Abstract
Background
Dickeya zeae is the causal agent of maize and rice foot rot diseases, but recently it was also found to infect banana and cause severe losses in China. Strains from different sources showed significant diversity in nature, implying complicated evolution history and pathogenic mechanisms.
Results
D. zeae strains were isolated from soft rot banana plants and ornamental monocotyledonous Clivia miniata. Compared with D. zeae strain EC1 isolated from rice, clivia isolates did not show any antimicrobial activity, produced less extracellular enzymes, had a much narrow host ranges, but released higher amount of extracellular polysaccharides (EPS). In contrast, the banana isolates in general produced more extracellular enzymes and EPS than strain EC1. Furthermore, we provided evidence that the banana D. zeae isolate MS2 produces a new antibiotic/phytotoxin(s), which differs from the zeamine toxins produced by rice pathogen D. zeae strain EC1 genetically and in its antimicrobial potency.
Conclusions
The findings from this study expanded the natural host range of D. zeae and highlighted the genetic and phenotypic divergence of D. zeae strains. Conclusions can be drawn from a series of tests that at least two types of D. zeae strains could cause the soft rot disease of banana, with one producing antimicrobial compound while the other producing none, and the D. zeae clivia strains could only infect monocot hosts. D. zeae strains isolated from different sources have diverse virulence characteristics.
Electronic supplementary material
The online version of this article (10.1186/s12866-018-1300-y) contains supplementary material, which is available to authorized users.
Keywords: Dickeya zeae, Virulence factor, Pathogenicity, Host range
Background
Dickeya species (spp.), is one of the top ten important bacterial phytopathogens in the world, and has been listed as a plant quarantine pest in China since 2007 [1, 2]. There are currently 8 species in this genus, including D. dianthicola, D. dadantii, D. zeae, D. chrysanthemi, D. paradisiaca, D. solani, D. aquatic and D. fangzhongdai [3–6], and among them, D. dadantii, D. zeae and D. solani usually cause devastating disease, resulting in a considerable loss in crop yield, especially on potato, rice and banana [7–12].
Being located in a divergent evolutionary branch, D. zeae bacteria were reported to infect a wide range of plants all over the world, including 4 kinds of natural dicotyledonous hosts such as potato, tobacco, Chrysanthemum and Philodendron, and 6 kinds of natural monocotyledonous hosts such as maize, rice, banana, pineapple, Brachiaria and hyacinth (Additional file 1) [3, 8, 10, 12–32], besides, other 32 kinds of plants were reported as artificial hosts of D. zeae (Additional file 1) [13, 33, 34].
Latent infection appears to be a common trait of D. zeae. For instance, rice foot rot disease occurred severely in Jiangsu Province in 1980s resulting in about 90% losses on rice yield [35], but inactive for about 10 years till in 2000s, outbreak occurred in Fujian, Hunan, Guizhou, and Shandong provinces with different disease incidences ranging from 15 to 100% [36–38]. The disease currently occurs in Guangdong Province occasionally and sporadically. However, banana soft rot disease also caused by D. zeae has become a severe problem in Guangdong Province since 2009, with over 6000 ha of banana plantation being infected from 2010 to 2012 [10, 12]. The disease is now spreading to the major banana plantation fields in China in Provinces of Fujian, Yunnan, Hainan and Guangxi. Study on D. zeae banana strain is rare and its pathogenicity mechanisms are unclear.
Among the Dickeya spp., D. dadantii is perhaps the most characterized representative. The pathogen produces a range of virulence factors including cell wall degrading enzymes, type III secretion system (T3SS), siderphores, and indigoidine pigment, which collectively contribute to bacterial virulence [39–42]. Different from other Dickeya species, D. zeae can infect both dicots and monocots [7], indicating the existence of additional virulence factors. Genetic analysis and genome sequence comparison identified a zms gene cluster in D. zeae rice strains, which encodes the biosynthesis of zeamine phytotoxins capable of inhibiting rice seeds germination and growth [11, 43, 44]. Characterization of the D. zeae rice isolate EC1 also unveiled a quorum sensing (QS) system that produces and senses acyl homoserine lactone (AHL) signal to regulate expression of virulence associated genes, as well as a MarR family transcriptional regulator SlyA, and hereafter, to influence cell motility and biofilm formation [7, 44]. In addition, strain EC1 also relies on a Fis transcriptional regulator to directly regulate the expression of zms genes and production of cell wall degrading enzymes [45]. However, many detailed virulence regulatory mechanisms of D. zeae still remain unknown, especially in the banana strains, and few virulence factors related to host specificity have been determined.
In this study, D. zeae strains from rice, banana and monocot ornamental clivia plants that respectively cause severe bacterial rot disease in fields were collected to explore their phylogenetic relationship, and virulence differentials among strains were investigated by comparing the production of major virulence factors including extracellular enzymes, extracellular polysaccharides (EPS) and phytotoxins, nematode-killing activity and pathogenicity on many reported host plants. The findings from this study may present a new insight and clues for the control of bacterial soft rot diseases on crops.
Results
Strains isolated from soft rot banana and clivia plants were classified as D. zeae based on phylogenetic analysis
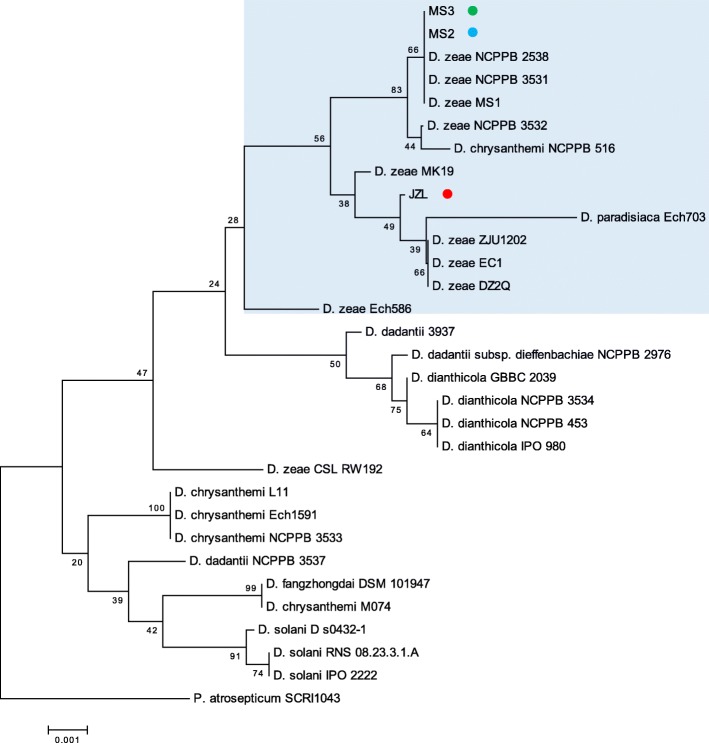
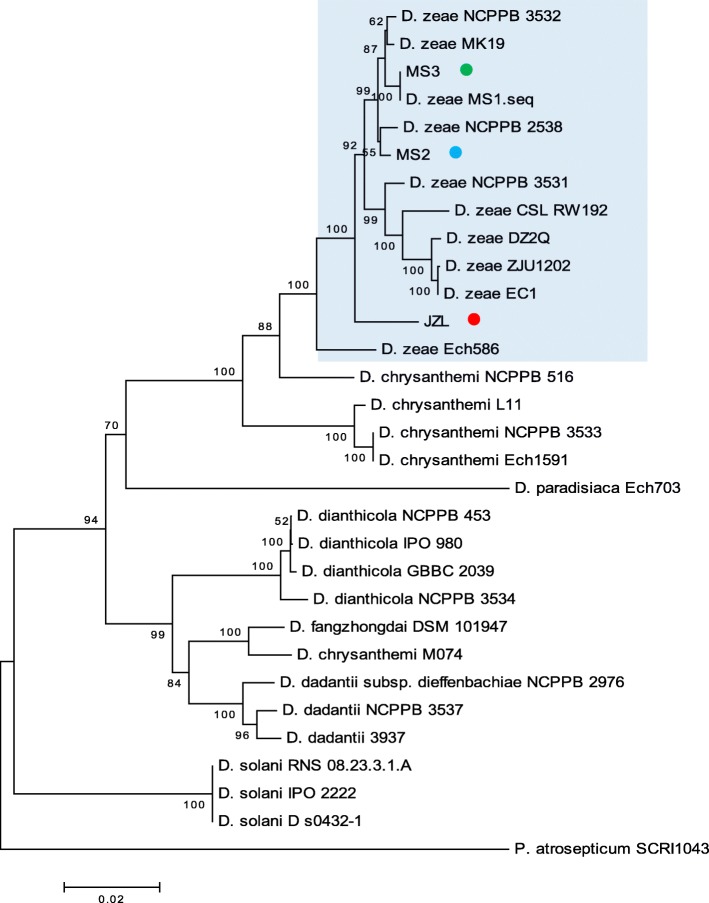
D. zeae was reported to be the causal agent of banana soft rot disease in Guangzhou, China [10, 12]. In this study, strains MS2 and MS3 that caused severe soft rot disease were respectively isolated from the basal pseudostems of banana plants in Nansha and Panyu Districts in Guangzhou city in 2012; and strains JZL1, JZL2 and JZL7 were isolated from the decayed centre leaves of clivia plants collected at Fangcun flower market in Guangzhou in 2017. The pure cultures of these isolates were inoculated to the corresponding banana and clivia plants, and typical soft rot symptoms were noticed (data not shown), validating their roles as banana and clivia pathogens, respectively. To identify the taxonomic status of these pathogens, MLSA analysis was performed based on the partial sequences of 16S rRNA gene, and the other four housekeeping genes including atpD, gyrB, infB and rpoB. Results showed that the three JZL strains contain identical housekeeping gene sequences, thus were designated as strain JZL. The alignment results showed that strains JZL, MS2 and MS3 clustered in the same branch with known D. zeae strains both in the 16S rDNA tree (Fig. 1) and the joint phylogenetic tree built on the concatenated nucleotide sequences of atpD, gyrB, infB and rpoB from 30 Dickeya species and strains (Fig. 2).
