ml−1), Hubb (5 μg ml−1) or peptides 1,2 or 3 (10 μg ml−1). Microtitre plates were blocked with 1% (w/v) bovine serum albumin (BSA) in phosphate‐buffered saline buffer (PBS; 200 μl) for 2 h. After washing, diluted primary antibody (100 μl) was added to triplicate wells and incubated shaken for 1·5 h at 37°C. Identical primary antibody application microtitre plate templates were used for serum samples for the BfUbb and Hubb plates. Microtitre plates were drained and washed five times with PBS with Tween 20 (0·05% w/v), followed by five 5‐min washes; 100 μl of either goat anti‐rabbit IgG H&L alkaline phosphatase or goat anti‐human IgG Fc alkaline phosphatase conjugate (Abcam, Cambridge, UK) diluted in 1% (w/v) BSA in PBS was added and the plates incubated shaken for 1 h at 37oC. The microtitre plates were drained and washed five times with PBS with Tween 20 (0·05% w/v), followed by three 5‐min washes. p‐Nitrophenyl‐phosphate substrate (50 μl) was added, the plates incubated shaken for 1 h at room temperature, the reaction stopped with 2 M NaOH and the plates read in at 405nm in an ELISA reader (Biotek, Winooski, VT, USA). Data for the rabbit antiserum BfUbb and Hubb ELISA experiments represent the mean of a minimum of two replicate experiments, and for the human serum samples data points represent the mean of three triplicate wells. Selected human serum titration experiments were repeated on separate occasions.
Human serum samples
Anonymized serum samples, taken during routine clinical practice and surplus to analytical diagnostic requirements, were obtained from the Regional Immunology Laboratory, Belfast Health and Social Care Trust, Northern Ireland, UK and stored at –80°C (Table 1). As the samples were unlinked and anonymized, and therefore untraceable, ethical approval was not a requirement. Serum samples were collected during 2016 and 2017 from patients who had tested positive for the first time for suspected autoimmune diseases, such as systemic lupus erythematosus (SLE) (autoimmune antibody category); anti‐cyclic citrullinated peptide (anti‐CCP) antibody‐positive (rheumatoid arthritis category); anti‐tissue transglutaminase (anti‐TTG) antibody‐positive (coeliac category); and oligoclonal banding positive on paired cerebrospinal fluid and serum samples (multiple sclerosis category). In addition, patient sera which had been tested for specific IgE to house dust, animal dander or pollen, but had been found to be negative (allergy test‐negative category), were also collected. Anonymous serum samples from patients diagnosed with ulcerative colitis were purchased from Sera Laboratories International (Haywards Heath, UK). Unlinked anonymized serum samples, surplus to requirements from healthy volunteers participating in a short‐term dietary intervention study approved by the Research Ethics Committee of the School of Medicine and Dentistry, Queen’s University Belfast with written informed consent 13, were also obtained.
Table 1.
Human serum samples
| Sample category | Samples (n) | Laboratory tests | Average age (years) | Females n (%) | |
|---|---|---|---|---|---|
| Coeliac | 99 | IgA anti‐tissue transglutaminase and/or | 43 | 60 (61%) | |
| IgA anti‐endomysial antibodies | |||||
| Rheumatoid arthritis | 83 | anti‐cyclic citrullinated peptide antibodies | 54 | 53 (64%) | |
| Multiple sclerosis | 36 | IgG oligoclonal band detection | 46 | 20 (56%) | |
| Autoimmune antibodies | 134 | Anti‐nuclear antibodies | Anti‐double‐stranded DNA antibodies | 57 | 98 (72%)* |
| Anti‐Ro52 and/or Ro60 antibodies | |||||
| Anti‐La antibodies | |||||
| Anti‐Sm antibodies | |||||
| Anti‐ribonuclear protein antibodies | |||||
| Anti‐Scl‐70 antibodies | |||||
| Anti‐Centromere protein B antibodies | |||||
| Anti‐Chromatin antibodies | |||||
| Anti‐Ribosomal P antibodies | |||||
| 3 | Anti‐cardiolipin and/or | ||||
| Anti‐beta‐2‐glycoprotein antibodies | |||||
| Allergy test negative | 37 | Anti‐house dust mite, animal mix, grass pollen mix | 50 | 23 (62%) | |
| Ulcerative colitis | 20 | Not applicable | 46 | 13 (65%) | |
| Healthy volunteer | 61 | Not applicable | 40 | 38 (59%) | |
| *one sex unknown |
Statistical analysis
Analysis of the relationship between the proportion of human serum samples with an ELISA optical density (OD)405 nm reading of > 2·0 or > 2·5 in the serum categories autoimmune antibodies, coeliac, rheumatoid arthritis, multiple sclerosis, allergy test‐negative and ulcerative colitis versus healthy volunteers was by two‐sided Fisher’s exact test (P = 0·05) with IBM spss statistics version 22 (IBM Corporation, Armonk, NY, USA).
Results
Molecular modelling and comparison of the immune‐reactivity of BfUbb and human ubiquitin
Comparison of the reactivity of rabbit polyclonal antiserum raised against human ubiquitin with recombinant BfUbb and human ubiquitin (Hubb) by ELISA revealed similar reactivity (Fig. 1a). The reactivity of antiserum raised against recombinant BfUbb with Hubb, however, was consistently lower than the reaction with recombinant BfUbb (Fig. 1b).
Figure 1.
