Abstract
The gut-brain-axis refers to the bidirectional communication between the enteric nervous system and the central nervous system. Mounting evidence supports the premise that the intestinal microbiota plays a pivotal role in its function and has led to the more common and perhaps more accurate term gut-microbiota-brain axis. Numerous studies have identified associations between an altered microbiome and neuroimmune and neuroinflammatory diseases. In most cases, it is unknown if these associations are cause or effect; notwithstanding, maintaining or restoring homeostasis of the microbiota may represent future opportunities when treating or preventing these diseases. In recent years, several studies have identified the diet as a primary contributing factor in shaping the composition of the gut microbiota, and in turn, the mucosal and systemic immune systems. In this review, we will discuss the potential opportunities and challenges with respect to modifying and shaping the microbiota through diet and nutrition in order to treat or prevent neuroimmune and neuroinflammatory disease.
Keywords: Microbiome, neurocognitive, gut-microbiota-brain axis, SCFA, neurotrophic, vitamin, oxidative stress, polyphenols, myalgic encephalomyelitis, Parkinson's disease, Alzheimer’s Disease, autism, multiple sclerosis, schizophrenia
1. Introduction
The human body is an ecosystem supporting trillions of microorganisms that live primarily, although not exclusively, within the gastrointestinal track [1]. For many years, it was widely believed the microbiota was mostly comprised of commensal bacteria that do not harm the host nor necessarily impart any significant health benefit. A notable exception to this point of view was based on observations that commensal bacteria compete with pathogenic species for nutrients and sites for colonization and, therefore, a compromised gut microbiota can lead to pathogenic intestinal infection [2]. For instance, the colonization of the gut by the bacteria Clostridium difficile can occur as a result of reduced microbiota competition during the course of long-term antibiotic therapy. In fact, the treatment of C. difficile infection by fecal transplantation is an excellent example where manipulating the gut microbiota has a direct and verifiable benefit for treating human disease [3].
In contrast to the aforementioned example, a perturbed microbiota may occur as a result of a disease process. For instance, gastrointestinal (GI)-associated CD4 T cells are the primary targets of infection by the human immunodeficiency virus (HIV)[4]. Alterations in mucosal immunity, including bacterial translocation, and subsequent chronic systemic inflammation are common and associate with HIV progression [5, 6]. Furthermore, components of this pathological process persist, despite viral suppression during highly active antiretroviral therapy (HAART) [7–9].
Previous studies have reported significant differences in the gut microbiome of HIV cases when compared to controls. Shifts in bacterial populations toward those with proinflammatory potential, such as Staphylococcus spp., Pseudomonas spp., and Enterobacteriaceae family members are commonly reported [10, 11]. In fact, increased Prevotella in the stool of HIV-infected individuals has been reported by several groups [12–14]. Vujkovic-Cvijin and coworkers observed that a dysbiotic mucosal-adherent bacterial population, enriched in Proteobacteria and depleted of Bacteroidia members, was associated with markers of mucosal immune disruption, as well as T cell activation, and chronic inflammation in HIV-infected subjects [15]. They additionally reported an upregulation of kynurenine pathway components. The kynurenine pathway, also known as the indoleamine 2,3-dioxygenase (IDO), pathway contributes to metabolic immune regulation by catabolizing the essential amino acid L-tryptophan and has been associated with inflammation, neurodegenerative diseases, and depression. Importantly, some of products of this pathway, such as quinolinic acid, are neurotoxic and have been associated neurological pathology in HIV infection [16].
With respect to C. difficile infection, the benefit of modifying the microbiota through fecal transplantation is fairly straightforward. In the case of HIV-infection, the situation is less obvious, although early studies suggest that altering the microbiota may influence systemic immune responses. Hensley-McBain et al. showed that macaques infected with simian immunodeficiency virus (SIV), the animal model of HIV infection, displayed significant increases in the number of peripheral Th17 and Th22 cells and reduced CD4 T cell activation in GI tissues after receiving antibiotics, followed by fecal transplantation. However, others reported that human subjects with HIV infection showed no significant change, post-fecal transplantation [17]. Albeit, the authors acknowledge that, unlike the macaques in the Hensley-McBain et al. study, the human subjects were not preconditioned with antibiotics, so depleting the previous microbiota may be in important step prior to microbiota transplantation.
At this time, all aspects of altering the microbiota are not fully understood in most cases. However, as our understanding improves with respect to the contributions of specific bacterial groups, rational modification of the microbiota may ultimately become an effective way of modifying diseases associated with an altered microbiota.
2. The gut-microbiota-brain axis
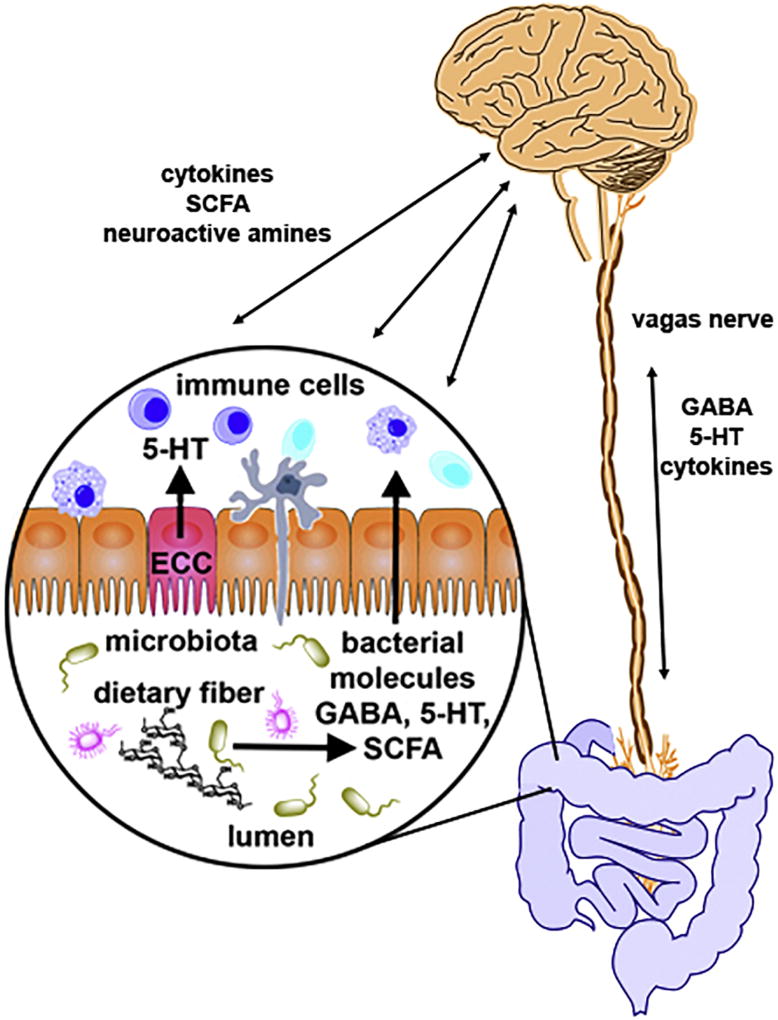
Most neuroimmune diseases are characterized by a spectrum of symptoms and the pathophysiology of these diseases cannot typically be defined by an individual organ or system (such as neurological); instead, a more systemic point of view must be considered. Indeed, it is increasingly evident that the gut microbiota dramatically influences systemic immunity, including the host’s neuroimmune status, both beneficially and adversely. The so-called “gut-microbiota-brain” axis dictates that biochemical signaling occurs between the enteric nervous system (ENS) of the GI tract and the central nervous system (CNS) and principally involves the intestinal microbiota [18]. This signaling can occur directly via the vagus nerve or indirectly, through chemical signals that are released into the periphery and act in an endocrine manner (Figure 1) [19–22].
Figure 1. Representation of the bidirectional communication between the gut-microbiota-brain axis.
Signaling can occur directly via the vagus nerve, through signaling molecules such as GABA (γ-Aminobutyric acid), serotonin (5-hydroxytryptamine, 5-HT) and antiinflammatory cytokines. Conversely, signaling can occur indirectly, through chemical messengers that are released into the periphery and act in an endocrine manner including GABA, 5-HT, produced by enterochromaffin cells (ECC) and short chain fatty acids (SCFA), such as butyrate and propionate, produced through bacterial fermentation of nondigestible fiber.
When an imbalance of the gut microbiota occurs and results in an increase in noncommensal microbes (dysbiosis), homeostasis of the gut microbiota is disrupted. Signaling between the gut-brain axis is also impacted, potentially leading to neurological and neuroimmune abnormalities. Indeed, dysbiosis is commonly associated with a compromised gut epithelium and the subsequent bacterial translocation may result in systemic inflammation and innate immune activation such as the upregulation of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α) [23, 24]. Furthermore, it is important to appreciate that systemic inflammation can promote neuroinflammation across the blood-brain barrier. In point of fact, it has been shown that a single intraperitoneal injection of TNF-α in mice increased serum and brain levels of the proinflammatory cytokines TNF-α, IL-6, and MCP-1, in a dose- and time-dependent manner [25].
3. Gut microbiota and neurotrophic factors
Biogenic amines such as catecholamines (CA), which include epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine, as well as other neuroactive amines like gamma-aminobutyric acid (GABA) and serotonin (5-hydroxytryptamine, 5-HT) [26–29] interact with several host systems to maintain homeostasis [26, 27]. Strikingly, over 90% of the body’s 5-HT is produced in the gut, and through the engagement of at least 14 different receptor subtypes [30], modulates the digestive system [31], the nervous system [32], the immune system [33], and cardiac function [34]. Using an animal model, Yano and coworkers showed that the gut microbiota significantly contributes to the level of colon and blood 5-HT, primarily through elevating its synthesis by host colonic enterochromaffin cells [35]. Furthermore, utilizing specific pathogen-free mice, germ-free mice, and gnotobiotic mice, Asano et al. showed that CA levels in the gut lumen were lower in germ-free mice than in specific pathogen-free mice and, moreover, CA levels correlated with Clostridium-associated β-glucuronidase activity [29], directly implicating a specific genus of bacteria. Although it is widely accepted that dopamine levels are primarily synthetized in the CNS, a study in rats showed evidence that the gut lumen also contributes to dopamine production to some extent [29]. Another study showed that administering Lactobacillus to germ-free mice not only increased the levels of 5-HT, but significantly increased the level of dopamine in the striatum, raising the possibility of using bacterial transplants for the treatment of Parkinson’s disease (PD) [36]. Also, enzymes that regulate dopamine synthesis can be modulated by gut microbiota through the microbiota-gut-brain axis [37].
In addition to the host’s production of biogenic amines, which may occur as a result of host-bacterial interactions, it has been reported that a number of gut-associated bacteria have the capacity to directly produce these signaling molecules. For example, Pessione et al. reported that Lactobacillus spp. and Enterococcus produce and release histamine and tyramine into the intestinal lumen [38]. Also, Escherichia coli and Pseudomonas have been reported to produce endogenous GABA [39] and Bifidobacteria has been reported to produce melatonin [40].
4. The gut microbiota, mucosal immunity, and neuroinflammation
Our current understanding supports the premise that the microbiota plays a pivotal role in maintaining mucosal immune competence and GI integrity. Indeed, previous studies show that animals who develop under germ-free conditions display extensive deficits in the development of the gut-associated lymphoid tissues (GALT), suggesting that commensal bacteria are important, not only for maintaining gastrointestinal health, but are also critical for the proper development of mucosal immunity. Several observations support the premise that the microbiota can influence the inflammatory state of intestinal epithelium and, in turn, its integrity. For instance, Bacteroides fragilis as well as some members of the genus Clostridia promote an antiinflammatory state through the production of antiinflammatory cytokines, such as IL-10 and IL-13, while some pathogenic bacteria, including Salmonella typhimurium and C. difficile, drive the production of inflammatory cytokines [41, 42].
The innate immune system senses microorganisms of the gut, primarily through their interaction with pattern recognition receptors, and their engagement by bacterial products is essential for maintaining intestinal homeostasis [43–45]. The structural constituents of bacterial cell walls persistently stimulate the innate immune system to produce inflammatory cytokines, thus generating a basal state of low-level immune activation that originates at the intestinal mucosal surface and affects the entire body [46]. To this end, a compromised gut epithelium and the subsequent bacterial translocation exacerbates this process, resulting in greater systemic inflammation and innate immune activation including the upregulation of inflammatory cytokines [23, 24]. The upregulation of these inflammatory mediators can promote pathological responses including sickness behavior, neurocognitive dysfunction, sleep abnormalities, and chronic fatigue [47, 48].
