Abstract
The bacterial second messenger (3ʹ–5ʹ)-cyclic-di-guanosine-monophosphate (CDG) is a promising mucosal adjuvant candidate that activates balanced Th1/Th2/Th17 responses. We showed previously that CDG activates stimulator of IFN genes (STING)-dependent IFN-I production in vitro. However, it is unknown whether STING or IFN-I is required for the CDG adjuvant activity in vivo. In this study, we show that STING−/− mice (Tmem173<tm1Camb>) do not produce Ag-specific Abs or Th1/Th2/Th17 cytokines during CDG/ Ag immunization. Intranasal administration of CDG did not induce TNF-α, IL-1β, IL-6, IL-12, or MCP-1 production in STING−/− mice. Surprisingly, we found that the cytokine and Ab responses were unaltered in CDG/Ag-immunized IFNAR−/− mice. Instead, we found that CDG activates STING-dependent, IFN-I–independent TNF-α production in vivo and in vitro. Furthermore, using a TNFR1−/− mouse, we demonstrate that TNF-α signaling is critical for CDG-induced Ag-specific Ab and Th1/Th2 cytokine production. This is distinct from STING-mediated DNA adjuvant activity, which requires IFN-I, but not TNF-α, production. Finally, we found that CDG activates STING-dependent, but IRF3 stimulation–independent, NF-κB signaling. Our results established an essential role for STING-mediated TNF-α production in the mucosal adjuvant activity of CDG in vivo and revealed a novel IFN-I stimulation–independent STING–NF-κB–TNF-α pathway.
Mucosal vaccination induces protective immune responses in the mucosal and systemic immune compartments. A bacterial second messenger, (3ʹ–5ʹ)-cyclic-di-guanosinemonophosphate (CDG), exhibits strong immunostimulatory properties in mouse and human cells. Particularly, CDG can activate human immature dendritic cells (DCs) and enhances DCs’ ability to stimulate T cells (1). In 2007, Ebensen et al. (2) demonstrated that CDG has mucosal adjuvant activity, inducing efficient Ag-specific secretory IgA production in the lung and vagina. Later groups demonstrated that the mucosal immune response induced by CDG translates into protective immunity against various pathogen infections, including H5N1 influenza (3), Acinetobacter baumannii (4), Staphylococcus aureus (5), Klebsiella pneumoniae (6), and Streptococcus pneumoniae (7, 8). Notably, the mucosal adjuvant activity of CDG is comparable to cholera toxin, the “gold standard” of mucosal adjuvanticity (8). CDG seems to have a better safety profile than cholera toxin (8) and elicits a balanced Th1/Th2/Th17 response (9, 10). Thus, CDG is a promising mucosal vaccine adjuvant candidate.
The mechanism by which CDG acts as a mucosal adjuvant is unknown. CDG activates broad immune responses, including the production of Th1/Th2/Th17 cytokines (9), proinflammatory cytokines, such as IL-1β and TNF-α (2, 6, 8, 9), and chemokines (2, 4, 6, 8, 9), as well as the recruitment of leukocytes (1, 4, 6, 8). IFN-I is also produced (11). However, it is not clear how the host responds to CDG in vivo, what cell type is the main responder to CDG in vivo, or the molecular mechanism that CDG uses to activate these cells. Furthermore, it is completely unknown which observed response in vivo is relevant to the adjuvant activity of CDG. A concentrated effort is needed to separate the adjuvant activity of CDG from the potential toxicity to be used in human mucosal vaccination.
Stimulator of IFN genes (STING) (12), also known as MPYS (13) and TMEM173, appears to be a universal receptor for cyclic dinucleotides, including the bacterial second messengers (3ʹ–5ʹ)cyclic-di-adenosine-monophosphate (CDA) and CDG, as well as the newly identified metazoan second messenger cyclic-GMP– AMP (cGAMP) (14). STING is absolutely required for cyclic dinucleotide-induced IFN-I production (15, 16). Structural studies revealed that cyclic dinucleotides interact with the cytoplasmic tail of STING, activating the TBK1–IRF3–IFN-I signaling axis (17, 18). It is unknown whether STING or IFN-I is required for the mucosal adjuvant activity of these cyclic dinucleotides in vivo.