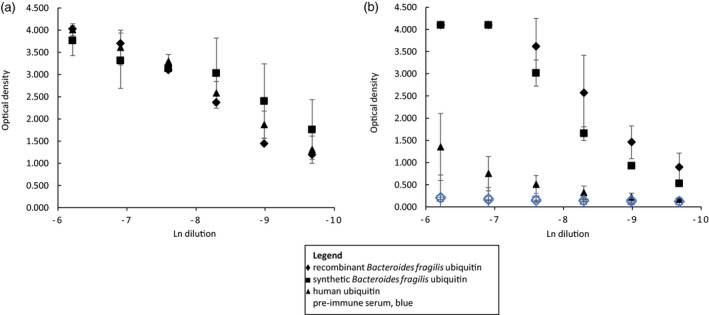
Comparison of enzyme‐linked immunosorbent assay (ELISA) reaction of rabbit polyclonal antiserum with Bacteroides fragilis and human ubiquitin. (a) Anti‐recombinant human ubiquitin (Hubb) antiserum and (b) anti‐recombinant B. fragilis ubiquitin (rBfUbb) antiserum with rBfUbb (diamond), synthetic Bacteroides fragilis ubiquitin (sBfUbb; square) and Hubb (triangle). Black, immune serum; blue, preimmune serum. Initial dilution 1/500. Mean of two or more replicate experiments ± SD.
These data suggest that BfUbb and Hubb may have both shared and unique epitopes.
The amino acid sequence of ubiquitin is highly conserved in eukaryotes; mammals have identical ubiquitin sequences and, for example, Saccharomyces cerevisiae has only three amino acid differences. As the BfUbb sequence shares 63% identity with the mammalian ubiquitin sequence (Fig. 2a), the potential for protein structural similarity of BfUbb with mammalian ubiquitin was analysed using the Phyre 2 web portal for protein modelling, prediction and analysis 9. The analyses revealed the potential for structural similarity between BfUbb and Hubb, with 99·8% confidence that the sequence match is true homology and therefore that the structure is predicted correctly (Fig. 2b,c). This is consistent with the hypothesis that the two proteins have potentially cross‐reactive epitopes. There are, however, a number of key amino acid differences that may form distinct epitopes. Notably, there is a lysine at position 11 in human ubiquitin which has been replaced by a tryptophan residue in BfUbb (Fig. 2a). These residues are surface exposed in the predicted structure (Fig. 2b,c). The amino acids at the C‐terminus diverge (Fig. 2a) and may form unique epitopes. Therefore, the immune reactivity of three short synthetic peptides (Fig. 2a) from the N‐terminal sequence inclusive of the tryptophan residue and the divergent C‐terminal sequence were compared with synthetic whole molecule BfUbb (sBfUbb) to determine if these sequences contained conserved or divergent epitopes (Fig. 3). The pattern of reactivity of the rabbit polyclonal anti‐BfUbb and anti‐human ubiquitin antiserum with the peptides was similar to that observed with the whole human ubiquitin and BfUbb proteins. Reaction with the anti‐human ubiquitin antiserum was higher than the reaction with the anti‐BfUbb antiserum. This suggests that these peptides do not contain the immunodominant BfUbb epitopes recognized by the anti‐BfUbb antiserum.
Figure 2.
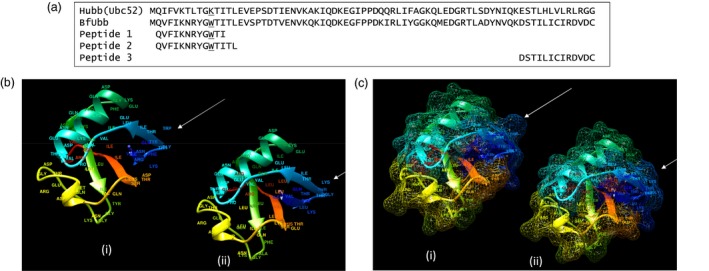
Comparison of BfUbb and Hubb structural models. (a) Amino acid sequence alignment of Hubb, BfUbb and synthetic peptide sequences. Tryptophan and lysine underlined. Molecular graphic of (b) ribbon model and (c) mesh surface model (i) BfUbb modelled on human ubiquitin (ii) Human ubiquitin. Arrow: position of tryptophan 11 in BfUbb and corresponding lysine 11 in human ubiquitin. Rainbow‐coloured; N terminus (blue), C terminus (red).
Figure 3.
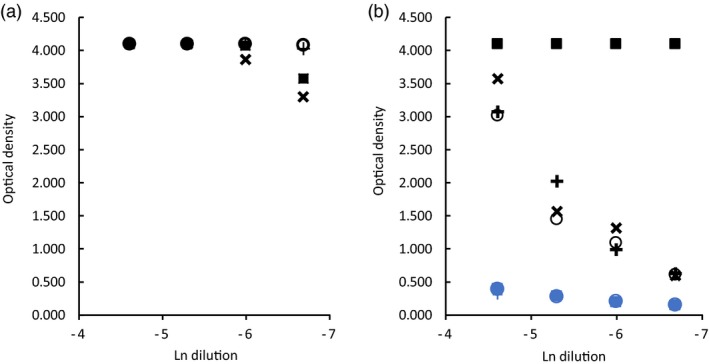
Comparison of enzyme‐linked immunosorbent assay (ELISA) reaction of rabbit polyclonal antiserum with BfUbb and synthetic peptides. (a) Anti‐recombinant human ubiquitin (Hubb) antiserum and (b) anti‐recombinant B. fragilis ubiquitin (rBfUbb) antiserum with: synthetic Bacteroides fragilis ubiquitin (sBfUbb, square); peptide 1‐ QVFIKNRYGWTI (cross); peptide 2‐ QVFIKNRYGWTITL (circle); peptide 3‐ DSTILICIRDVDC (x). Black, immune serum; blue, preimmune serum. Initial dilution 1/100.