In addition to the previous examples that largely rely on innate immune responses, cell-mediated immunity is also influenced by the gut microbiota. Notably, the majority of Th1 and Th17 cells reside in the small intestine and differentiate as a result of signals associated with the gut microbiota [49, 50]. Failure to maintain the proper balance of these T cells leads to increased bacterial translocation and innate immune activation [51, 52]. Importantly, current estimates suggest that more than 60% of all T-cells reside within the small intestine [53] underscoring the potential contribution of the gut to systemic immunity. Therefore, a compromised gut may have a profound and complex influence on the host’s neuroimmune system.
Alterations in gut microbiota can also indirectly affect mucosal immunity by adversely dysregulating energy homeostasis and promoting oxidative stress. For instance, hydrogen sulfide (H2S), which is produced during the course of anaerobic respiration by bacteria such as Prevotella, is associated with mitochondrial dysfunction, epithelial damage, and increased intestinal inflammation [54, 55]. Furthermore, elevated lactic acid production from bacteria such as Enterococcus and Streptococcus spp. can also contribute to GI pathology by promoting mitochondrial dysfunction and enhance oxidative stress. These examples are but a few of the putative mechanisms whereby the microbiota impacts neuroimmune and neuroinflammatory processes.
It is important to bear in mind that the composition of the gut microbiota is influenced by a combination of factors including genetics, diet, antibiotic use, and disease, all of which may act in concert and these factors will need to be considered [56, 57]. Additionally, most investigations into the composition of disease-associated microbiomes have relied on 16S ribosomal analysis, which primarily identify families of bacteria, but few studies have conducted a comprehensive survey at the species level. In one of the few instances, Li and coworkers conducted a comprehensive survey by combining 249 newly sequenced samples from the Metagenomics of the Human Intestinal Tract project with 1,018 previously sequenced samples to create a cohort from three continents and concluded that almost 10 million unique bacterial genes are potentially represented [58]. These data emphasize the potential diversity of the human microbiota and challenges that lay ahead with respect to understanding the contributions of specific bacterial species. However, as our knowledge increases as to the contributions of each species, with respect to neuroimmune and neuroinflammatory diseases, the rational modulation of the intestinal microbiota will ultimately be within our grasp.
5. Neuroimmune and neuroinflammatory diseases associated with alterations of the gut microbiota
5.1 Parkinson's disease
Parkinson’s disease (PD) is a devastating, neurodegenerative disorder characterized by the progressive degeneration of axons that project from midbrain dopamine neurons to the striatum. Pathologically, PD is characterized by an accumulation of intracellular, protein aggregates termed “Lewy bodies” in midbrain dopamine neurons. The progressive loss of midbrain dopamine neurons leads to the onset of clinical symptoms, including the presence of tremors and bradykinesia. Additionally, the loss of posture/balance and the onset of dementia are observed in late-stage PD.
PD is also characterized by the presence of non-motor symptoms. In fact, it is generally accepted that the majority of individuals who suffer with PD also suffer from gastrointestinal comorbidities, of which constipation is considered the most prominent [59]. Specifically, PD cases show signs of gastrointestinal dysmotility including delayed gastric emptying and constipation. Indeed, approximately 50% of PD cases suffer from severe constipation and show comorbidity with bowel-related disorders including Crohn’s disease and inflammatory bowel syndrome. Additionally, constipation can occur 20 years before the onset of motor symptoms in PD. These observations suggest that constipation represent an early pathological event that precedes the onset of neurological and motor symptoms in PD by 10–15 years. As previously mentioned, the gut lumen can contribute to the production of dopamine [29]. Therefore, given the observation that the ENS produces some level of dopamine and that PD symptoms are caused by a reduction in dopamine, it is conceivable that gut pathology observed in the majority of PD cases is a major risk factor that can exacerbate the depletion of dopamine and worsen PD neuropathology.
PD cases show altered gut homeostasis including increased oxidative stress which contributes to barrier and intestinal permeability, leading to a leaky gut and systemic low-grade inflammation [60–62]. While the pathophysiological mechanisms that contribute to altered gut homeostasis in PD are not known, mounting evidence suggest that early alterations in the microbiome are associated with constipation and gut-related disorders. Consistent with this model, Lai et al. reported that a diagnosis of IBS is associated with an increase in risk of PD [63, 64]. Other studies have shown that PD cases present with altered microbiomes including an over-abundance of a number of bacterial groups, including Bacteroidetes, Lactobacillaceae, Faecalibacterium prausnitzii, Enterococcaceae, [65] Prevotella, [65, 66] and Clostridium spp. [67]. Moreover, there is strong evidence that the microbiota is altered early during the course of disease. One recent study showed that the prevalence of Bifidobacterium and Bacteroides fragilis is decreased, along with an increase in Lactobacilli gasseri and Enterobacteriaceae [66, 67]. Notably, alterations in the gut microbiome in PD cases are significantly associated with worsened PD-associated symptoms [68].
Although it is not known if an altered microbiota is the initiating factor of PD, a major drive of etiology or consequence of disease progression, there is some evidence that suggests an altered gut microbiome contributes to PD pathophysiology. Importantly, there is convincing evidence of strong interactions between the ENS and the CNS via a braingut/enteric axis in PD. Like the brain, significant accumulation of Lewy bodies has been observed in the ENS of PD cases suggesting that the aberrant accumulation of protein aggregates contributes to neurodegeneration of the ENS and gut pathology [69] which predates the PD symptoms by 10 to 20 years [70]. Furthermore, fecal transplantation studies have compellingly shown that an altered microbiota contributes to PD symptoms. Strikingly, transplanting microbiota from six PD cases worsened the motor symptoms in α-synuclein expressing mice [71]. Conversely, depleting the gut microbiota in this transgenic PD mouse model ameliorated the symptoms of PD, reduced the aggregation of Lewy bodies in the CNS, and reversed constipation. Finally, an altered microbiome in PD may contribute to an increase in oxidative stress caused by overactive macrophages, which lead to increase wall permeability and enhances the aggregation of α-synuclein and in an in vivo chemical model of PD [69, 72, 73]. Overall, this data suggests causation between altered gut microbiome and PD symptoms.
To date, little is known about the molecular causes of altered gut homeostasis in PD. A reduction in the level of neurotrophins, including brain-derived neurotrophic factor (BDNF), may contribute to deregulated gut homeostasis and constipation in PD based on the following evidence. BDNF is one of the most abundant neurotrophins produced in the gut to support normal brain development, neuronal survival and the differentiation of midbrain dopamine neurons [74]. Furthermore, BDNF can exert strong antiinflammatory processes in models of immune-graft rejection, allergy and experimental meningitis models suggesting that BDNF can regulate the immune system. [75–77]. Based on studies performed in postmortem brain tissue, midbrain dopamine neurons in PD show a significant reduction in neurotrophic factors such as BDNF and of the BDNF receptor TrkB [78–80] suggesting that a decrease in BDNF reduces neuronal survival and increases the susceptibility of dopamine neurons to oxidative stress.
A proper level of BDNF is critical for the expression and proper function of the N-methyl-D-aspartate (NMDA) receptor in the CNS and ENS [81]. Given that a low level of BDNF in the CNS and the ENS in PD has been well-documented, it has been postulated that a reduction in the level of membrane-bound NMDA receptor may contribute to alterations in gut homeostasis and changes in CNS function, via affecting the kynurenine pathway [81]. Furthermore, as the gut microbiome produces a significant level of BDNF to support normal gut function [81], these data suggest that a low BDNF level plays a pivotal role in neurodegeneration of dopamine neurons in the CNS and ENS, gut pathology and inflammation in PD.
Finally, there is experimental evidence that suggest that supplementation of exogenous recombinant human BDNF, or of compounds that increase BDNF levels, may ameliorate constipation, oxidative stress and clinical symptoms in PD. For instance, Vidal-Martinez et al. showed that transgenic mice that overexpress mutant α-synuclein (A53T), a genetic model of PD, shows decreased gut motility compared to wild-type mice whereas treating mice with AN121, an antagonist of the BDNF receptor, reversed the ameliorative effects of BDNF on constipation [82]. Overall, these data suggest that low levels of BDNF and a decline in BDNF-mediated signaling contributes to gut pathology and subsequent neurodegeneration of dopamine neurons in PD.
5.2 Myalgic encephalomyelitis
Many chronic diseases are characterized by systemic immune activation, gastrointestinal issues and neurocognitive abnormalities. One such example, myalgic encephalomyelitis (ME), is a heterogeneous disorder often identified by incapacitating post-exertional fatigue, not relieved by rest, accompanied by neurological symptoms (e.g. brain fog, modest brain atrophy, and gradual decline in cognitive function), and inflammatory sequelae [83]. GI abnormalities are also commonly reported by those with ME, and, in fact, are so prevalent and their symptoms overlap with irritable bowel syndrome (IBS) to such an extent that many individuals diagnosed with ME report that they received a previous diagnosis of IBS [84]. This assertion is supported by studies conducted by Maes and coworkers, who reported that a majority of subjects with ME (59.6% vs. 17.7%) experienced GI symptoms and that these symptoms strongly associated with a diagnosis of IBS [85].
Consistent with a GI involvement in the pathophysiology of ME, several studies have reported alterations of the ME microbiota and microbiome. For instance, using culture- and metabolomic-based analyses, Sheedy and colleagues reported significantly increased proportions of D-lactic acid-producing Enterococcus and Streptococcus spp. in fecal samples of subjects with ME [86]. Recently, Wallis et al. conducted a systematic literature review to examine similarities between ME and acute D-Lactic acidosis and concluded that high levels of D-lactate may play a role in the neurological comorbidity in ME [87]. However, as there have not been any robust clinical studies to evaluate the circulating levels of D-lactate in ME subjects, the contribution of D-lactate in the neurological comorbidity of ME remains to be established.
In the first published metagenomics analysis of an ME cohort, Fremont et al. observed that subjects with ME displayed an overall gut microbiome that is different from non-ME subjects when geographically controlled [88]. In this study, stool from ME cases and controls from Belgium and Norway were analyzed by pyrosequencing, which revealed that Belgian cases and controls differed, as did Norwegian cases and controls. Interestingly, Belgian and Norwegian ME cases differed from each other, as did Belgian and Norwegian controls. Not only does this study articulate an association between ME and alterations in the gut microbiome, these data emphasize the potential for geographic differences as well as disease differences when investigating disease associations with an altered microbiome. Variations in the gut microbiota of ME cases were later confirmed by Giloteaux and colleagues, who showed that ME is characterized by dysbiosis, bacterial translocation and an altered microbiome, and additionally reported that overall bacterial diversity was lower in the ME cases when compared to controls. In particular, they observed large reduction in the relative abundance and diversity of members of the Firmicute phylum.
In a later study, it was revealed by Nagy-Szakal and coworkers that ME cases without IBS can be differentiated from those with IBS based on their microbiome profile [89]. Specifically, ME cases with IBS were identified by an increase of unclassified Alistipes and decreased Faecalibacterium whereas increased unclassified Bacteroides abundance and decreased Bacteroides vulgatus were more prevalent in ME cases without IBS. It was also revealed that the severity of symptoms such as pain, fatigue, and reduced motivation were correlated with the abundance of specific bacterial taxa [89]. When taken together, these studies strongly imply that an altered microbiome profile is common among those with ME and, additionally, may differentiate specific subgroups.
Although not all individual with ME report GI comorbidity, previous research suggests that gut pathology may not be a requisite for alterations in the microbiota. For example, Shaukla and colleagues observed differences in gut and plasma microbiome following an exercise challenge that was not recapitulated in controls subjects [90]. Interestingly, as previously indicated, on average, the composition of microbiome of those with ME is reported to be less diverse; however, following exercise challenge, an increase in relative abundance of six of the nine major bacterial phyla/genera was observed in ME cases compared to only two of the nine in controls. Previous studies in animal models [91, 92] as well as humans [93], suggest that exercise is associated with increased microbiota diversity, as well as an expansion of beneficial bacteria, such as butyrate-producing species. However, the increased microbiota diversity observed in ME subjects was observed concurrently with an increase in bacterial products in the blood, suggesting bacterial translocation is associated with strenuous exercise in ME. Although, it is yet to be determined whether or not the increased bacterial diversity induced by exercise is beneficial or pathological for those with ME [94]. Further studies will be required to determine if the bacterial translocation and potentially associated systemic inflammation observed during strenuous exercise in ME contributes to the manifestation of exercise intolerance; however, this study does introduce the intriguing possibility of a connection between the gut microbiota, intestinal integrity and systemic inflammation observed in ME.