In this study, using STING−/−, IFNAR−/−, and TNFR1−/− mice, we found that STING-mediated TNF-α, but not IFN-I, signaling is essential for the mucosal adjuvant activity of CDG. This is distinct from the STING-mediated adjuvant activity of a DNA vaccine that requires IFN-I, but not TNF-α, signaling (19). Thus, our results reveal that STING-mediated DNA and CDG sensing can be dissociated in vivo.
Materials and Methods
Mice
Six- to twelve-week-old female mice were used for all experiments. IFNAR−/− and STING−/− mice (Tmem173<tm1Camb>.) were described previously (15, 20). TNFR1−/− mice were from The Jackson Laboratory (C57BL/6-Tnfrsf1atm1Imx/J). All mice were on a C57B6/L background. Mice were housed and bred in the Animal Research Facility at Albany Medical College. All experiments with mice were performed in accordance with the regulations and approval of Albany Medical College and the Institutional Animal Care and Use Committee.
Immunization and sample collection
Mice were immunized with three doses (14 d apart) of OVA (20 μg, InvivoGen; cat. code vac-efova), with or without CDG (5 μg, InvivoGen; cat. code vac-cdg) (Fig. 1A). Mice (n = 4/group) were vaccinated intranasally (i.n.) with adjuvanted Ag or nonadjuvanted Ag (20 μg OVA/ dose). For i.n. vaccination, animals were anesthetized using isoflurane in an E-Z Anesthesia system (Euthanex, Palmer, PA). Ag, with or without CDG, was administered drop-wise at 5 μl/nostril. Sera and nasal washes were collected 14 d after the last immunization.
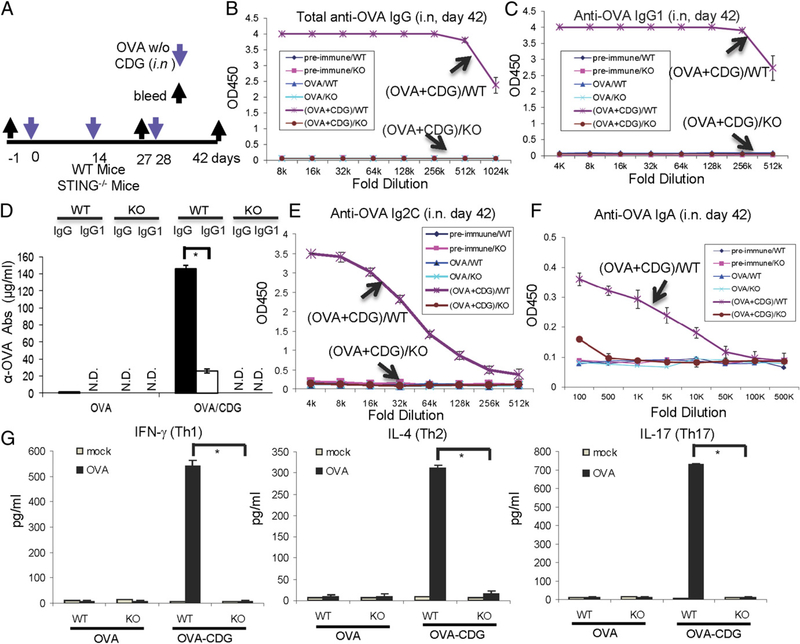
FIGURE 1.
STING is essential for the mucosal adjuvant activity of CDG. (A) Outline of the study. Mice were vaccinated i.n. with three doses (14 d apart) of OVA (20 μg) together with CDG (5 μg) or OVA alone (20 μg). Blood and nasal wash samples were collected 14 d after the last immunization (day 42). (B–F) Each group has four mice. Sera or nasal washes from the four mice in the same group were pooled. OVA-specific Abs in the sera and nasal washes were measured by ELISA, as described in Materials and Methods (n = 3). (G) Splenocytes were collected 14 d after the last immunization and were activated in vitro with OVA (100 μg/ml) for 4 d. Supernatants from the same group were pooled together. Th1 (IFN-γ), Th2 (IL-4), and Th17 (IL-17) cytokines were measured by ELISA (n = 3). Data are mean ± SE from three independent experiments. *p, 0.05.