Fig. 1.
Phylogenetic tree based on the 16S rRNA sequences of Dickeya species. Consensus sequences were aligned with ClustalW and trimmed in size of 654 bp. Bootstrap value after 1000 replicates is expressed as percentages. Pectobacterium atrosepticum SCRI1043 is included as an outgroup. Bar, 0.1% substitution rate per site
Fig. 2.
Joint phylogenetic tree based on the concatenated nucleotide sequences of atpD, gyrB, infB and rpoB of Dickeya strains. Consensus sequences were aligned with ClustalW and trimmed in the following sizes: atpD, 642 bp; gyrB, 745 bp; infB, 1042 bp; rpoB, 1000 bp. All the sequences of a same strain were assembled for constructing the joint Neighbor-joining tree. Bootstrap values after 1000 replicates are expressed as percentages. P. atrosepticum SCRI1043 was included as an outgroup. Bar, 2% substitution rate per site
The 16S rDNA sequences of strains MS2 and MS3 are fully identical to those of D. zeae MS1 (from banana) [12], 99% identical to those of D. zeae rice isolates EC1 [11, 43], ZJU1202 [19] and DZ2Q [20]. Strains MS2 and MS3 had the identical atpD and gyrB sequences, showing 99% identity to their counterparts of strain MS1. In contrast, while strains MS3 and MS1 contain the same infB and rpoB gene sequences, strain MS2 shared 99% identity to those of strain MS1. Cumulatively, these data indicate that strains MS2 and MS3 are members of D. zeae, closely related to the previously identified D. zeae strain MS1 [12].
The 16S rDNA sequence of strain JZL is 99% identical to those of D. zeae strains EC1, Ech586 (from Philodendron) and MK19 (from river water), and 98% identical to that of strain MS2. The assembled sequence of atpD, gyrB, infB and rpoB (3429 bp) of MS2 is 99% identical to those of strains MS1 and MS3, 98% identical to that of strain EC1, and 97% identical to that of strain Ech586; and the assembled sequence of strain JZL is 97% identical to those of strains EC1 and Ech586, and 98% identical to those of strains MS1, MS2, MS3 and MK19. In summary, these data establish that strain JZL also belongs to the species D. zeae. To our knowledge, this is the first report indicating clivia as another natural host plant of D. zeae. Thereafter, the natural host range of D. zeae is expanded.
D. zeae strains in Asian countries were usually isolated from monocots
D. zeae was reported to infect both monocots and dicots [7, 11]. In most cases, it was isolated from monocot natural hosts, such as maize, rice, banana, pineapple, Brachiaria and hyacinth [3, 10, 13, 14, 16, 18, 21, 23–26]. By analysing the distribution of D. zeae, we found that it was most geographically originated from southeast Asian countries, especially the southeast of China, on rice, maize and banana (Additional files 1 and 2). Apart from China, D. zeae was also isolated from Japan, South Korea, North Korea, Philippines, India, Indonesia and Bangladesh on rice plants, and from South Korea, Japan, Thailand and India on maize host. It was currently only found in China on banana host, and in Malaysia on pineapple (Additional file 2). Given that pineapple heart rot disease was also found in Philippines and Hawaii, the pathogen was probably a novel species different from D. zeae [21, 27]. These geographical distribution and natural host range findings suggest that D. zeae may have a high level of host specificity and geographic related evolution history.
D. zeae strain JZL showed a significantly narrower host range than other D. zeae strains
To evaluate the host ranges of the D. zeae isolates, we performed pathogenicity tests on various reported hosts of D. zeae. Results showed that strains MS2 and MS3 could infect all the tested host plants similar to strain EC1, while JZL strains could not infect dicotyledonous plants including Cucumis sativus, Benincasa hispida, Brassica pekinensis, Raphanus sativus, Daucus carota, Solanum tuberosm, Lycopersicon esculentum, Solanum melongena and Capsicum annuum, but infect monocots including Oryza sativa, Musa sapientum, Clivia miniata, Zingiber officinale, Gladiolus gandavensis, Colocasia esculenta and Alocasia macrorrhiza (Table 1, Additional file 3), suggesting a significantly narrower host range than the other strains isolated from rice (strain EC1) and banana (strains MS2 and MS3). In addition, the soft rot symptoms on monocots caused by strains JZLs developed more slowly than those caused by the others except on clivia, and strains JZLs could not infect onion (Allium cepa) (Table 1, Additional file 3).
Table 1.
Pathogenicity tests of D. zeae strains on some dicotyledonous and monocotyledonous plants
| Inoculated plant | Inoculation amount, time | Diseased area (mm2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Class | Species | Organ | EC1 | MS2 | MS3 | JZL1 | JZL2 | JLZ7 | |
| Dicots | Cucumis sativus | Fruit | 2 μL, 24 h | 57.22 ± 7.70 | 107.97 ± 42.00 | 65.46 ± 7.88 | 0 | 0 | 0 |
| Benincasa hispida | Fruit | 2 μL, 24 h | 1140.57 ± 15.55 | 1336.50 ± 3.32 | 1252.61 ± 18.71 | 0 | 0 | 0 | |
| Brassica pekinensis | Petiole | 2 μL, 12 h | 56.81 ± 3.14 | 49.83 ± 2.73 | 42.40 ± 3.17 | 0 | 0 | 0 | |
| Raphanus sativus | Tuber | 2 μL, 24 h | 156.89 ± 2.60 | 106.72 ± 2.03 | 99.84 ± 9.76 | 0 | 0 | 0 | |
| Daucus carota | Tuber | 2 μL, 24 h | 274.83 ± 18.74 | 266.70 ± 8.98 | 231.42 ± 14.11 | 0 | 0 | 0 | |
| Solanum tuberosm | Tuber | 2 μL, 24 h | 173.33 ± 4.28 | 211.05 ± 20.54 | 171.45 ± 6.07 | 0 | 0 | 0 | |
| Lycopersicon esculentum | Fruit | 100 μL, 2 d | 908.22 ± 5.95 | 904.75 ± 9.80 | 945.81 ± 13.77 | 0 | 0 | 0 | |
| Solanum melongena | Fruit | 100 μL, 2 d | 66.52 ± 2.95 | 61.83 ± 1.67 | 86.72 ± 0.85 | 0 | 0 | 0 | |
| Capsicum annuum | Fruit | 2 μL, 24 h | 101.79 ± 8.81 | 106.65 ± 10.76 | 191.42 ± 10.55 | 0 | 0 | 0 | |
| Monocots | Oryza sativa | Stem | 200 μL, 7 d | 720.62 ± 21.48 | 575.71 ± 29.53 | 539.35 ± 17.77 | 452.67 ± 19.53 | 413.01 ± 12.64 | 499.85 ± 12.09 |
| Musa sapientum | Stem | 200 μL, 7 d | 1110.83 ± 19.23 | 1358.89 ± 17.78 | 1201.88 ± 19.91 | 407.90 ± 12.48 | 377.89 ± 13.71 | 464.23 ± 11.06 | |
| Clivia miniata | Leaf | 200 μL, 24 h | 1643.86 ± 6.94 | 2195.85 ± 7.06 | 1239.95 ± 7.55 | 1807.41 ± 9.52 | 1693.21 ± 20.27 | 1594.90 ± 14.66 | |
| Allium cepa | Bulb | 2 μL, 24 h | 185.83 ± 21.68 | 194.27 ± 18.73 | 119.90 ± 11.29 | 0 | 0 | 0 | |
| Zingiber officinale | Tuber | 2 μL, 24 h | 134.37 ± 14.17 | 86.92 ± 6.75 | 87.31 ± 12.23 | 47.44 ± 7.16 | 65.81 ± 6.11 | 81.33 ± 13.96 | |
| Gladiolus gandavensis | Stem | 200 μL, 7 d | 254.16 ± 9.05 | 168.17 ± 9.23 | 171.59 ± 5.92 | 36.75 ± 1.62 | 36.38 ± 2.39 | 36.85 ± 3.05 | |
| Colocasia esculenta | Tuber | 2 μL, 5 d | 461.847 ± 15.55 | 247.31 ± 15.31 | 117.34 ± 8.00 | 81.83 ± 6.00 | 40.46 ± 5.54 | 79.48 ± 3.91 | |
| Alocasia macrorrhiza | Stem | 200 μL, 7 d | 2860.90 ± 65.15 | 2763.17 ± 30.46 | 2633.75 ± 35.94 | 1316.86 ± 17.97 | 1430.45 ± 32.58 | 1381.58 ± 15.23 | |
D. zeae clivia strains showed weak aggressiveness on potato and cabbage, but were comparable with rice and banana strains on banana and clivia
To understand whether the decreased virulence of D. zeae clivia strains on hosts was due to their weak aggressiveness, we performed pathogenicity tests on two dicotyledonous and two monocotyledonous plants in equal weight, and calculated the numbers of bacterial cells invading into the plant tissues. Results showed that the JZL strains could not propagate in potato or cabbage tissues after inoculation for 12 h and 24 h, while EC1, MS2 and MS3 strains grew rapidly in potato in 12 h, and slowly in cabbage (Fig. 3a and b). In banana tissues, the cell number of all the strains were similar no matter at 12 h or 24 h post inoculation (Fig. 3c). In clivia, JZL strains initially grew slower than strains EC1, MS2 and MS3, but caught up at 24 h post inoculation (Fig. 3d).
Fig. 3.
