Abstract
Our ability to modulate and observe neuronal activity in defined neurons in freely moving animals has revolutionized neuroscience research in recent years. Findings in the lateral hypothalamus (LHA) highlighted the existence of many neuronal circuits that regulate distinct phenotypes of feeding behavior, emotional valence, and locomotor activity. Several of these neuronal circuits do not fit into a common model of neuronal integration and highlight the need to improve working models for complex behaviors. This review will specifically focus on recent literature that distinguishes LHA circuits based on their molecular and anatomical characteristics and studies their role in feeding, associated behaviors (e.g., arousal and locomotion), and emotional states (e.g., emotional valences).
The central nervous system (CNS) is responsible for generating feelings of hunger and controlling food intake behaviors in response to internal and external stimuli (1). To understand the central processes involved in the pathogenesis of obesity and to identify potential drug targets, researchers have focused their studies on understanding hypothalamic functions. In the past decades, our understanding of hypothalamic modulations of many behaviors and physiological adaptations (including reproductive, sexual, aggression, social, thermoregulation, and food intake) has evolved tremendously (2–10).
Early research distinguished the hypothalamus into feeding and satiety centers, located in the lateral hypothalamic area (LHA) and the ventromedial hypothalamus, respectively, because electric stimulation or ablation showed robust and opposing changes in feeding behavior (11–14). The LHA also elicited robust electric self-stimulation (15), and the important interaction of reward, learning, and food intake was suggested (16). However, the neuronal origin that contributed to the observed feeding behaviors remained a matter of debate, because electric lesions and stimulations could modulate local cell bodies as well as fibers of passage (14). A major move forward came with methodological advances in immunohistochemistry and pathway tracing that allowed an unprecedented resolution of hypothalamic circuits and confirmed a rich LHA interconnection (17). With the discovery of individual neurons within the arcuate nucleus (ARC) that harbor their own sets of neurons to drive opposing feeding behaviors (18, 19), the idea of feeding and satiety centers has been replaced with opposing acting feeding and satiety neurons.
Over the past decade, a revolution took place in neuroscience research, with many exciting new technologies that allow the live observation or remote control of neuronal activity from identified neurons and their specific projection sites in freely behaving animals. This development resulted in a plethora of newly discovered neuronal populations and circuits that contribute to energy homeostasis and connect many central sites to feeding behavior or aspects of feeding behavior (20–24).
In the LHA, γ-aminobutyric acid (GABA)ergic and glutamatergic neuronal populations were recently identified according to their opposing effects on feeding and satiety. However, several LHA-induced behaviors are inconsistent with ARC feeding circuits. This inconsistency raises the question of whether and how LHA feeding circuits integrate into well-studied ARC feeding circuits. This review specifically focuses on recent literature of distinct GABAergic and glutamatergic LHA neuronal circuits, their role in feeding behavior, feeding-associated behaviors (emotional valences, arousal, and locomotion) and their relation to ARC feeding circuits. Finally, we highlight remaining gaps in our understanding of LHA feeding circuits.
Distinguishing Feeding Behaviors
Energy intake and energy expenditure are both highly variable and modulated parameters that ensure body weight maintenance. Energy intake and food intake are loosely defined terms that can refer to a variety of ingestive behaviors that have been well characterized in the past (1, 25). New molecular genetic methods that modulate or monitor acute changes in neuronal activation (e.g., optogenetics or fiber photometry) have more extensively focused on real-time behavioral changes (e.g., short-term food intake, food seeking, food consumption, positive and negative valences associated with feeding and energy status), in contrast to measuring daily food intake, and a clear distinction of these terminologies is warranted.
In this review, we use the term metabolic food intake to represent the daily caloric food intake necessary to fulfill metabolic needs and to maintain a stable body weight. This is in contrast to acute feeding behaviors, which contribute to metabolic food intake (Fig. 1) (1, 25) and can be distinguished into appetitive feeding (food seeking, foraging, food approach) and consumatory feeding (actual food consumption, meal, feeding bout). The decision to eat and how much to eat or not to eat is influenced by circadian time (sleep-wake cycle, arousal), external and internal factors that modulate the internal need states and determine whether we feel hungry or satiated. External factors include possible danger (e.g., predators), availability of food, and recognition of food sources. Internal factors are available energy stores, associated changes in circulating nutrients (glucose, fatty acids, and amino acids), and hormones (e.g., leptin, ghrelin, asprosin) and learned emotional valences that are associated with a food source (23, 26–28).
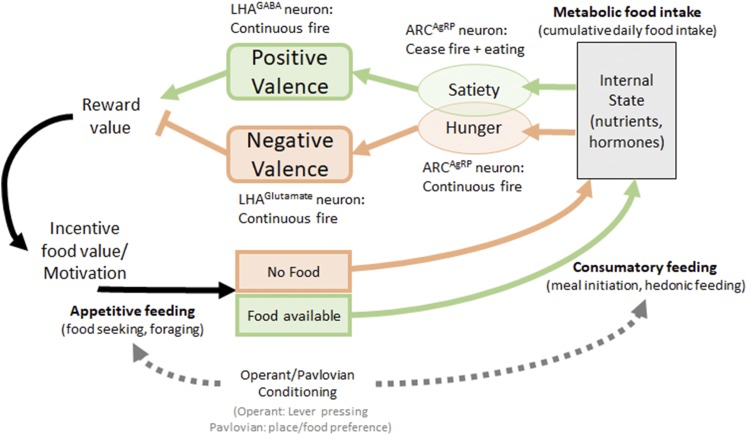
Figure 1.
Model of feeding behavior regulation. The internal state informs the body about energy need states, such as hunger and satiety, and is induced by changes in neuronal activity of ARCAgRP neurons. Hunger and satiety are associated with negative or positive valences and play an integral role in modulating reward value or the incentive value of food and change the motivation to seek out food (e.g., lever pressing in an operant conditioning task). Activation of select neuronal populations in the ARC and LHA modulates both emotional valences and feeding behaviors, depending on activation timing and activation context.