To further investigate the cross‐reactivity and to determine potential shared epitopes, the reaction of the polyclonal antisera with libraries of linear peptides was examined. Four series of peptides were synthesized with sequences derived from BfUbb, human ubiquitin and two BfUbb/human ubiquitin chimaeric sequences (Fig. 4a). These were tested with the polyclonal antisera using PepScan technology 12. For each of the BfUbb, human ubiquitin and the two chimaeric sequences, 61 peptides, each 15 amino acids long with an overlap of 14 amino acids, and 52 peptides of 24 amino acids with an overlap of 23 amino acids were tested. The BfUbb‐derived peptide sequences are indicated in the x‐axis labels of Fig. 4b,c. Probing of the arrays with anti‐Hubb antiserum revealed two potential shared linear epitopes in the BfUbb and Hubb sequences (Fig. 4a–d). The putative BfUbb O‐ glycosylation sites DTV and DST are not within either of these epitopes (Fig. 4d). The anti‐rBfUbb antiserum did not have a detectable reaction with the shared epitopes identified by the anti‐Hubb antiserum (Fig. 4b,c). This suggests that there are too few antibodies to the shared linear epitopes present in the anti‐rBfUbb antiserum to be detectable in this assay. This is in accordance with the lower reaction of the anti‐BfUbb antiserum to Hubb in the whole molecule titration assay (Fig. 1b). It also suggests that the immunodominant BfUbb epitopes recognized by the anti‐BfUbb antiserum are conformational epitopes. At a 1/500 dilution, the anti‐rBfUbb reacted with the 24‐mer GWTITLEVSPTDTVENVKQKIQDK from BfUbb, which suggests a potential unique conformational epitope (Fig. 4c, top panel). The position of the 24‐mer peptide relative to the shared epitopes identified by the anti‐Hubb antiserum is illustrated in the Supporting information, Fig. S1.
Figure 4.
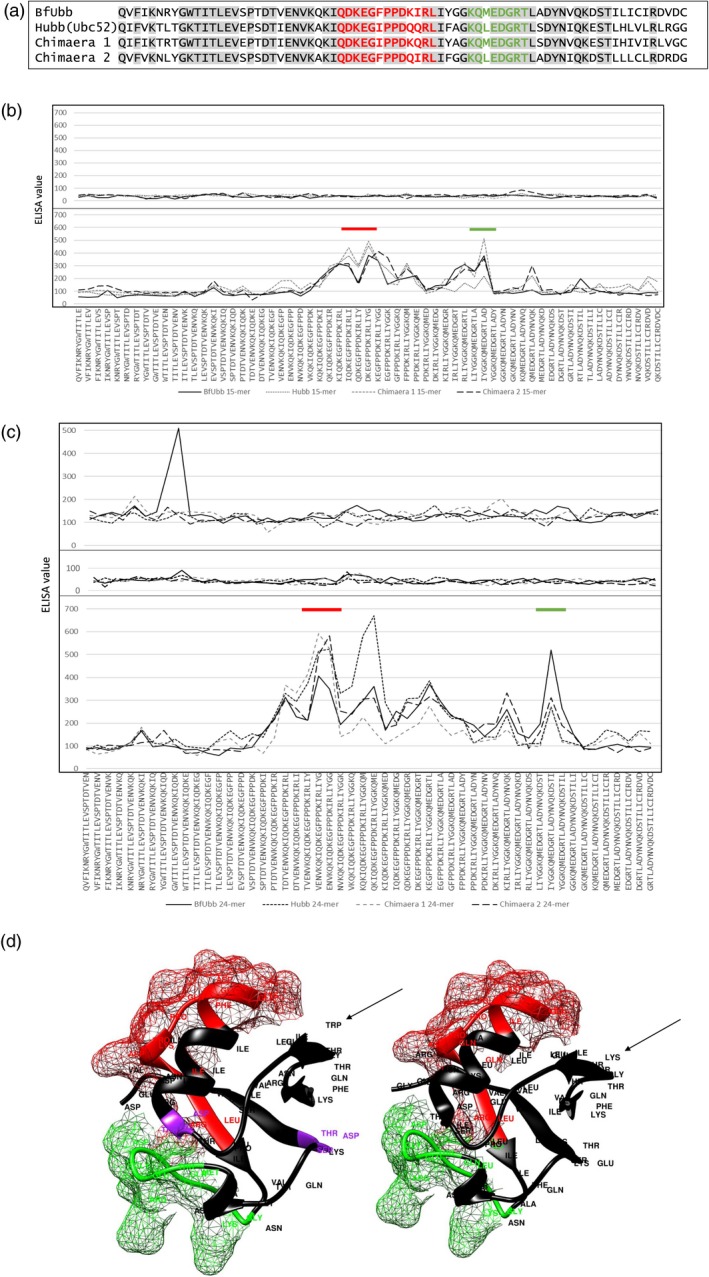
Reaction of rabbit polyclonal anti‐BfUbb and anti‐Hubb antiserum with linear peptide libraries derived from BfUbb and Hubb sequences. (a) Bacteroides fragilis (BfUbb), human (Hubb) ubiquitin amino acid sequences and BfUbb/Hubb chimaeric sequences used to generate the 15‐mer and 24‐mer PepScan linear peptide libraries. Sequence identity, grey shading; linear epitopes recognised by the anti‐rHubb antiserum, red and green. (b) Plots of enzyme‐linked immunosorbent assay (ELISA) results for 15‐mer PepScan linear peptide arrays probed with anti‐rBfUbb (top) and anti‐rHubb (bottom) 1/2000 dilution rabbit polyclonal anti‐sera. The BfUbb peptide sequences are indicated in the x‐axis labels; the equivalent Hubb and chimaeric peptide sequences can be derived from the alignments in (a). (c) Plots of ELISA results for 24‐mer PepScan linear peptide arrays probed with anti‐rBfUbb (top: 1/500 dilution; middle: 1/2000 dilution) and anti‐rHubb (bottom: 1/2000 dilution) rabbit polyclonal antisera. The BfUbb peptide sequences are indicated in the x‐axis labels; the equivalent Hubb and chimaeric peptide sequences can be derived from the alignments in (a). Bars indicate regions containing linear epitopes recognized by the anti‐rHubb antiserum (d). Molecular graphic of BfUbb (left) and Hubb (right) ribbon models; shared linear epitopes (red and green) showing mesh surface model. Putative BfUbb glycosylation sites, purple. Arrow: position of tryptophan 11 in BfUbb and corresponding lysine 11 in human ubiquitin.