5.3 Schizophrenia
The role of the human microbiome in schizophrenia is largely undiscovered, but many argue that it’s an endeavor worth pursuing [95]. Currently there are limited published studies involving the fecal and orophyngeal microbiome in those with schizophrenia. One such oropharyngeal investigation using metagenomic analysis (16 adults with schizophrenia, 16 non-psychiatric controls) found differences at both the phylum and the genus levels. Samples from subjects with schizophrenia had less overall diversity of species compared to controls, and an increased number of metabolic pathways representing metabolite transport systems, including siderophores (iron-chelating compounds secreted by microorganisms such as bacteria and fungi), glutamate and vitamin B12; this is in contrast to carbohydrate, lipid pathways and energy metabolism which were abundant in controls [96]. Another study of the oropharyngeal microbiome (41 adults with schizophrenia, 33 non-psychiatric controls) assessed bacteriophages (viruses that infect bacteria and alter their metabolism) and found that the Lactobacillus phage phi adh was significantly more abundant in schizophrenia cases than in controls [97].
One small study that used fecal samples to investigate the role of the gut microbiome in first episode psychosis (FEP) (28 cases, 16 controls) reported an elevation of the Lactobacillus group and a positive correlation with the severity of psychotic symptoms in multiple domains. Differences in the microbiota were also associated with poorer treatment response in one FEP subgroup [98]. Another case-control study looked at exposures to fungal members of the gut microbiome, including the yeast species, Candida albicans. Severance et al. investigated antifungal IgG antibody responses of participants with bipolar disorder (n=270) and schizophrenia (n=261) revealing sex-specific differences; C. albicans seropositivity conferred increased odds for a schizophrenia diagnosis in males, while C. albicans seropositivity in females was associated with higher odds of cognitive impairment (lower cognitive scores) [99]. In a follow-up, 56 of the outpatients with schizophrenia were enrolled in a longitudinal, double-blind, placebo-controlled study, showing sex-specific effects; probiotic treatment significantly reduced levels of C. albicans antibodies over the 14-week study period in males, but not in females [100].
The maternal immune activation (MIA) mouse model mimics neurodevelopmental disorders such as autism or schizophrenia by administering immune stimulants [such as the endotoxin lipopolysaccharide (LPS), mimicking a bacterial infection, or the double-stranded RNA molecule, polyinosinic:polycytidylic acid (poly I:C), mimicking a viral infection] to the pregnant mouse to induce behavior change in the offspring. This model is based on the theory that it is not the infectious agent, but rather the maternal immune activation that causes the behavior change in the offspring. The gut microbiome likely plays a pivotal role in the MIA model, as offspring have gastrointestinal abnormalities and altered microbiota, similar to humans with autism and schizophrenia [101]. Additionally, one promising MIA study gave the probiotic Bacteriodes fragilis to MIA offspring, and by doing so, corrected their intestinal permeability deficits and reversed some of their behaviors (communicative, stereotypic, anxiety-like and sensorimotor); however, social deficits persisted. After B. fragilis treatment, two serum metabolites normalized, 4-ethylphenylsulfate (4EPS) and indolepyruvate; notably, 4EPS is structurally similar to p-cresol, the putative autism spectrum disorders (ASD) biomarker, and indolepyruvate is theorized to be a metabolic byproduct of gut bacteria [101]. A recent MIA mouse study demonstrated that IL-6 in the placenta activates inflammatory signals to influence fetal brain development and behavior [102].
5.4 Autism spectrum disorders
As with other neurological disorders, such as PD and Alzheimer’s disease (AD), ASD are exceedingly comorbid with GI symptoms such as constipation, bloating and diarrhea, and several previous studies have reported that ASD often associate with an altered intestinal microbiome [103]. For instance, Finedgold et al. reported that the number of Clostridial species found in the stool samples of children with ASD was greater than in the stool samples of control children [104]. Also, Tomova and coworkers reported that microbiomes of children with ASD exhibit a significant decrease in the Bacteroidetes/Firmicutes ratio and an elevation in the amount of Lactobacillus species as well as the Desulfovibrio species when compared to siblings and healthy controls [105].
Although the precise mechanism connecting the microbiota to the neuropathology of ASD is not fully elucidated, recent studies by Golubeva et al. support that social behavior deficits in the BTBR T+Itpr3tf/J mouse model of ASD are associated with microbiota-related alterations in bile acid and tryptophan metabolism [106]. Particularly, they observed that tissue levels of 5-HT were decreased in the small and large intestine of BTBR mice by 50% when compared to control mice. Furthermore, this decrease coincided with decreased tryptophan hydroxylase 1 (Tph1) transcription and increased transporter (Sert) transcription. These observations were associated with GI distress, intestinal permeability, and changes in ENS morphology.
A recent clinical trial was conducted by Kang and coworkers to investigate the potential benefits of fecal transplantation in autistic children [107]. After a two-week pretreatment with antibiotics to deplete the existing microbiota, subjects were treated with an initial bolus of transplant bacteria, followed by a lower daily maintenance dose for seven to eight weeks. Upon completion of the treatment, a significant improvement in gastrointestinal symptoms was observed, including constipation, diarrhea, indigestion, and abdominal pain. They additionally reported that behavioral symptoms, as indicated using the PGI-II assessment, showed significant improvements which were maintained at eight-weeks post-treatment. Finally, microbiome pyrosequencing confirmed that the donor transplant was partially maintained as well as beneficial changes in the gut environment.
In order to explore the possibility that microbiome-driven behavioral changes are accompanied by corresponding changes in neurological tissue, Ong et al. utilized diffusion tensor imaging to show that over-all changes in white matter structural integrity occurred in a diet-dependent manner [108]. They further reported that the changes in diet were accompanied by changed in the microbiome. These studies provide compelling evidence that modifying the microbiota through diet, may represent an effective strategy to treat neurological pathology; however, it should be pointed out that other investigators have reported subjects with AD are also characterized by alterations in their oral microbiome, raising the possibility that more than just the gut microbiota may be involved in these observations [109].
5.5 Multiple sclerosis
Multiple sclerosis (MS) is a severely debilitating autoimmune disease, characterized by chronic inflammation of the CNS, leading to demyelination. Although the etiology of MS is presently unknown, genetics and environmental factors are thought to play an important role [110, 111]. In addition to a host of neurological symptoms, individuals with MS commonly experience GI aberrations [112]. In fact, a survey-based study revealed that approximately two thirds of individuals with MS reported GI complaints that persist for at least six months, which include diarrhea, constipation, and fecal incontinence [113].
Several previous studies have reported that subjects with MS have an altered microbiome when compared to age- and gender-matched controls [114–116]. For instance, Miyake and coworkers conducted a longitudinal study to compare the gut microbiota of Japanese subject with relapsing-remitting MS (RRMS) to that of healthy controls and observed that 21 species of bacteria exhibited significant changes in relative abundance in addition to observing an overall moderate dysbiosis in the RRMS cohort. However, in contrast to other disease, such as inflammatory bowel disorders, which show reduced diversity [117, 118], they reported that RRMS cases displayed similar bacterial diversity to that of controls.
Perhaps the most compelling evidence for a microbiota-MS connection arise from observations conducted using the classical MS mouse model, experimental autoimmune encephalomyelitis (EAE). Lee and colleagues reported that intestinal microbiota significantly influence the balance between proinflammatory and antiinflammatory and immune responses during the induction of EAE [119]. Specifically, they observed that mice, reared under germ-free conditions developed an attenuated form of EAE characterized by decreased levels of the proinflammatory cytokines IL-17A and IFN-γ in the intestine and spinal cord with an concomitant increase in CD4+CD25+Foxp3+ regulatory T cells (Tregs). The authors of this study additionally showed that specific pathogen-free mice that harbor segmented filamentous bacteria fully developed EAE, thus providing compelling evidence that the bacterial composition of the gut can influence neurologic inflammation in MS.
Subsequent to this study, Haghikia and coworkers showed that long-chain fatty acids (LCFAs) promote polarization of naive T cells toward a Th1 and Th17 differentiation and impaired their intestinal sequestration via the p38-MAPK signaling pathway. In contrast, EAE mice treated with short-chain fatty acids (SCFAs) displayed increased differentiation and proliferation of Tregs, and an accompanying resolution of EAE pathology. It is noteworthy that microbiome survey studies of other neuroimmune disease such as ME [120] and autoimmune diseases, such as Crohn’s disease [121], are characterized by reduced levels of butyrate-producing bacteria. These data suggest that rationally modifying the gut microbiota through diet, in order to promote the growth and maintenance of SCFAproducing bacterial, represents a potential strategy for treating neuroinflammatory disease.
5.6 Alzheimer’s disease
Alzheimer’s disease is the most common neurodegenerative disorder and is typically associated with a toxic buildup of β-amyloid plaques and hyperphosphorylated and misfolded tau protein in the brain [122]. As with PD cases, recent evidence suggest that alterations in the microbiome of those with AD may be associated with or contribute to the pathophysiology of AD [123]. Recently, Minter et al. reported that antibiotic treatment of a murine model of AD lead to reduced amyloidosis [124]. Subsequent to this study, Kobayashi and colleagues reported that oral administration of Bifidobacterium breve strain A1 to a mouse model of AD (intracerebroventricularly administered Aβ25–35) resulted in reversal of cognitive impairment [125]. This study raises the possibility that an altered microbiome contributes to the neuropathological progression in AD. The authors of this study additionally reported that transcriptional profiling of the hippocampus revealed that a total of 305 genes (247 upregulated, 58 downregulated) were differentially expressed in the AD animal model when compared to non-AD mice and that most of the differentially expressed genes were involved in immune response. Strikingly, upon treatment with B. breve A, the transcriptional profiles AD mice differed from non-AD mice by only two genes, suggesting that B. breve A could modulate excessive AD-associated immune responses and underscores the relevance of the altered microbiome the progression of AD pathology.
Recently, an association between an altered microbiome and the presentation of AD was demonstrated in human subjects by Vogt and coworkers [126]. In this report, it was showed that the gut microbiome of AD cases contains less microbial diversity and was compositionally distinct from age- and sex-matched controls. It has also been shown that a significant reduction in BDNF and of neuroprotective signaling is observed in postmortem brain tissue as well as in vivo models of AD and this has been suggested to contribute to the overt and progressive neurodegeneration of hippocampal neurons [127]. In light of recent evidence showing that BDNF levels are regulated by the gut microbiome and the neuroprotective role of BDNF, it would be relevant to understand whether the altered microbiota in AD cases can contribute to a reduction of BDNF, and thereby, exacerbate AD neuropathology. As mentioned in the PD section, a reduction in BDNF can also exacerbate oxidative stress and alter gut homeostasis in AD cases. In addition to supporting a role for the microbiota in the pathophysiology of AD, these murine and human studies suggest that the progression and presentation of AD may be modified through the modification of the microbiota. Nonetheless, it has been suggested that pathology of AD commences as early as 20 years prior to the manifestation of overt symptoms [128]; therefore, in light of this protracted prodromal phase, modifying AD progression after symptoms are manifest may prove to be impractical. It is possible that the greatest opportunities in modifying the microbiota will be in the proactive prevention of AD for those with a hereditary predisposition.
6. Modifying the Microbiota through the use of prebiotics and probiotics
6.1 Prebiotics
Modulation of the microbiota is an evolving strategy as a part of comprehensive approach to lifestyle wellness [129]. The diverse ecosystem that the microbial organisms inhabit in the gut allow for potential targets to maintain or improve health as well as treat disease. Advances in microbiome research utilizing high-throughput RNA sequencing has improved our knowledge of the composition of the microbiota and the substances that influence their colonizing abilities. Therefore, these methods can be used as a prognostic tool to follow the effects of dietary interventions on the composition of these microbial populations in a longitudinal manner [130, 131]. Prebiotics, are a class of compounds that has been recognized for their ability to manipulate the host’s microbiota. Whereas probiotics are live microorganisms, prebiotics are nonviable substrates that serve as nutrients for beneficial microorganisms harbored by the host. It is important to note that prebiotics differ from most dietary fibers such as pectins, cellulose and xylans, which encourage growth of a wide variety of gut microorganisms. A prebiotic is more specific in that it is not broadly metabolized, but elicits a metabolism biased towards health-promoting microorganisms within the indigenous ecosystem. Simpson and Campbell provide a comprehensive review of microbiota interaction and compare studies on fiber and prebiotics [130].
The first prebiotics assessed in humans and used commercially stimulated Lactobacillus and Bifidobacterium but not pathogens such as Clostridium or Escherichia [132]. In 2004, the definition of prebiotics was altered to “selectively fermented ingredients that allow specific changes, both in composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health” [131]. It is important to note that the prebiotic is selectively utilized by microorganisms and can lead to an overall health benefit for the host. However, if additional microorganisms that have pathogenic effects have enhanced function or growth and lead to a negative consequence for the host then this substrate can no longer be called a prebiotic. This distinction makes it important to determine both function and composition of the gut microbiota involved. Furthermore, prebiotics should not cause gas formation, unfavorable changes in bowel habits, or any type of negative symptom for the human host [133].