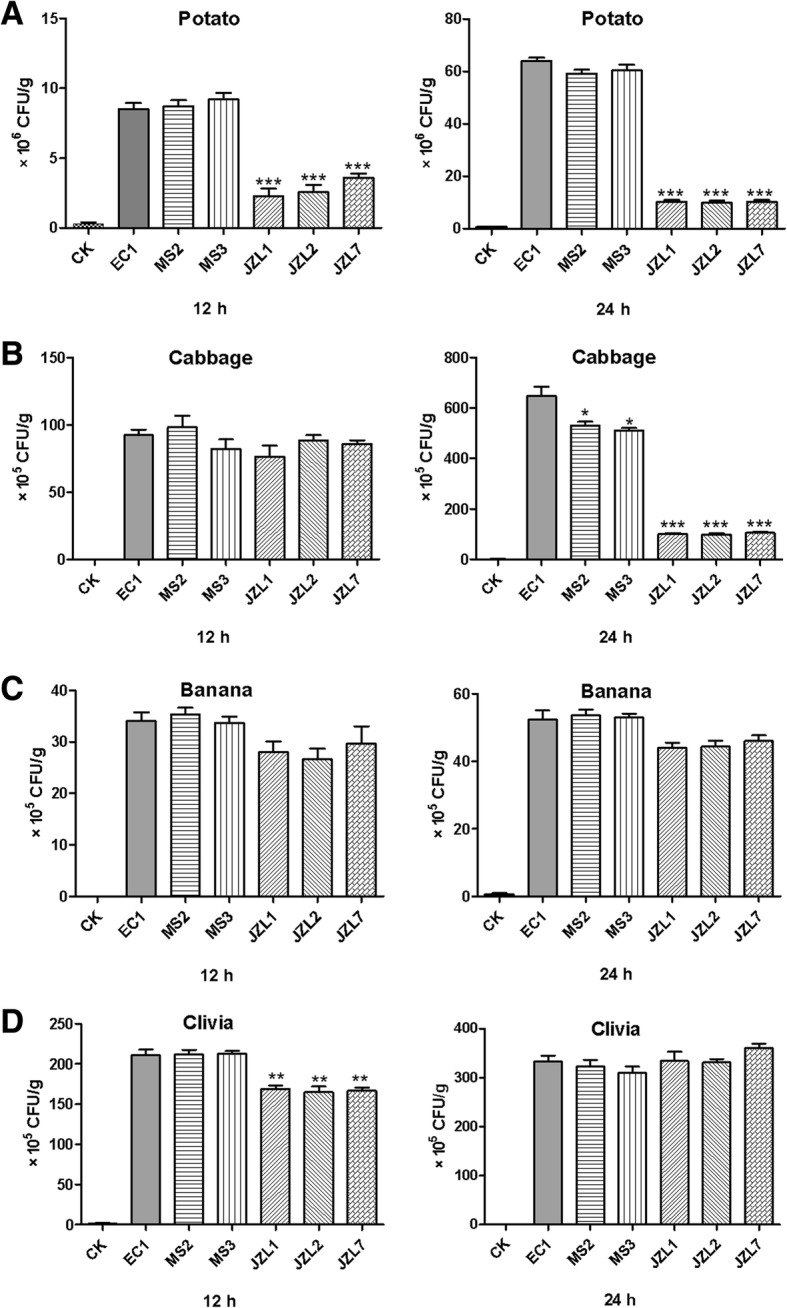
The number of bacterial cells of all the tested D. zeae strains invading into potato (a), cabbage (b), banana (c) and clivia (d), respectively. Healthy plant materials were surface-sterilized and inoculated with 2 μL of bacterial overnight cultures (OD600 = 2.0) in LB medium, and incubated at 28 °C. Tissues were taken out after 12 h and 24 h, respectively. And the diseased and surrounding healthy tissues in equal weight were cut and ground, and then added with 10 mL of sterilized 0.85% NaCl solution, stirred evenly, and 1 mL of which was diluted in series gradients, and 100 μL in each gradient was spread evenly onto LB agar plates in triplicates and kept at 28 °C for 24 h. Colonies between 30 to 300 CFU were counted. LB medium was used as a negative control. Each assay was repeated three times with duplicates
D. zeae strains from clivia and banana differ in production of extracellular enzymes and polysaccharides
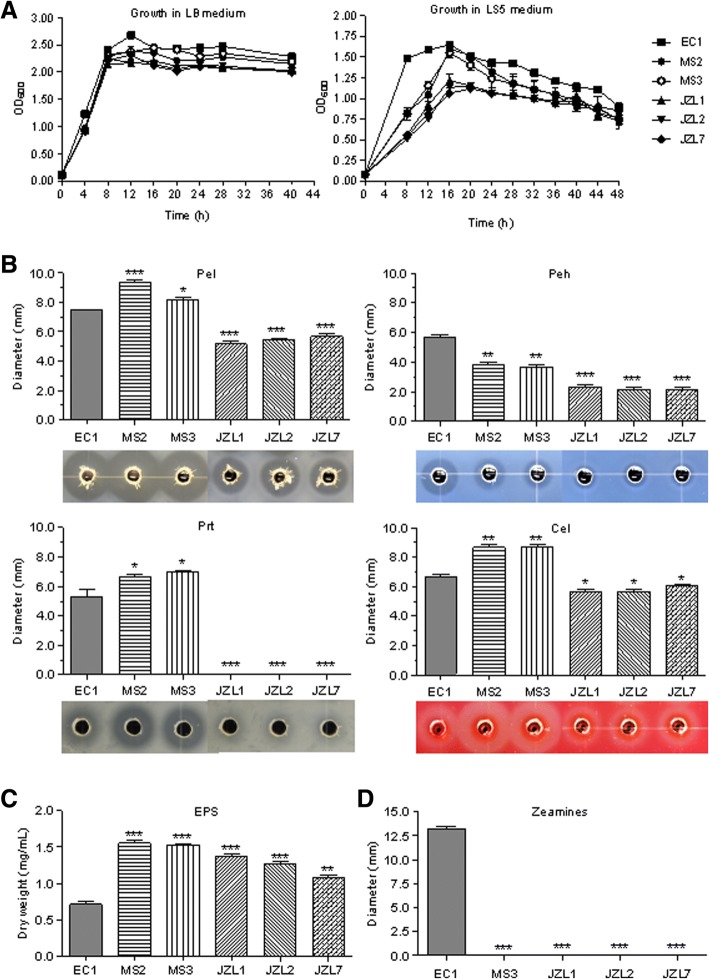
From the above results of pathogenicity tests, we inferred that the narrower host range of JZL strains may be due to its less virulence than the other strains, and strains EC1, MS2 and MS3 probably possess some additional virulence factor(s) than the three JZL strains. To compare the major virulence factors produced by different D. zeae strains, we first measured their growth dynamic process. Results showed that all the strains shared a similar growth pattern reaching the peak from 12 h to 16 h both in LB and LS5 media, though the cell density of JZL strains could not reach OD600 = 1.3 in LS5 medium (Fig. 4a). We then measured the production of cell wall degrading enzymes and extracellular polysaccharides (EPS), which are two categories of virulence factors produced by the rice pathogen D. zeae strain EC1 [44, 59]. Results showed that JZL strains produced lower amount of pectate lyases (Pel), polygalacturonases (Peh), proteases (Prt) and cellulases (Cel) than the others, especially hardly any protein degrading zone was visible. In contrast, the two banana strains, MS2 and MS3, produced higher amounts of the above-mentioned extracellular enzymes than those produced by the rice strain EC1 except polygalacturonases (Fig. 4b). In a somewhat different pattern to the production of cell wall degradation enzymes, the rice pathogen strain EC1 produced significantly less EPS than the MS strains from banana and the JZL strains from clivia plants (Fig. 4c).
Fig. 4.
Major virulence factors produced by D. zeae strains. a Growth curves of D. zeae strains in LB and LS5 media. b Extracellular cell wall degrading enzymes produced by D. zeae strains. Samples of 40 μL bacterial cells (OD600 = 1.8) were added to the assay plate wells (4 mm in diameter) and incubated at 28 °C. Pel and Peh assay plates were treated with 4 N HCl after 11 h and 14 h respectively. Cel assay plate was stained with 0.1% (w/v) Congo Red for 15 min after 14 h, and decolored with 1 M NaCl twice. Prt assay plate was taken photos after 24 h without any further treatment. c Production of extracellular polysaccharides of D. zeae strains. Samples of 3 mL bacterial cultures (OD600 = 1.8) were applied into 300 mL LB medium and grown with shaking at 200 r/m for 12 h, which were centrifuged at 8000 rpm for 40 m, and then at 4000 rpm for 20 m to obtain 250 mL supernatants. Double volumes of absolute ethanol were added to the supernatants, mixed thoroughly, stored at 4 °C overnight for precipitation, and centrifuged at 8000 rpm for 40 m. Finally, supernatants were discarded and pellets were weighed after drying at 55 °C overnight. d Phytotoxins produced by D. zeae strains. The bioassay plate was prepared as previously described [44]. Samples of 20 μL of bacterial cultures (OD600 = 1.5 in LS5 medium) were added into the toxin bioassay plate wells (4 mm in diameter), and incubated overnight at 37 °C
D. zeae banana strain MS2 produces novel antibiotic-like toxin(s) different from zeamines with slightly weaker ability to inhibit rice seed germination and kill nematodes
Our previous study found that D. zeae rice strain EC1 produces zeamines as major virulence factors in both rice plants and potato tubers [11, 60]. The phytotoxins are encoded by a zms gene cluster only found in D. zeae rice strains including ZJU1202 [19] and DZ2Q [20], some D. solani and Serratia plymuthica strains [43, 61], but were absent in other D. zeae strains isolated from other hosts including strain MS1 from banana [12, 43]. The results from this study showed that JZL strains could not produce zeamines, neither could strain MS3 (Fig. 4d), consistent with our previous findings [43].
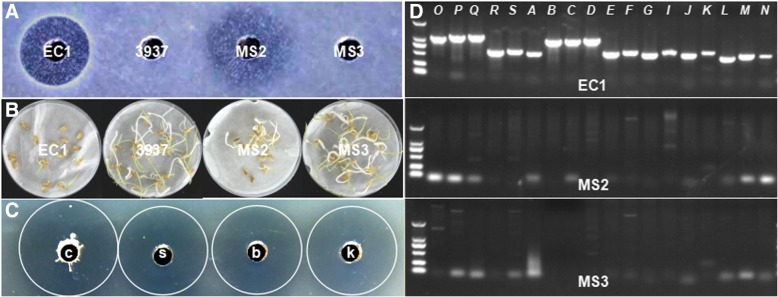
Surprisingly, strain MS2 produced an antibiotic-like toxin(s) to inhibit the growth of E. coli DH5α, resulting in a halo with a vague edge, quite different from the halo with a clear edge caused by strain EC1 (Fig. 5a). Given that zeamines are able to inhibit rice seed germination, we tested the inhibitory activity of strain MS2 against rice seeds. The results indicated that strain MS2 had a moderate inhibitory effect on seed germination, with an inhibitory rate being about 50%, less than that of 87% by strain EC1 (Fig. 5b). Consistent with its negative antimicrobial activity phenotype (Fig. 3d, Fig. 5a), strain MS3 had no effect on seed germination (Fig. 5b). It is inferred that the inhibitory activity against seed germination is probably due to the toxin(s) produced by strain MS2, which is supposed to be a kind of extracellular nonprotein metabolite since it did not loss the activity after boiling at 100 °C for 10 min or treating with Protease K at 37 °C for 30 min (Fig. 5c).
