Abstract
Background
It is unclear whether variations in thyroid status within or near the reference range affect energy expenditure, body mass, or body composition.
Methods
138 subjects treated with levothyroxine (LT4) for hypothyroidism with normal TSH levels underwent measurement of total, resting, and physical activity energy expenditure; thermic effect of food; substrate oxidation; dietary intake; and body composition. They were assigned to receive an unchanged, higher, or lower LT4 dose in randomized, double-blind fashion, targeting one of three TSH ranges (0.34 to 2.50, 2.51 to 5.60, or 5.61 to 12.0 mU/L). The doses were adjusted every 6 weeks to achieve target TSH levels. Baseline measures were reassessed at 6 months.
Results
At study end, the mean LT4 doses and TSH levels were 1.50 ± 0.07, 1.32 ± 0.07, and 0.78 ± 0.08 µg/kg (P < 0.001) and 1.85 ± 0.25, 3.93 ± 0.38, and 9.49 ± 0.80 mU/L (P < 0.001), respectively, in the three arms. No substantial metabolic differences in outcome were found among the three arms, although direct correlations were observed between decreases in thyroid status and decreases in resting energy expenditure for all subjects. The subjects could not ascertain how their LT4 dose had been adjusted but the preferred LT4 dose they perceived to be higher (P < 0.001).
Conclusions
Altering LT4 doses in subjects with hypothyroidism to vary TSH levels in and near the reference range did not have major effects on energy expenditure or body composition. Subjects treated with LT4 preferred the perceived higher LT4 doses despite a lack of objective effect. Our data do not support adjusting LT4 doses in patients with hypothyroidism to achieve potential improvements in weight or body composition.
LT4 doses were adjusted in subjects with hypothyroidism to achieve low-normal, high-normal, or mildly elevated TSH levels. No major effects were seen on energy expenditure or body composition.
Thyroid hormone plays a critical role in determining energy expenditure, body mass, and body composition, which becomes evident in overt thyroid dysfunction (1). However, when thyroid status varies within and just outside the reference range (high or low), the effects on body weight and metabolic function are less clear (2–7). In the absence of consensus, many patients with weight concerns and mild TSH elevations have been treated with levothyroxine (LT4). Also, the LT4 doses have often been further increased in patients with hypothyroidism to treat unwanted weight gain or complaints of altered body composition.
To address this issue, we recruited subjects with primary hypothyroidism receiving replacement doses of LT4, who underwent extensive testing of energy expenditure, dietary intake, and body composition. We then adjusted their LT4 doses in a blinded fashion over 6 months to achieve one of three TSH ranges (low-normal, high-normal, or mildly elevated) and repeated the tests. We hypothesized that lowering the LT4 doses to increase the TSH levels in these ranges would adversely affect energy expenditure and body composition.
Experimental Subjects
A total of 197 subjects with hypothyroidism receiving LT4 monotherapy were recruited from our clinics through a review of the electronic health records and by flyers. All the patients had had hypothyroidism diagnosed as adults and had had elevated TSH levels in the past. The LT4 doses had been stable for ≥3 months. None had acute or chronic illnesses or were taking medications that could affect the thyroid hormone levels, mood, or cognition. Stable doses of an oral contraceptive or estrogen therapy were allowed. Testing was performed during the first 14 days after onset of menstrual bleeding or an oral contraceptive cycle in the premenopausal women.
Materials and Methods
The Oregon Health & Science University (OHSU) institutional review board approved the protocol, and all the subjects provided written informed consent.
Screening visit
The subjects were screened for general health, medication use, thyroid status, and mood or cognitive disorders using history taking, physical examination, and laboratory tests.
Run-in visits
The subjects taking branded LT4 with normal screening TSH levels proceeded directly to the baseline visit. Those subjects with abnormal screening TSH levels or who had been taking generic LT4 were prescribed branded LT4 and underwent run-in visits every 6 weeks with LT4 dose adjustments until the doses were stable with normal TSH levels for 3 months.
Baseline visit
Within 6 weeks of the screening or final run-in visit, the subjects returned for a baseline visit. The subjects were required to be fasting and to have refrained from taking their LT4 dose that morning. Serum TSH, free T4 (fT4), and free T3 (fT3) levels were obtained. The following measurements were performed.
Anthropometric measurements
Weight was measured using a digital scale (Model 5002; Scale-Tronix, Wheaton, IL) to the nearest 0.01 kg. Height was measured without shoes using a wall-mounted stadiometer (Harpenden Stadiometer; Holtain, Crymych, UK).
Total energy expenditure measured using doubly labeled water
The subjects consumed water enriched with stable isotopes for hydrogen (2H; Sigma Aldrich, St. Louis, MO) and oxygen (18O; Cambridge Isotope Laboratories, Andover, MA). Owing to limitations in the availability of doubly labeled water, only 65 subjects could complete the total energy expenditure (TEE) measurements. Each subject drank a premixed dose of water that provided 1.7 g of 2H218O/kg body weight. Spot urine samples were collected before the dose, at 2, 3, and 4 hours, and at 7 days after the dose. TEE was calculated from the 2H/1H ratio in hydrogen gas and 18O/16O ratio in carbon dioxide gas using standard techniques (Europa 20/20 isotope ratio mass spectrometer; Dale Schoeller, PhD, University of Wisconsin, Madison, WI) (8, 9). The within-subject coefficient of variation (CV) was 0.2% for 2H218O and 2% for 2H2O. The TEE intrasubject variation was 7.8%.
Resting energy expenditure measured using indirect calorimetry
Indirect calorimetry was performed at 21.1°C after the participant had fasted for 12 hours and abstained from substantial physical activity for 24 hours using standard techniques (VMax Encore 29N Indirect Calorimeter; SensorMedics Viasys Health Care, Yorba Linda, CA). Expired air was sampled and analyzed for the volume of oxygen consumed and the volume of carbon dioxide produced each minute for 30 minutes. Resting energy expenditure (REE) was calculated using the modified Weir equation, and macronutrient oxidation was estimated using the equations of Jequier and 24-hour urine nitrogen measurements (10).
Thermic effect of food
The thermic effect of food (TEF) was determined using indirect calorimetry immediately after the REE had been measured (11). Owing to limitations in subject availability for these 6-hour visits, 80 subjects completed the TEF measurements. Each participant consumed a standard liquid meal (Ensure; Ross Laboratories, Columbus, OH). The caloric content was 14% protein, 31.5% fat, and 54.5% carbohydrate and provided calories equivalent to 35% of the individual’s REE. The postprandial energy expenditure was measured for 15 minutes every 30 minutes for 6 hours using the procedure described for REE. The 6-hour area under the curve was calculated using the trapezoidal method. The result was multiplied by 3.5, a constant representing the typical consumption of three meals and one snack daily, to estimate the total 24-hour TEF.
Body composition measured using dual energy X-ray absorptiometry
Body composition was measured using dual energy X-ray absorptiometry (Hologic QDR Discovery A Densitometer; Hologic, Bedford, MA) following standard procedures. Visceral adipose tissue was estimated using the Hologic Horizon DXA System software by a single, trained operator (12).
Dietary intake
Three 24-hour food recall interviews were conducted by telephone within 1 week of the testing visit by bionutritionists trained in the Nutrition Data System for Research, a software application for the collection of dietary recall information in a standardized fashion (13).
Physical activity measured using accelerometry
The subjects wore a small multidirectional accelerometer (Actical; MiniMitter, Bend, OR) at the waist for 7 consecutive days within 2 weeks of the testing visit, except during sleep. The data were converted into energy expended and accumulated time during sedentary and light, moderate, and vigorous activities using standard analyses.
Randomization
Immediately after the baseline visit, the subjects were randomized to one of three arms: Low-normal TSH (0.34 to 2.50 mU/L), high-normal TSH (2.51 to 5.60 mU/L), or mildly elevated TSH (5.61 to 12.0 mU/L). These were determined from the OHSU TSH assay reference range, with debate regarding restricting the upper limit to 2.50 mU/L to conform to a Gaussian distribution (14) and our intention to restrict elevated TSH levels to the subclinical hypothyroid range. Randomization was stratified by whether the subject’s baseline TSH was low-normal or high-normal (<2.5 mU/L or >2.5 mU/L).
LT4 dosing
With consideration of the baseline TSH levels, the dispensing physician (K.G.S.) initially determined whether subjects should continue taking their usual LT4 dose or receive a different dose to achieve the assigned target TSH ranges. If a different dose was indicated, the subject’s usual dose was altered by 25 to 50 µg, depending on the difference between the initial and target TSH levels. The primary investigator (M.H.S.), research assistants, and subjects were unaware of the treatment assignment and LT4 doses. The OHSU research pharmacy dispensed 6-week supplies of LT4 pills in opaque gel capsules to maintain blinding.
LT4 dose adjustments
At 6, 12, and 18 weeks, the subjects returned for brief interim visits. The primary investigator assessed the clinical effects and determined whether the subject could comfortably continue the study. The subjects were asked to keep a daily diary of when they took their pills, which was revised at each visit, and compliance was encouraged at each review. The TSH levels from these visits were reviewed by the dispensing physician (K.G.S.), who adjusted the LT4 doses if the interim TSH level was not in the target range. The LT4 doses were adjusted by 12.5 to 50 µg, depending on the difference between the interim and target TSH levels, and the research pharmacy dispensed new 6-week supplies. Additional interim visits were allowed if the TSH level was not in the target range at 18 weeks. Once the TSH was in the target range, no further interim visits were scheduled, and the subject proceeded to the end-of-study visit.