There are many fermentable carbohydrates that have a prebiotic effect, but the dietary prebiotics most extensively documented to have health benefits in humans are the non-digestible oligosaccharides fructans and galactans, which are preferentially utilized by Bifidobacteria [133, 134]. In contrast to many other genera of bacteria, Bifidobacteria have the β-fructanosidase and β-galactosidase enzymes necessary to digest the linkage bonds in fructan and galactan oligosaccharides as well as the transport machinery to necessary capture and deliver the substrates into their cytoplasm.
Interestingly, the first oligosaccharides identified to have prebiotic effects and positively impact gastrointestinal health are present in human milk. Human milk oligosaccharides are particularly important for the development of the newborn baby’s intestinal microbiota and metabolic and immunologic systems, which have consequences for health in early development as well as later in life. Human milk oligosaccharides, after fucosylation and sialylation, prevents adhesion of pathogens to the neonate’s intestinal epithelium by a competitive mechanism which infers protection from infection [129, 135, 136].
Ultimately, utilizing prebiotics as an intervention to improve health and reduce risk of disease is the goal. The approach that is the most effective are those that rely on prevention and recognition that life strategies adopted in early in life, which, when maintained, will promote a viably diverse and durable microbiota that will promote greatest potential to benefit the health of the host.
6.2 Probiotics
Probiotics are preparations of live or attenuated microorganisms, such as bacteria or yeast, which may afford certain health benefits when consumed. Several different probiotic preparations are commercially available and differ from one manufacturer to another in a number of ways, including bacterial composition, number of organisms, and biological activity. Many health benefits are attributed to probiotics, such as improving or supporting immunity, competitively inhibiting noncommensal bacteria growth and providing many of the essential vitamins and cofactors necessary for human health, that are not endogenously produced by the host [137–140].
Various food preparations, such as yogurt and some fermented foods, are natural sources of probiotics. Pu et al. conducted a clinical trial to evaluate the efficacy of yogurt containing Lactobacillus paracasei strain N1115, to prevent acute infection in elderly subjects and observed a significant benefit, potentially through an enhancement of the T-cell-mediated natural immune defense [141]. Indeed, N1115 is reported to exhibit substantial resistance to acid and bile stresses and also stimulates macrophages to produce IL-10, IL-6, and TNF-α [142]. Bercik and coworkers reported that mice infected with the nematode parasite Trichuris muris, displayed anxiety-like behavior which correlated with decreased level of BDNF. However, upon treatment with the common probiotic Bifidobacterium longum, the anxiety-like behavior was reversed and BDNF levels were normalized [21]. Also, Messaoudi et al. evaluated the efficacy of a probiotic formulation containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175, to reduce anxiety in rats and also its possible effects on anxiety, depression, stress and coping strategies in healthy human volunteers [143]. The authors of that study concluded that the probiotic formulation exhibited anxiolytic-like activity in rats and provided beneficial psychological effects in healthy human subjects.
An increasing number of studies support the notion that probiotics have significant benefit in maintaining homeostasis of the CNS. However, most of these studies are based on indirect evidence. In an effort to reveal the biological underpinnings of the gut-brain axis, researchers in the laboratory of John Cryan, show that protracted treatment of mice with the lactic acid bacteria Lactobacillus rhamnosus induced alterations in the mRNA which codes for the GABA B1b subunit in specific regions in the brain [20]. They additionally showed that the probiotic reduced stress-induced corticosterone and anxiety- and depression-related behavior. Importantly, the neurochemical and behavioral effects were not observed in mice upon vagotomy, unequivocally showing a role for bacteria in the bidirectional communication between the gut and the brain via the vagus nerve.
The term psychobiotics, initially coined by Dinan et al. is typically defined as any live organism that, when ingested in adequate amounts, produces a health benefit in patients suffering from psychiatric illness [144]. Accordingly, psychobiotics are, by definition, a subgroup of probiotics with the added emphasis on mental illness. Most psychobiotics are capable of producing or promoting the endogenous synthesis of neurotransmitters such GABA, catecholamines, and 5-HT, all of which influence the brain-gut axis and mental health. A list of biogenic amines implicated in neuroimmune disease pathology as well as the microbes that associate with changes in the respective neurotransmitter are given in Table 1.
Table 1.
Biogenic amines implicated in neuroimmune disease pathology and microbes that associate with changes in the respective biogenic amines.
| Neurotransmitter | Function | Neuroimmune Disease Association |
Microbe family or genus implicated* |
Reference |
|---|---|---|---|---|
| Brain-derived neurotrophic factor (BDNF) | Neurotrophin | Alzheimer’s Disease | Bifidobacterium breve, Bifidobacterium longum | [21, 145–152] |
| Autism | ||||
| Multiple Sclerosis | ||||
| Myalgic Encephalomyelitis | ||||
| Parkinson’s Disease | ||||
| Schizophrenia | ||||
|
| ||||
| Dopamine | Neurotransmitter, precursor of epinephrine and norepinephrine | Alzheimer’s Disease | Bacillus cereus and Serratia | [153–161] |
| Autism | ||||
| Multiple Sclerosis | ||||
| Myalgic Encephalomyelitis | ||||
| Parkinson’s Disease | ||||
| Schizophrenia | ||||
|
| ||||
| Gamma-aminobutyric acid (GABA) | Inhibitory neurotransmitter | Alzheimer’s Disease | Bifidobacterium dentium, Lactobacillus rhamnosus, Escherichia coli, Pseudomonas | [162–167] |
| Autism | ||||
| Multiple Sclerosis | ||||
| Schizophrenia | ||||
|
| ||||
| Glutamate | Excitatory neurotransmitter | Alzheimer’s Disease | Lactobacillus plantarum, Bifidobacteria, Lactobacilli | [147, 166, 168–171] |
| Autism | ||||
| Myalgic Encephalomyelitis | ||||
| Schizophrenia | ||||
|
| ||||
| Histamine | Neurotransmitter, regulates physiologic function in the gut | Alzheimer’s Disease | Lactobacillus vaginalis, Morganella morganii | [172] |
| Parkinson’s Disease | ||||
| Schizophrenia | ||||
|
| ||||
| Melatonin | Hormone, regulates synchronization of the circadian rhythm | Alzheimer’s Disease | Bifidobacteria Firmicutes Verrucomicrobia Enterobacter aerogenes, Escherichia coli | [40, 173–181] |
| Autism | ||||
| Myalgic Encephalomyelitis | ||||
| Multiple Sclerosis | ||||
| Parkinson’s Disease | ||||
| Schizophrenia | ||||
|
| ||||
| Norepinephrine | Hormone and neurotransmitter | Alzheimer’s Disease | Escherichia, Bacillus, Saccharomyces | [182–187] |
| Autism | ||||
| Myalgic Encephalomyelitis | ||||
| Multiple Sclerosis | ||||
| Parkinson’s Disease | ||||
| Schizophrenia | ||||
|
| ||||
| Serotonin (5-HT) | Monoamine neurotransmitter | Alzheimer’s Disease | Candida, Streptococcus, Escherichia coli, Enterococcus, L. bolteae, L. hathewayi, F. plautii, Lactobacillus Plantarum, Lactococcus lactis subsp. Cremoris, L. lactis subsp. Lactis, Streptococcus thermophiles, Morganella morganii, Hafnia alvei | [188–196] |
| Autism | ||||
| Multiple Sclerosis | ||||
| Myalgic Encephalomyelitis | ||||
| Parkinson’s Disease | ||||
| Schizophrenia | ||||
Note
The listed microbes are reported to influence the production of the respective neurotransmitters implicated in neuroimmune disease but are not necessary implicated in the referenced neuroimmune disease directly.
As early as 2005, Logan and Katzman proposed the use of probiotics for the treatment of major depressive disorders [197]. Later studies, using animal models, supported the notion that certain psychobiotics possess antidepressant or anxiolytic properties. For instance, Barrett et al. reported that specific strains of Lactobacillus and Bifidobacterium produce GABA [167], the principal inhibitory neurotransmitter in the brain and which plays an essential role in anxiety and depression [198, 199]. In addition to producing and promoting the production of neuroactive substance, psychobiotics also can act on the brain through epigenetic modulation [26], by reducing inflammation [200, 201], and by influencing the body’s stress response via the hypothalamic-pituitary-adrenal (HPA) axis [202]. It should be noted that some prebiotics support the growth of psychobiotics and for this reason, some researchers have suggested that these prebiotics be classified as psychobiotics [203].
7. Fatty acids and polyphenols
7.1 Short chain and omega-3 fatty acids
Short-chain fatty acids are the end-products of fermentation of nondigestible carbohydrates by intestinal microbiota and have antiinflammatory and histone deacetylase-inhibiting properties [204]. As they are critical for homeostasis of the GI tract, they also represent important players in the gut-microbiota-brain axis. The three principal SCFAs produced by the intestinal microbiota, acetate, propionate, and butyrate are important for colonic health and have been implicated in protection against colitis and colorectal cancer [205–208]. Although all three are taken up by the colonic mucosa, previous studies suggest that butyrate is transported preferentially and appears to be the preferred energy source for colonocytes [209, 210]. The production of propionate is primarily restricted to anaerobic bacteria family of Clostridiales; however, the bacteria responsible for the production of the other principal SCFAs are more broadly distributed [211]. In addition to the production of SCFAs through anaerobic formation in the gut, a significant amount is found in dairy products such as whole milk and cheese [212].
Given that SCFAs are necessary for proper intestinal function and are primarily made by intestinal bacteria, perturbations in the gut microbiota may have profound effects on the gut-microbiota-brain axis. As previously articulated, several neurological disorders are characterized by intestinal comorbidity. An altered mucosal immune environment may lead to changes in the microbiota, therefore, it is reasonable to assume that restoring homeostasis of the mucosal immune system may be a first step in establishing and maintaining a healthy microbiota profile. Accordingly, introduction of SCFAs may represent one method of indirectly modifying the microbiota, and in turn the gut-microbiota-brain axis. To the best of our knowledge, no previous human clinical trials have been conducted to assess the benefit of SCFA supplementation in the treatment of neurodegenerative or neuroimmune disorders. Albeit, because of their histone deacetylase-inhibiting properties, it has been suggested that they may be of benefit in treating disease such as Huntington's disease, Parkinson's disease and amyotrophic lateral sclerosis [213].
In addition to the benefits imparted by SCFAs, several studies have reported that omega-3 polyunsaturated fatty acids (n-3-PUFAs), primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), may improve or prevent some neurological and neuroimmune disorders. For instance, Jiang et al. reported that supplementation with DHA enhanced serotoninergic and dopaminergic neurotransmission, and decreases the levels of several hypothalamic–pituitary adrenal hormones in mice, suggesting that DHA may be efficacious in treating depression [214]. Also, a number of clinical studies have shown n-3-PUFA treatments to benefit subjects with AD [215–218]. While the exact mechanisms underlying such effects are a matter of ongoing investigations, previous studies show that n-3-PUFAs are required for normal neuronal function. In fact, postmortem AD brain biopsies have been shown to exhibit lower DHA levels. Yassine and coworkers reported that the AD risk allele apolipoprotein ε4 (APOE ε4) and lower CSF Aβ42 levels were associated with decreased transport of DHA to cerebrospinal fluid and concluded that brain amyloid pathology may limit the transport of DHA to the brain [219].
Recently, Watson et al. conducted a randomized trial to investigate the effect of n-3-PUFA supplements on the human intestinal microbiota and observed that supplementation induces a reversible increase in several SCFA-producing bacteria, including Bifidobacterium, Roseburia and Lactobacillus [220]. These observations suggest that n-3-PUFA supplementation may represent an effective way to modify the productions of SCFAs, and in turn, improve GI homeostasis. Consistent with this, Ramos-Romero et al. reported that supplementation with n-3-PUFA modified the populations of Lactobacillus, Bifidobacterium and SCFAs in rats [221]; and Pusceddu and colleagues showed EPA and DHA supplementation alters the gut microbiota composition of both neurodevelopmentally normal and early-life stressed Sprague-Dawley female rats [222]. Although DHA and EPA are available commercially as purified supplements, they are present in high quantities in fish, especially cold-water fatty fish, such as salmon, mackerel, tuna, herring, and sardines
7.2 Polyphenols
Polyphenols are large organic molecules that contain at least one hydroxyl group attached to the carbon atom of an aromatic ring. They are naturally occurring in many plants and fruit and are largely responsible for their brilliant color. Seasonings are probably the highest sources of polyphenols, followed by seeds, vegetables, and fruits. Polyphenols are classified according to their structure as either flavonoids or non-flavonoids with the non-flavonoids being further subclassified as either phenolic acids, stilbenes, and lignans. They have been the focus of a significant body of research for their protective effects against cancer, cardiovascular disease, diabetes, and Alzheimer’s disease, as well as for their antiaging properties [223–226].