Fig. 5.
Phytotoxins produced by Dickeya strains. a, Bioassay of toxin production. b, Inhibitory activity of toxins from Dickeya strains against rice seed germination. Bacteria were grown in LB medium till OD600 = 1.5, and 20 seeds of rice variety CO39 were added to 5 ml of every bacterial culture and incubated at room temperature for 5 h, which were then cleaned and transferred onto a Petri dish with filter paper on it, and then incubated at 28 °C under 16 h light and 8 h dark conditions. Rice seeds incubated with same amount of D. dadantii 3937 were used as a control. c, The toxin produced by MS2 is an extracellular nonprotein metabolite. Strain MS2 was grown in LS5 medium till OD600 = 1.5 (c), and supernatant of the culture (s) was collected, which was treated by boiling at 100 °C for 10 min (b) or digestion with protease K at 37 °C for 30 min (k). Finally, the inhibition activity against the growth of E. coli DH5α was measured. d, PCR detection of zeamines biosynthesis genes from zmsO to zmsN. Based on the coding sequences of zms gene cluster in strain EC1 [43], 18 pairs of primers corresponding to zmsO to zmsN were designed to detect the zeamine biosynthsis gene cluster in D. zeae strains, which are presented in Additional file 4. The DNA marker is DL2000
To define whether the phytotoxin(s) produced by strain MS2 are zeamines or derivatives, we detected the zeamine biosynthesis genes from zmsO to zmsN based on their DNA sequences in strain EC1 [43], and results showed that no corresponding bands could be amplified if using the genomic DNA of strain MS2 or MS3 as a template (Fig. 5d). In combination with the sequencing results of MS2 genome (unpublished results), we confirmed that the phytotoxin(s) produced by D. zeae MS2 is probably novel, encoded by biosynthesis genes different from the zms gene cluster [43].
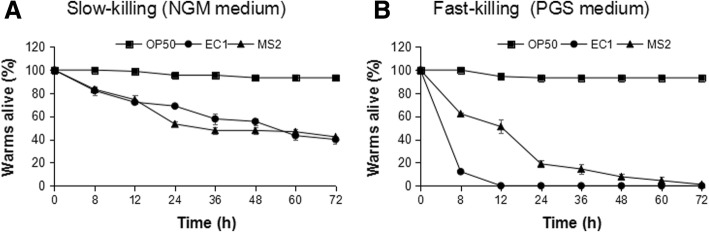
To investigate whether this phytotoxin(s) is toxic to nematodes, like zeamines produced by S. plymuthica A153 [62], we tested the nematode-killing dynamics of the two strains producing toxins. The results showed that both strains EC1 and MS2 had nematocidal activities, with similar slow-killing activity on NGM medium (Fig. 6a), whereas, strain EC1 had faster speed in killing C. elegans on PGS medium than strain MS2 (Fig. 6b). We inferred that the toxins produced by strains MS2 and EC1 may have similar killing effect on nematodes in the NGM medium. In the fast-killing assay, the worms treated with strain EC1 quickly became immobilized, and were completely dead in 12 h after treatment, while worms on strain MS2 lawn seemed more energetic and remained alive for a longer time compared with those on strain EC1 lawn, with only about 51% of worms died at 12 h, and 100% death occurred at 72 h after treatment (Fig. 6b), suggesting that the toxin(s) produced by strain MS2 was less toxic to C. elegans than zeamines produced by strain EC1 in the PGS medium.
Fig. 6.
Worm-killing assay of D. zeae strains towards C. elegans. a Slow-killing; b Fast-killing. Strains EC1 and MS2 were grown in LB medium at 28 °C and the C. elegans food-source E. coli OP50 (negative control) at 37 °C overnight, and 50 μL of the liquid culture was spotted onto the center of NGM (slow-killing) (a) or PGS (fast-killing) (b) agar plates and allowed to dry thoroughly. In the slow-killing assay, 50 μM of floxuridine (FudR, Sigma) was added into NG agar to inhibit hatching of nematode eggs [58]. The plates containing bacteria were incubated at 28 °C and 37 °C respectively overnight and cooled for at least 2 h at room temperature before adding 30 L4 stage or adult hermaphrodite worms. The plates were kept at 20 °C, and live worms were scored
Furthermore, the inhibition activity of Dickeya strains against some important pathogenic microorganisms was measured. Results showed that D. dadantii 3937 had little antimicrobial activity against all the tested microorganisms (Table 2). For the tested pathogenic bacteria, D. zeae EC1 showed the strongest antimicrobial effect with obvious antimicrobial halo zone on the pathogens except Ralstonia solanacearum EP1, while strains isolated from banana had little antibacterial activity except that strain MS2 inhibited the growth of E. coli DH5α (Table 2). The antifungal activity of EC1 was strongest, followed by strains MS2 and MS3, and the antagonistic activity of the D. zeae strains isolated from banana indicated that it is not the new toxin produced by MS2 that has the antifungal activity, since strain MS3 showed a similar antifungal activity as strain MS2 (Table 2).
Table 2.
Inhibition activity of Dickeya strains against some pathogenic microorganisms
| Microorganism | Description | Source or reference | Inhibition activity (mm) | |||
|---|---|---|---|---|---|---|
| EC1 | MS2 | MS3 | 3937 | |||
| Escherichia coli DH5α | Indicator for toxin antagonism | Lab storage | 5.23 ± 0.23 | 7.51 ± 0.34 | 0 | 0 |
| Ralstonia solanacearum EP1 | Pathogen of eggplant bacterial wilt | [68] | 0 | 0 | 0 | 0 |
| Xanthomonas campestris pv. campestris Xc1 | Pathogen of crucifers black rot | [69] | 9.68 ± 0.36 | 0 | 0 | 0 |
| Pseudomonas aeruginosa PAO1 | Pathogen of cystic fibrosis | [70] | 4.04 ± 0.43 | 0 | 0 | 0 |
| Fusarium oxysporum f.sp. cubense FOC4 | Pathogen of banana wilt | [71] | 6.17 ± 0.33 | 3.95 ± 0.39 | 3.32 ± 0.22 | 0 |
| Rhizoctonia solani AG-1 IA | Pathogen of rice sheath blight | [72] | 4.07 ± 0.23 | 1.31 ± 0.09 | 0.70 ± 0.15 | 0 |
| Magnaporthe oryzae B157 | Pathogen of rice blast | [73] | 4.70 ± 0.28 | 1.98 ± 0.34 | 1.50 ± 0.31 | 0 |
| Peronophythora litchi | Pathogen of litchi downy blight | [74] | 11.69 ± 0.34 | 8.71 ± 0.15 | 3.48 ± 0.21 | 3.72 ± 0.22 |
| Colletotrichum capsici | Pathogen of capsicum anthracnose | Lab storage | 5.45 ± 0.14 | 5.26 ± 0.13 | 4.79 ± 0.10 | 0 |
| C. gloeosporioides | Pathogen of mango anthracnose | Lab storage | 4.91 ± 0.10 | 4.06 ± 0.20 | 3.59 ± 0.42 | 0 |
| Sporisorium scitamineum | Pathogen of sugarcane smut | [75] | 2.60 ± 0.06 | 2.27 ± 0.54 | 1.51 ± 0.22 | 0 |
D. zeae JZL strains have stronger cell motility than other strains
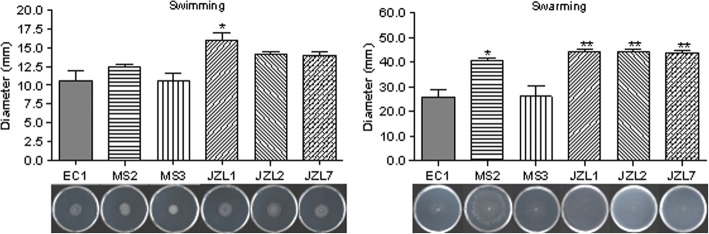
Bacterial motility is of important pathological significance during the early stage of infection. In this study, the mobile capability of D. zeae strains was measured including swimming and swarming motility. Results showed that all the strains were capable of swimming and swarming, but in general, the strains isolated from clivia swarmed and swam faster than the other strains, except that strain JZL7 swarmed similar to strain MS2 (Fig. 7). Given that JZL strains were weaker than the other three strains in production of various virulence factors but showed comparable virulence against Clivia miniata (Fig. 4, Table 1, Additional file 3), we reasoned that the cell motility of JZL strains might contribute substantially in their pathogenicity against the clivia plants, which awaits further investigations.
Fig. 7.
Cell motility of D. zeae strains. One microlitre of bacterial culture (OD600 = 1.5 in LB medium) was spotted onto the centre of a plate containing about 20 mL of semisolid swimming or swarming medium, which was then incubated at 28 °C for 20 h before measurement of the diameters of bacterial motility zone
Discussion
D. zeae is a kind of pectinolytic bacteria causing soft rot disease on plants all over the world. It usually causes great agricultural economic losses in tropical, subtropical and temperate regions, especially in Southeast Asian countries such as Japan, Philippines, Bangladesh, China, India, Indonesia, South Korea, North Korea and Malaysia on maize, rice and banana (Additional file 1) [3, 14, 15, 19, 24, 26]. So far, the host range of D. zeae includes 25 dicotyledonous plants, and 18 monocotyledonous plants including the natural host clivia expanded in this study (Additional file 1).