End-of-study visit
Approximately 6 weeks after the final interim visit, the baseline measurements were repeated. At this visit, the TSH, fT4, and fT3 levels were measured, and this TSH level was subsequently used to assign subjects to the actual end-of-study TSH arms for the purposes of data analysis (details provided in the Statistical analysis section). The subjects were then returned to their usual LT4 dose or the dose that had led to better TSH control during the study in accordance with subject preference.
Statistical analysis
TSH was measured using immunochemiluminometric assay (Beckman Coulter; functional sensitivity, 0.02 mU/L; normal range, 0.34 to 5.60 mU/L; interassay CV, 5% at 0.70 mU/L). fT4 was measured using direct equilibrium dialysis (Quest Diagnostics; sensitivity, 0.08 ng/dL; normal range, 0.8 to 2.7 ng/dL; interassay CV, 6.8% at 0.3 ng/dL and 1.6% at 3.8 ng/dL). fT3 was measured using tracer dialysis (Quest Diagnostics; sensitivity, 25 pg/dL; normal range, 210 to 440 pg/dL; interassay CV, 4%). The TSH levels were measured at the time of testing, with stable assay characteristics during the study. The fT4 and fT3 levels were batched and analyzed at the end of the study. All samples were run in duplicate.
Differences among treatment arms were analyzed using multiple linear regression models adjusted for age, sex/estrogen status, baseline TSH (low-normal vs high-normal), SD of TSH values at the interim and last visits, baseline LT4 dose (µg/kg), duration of LT4 (years), duration taking the LT4 dose (years), and baseline value of the outcome variable. Incorporating the baseline values of each outcome allowed each subject to serve as their own control. The TEF models were also adjusted for the end-of-study lean body mass (LBM; kg), percentage of fat, and meal size. A Tukey post hoc multiple comparison procedure was used to determine which arms had statistically significant differences and adjust the P values of the pairwise arm comparisons for multiple testing. Because not all outcomes were independent, eight groups of related measures were created, and Bonferroni and false discovery rate (FDR) multiple testing adjustments were applied to all the related individual Tukey adjusted P values comparing the three arms within a group. The groups were TEE, REE, TEF, physical activity energy expenditure (PAEE), physical activity measures, substrate oxidation, body composition, and diet. The Bonferroni adjustment controlled for the familywise type I error rate or the probability of at least one false rejection of a null hypothesis for all tests within a group. The FDR is less conservative because it controls for the proportion of incorrect false-positive results among just the rejected null hypotheses within a group of tests. Analyses were conducted as intention-to-treat and by the actual TSH arm the subjects had achieved at the end of the study. We also examined relationships between the outcomes and TSH, fT4, or fT3 using the same regression analyses but substituting in the separate models the selected hormone for the categorical arms variable. All analyses were conducted in R, version 3.3.2 (R Foundation, Vienna, Austria) (15).
Results
Demographic, clinical, and thyroid status parameters
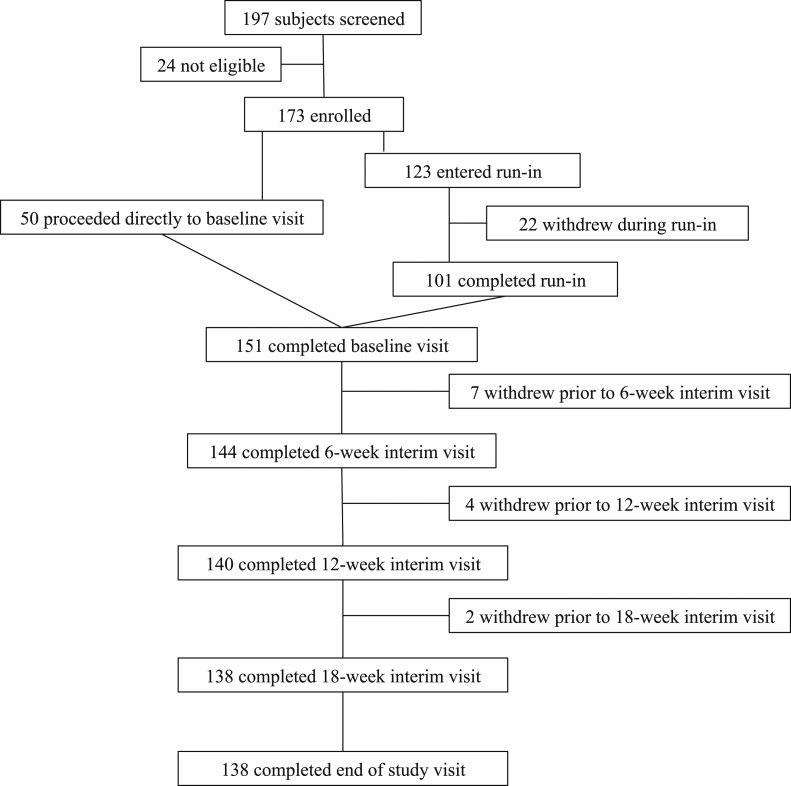
A flowchart of the study design and subject enrollment is provided in Fig. 1. Of the 197 subjects initially screened, 24 were excluded because of abnormal laboratory test results (low-density lipoprotein >160 mg/dL, n = 11; glucose >120 mg/dL, n = 2; elevated serum calcium, n = 1), abnormal electrocardiographic findings (n = 3), TSH out of range (n = 5), or medical issues (n = 2). Of the 173 included subjects, 50 were taking branded LT4 and had normal screening TSH levels and proceeded directly to the baseline visit. The remaining 123 subjects were taking generic LT4 and/or had had abnormal screening TSH levels and proceeded to the run-in phase. Of the 173 subjects, 151 completed the baseline visit and 22 withdrew during the run-in phase (personal issues, n = 17; started taking other medications, n = 2; started a weight loss diet, n = 1; or medical issues, n = 2). Also, 13 subjects withdrew before the final visit (personal issues, n = 5; medical issues, n = 6; pregnancy, n = 1; or started weight loss diet, n = 1). Of these 13 subjects, seven withdrew before the 6-week interim visit, four before the 12-week interim visit, and two before the 18-week interim visit. The subjects who had been excluded or had withdrawn from the study were not different from the study population in demographic or clinical attributes.
Figure 1.
Flowchart of study design and enrollment.
A total of 138 subjects completed the study (125 women and 13 men). The age range was 27 to 70 years. They were receiving LT4 therapy for primary hypothyroidism (n = 112), hypothyroidism after iodine-131 therapy for Graves disease (n = 17), postpartum thyroiditis leading to permanent hypothyroidism (n = 3), or after thyroid surgery (n = 6). The mean duration of LT4 use was 12 years (range, 5 months to 50 years). The mean duration of the current LT4 dose was 1.6 years. The baseline neurocognitive and metabolic data from these subjects and the neurocognitive results from the intervention have been previously reported (16–18).
During the run-in phase, 92 subjects (67%) switched from generic to branded LT4, and 36 (26%) required an LT4 dose adjustment. The proportion of subjects requiring a run-in or dose adjustment did not differ among the three arms (P = 0.50). At baseline, 87 subjects (63%) had low-normal TSH and 51 (37%) had high-normal TSH levels. Nineteen subjects (14%) did not require LT4 dose adjustments at the interim visits after the initial randomization, and 119 (86%) required one to five additional dose adjustments (mean 2.1). Seventy subjects (51%) were within the target TSH range at the 6-week interim visit, 81 (59%) were within the target range at the 12-week interim visit, and 83 (60%) were within the target range at the 18-week interim visit. During the interim visits, 40 subjects who had achieved the target TSH level had at least one subsequent interim TSH level outside their target range (11 in arm 1, 15 in arm 2, 14 in arm 3). Forty-five subjects (33%) had not achieved their intended target TSH range at the end of the study (17%, 64%, and 16% in the low-normal, high-normal, and mildly elevated TSH arms). It was especially difficult for the subjects in the high-normal TSH arm, because small changes in the TSH levels near the lower or upper cutoffs of this arm moved the subjects into one of the other two arms. Therefore, we conducted two separate analyses, one as an intention-to-treat by randomized arm and one using the actual TSH levels at the end-of-study visit. The results are presented for the intention-to-treat analysis, followed by the actual end-of-study arm analysis.