Several polyphenolics are found in green and black tea, and many excellent reviews are available that address the putative health benefits of tea-derived polyphenolics [227–230]. Although research that addresses their influence on the microbiome is less developed than that for other health benefits, previous studies do support the premise that these molecules have the capacity to influence the microbiota. For instance, Ankolekar et al. investigated nine tea extracts and concluded that gallic acid, quercetin, and tea catechins (including catechin, epicatechin, and epigallocatechin) have the capacity to inhibit H. pylori without affecting the beneficial lactic acid bacteria [231]. Additionally, Wang et al. reported that mice infected with E. coli O157:H7, displayed improve immune function and increased microbiota diversity upon treatment of mice with fuzhuan brick-tea extract [232]. However, Janssens and colleagues reported that long-term green tea supplementation does not change the human gut microbiome profile [233]. These studies suggest that tea-derived polyphenolics may impact pathogenic bacteria without altering the normal gut flora; however, further studies will be required with purified polyphenolics in order to make definitive determinations.
Red wine, is also a significant source of polyphenols that have been shown to influence the intestinal microflora, as well as oxidative damage and gene expression profiles of colonic mucosa. For instance, Rodrıguez Vaquero and coworkers showed that polyphenols of different wines have antibacterial properties with E. coli being most sensitive bacterium and Flavobacterium sp. showing the most resistance [234]. Also, Dolara et al. reported that polyphenols from red wine (50 mg/kg) inhibited colon cancer in a rat model [235]. They further reported that the microbiome profile was shifted in polyphenol-treated rats when compared to the control rats and that the rats not treated with carcinogens, produced a significant decrease in the basal level of DNA oxidative damage of the colon mucosa. Finally, they observed that the transcription of genes involved in inflammatory response and steroid metabolism were downregulated in colon mucosa of polyphenols-treated rats. While these studies clearly underscore the potential benefits of polyphenols in modifying and modulating the gut microbiota, most of these studies have been carried out using animal models. Therefore, more human subject studies will be required to fully appreciate their benefit.
8. Vitamins
7.2 Vitamin A
The microbiota utilizes dietary vitamins and minerals and, accordingly, these micronutrients represents potential mechanisms to modify the gut microbiota. For instance, vitamin A (vA) plays an important role in neurological function as well as regulating the central nervous system development [236, 237]. Additionally, vA, through its metabolite retinoic acid, is an important factor that promotes intestinal immunity [238] and maintains mucosal epithelial integrity [239]. vA supplementation has been shown to be efficacious for a number of disease characterized by altered microbiome profiles and neuroimmune abnormalities. For instance, Liu et al. reported that autistic children who received vA intervention displayed a significant increase in Bacteroidetes/Bacteroidales and a decrease in Bifidobacterium [240]. Additionally, they reported that vA intervention results in significant changes in autism-related biomarkers. Indeed, vA has been shown to influence commensal GI bacterial profiles. For instance, Lee and Ko reported that vA supplementation significantly increased the GI levels of Lactobacillus sp. during norovirus infection, and was associated with decreased viral load [241]. Finally, in a recent report, Hibberd and coworkers utilized a humanized microbiota mouse model to evaluate the effects of micronutrient deficiencies in humans showed that acute vA deficiency led to the largest impact [242].
7.3 Vitamin D
Several neuroimmune and neuroinflammatory diseases characterized by putative microbiome alterations, such as multiple sclerosis, autism and Alzheimer’s disease, have been associated with Vitamin D (vD) deficiency [243–245]. For instance, Shen and Ji conducted a meta-analysis of the existing literature and reported that subjects deficient for 25-hydroxyvitamin D (< 50 nmol/L) were at increased risk of developing AD by 21 % compared with those with vD levels greater than 50 nmol/L [245]. As an additional example, Mostafa and AL-Ayadhi evaluated serum 25-hydroxyvitamin D levels in 50 autistic children and 30 healthy-matched controls and determined that the autistic children had significantly lower levels than healthy children (P < 0.001) with 40% and 48% being vitamin D deficient and insufficient, respectively [246]. While direct evidence for the efficacy of vD in altering the microbiota in neuroimmune and neuroinflammatory diseases is limited, a substantial body of evidence supports its role in maintaining GI homeostasis, and potentially the microbiota, by regulating mucosal inflammatory responses [247], modulating pattern recognition receptors [248] and maintaining intestinal barrier function [249, 250].
The potential benefits of using vD to modulate the microbiota in the context of neuroimmune and neuroinflammatory disease is supported by indirect evidence. For instance, NOD2 (nucleotide-binding oligomerization domain 2), which recognizes bacterial-derived LPS, has been reported as susceptibility gene contributing to the development of Crohn’s disease (CD) [251]. Dionne and coworkers showed that when monocyte-derived dendritic cells isolated from subjects with CD are treated with the hormonal form of vD, 1,25-dihydroxy vitamin D (1,25D) a decrease in Toll-like receptor (TLR)-induced cytokine production is observed as well as NOD2-associated NF-kappa-B activation [248]. Additionally, previous studies have shown that 1,25D induces the transcription of genes that encode antimicrobial peptides [252]. Earlier studies showed that the promoters of the human cathelicidin antimicrobial peptide and defensin beta2 genes contain consensus vD response elements that mediate 1,25D-dependent gene expression [253].
Recently, Wang et al. conducted genome-wide association study (GWAS) to investigate potential genetic contributions to variations in the gut microbiota and identified polymorphisms in the vD receptor (VDR) as a significant contributing factor [254]. Although these observations may suggest that vD may play a role in disease characterized by alterations in the microbiota, future studies will be required to determine if supplementing vD as a means to treat these diseases is efficacious.
Although vD is typically supplemented as 25-hydroxyvitamin D, 1,25D is primarily the biologically active form [255]. It is largely formed in the kidneys but is also generated locally by many other body tissues. It is noteworthy that Bora et al. recently shown that germ-free mice are deficient 1,25-hydroxyvitamin D [256], suggesting that not only can vD modulate the gut microbiota, a healthy microbiome is likely necessary for vD homeostasis.
7.4 B Vitamins
In humans, B vitamins are acquired through diet or from the gut microbiota and their deficiencies are often found in patients with intestinal malabsorption [257–259]. Additionally, B vitamin deficiencies lead to deleterious neurological effects including polyneuropathy, diabetic polyneuropathy, optic atrophy, myelopathy and cerebellar ataxia [260, 261]. Recent studies have shown that the ability of the microbiota to synthesize B vitamins increases as the microbial community of the gut matures early in life [262].
Vitamin B12 is made in significant quantities by commensal bacteria in the large intestine; however, the necessary transport receptors in humans are primarily in the small intestine suggesting that the B12 produced by the microbiota are primarily consumed by the microbiota [263]. Accordingly, B12 supplementation may represent an effective way to modulate the gut microbiota, particularly in the small intestine [264].
8. Conclusions
Over the last decade, it has become evident that the GI microbiota is a key regulator of the gut-brain axis and several lines of well-accepted evidence support the premise that it influences human health and disease. Stress-related behaviors, including those relevant to anxiety and depression as well as neuroinflammatory and neuroimmune disease have all been implicated in dysregulation of the GI microbiota. Additionally, if we acknowledge that an altered microbiota may contribute the development of disease, we must also acknowledge that some dietary factors may change the microbiota in a way that negatively impacts human health. Indeed, previous studies have shown that artificial sweeteners, when given to laboratory animals, raise blood sugar levels potentially leading to insulin resistance [265–267]. Moreover, this observation is directly linked to changes in the microbiota in that non-absorbable antibiotics can reverse this observation. Additionally, several studies have shown that a high fat diet is associated with a decrease in butyrate-producing bacteria, and increased gastrointestinal inflammation [268, 269].
Numerous studies have now identified alterations in the gut microbiota in a wide range of neuroimmune diseases, although, in most instances, it has yet to be determined if the aberrant microbiota contributes to the disease or is a result of the disease [88, 270–273]. As evidence mounts connecting the gut microbiota to neuroimmune and neuroinflammatory disease, the possibility for altering the microbiota as a treatment strategy is a logical progression in an age of translational medicine.
Acknowledgments
We would like to thank Carli Kinnie, for her assistance in editing this manuscript. This work was supported by an NIH grant GM103554 (COBRE in Biology of Cell Signaling Across Membranes to RKD), by a 2016 Solve ME/CFS Ramsey Award (to RKD).
Abbreviations
- 4EPS
4-ethylphenylsulfate
- 5-HT
5-hydroxytryptamine
- AD
Alzheimer’s disease
- ASD
Autism spectrum disorders
- BDNF
Brain-derived neurotrophic factor
- CA
Catecholamines
- CD
Crohn's disease
- CNS
Central nervous system
- DHA
Docosahexaenoic acid
- EAE
Experimental autoimmune encephalomyelitis
- ENS
Enteric nervous system
- EPA
Eicosapentaenoic acid
- FEP
First episode psychosis
- GABA
Gamma-aminobutyric acid
- GALT
Gut-associated lymphoid tissues
- GI
Gastrointestinal
- HIV
Human immunodeficiency virus
- HAART
Highly active antiretroviral therapy
- IBS
Irritable bowel syndrome
- IDO
Indoleamine 2,3-dioxygenase
- IL
Interleukin
- LCFAs
Long-chain fatty acids
- ME
Myalgic encephalomyelitis
- MIA
Maternal immune activation
- MS
Multiple sclerosis
- NMDA
N-methyl-D-aspartate
- PD
Parkinson’s disease
- poly I:C
Polyinosinic:polycytidylic
- RRMS
Relapsing-remitting MS
- SCFAs
Short-chain fatty acids
- SIV
Simian immunodeficiency virus
- TLF
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
- Tregs
Regulatory T cells
- vA
Vitamin A
- vD
Vitamin D
- VDR
Vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
All authors read and approved the final manuscript. The authors declare that there are no conflicts of interests.