By comparing the geographical distribution of D. zeae, we found that this species is centralized on monocot hosts in Asia (Additional file 1), similar to the situation that D. solani bacteria were mostly found in European countries and Israel on potato [10, 63]. The centralized distribution of D. zeae is probably due to the growth environment or host specificity related to bacterial evolution and adaptation during plant-pathogen interactions. According to our previous study, we found that the genome sequences of D. zeae rice strains EC1 and ZJU1202, both isolated from Guangdong Province, share 99.962% identity, while strain EC1 is only 95.863% identical to the rice strain DZ2Q isolated from Italy, and interestingly, all these three rice strains harbor an unique zms gene cluster encoding zeamines, which was absent in other sequenced D. zeae strains isolated from other sources [11, 43], suggesting that this zms gene cluster is probably related to their rice host specificity. In this study, we also found that strains MS2 and MS3, both isolated from banana, could not produce zeamines (Fig. 5), but strain MS2 produces another type of antimicrobial compounds with moderate inhibition activities on rice seed germination and nematicidal activity (Figs. 5 and 6), while strain MS3 produces none, in addition, strain MS2 showed higher pectinolytic activity, faster motility, and stronger virulence than strain MS3, indicating the complicated and dynamic process of D. zeae evolution.
We also isolated D. zeae strains from ornamental monocot plant Clivia miniata and validated them as the causal agents of clivia soft rot disease. Although MS2, MS3 and JZL strains are in a same big branch of D. zeae, they fall in different clades of the 16S rDNA tree and the joint phylogenetic tree (Figs. 1 and 2). Given that 16S rDNA gene sequence is insufficient to assign the taxonomy of a bacterium owing to its polyphyletic nature not only in a same family, but also in a same genus [64], we consider that the joint phylogenetic tree based on MLSA analysis is more reliable. Phenotypic characteristics of virulence factors indicated that D. zeae clivia strains JZLs produce remarkably less extracellular degrading enzymes than other strains, especially proteases (Fig. 4), probably responsible for the significantly narrower host range and the extremely less virulence on the tested hosts except clivia (Table 1). In contrast, D. zeae strains JZLs caused a comparable disease severity on clivia with the rice and banana strains (Table 1), which may suggest that these JZL strains have stronger affinity on their natural host plant than non-natural host.
The differences in production of virulence factors and bacterial aggressiveness among strains suggest that D. zeae strains causing soft rot disease are highly diverse whether from different sources or from the same host. Indeed, it has already been reported that D. solani strains from different climatic conditions, despite their genotypic homogeneity, behave very differently under particular environmental conditions, especially the incubation temperature [65]. The diversity in virulence factors and infection ability is not solely specific to certain species, rather, it is strain characteristic [66, 67].
The narrower host range and the lower pathogenicity of D. zeae JZL strains may be attributed to the loss of some important pathogenic islands during the evolution along with host clivia. Actually, in a long period of evolution and interaction with host plants, gene deletions and additional acquisition frequently occur, for instance, the zms gene cluster in D. zeae rice strains associated with the rice host specificity [11, 43, 60], and probably the genes encoding the antimicrobial compound(s) in strain MS2. Aggressiveness of D. zeae strains showed that EC1, MS2 and MS3 strains grew faster in potato and clivia than in cabbage and banana (Fig. 3), probably because the former two plants could provide more carbon sources for the bacterial growth. And the cell numbers of all the strains in banana tissues seemed comparable (Fig. 3c), while the disease degree caused by JZL strains was obviously lower than that caused by the other strains (Table 1, Additional file 3). We considered that the decreased virulence of JZL strains on monocotyledonous hosts is unrelated to the bacterial growth speed, which was also verified in Fig. 4a, but due to the reduced production of CWDEs (Fig. 4b).
Moreover, the antimicrobial compound(s) produced by strain MS2 is one of the major virulence factors inhibiting rice seed germination and killing nematodes. Genes encoding this new toxin(s) are being identified and the effects on rice seeds are being investigated. Whole genome comparison of these Dickeya zeae strains will be helpful for identification of the host-specific genes among D. zeae strains and revealing the interactions between pathogens and hosts.
Conclusions
In this study, we identified the causal agents respectively from soft rot banana and clivia plants as Dickeya zeae. To our knowledge, this is the first time reporting clivia as the natural host of D. zeae. After comparing the virulence factors produced by different D. zeae strains isolated from rice, banana and clivia, we concluded that at least two types of D. zeae strains cause soft rot disease of banana, one of which produces a novel phytotoxin different from zeamines, while the other produces none, and the clivia isolates only infect monocots because of their weak aggressiveness on dicots. The virulence differentials of strains may provide targets for controlling bacterial soft rot diseases caused by Dickeya.
Methods
D. zeae strains collection, isolation and identification
Bacterial strains used in this study were listed in Additional file 4. D. zeae strain EC1 was isolated from diseased rice stem foot [11]. Strains MS2 and MS3 were isolated from the basal pseudostems of banana plants with soft rot disease respectively collected from Nansha and Panyu Districts in Guangzhou, Guangdong Province in 2012. D. zeae JZL strains were isolated from Clivia miniata (belonging to Amaryllidaceae) plants with soft rot symptoms at Fangcun flower market in Guangzhou city, Guangdong Province, China in 2017. D. dadantii 3937 isolated from African violets was also used for testing the virulence factors as a control.
MS2, MS3 and JZL strains that fulfilled Koch’s postulates were identified by sequencing of 16S rRNA gene using 27F/1492R primers [46], and atpD, gyrB, infB and rpoB genes using primers and PCR conditions listed by Brady et al. [47]. DNA fragments were amplified using EasyTaq DNA polymerase (Transgen) and cloned into pUC19-T vector for sequencing. The sizes of the resultant amplicons were as follows: 16S rRNA, 1507 bp; atpD, 884 bp; gyrB, 974 bp; infB, 1124 bp; rpoB, 1090 bp.
Multilocus sequences analysis (MLSA) and phylogenetic analysis
Sequence similarities of all genes were determined by using BLASTn program, and all the sequences of related strains were obtained from GenBank database. Consensus sequences were aligned with ClustalW and trimmed in the following sizes: 16S rRNA, 654 bp; atpD, 642 bp; gyrB, 745 bp; infB, 1042 bp; rpoB, 1000 bp. All the sequences of a same strain except 16S rRNA were assembled for constructing a joint phylogenetic tree. Neighbor-joining trees were constructed by bootstrap analysis with 1000 replicates using MEGA5 package [48]. The GenBank accession numbers of 16S rRNA, atpD, gyrB, infB and rpoB gene sequences of strain MS2 are MF973080, MG018807, MG018810, MG018813 and MG018816, respectively, those of strain MS3 are MF973081, MG018808, MG018811, MG018814 and MG018817, respectively, and those of strain JZL are MF973082, MG018809, MG018812, MG018815 and MG018818, respectively.
Pathogenicity assay against monocotyledonous and dicotyledonous plants
The tested strains were grown in LB medium till OD600 = 1.5, and different plant organs of monocotyledons and dicotyledons listed in Table 1 were selected using different inoculation methods. For rice (Oryza sativa), banana (Musa sapientum ABB), Gladiolus gandavensis and Alocasia macrorrhiza, every 200 μL of bacterial cultures was injected into the basal stems of the seedlings, and into the bases of clivia leaves. For radish (Raphanus sativus), carrot (Daucus carota), potato (Solanum tuberosm), Zingiber officinale, and Colocasia esculenta, tubers were washed with tap water and dried with a paper towel, subsequently, surface-sterilized with 70% ethanol and then sliced evenly about 5 mm in thickness. Each slice was placed in a tray with moistened filter paper. Other plant materials to be inoculated were surface-sterilized. Bacterial cells of 2 μL were applied to the inoculated parts after piercing them with pipette tips except eggplant (Solanum melongena) and tomoto (Lycopersicon esculentum) inoculated with 100 μL of bacterial cultures. All trays were kept at 28 °C till symptoms appeared. Same volume of LB medium was inoculated as a negative control. Each assay was repeated three times with triplicates. The area of lesions were measured using Image J software.
Tests on aggressiveness of D. zeae strains
The tested strains were grown in LB medium till OD600 = 2.0. Healthy potato tubers, cabbage petiole, banana pseudostems and clivia leaves were selected, washed with tap water, surface-sterilized with 70% ethanol, and placed onto the moistened filter paper in plates in biosafety cabinet under UV sterilization for 15 min. The plant tissues were pierced and inoculated with 2 μL of bacterial cultures. Plates were incubated at 28 °C. Same volume of LB medium was inoculated as a negative control. Tissues were taken out after 12 h and 24 h of incubation, respectively, where the diseased and surrounding healthy tissues in equal weight were cut and ground, then added with 10 mL of sterilized 0.85% NaCl solution, stirred evenly, and 1 mL of which was taken and diluted into 103, 104, and 105 folds, and then 100 μL in each dilution gradient was spread evenly onto LB agar plates in triplicates. Plates were kept at 28 °C for 24 h, and colonies between 30 to 300 CFU (Colony-Forming Unit) were counted. Each assay was repeated three times with duplicates.
Measurement of bacterial growth curves
Bacterial strains to be tested were grown in LB medium overnight at 28 °C. All the bacterial cultures were adjusted to OD600 = 2.0 and diluted into fresh LB medium in 1:100 ratio. Dilutions were mixed thoroughly and aliquots of 500 μL were transferred into 2.0 mL tubes. Bacteria were grown with shaking at 200 r/min under 28 °C and cell density was measured at 0, 4, 8, 12, 16, 20, 24, 28 and 40 h respectively. The experiment was repeated three times in triplicate.