Emotional valence is a measure of pleasure associated with an internal state or related context and can modulate the motivation to pursue a food reward [for further review, see Berridge and Kringelbach (29)]. These changes in motivation modulate learned Pavlovian and operant behavior and are used to measure emotional valences in conditioned place and food preference tasks (Pavlovian conditioning) or lever pressing for rewards (operant conditioning) (30–33). Importantly, emotional valences are modulated by internal state changes and are powerful enough to reverse the rewarding effect and incentive value of food, as measured in food reactivity tests (34) or in Pavlovian and operant conditioning tasks (32, 33). Food intake measured in conditioned tasks and with palatable foods (e.g., rich in sugar or fat) is called hedonic food intake (35), to emphasize the associated positive valence, and typically measures short-term feeding episodes. Although metabolic food intake is composed of these short-term feeding events, the distinction is important because the regulation of acute feeding behavior seems distinct from long-term regulation of metabolic food intake. For example, increased hedonic food intake episodes may be compensated throughout the day by reduced chow intake. Furthermore, only neuronal circuits that ultimately can modulate metabolic food intake and body weight are attractive targets to treat obesity.
ARC Neurons Mediate Hunger Valences to the LHA
The ARC mediates metabolic need states via two opposing sets of ARC neurons: anorexigenic pro-opiomelanocortin (POMC) expressing neurons and orexigenic agouti-related peptide (AgRP) expressing neurons (18, 19, 36). During metabolic need states, such as fasting, humoral signals change dramatically (e.g., the adipose tissue–derived hormone leptin decreases to signal depletion of body fat stores, whereas the gut hormone ghrelin increases before meals) (37–40). ARCPOMC and ARCAgRP neurons are differentially regulated by leptin and ghrelin, resulting in neuronal silencing of ARCPOMC neurons and neuronal activation of ARCAgRP neurons in fasted, hungry mice (41–45).
The behavioral feeding responses to fasting can be mimicked by activation of ARCAgRP neurons alone (notably in the absence of a metabolic need state), strongly supporting ARCAgRP neurons as an integral way to communicate the feeling of hunger that is associated with metabolic need states (18, 19, 36, 46). The LHA receives strong input from ARCPOMC and ARCAgRP neurons, and both neurons and their peptides are able to modulate neuronal activity in LHA neurons (47–49). Importantly, ARCAgRP neurons mediate hunger-induced feeding behavior via several brain sites that include the paraventricular nucleus (PVN), bed nucleus of stria terminalis (BNST), and the LHA itself (33, 50).
Activation of ARCAgRP neurons results in long-term metabolic food intake changes that are able to cause body weight changes according to the activation level of ARCAgRP neurons and also increases the incentive value of food in a progressive ratio task (19, 46, 51). Thus, activation of ARCAgRP neurons and hunger information is identified by changes in metabolic food intake (24 hours food intake) and hedonic food intake (operant responding, palatable food intake). Importantly, hunger or activation of ARCAgRP neurons mediates a negative valence that will cause conditioned avoidance (32), which is in line with the idea of informing the body about a need state and motivating the animal to escape the need state, in this case by seeking and eating food. Conversely, ARCAgRP neuronal activation is quickly reversed by meal initiation (consumatory feeding) and even by the mere sight or approach of food (appetitive feeding) (42), indicating that this fast deactivation signal must be independent of the internal need state.
Additional clues that the feeling of hunger underlies a more complex regulatory system than initially thought came from the observation that ARCAgRP activation seemed to dynamically change emotional valences from positive (initial increased operant responding) to negative (decreased operant responding) with ongoing activation during operant testing (32). Indeed, additional studies highlighted that ARCAgRP neurons induced a positive valence with neuronal prestimulation where food remains absent, whereas operant testing (food accessibility) occurred without ongoing neuronal activation. This paradigm more authentically reproduced the physiological ARCAgRP activation pattern observed in the transition from fasting to refeeding. Prestimulation of ARCAgRP neurons in the absence of food caused strong and long-lasting (days) increases in appetitive (lever pressing) and consumatory (caloric intake) feeding responses and induced positive valence (food preference and place preference) (33). Taken together, these data indicate that activation of ARCAgRP neurons underlies modulation by fast sensory signals (seconds) and long-term internal signals (e.g., humoral factors). Furthermore, the timing and context of ARCAgRP neuronal firing dynamically change emotional valences from negative to positive. Finally, these prestimulation effects modulate appetitive and consumatory feeding via LHA circuits (as well as ARCAgRP > BNST and ARCAgRP > PVN circuits), even though metabolic feeding and emotional valences were not directly evaluated (33). Thus, it remains to be determined which LHA neurons could integrate ARCAgRP feeding circuits.
The LHA as a Central Interconnection Hub Within the CNS
The rodent hypothalamus is organized into well-defined nuclei with densely packed cell bodies (e.g., ARC, ventral and dorsomedial hypothalamus, PVN). The larger LHA is an exception with its organization into less dense neuronal cell bodies that allows the passage of neuronal processes via massive bundles known as the median forebrain bundle (mfb). The mfb reaches through the brain like a highway to connect neuronal cell bodies from caudally arising neurons (e.g., midbrain and brainstem) to forebrain structures and vice versa. Also, cell bodies within the LHA enter the mfb and receive innervation from mfb fibers (52). The fornix (fx) is another prominent fiber bundle within the LHA that carries neuronal fibers from the hippocampus to posterior sites, leading through the LHA, and extends anterior into the septal nuclei and nucleus accumbens (NAc). The fx is also widely used as an LHA landmark.
The mfb plays also an integral part in connecting key components of the limbic system. Dopaminergic (DA) neurons in the ventral tegmental area (VTA) propagate their signals from the VTA to the forebrain (striatum and NAc) and vice versa via the mfb (53). The NAc has been implicated as a key structure in translating motivation into motor function (54), and thus it is important for reward behavior. The LHA shows prominent interconnection with VTA circuits and receives innervation from the NAc, which is in line with many studies that connect LHA function to reward behavior and hedonic feeding (55–57).