The intention-to-treat analysis results showed that the subjects in the three arms did not differ in age, sex, estrogen status, ethnicity, body weight, or duration of the current LT4 dose (Table 1). The duration of LT4 treatment was longer in the high-normal TSH arm (P < 0.001). The mean LT4 doses at the end of the study were progressively lower in the three arms (1.50 ± 0.07, 1.32 ± 0.07, and 0.78 ± 0.08 µg/kg/d; P < 0.001), and the mean TSH levels were progressively greater (1.85 ± 0.25, 3.93 ± 0.38, and 9.49 ± 0.80 mU/L; P < 0.001). The mean fT4 levels were lower in the mildly elevated TSH arm (1.79 ± 0.06, 1.64 ± 0.07, and 1.34 ± 0.05 ng/dL; P < 0.001), and the mean fT3 levels were not significantly different statistically among the three arms (201.4 ± 6.0, 191.1 ± 6.2, and 184.1 ± 6.6 pg/dL; P = 0.15). Of the 138 subjects, 72 (52%) had low baseline fT3 levels (range, 118 to 209 pg/dL). At the end of the study, 28 subjects in the low-normal TSH arm (61%), 34 in the high-normal TSH arm (72%), and 34 in the mildly elevated TSH arm (76%) had low fT3 levels (range, 82 to 209 pg/dL).
Table 1.
Clinical Parameters and Thyroid Function Test Results at Baseline and End of Study by Intention to Treat
| Variable | Baseline | End of Study |
P Value | ||
|---|---|---|---|---|---|
| Arm 1 (Low-Normal TSH) | Arm 2 (High-Normal TSH) | Arm 3 (Mildly Elevated TSH) | |||
| Subjects, n | 138 | 46 | 47 | 45 | |
| Age, y | 49.2 ± 1 | 49.5 ± 1.7 | 50.9 ± 1.8 | 49.3 ± 1.6 | 0.77 |
| Sexa | |||||
| Female | 91% | 41 (89.1) | 41 (87.2) | 43 (95.6) | 0.45 |
| Male | 9% | 5 (10.9) | 6 (12.8) | 2 (4.4) | |
| Estrogen statusa | |||||
| Male | 9% | 5 (10.9) | 6 (12.8) | 2 (4.4) | 0.50 |
| Prenone | 39% | 19 (41.3) | 15 (31.9) | 20 (44.4) | |
| Preon | 9% | 4 (8.7) | 5 (10.6) | 4 (8.9) | |
| Postnone | 38% | 18 (39.1) | 19 (40.4) | 15 (33.3) | |
| Poston | 4% | 0 (0) | 2 (4.3) | 4 (8.9) | |
| Ethnicitya | |||||
| White | 92% | 44 (95.7) | 41 (87.2) | 42 (93.3) | 0.35 |
| Other | 8% | 2 (4.3) | 6 (12.8) | 3 (6.7) | |
| Body mass index, kg/m2 | 27.8 ± 0.5 | 28.6 ± 0.8 | 27.3 ± 0.8 | 27.6 ± 1.0 | 0.58 |
| LT4 duration,a y | 11.9 ± 0.8 | 9.9 ± 1.3 | 15.9 ± 1.7 | 9.7 ± 0.9 | < 0.001b,c |
| Current LT4 dose duration,a y | 1.63 ± 0.19 | 1.75 ± 0.35 | 1.50 ± 0.18 | 1.63 ± 0.40 | 0.86 |
| LT4 dose, µg/kg | 1.44 ± 0.04 | 1.50 ± 0.07 | 1.32 ± 0.07 | 0.78 ± 0.08 | < 0.001c,d |
| LT4 dose change,e µg/kg | NA | 0.14 ± 0.02 | −0.21 ± 0.03 | −0.64 ± 0.05 | < 0.001b,c,d |
| TSH, mU/L | 2.21 ± 0.13 | 1.85 ± 0.25 | 3.93 ± 0.38 | 9.49 ± 0.80 | < 0.001b,c,d |
| TSH change,e mU/L | −0.18 ± 0.33 | 1.60 ± 0.42 | 7.23 ± 0.86 | < 0.001c,d | |
| fT4, ng/dL | 1.67 ± 0.03 | 1.79 ± 0.06 | 1.64 ± 0.07 | 1.34 ± 0.05 | < 0.001c,d |
| fT4 change,e ng/dL | 0.13 ± 0.07 | −0.04 ± 0.07 | −0.32 ± 0.06 | < 0.001c,d | |
| fT3, pg/dL | 214 ± 4.2 | 201.4 ± 6.0 | 191.1 ± 6.2 | 184.1 ± 6.6 | 0.15 |
| f T3 change,e pg/dL | −18.9 ± 9.3 | −14.4 ± 6.9 | −32.4 ± 7.9 | 0.27 | |
Data presented as n, mean ± SEM, or n (%); differences between arms were tested using analysis of variance, and follow-up post hoc Tukey multiple comparisons were used to determine which arms had statistically significant differences at the 5% level.
Abbreviations: Postnone, postmenopausal, no hormonal treatment; Poston, postmenopausal with hormonal treatment; Prenone, premenopausal, no hormonal treatment; Preon, premenopausal with hormonal treatment.
Values at baseline for each arm.
Arm 1 vs arm 2.
Arm 2 vs arm 3.
Arm 1 vs arm 3.
Change variables provided as differences between end of study and baseline, stratified by treatment arm.
Body composition and energy expenditure by intention-to-treat analysis
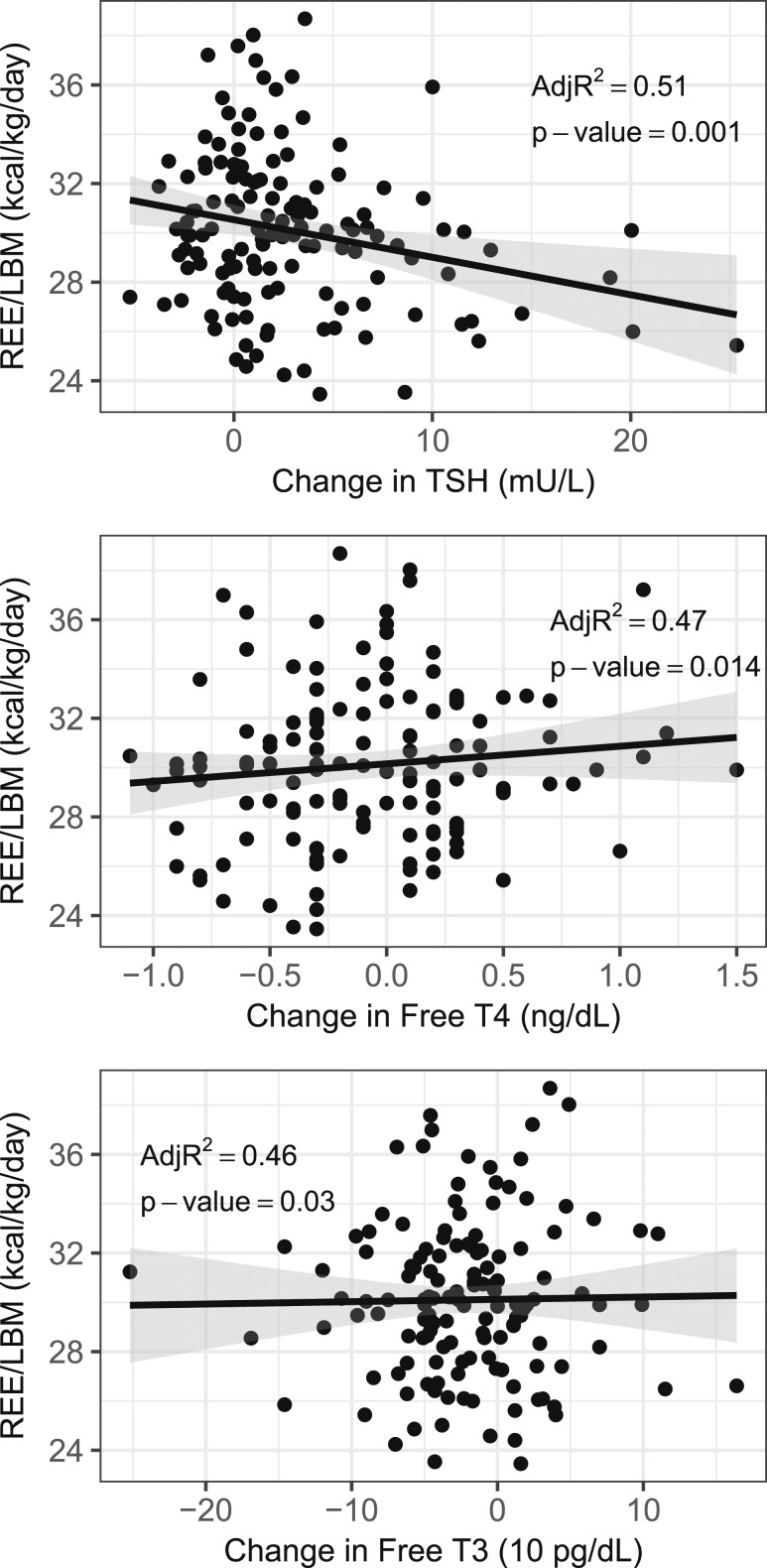
At the end of the study, no differences were found among the three arms in TEE, REE, oxidation rates, TEF variables, or body composition (Table 2), including total and visceral fat. Analyzing TSH, fT4, and fT3 as continuous variables (Table 3; Fig. 2), the REE/LBM correlated directly with increases in fT4 levels (P = 0.01) and correlated inversely with increases in TSH levels (P = 0.001). For each increase in fT4 of 1 ng/dL during the study, the REE/LBM increased on average by 1.22 kcal/kg/d. For each increase in TSH of 1 mU/L, the REE/LBM decreased on average by 0.15 kcal/kg/d. A trend was also seen for a direct correlation between REE/LBM and increases in fT3 levels, with an average 0.11 kcal/kg/d increase in REE/LBM for each 10 pg/dL increase in fT3. However, this difference was no longer statistically significant after Bonferroni or FDR correction (P = 0.03). REE correlated inversely with increases in TSH; however, this was only statistically significant using FDR and not Bonferroni correction (P = 0.03). Fat oxidation correlated inversely with increases in TSH (P = 0.004). Trends were found for direct correlations between LBM and increasing fT4 and fT3 and between LBM and increasing TSH. However, these were no longer statistically significant after Bonferroni or FDR correction (P = 0.02 to P = 0.03). Other trends that were no longer statistically significant after Bonferroni or FDR correction included direct correlations between TEE/LBM and increases in fT3 (P = 0.04) and inverse correlations between REE and increases in TSH (P = 0.03). No statistically significant correlations were found between the changes in TSH, fT4, or fT3 during the study and other measures.