Measurement of cell wall degrading enzymatic (CWDE) activities
The activities of cell wall degrading enzymes were measured using the medium recipe described previously [49–51]. Briefly, assay medium was prepared, and 30 mL of each medium was poured into a 12 × 12 cm square plate. Subsequently, wells (4 mm in diameter) were punched after solidification. Samples of 40 μL bacterial cells were added into the wells after they had grown to OD600 = 1.8. All plates were incubated at 28 °C. Pectate lyase (Pel) and polygalacturonase (Peh) assay plates were covered with 4 N HCl after 11 h and 14 h respectively. Cellulase (Cel) assay plate was stained with 0.1% (w/v) Congo Red for 15 min after 14 h, and treated with 1 M NaCl twice. Protease (Prt) activity was indicated by the transparent halos surrounding the wells after 24 h incubation. The experiment was repeated three times with duplicates.
Assay of extracellular polysaccharide (EPS) production
Extracellular polysaccharides (EPS) are one of the important virulence factors for bacterial phytopathogens and the main toxic factors leading to water-logging and wilting on plants after infection [52–54]. For measuring the production of EPS, single colonies of the tested strains were picked and transferred into 10 mL LB medium for culture overnight at 28 °C until OD600 = 1.8, afterwards, 3 mL of which was applied into 300 mL LB medium, and grown with shaking at 200 r/m for 12 h. Cultures were centrifuged at 8000 rpm for 40 m, and then at 4000 rpm for 20 m to obtain 250 mL supernatants. Double volumes of absolute ethanol were added to the supernatants, mixed thoroughly, stored at 4 °C overnight for precipitation, and subjected to centrifugation at 8000 rpm for 40 m. Finally, supernatants were discarded and pellets were weighed after drying at 55 °C overnight. The experiment was repeated three times in triplicate.
Toxin bioassay
The tested strains were streaked on LB plates and single colonies were transferred into LS5 medium [55] to grow at 28 °C overnight. Cell density was adjusted to OD600 = 1.5 prior to bioassay. The bioassay plate was prepared as previous described [44]. Wells of 4 mm in diameter were punched in the plate and 20 μL of the bacterial cultures were added into the wells. The plate was incubated overnight at 37 °C.
To preliminarily determine the chemical property of the toxin, supernatant of strain MS2 overnight culture was collected, and treated by boiling at 100 °C for 10 min and by digestion with protease K at 37 °C for 30 min, respectively. Finally, the inhibition activity against the growth of E. coli DH5α was measured. The experiment was repeated three times in triplicate.
Inhibition of phytotoxins against rice seed germination
Bacteria were grown in LB medium till OD600 = 1.5, and 20 seeds of rice variety CO39 were added to 5 ml of every bacterial culture and kept at room temperature for 5 h, then taken out to clean in sterilized water and transferred onto the moistened filter paper in a Petri dish, which was then kept at 28 °C under 16 h light and 8 h dark conditions. Rice seeds incubated with same amount of D. dadantii 3937 were used as a control. The experiment was repeated three times in triplicate.
Detection of zeamine biosynthesis gene cluster
Based on the coding sequences of zms gene cluster in strain EC1 [43], we designed 18 pairs of primers (Additional file 5) corresponding to zmsO to zmsN to detect the zeamine biosynthsis gene cluster in D. zeae strains, which are presented in Additional file 4. DNA fragments were amplified using EasyTaq DNA polymerase (Transgen) using conditions as following: 95 °C, 2 min; 30 cycles of 94 °C, 30 s, 55 °C, 30 s and 72 °C, 1 min; 72 °C, 5 min.
Antimicrobial activity assay
Pathogenic microorganisms used in this study were listed in Table 2, in which, bacterial pathogens were grown in LB medium overnight and fungi were grown on PDA medium at 28 °C until colonies covered the Petri dishes. For antibacterial activity, the same method of toxin bioassay against E. coli described in previous section was used against other bacterial pathogens, and the diameters of the antibacterial halos were measured. For antifungal activity, fungal dishes in diameter of 4 mm were punched and placed onto the center of PDA plate, and 2 μL of EC1, MS2, MS3 and 3937 strain overnight cultures were respectively spotted onto each of the four sides of the fungal dishes (3 cm away from the edge of the fungal dishes). Plates were incubated at 28 °C until fungal colonies on blank plates covered the petri dishes, and the distance between bacterial colonies and the hyphal edge of the tested fungi was measured. The experiment was repeated three times.
Nematode killing activity
Wild type Caenorhabditis elegans were maintained according to the methods as previously described [56], and the medium recipe for worm-killing assays was referred to in literature reported by Tan et al. [57]. D. zeae strains EC1 and MS2 were routinely grown in LB medium at 28 °C and the C. elegans food-source E. coli OP50 at 37 °C overnight, and 50 μL of the liquid culture was spotted onto the center of PGS (fast-killing) or NGM (slow-killing) agar plates and allowed to dry thoroughly. In the slow-killing assay, 50 μM of floxuridine (FudR, Sigma) was added into NG agar to inhibit hatching of nematode eggs [58]. E. coil OP50 culture was used as a negative control. The plates containing bacteria were incubated at 28 °C and 37 °C respectively overnight and cooled for at least 2 h at room temperature before adding 30 L4 stage or adult hermaphrodite worms. The plates were kept at 20 °C, and live worms were scored. Each trial was repeated three times in triplicate.
Measurement of cell motility
To determine the cell motility, media for swimming (per litre contains 10 g bactotryptone, 5 g NaCl and 3 g agar) and swarming (per litre contains 5 g peptone, 3 g yeast extract and 4 g agar) assay were prepared. One microlitre of overnight bacterial culture (OD600 = 1.5) was spotted onto the centre of a plate containing about 20 mL of .each medium. The plates were incubated at 28 °C for 20 h before measurement of the diameters of bacterial motility zone. Each experiment was repeated at least three times in triplicate [44].
Statistic analysis
All the experiments were repeated in three times with duplicates or triplicates. For statistic analysis, GraphPad Prism 5.0 software was used to performed Student’s t-test, and the data of D. zeae strains were normalized to those of strain EC1. * indicates P < 0.05, ** indicates P < 0.001, and *** indicates P < 0.0001.
Additional files
The hosts and origins of D. zeae strains. (DOC 92 kb)
Natural hosts and distribution of D. zeae strains in southeast Asia. The map was drawn using Photoshop CS6 software and host plant icons were added on the corresponding locations of the map. (TIF 2891 kb)
The diseased symptoms of the tested strains on dicotyledonous and monocotyledonous hosts corresponding to Table 1. (PDF 6199 kb)
Bacterial strains used in this study. (DOC 36 kb)
Primers used in this study for detection of zeamines biosynthesis genes. (DOC 54 kb)
Acknowledgments
We are very grateful to Dr. Xiaoming Pu in Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences for his great help in the field investigation of banana soft rot disease.
Funding
This work was supported by a grant from the National Key Project for Basic Research of China (973 Program) (No. 2015CB150600) and by grants from the Science and Technology Planning Project of Guangdong Province, China (No. 2017A010105009), and Guangdong Province “Innovation and University Development” Project (No. 2016KZDXM026). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing this manuscript.
Availability of data and materials
The data sets supporting the results of this article are included within the article and its Additional files. In addition, specimens were collected and taken according to the guidelines of the Chinese “Biosafty Management Regulations for Pathogenic Microbiological Laboratory”.
Abbreviations
- AHL
Acyl homoserine lactone
- Cel
Cellulases
- CWDE
Cell wall degrading enzyme
- EPS
Extracellular polysaccharides
- MLSA
Multilocus sequences analysis
- Peh
Polygalacturonases
- Pel
Pectate lyases
- Prt
Proteases
- QS
Quorum sensing
Authors’ contributions
JZ conceived the study, drafted and coordinated the manuscript, MH, RC, WL, LF, LS, YX, JL and XF performed the experiments, MH and JZ analyzed the data, LZ and JZ revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Hu, Email: 845218367@qq.com.
Jieling Li, Email: 1397371751@qq.com.
Ruiting Chen, Email: 992866506@qq.com.
Wenjun Li, Email: 526374826@qq.com.
Luwen Feng, Email: 402893564@qq.com.
Lei Shi, Email: 790077381@qq.com.
Yang Xue, Email: 344213009@qq.com.
Xiaoyin Feng, Email: 1126400806@qq.com.
Lianhui Zhang, Email: lhzhang01@scau.edu.cn.
Jianuan Zhou, Email: jianuanzhou@scau.edu.cn.