Human serum reactivity with BfUbb and human ubiquitin
On the basis of the differential reactivity of the rabbit antisera to BfUbb and Hubb, the IgG ELISA reactivity of anonymous human antiserum with either human ubiquitin or sBfUbb whole molecule was examined. Serum samples surplus to diagnostic requirements from 393 individuals who had tested positive for the first time by the regional immunology laboratory for a variety of different conditions, 20 serum samples from individuals diagnosed with ulcerative colitis and 61 serum samples from healthy individuals were tested (Table 1). It is clear that some individuals have high ELISA OD readings for BfUbb or both BfUbb and Hubb (Fig. 5a,b). In contrast, no individuals had a high OD reading for Hubb and a low OD reading for BfUbb. The levels of autoantibodies detected in the immunology laboratory tests did not relate directly to the level of antibodies to either BfUbb of Hubb, nor did the distribution of antinuclear subtypes demonstrate any clear pattern of association with antibodies to BfUbb or Hubb (Supporting information, Table S1). The data indicate clearly, however, that the immune system of some individuals with autoimmune disorders has been exposed to BfUbb, which has resulted in the generation of IgG antibodies reactive with BfUbb epitopes not present in Hubb.
Figure 5.
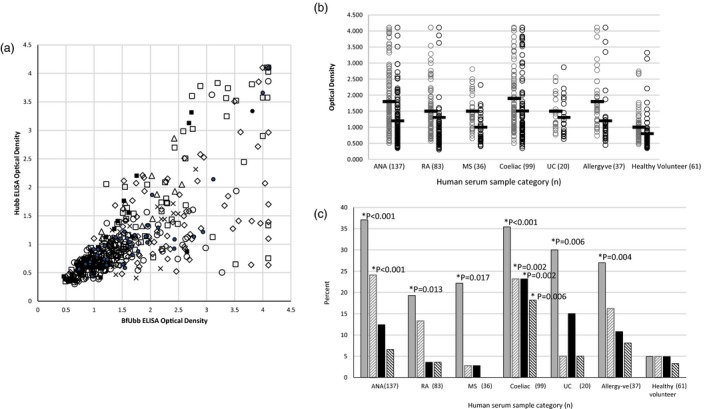
Comparison of immunoglobulin (Ig)G reaction of human serum samples with sBfUbb and Hubb by enzyme‐linked immunosorbent assay (ELISA). (a) Plot of optical density (OD) readings for BfUbb versus Hubb (1/90 serum dilution). Category: autoimmune antibodies (ANA), diamond; rheumatoid arthritis (RA), open circle; multiple sclerosis (MS), x; ulcerative colitis (UC), triangle; coeliac, open square; allergy test negative (allergy–ve), solid circle; healthy volunteer (HV), solid square. (b) Plot of OD reading versus serum category (1/90 serum dilution) for reaction with sBfUbb (grey) and Hubb (black). Bar = mean OD reading. (C) Percentage within sample category (1/90 serum dilution) with an OD > 2 (solid fill) and OD > 2·5 (diagonal stripes); sBfUbb (grey), Hubb (black). *Indicates statistically significant difference with the healthy volunteer samples by Fisher’s exact test (two‐sided).
To determine if there was any significant difference in the number of individuals with higher titres of specific antibodies in the different human serum categories, the proportion of samples with an OD reading above either 2·0 or 2·5 was determined (Fig. 5c). The difference in the proportion of each of the categories was compared for statistical significance with the healthy volunteer samples (P = 0·05). The greatest proportion of patients with high levels of IgG reactive with BfUbb was in the category which included 134 samples positive for anti‐nuclear antibodies and three patients with anti‐cardiolipin and anti‐beta‐2‐glycoprotein antibodies, with 37% (46 of 137) of samples having an OD > 2·0 and 24% (33 of 137) and OD > 2·5. The proportion reactive with Hubb, however, was not significantly different to the healthy volunteers (Fig. 5c). This indicates that individuals who had been referred for immunology laboratory testing for suspected autoimmune disease were statistically more likely to have high levels of BfUbb‐reactive antibodies than healthy individuals. Included within this group were two individuals with antibodies to beta‐2‐glycoprotein and cardiolipin.
The proportion of individuals in the categories rheumatoid arthritis, multiple sclerosis, coeliac, ulcerative colitis and allergy test‐negative were statistically significantly higher (P = 0·05) than the healthy volunteer group at an OD > 2·0, but not an OD > 2·5. Of the individuals referred for testing for anti‐cyclic citrullinated peptide antibodies, 19% (16 of 83) had an OD > 2 of anti‐BfUbb antibodies and 13% (11 of 83) an OD > 2·5. Individuals referred for testing for suspected multiple sclerosis, with a positive test for oligoclonal band detection, were least likely to have a high reaction to either BfUbb or Hubb; indeed, only one of 36 had an OD > 2·5 and none of the serum samples from these individuals reached saturation OD (> 4·1) in the ELISA test. Similarly, none of the ulcerative colitis samples reached saturation and one of 20 had an OD > 2·5. Interestingly, three of the individuals who had been referred for allergy to dust mites, mixed pollen and mixed animal antigens, but in whom specific IgE had not been detected (n = 37), had OD > 3·0 for both BfUbb and Hubb. The pattern of reaction with individuals who tested positive for IgA anti‐tissue transglutaminase antibodies, indicative of coeliac disease, differed from the other categories. The proportion of individuals with both anti‐BfUbb and Hubb IgG OD > 2·0 and OD > 2·5 was significantly higher (P < 0·006) than the healthy volunteers.