Bronchoalveolar lavage
Mice were sacrificed at the indicated time by CO2 asphyxiation, and lungs were lavaged twice with 0.8 ml ice-cold PBS. The bronchoalveolar lavage fluid (BALF) was centrifuged at 2000 × g for 1 min. Collected cells were analyzed by flow cytometry. The supernatant was collected and stored at −80°C for cytokine analysis.
Cell culture
Bone marrow–derived macrophages (BMDMs) (15) was generated as previously described. For bone marrow–derived DCs (BMDCs), bone marrow cells were cultured for 7 d in DMEM supplemented with 10% FBS, sodium pyruvate (1 mM), L-glutamine (2 mM), 1% penicillin/streptomycin, 2-ME (50 μM), HEPES buffer (10 mM), 1% nonessential amino acids, and ~3% GMCSF (B78hi/GMCSF.1 cell line hybridoma supernatants). During culture, media were changed on days 2 and 4. Differentiation into DCs was verified on day 7 by flow cytometric analysis of surface CD11c levels (~70–80%).
Detection of Ag-specific IgG in serum
The Ag-specific Abs in serum samples were determined by ELISA. The anti–IgG-HRP Abs used were anti-mouse IgG-HRP (cat. no. 030–05), anti mouse IgG1-HRP (cat. no. 1070–05), anti-mouse IgG2C-HRP (cat. no. 1079–05), and anti-mouse IgA-HRP (cat. no. 1040–05; all from Southern Biotech). Total anti-OVA IgG and IgG1 were quantified using a mouse anti-OVA IgG kit (cat. no. 3011) and an anti-OVA IgG1 kit (cat. no. 3013; both from Chondrex, Redmond, WA).
ELISAs
Cytokine concentrations were measured using commercial ELISA kits, according to the manufacturer’s instructions. The ELISA kits used were MCP-1 (cat. no. 88–7391), IL-2 (cat. no. 88–7024), IL-4 (cat. no. 88–7044), IL-6 (cat. no. 88–7064), IL-12/p70 (cat. no. 88–7921), IL-17A (cat. no. 88– 7371), TNF-α (cat. no. 88–7324), IL-1β (cat. no. 88–7013), and IFN-γ (cat. no. 88–7314; all from eBioscience), as well as an IFN-α ELISA kit (PBL InterferonSource; cat. no. 42120).
In vitro activation of lymphocytes
Splenocytes from OVA- or OVA/CDG-immunized mice (100 μl, 2 × 106 cells/ml) were seeded in a U-bottom 96-well plate and stimulated with OVA (100 μg/ml) for 4 d. Cell supernatants were removed and stored at −80°C for later cytokine measurement.
Flow cytometric assay
Cells were stained with FITC-CD80 (BD Pharmingen; clone 30-F11), PECD86 (BD Pharmingen; clone A3–1), allophycocyanin-Ly6C (BD Pharmingen; clone AL-21), allophycocyanin–CY7–CD11b (BD Pharmingen; clone M1/70), allophycocyanin-F4/80 (Caltag; clone BM8), or PE-Ly6G (BD Pharmingen; clone 1A8). Live cells were gated. FACS was performed using a FACScan flow cytometer (BD Biosciences) and analyzed using FlowJo software (CellQuest).