Table 2.
End-of-Study Energy Expenditure and Body Composition Measures for Each Arm by Intention to Treat
| Measurea | Arm 1 (Low-Normal TSH; n = 46) | Arm 2 (High-Normal TSH; n = 47) | Arm 3 (Mildly Elevated TSH; n = 45) | Arm 2 vs Arm 1b | Arm 3 vs Arm 1b | Arm 3 vs Arm 2b | |||
|---|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) | P Valuec | Difference (95% CI) | P Valuec | Difference (95% CI) | P Valuec | ||||
| TEE, kcal/d | 2239 ± 57 | 2361 ± 155 | 2201 ± 71 | −31.9 (−243.0 to 179.2) | 0.93 | −21.6 (−234.7 to 191.4) | 0.97 | 10.3 (−185.8 to 206.4) | > 0.99 |
| TEE/LBM, kcal/kg/d | 51.1 ± 1.5 | 53.0 ± 1.6 | 50.6 ± 1.1 | −0.07 (−4.39 to 4.25) | > 0.99 | −0.83 (−5.16 to 3.50) | 0.89 | −0.76 (−4.75 to 3.23) | 0.89 |
| REE, kcal/d | 1397 ± 36 | 1333 ± 33 | 1298 ± 33 | −17.8 (−80.8 to 45.2) | 0.78 | −31.7 (−99.0 to 35.6) | 0.51 | −13.9 (−76.9 to 49.1) | 0.86 |
| REE/LBM, kcal/kg/d | 30.3 ± 0.4 | 30.0 ± 0.4 | 30.0 ± 0.5 | −0.51 (−1.79 to 0.76) | 0.61 | −0.69 (−2.06 to 0.67) | 0.45 | −0.18 (−1.46 to 1.09) | 0.94 |
| CHO oxidation, g/d | 133 ± 11 | 137 ± 12 | 149 ± 11 | 8.9 (−34.6 to 52.3) | 0.88 | 31.5 (−14.6 to 77.5) | 0.24 | 22.6 (−19.1 to 64.4) | 0.41 |
| Fat oxidation, g/d | 66.9 ± 4.4 | 63.1 ± 5.6 | 50.0 ± 4.5 | −0.97 (−19.52 to 17.57) | > 0.99 | −17.3 (−37.4 to 2.8) | 0.11 | −16.3 (−35.0 to 2.4) | 0.10 |
| Protein oxidation, g/d | 62.6 ± 3.3 | 54.2 ± 2.8 | 55.6 ± 2.7 | −8.1 (−16.9 to 0.7) | 0.08 | −2.6 (−11.9 to 6.7) | 0.79 | 5.5 (−3.2 to 14.2) | 0.29 |
| TEF, kcal/d | 136 ± 13 | 147 ± 28 | 125 ± 12 | 23.1 (−42.7 to 88.8) | 0.68 | −11.6 (−74.8 to 51.5) | 0.90 | −34.7 (−91.3 to 21.9) | 0.31 |
| TEF peak energy, kcal | 34.7 ± 1.0 | 34.0 ± 1.6 | 33.1 ± 1.1 | 0.65 (−1.76 to 3.05) | 0.80 | −0.35 (−2.67 to 1.98) | 0.93 | −0.99 (−3.05 to 1.07) | 0.48 |
| TEF time to peak, h | 1.4 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2 | −0.01 (−0.90 to 0.87) | > 0.99 | 0.30 (−0.55 to 1.15) | 0.67 | 0.32 (−0.45 to 1.08) | 0.58 |
| LBM, kg | 46.2 ± 1.1 | 45.0 ± 1.4 | 43.8 ± 1.3 | 0.57 (−0.15 to 1.28) | 0.15 | 0.66 (−0.11 to 1.42) | 0.11 | 0.09 (−0.63 to 0.81) | 0.95 |
| Fat mass, kg | 31.0 ± 1.6 | 27.9 ± 1.6 | 30. 0 ± 2.0 | −0.15 (−1.45 to 1.14) | 0.96 | 0.61 (−0.77 to 1.99) | 0.54 | 0.77 (−0.52 to 2.06) | 0.34 |
| BMI, kg/m2 | 28.6 ± 0.8 | 27.3 ± 0.8 | 27.6 ± 1.0 | 0.09 (−0.52 to 0.70) | 0.93 | 0.42 (−0.23 to 1.08) | 0.28 | 0.33 (−0.28 to 0.94) | 0.40 |
| Fat mass, % | 38.2 ± 1.0 | 36.4 ± 1.2 | 38.1 ± 1.1 | −0.55 (−1.54 to 0.44) | 0.39 | 0.14 (−0.92 to 1.20) | 0.95 | 0.69 (−0.30 to 1.68) | 0.23 |
| Visceral fat, g in abdominal ROI | 584 ± 49 | 491 ± 46 | 489 ± 43 | 7.9 (−42.5 to 58.3) | 0.93 | 23.9 (−30.0 to 77.9) | 0.55 | 16.0 (−34.0 to 66.0) | 0.73 |
Data presented as unadjusted mean ± SEM.
Abbreviations: CHO, carbohydrate; ROI, region of interest.
TEE values were available for 19 subjects in the low-normal, 23 in the high-normal, and 23 in the mildly elevated arms; REE values were available for all subjects; substrate oxidation values were available for all subjects, except for 4 missing CHO values and 1 missing fat value in the low-normal arm, 1 missing CHO and 1 missing fat value in the high-normal arm, and 1 missing fat value in the mildly elevated arm; TEF values were available for 23 in the low-normal, 26 in the high-normal, and 31 in the mildly elevated arm; and body composition values were available for all subjects.
Multiple linear regression models were adjusted for age, estrogen status, baseline TSH (low normal vs high normal), SD of TSH values at all but first visit, baseline LT4 dose, duration of LT4, duration of LT4 dose, and baseline value of outcome. The TEF models were also adjusted for end of study LBM, fat percentage, and meal size.
P values were adjusted using Tukey multiple comparison procedure for pairwise comparisons between arms.
Table 3.
Correlations Between Changes in Thyroid Hormone Levels With Energy Expenditure and Body Composition Measures at End of Study
| Measurea | fT4 |
fT3 |
TSH |
|||
|---|---|---|---|---|---|---|
| Coefficientb | P Valuec | Coefficientb | P Valuec | Coefficientb | P Valuec | |
| TEE, kcal/d | −8.23 (−144.09 to 127.62) | 0.90 | 2.53 (−12.55 to 17.61) | 0.74 | −3.38 (−17.61 to 10.85) | 0.64 |
| TEE/LBM, kcal/kg/d | 0.78 (−2.00 to 3.57) | 0.57 | 0.31 (0.01 to 0.61) | 0.04 | −0.19 (−0.48 to 0.09) | 0.18 |
| REE, kcal/d | 35.7 (−12.7 to 84.1) | 0.15 | 1.09 (−3.88 to 6.06) | 0.66 | −5.03 (−9.62 to −0.44) | 0.03e,f |
| REE/LBM, kcal/kg/d | 1.22 (0.25 to 2.19) | 0.01d,e,f | 0.11 (0.01 to 0.21) | 0.03 | −0.15 (−0.24 to −0.06) | 0.001d,e,f |
| CHO oxidation, g/d | −1.21 (−34.11 to 31.69) | 0.94 | −1.83 (−5.29 to 1.62) | 0.30 | 1.67 (−1.48 to 4.81) | 0.30 |
| Fat oxidation, g/d | 8.71 (−5.85 to 23.27) | 0.24 | 1.41 (−0.07 to 2.9) | 0.06 | −2.00 (−3.34 to −0.66) | 0.004d,e,f |
| Protein oxidation, g/d | 1.32 (−5.52 to 8.15) | 0.70 | 0.20 (−0.49 to 0.89) | 0.57 | −0.03 (−0.68 to 0.63) | 0.94 |
| TEF, kcal/d | 14.4 (−24.7 to 53.5) | 0.46 | −0.71 (−5.76 to 4.33) | 0.78 | −0.26 (−4.29 to 3.77) | 0.90 |
| TEF peak energy, kcal | 0.81 (−0.63 to 2.24) | 0.26 | −0.13 (−0.31 to 0.06) | 0.18 | −0.01 (−0.16 to 0.14) | 0.87 |
| TEF time to peak, h | −0.10 (−0.61 to 0.41) | 0.69 | −0.06 (−0.13 to 0.01) | 0.08 | 0.02 (−0.03 to 0.07) | 0.44 |
| LBM, kg | −0.60 (−1.15 to −0.06) | 0.03 | −0.06 (−0.12 to −0.01) | 0.02 | 0.06 (0.005 to 0.11 | 0.03 |
| Fat mass, kg | 0.28 (−0.72 to 1.28) | 0.58 | 0.05 (−0.05 to 0.15) | 0.34 | −0.03 (−0.12 to 0.07) | 0.61 |
| BMI, kg/m2 | −0.10 (−0.57 to 0.38) | 0.68 | 0.00 (−0.05 to 0.05) | 0.95 | 0.02 (−0.03 to 0.06) | 0.50 |
| Fat mass, % | 0.74 (−0.01 to 1.49) | 0.05 | 0.07 (−0.01 to 0.15) | 0.10 | −0.06 (−0.13 to 0.02) | 0.14 |
| Visceral fat, g in abdominal ROI | 5.46 (−32.77 to 43.69) | 0.78 | −2.45 (−6.35 to 1.46) | 0.22 | −0.64 (−4.37 to 3.10) | 0.74 |
Data presented as correlation (95% CI).