Selected serum samples from each category were titrated by doubling dilution and tested for reactivity with BfUbb and Hubb. Examples of the titration patterns are illustrated in Fig. 6a–d: (a) and (b) illustrate a dominant reaction to BfUbb unique epitopes, (c) illustrates reaction to cross‐reactive epitopes and (d) a lack of reactivity. Individuals with the same positive immunology test result could have different titration patterns, as illustrated in Fig. 6b–d serum samples, which were from three different anti‐ribosomal P protein‐positive individuals. Further example titrations are presented in Supporting information, Fig. S2 to illustrate that both an immunodominant reaction to BfUbb and cross‐reaction patterns were present in different serum sample categories. Some samples had an OD > 2 at dilutions greater than 1/400.
Figure 6.
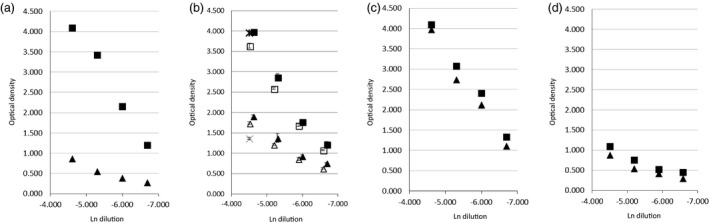
Example titrations of the immunoglobulin (Ig)G reactivity of human serum samples; comparison of Hubb (triangle) and BfUbb (square). Immunology laboratory test positive for: (a) IgG anti‐cardiolipin and beta2glycoprotein and (b–d) anti‐ ribosomal P antibody. Initial dilution 1/90. In (b) replicate experiments with 1/100 initial dilution and a 1/90 dilution single point replicate (BfUbb, star; Hubb, X) are also illustrated. Error bars [standard deviation (s.d.)].
The reaction to the 12‐mer BfUbb N terminal peptide 1 was also examined in a selection of serum samples. Figure 7a is a human serum sample with an immunodominant reaction to BfUbb, while Fig. 7b illustrates reaction to cross‐reactive epitopes. As with the rabbit polyclonal antiserum (Fig. 3), a similar reaction with human ubiquitin and peptide 1 was observed (Fig. 7 and Supporting information, Fig. S3). This indicates that the N‐ terminal linear peptide contains an epitope which can also be cross‐reactive in humans.
Figure 7.
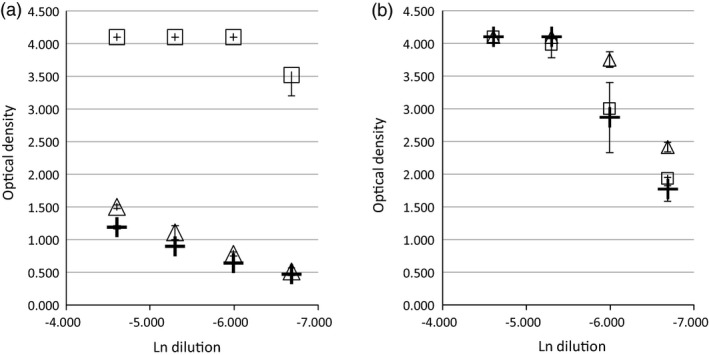
Example titrations of the immunoglobulin (Ig)G reactivity of human serum samples; comparison of whole molecule BfUbb (square), Hubb (triangle) and peptide 1‐QVFIKNRYGWTI (cross). Initial dilution 1/100. Immunology laboratory test positive for (a) anti‐cardiolipin antibody and (b) anti‐tissue transglutaminase and endomysial antibody [standard deviation (s.d.)].
Discussion
It is evident that BfUbb and Hubb have potentially highly similar molecular structures which are sufficient to result in cross‐reacting epitopes. Our data clearly demonstrate an immune response to BfUbb in human serum samples; some individuals have higher titre antiserum reactive with BfUbb when compared with human ubiquitin. This indicates that both unique BfUbb epitopes and epitopes shared with Hubb can be recognized. The reaction of the human serum samples to the BfUbb peptide 1 provides clear evidence that there is the potential for antibodies to both BfUbb and Hubb within human serum (Fig. 7). Patients referred for first‐time serum testing to the regional immunology laboratory for a variety of different conditions are more likely to have antibodies to BfUbb than healthy volunteers, which suggests a potential relationship with human disease. It is interesting that the multiple sclerosis and ulcerative colitis patients did not exhibit the high levels of antibodies observed in the other autoimmune conditions; this may be related to differences in the immunological pathogenesis and dominant hypersensitivity mechanisms in these different disorders. Muller and colleagues 14 reported that 79% of 161 patients with systemic lupus erythematosus (SLE) had antibodies reactive with human ubiquitin and a synthetic peptide composed of residues 22–45. One of the two Hubb‐BfUbb cross‐reactive epitopes that we identified is within this 22–45 residue region. Muller and colleagues also detected ubiquitin‐specific antibodies in 16% of rheumatoid arthritis and 15% of scleroderma patients. These authors studied successive serum samples from seven patients during active and inactive SLE phases and reported that anti‐ubiquitin antibodies were detected before anti‐DNA antibodies and observed a reaction with ubiquitin–histone complexes. Our observed titration pattern for BfUbb and Hubb may also represent different stages in antibody production with an initial reaction where unique BfUbb epitopes predominate (Fig. 6a), followed by a progression towards, and concomitant increase in, antibodies to BfUbb/Hubb cross‐reactive epitopes. The titration pattern in Fig. 6b could represent an early stage of this progression.