In vitro CDG- or IFN-stimulating DNA activation
CDG- or IFN-stimulating DNA (ISD) activation was done in 24-well plates or in a 12-well plate when isolating nuclear proteins. BMDMs or BMDCs were seeded at 0.5 × 106 cell/well in a 24-well plate in 1 ml BMDM or BMDC medium overnight. The next day, the following solutions were prepared for each well of stimulation: (a) 50 μl DMEM (Life Technologies; cat. no. 11965) plus 2 μg CDG (InvivoGen; cat. code tlrl-cdg) or ISD (InvivoGen; cat. code tlrl-isdn) or (b) 50 μl DMEM plus 2 ml Lipofectamine 2000 (Invitrogen; cat. no. 11668). Solutions were vortexed for 1 sec and incubated at room temperature for 5 min. Solution (a) was added to solution (b), vortexed for 2 s, and incubated at room temperature for 20 min. At the end of the incubation, an additional 100 μl complete 10% medium was added to the combined solution. A total of 200 μl CDG or ISD solution was introduced to the cells at a final concentration of 10 μg/ml.
Western blot
Cells were lysed in RIPA buffer (15), and whole cell lysates (WCL) were loaded directly on a 10% Mini-PROTEAN TGX precast gel (Bio-Rad; cat. no. 456–1035). The following Abs were used: anti–α-tubulin (Rockland; cat. no. 200–301-880), anti–IκB-α (Santa Cruz; cat. no. 371), anti–p-IRF3 (S396) (Cell Signaling Technology; cat. code 4D4G), anti-TBK1 (Cell Signaling Technology; cat. code D1B4), anti-iNOS (BD Transduction Laboratories; cat. no. 610332), anti–NF-κB p65 (Santa Cruz; cat. code sc-372), anti-IRF3 (Cell Signaling Technology; cat. code D83B9), and anti-histone H3 (Cell Signaling Technology; cat. code D1H2).
Isolation of nucleus proteins
BMDMs or BMDCs were washed once in ice-cold PBS. Cells (~106 cells) were resuspended in 400 μl hypotonic lysis buffer (20 mM Tris-HCl [pH 7.4], 3 mM MgCl2, 10 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, and protease inhibitors) and left on ice for 15 min. At the end of the incubation, Nonidet P-40 was added to a final concentration of 0.6%, and the suspension was vortexed vigorously for 15 s. The nuclei were recovered by centrifugation (3000 rpm for 10 min, at 4°C). The supernatant was removed, and the pellet, which is the nuclear fraction, was resuspended in 18 ml extraction buffer (100 mM Tris-HCl [pH 7.4], 10% glycerol, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.1% SDS, 300 mM NaCl, 0.5% deoxycholate, 0.5 mM DTT, 1 mM PMSF, and protease inhibitors) and kept at −80°C overnight. The next day, the nuclei were thawed on ice and centrifuged for 30 min at 14,000 × g at 4°C. The supernatant is the nuclear extraction.
Chemical inhibitor treatment
BMDMs or BMDCs were treated with 1 μM Bx-795 (InvivoGen; cat. code tlrl-bx7) or 4 μg/ml MG-132 (Selleckchem; cat. code S2619) at 37°C in culture for 2 h. Medium was changed, and cells were activated for the indicated length of time. Cytokine and NO were measured in the supernatant. Cells were lysed for Western blot analysis.
Statistical analysis
The statistical significance was determined using the unpaired student t test (GraphPad Prism 5.0; GraphPad).
Results
The mucosal adjuvant activity of CDG depends on STING
Cytosolic sensing of CDG activates STING-mediated IFN-I production. To investigate the role of STING in CDG-induced mucosal adjuvant activity, we immunized STING−/− (Tmem173<tm1Camb.>) mice (15) with CDG plus OVA or OVA alone (i.n.) and measured the OVA-specific IgG response in blood (Fig. 1A). We found that CDG failed to stimulate an OVA-specific IgG response in STING−/− mice (Fig. 1B). CDG-adjuvanted vaccination generated balanced Th1, Th2, and Th17 responses (9). The Th1 immune response is associated with the generation of IgG2C Abs in C57BL/6 mice, whereas the Th2 humoral immune response is associated with IgG1 production. We found that CDG-induced OVA-specific IgG1 and IgG2C responses are completely absent in STING−/− mice (Fig. 1C–E). Nasal IgA production is also absent in STING−/− mice (Fig. 1F). The ex vivo recall assay in splenocytes from OVA/CDG-immunized wild-type (WT) mice showed balanced Th1 (IFN-γ), Th2 (IL-4), and Th17 (IL-17) cytokine production (Fig. 1G). Importantly, all of these responses were absent in splenocytes from OVA/CDG-immunized STING−/− mice (Fig. 1G). We concluded that STING is essential for the mucosal adjuvant activity of CDG, including CDG-induced Th1/Th2/Th17 responses, in vivo.