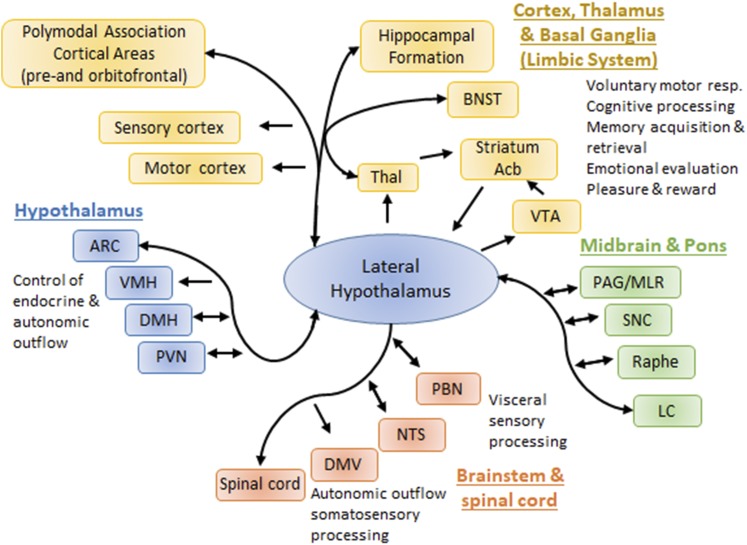
Another unique feature of the LHA is its enormous interconnection with other central sites and number of distinct nuclei that have been distinguished (58, 59). Therefore, the LHA interconnects with all other major output systems of the brain, including the autonomic nervous system via the brainstem, spinal cord, and PVN, the neuroendocrine axis via the PVN and other hypothalamic sites, and the cognitive processing, memory, and emotional valences via the cortex, thalamus, and limbic system (Fig. 2). Based on projection patterns or functional studies, the LHA is further implicated in a variety of behaviors such as feeding (60), foraging and exploration (58, 61), sleep/wake states and arousal (62, 63), and anxiety (64, 65).
Figure 2.
LHA central connectivity. The LHA interconnects to all major output systems of the brain (endocrine and autonomic control, behavior, learning, and memory). The relative importance of LHA targets and inputs for feeding and associated behavior is not fully understood and requires systematic and unbiased comparison of LHA connections. Acb, nucleus accumbens; DMH, dorsomedial nucleus of the hypothalamus; DMV, dorsal motor nucleus of vagus; LC, locus coeruleus; NTS, nucleus of the solitary tract; PAG/MLR, periaqueductal gray/mesencephalic locomotor region; PBN, parabrachial nucleus; SNC, substantia nigra pars compacta; Thal, thalamus; VMH, ventromedial hypothalamus.
Thus, the interconnected organization of the LHA is well suited to orchestrate environmental (sight, small, taste, temperature, cognition) and metabolic (blood glucose, circulating hormones, inflammation) stimuli into appropriate contextual responses from autonomic function, the neuroendocrine axis, and behavior [for detailed review, see Berthoud and Münzberg (53)]. A more detailed study of small LHA subdivisions has further highlighted that each of these sites shows a high degree of interconnectivity within and outside the LHA (59, 66, 67). Despite the massive interconnectivity, anatomical projection specificity exists for limbic inputs to the LHA, with possible functional relevance to modulate motivated behaviors (68, 69). Yet a consistent model of information flow and integration across various LHA inputs and outputs that can predict the modulation of food intake is not in place.
GABA and Glutamate LHA Neurons Mediate Different Aspects of Feeding Behavior
Recent work in the LHA highlighted the role of opposing acting neurons in feeding behavior based on their neurotransmitter characteristic of excitatory glutamate or inhibitory GABA. The transporters for glutamate or GABA, vesicular glutamate transporter-2, and vesicular GABA transporter, respectively, have been most useful to visualize and manipulate these two neuronal populations in many central sites (70, 71). The LHA shows wide distribution of both stimulatory glutamate (LHAglutamate) and inhibitory GABA (LHAGABA) neurons that have opposing roles in metabolic and hedonic feeding and promote emotional valences in animals that modulate conditioned preferences (57).
Emotional valences and feeding behavior
As outlined earlier, activation of ARCAgRP neurons can induce positive and negative valences depending on the timing of stimulation and availability of food during stimulation. In a continuous stimulation paradigm that induced a negative valence in ARCAgRP neurons, LHAGABA neurons promote food intake in conjunction with a positive valence (72), whereas LHAglutamate neurons inhibit food intake in conjunction with a negative valence (73). These data indicate that ARCAgRP-induced feeding behavior overall may differ from LHAGABA-induced feeding behavior, at least with regard to the associated emotional valence. On the other hand, the opposing effects of LHAGABA and LHAglutamate neurons to modulate food intake and emotional valance fit well into the overarching model for hunger-driven food intake (Fig. 1). Furthermore, prestimulation paradigms in ARCAgRP neurons will induce hedonic and metabolic feeding that are associated with a positive valence and involve interaction with LHA neurons (33).
The dynamic nature of emotional valences is also demonstrated when they compete with other motivational states, such as anxiety or social interactions. Fasted mice (physiological hunger) and activation of ARCAgRP neurons would overcome competing motivational states such as anxiety and sexual or social interaction to gain access to food. Yet this effect was not observed when LHAGABA neurons were activated (74), arguing again that ARCAgRP- and LHAGABA-induced food intake may represent divergent feeding circuits.
Neuronal connectivity is another variable that contributes to the complexity of behavioral and emotional modulations. Positive and negative valences of both LHA populations are reproduced by selective stimulation of their projections to the VTA, where LHAGABA > VTA afferents stimulate VTAdopamine neurons (by disinhibition of VTAGABA interneurons), whereas LHAglutamate > VTA afferents inhibit VTAdopamine neurons (stimulation of VTAGABA interneurons) (75). However, these LHAGABA > VTA projections mediate only metabolic feeding, not hedonic feeding, and LHAglutamate > VTA afferents have no effect on food intake. Instead, LHAglutamate neurons mediate negative valence (and inhibition of food intake) via the lateral habenula (76), thus supporting the existence of several redundant and possibly independent-acting circuits that modulate feeding and emotional valences.