Molecular mimicry by infectious agents is hypothesized to be a trigger for the development of autoimmunity. It is proposed that exposure of the immune system to exogenous molecules with a sufficiently similar structure either breaks tolerance to self‐antigens or activates pre‐existing but unreactive immune system cells 15, 16. Viral and bacterial molecules from pathogens have been associated with triggering autoimmune diseases, but conclusive proof of a direct relationship has remained elusive in many instances 17. More recently, bacterial population shifts in the intestinal microbiota from a state of symbiosis to dysbiosis have been linked to many conditions, including autoimmune disease. For example, animal models implicate bacteria in the triggering of rheumatoid arthritis (e.g. 18). The mechanistic details of the microbiota associations, however, cannot be determined by metagenomics or metataxonomics alone, as such studies only imply correlations.
Clearly, as B. fragilis are members of the GI tract microbiota, conditions, diseases or infections that impact upon the integrity of the gut epithelium will be important in the exposure of the immune system to ubiquitin. Some isolates of B. fragilis produce a zinc‐dependent metalloprotease enterotoxin (BFT) that has been associated with childhood diarrhoea, colonic inflammation and potentially colon cancer 1. The enterotoxin increases colonic epithelial permeability by inducing changes to and dissolution of the epithelial tight junctions, thought to be caused by BFT cleavage of the zonula adherens protein E‐cadherin. Carriage of BFT‐positive B. fragilis is not always associated with acute diarrhoeal disease, as enterotoxigenic B. fragilis can be isolated from healthy individuals 1. The reasons for this are unknown, but the BFT protein can undergo spontaneous autodigestion. The BFT gene is located within a potentially mobile genetic element of approximately 6‐kbp in size with 12 bp direct repeats at either end 1. This BFT element is variably present in large genomic islands, including integrative and conjugative genetic elements, that are transferred horizontally within the B. fragilis pan‐genome 19. There is the potential for more than one copy of the BFT gene to be present at different chromosomal locations. The carriage of BFT genes and therefore ability to produce the enterotoxin is highly variable among B. fragilis strains. The variable exposure of individuals to BfUbb, and therefore the variable development of anti‐BfUbb antibodies, could be dependent upon colonization by B. fragilis carrying both the BfUbb gene and the BFT gene. GI tract infections caused by other organisms that affect GI tract permeability could impact equally upon BfUbb exposure.
In addition, B. fragilis has a highly complex and dynamic interaction with the immune system due to the ON–OFF–ON variable production of at least 11 molecularly and antigenically different polysaccharides within an individual strain. B. fragilis contains a great diversity in capsule biosynthesis with multiple polysaccharide biosynthesis operons within the pan‐genome; for example, a total of 28 different polysaccharide biosynthesis operons were identified in the first three genomes sequenced 19, 20. One of these variably produced polysaccharides, PSA, has been reported to influence regulatory T cell (Treg) development, but PSA is variably produced within a B. fragilis population 21. In addition, not all B. fragilis strains carry the PSA biosynthesis operon. These variable polysaccharides coat the bacterial cell surface and the OMV 8 with which BfUbb is also associated 4.
Factors which influence the potential delivery of BfUbb to immune system cells and subsequent immune system events could therefore include the damage of GI tract epithelium by B. fragilis enterotoxin or other GI tract pathogens and the types of B. fragilis polysaccharides being produced within the population and present on OMVs. Activation of Tregs by interleukin (IL‐10) is dependent upon the engulfment of B. fragilis OMV by dendritic cells 2. While the evolution of this commensal relationship has provided cues for immune regulation, it also provides the opportunity for presentation of potential antigens that mimic host molecules. Although BfUbb appears to function as a competitive inhibitor of other B. fragilis strains in the gut 22, our data show that the similarity to eukaryotic ubiquitin can lead to the generation of antibodies against self‐epitopes. This might go beyond proteins and include other molecules such as cardiolipin, which is present in the outer membranes of many Gram‐negative bacteria 23, including B. fragilis. Interestingly, one of the human serum samples with a high titre reaction to BfUbb also had anti‐cardiolipin antibodies (Fig. 6a).
A loss of epithelial integrity may precede gluten sensitivity in individuals with a genetic predisposition to coeliac disease 24. Interestingly, there is an increased risk of coeliac disease development with multiple gastrointestinal infections 25. The high incidence of anti‐BfUbb and anti‐Hubb antibodies in the coeliac serum category could therefore reflect early exposure to BfUbb and could be an additional aetiological factor for coeliac disease development. Repeated GI infections leading to BfUbb exposure could also be a factor in individuals with genetic predispositions to other diseases.
Extracellular ubiquitin is detectable in serum at nanogram ml−1 concentrations 26 in healthy individuals. Slightly elevated nanogram ml−1 concentrations have been associated with a number of diseases. Examples include liver cirrhosis, type 2 diabetes, sepsis, leukaemia, rheumatoid arthritis, allergy to Hymenoptera venom, schistosomiasis, acute viral hepatitis and patients undergoing haemodialysis. While a major source of extracellular ubiquitin is likely to be cell lysis, there is also evidence of active cellular secretion of ubiquitin. Extracellular ubiquitin may play a role in immune system regulation 27. It has also been proposed that extracellular ubiquitin masks cell debris, thus preventing an autoimmune response to the debris molecules 28. Clearly anti‐ubiquitin antibodies may play a role in systemic autoimmune rheumatic diseases, as well as other conditions.
Extracellular ubiquitin–proteasome interaction in mammals and other animals is necessary for sperm–egg penetration and therefore essential for successful fertilization 29. Concentrations of ubiquitin in human seminal plasma of between 1·8 and 19·1 μg ml−1 have been reported 30. A monoclonal antibody specific for polyubiquitin was shown to inhibit fertilization in an Ascidian model system 31, and it is recognized that inhibition of extracellular ubiquitin–proteasome activity results in infertility in mammals 29. In addition, anti‐sperm antibodies can cause infertility in both men and women 32. High levels of anti‐ubiquitin antibodies could therefore potentially impact on fertility.