References
- 1.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol. 2003;17:741–54. doi: 10.1016/s1521-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 3.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–16. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boichuk SV, Khaiboullina SF, Ramazanov BR, Khasanova GR, Ivanovskaya KA, Nizamutdinov EZ, et al. Gut-Associated Plasmacytoid Dendritic Cells Display an Immature Phenotype and Upregulated Granzyme B in Subjects with HIV/AIDS. Frontiers in immunology. 2015;6:485. doi: 10.3389/fimmu.2015.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 8.Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauce D, Larsen M, Fastenackels S, Pauchard M, Ait-Mohand H, Schneider L, et al. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117:5142–51. doi: 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumbarello M, Tacconelli E, Caponera S, Cauda R, Ortona L. The impact of bacteraemia on HIV infection. Nine years experience in a large Italian university hospital. J Infect. 1995;31:123–31. doi: 10.1016/s0163-4453(95)92110-9. [DOI] [PubMed] [Google Scholar]
- 11.Manfredi R, Calza L, Chiodo F. Enteric and disseminated Campylobacter species infection during HIV disease: a persisting but significantly modified association in the HAART era. Am J Gastroenterol. 2002;97:510–1. doi: 10.1111/j.1572-0241.2002.05522.x. [DOI] [PubMed] [Google Scholar]
- 12.Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329–39. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–94. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Science translational medicine. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–9. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 17.Vujkovic-Cvijin I, Rutishauser RL, Pao M, Hunt PW, Lynch SV, McCune JM, et al. Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals. Gut Microbes. 2017;8:440–50. doi: 10.1080/19490976.2017.1334034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gareau MG. Microbiota-gut-brain axis and cognitive function. Advances in experimental medicine and biology. 2014;817:357–71. doi: 10.1007/978-1-4939-0897-4_16. [DOI] [PubMed] [Google Scholar]
- 19.Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, et al. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology. 2009;137:262–73. doi: 10.1053/j.gastro.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 20.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–12 e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304:G211–20. doi: 10.1152/ajpgi.00128.2012. [DOI] [PubMed] [Google Scholar]
- 23.Yue C, Ma B, Zhao Y, Li Q, Li J. Lipopolysaccharide-induced bacterial translocation is intestine site-specific and associates with intestinal mucosal inflammation. Inflammation. 2012;35:1880–8. doi: 10.1007/s10753-012-9510-1. [DOI] [PubMed] [Google Scholar]
- 24.Asfaha S, MacNaughton WK, Appleyard CB, Chadee K, Wallace JL. Persistent epithelial dysfunction and bacterial translocation after resolution of intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G635–44. doi: 10.1152/ajpgi.2001.281.3.G635. [DOI] [PubMed] [Google Scholar]
- 25.Biesmans S, Bouwknecht JA, Ver Donck L, Langlois X, Acton PD, De Haes P, et al. Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice. BioMed research international. 2015;2015:716920. doi: 10.1155/2015/716920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes, brain, and behavior. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 27.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Advances in experimental medicine and biology. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 28.Mazzoli R, Pessione E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front Microbiol. 2016;7:1934. doi: 10.3389/fmicb.2016.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–95. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 30.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–54 e4. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–86. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS chemical neuroscience. 2013;4:48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–30. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu WH, Chuang HL, Huang YT, Wu CC, Chou GT, Wang S, et al. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res. 2016;298:202–9. doi: 10.1016/j.bbr.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 37.Nair AT, Ramachandran V, Joghee NM, Antony S, Ramalingam G. Gut Microbiota Dysfunction as Reliable Non-invasive Early Diagnostic Biomarkers in the Pathophysiology of Parkinson's Disease: A Critical Review. J Neurogastroenterol Motil. 2018;24:30–42. doi: 10.5056/jnm17105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pessione E, Mazzoli R, Giuffrida MG, Lamberti C, Garcia-Moruno E, Barello C, et al. A proteomic approach to studying biogenic amine producing lactic acid bacteria. Proteomics. 2005;5:687–98. doi: 10.1002/pmic.200401116. [DOI] [PubMed] [Google Scholar]
- 39.Richard HT, Foster JW. Acid resistance in Escherichia coli. Adv Appl Microbiol. 2003;52:167–86. doi: 10.1016/s0065-2164(03)01007-4. [DOI] [PubMed] [Google Scholar]
- 40.Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186–94. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 41.Flegel WA, Muller F, Daubener W, Fischer HG, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659–66. doi: 10.1128/iai.59.10.3659-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel S, McCormick BA. Mucosal Inflammatory Response to Salmonella typhimurium Infection. Frontiers in immunology. 2014;5:311. doi: 10.3389/fimmu.2014.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 45.Vlantis K, Polykratis A, Welz PS, van Loo G, Pasparakis M, Wullaert A. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut. 2016;65:935–43. doi: 10.1136/gutjnl-2014-308323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–76. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNutt MD, Liu S, Manatunga A, Royster EB, Raison CL, Woolwine BJ, et al. Neurobehavioral effects of interferon-alpha in patients with hepatitis-C: symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology. 2012;37:1444–54. doi: 10.1038/npp.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchetti G, Cozzi-Lepri A, Tincati C, Calcagno A, Ceccherini-Silberstein F, De Luca A, et al. Immune activation and microbial translocation in liver disease progression in HIV/hepatitis co-infected patients: results from the Icona Foundation study. BMC Infect Dis. 2014;14:79. doi: 10.1186/1471-2334-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Curr Opin Immunol. 1993;5:247–52. doi: 10.1016/0952-7915(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 54.Beaumont M, Andriamihaja M, Lan A, Khodorova N, Audebert M, Blouin JM, et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic Biol Med. 2016;93:155–64. doi: 10.1016/j.freeradbiomed.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 55.Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond) 2010;7:79. doi: 10.1186/1743-7075-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834–41. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 59.Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. The Lancet Neurology. 2015;14:625–39. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 60.Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, et al. Pathological lesions in colonic biopsies during Parkinson's disease. Gut. 2008;57:1741–3. doi: 10.1136/gut.2008.162503. [DOI] [PubMed] [Google Scholar]
- 61.Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson's Disease. PLoS One. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci. 2012;39:185–8. doi: 10.1017/s0317167100013202. [DOI] [PubMed] [Google Scholar]
- 63.Lai SW, Liao KF, Lin CL, Sung FC. Irritable bowel syndrome correlates with increased risk of Parkinson's disease in Taiwan. European journal of epidemiology. 2014;29:57–62. doi: 10.1007/s10654-014-9878-3. [DOI] [PubMed] [Google Scholar]
- 64.Mertsalmi TH, Aho VTE, Pereira PAB, Paulin L, Pekkonen E, Auvinen P, et al. More than constipation - bowel symptoms in Parkinson's disease and their connection to gut microbiota. European journal of neurology. 2017;24:1375–83. doi: 10.1111/ene.13398. [DOI] [PubMed] [Google Scholar]
- 65.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism & related disorders. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Movement disorders : official journal of the Movement Disorder Society. 2015;30:350–8. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 67.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. Colonic bacterial composition in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2015;30:1351–60. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 68.Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, et al. Progression of Parkinson's disease is associated with gut dysbiosis: Two-year follow-up study. PLoS One. 2017;12:e0187307. doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawkes DB, Slessor KE, Bernhardt PV, De Voss JJ. Cloning, expression and purification of cindoxin, an unusual Fmn-containing cytochrome p450 redox partner. Chembiochem. 2010;11:1107–14. doi: 10.1002/cbic.201000119. [DOI] [PubMed] [Google Scholar]
- 71.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167:1469–80 e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cote M, Bourque M, Poirier AA, Aube B, Morissette M, Di Paolo T, et al. GPER1-mediated immunomodulation and neuroprotection in the myenteric plexus of a mouse model of Parkinson's disease. Neurobiology of disease. 2015;82:99–113. doi: 10.1016/j.nbd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Cote M, Poirier AA, Aube B, Jobin C, Lacroix S, Soulet D. Partial depletion of the proinflammatory monocyte population is neuroprotective in the myenteric plexus but not in the basal ganglia in a MPTP mouse model of Parkinson's disease. Brain Behav Immun. 2015;46:154–67. doi: 10.1016/j.bbi.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Pei Y, He X, Xie Z. Survival and differentiation of dopaminergic neurons can be regulated by soluble factors from cortex in vitro. Neuroreport. 2004;15:1847–50. doi: 10.1097/00001756-200408260-00002. [DOI] [PubMed] [Google Scholar]
- 75.Reinshagen M, von Boyen G, Adler G, Steinkamp M. Role of neurotrophins in inflammation of the gut. Curr Opin Investig Drugs. 2002;3:565–8. [PubMed] [Google Scholar]
- 76.Araujo AV, Santos C, Contente H, Branco C. Air everywhere: colon perforation after colonoscopy. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-219178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci. 2014;8:430. doi: 10.3389/fncel.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benisty S, Boissiere F, Faucheux B, Agid Y, Hirsch EC. trkB messenger RNA expression in normal human brain and in the substantia nigra of parkinsonian patients: an in situ hybridization study. Neuroscience. 1998;86:813–26. doi: 10.1016/s0306-4522(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 79.Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, et al. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp Neurol. 2000;166:127–35. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 80.Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson's disease substantia nigra. Neuroreport. 1999;10:557–61. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- 81.Maqsood R, Stone TW. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem Res. 2016;41:2819–35. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 82.Vidal-Martinez G, Vargas-Medrano J, Gil-Tommee C, Medina D, Garza NT, Yang B, et al. FTY720/Fingolimod Reduces Synucleinopathy and Improves Gut Motility in A53T Mice: CONTRIBUTIONS OF PRO-BRAIN-DERIVED NEUROTROPHIC FACTOR (PRO-BDNF) AND MATURE BDNF. The Journal of biological chemistry. 2016;291:20811–21. doi: 10.1074/jbc.M116.744029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devanur LD, Kerr JR. Chronic fatigue syndrome. J Clin Virol. 2006;37:139–50. doi: 10.1016/j.jcv.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 84.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Archives of internal medicine. 2000;160:221–7. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 85.Maes M, Leunis JC, Geffard M, Berk M. Evidence for the existence of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) with and without abdominal discomfort (irritable bowel) syndrome. Neuro Endocrinol Lett. 2014;35:445–53. [PubMed] [Google Scholar]
- 86.Sheedy JR, Wettenhall RE, Scanlon D, Gooley PR, Lewis DP, McGregor N, et al. Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo. 2009;23:621–8. [PubMed] [Google Scholar]
- 87.Wallis A, Ball M, McKechnie S, Butt H, Lewis DP, Bruck D. Examining clinical similarities between myalgic encephalomyelitis/chronic fatigue syndrome and D-lactic acidosis: a systematic review. Journal of translational medicine. 2017;15:129. doi: 10.1186/s12967-017-1229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fremont M, Coomans D, Massart S, De Meirleir K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe. 2013;22:50–6. doi: 10.1016/j.anaerobe.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Nagy-Szakal D, Williams BL, Mishra N, Che X, Lee B, Bateman L, et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017;5:44. doi: 10.1186/s40168-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, et al. Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) PLoS One. 2015;10:e0145453. doi: 10.1371/journal.pone.0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, et al. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. 2008;72:572–6. doi: 10.1271/bbb.70474. [DOI] [PubMed] [Google Scholar]
- 92.Queipo-Ortuno MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8:e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–20. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 94.Snell CR, Stevens SR, Davenport TE, Van Ness JM. Discriminative validity of metabolic and workload measurements for identifying people with chronic fatigue syndrome. Phys Ther. 2013;93:1484–92. doi: 10.2522/ptj.20110368. [DOI] [PubMed] [Google Scholar]
- 95.Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Molecular psychiatry. 2014;19:1252–7. doi: 10.1038/mp.2014.93. [DOI] [PubMed] [Google Scholar]
- 96.Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr Bull. 2015;41:1153–61. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 99.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016;2:16018. doi: 10.1038/npjschz.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017;62:41–5. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav Immun. 2017;62:11–23. doi: 10.1016/j.bbi.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(Suppl 2):S160–8. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 104.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 105.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015;138:179–87. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 106.Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, et al. Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine. 2017 doi: 10.1016/j.ebiom.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ong IM, Gonzalez JG, McIlwain SJ, Sawin EA, Schoen AJ, Adluru N, et al. Gut microbiome populations are associated with structure-specific changes in white matter architecture. Translational psychiatry. 2018;8:6. doi: 10.1038/s41398-017-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiao Y, Wu M, Feng Y, Zhou Z, Chen L, Chen F. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Scientific reports. 2018;8:1597. doi: 10.1038/s41598-018-19982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ascherio A. Environmental factors in multiple sclerosis. Expert Rev Neurother. 2013;13:3–9. doi: 10.1586/14737175.2013.865866. [DOI] [PubMed] [Google Scholar]