References
- 1.Liu G. List of Plant Quarantine Pests in the People’s Republic of China. Pestic Mark Inf. 2007;13:40–41. [Google Scholar]
- 2.Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samson R, Legendre JB, Christen R, Fischer-Le Saux M, Achouak W, Gardan L. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int J Syst Evol Microbiol. 2005;55(4):1415–1427. doi: 10.1099/ijs.0.02791-0. [DOI] [PubMed] [Google Scholar]
- 4.Brady C, Cleenwerck I, Denman S, Venter SN, Rodriguez-Palenzuela P, Coutinho TA, Devos P. Proposal to reclassify Brenneria quercina (Hildebrand and Schroth 1967) Hauben et al. 1999 into a new genus, Lonsdalea gen. nov., as Lonsdalea quercina comb. nov., descriptions of Lonsdalea quercina subsp. quercina comb. nov., Lonsdalea quercina subsp. iberica subsp. nov. and Lonsdalea quercina subsp. britannica subsp. nov., emendation of the description of the genus Brenneria, reclassification of Dickeya dieffenbachiae as Dickeya dadantii subsp. dieffenbachiae comb. nov., and emendation of the description of Dickeya dadantii. Int J Syst Evol Microbiol. 2012;62(7):1592–1602. doi: 10.1099/ijs.0.035055-0. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson N, Devos P, Pirhonen M, Elphinstone J. Dickeya aquatica sp nov., isolated from waterways. Int J Syst Evol Microbiol. 2014;64(7):2264–2266. doi: 10.1099/ijs.0.058693-0. [DOI] [PubMed] [Google Scholar]
- 6.Tian Y, Zhao Y, Yuan X, Yi J, Fan J, Xu Z, Hu B, De Boer SH, Li X. Dickeya fangzhongdai sp. nov., a plant-pathogenic bacterium isolated from pear trees (Pyrus pyrifolia) Int J Syst Evol Microbiol. 2016;66(8):2831–2835. doi: 10.1099/ijsem.0.001060. [DOI] [PubMed] [Google Scholar]
- 7.Hussain MB, Zhang HB, Xu JL, Liu Q, Jiang Z, Zhang LH. The acyl-homoserine lactone-type quorum-sensing system modulates cell motility and virulence of Erwinia chrysanthemi pv. zeae. J Bacteriol. 2008;190(3):1045–1053. doi: 10.1128/JB.01472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sławiak M, van Beckhoven JRCM, Speksnijder AGCL, Czajkowski R, Grabe G, van der Wolf JM. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur J Plant Pathol. 2009;125:245–261. doi: 10.1007/s10658-009-9479-2. [DOI] [Google Scholar]
- 9.Lin BR, Shen HF, Pu XM, Tian XS, Zhao WJ, Zhu SF, Dong MM. First report of a soft rot of banana in Mainland China caused by a Dickeya sp. (Pectobacterium chrysanthemi) Plant Dis. 2010;94:640. doi: 10.1094/PDIS-94-5-0640C. [DOI] [PubMed] [Google Scholar]
- 10.Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, Tsror L, Elphinstone JG. Dickeya species: an emerging problem for potato production in Europe. Plant Pathol. 2011;60:385–399. doi: 10.1111/j.1365-3059.2011.02427.x. [DOI] [Google Scholar]
- 11.Zhou JN, Zhang HB, Wu J, Liu QG, Xi PG, Lee J, Liao JL, Jiang ZD, Zhang LH. A novel multi-domain polyketide synthase is essential for zeamine antibiotics production and the virulence of Dickeya zeae. Mol Plant-Microbe Interact. 2011;24(10):1156–1164. doi: 10.1094/MPMI-04-11-0087. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JX, Shen HF, Pu XM, Lin BR. Identification of Dickeya zeae as a casual agent of bacterial soft rot in banana in China. Plant Dis. 2014;98(4):436–442. doi: 10.1094/PDIS-07-13-0711-RE. [DOI] [PubMed] [Google Scholar]
- 13.Sinha SK, Prasad M. Bacterial stalk rot of maize, its symptoms and host-range. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1977;132:81–88. doi: 10.1016/s0044-4057(77)80037-3. [DOI] [PubMed] [Google Scholar]
- 14.Goto M. Bacterial Foot Rot of Rice Caused by a Strain of Erwinia-Chrysanthemi. Phytopathology. 1979;69(3):213–216. doi: 10.1094/Phyto-69-213. [DOI] [Google Scholar]
- 15.Liu QG, Wang ZZ. Infection characteristics of Erwinia chrysanthemi pv. zeae on rice. J S China Agric Univ. 2004;25:55–57. [Google Scholar]
- 16.Jafra S, Przysowa J, Gwizdek-Wiśniewska A, van der Wolf JM. Potential of bulb-associated bacteria for biocontrol of hyacinth soft rot caused by Dickeya zeae. J Appl Microbiol. 2008;106:268–277. doi: 10.1111/j.1365-2672.2008.04000.x. [DOI] [PubMed] [Google Scholar]
- 17.Stead DE, Parkinson N, Bew J, Hennessy J, Wilson JK, Elphinstone JE. The first record of Dickeya zeae in the UK. Plant Pathol. 2010;59:401. doi: 10.1111/j.1365-3059.2009.02113.x. [DOI] [Google Scholar]
- 18.Myung IS, Jeong IH, Moon SY, Kim WG, Lee SW, Lee YH, Lee YK, Shim HS, Ra DS. First report of bacterial stalk rot of sweet corn caused by Dickeya zeae in Korea. New Dis Rep. 2010;22:236–250. [Google Scholar]
- 19.Li B, Shi Y, Ibrahim M, Liu H, Shan C, Wang Y, Kube M, Xie GL, Sun G. Genome sequence of the rice pathogen Dickeya zeae strain ZJU1202. J Bacteriol. 2012;194(16):4452–4453. doi: 10.1128/JB.00819-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertani I, Passos da Silva D, Abbruscato P, Piffanelli P, Venturi V. Draft genome sequence of the plant pathogen Dickeya zeae DZ2Q, isolated from rice in Italy. Genome Announc. 2013;1(6):e00905–e00913. doi: 10.1128/genomeA.00905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrero G, Schneider KL, Jenkins DM, Alvarez AM. Phylogeny and classification of Dickeya based on multilocus sequence analysis. Int J Syst Evol Microbiol. 2013;63:3524–3539. doi: 10.1099/ijs.0.046490-0. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard L, Saddler GS, Parkinson NM, Bertrand V, Elphinstone JG. Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathol. 2012;62:587–596. doi: 10.1111/j.1365-3059.2012.02678.x. [DOI] [Google Scholar]
- 23.Pritchard L, Humphris S, Saddler GS, Elphinstone JG, Pirhonen M, Toth IK. Draft genome sequences of 17 isolates of the plant pathogenic bacterium dickeya. Genome Announc. 2013;1(6):e00978–e00913. doi: 10.1128/genomeA.00978-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JX, Lin BR, Shen HF, Pu XM. Genome sequnence of the banana pathogen Dickeya zeae strain MS1, which causes bacteria soft rot. Genome Announc. 2013;1(3):e00317–e00313. doi: 10.1128/genomeA.00317-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Cisneros BA, Juarez-Lopez G, Valencia-Torres N, Duran-Peralta E, Mezzalama M. First report of bacterial stalk rot of maize caused by Dickeya zeae in Mexico. Plant Dis. 2014;98(9):1267. doi: 10.1094/PDIS-02-14-0198-PDN. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran K, Manaf UA, Zakaria L. Molecular characterization and pathogenicity of Erwinia spp. associated with pineapple [Ananas comosus (L.) Merr.] and papaya (Carica papaya L.) J Plant Protection Res. 2015;55(4):396–404. doi: 10.1515/jppr-2015-0053. [DOI] [Google Scholar]
- 27.Kumar A, Hunjan MS, Kaur H, Dhillon HK, Singh PP. Biochemical responses associated with resistance to bacterial stalk rot caused by Dickeya zeae in maize. J Phytopathol. 2017;165:822–832. doi: 10.1111/jph.12622. [DOI] [Google Scholar]
- 28.Reifschneider FJB, Lopes CA. Bacterial top and stalk rot of maize Zea mays in brazi. Plant Dis. 1982;66(6):519–520. doi: 10.1094/PD-66-519. [DOI] [Google Scholar]
- 29.Masumi M, Izadpanah K. Occurrence of bacterial stalk rot of maize in Fars Province. Iranian J Plant Pathol. 1988;24(1–4):29–30. [Google Scholar]
- 30.Takeuchi T, Kodama F. Bacterial stalk rot of corn caused by Erwinia chrysanthemi pv. zeae (Sabet) Victoria, Arboleda et Muñoz occurred in Hokkaido, Japan. 1992. pp. 42–44. [Google Scholar]
- 31.Zheng YN. Occurrence and control of bacterial stalk rot of maize. J Anhui Agric Sci. 2006;34(10):2128–2127. [Google Scholar]
- 32.El-Helaly AF, Abo-El-Dahab MK, El-Goorani MA, Gabr MR. Identification of Erwinia sp., causing stalk rot of maize in Egypt. Zentralbl Bakteriol Naturwiss. 1978;133(7–8):680–685. doi: 10.1016/s0323-6056(78)80073-1. [DOI] [PubMed] [Google Scholar]
- 33.Wei G, Huang YL, Huang XS. Infection way and hosts of rice foot rot bacteria. Guangxi Agric Sci. 1986;6:32–34. [Google Scholar]
- 34.Liu QG, Zhang Q, Wei CD. Advances in Research of Rice Bacterial Foot Rot. Sci Agric Sin. 2013;46(14):2923–2931. [Google Scholar]
- 35.Zhou Y, Zhai YC, Cao BH. Rice bacterial foot rot seriously happened in Rudong County, Jiangsu Province. Plant Prot. 1989;6:51. [Google Scholar]
- 36.Yang MH. Serious rice bacterial foot rot occurred in Taining County of Fujian Province in 1999. Plant Prot Technol Ext. 2000;20(2):41. [Google Scholar]
- 37.Li CY. Occurrence and control measures of rice bacterial foot rot in Anshun. Plant Doct. 2007;20(4):8. [Google Scholar]
- 38.Xue NQ, Liu Y. Occurrence and control of rice bacterial foot rot. Shandong Agric Sci. 2008;3:102–103. [Google Scholar]
- 39.Collmer A, Bauer DW. Erwinia chrysanthemi and Pesudominas syringae: plant pathogens trafficking in extracellular virulence proteins. Curr Top Microbiol Immunol. 1994;192:43–78. doi: 10.1007/978-3-642-78624-2_3. [DOI] [PubMed] [Google Scholar]
- 40.Reverchon S, Rouanet C, Expert D, Nasser W. Charaterization of indigoidine biosynthetic genes in Erwinia chrysanthemi. Mol Microbiol. 2002;29:1407–1418. doi: 10.1046/j.1365-2958.1998.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franza T, Mahé B, Expert D. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Mol Microbiol. 2005;55:261–275. doi: 10.1111/j.1365-2958.2004.04383.x. [DOI] [PubMed] [Google Scholar]