Taken together, the opposing effects of LHAGABA and LHAglutamate neurons fit well into an internal state–driven model of food intake and emotional valences that integrates feeding signals from the ARC. However, it remains unclear how the dynamic change of emotional valences promoted by ARCAgRP neurons fits into these circuits. Even though ARCAgRP > LHA afferents were confirmed to induce acute and operant feeding (32, 33), the associated negative valence was not evaluated for specific ARCAgRP projections. ARCAgRP > LHA afferents may also target subsets of LHA populations that would explain diverse behavioral phenotypes (e.g., the suprafornical LHA region, which receives many inputs relevant for feeding behavior) (66).
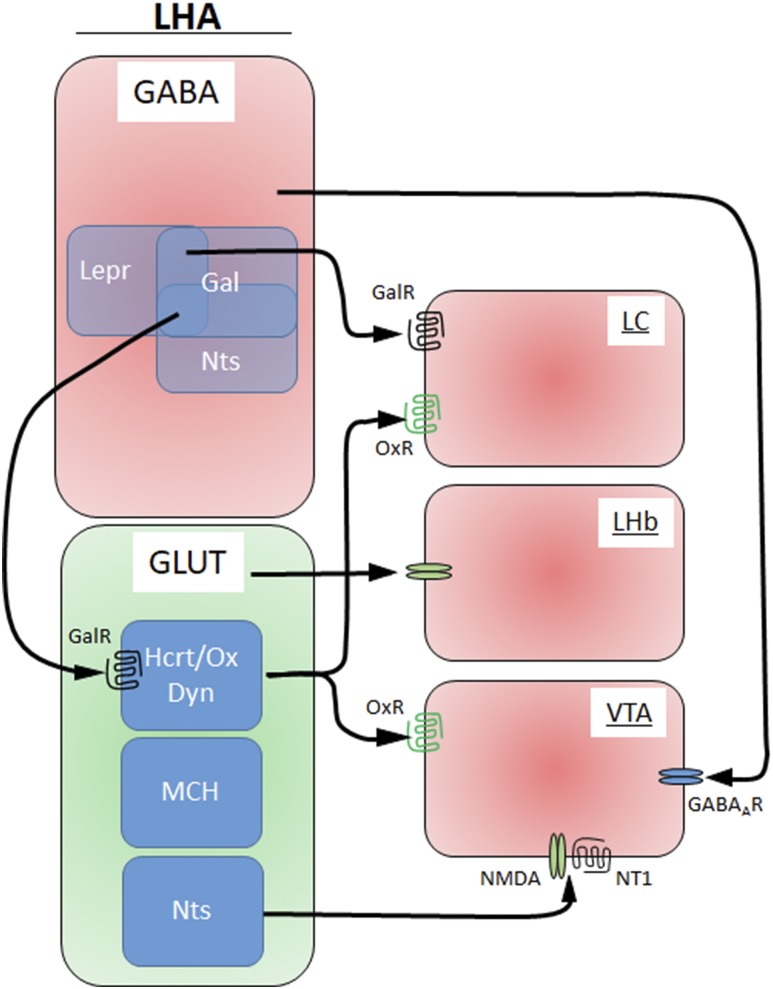
Overall, the molecular and anatomical diversity within the population of LHAGABA and LHAglutamate neurons must be considered, which probably combines molecularly heterogeneous neurons, and divergent projection patterns, specifically considering the strong interconnectivity of the LHA as a whole as well as small LHA subdivisions (59, 66, 67). Indeed, LHAGABA and LHAglutamate neurons are distinguishable into several neuronal subpopulations, according to their expression of hormone receptors, neuropeptide expression, projection sites, or their feeding-related activation pattern (Fig. 3).
Figure 3.
GABA and glutamate subpopulations in the LHA. The LHA distinguishes stimulatory glutamatergic (GLUT) neurons and inhibitory GABAergic neurons. Additional subpopulations are found within each group with distinct projection sites. Dyn, dynorphin; GABAA, GABA-receptor; Gal, galanin; GalR, galanin receptor; Hcrt/Ox, hypocretin/orexin; LHb, lateral habenual; MCH, melanin concentrating hormone; NMDA, N-methyl-d-aspartate; NT1, neurotensin receptor-1; Nts, neurotensin; OxR, hypocretin/orexin/receptor.
Consumatory and appetitive LHA neurons
LHAGABA neurons are composed of discrete neuronal populations that are activated with appetitive or consumatory feeding behaviors (72). The distinction was made possible by the use of an implantable gradient-index lens to visualize individual neurons and simultaneously detect calcium-sensitive changes in neuronal activity. Such studies are missing in LHAglutamate neurons. However, LHAglutamate subpopulations such as hypocretin/orexin (Hcrt/ox) (LHAHcrt/ox)-expressing neurons are activated with arousal and food rewards (77–79), whereas lateral habenula–projecting LHAglutamate neurons suppress consumatory feeding and promote aversion (76). Thus, LHAglutamate neurons may similarly dissociate into neuronal subgroups based on their feeding-related activation patterns (e.g., consumatory vs appetitive feeding). The LHA is typically viewed as an integrator of hypothalamic signals, yet the dissociation of neurons that are associated with appetitive vs consumatory feeding indicates further distinction of feeding circuits at the level of the LHA. Future studies in ARCAgRP neurons and other feeding-related populations should elucidate how common the distinction into appetitive and consumatory responsive neurons is throughout the hypothalamus.
Metabolic and hedonic feeding mediated by LHA neurons
Ablation of LHAGABA and LHAglutamate neurons have opposing effects on metabolic and hedonic feeding, sufficient to cause body weight changes (72, 76), and both densely innervate the VTA. LHA projections to the VTA and subsequent regulation of DA neurons are proposed as a key component of hedonic feeding (72, 75, 80, 81). Thus, the failure of LHA > VTA projections to modulate hedonic feeding after optogenetic stimulation was surprising (75). However, it was noted earlier that chemical lesions or blockade of dopamine signaling in the NAc had strong effects on locomotion but did not affect food intake (82, 83), so that dopamine neurons may promote the motivational aspects of feeding rather than influencing food intake per se (84).