Abbreviations: CHO, carbohydrate; ROI, region of interest.
TEE values were available for 65 subjects; REE values were available for all subjects; substrate oxidation values were available for all subjects, except for 5 missing CHO and 3 missing fat values; TEF values were available for 80 subjects; body composition values were available for all subjects, except for one missing visceral fat value.
Correlations (95% CI) were modeled using multiple linear regressions, with separate models for each hormone. Positive coefficients indicate the measure increased with increasing hormone levels and negative coefficients indicate the measure decreased with increasing hormone levels; the magnitude of the coefficient indicates the estimated change in the outcome for each 1-unit increase in the study change (end of study minus baseline) for fT4 or TSH and a 10-unit increase in the study change for fT3.
P values were adjusted for age, estrogen status, SD of TSH values at all but first visit, baseline LT4 dose, duration of LT4, duration of LT4 dose, baseline hormone value, change in hormone value, and baseline value of outcome. The TEF models were also adjusted for end-of-study LBM, fat percentage, and meal size.
P value was still statistically significant at 0.05 when applying multiple testing adjustments and grouping outcomes from the same test for the Bonferroni correction.
P value was still statistically significant at 0.05 level when applying multiple testing adjustments and grouping outcomes from the same test for the FDR correction.
Statistically significant coefficient with corresponding P values; for each set of related outcome measures (TEE, REE, substrate oxidation, TEF, and body composition), multiple testing adjustments were applied to all the individually related P values (data not shown) from models adjusting for the same hormone type.
Figure 2.
Correlations between changes in serum (Top) TSH, (Middle) fT4, and (Low) fT3 levels during the study with REE/LBM in subjects treated with LT4.
Dietary intake and physical activity by intention-to-treat analysis
At the end of the study, the daily moderate or vigorous PAEE was greater in arm 2 than in arm 1 (401 kcal/d vs 352 kcal/d; P = 0.002; Tables 4 and 5). Also, the percentage of daily time spent in moderate or vigorous activity was greater in arm 2 than in arms 1 and 3 (15.6% vs 12.9% or 12.2%, respectively; P = 0.001). Trends were found toward greater total daily PAEE, more time spent in moderate or vigorous activity, and less time spent in sedentary activities in arm 2, which were statistically significant after FDR but not after Bonferroni correction (P = 0.004 to P = 0.01). No other differences were found among the three arms in daily energy intake or physical activity outcomes. Analyzing TSH, fT4, and fT3 as continuous variables (Table 5), we found no correlations between any thyroid hormone level and outcomes.
Table 4.
End-of-Study Dietary Intake and Physical Activity Measures for Each Arm by Intention to Treat
| Measurea | Arm 1 (Low-Normal TSH; n = 46) | Arm 2 (High-Normal TSH; n = 47) | Arm 3 (Mildly Elevated TSH; n = 45) | Arm 2 vs Arm 1b |
Arm 3 vs Arm 1b |
Arm 3 vs Arm 2b |
|||
|---|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) | P Value | Difference (95% CI) |
P Value | Difference (95% CI) | P Value | ||||
| Daily energy intake, kcal/kg/d | 26.8 ± 1.3 | 28.6 ± 1.6 | 27.4 ± 1.2 | 1.3 (−2.1 to 4.7) | 0.64 | 1.2 (−2.4 to 4.8) | 0.71 | −0.1 (−3.5 to 3.3) | > 0.99 |
| CHO intake, % | 45.2 ± 1.2 | 46.0 ± 1.2 | 46.9 ± 1.2 | 0.96 (−2.83 to 4.75) | 0.82 | 1.11 (−3.00 to 5.22) | 0.8 | 0.15 (−3.64 to 3.93) | > 0.99 |
| Fat intake, % | 36.1 ± 0.8 | 34.1 ± 1.0 | 34.5 ± 0.9 | −0.46 (−3.21 to 2.29) | 0.92 | −0.14 (−3.07 to 2.78) | > 0.99 | 0.31 (−2.37 to 2.99) | 0.96 |
| Protein intake, % | 16.0 ± 0.5 | 16.4 ± 0.7 | 15.4 ± 0.6 | −0.74 (−2.71 to 1.23) | 0.65 | −0.87 (−2.96 to 1.23) | 0.59 | −0.13 (−2.11 to 1.85) | 0.99 |
| Daily PAEE, kcal/d | |||||||||
| Total | 524 ± 29 | 574 ± 38 | 503 ± 33 | 110 (30 to 191) | 0.004c | 20.2 (−67.0 to 107.5) | 0.85 | −90.0 (−172.2 to −7.9) | 0.03 |
| Per kg of LBM | 11.6 ± 0.7 | 12.6 ± 0.6 | 11.4 ± 0.6 | 2.3 (0.5 to 4.0) | 0.006c | 0.32 (−1.55 to 2.19) | 0.91 | −2.0 (−3.7 to −0.2) | 0.03 |
| Light | 173 ± 7 | 172 ± 8 | 164 ± 9 | 6.4 (−12.5 to 25.2) | 0.70 | 0.32 (−20.17 to 20.8) | > 0.99 | −6.0 (−25.2 to 13.1) | 0.73 |
| Moderate/vigorous | 352 ± 25 | 401 ± 31 | 339 ± 26 | 99.9 (32.0 to 167.7) | 0.002c,d | 20.2 (−53.3 to 93.6) | 0.79 | −79.7 (−149.0 to −10.4) | 0.02 |
| Duration of daily activity, min | |||||||||
| Sedentary | 575 ± 16 | 530 ± 17 | 591 ± 16 | −18.6 (−63.6 to 26.5) | 0.59 | 38.1 (−10.7 to 86.8) | 0.16 | 56.6 (11.4 to 101.8) | 0.01c |
| Light | 200 ± 8 | 207 ± 7 | 196 ± 7 | 12.2 (−9.5 to 33.9) | 0.38 | 3.4 (−20.1 to 27.0) | 0.94 | −8.8 (−30.8 to 13.3) | 0.61 |
| Moderate/vigorous | 114 ± 8 | 135 ± 9 | 109 ± 7 | 32.2 (8.4 to 56.0) | 0.005c | 9.3 (−16.1 to 34.6) | 0.66 | −22.9 (−46.8 to 0.9) | 0.06 |
| Proportion of time spent in daily activity, % | |||||||||
| Sedentary | 64.3 ± 1.5 | 60.5 ± 1.6 | 65.6 ± 1.4 | −3.9 (−7.9 to 0.1) | 0.06 | 1.0 (−3.4 to 5.4) | 0.85 | 4.9 (0.8 to 9.0) | 0.01 |
| Light | 22.8 ± 0.8 | 23.9 ± 0.8 | 22.1 ± 0.8 | 0.14 (−2.13 to 2.40) | 0.99 | −1.0 (−3.5 to 1.4) | 0.57 | −1.2 (−3.5 to 1.1) | 0.44 |
| Moderate/vigorous | 12.9 ± 0.9 | 15.6 ± 1.0 | 12.2 ± 0.8 | 3.5 (1.2 to 5.8) | 0.001c,d | −0.01 (−2.48 to 2.45) | > 0.99 | −3.5 (−5.8 to −1.2) | 0.001c,d |
Data presented as unadjusted mean ± SEM.
Abbreviation: CHO, carbohydrate.
Dietary measures were available for all but 1 value in the mildly elevated arm; physical activity measures were available for all subjects but 1 in the low-normal, 1 in the high-normal, and 2 in the mildly elevated arms.
Multiple linear regression models were adjusted for age, estrogen status, baseline TSH (low-normal vs high-normal), SD of TSH values at all but the first visit, baseline LT4 dose, duration of LT4, duration of LT4 dose, and baseline value of outcome; P values were adjusted using Tukey multiple comparison procedure for pairwise comparisons between arms. For each set of related outcome measures (diet, PAEE, and physical activity measures), Bonferroni and FDR multiple testing adjustments were applied to all related individual Tukey-adjusted P values comparing the three arms (data not shown).