- 42.Yap MN, Yang CH, Barak JD, Jahn CE, Charkowski AO. The Erwinia chrysanthemi type III secretion system is required for multicellular behavior. J Bacteriol. 2005;187:639–648. doi: 10.1128/JB.187.2.639-648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou JN, Cheng YY, Lv MF, Liao LS, Chen YF, Gu YF, Liu SY, Jiang ZD, Xiong YY, Zhang LH. The complete genome sequence of Dickeya zeae EC1 reveals substantial divergence from other Dickeya strains and species. BMC Genomics. 2015;16:571. doi: 10.1186/s12864-015-1545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou JN, Zhang HB, Lv MF, Chen YF, Liao LS, Cheng YY, Liu SY, Chen SH, He F, Cui ZN, Jiang ZD, Chang CQ, Zhang LH. SlyA regulates phytotoxin production and virulence in Dickeya zeae EC1. Mol Plant Pathol. 2016;17(9):1398–1408. doi: 10.1111/mpp.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv MF, Chen YF, Liao LS, Liang ZB, Shi ZR, Tang YX, Ye SX, Zhou JN, Zhang LH. Fis is a global regulator critical for modulation of virulence factor production and pathogenicity of Dickeya zeae. Sci Rep. 2018;8(1):341. doi: 10.1038/s41598-017-18578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coenye T, Falsent E, Vananneyt M, Hostef B, Govant JRW, Kersters K, Vandamme P. Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int. J Syst Bacteriol. 1999;49:405–413. doi: 10.1099/00207713-49-2-405. [DOI] [PubMed] [Google Scholar]
- 47.Brady C, Cleenwerck I, Venter SN, Vancanneyt M, Swings J, Coutinho TA. Phylogeny and identification of Pantoea species associated with plants, humans and the natural environment based on multilocus sequence analysis (MLSA) Syst Appl Microbiol. 2008;31:447–460. doi: 10.1016/j.syapm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Tamura K, Peterson D, Peterson N, Steche G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barras F, Thurn KK, Chatterjee AK. Resolution of four pectate lyase structural genes of Erwinia chrysanthemi (EC16) and characterization of the enzymes produced in Escherichia coli. Mol Gen Genet. 1987;209:319–325. doi: 10.1007/BF00329660. [DOI] [PubMed] [Google Scholar]
- 50.Scott-Craig JS, Panaccione DG, Cervone F, Walton JD. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell. 1990;2:1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee A, Cui Y, Liu Y, Dumenyo CK, Chatterjee AK. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-L-homoserine lactone. Appl Environ Microbiol. 1995;61(5):1959–1967. doi: 10.1128/aem.61.5.1959-1967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayward AC. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol. 1991;29:65–108. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- 53.Kao CC, Barlow E, Sequeira L. Extracellular polysaccharide is required of wild-type virulence of Pseudomonas solanacearum. J Bacteriol. 1992;174(3):1068–1071. doi: 10.1128/jb.174.3.1068-1071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Condemine G, Castillo A, Passeri F, Enard C. The PecT repressor coregulates synthesis of exopolysaccharides and virulence factors in Erwinia chrysanthemi. Mol Plant-Microbe Interact. 1992;12(1):45–52. doi: 10.1094/MPMI.1999.12.1.45. [DOI] [PubMed] [Google Scholar]
- 55.Liao LS, Cheng YY, Liu SY, Zhou JN, An SW, Lv MF, Chen YF, Gu YF, Chen SH, Zhang LH. Production of novel antibiotics zeamines through optimizing Dickeya zeae fermentation conditions. PLoS One. 2014;9(12):e116047. doi: 10.1371/journal.pone.0116047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stiernagle T. Maintenance of C. elegans. In: Fay D, editor. C. elegans: a practical approach. Oxford: Oxford University Press; 1999. pp. 1–11. [Google Scholar]
- 57.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Pro Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houthoofd K, Braeckman BP, Vanfleteren JR. The hunt for the record life span in Caenorhabditis elegans. J Gerontol A-Biol Sci Med Sci. 2004;59:408–410. doi: 10.1093/gerona/59.5.B408. [DOI] [PubMed] [Google Scholar]
- 59.Chen YF, Lv MF, Liao LS, Gu YF, Liang ZB, Shi ZR, Liu SY, Zhou JN, Zhang LH. Genetic modulation of c-di-GMP turnover affects multiple virulence traits and bacterial virulence in rice pathogen Dickeya zeae. PLoS One. 2016;11(11):e0165979. doi: 10.1371/journal.pone.0165979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng YY, Liu XL, An SW, Chang CQ, Zou YQ, Huang LH, Zhong J, Liu QG, Jiang ZD, Zhou JN, Zhang LH. A nonribosomal peptide synthase containing a stand-Alone condensation domain is essential for phytotoxin zeamine biosynthesis. Mol Plant-Microbe Interact. 2013;26:1294–1301. doi: 10.1094/MPMI-04-13-0098-R. [DOI] [PubMed] [Google Scholar]
- 61.Masschelein J, Mattheus W, Gao LJ, Moons P, Van Houdt R, Uytterhoeven B, Lamberigts C, Lescrinier E, Rozenski J, Herdewijn P, Aertsen A, Michiels C, Lavigne R. A PKS/NRPS/FAS hybrid gene cluster from Serratia plymuthica RVH1 encoding the biosynthesis of three broad spectrum, zeamine-related antibiotics. PLoS One. 2013;8(1):e54143. doi: 10.1371/journal.pone.0054143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hellberg JEEU, Matilla MA, Salmond GPC. The broad-spectrum antibiotic, zeamine, kills the nematode worm Caenorhabditis elegans. Front Microbiol. 2015;6:137. doi: 10.3389/fmicb.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Wolf JM, Nijhuis EH, Kowalewska MJ, Saddler GS, Parkinson N, Elphinstone JG, Pritchard L, Toth IK, Lojkowska E, Potrykus M, Waleron M, de Vos P, Cleen-Werck I, Pirhonen M, Garlant L, Helias V, Pothier JF, Pflüger V, Duffy B, Tsror L, Manulis S. Dickeya solani sp. nov., a pectinolytic plant pathogenic bacterium isolated from potato (Solanum tuberosum) Int J Syst Evol Microbiol. 2014;64:768–774. doi: 10.1099/ijs.0.052944-0. [DOI] [PubMed] [Google Scholar]
- 64.Liu SY, Tang YX, Wang DC, Lin NQ, Zhou JN. Identification and characterization of a new Enterobacter onion bulb decay caused by Lelliottia amnigena in China. App Micro Open Access. 2016;2:114. [Google Scholar]
- 65.Golanowska M, Kielar J, Łojkowska E. The effect of temperature on the phenotypic features and the maceration ability of Dickeya solani strains isolated in Finland, Israel and Poland. Eur J Plant Pathol. 2017;147(4):803–817. doi: 10.1007/s10658-016-1044-1. [DOI] [Google Scholar]
- 66.Potrykus M, Golanowska M, Hugouvieux-Cotte-Pattat N, Lojkowska E. Regulators involved in Dickeya solani virulence, genetic conservation, and functional variability. Mol Plant-Microbe Interact. 2014;27:700–711. doi: 10.1094/MPMI-09-13-0270-R. [DOI] [PubMed] [Google Scholar]
- 67.Alič Š, Naglič T, Tušek-Žnidarič M, Peterka M, Ravnika M, Dreo T. Putative new species of the genus Dickeya as major soft rot pathogens in Phalaenopsis orchid production. Plant Pathol. 2017;66(8):1357–1368. doi: 10.1111/ppa.12677. [DOI] [Google Scholar]
- 68.Li P, Wang DC, Yan JL, Zhou JN, Deng YY, Jiang ZD, Cao BH, He ZF, Zhang LH. Genomic analysis of phylotype I strain EP1 of Ralstonia solanacearum species complex reveals substantial divergence from other Ralstonia solanacearum strains. Front Microbiol. 2016;7:719. doi: 10.3389/fmicb.2016.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng YY, Wu J, Yin WF, Li P, Zhou JN, Chen SH, He F, Cai J. Diffusible signal factor family signals provide a fitness advantage to Xanthomonas campestris pv. campestris in interspecies competition. Environ Microbiol. 2016;18(5):1534–1545. doi: 10.1111/1462-2920.13244. [DOI] [PubMed] [Google Scholar]
- 70.Zhou L, Wang J, Zhang LH. Modulation of bacterial Type III secretion system by a spermidine transporter dependent signaling pathway. PLoS One. 2007;2(12):e1291. doi: 10.1371/journal.pone.0001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li MH, Xie XL, Lin XF, Shi JX, Ding Z, Ling JF, Xi PG, Zhou JN, Leng YQ, Zhong SB, Jiang ZD. Functional characterization of the gene FoOCH1 encoding a putative 4 a-1, 6-mannosyltransferase in Fusarium oxysporum f. sp. cubense. Fungal Genet Biol. 2014;65(4):1–13. doi: 10.1016/j.fgb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Shu CW, Zou CJ, Chen JL, Tang F, Yi RH, Zhou EX. Genetic diversity and population structure of Rhizoctonia solani AG-1 IA, the causal agent of rice sheath blight, in South China. Can J Plant Pathol. 2014;36(2):179–186. doi: 10.1080/07060661.2014.913685. [DOI] [Google Scholar]
- 73.Zhang SL, Liang ML, Naqvi NI, Lin CX, Qian WQ, Zhang LH, Deng YZ. Phototrophy and starvation-based induction of autophagy upon removal of Gcn5-catalyzed acetylation of Atg7 in Magnaporthe oryzae. Autophagy. 2017;13:1318–1330. doi: 10.1080/15548627.2017.1327103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liao LS, Zhou JN, Wang HS, He F, Liu SY, Jiang ZD, Chen SH, Zhang LH. Control of litchi downy blight by zeamines produced by Dickeya zeae. Sci Rep. 2015;5(1):1519. doi: 10.1038/srep15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu SY, Lin NQ, Chen YM, Liang ZB, Liao LS, Lv MF, Chen YF, Tang YX, He F, Chen SH, Zhou JN, Zhang LH. Biocontrol of sugarcane smut disease by interference of fungal sexual mating and hyphal growth using a bacterial isolate. Front Microbiol. 2017;8:778. doi: 10.3389/fmicb.2017.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The hosts and origins of D. zeae strains. (DOC 92 kb)
Natural hosts and distribution of D. zeae strains in southeast Asia. The map was drawn using Photoshop CS6 software and host plant icons were added on the corresponding locations of the map. (TIF 2891 kb)
The diseased symptoms of the tested strains on dicotyledonous and monocotyledonous hosts corresponding to Table 1. (PDF 6199 kb)
Bacterial strains used in this study. (DOC 36 kb)
Primers used in this study for detection of zeamines biosynthesis genes. (DOC 54 kb)
Data Availability Statement
The data sets supporting the results of this article are included within the article and its Additional files. In addition, specimens were collected and taken according to the guidelines of the Chinese “Biosafty Management Regulations for Pathogenic Microbiological Laboratory”.