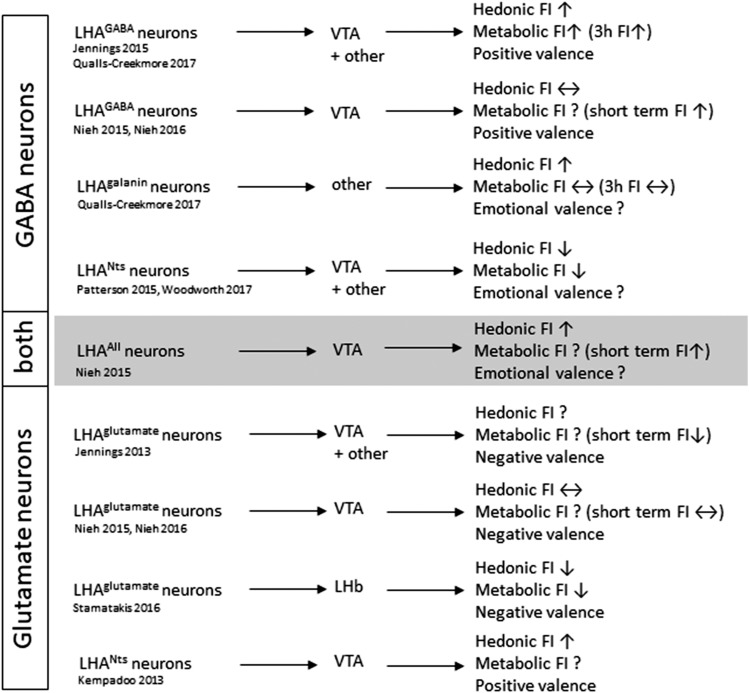
The comparison of LHA neurons with and without VTA projections gave further insights into alternative circuits that promote hedonic feeding (Fig. 4). LHAGABA neurons are composed of long-form leptin receptor (Lepr)– (85), neurotensin (Nts)- (86), and galanin- (87) expressing neurons, and all of these innervate LHAHcrt/ox neurons and are well associated with food reward (78, 86, 88). Nts and galanin are coexpressed in many LHA neurons (53); however, LHANts and LHAgalanin neurons are further separated by their distinct projection profiles (86, 88, 89). Only LHANts, but not LHAgalanin neurons, innervate the VTA, whereas LHAgalanin but not LHANts neurons innervate the locus coeruleus (LC) (86, 88, 89) (Fig. 3). In line with these differential projection profiles, neuronal activation induced clearly distinguishable feeding responses. Activation of LHAgalanin neurons (no VTA projections) is sufficient to enhance food reward, similar to activation of overall LHAGABA neurons, but had no effect on metabolic food intake (90). In contrast, activation of LHANts neurons (including substantial VTA-projecting neurons) suppressed metabolic food intake and had no effect on palatable food intake (91). This demonstrates that LHAGABA neurons are composed of several neuronal subsets that drive different aspects of food intake. Of particular interest is that LHAgalanin neurons increase hedonic feeding without affecting metabolic feeding, further demonstrating that several possibly redundant and independently regulated circuits can be distinguished based on their feeding behavior, at least in experimental settings.
Figure 4.
Diverse LHAGABA and LHAglutamate circuits and their associated feeding behavior and emotional valence. Behavioral changes follow neuronal activation or projection-specific activation of select LHA circuits.
Furthermore, LHANts > VTA projecting neurons drive rewarding valences and self-stimulation via Nts receptor-1 and N-methyl-d-aspartate glutamate receptors (80), indicating that VTA-projecting LHANts neurons also include glutamatergic neurons. Similarly, LHAHcrt/ox neurons (distinct from LHANts neurons) contribute to glutamatergic VTA inputs that promote hedonic feeding (55, 92, 93), whereas overall LHAglutamate > VTA projections have no effect on food intake (75). Again, these inconsistent data suggest a more complex organization of LHA > VTA inputs, with distinct circuits that promote discrete sets of behavioral phenotypes and may not require direct interaction with the VTA. Such indirect interaction could involve inhibitory inputs to LHAHcrt/Ox neurons (94), even though enhanced reward behavior has been associated with activation of LHAHcrt/Ox neurons (78, 95).
Furthermore, it remains unclear how neuropeptides and humoral inputs contribute to the observed behaviors at a circuit level. The anorexigenic (leptin, ghrelin, POMC-derived peptides) and orexigenic (Nts, galanin, Hcr/Ox, neuropeptide Y, AgRP) properties of many neuropeptides and hormones have been well described [for review, see Berthoud and Münzberg (53)]. Furthermore, many gut hormones that were first described to act in the brainstem were found to also interact with hypothalamic and limbic sites [for review, see Williams (96) and Grill and Hayes (97)], suggesting further complexity to modulate feeding circuits. However, there has been little progress at the circuit levels to integrate neuropeptide and humoral impact. Some data exist on melanocortin-4 receptors [(MC4Rs) which are stimulated by POMC-derived neuropeptides and inhibited by AgRP] that highlight MC4R-dependent (ARCAgRP > PVN) and MC4R-independent (ARCAgRP > LHA) feeding circuits (98). Also, the different temporal effects of neuropeptide Y, AgRP, and GABA, or G-protein coupled receptor signaling (Gsvs Gq), have been addressed for ARCAgRP-induced feeding behavior but with no context for how it may affect emotional valences or LHA feeding circuits (51, 99). Furthermore, the role of LHAHcrt/ox neurons and Hcrt/Ox peptide itself has not been addressed on a circuit level. These studies are further complicated because new studies using single-cell quantitative PCR suggest that at least some LHAHcrt/ox neurons may have GABA and glutamatergic characteristics and can express galanin mRNA (100), despite histologic evidence that these populations do not overlap (87).