P value was still statistically significant at the 0.05 level when applying multiple testing adjustments and grouping outcomes from the same test together for the Bonferroni correction.
P value was still statistically significant at the 0.05 level when applying multiple testing adjustments and grouping outcomes from the same test together for the FDR correction.
Table 5.
Correlations Between Changes in Thyroid Hormone Levels and Dietary Intake and Physical Activity at End of Study
| Measurea | fT4 |
fT3 |
TSH |
|||
|---|---|---|---|---|---|---|
| Coefficientb | P Valuec | Coefficientb | P Valuec | Coefficientb | P Valuec | |
| Daily energy intake, kcal/kg/d | −0.09 (−2.71 to 2.54) | 0.95 | −0.04 (−0.31 to 0.23) | 0.77 | 0.11 (−0.14 to 0.36) | 0.39 |
| CHO intake, % | −0.43 (−3.35 to 2.49) | 0.77 | 0.11 (−0.18 to 0.41) | 0.46 | −0.03 (−0.31 to 0.25) | 0.83 |
| Fat intake, % | 1.52 (−0.54 to 3.58) | 0.15 | −0.07 (−0.28 to 0.14) | 0.51 | −0.03 (−0.23 to 0.17) | 0.77 |
| Protein intake, % | 0.18 (−1.36 to 1.71) | 0.82 | −0.08 (−0.23 to 0.08) | 0.31 | −0.02 (−0.17 to 0.13) | 0.78 |
| Daily PAEE, kcal/d | ||||||
| Total | −41.5 (−107.0 to 24.0) | 0.21 | −0.99 (−7.82 to 5.85) | 0.78 | 0.21 (−6.02 to 6.43) | 0.95 |
| Per kg of LBM | −0.62 (−2.04 to 0.79) | 0.38 | 0.03 (−0.11 to 0.18) | 0.65 | −0.01 (−0.15 to 0.12) | 0.85 |
| Light | −6.77 (−20.99 to 7.45) | 0.35 | −0.83 (−2.29 to 0.62) | 0.26 | −0.10 (−1.51 to 1.31) | 0.89 |
| Moderate/vigorous | −33.3 (−89.1 to 22.6) | 0.24 | −0.01 (−5.82 to 5.80) | > 0.99 | 0.59 (−4.67 to 5.86) | 0.82 |
| Duration of daily activity, min | ||||||
| Sedentary | −13.7 (−48.8 to 21.5) | 0.44 | −0.43 (−4.10 to 3.23) | 0.82 | 1.33 (−2.11 to 4.77) | 0.44 |
| Light | −4.51 (−21.15 to 12.14) | 0.59 | −0.39 (−2.08 to 1.30) | 0.65 | −0.19 (−1.84 to 1.45) | 0.82 |
| Moderate/vigorous | −1.80 (−20.87 to 17.27) | 0.85 | 1.17 (−0.79 to 3.14) | 0.24 | 0.16 (−1.67 to 1.99) | 0.86 |
| Proportion of time spent in daily activity, % | ||||||
| Sedentary | 0.09 (−3.15 to 3.34) | 0.95 | −0.04 (−0.37 to 0.30) | 0.83 | 0.06 (−0.26 to 0.37) | 0.71 |
| Light | 0.25 (−1.46 to 1.95) | 0.78 | −0.04 (−0.21 to 0.14) | 0.69 | −0.06 (−0.23 to 0.11) | 0.49 |
| Moderate/vigorous | −0.24 (−2.17 to 1.70) | 0.81 | 0.08 (−0.12 to 0.28) | 0.45 | 0.00 (−0.18 to 0.18) | 0.98 |
Data presented as correlation (95% CI).
Abbreviation: CHO, carbohydrate.
Dietary measures were available for 137 subjects; physical activity measures were available for 134 subjects.
Correlations (95% CIs) were modeled using multiple linear regressions, with separate models for each hormone. Positive coefficients indicate the measure increased with increasing hormone levels, and negative coefficients indicate the measure decreased with increasing hormone levels. The magnitude of the coefficient indicates the estimated change in the outcome for each 1-unit increase in the study change (end of study minus baseline) for fT4 or TSH and a 10-unit increase in the study change for fT3.
P values were adjusted for age, estrogen status, SD of TSH values at all but the first visit, baseline LT4 dose, duration of LT-4, duration of LT4 dose, baseline hormone value, change in hormone value, and baseline value of outcome.
Analyses by actual TSH arm at end of study
Using actual TSH arm at the end of the study, 57 subjects had TSH levels in the low-normal range, 28 in the high-normal range, and 53 in the mildly elevated range (Supplemental Table 1). The subjects did not differ in terms of any baseline demographic, clinical, or thyroid hormone variables. The mean LT4 doses at the end of the study were progressively lower in the three arms (1.52 ± 0.06, 1.10 ± 0.10, and 0.92 ± 0.08 µg/kg/d; P < 0.001). The mean TSH levels were progressively higher (1.34 ± 0.08, 3.74 ± 0.12, and 9.74 ± 0.63 mU/L; P < 0.001). The mean fT4 and fT3 levels were lower in the mildly elevated TSH arm (1.89 ± 0.06, 1.44 ± 0.08, and 1.35 ± 0.04 ng/dL, P < 0.001; and 206.2 ± 5.6, 196.0 ± 7.7, and 175.2 ± 5.4 pg/dL, P < 0.001, respectively). In the low-normal, high-normal, and mildly elevated TSH arms, 33 (72%), 19 (40%), and 44 (98%) subjects had low fT3 levels (range, 82 to 209 pg/dL), respectively.
Using the actual TSH arm at end of study, the LBM was 1 kg greater in arm 2 than in arm 1 after adjustment for covariates (P = 0.003). Trends toward a greater BMI in arm 2 were found (P = 0.03) and lower LBM, lower fat oxidation rates, and longer time to peak TEF in arm 3 (P = 0.01 to P = 0.03), which were not statistically significant after Bonferroni or FDR correction. No other differences were found among the three arms in the measures of energy expenditure, body composition, energy intake, or physical activity (Supplemental Tables 2 and 3).
Subjects’ perceptions of LT4 dose
At the final study visit, the subjects were asked whether they thought their LT4 dose at the end of the study was greater, lower, or unchanged from the start of the study and which of the two doses they preferred. The subjects were not able to accurately ascertain the changes in their LT4 dose (P = 0.55). However, most preferred whichever LT4 dose they thought was greater (P < 0.001): 68% preferred the dose at the end of the study when they thought their dose had been increased during the study, and 96% preferred the dose at the beginning of the study when they thought their dose had been lowered during the study. These data have been previously reported (Supplemental Table 4) (18).
Effect size calculations
When the present study was designed, few data were available to guide the power calculations. We relied on unreported cross-sectional data from our previous studies, and the only reported interventional study of altering LT4 doses in subjects with hypothyroidism (19). In the latter study, the REE decreased by 4% in nine subjects when their LT4 doses were decreased to achieve TSH changes in a range similar to what we had intended, which was statistically significant. Our calculations using these data indicated sufficient power to detect a similar change in REE/LBM, our primary outcome variable, with our sample size. Recognizing the limitations of this calculation, we also performed a post hoc effect size calculation for REE/LBM using our results. To achieve 80% power at a 5% level of significance, our study would have required 201 subjects to find a statistically significant difference between arms 1 and 3 and 1236 subjects to find a statistically significant difference between arms 1 and 2.
Discussion
In the present cohort of subjects treated with LT4, we found only limited evidence that altering the LT4 doses in a randomized, blinded fashion to achieve TSH levels in the low-normal, high-normal, or mildly elevated range affected metabolic function or body composition over 6 months. No consistent differences were found among the three TSH arms in energy expenditure, dietary intake, substrate oxidation, or body composition outcomes. However, using TSH, fT4, and fT3 as continuous measures, we found small, but statistically significant, correlations between increases in thyroid status in and near the reference range and increases in REE/LBM, suggesting that lowering LT4 doses might decrease REE. Despite this finding, the body weight and composition were not affected by altering the LT4 doses.
Unwanted weight gain is a common complaint among patients receiving LT4. If a screening TSH level is in the high normal or slightly elevated range, a diagnosis of subclinical hypothyroidism is often made. However, studies have found minor or no associations between body weight or BMI and subclinical hypothyroidism (2, 20) or have failed to demonstrate substantial effects of LT4 treatment on body weight (21), suggesting that these small shifts in thyroid status do not contribute to weight gain. Beyond measuring body weight, little is known regarding the relationships between subclinical thyroid disease and energy expenditure or body composition. The largest observational study to date reported the body composition for 427 older adults with subclinical hypothyroidism and 2864 euthyroid individuals (2). Subclinical hypothyroidism was not associated with differences in weight, lean mass, fat mass, or the percentage of fat at baseline or after 6 years of follow-up. To date, only three small interventional studies have investigated these parameters in subjects with subclinical hypothyroidism (n = 30 to 55) (22–24). LT4 treatment of ≤12 months in previously untreated patients did not affect REE, sleeping energy expenditure, or body composition.