As our data were generated from a pilot study of anonymous serum samples, the clinical details of the patients are unknown. Therefore, these individuals may have had conditions or symptoms not related to the tests for which they had been referred. The consequences of circulating serum antibodies predominantly reactive with only BfUbb are currently unknown; however, the levels of antibodies to Hubb detected in our study could clearly be highly significant for health.
In conclusion, we have shown that the homologue of human ubiquitin BfUbb, produced uniquely by Bacteroides fragilis, a normal resident bacterium in the human gut, can generate IgG antibodies in humans. We also demonstrate that the close sequence and structural similarity of BfUbb and human ubiquitin results in cross‐reactive epitopes. Antibodies to ubiquitin may potentially have multiple impacts on human health, depending on co‐morbidities. The timing and route of exposure to BfUbb could be critical in disease processes. It will therefore be important to determine if an immune reaction to BfUbb is a consequence and/or a driver of disease. Resolving this conundrum could identify entirely novel disease patterns. It could also significantly improve our current understanding of the conditions for which the patients in this study were referred for testing.
Author contributions
S. P., Principal Investigator; study concept, design and execution; carried out experiments, data collection, analysis and interpretation; prepared the figures; wrote the paper. L. S. contributed to study and laboratory protocol design; carried out experiments and data collection; commented on the paper. J. D. M. E. was responsible for human serum sample acquisition; contributed to study design; commented on the paper; and G. B., contributed to study design and commented on the paper.
Disclosure
The authors have no conflicts of interest.
Supporting information
Fig. S1. Molecular graphic of BfUbb shared Hubb epitopes and putative BfUbb unique epitope containing region. BfUbb ribbon model; linear epitopes shared with Hubb (red and green) and putative unique BfUbb epitope region (blue) showing (a) solid and (b) mesh surface models. The position of the tryptophan 11 residue is indicated (arrow).
Fig. S2. Example titrations of the IgG reactivity of human serum samples; comparison of Hubb (triangle) and BfUbb (square). Immunology Laboratory test positive for: (i) and (ii) anti‐ tissue transglutaminase and endomysial antibody; (iii) and (iv) anti‐cyclic citrullinated peptide antibody; (v) and (vi) allergy test negative; (vii) detection of oligoclonal bands; (viii) ulcerative colitis patient sample; (ix) anti‐Scl‐70 antibody; (x) anti‐La antibody and (xi) anti‐Ro52 and Ro60 antibody. Initial dilution 1/90. In (ii, iv, vi, ix and xi) replicate experiments with 1/100 initial dilution and a 1/90 dilution single point replicate (BfUbb, star; Hubb, X) are also illustrated. Error bars (SD).
Fig. S3. Example titrations of the IgG reactivity of human serum samples; comparison of whole molecule BfUbb (square), Hubb (triangle) and Peptide 1‐QVFIKNRYGWTI (cross). Initial dilution 1/100. Immunology Laboratory test positive for (i) anti‐dsDNA, ‐chromatin, ‐Ro 52, ‐Ro 60 and –centromere antibody and (ii) anti‐cyclic citrullinated peptide antibody. Error bars (SD).
Table S1. Comparison of ELISA IgG reaction to BfUbb and Hubb with Immunology Laboratory test results
Acknowledgements
This work was supported by Belfast Health and Social Care Trust Immunology Department Charitable funds Reference 2118. We thank the staff of the Regional Immunology Laboratory, Belfast Health and Social Care Trust, Northern Ireland, UK for collecting the human serum samples.
References
- 1. Patrick S. Bacteroides In: Tang Y, Sussman M, Liu D, Poxton I, Schwartzman J, eds. Molecular medical microbiology. London: Academic Press; 2015:917–44. [Google Scholar]
- 2. Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012; 12:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swidsinski A, Ladhoff A, Pernthaler A et al Mucosal flora in inflammatory bowel disease. Gastroenterology 2002; 122:44–54. [DOI] [PubMed] [Google Scholar]
- 4. Patrick S, Jobling KL, O’Connor D, Thacker Z, Dryden DTF, Blakely GW. A unique homologue of the eukaryotic protein‐modifier ubiquitin present in the bacterium Bacteroides fragilis, a predominant resident of the human gastrointestinal tract. Microbiology 2011; 157:3071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patrick S, Blakely GW. Crossing the eukaryote–prokaryote divide: a ubiquitin homolog in the human commensal bacterium Bacteroides fragilis . Mob Genet Elements 2012; 2:149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin–proteasome system and onto human diseases and drug targeting. Biochim Biophys Acta 2012; 1824:3–13. [DOI] [PubMed] [Google Scholar]
- 7. Hochstrasser M. Origin and function of ubiquitin‐like proteins. Nature 2009; 458:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patrick S, McKenna JP, O'Hagan S, Dermott E. A comparison of the haemagglutinating and enzymic activities of Bacteroides fragilis whole cells and outer membrane vesicles. Microb Pathog 1996; 20:191–202. [DOI] [PubMed] [Google Scholar]
- 9. Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015; 10:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pettersen EF, Goddard TD, Huang CC et al UCSF Chimera – a visualization system for exploratory research and analysis. J Comput Chem 2004; 25:1605–12. [DOI] [PubMed] [Google Scholar]
- 11. Slootstra JW, Puijk WC, Ligtvoet GJ, Langeveld JP, Meloen RH. Structural aspects of antibody–antigen interaction revealed through small random peptide libraries. Mol Divers 1996; 1:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Timmerman P, Puijk WC, Meloen RH. Functional reconstruction and synthetic mimicry of a conformational epitope using CLIPS technology. J Mol Recog 2007; 20:283–99. [DOI] [PubMed] [Google Scholar]