Arousal, Locomotion, and Anxiety
Beyond feeding, the LHA plays an important role in arousal, which accompanied the discovery of LHAHcrt/Ox neurons (101, 102). LHAHcrt/Ox neurons are critical for arousal, and their activation level modifies sleep/wake states via their projections to the LC (103). LHAHcrt/Ox neurons are implicated in hedonic food intake (77–79, 93), but reported effects on metabolic feeding are minimal (104). Therefore, the feeding effects via LHAHcrt/Ox neurons may be an indirect result of the heightened arousal, because low arousal or even sleep is incompatible with feeding behavior. Indeed, recent studies further emphasized the role of LHAHcrt/Ox neurons in arousal, indicated by enhanced locomotor activity. Activation of glutamatergic LHAHcrt/ox neurons induces robust locomotor activity (104, 105), which was sufficient to blunt high-fat diet–induced weight gain in mice but had no effect on metabolic feeding (105). LHAHcrt/Ox neuronal stimulation also influences LHA circuitries (e.g., activation of LHAGABA neurons), which similarly induces robust locomotion (75, 90, 106).
Currently, our understanding of locomotor activity and its relevance for diverse behavior is not well defined. Locomotion is easily evaluated by measuring infrared beam breaks, but it fails to reflect the underlying motivation that causes locomotor events. For instance, activation of LHAGABA neurons (strong VTA projections) or LHAgalanin neurons (no VTA projections) both induced very similar home cage locomotor activity and energy expenditure (90), but locomotion was unchanged in stress-related open field settings after LHAGABA activation (107). More thorough testing revealed distinct locomotor modalities. Activation of LHAgalanin neurons increased the overall occurrence of normal mouse behavior with frequent transitions across diverse behaviors (grooming, exploration, digging, feeding), and this behavior was associated with an anxiolytic valence, possibly reflecting increased arousal and willingness to explore. In striking contrast, activation of LHAGABA neurons induced repetitive behavior with compulsive gnawing or digging and few behavioral transitions, which was associated with anxiogenic valence (90). LHAGABA > VTA projections also mediated repetitive gnawing and licking behavior (75, 81), which can lead to noncaloric consumatory intake (107), suggesting that LHAGABA > VTA circuits are responsible for compulsive locomotion. Furthermore, activation of LHANts neurons increases locomotor activity but is accompanied by decreased feeding behavior (91), in striking contrast to activation of LHAGABA and LHAgalanin neurons that increased metabolic or hedonic feeding (90). An increase in locomotor activity is also in line with food-seeking and foraging behavior, which may be difficult to interpret properly in the absence of food-directed consumatory behavior. However, it is conceivable that foraging behavior may be more clearly defined by increased locomotor activity, coupled with reduced anxiety, consistent with findings that physiological hunger and ARCAgRP stimulation are able to overcome fear of predator odor for the sake of finding food (74).
Locomotion inherently intertwines with almost all behaviors (e.g., feeding, learning tasks, arousal, drinking, anxiety, social interaction), and it is difficult to dissociate locomotor function from ingestive behavior. This effect has been also noted for the mesolimbic dopamine system, where VTADA neurons are tightly connected with food reward and the motivation to work for food. However, deletion of DA neurons or inputs to the NAc alone only diminishes locomotor activity but is not sufficient to modulate food intake (82, 83). Such depletion may result in reduced effort to obtain a food reward but would not change intake of freely available food (84). Similarly, compulsive locomotion as observed after activation of LHAGABA neurons or its projections to the VTA may interfere or even mask hedonic feeding. For example, chemogenetic activation of LHAGABA neurons required individual dose-response analysis to unmask increased operant responding, due to overwhelming compulsive locomotor behavior with higher stimulation doses (90); this response was also noted as a confounding effect with LHAGABA > VTA stimulation and the inability to increase compulsive operant responding (75, 81).
Thus, locomotor activity can be associated with different feeding and non–food-related behavior, with diverse influences on emotional valences. An important task for future research is a better classification of contextual behavior that defines locomotor activity as it relates to feeding behavior, behavioral setting, and emotional valences [e.g., by improving the visualization of complex behavior analysis, as elegantly shown by Sterley et al. (108)]. Furthermore, projection-specific studies of feeding and associated behaviors (e.g., emotional valence), as performed comprehensively for feeding responses via ARCAgRP neurons (50), will allow evaluation if select feeding circuits integrate into each other or build independent feeding circuits. Particularly for the LHA, the VTA-centric view to modulating feeding behavior requires an unbiased evaluation of the targets and their relative contribution to feeding and associated behaviors.
Conclusion and Perspective
CNS circuits that modulate feeding behavior ultimately need to modulate the final motor neurons that allow feeding behavior coordination for food approach and consumption (holding, biting, chewing, and swallowing of food). The need to coordinate many behavioral components and sensory inputs (e.g., metabolic state, emotional state, sight and smell of food) has led to the overall view that these components must be integrated at various levels from central circuits to final output systems (neuroendocrine release, autonomic outflow, and motor function and behavior).
From an anatomical perspective, the impressive interconnectivity of the LHA with other central sites and its ability to sense and respond to physiological state changes make the LHA an ideal integration center for all components of feeding behavior [for further review, see Berthoud and Münzberg (53) and Petrovich (56)]. In contrast, some LHA populations (e.g., LHANts and LHAgalanin neurons) show limited extra-LHA projection sites and modulation of distinct feeding behaviors.
We have highlighted that LHAGABA and LHAglutamate neurons consist of diverse neuronal populations based on neurotransmitter and neuropeptide expression patterns, extrahypothalamic projection patterns, and the behaviors elicited by neuronal activation. These data suggest that the LHA not only plays an integrating role for internal and external stimuli but also dissociates behavioral components (e.g., appetitive, consumatory, hedonic, metabolic food intake). Thus, one future task is to identify additional genetic markers that identify more homogeneous LHA subpopulations [e.g., as recently performed with single–LHA neuron analysis (100)] that allow identification of more specific neuronal markers.