Owing to the difficulties in recruiting subjects with de novo subclinical hypothyroidism for randomized, placebo-controlled studies, we adopted an alternate approach to investigating the effects on metabolic parameters by inducing subclinical hypothyroidism in subjects treated with LT4. To the best of our knowledge, our study is the largest and most comprehensive interventional study to date to investigate metabolic effects of variations in LT4 dosing in human subjects. Our results were largely negative, similar to the results of the summarized studies. However, in a secondary analysis, we found that increases in REE/LBM correlated directly with increases in fT4 and fT3 levels and inversely with increases in TSH levels across the achieved range of TSH levels when we altered the LT4 doses. The correlations were stronger for fT4 than for fT3; this implies that circulating fT4 levels are more relevant than fT3 levels, perhaps because fT4 serves as the circulating prohormone for tissue-specific intracellular conversion to T3. Our data suggest that REE is influenced by small differences in thyroid hormone levels, including in the subclinical hypothyroid range (lower REE). Supporting this idea, we have previously reported cross-sectional data from the present cohort, which showed a robust direct correlation between the baseline fT3 levels and REE/LBM (17). One small reported study adopted a similar approach to our intervention, altering the LT4 doses at 6- to 8-week intervals in nine subjects with hypothyroidism (19). Lowering the LT4 doses and increasing the TSH levels across a range of 0.12 to 11.0 mU/L led to a 4% decrease in REE/fat-free mass. This was a slightly greater change than the 2.2% difference in REE/LBM between arms 1 and 3 at the end of the present study, although within a similar range. The TSH range was broader in that study, and the study period was much shorter, which could also explain the greater difference. It is possible that with a longer period, compensatory mechanisms occur in those with subclinical hypothyroidism to mitigate decreases in energy expenditure.
Although we found direct correlations between increasing thyroid status and greater REE/LBM when we increased the LT4 doses, we did not find effects on body composition, except for trends toward reduced LBM. Compensations in non-REE metabolism (e.g., TEF, PAEE, and nonactivity thermogenesis) might maintain daily TEE, preventing weight loss or improvements in body composition, despite an increasing REE. Alternatively, the 6-month duration of our study might not have been long enough for effects on body weight or composition to become established. We did not find differences in TEE, TEF, or PAEE, except for a trend toward a direct correlation between fT3 and TEE/LBM. However, our sample size for the TEE and TEF measurements was limited, and we could have missed subtle compensatory changes. Finally, it is also possible that body adiposity could generate higher fT3 levels (25), perhaps via insulin-stimulated deiodinase activity (26) or an expanded bile acid pool (27, 28). In that case, changes in fT3 levels could be a consequence of, rather than a direct contributor to, body weight and composition. Thus, independently altering the fT3 levels might not have major effects on body composition. In any case, although our intervention increased fT4 levels and decreased TSH levels as expected across the three study arms, we only saw minor changes in serum fT3 levels. Taken together, these data argue against treating subclinical hypothyroidism to improve weight or body composition, regardless of the small effects we found in REE/LBM.
In addition to the controversy regarding treating mild hypothyroidism, debate has ensued in the thyroid field regarding the clinical relevance of variations in thyroid status within the TSH reference range (29, 30). Some experts have argued that the upper limit of the TSH reference range should be lowered to 2.5 mU/L (14). We addressed this question in arms 1 and 2 of the present study and found few differences in metabolic outcomes, with the exception of the correlations between thyroid hormone levels and the outcomes described across all three arms. The reported data regarding metabolic measures in subjects treated with LT4 are sparse. Some observational studies have reported minor correlations between reference range thyroid function in euthyroid subjects and weight or BMI (30, 31). However, our recent cross-sectional study of the present LT4-treated cohort showed direct correlations between fT3 levels and REE/LBM, BMI, fat mass, and visceral fat mass (17). Very few interventional studies on this issue have been reported. The study by al-Adsani et al. (19) found statistically significant short-term effects of altering thyroid status within the reference range on REE. However, three studies did not find effects on weight or body composition when euthyroid subjects were given LT4 to lower their TSH levels within the reference range (32) or subjects with hypothyroidism were given LT4 doses targeting a high-normal vs low-normal TSH (33, 34). It is clear from our present study that varying the LT4 dosage within the TSH reference range does not affect weight or improve body composition in subjects with hypothyroidism; therefore, lowering the TSH levels to <2.50 mU/L is unlikely to benefit these outcomes in patients with hypothyroidism.
A major strength of our study was the blinded nature of our intervention. When we queried our subjects, they could not accurately identify how or whether their LT4 dose had been altered and most preferred whichever dose they perceived to be the higher dose, confirming an intrinsic bias toward higher LT4 doses. Studies have indicated that self-knowledge of a thyroid disorder impairs well-being regardless of the TSH level (35, 36), which could also have biased unblinded studies.
We found a high prevalence of low serum fT3 levels in our subjects at baseline, which has been previously reported [reviewed by Jonklaas et al. (37)]. When we lowered the LT4 doses, we did find decreasing fT3 levels, confirming that some correlation exists between the LT4 dose and fT3 level. However, the fT3 levels were only 5% lower in arm 2 and 15% lower in arm 3 compared with that in arm 1 (actual arm achieved by subjects; Supplemental Table 1). This indicates that increasing LT4 doses for patients with hypothyroidism leads to only modest increases in fT3 levels, and many subjects will continue to have low fT3 levels despite TSH levels in the low-normal range. A number of randomized, controlled studies have replaced some of a hypothyroid subject’s LT4 with liothyronine (LT3), but none found clinically important differences in weight (38). One recent cross-over study did report that subjects with hypothyroidism lost an average of 1.5 kg after 6 weeks when LT3 had been completely substituted for their LT4 dose (39), and a second study reported an average 1.2-kg weight loss after 16 weeks when desiccated thyroid extract was substituted (40). Further studies are needed to investigate whether longer-term treatment with higher doses of LT4 or the addition of LT3 might improve body weight or composition.
Our study also had several limitations. Our original power calculations were based on limited reported data (19), and our outcomes proved to be less affected by altered LT4 doses than reported in that study. We also performed an effect size calculation, which showed that large numbers of subjects would be needed to reach statistical significance, especially for comparisons within the TSH reference range. The small magnitude of our effects raises the question of whether clinically meaningful alterations would be found with a larger sample size, although small changes in energy expenditure could be clinically relevant if aggregated over a longer period than the 6-month duration of our study. We did not provide standardized meals before measuring the REE, which could have increased the variability of this measure. We were only able to perform TEE and TEF measurements for a subset of the subjects owing to isotope availability (TEE) and subject time constraints (TEF). This further limited our conclusions regarding those two outcomes. Thus, we regard our results as providing a foundation for further studies with larger numbers of participants treated to targets for longer periods. We did not include an untreated euthyroid control group; thus, we could not ascertain whether our subjects had decrements in metabolic function or body composition at baseline compared with the general population. However, we previously found slightly lower REE in subjects treated with LT4 with normal TSH levels compared with euthyroid control subjects, with no differences in other metabolic parameters or body composition (41). We performed a large number of correlations, although we accounted for this in our analyses. It is possible that some of our minor findings resulted from chance. Most of our subjects were white women and were younger and slimmer than the U.S. population overall, limiting the generalizability of our findings to other groups, including men and those of other ethnic groups. The subjects less satisfied with their weight might have preferentially volunteered, introducing a selection bias. Our subjects were heterogeneous in terms of thyroid diagnosis and length of LT4 treatment. In particular, most of the subjects had primary hypothyroidism and probably had some degree of residual endogenous thyroid hormone production, which could have limited the effects. We limited our study to 6 months to optimize subject retention, recognizing that this would be sufficient time to observe changes in our energy expenditure outcomes but perhaps not for body composition. More subtle changes might require longer follow-up period to determine whether they are sustained and meaningful. Many of our subjects experienced variations in TSH levels at the interim visits and required LT4 dose adjustments, which we accounted for in our analysis. Because of this variation, a substantial number of subjects were not within their target TSH ranges at interim visits, and some subjects had subsequent TSH values outside their target ranges after first achieving their target TSH levels. Therefore, many subjects were exposed to their intended treatment arm for less than the 6-month duration of the study. We allowed for an additional interim visit for those subjects who had not reached their target TSH level at the 18-week visit, which improved the number of subjects achieving the target TSH levels but added variation to the study duration. One-third of our subjects had not achieved the target TSH level at the end of the study, especially in the high-normal TSH group. To address this, we conducted separate intention-to-treat and actual end-of-study analyses and analyses using changes in TSH and thyroid hormones as continuous variables. In addition, regardless of the ultimate TSH attained, the LT4 doses were altered in each arm consistent with the study design. Because patients often request changes in their LT4 dose regardless of their TSH levels, an interpretation of our results according to the LT4 dose adjustments is a valuable perspective for clinical practice. We attempted to collect blood samples at a consistent time of day; however, this was not always possible. In healthy subjects and subjects treated with LT4, the TSH levels decrease slightly between 7:00 and 9:00 am and then remain stable until evening (42).