- 111.Forte GI, Ragonese P, Salemi G, Scola L, Candore G, D'Amelio M, et al. Search for genetic factors associated with susceptibility to multiple sclerosis. Ann N Y Acad Sci. 2006;1067:264–9. doi: 10.1196/annals.1354.034. [DOI] [PubMed] [Google Scholar]
- 112.Ghezzi A, Zaffaroni M, Caputo D, Marforio S, Montanini R. Cerebral evoked potentials and cerebrospinal fluid examination in progressive spastic paraparesis (possible multiple sclerosis) Riv Neurobiol. 1984;30:544–50. [PubMed] [Google Scholar]
- 113.Levinthal DJ, Rahman A, Nusrat S, O'Leary M, Heyman R, Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int. 2013;2013:319201. doi: 10.1155/2013/319201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Scientific reports. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10:e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. 2015;63:729–34. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–93. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–11. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 108(Suppl 1):4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4:30. doi: 10.1186/s40168-016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–7. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 122.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctot KL, et al. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer's Disease. Curr Pharm Des. 2016;22:6152–66. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 124.Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1DeltaE9 murine model of Alzheimer's disease. Scientific reports. 2017;7:10411. doi: 10.1038/s41598-017-11047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Scientific reports. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer's disease. Scientific reports. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dagda RK, Das Banerjee T. Role of protein kinase A in regulating mitochondrial function and neuronal development: implications to neurodegenerative diseases. Rev Neurosci. 2015;26:359–70. doi: 10.1515/revneuro-2014-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–92. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 130.Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158–79. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics--approaching a definition. Am J Clin Nutr. 2001;73:361S–4S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 132.Depeint F, Tzortzis G, Vulevic J, I'Anson K, Gibson GR. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr. 2008;87:785–91. doi: 10.1093/ajcn/87.3.785. [DOI] [PubMed] [Google Scholar]
- 133.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 134.Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015;32:42–6. doi: 10.1016/j.copbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 135.Kulinich A, Liu L. Human milk oligosaccharides: The role in the fine-tuning of innate immune responses. Carbohydr Res. 2016;432:62–70. doi: 10.1016/j.carres.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 136.Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr. 2005;135:1304–7. doi: 10.1093/jn/135.5.1304. [DOI] [PubMed] [Google Scholar]
- 137.Andriulli A, Neri M, Loguercio C, Terreni N, Merla A, Cardarella MP, et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S218–23. doi: 10.1097/MCG.0b013e31817fadd6. [DOI] [PubMed] [Google Scholar]
- 138.Whorwell PJ. Do probiotics improve symptoms in patients with irritable bowel syndrome? Therap Adv Gastroenterol. 2009;2:37–44. doi: 10.1177/1756283X09335637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pontier-Bres R, Prodon F, Munro P, Rampal P, Lemichez E, Peyron JF, et al. Modification of Salmonella Typhimurium motility by the probiotic yeast strain Saccharomyces boulardii. PLoS One. 2012;7:e33796. doi: 10.1371/journal.pone.0033796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li P, Gu Q, Wang Y, Yu Y, Yang L, Chen JV. Novel vitamin B12-producing Enterococcus spp. and preliminary in vitro evaluation of probiotic potentials. Appl Microbiol Biotechnol. 2017;101:6155–64. doi: 10.1007/s00253-017-8373-7. [DOI] [PubMed] [Google Scholar]
- 141.Pu F, Guo Y, Li M, Zhu H, Wang S, Shen X, et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin Interv Aging. 2017;12:1223–31. doi: 10.2147/CIA.S141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S, Zhu H, He F, Luo Y, Kang Z, Lu C, et al. Whole Genome Sequence of the Probiotic Strain Lactobacillus paracasei N1115, Isolated from Traditional Chinese Fermented Milk. Genome Announc. 2014;2 doi: 10.1128/genomeA.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 144.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–6. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 145.O'Sullivan E, Barrett E, Grenham S, Fitzgerald P, Stanton C, Ross RP, et al. BDNF expression in the hippocampus of maternally separated rats: does Bifidobacterium breve 6330 alter BDNF levels? Benef Microbes. 2011;2:199–207. doi: 10.3920/BM2011.0015. [DOI] [PubMed] [Google Scholar]
- 146.Xiong P, Zeng Y, Wu Q, Han Huang DX, Zainal H, Xu X, et al. Combining serum protein concentrations to diagnose schizophrenia: a preliminary exploration. J Clin Psychiatry. 2014;75:e794–801. doi: 10.4088/JCP.13m08772. [DOI] [PubMed] [Google Scholar]
- 147.Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–16. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 149.Bryn V, Halvorsen B, Ueland T, Isaksen J, Kolkova K, Ravn K, et al. Brain derived neurotrophic factor (BDNF) and autism spectrum disorders (ASD) in childhood. Eur J Paediatr Neurol. 2015;19:411–4. doi: 10.1016/j.ejpn.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 150.Jukkola P, Gu Y, Lovett-Racke AE, Gu C. Suppression of Inflammatory Demyelinaton and Axon Degeneration through Inhibiting Kv3 Channels. Front Mol Neurosci. 2017;10:344. doi: 10.3389/fnmol.2017.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moriya J, Chen R, Yamakawa J, Sasaki K, Ishigaki Y, Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull. 2011;34:354–9. doi: 10.1248/bpb.34.354. [DOI] [PubMed] [Google Scholar]
- 152.Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. Journal of neurology. 2010;257:540–5. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- 153.Storga D, Vrecko K, Birkmayer JG, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203:29–32. doi: 10.1016/0304-3940(95)12256-7. [DOI] [PubMed] [Google Scholar]
- 154.Chung SJ, Lee JJ, Ham JH, Ye BS, Lee PH, Sohn YH. Striatal Dopamine Depletion Patterns and Early Non-Motor Burden in Parkinsons Disease. PLoS One. 2016;11:e0161316. doi: 10.1371/journal.pone.0161316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, et al. Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun. 2013;31:153–60. doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kriete T, Noelle DC. Dopamine and the development of executive dysfunction in autism spectrum disorders. PLoS One. 2015;10:e0121605. doi: 10.1371/journal.pone.0121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Warren N, O'Gorman C, Lehn A, Siskind D. Dopamine dysregulation syndrome in Parkinson's disease: a systematic review of published cases. J Neurol Neurosurg Psychiatry. 2017;88:1060–4. doi: 10.1136/jnnp-2017-315985. [DOI] [PubMed] [Google Scholar]
- 158.Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Translational psychiatry. 2018;8:30. doi: 10.1038/s41398-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Levite M, Marino F, Cosentino M. Dopamine, T cells and multiple sclerosis (MS) J Neural Transm (Vienna) 2017;124:525–42. doi: 10.1007/s00702-016-1640-4. [DOI] [PubMed] [Google Scholar]
- 160.Taj A, Jamil N. Bioconversion of Tyrosine and Tryptophan Derived Biogenic Amines by Neuropathogenic Bacteria. Biomolecules. 2018;8 doi: 10.3390/biom8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574–81. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 162.Paula-Lima AC, Brito-Moreira J, Ferreira ST. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer's disease. J Neurochem. 2013;126:191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 163.Fuhrer TE, Palpagama TH, Waldvogel HJ, Synek BJL, Turner C, Faull RL, et al. Impaired expression of GABA transporters in the human Alzheimer's disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. Neuroscience. 2017;351:108–18. doi: 10.1016/j.neuroscience.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 164.Cao G, Edden RAE, Gao F, Li H, Gong T, Chen W, et al. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur Radiol. 2018;28:1140–8. doi: 10.1007/s00330-017-5064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoftman GD, Dienel SJ, Bazmi HH, Zhang Y, Chen K, Lewis DA. Altered Gradients of Glutamate and Gamma-Aminobutyric Acid Transcripts in the Cortical Visuospatial Working Memory Network in Schizophrenia. Biol Psychiatry. 2018;83:670–9. doi: 10.1016/j.biopsych.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.El-Ansary A, Bacha AB, Bjorklund G, Al-Orf N, Bhat RS, Moubayed N, et al. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab Brain Dis. 2018 doi: 10.1007/s11011-018-0212-8. [DOI] [PubMed] [Google Scholar]
- 167.Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 168.Lakhan SE, Caro M, Hadzimichalis N. NMDA Receptor Activity in Neuropsychiatric Disorders. Front Psychiatry. 2013;4:52. doi: 10.3389/fpsyt.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith AK, Fang H, Whistler T, Unger ER, Rajeevan MS. Convergent genomic studies identify association of GRIK2 and NPAS2 with chronic fatigue syndrome. Neuropsychobiology. 2011;64:183–94. doi: 10.1159/000326692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schlauch KA, Khaiboullina SF, De Meirleir KL, Rawat S, Petereit J, Rizvanov AA, et al. Genome-wide association analysis identifies genetic variations in subjects with myalgic encephalomyelitis/chronic fatigue syndrome. Translational psychiatry. 2016;6:e730. doi: 10.1038/tp.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zareian M, Ebrahimpour A, Bakar FA, Mohamed AK, Forghani B, Ab-Kadir MS, et al. A glutamic acid-producing lactic acid bacteria isolated from Malaysian fermented foods. Int J Mol Sci. 2012;13:5482–97. doi: 10.3390/ijms13055482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu W, Chen Z. The roles of histamine and its receptor ligands in central nervous system disorders: An update. Pharmacol Ther. 2017;175:116–32. doi: 10.1016/j.pharmthera.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 173.Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62 doi: 10.1111/jpi.12399. [DOI] [PubMed] [Google Scholar]
- 174.Paulose JK, Wright JM, Patel AG, Cassone VM. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS One. 2016;11:e0146643. doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wen J, Ariyannur PS, Ribeiro R, Tanaka M, Moffett JR, Kirmani BF, et al. Efficacy of N-Acetylserotonin and Melatonin in the EAE Model of Multiple Sclerosis. J Neuroimmune Pharmacol. 2016;11:763–73. doi: 10.1007/s11481-016-9702-9. [DOI] [PubMed] [Google Scholar]
- 176.Alvarez-Sanchez N, Cruz-Chamorro I, Diaz-Sanchez M, Sarmiento-Soto H, Medrano-Campillo P, Martinez-Lopez A, et al. Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J Pineal Res. 2017;63 doi: 10.1111/jpi.12442. [DOI] [PubMed] [Google Scholar]
- 177.Hoppe JB, Frozza RL, Horn AP, Comiran RA, Bernardi A, Campos MM, et al. Amyloid-beta neurotoxicity in organotypic culture is attenuated by melatonin: involvement of GSK-3beta, tau and neuroinflammation. J Pineal Res. 2010;48:230–8. doi: 10.1111/j.1600-079X.2010.00747.x. [DOI] [PubMed] [Google Scholar]
- 178.Anderson G, Maes M. Melatonin: an overlooked factor in schizophrenia and in the inhibition of anti-psychotic side effects. Metab Brain Dis. 2012;27:113–9. doi: 10.1007/s11011-012-9307-9. [DOI] [PubMed] [Google Scholar]
- 179.Paul R, Phukan BC, Justin Thenmozhi A, Manivasagam T, Bhattacharya P, Borah A. Melatonin protects against behavioral deficits, dopamine loss and oxidative stress in homocysteine model of Parkinson's disease. Life Sci. 2018;192:238–45. doi: 10.1016/j.lfs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 180.van Heukelom RO, Prins JB, Smits MG, Bleijenberg G. Influence of melatonin on fatigue severity in patients with chronic fatigue syndrome and late melatonin secretion. European journal of neurology. 2006;13:55–60. doi: 10.1111/j.1468-1331.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 181.Braam W, Ehrhart F, Maas A, Smits MG, Curfs L. Low maternal melatonin level increases autism spectrum disorder risk in children. Research in developmental disabilities. 2018 doi: 10.1016/j.ridd.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 182.Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, et al. Targeting norepinephrine in mild cognitive impairment and Alzheimer's disease. Alzheimers Res Ther. 2013;5:21. doi: 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vazey EM, Aston-Jones G. The emerging role of norepinephrine in cognitive dysfunctions of Parkinson's disease. Front Behav Neurosci. 2012;6:48. doi: 10.3389/fnbeh.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer's disease. Biol Psychiatry. 1999;46:1243–52. doi: 10.1016/s0006-3223(99)00232-2. [DOI] [PubMed] [Google Scholar]
- 185.Strahler J, Fischer S, Nater UM, Ehlert U, Gaab J. Norepinephrine and epinephrine responses to physiological and pharmacological stimulation in chronic fatigue syndrome. Biol Psychol. 2013;94:160–6. doi: 10.1016/j.biopsycho.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 186.Rajda C, Bencsik K, Fuvesi J, Seres E, Vecsei L, Bergquist J. The norepinephrine level is decreased in the lymphocytes of long-term interferon-beta-treated multiple sclerosis patients. Mult Scler. 2006;12:265–70. doi: 10.1191/135248506ms1269oa. [DOI] [PubMed] [Google Scholar]
- 187.Lake CR, Ziegler MG, Murphy DL. Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Arch Gen Psychiatry. 1977;34:553–6. doi: 10.1001/archpsyc.1977.01770170063005. [DOI] [PubMed] [Google Scholar]
- 188.Shishov VA, Kirovskaia TA, Kudrin VS, Oleskin AV. [Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12] Prikl Biokhim Mikrobiol. 2009;45:550–4. [PubMed] [Google Scholar]
- 189.Kuley E, Ozogul F, Ozogul Y, Akyol I. The function of lactic acid bacteria and brine solutions on biogenic amine formation by foodborne pathogens in trout fillets. Food Chem. 2011;129:1211–6. doi: 10.1016/j.foodchem.2011.05.113. [DOI] [PubMed] [Google Scholar]
- 190.Özoğul F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur Food Res Technol. 2004;219:465–9. [Google Scholar]