The finding that complex behavior such as food intake involves distinct subsets of neurons that respond to select behavioral components (appetitive vs consumatory food intake) also shows that we may be just beginning to properly define and categorize complex behavior. Specifically, as other central sites have found similar behavioral dissociations of complex behavior, prey killing can be dissected into prey pursuit (appetitive behavior) via amygdala > brainstem projecting neurons and a killing bite (this fits the category of aggressive behavior and consumatory behavior) via amygdala midbrain projecting neurons (109). This task is particularly difficult, because most behaviors include some form of locomotion, and interpretation is particularly prone to investigator bias, depending on the system investigated (e.g., food intake, aggression, anxiety, arousal). Therefore, the compulsive locomotor behavior that has been noted by some investigators when stimulating LHAGABA neurons has been interpreted as consumatory (gnawing or food intake), increased arousal, repetitive behavior, or anxiety associated, depending on the experimental approaches or context of study (72, 81, 90, 107, 110). This finding highlights an important need for future studies to find a more systematic way to classify locomotor behavior and identify the main behavioral drive (e.g., food intake vs anxiety) that underlies the change in locomotion.
Additionally, it is important to account for the internal (physiological and emotional status) and external (environment) context of the animal when interpreting behavioral outcomes. The discussed studies strengthen the view that positive and negative valences are an important motivating aspect for feeding behavior. We have only begun to unravel the importance of timing for neuronal firing (prestimulation vs continuous) and the environmental context when neuronal firing occurs (e.g., food availability, competing need states such as anxiety, social interaction, or mating). In the future it will be important to be more conscientious about the dynamic changes that are possible when it comes to emotional valences and associated changes in motivational drives for feeding.
Acknowledgments
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants T32-DK064584 (to E.Q.-C.), P20-GM103528 (to H.M.), and R01-DK092587 (to H.M.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AgRP
agouti-related-peptide
- ARC
arcuate nucleus
- BNST
bed nucleus of stria terminalis
- CNS
central nervous system
- DA
dopaminergic
- fx
fornix
- GABA
γ-aminobutyric acid
- Hcrt/ox
hypocretin/orexin
- LC
locus coeruleus
- Lepr
leptin receptor
- LHA
lateral hypothalamus
- MC4R
melanocortin-4 receptor
- mfb
median forebrain bundle
- NAc
nucleus accumbens
- Nts
neurotensin
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- VTA
ventral tegmental area
References
- 1. Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26(4):393–428. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Rønnekleiv OK, Kelly MJ. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30(6):630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, Lin D. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat Neurosci. 2017;20(11):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ Jr., Borius M, Inoue S, Chiang MC, Bender KJ, Ganguli S, Shah NM. Social control of hypothalamus-mediated male aggression. Neuron. 2017;95:955–970, e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud HR, Morrison CD, Derbenev AV, Zsombok A, Münzberg H. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J Neurosci. 2016;36(18):5034–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans MC, Anderson GM. Neuroendocrine integration of nutritional signals on reproduction. J Mol Endocrinol. 2017;58(2):R107–R128. [DOI] [PubMed] [Google Scholar]
- 8. Clarke IJ, Arbabi L. New concepts of the central control of reproduction, integrating influence of stress, metabolic state, and season. Domest Anim Endocrinol. 2016;56(suppl):S165–S179. [DOI] [PubMed] [Google Scholar]
- 9. Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andermann ML, Lowell BB. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95(4):757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24(2):123–140. [PMC free article] [PubMed] [Google Scholar]
- 12. Balagura S, Devenport LD. Feeding patterns of normal and ventromedial hypothalamic lesioned male and female rats. J Comp Physiol Psychol. 1970;71(3):357–364. [DOI] [PubMed] [Google Scholar]
- 13. Becker EE, Kissileff HR. Inhibitory controls of feeding by the ventromedial hypothalamus. Am J Physiol. 1974;226(2):383–396. [DOI] [PubMed] [Google Scholar]
- 14. Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77(2):323–324. [DOI] [PubMed] [Google Scholar]
- 15. Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–427. [DOI] [PubMed] [Google Scholar]
- 16. Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135(3501):375–377. [DOI] [PubMed] [Google Scholar]
- 17. Swanson LW. The neuroanatomy revolution of the 1970s and the hypothalamus. Brain Res Bull. 1999;50(5–6):397. [DOI] [PubMed] [Google Scholar]
- 18. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke RE, Verdejo-Garcia A, Andrews ZB. The role of corticostriatal-hypothalamic neural circuits in feeding behaviour: implications for obesity [published online ahead of print 28 April 2018] J Neurochem. doi: 10.1111/jnc.14455. [DOI] [PubMed]
- 21. Sternson SM, Eiselt AK. Three pillars for the neural control of appetite. Annu Rev Physiol. 2017;79(1):401–423. [DOI] [PubMed] [Google Scholar]
- 22. Zhang N, Bi S. Effects of physical exercise on food intake and body weight: role of dorsomedial hypothalamic signaling. Physiol Behav. 2018;192:59–63. [DOI] [PubMed] [Google Scholar]
- 23. Münzberg H, Qualls-Creekmore E, Yu S, Morrison CD, Berthoud HR. Hedonics act in unison with the homeostatic system to unconsciously control body weight. Front Nutr. 2016;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 2018;27(1):42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37(4):261–283. [DOI] [PubMed] [Google Scholar]
- 26. Berthoud HR, Münzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc. 2012;71(3):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, Jia P, Zhao Z, Farias M, Wu Q, Milewicz DM, Sutton VR, Moore DD, Butte NF, Krashes MJ, Xu Y, Chopra AR. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23(12):1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poher AL, Tschöp MH, Müller TD. Ghrelin regulation of glucose metabolism. Peptides. 2018;100:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yiin YM, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and free-feeding rats. Physiol Behav. 2005;84(2):217–231. [DOI] [PubMed] [Google Scholar]
- 31. Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. [DOI] [PubMed] [Google Scholar]
- 32. Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Y, Lin YC, Zimmerman CA, Essner RA, Knight ZA. Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife. 2016;5:e18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cabanac M, Lafrance L. Postingestive alliesthesia: the rat tells the same story. Physiol Behav. 1990;47(3):539–543. [DOI] [PubMed] [Google Scholar]
- 35. Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91(5):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. [DOI] [PubMed] [Google Scholar]
- 37. Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89(1):71–84. [DOI] [PubMed] [Google Scholar]
- 38. Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21(3):263–307. [DOI] [PubMed] [Google Scholar]
- 39. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. [DOI] [PubMed] [Google Scholar]
- 40. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. [DOI] [PubMed] [Google Scholar]