In conclusion, we found few differences in metabolic function or body composition in subjects with hypothyroidism when the LT4 doses were altered in a randomized, blinded fashion over 6 months to achieve TSH levels in the low-normal, high-normal, or mildly elevated range. We did, however, find correlations between changes in thyroid status and REE/LBM and opposing trends in LBM. Although these relationships did not translate into statistically significant differences in body composition during 6 months of treatment, longer studies might be warranted. Because our study did not preselect subjects who might be more likely to respond to alterations in thyroid hormone therapy, future studies could focus on targeted populations, such as symptomatic subjects, subjects with low fT3 levels, or subjects with genetic polymorphisms that affect thyroid hormone metabolism or action [reviewed by Wiersinga (43)]. Until such time, however, the use of higher LT4 doses in the treatment of patients with hypothyroidism who report weight gain or in the hopes of inducing beneficial changes in body composition is not supported by our findings.
Supplementary Material
Acknowledgments
We would like to thank the staff of the OHSU Clinical and Translational Research Center for excellent patient care and research support and the Biostatistics & Design Program for data analysis expertise.
Financial Support : This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01 DK075496 to M.H.S.) and National Center for Advancing Translational Sciences (Grant UL1 RR024120; OHSU Clinical and Translational Science Award).
Clinical Trial Information: ClinicalTrials.gov no. NCT00565864 (registered 30 November 2007).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CV
coefficient of variation
- FDR
false discovery rate
- fT3
free T3
- fT4
free T4
- LBM
lean body mass
- LT3
liothyronine
- LT4
levothyroxine
- OHSU
Oregon Health & Science University
- PAEE
physical activity energy expenditure
- REE
resting energy expenditure
- TEF
thermic effect of food
References
- 1. McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garin MC, Arnold AM, Lee JS, Tracy RP, Cappola AR. Subclinical hypothyroidism, weight change, and body composition in the elderly: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2014;99(4):1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren R, Jiang X, Zhang X, Guan Q, Yu C, Li Y, Gao L, Zhang H, Zhao J. Association between thyroid hormones and body fat in euthyroid subjects. Clin Endocrinol (Oxf). 2014;80(4):585–590. [DOI] [PubMed] [Google Scholar]
- 4. Spadafranca A, Cappelletti C, Leone A, Vignati L, Battezzati A, Bedogni G, Bertoli S. Relationship between thyroid hormones, resting energy expenditure and cardiometabolic risk factors in euthyroid subjects. Clin Nutr. 2015;34(4):674–678. [DOI] [PubMed] [Google Scholar]
- 5. Tiller D, Ittermann T, Greiser KH, Meisinger C, Agger C, Hofman A, Thuesen B, Linneberg A, Peeters R, Franco O, Heier M, Kluttig A, Werdan K, Stricker B, Schipf S, Markus M, Dörr M, Völzke H, Haerting J. Association of serum thyrotropin with anthropometric markers of obesity in the general population. Thyroid. 2016;26(9):1205–1214. [DOI] [PubMed] [Google Scholar]
- 6. Bjergved L, Jørgensen T, Perrild H, Laurberg P, Krejbjerg A, Ovesen L, Rasmussen LB, Knudsen N. Thyroid function and body weight: a community-based longitudinal study. PLoS One. 2014;9(4):e93515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin JA, Mo EY, Kim ES, Moon SD, Han JH. Association between lower normal free thyroxine concentrations and obesity phenotype in healthy euthyroid subjects. Int J Endocrinol. 2014;2014:104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118(11):1278–1289. [DOI] [PubMed] [Google Scholar]
- 9. Compher C, Frankenfield D, Keim N, Roth-Yousey L; Evidence Analysis Working Group . Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881–903. [DOI] [PubMed] [Google Scholar]
- 10. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. [DOI] [PubMed] [Google Scholar]
- 11. Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63(2):164–169. [DOI] [PubMed] [Google Scholar]
- 12. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring). 2012;20(5):1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 14. Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90(9):5483–5488. [DOI] [PubMed] [Google Scholar]
- 15. Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team (2015). nlme: Linear and Nonlinear Mixed Effects Models. R package, version 3.1-120. Available at:http://CRAN.R-project.org/package=nlme. Accessed 2 August 2017.
- 16. Samuels MH, Kolobova I, Smeraglio A, Niederhausen M, Janowsky JS, Schuff KG. Effects of thyroid function variations within the laboratory reference range on health status, mood and cognition in levothyroxine treated subjects. Thyroid. 2016;26(9):1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samuels MH, Kolobova I, Antosik M, Niederhausen M, Purnell JQ, Schuff KG. Thyroid function variation in the normal range, energy expenditure and body composition in L-T4 treated subjects. J Clin Endocrinol Metab. 2017;102(7):2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab. 2018;103(5):1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82(4):1118–1125. [DOI] [PubMed] [Google Scholar]
- 20. Asvold BO, Bjøro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94(12):5023–5027. [DOI] [PubMed] [Google Scholar]
- 21. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, Sattar N, Aubert CE, Aujesky D, Bauer DC, Baumgartner C, Blum MR, Browne JP, Byrne S, Collet TH, Dekkers OM, den Elzen WPJ, Du Puy RS, Ellis G, Feller M, Floriani C, Hendry K, Hurley C, Jukema JW, Kean S, Kelly M, Krebs D, Langhorne P, McCarthy G, McCarthy V, McConnachie A, McDade M, Messow M, O’Flynn A, O’Riordan D, Poortvliet RKE, Quinn TJ, Russell A, Sinnott C, Smit JWA, Van Dorland HA, Walsh KA, Walsh EK, Watt T, Wilson R, Gussekloo J; TRUST Study Group . Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376(26):2534–2544. [DOI] [PubMed] [Google Scholar]
- 22. Kong WM, Sheikh MH, Lumb PJ, Naoumova RP, Freedman DB, Crook M, Doré CJ, Finer N. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med. 2002;112(5):348–354. [DOI] [PubMed] [Google Scholar]
- 23. Ulas T, Buyukhatipoglu H, Eren MA, Dal MS, Torun A, Aydogan T, Demir ME, Turan MN. Evaluation of sleeping energy expenditure using the SenseWear armband in patients with overt and subclinical hypothyroidism. Clin Invest Med. 2012;35(3):E126–E131. [DOI] [PubMed] [Google Scholar]
- 24. Teixeira PF, Cabral MD, Silva NA, Soares DV, Braulio VB, Couto AP, Henriques JL, Costa AJ, Buescu A, Vaisman M. Serum leptin in overt and subclinical hypothyroidism: effect of levothyroxine treatment and relationship to menopausal status and body composition. Thyroid. 2009;19(5):443–450. [DOI] [PubMed] [Google Scholar]
- 25. Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, Chen KY, Simchowitz L, Celi FS. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lartey LJ, Werneck-de-Castro JP, O-Sullivan I, Unterman TG, Bianco AC. Coupling between nutrient availability and thyroid hormone activation. J Biol Chem. 2015;290(51):30551–30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. [DOI] [PubMed] [Google Scholar]
- 28. Bennion LJ, Grundy SM. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975;56(4):996–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100(3):1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98(9):3562–3571. [DOI] [PubMed] [Google Scholar]
- 31. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 2016;101(12):4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dubois S, Abraham P, Rohmer V, Rodien P, Audran M, Dumas JF, Ritz P. Thyroxine therapy in euthyroid patients does not affect body composition or muscular function. Thyroid. 2008;18(1):13–19. [DOI] [PubMed] [Google Scholar]
- 33. Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D, Gillett MJ, Gilbert R, Tanner M, Stuckey BG. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab. 2006;91(7):2624–2630. [DOI] [PubMed] [Google Scholar]
- 34. Boeving A, Paz-Filho G, Radominski RB, Graf H, Amaral de Carvalho G. Low-normal or high-normal thyrotropin target levels during treatment of hypothyroidism: a prospective, comparative study. Thyroid. 2011;21(4):355–360. [DOI] [PubMed] [Google Scholar]
- 35. Panicker V, Evans J, Bjøro T, Asvold BO, Dayan CM, Bjerkeset O. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjects on and not on T4: findings from the HUNT study. Clin Endocrinol (Oxf). 2009;71(4):574–580. [DOI] [PubMed] [Google Scholar]
- 36. van de Ven AC, Netea-Maier RT, de Vegt F, Ross HA, Sweep FC, Kiemeney LA, Hermus AR, den Heijer M. Is there a relationship between fatigue perception and the serum levels of thyrotropin and free thyroxine in euthyroid subjects? Thyroid. 2012;22(12):1236–1243. [DOI] [PubMed] [Google Scholar]
- 37. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jonklaas J. Risks and safety of combination therapy for hypothyroidism. Expert Rev Clin Pharmacol. 2016;9(8):1057–1067. [DOI] [PubMed] [Google Scholar]
- 39. Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, Pucino F. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96(11):3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):1982–1990. [DOI] [PubMed] [Google Scholar]
- 41. Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid. 2016;26(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013;34(5):619–657. [DOI] [PubMed] [Google Scholar]
- 43. Wiersinga WM. Therapy of endocrine disease: T4 + T3 combination therapy: is there a true effect? Eur J Endocrinol. 2017;177(6):R287–R296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.