- 191.Claeysen S, Bockaert J, Giannoni P. Serotonin: A New Hope in Alzheimer's Disease? ACS chemical neuroscience. 2015;6:940–3. doi: 10.1021/acschemneuro.5b00135. [DOI] [PubMed] [Google Scholar]
- 192.Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, et al. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell Mol Gastroenterol Hepatol. 2017;3:218–30. doi: 10.1016/j.jcmgh.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vermeiren Y, Janssens J, Van Dam D, De Deyn PP. Serotonergic Dysfunction in Amyotrophic Lateral Sclerosis and Parkinson's Disease: Similar Mechanisms, Dissimilar Outcomes. Front Neurosci. 2018;12:185. doi: 10.3389/fnins.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dinan TG, Majeed T, Lavelle E, Scott LV, Berti C, Behan P. Blunted serotonin-mediated activation of the hypothalamic-pituitary-adrenal axis in chronic fatigue syndrome. Psychoneuroendocrinology. 1997;22:261–7. doi: 10.1016/s0306-4530(97)00002-4. [DOI] [PubMed] [Google Scholar]
- 195.Malinova TS, Dijkstra CD, de Vries HE. Serotonin: A mediator of the gut-brain axis in multiple sclerosis. Mult Scler. 2017:1352458517739975. doi: 10.1177/1352458517739975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fjukstad KK, Engum A, Lydersen S, Dieset I, Steen NE, Andreassen OA, et al. Metabolic risk factors in schizophrenia and bipolar disorder: The effect of comedication with selective serotonin reuptake inhibitors and antipsychotics. Eur Psychiatry. 2018;48:71–8. doi: 10.1016/j.eurpsy.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 197.Logan AC, Katzman M. Major depressive disorder: probiotics may be an adjuvant therapy. Med Hypotheses. 2005;64:533–8. doi: 10.1016/j.mehy.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 198.Catavero C, Bao H, Song J. Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 2018;371:33–46. doi: 10.1007/s00441-017-2668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sibbe M, Kulik A. GABAergic Regulation of Adult Hippocampal Neurogenesis. Molecular neurobiology. 2017;54:5497–510. doi: 10.1007/s12035-016-0072-3. [DOI] [PubMed] [Google Scholar]
- 200.Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, et al. Oral Administration of the Probiotic Strain Escherichia coli Nissle 1917 Reduces Susceptibility to Neuroinflammation and Repairs Experimental Autoimmune Encephalomyelitis-Induced Intestinal Barrier Dysfunction. Frontiers in immunology. 2017;8:1096. doi: 10.3389/fimmu.2017.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Luna RA, Foster JA. Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr Opin Biotechnol. 2015;32:35–41. doi: 10.1016/j.copbio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 203.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–81. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–76. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34:386–91. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Archer SY, Meng S, Shei A, Hodin RA. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci U S A. 1998;95:6791–6. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39:164–71. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- 208.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Clausen MR, Mortensen PB. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995;37:684–9. doi: 10.1136/gut.37.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ritzhaupt A, Ellis A, Hosie KB, Shirazi-Beechey SP. The characterization of butyrate transport across pig and human colonic luminal membrane. J Physiol. 1998;507(Pt 3):819–30. doi: 10.1111/j.1469-7793.1998.819bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–35. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–32. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 213.Hahnen E, Hauke J, Trankle C, Eyupoglu IY, Wirth B, Blumcke I. Histone deacetylase inhibitors: possible implications for neurodegenerative disorders. Expert Opin Investig Drugs. 2008;17:169–84. doi: 10.1517/13543784.17.2.169. [DOI] [PubMed] [Google Scholar]
- 214.Jiang LH, Liang QY, Shi Y. Pure docosahexaenoic acid can improve depression behaviors and affect HPA axis in mice. Eur Rev Med Pharmacol Sci. 2012;16:1765–73. [PubMed] [Google Scholar]
- 215.Irving GF, Freund-Levi Y, Eriksdotter-Jonhagen M, Basun H, Brismar K, Hjorth E, et al. Omega-3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer's disease: the omega-3 Alzheimer's disease study. J Am Geriatr Soc. 2009;57:11–7. doi: 10.1111/j.1532-5415.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 216.Freund Levi Y, Vedin I, Cederholm T, Basun H, Faxen Irving G, Eriksdotter M, et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: the OmegAD study. J Intern Med. 2014;275:428–36. doi: 10.1111/joim.12166. [DOI] [PubMed] [Google Scholar]
- 217.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, et al. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28:845–55. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 218.Serini S, Bizzarro A, Piccioni E, Fasano E, Rossi C, Lauria A, et al. EPA and DHA differentially affect in vitro inflammatory cytokine release by peripheral blood mononuclear cells from Alzheimer's patients. Curr Alzheimer Res. 2012;9:913–23. doi: 10.2174/156720512803251147. [DOI] [PubMed] [Google Scholar]
- 219.Yassine HN, Rawat V, Mack WJ, Quinn JF, Yurko-Mauro K, Bailey-Hall E, et al. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer's disease. Alzheimers Res Ther. 2016;8:25. doi: 10.1186/s13195-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2017 doi: 10.1136/gutjnl-2017-314968. [DOI] [PubMed] [Google Scholar]
- 221.Ramos-Romero S, Hereu M, Molinar-Toribio E, Almajano MP, Mendez L, Medina I, et al. Effects of the combination of omega-3 PUFAs and proanthocyanidins on the gut microbiota of healthy rats. Food Res Int. 2017;97:364–71. doi: 10.1016/j.foodres.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 222.Pusceddu MM, El Aidy S, Crispie F, O'Sullivan O, Cotter P, Stanton C, et al. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS One. 2015;10:e0139721. doi: 10.1371/journal.pone.0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5:3779–827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thakur VS, Gupta K, Gupta S. The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr Pharm Biotechnol. 2012;13:191–9. doi: 10.2174/138920112798868584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients. 2014;6:6020–47. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer's disease prevention and treatment. Biochim Biophys Acta. 2015;1852:1202–8. doi: 10.1016/j.bbadis.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 228.Khokhar S, Magnusdottir SG. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United kingdom. J Agric Food Chem. 2002;50:565–70. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- 229.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Matsui T. Condensed catechins and their potential health-benefits. Eur J Pharmacol. 2015;765:495–502. doi: 10.1016/j.ejphar.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 231.Ankolekar C, Johnson D, Pinto Mda S, Johnson K, Labbe R, Shetty K. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J Med Food. 2011;14:1321–9. doi: 10.1089/jmf.2010.0237. [DOI] [PubMed] [Google Scholar]
- 232.Wang Y, Xu A, Liu P, Li Z. Effects of Fuzhuan Brick-Tea Water Extract on Mice Infected with E. coli O157:H7. Nutrients. 2015;7:5309–26. doi: 10.3390/nu7075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Janssens PL, Penders J, Hursel R, Budding AE, Savelkoul PH, Westerterp-Plantenga MS. Long-Term Green Tea Supplementation Does Not Change the Human Gut Microbiota. PLoS One. 2016;11:e0153134. doi: 10.1371/journal.pone.0153134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rodríguez Vaqueroab MJ, Albertob MR, Mancade Nadra MC. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18 [Google Scholar]
- 235.Dolara P, Luceri C, De Filippo C, Femia AP, Giovannelli L, Caderni G, et al. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res. 2005;591:237–46. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 236.Haushalter C, Asselin L, Fraulob V, Dolle P, Rhinn M. Retinoic acid controls early neurogenesis in the developing mouse cerebral cortex. Developmental biology. 2017;430:129–41. doi: 10.1016/j.ydbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 237.Park JC, Jeong WJ, Kim MY, Min D, Choi KY. Retinoic-acid-mediated HRas stabilization induces neuronal differentiation of neural stem cells during brain development. J Cell Sci. 2016;129:2997–3007. doi: 10.1242/jcs.184366. [DOI] [PubMed] [Google Scholar]
- 238.Kunisawa J, Kiyono H. Vitamin-mediated regulation of intestinal immunity. Frontiers in immunology. 2013;4:189. doi: 10.3389/fimmu.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pacific journal of allergy and immunology. 2015;33:71–89. [PubMed] [Google Scholar]
- 240.Liu J, Liu X, Xiong XQ, Yang T, Cui T, Hou NL, et al. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders - a pilot study. BMC microbiology. 2017;17:204. doi: 10.1186/s12866-017-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee H, Ko G. New perspectives regarding the antiviral effect of vitamin A on norovirus using modulation of gut microbiota. Gut Microbes. 2017:1–5. doi: 10.1080/19490976.2017.1353842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW, et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Science translational medicine. 2017;9 doi: 10.1126/scitranslmed.aal4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kocovska E, Gaughran F, Krivoy A, Meier UC. Vitamin-D Deficiency As a Potential Environmental Risk Factor in Multiple Sclerosis, Schizophrenia, and Autism. Front Psychiatry. 2017;8:47. doi: 10.3389/fpsyt.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dursun E, Alaylioglu M, Bilgic B, Hanagasi H, Lohmann E, Atasoy IL, et al. Vitamin D deficiency might pose a greater risk for ApoEvarepsilon4 non-carrier Alzheimer's disease patients. Neurol Sci. 2016;37:1633–43. doi: 10.1007/s10072-016-2647-1. [DOI] [PubMed] [Google Scholar]
- 245.Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta-analysis. Nutr J. 2015;14:76. doi: 10.1186/s12937-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mostafa GA, Al-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin D in children with autism: relation to autoimmunity. J Neuroinflammation. 2012;9:201. doi: 10.1186/1742-2094-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–36. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dionne S, Calderon MR, White JH, Memari B, Elimrani I, Adelson B, et al. Differential effect of vitamin D on NOD2- and TLR-induced cytokines in Crohn's disease. Mucosal Immunol. 2014;7:1405–15. doi: 10.1038/mi.2014.30. [DOI] [PubMed] [Google Scholar]
- 249.Zhang YG, Wu S, Sun J. Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers. 2013;1 doi: 10.4161/tisb.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, et al. Tight junction proteins claudin-2 and-12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–21. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Balasubramanian I, Gao N. From sensing to shaping microbiota: insights into the role of NOD2 in intestinal homeostasis and progression of Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2017;313:G7–G13. doi: 10.1152/ajpgi.00330.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 253.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 254.Wang J, Thingholm LB, Skieceviciene J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nature genetics. 2016;48:1396–406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bikle D. Vitamin D: Production, Metabolism, and Mechanisms of Action. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext. South Dartmouth (MA): 2000. [PubMed] [Google Scholar]
- 256.Bora SA, Kennett MJ, Smith PB, Patterson AD, Cantorna MT. The Gut Microbiota Regulates Endocrine Vitamin D Metabolism through Fibroblast Growth Factor 23. Frontiers in immunology. 2018;9:408. doi: 10.3389/fimmu.2018.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ao M, Tsuji H, Shide K, Kosaka Y, Noda A, Inagaki N, et al. High prevalence of vitamin B-12 insufficiency in patients with Crohn's disease. Asia Pac J Clin Nutr. 2017;26:1076–81. doi: 10.6133/apjcn.022017.13. [DOI] [PubMed] [Google Scholar]
- 258.Erzin Y, Uzun H, Celik AF, Aydin S, Dirican A, Uzunismail H. Hyperhomocysteinemia in inflammatory bowel disease patients without past intestinal resections: correlations with cobalamin, pyridoxine, folate concentrations, acute phase reactants, disease activity, and prior thromboembolic complications. J Clin Gastroenterol. 2008;42:481–6. doi: 10.1097/MCG.0b013e318046eab0. [DOI] [PubMed] [Google Scholar]
- 259.Phelip JM, Ducros V, Faucheron JL, Flourie B, Roblin X. Association of hyperhomocysteinemia and folate deficiency with colon tumors in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:242–8. doi: 10.1002/ibd.20309. [DOI] [PubMed] [Google Scholar]
- 260.Russo C, Morabito F, Luise F, Piromalli A, Battaglia L, Vinci A, et al. Hyperhomocysteinemia is associated with cognitive impairment in multiple sclerosis. Journal of neurology. 2008;255:64–9. doi: 10.1007/s00415-007-0668-7. [DOI] [PubMed] [Google Scholar]
- 261.Langohr HD, Petruch F, Schroth G. Vitamin B 1, B 2 and B 6 deficiency in neurological disorders. Journal of neurology. 1981;225:95–108. doi: 10.1007/BF00313323. [DOI] [PubMed] [Google Scholar]
- 262.Hu J, Nie Y, Chen J, Zhang Y, Wang Z, Fan Q, et al. Gradual Changes of Gut Microbiota in Weaned Miniature Piglets. Front Microbiol. 2016;7:1727. doi: 10.3389/fmicb.2016.01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Seetharam B, Alpers DH. Absorption and transport of cobalamin (vitamin B12) Annual review of nutrition. 1982;2:343–69. doi: 10.1146/annurev.nu.02.070182.002015. [DOI] [PubMed] [Google Scholar]
- 264.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell metabolism. 2014;20:769–78. doi: 10.1016/j.cmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 2015;6:149–55. doi: 10.1080/19490976.2015.1017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS One. 2017;12:e0178426. doi: 10.1371/journal.pone.0178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 268.Xiao L, Sonne SB, Feng Q, Chen N, Xia Z, Li X, et al. High-fat feeding rather than obesity drives taxonomical and functional changes in the gut microbiota in mice. Microbiome. 2017;5:43. doi: 10.1186/s40168-017-0258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jakobsdottir G, Xu J, Molin G, Ahrne S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One. 2013;8:e80476. doi: 10.1371/journal.pone.0080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brandscheid C, Schuck F, Reinhardt S, Schafer KH, Pietrzik CU, Grimm M, et al. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer's Mouse Model. Journal of Alzheimer's disease : JAD. 2017;56:775–88. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- 272.Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Colpitts SL, Kasper LH. Influence of the Gut Microbiome on Autoimmunity in the Central Nervous System. J Immunol. 2017;198:596–604. doi: 10.4049/jimmunol.1601438. [DOI] [PubMed] [Google Scholar]