- 13. Graydon R, Hogg RE, Chakravarthy U, Young IS, Woodside JV. The effect of lutein‐ and zeaxanthin‐rich foods v. supplements on macular pigment level and serological markers of endothelial activation, inflammation and oxidation: pilot studies in healthy volunteers. Br J Nutr 2012; 108:334–42. [DOI] [PubMed] [Google Scholar]
- 14. Muller S, Briand JP, Van Regenmortel MH. Presence of antibodies to ubiquitin during the autoimmune response associated with systemic lupus erythematosus. Proc Natl Acad Sci USA 1988; 85:8176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med 1999; 341:2068–74. [DOI] [PubMed] [Google Scholar]
- 16. Christen U, von Herrath MG. Induction, acceleration or prevention of autoimmunity by molecular mimicry. Mol Immunol 2004; 40:1113–20. [DOI] [PubMed] [Google Scholar]
- 17. Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol 2012; 42:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogier R, Evans‐Marin H, Manasson J et al Alteration of the intestinal microbiome characterizes preclinical inflammatory arthritis in mice and its modulation attenuates established arthritis. Sci Rep 2017; 7:15613,017–15802‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Husain F, Tang K, Veeranagouda Y, Boente R, Patrick S, Blakely G et al Novel large‐scale chromosomal transfer in Bacteroides fragilis contributes to its pan‐genome and rapid environmental adaptation. Microb Genom 2017; 3:10.1099/mgen.0.000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patrick S, Blakely GW, Houston S et al Twenty‐eight divergent polysaccharide loci specifying within‐ and amongst‐strain capsule diversity in three strains of Bacteroides fragilis . Microbiology 2010; 156:3255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patrick S, Ingram RB, Schneiders T, Fitzgerald DC. Microbial regulation of gastrointestinal immunity in health and disease In: Constantinescu CS, Arsenescu RI, Arsenescu V, eds. Neuro‐Immuno‐Gastroenterol Switzerland: Springer; 2016:39–52. [Google Scholar]
- 22. Chatzidaki‐Livanis M, Coyne MJ, Roelofs KG, Gentyala RR, Caldwell JM, Comstock LE. Gut symbiont Bacteroides fragilis secretes a eukaryotic‐like ubiquitin protein that mediates intraspecies antagonism. MBio 2017; 8:10.1128/mBio. 01902–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 2009; 1788:2084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cukrowska B, Sowinska A, Bierla JB, Czarnowska E, Rybak A, Grzybowska‐Chlebowczyk U. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota – key players in the pathogenesis of celiac disease. World J Gastroenterol 2017; 23:7505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ritter J, Zimmermann K, Johrens K et al T‐cell repertoires in refractory coeliac disease. Gut 2018; 67:644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takada K, Nasu H, Hibi N et al Serum concentrations of free ubiquitin and multiubiquitin chains. Clin Chem 1997; 43:1188–95. [PubMed] [Google Scholar]
- 27. Sixt SU, Dahlmann B. Extracellular, circulating proteasomes and ubiquitin – incidence and relevance. Biochim Biophys Acta 2008; 1782:817–23. [DOI] [PubMed] [Google Scholar]
- 28. Weil R. Does antigen masking by ubiquitin chains protect from the development of autoimmune diseases? Front Immunol 2014; 5:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sutovsky P. Sperm proteasome and fertilization. Reproduction 2011; 142:1–14. [DOI] [PubMed] [Google Scholar]
- 30. Lippert TH, Seeger H, Schieferstein G, Voelter W. Immunoreactive ubiquitin in human seminal plasma. J Androl 1993; 14:130–1. [PubMed] [Google Scholar]
- 31. Sawada H, Sakai N, Abe Y et al Extracellular ubiquitination and proteasome‐mediated degradation of the ascidian sperm receptor. Proc Natl Acad Sci USA 2002; 99:1223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bohring C, Krause W. Characterization of spermatozoa surface antigens by antisperm antibodies and its influence on acrosomal exocytosis. Am J Reprod Immunol 2003; 50:411–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Molecular graphic of BfUbb shared Hubb epitopes and putative BfUbb unique epitope containing region. BfUbb ribbon model; linear epitopes shared with Hubb (red and green) and putative unique BfUbb epitope region (blue) showing (a) solid and (b) mesh surface models. The position of the tryptophan 11 residue is indicated (arrow).
Fig. S2. Example titrations of the IgG reactivity of human serum samples; comparison of Hubb (triangle) and BfUbb (square). Immunology Laboratory test positive for: (i) and (ii) anti‐ tissue transglutaminase and endomysial antibody; (iii) and (iv) anti‐cyclic citrullinated peptide antibody; (v) and (vi) allergy test negative; (vii) detection of oligoclonal bands; (viii) ulcerative colitis patient sample; (ix) anti‐Scl‐70 antibody; (x) anti‐La antibody and (xi) anti‐Ro52 and Ro60 antibody. Initial dilution 1/90. In (ii, iv, vi, ix and xi) replicate experiments with 1/100 initial dilution and a 1/90 dilution single point replicate (BfUbb, star; Hubb, X) are also illustrated. Error bars (SD).
Fig. S3. Example titrations of the IgG reactivity of human serum samples; comparison of whole molecule BfUbb (square), Hubb (triangle) and Peptide 1‐QVFIKNRYGWTI (cross). Initial dilution 1/100. Immunology Laboratory test positive for (i) anti‐dsDNA, ‐chromatin, ‐Ro 52, ‐Ro 60 and –centromere antibody and (ii) anti‐cyclic citrullinated peptide antibody. Error bars (SD).
Table S1. Comparison of ELISA IgG reaction to BfUbb and Hubb with Immunology Laboratory test results