- 41. Münzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Björnholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG Jr. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160(5):829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390(6659):521–525. [DOI] [PubMed] [Google Scholar]
- 44. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. [DOI] [PubMed] [Google Scholar]
- 45. Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. [DOI] [PubMed] [Google Scholar]
- 46. Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. [DOI] [PubMed] [Google Scholar]
- 48. Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. [DOI] [PubMed] [Google Scholar]
- 49. Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402(4):442–459. [PubMed] [Google Scholar]
- 50. Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, Wess J. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun. 2016;7:10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206(1):49–81. [DOI] [PubMed] [Google Scholar]
- 53. Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2-3):69–97. [DOI] [PubMed] [Google Scholar]
- 55. Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. [DOI] [PubMed] [Google Scholar]
- 56. Petrovich GD. Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front Syst Neurosci. 2018;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol. 2005;493(3):412–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hahn JD, Swanson LW. Connections of the juxtaventromedial region of the lateral hypothalamic area in the male rat. Front Syst Neurosci. 2015;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mogenson GJ, Stevenson JA. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp Neurol. 1967;17(2):119–127. [DOI] [PubMed] [Google Scholar]
- 61. Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449(1-2):311–327. [DOI] [PubMed] [Google Scholar]
- 62. Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153(3):860–870. [DOI] [PubMed] [Google Scholar]
- 63. Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24(50):11439–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118(3):183–191. [DOI] [PubMed] [Google Scholar]
- 66. Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Brain Res Rev. 2010;64(1):14–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hahn JD, Swanson LW. Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J Comp Neurol. 2012;520(9):1831–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Niu JG, Yokota S, Tsumori T, Oka T, Yasui Y. Projections from the anterior basomedial and anterior cortical amygdaloid nuclei to melanin-concentrating hormone-containing neurons in the lateral hypothalamus of the rat. Brain Res. 2012;1479:31–43. [DOI] [PubMed] [Google Scholar]
- 69. Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct. 2016;221(6):2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5(5):383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S, Stuber GD. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160(3):516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341(6153):1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, Krashes MJ. Hunger-driven motivational state competition. Neuron. 2016;92(1):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, Izadmehr EM, Tye KM. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90(6):1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci. 2016;36(2):302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67(8):753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. [DOI] [PubMed] [Google Scholar]
- 79. Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl). 2013;226(1):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33(18):7618–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM. Decoding neural circuits that control compulsive sucrose seeking [published correction appears in Cell. 2015;161(6):1468–1471]. Cell. 2015;160(3):528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bakshi VP, Kelley AE. Dopaminergic regulation of feeding-behavior. 1. Differential-effects of haloperidol microinfusion into 3 striatal subregions. Psychobiology. 1991;19(3):223–232. [Google Scholar]
- 83. Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92(5):917–927. [DOI] [PubMed] [Google Scholar]
- 84. Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. [DOI] [PubMed] [Google Scholar]
- 85. Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG Jr. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG Jr. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14(3):313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Laque A, Zhang Y, Gettys S, Nguyen TA, Bui K, Morrison CD, Münzberg H. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am J Physiol Endocrinol Metab. 2013;304(9):E999–E1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Laque A, Yu S, Qualls-Creekmore E, Gettys S, Schwartzenburg C, Bui K, Rhodes C, Berthoud HR, Morrison CD, Richards BK, Münzberg H. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metab. 2015;4(10):706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Woodworth HL, Brown JA, Batchelor HM, Bugescu R, Leinninger GM. Determination of neurotensin projections to the ventral tegmental area in mice. Neuropeptides. 2018;68:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Qualls-Creekmore E, Yu S, Francois M, Hoang J, Huesing C, Bruce-Keller A, Burk D, Berthoud HR, Morrison CD, Münzberg H. Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J Neurosci. 2017;37(25):6053–6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Woodworth HL, Beekly BG, Batchelor HM, Bugescu R, Perez-Bonilla P, Schroeder LE, Leinninger GM. Lateral hypothalamic neurotensin neurons orchestrate dual weight loss behaviors via distinct mechanisms. Cell Reports. 2017;21(11):3116–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG Jr. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J Neurosci. 2014;34(34):11405–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1436–R1444. [DOI] [PubMed] [Google Scholar]
- 96. Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, Elmquist JK, Tannous BA, Krashes MJ, Lowell BB. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18(6):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18(4):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mickelsen LE, Kolling FW IV, Chimileski BR, Fujita A, Norris C, Chen K, Nelson CE, Jackson AC. Neurochemical heterogeneity among lateral hypothalamic hypocretin/orexin and melanin-concentrating hormone neurons identified through single-cell gene expression analysis. eNeuro. 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sakurai T. Orexins and orexin receptors: implication in feeding behavior. Regul Pept. 1999;85(1):25–30. [DOI] [PubMed] [Google Scholar]
- 103. Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology. 2014;85:451–460. [DOI] [PubMed] [Google Scholar]
- 105. Zink AN, Bunney PE, Holm AA, Billington CJ, Kotz CM. Neuromodulation of orexin neurons reduces diet-induced adiposity. Int J Obes. 2018;42(4):737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kosse C, Schöne C, Bracey E, Burdakov D. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc Natl Acad Sci USA. 2017;114(17):4525–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Navarro M, Olney JJ, Burnham NW, Mazzone CM, Lowery-Gionta EG, Pleil KE, Kash TL, Thiele TE. Lateral hypothalamus GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology. 2016;41(6):1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sterley TL, Baimoukhametova D, Füzesi T, Zurek AA, Daviu N, Rasiah NP, Rosenegger D, Bains JS. Social transmission and buffering of synaptic changes after stress. Nat Neurosci. 2018;21(3):393–403. [DOI] [PubMed] [Google Scholar]
- 109. Han W, Tellez LA, Rangel MJ Jr., Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell. 2017;168:311–324, e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM. A novel population of wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr Biol. 2016;26(16):2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]