STING is not required for alum-adjuvanted IgG responses
A previous report (21) showed that STING is also required for the adjuvant activity of DNA. We then asked whether STING−/− animals have abnormal responses to other adjuvants. Aluminum salts (alum) have been used as a human vaccine adjuvant since the 1930s. We immunized STING−/− mice with either alum-adjuvanted OVA (i.p.) or CDG-adjuvanted OVA (i.p.) and measured the production of OVA-specific IgG in serum. Although STING−/− failed to produce OVA-specific IgG in response to adjuvant CDG (i.p) (Supplemental Fig. 1A), they had no problem producing OVA-specific IgG when immunized with adjuvant alum (Supplemental Fig. 1B). This suggested that STING is required for the adjuvant activity of DNA or CDG. This is consistent with the recent report (22) that STING is dispensable for alum-adjuvanted IgG1 responses. Furthermore, STING−/− mice did not produce OVA-specific IgG1 and IgG2C when immunized with CDG/OVA via the i.p. route (Supplemental Fig. 1C, 1D). This demonstrated that STING mediates the adjuvant activity of CDG via the i.p. and i.n. routes.
STING is required for CDG-induced cytokine and chemokine production in vivo
Consistent with the essential role for STING in mediating the adjuvant activity of CDG, we found that STING−/− mice did not exhibit CDG-induced neutrophil recruitment in the peritoneal cavity during i.p. administration (Supplemental Fig. 1E). As a control, these mice had alum-induced neutrophil influx (Supplemental Fig. 1F). To characterize the in vivo events during i.n. CDG immunization further, we examined CDG-induced cytokine and chemokine production in BALF.
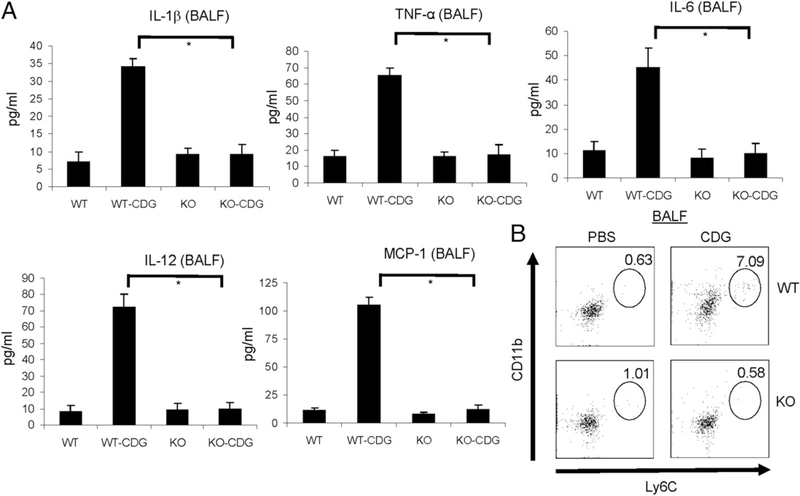
Intranasal administration of CDG induces cytokine and chemokine production in BALF (8). We found that there was no CDGinduced IL-1β, IL-6, IL-12p70, or TNF-α production in BALF from STING−/− mice (Fig. 2A). CDG administration (i.n.) also did not induce chemokine MCP-1 production (Fig. 2A) or the recruitment of Ly6ChiCD11b+ inflammatory monocytes in STING−/− mice (Fig. 2B). Thus, STING is essential for CDG-induced cytokine and chemokine production in vivo.
FIGURE 2.
STING is essential for CDG-induced cytokine and chemokine production in vivo. (A) Mice were administered CDG (10 μg) or PBS i.n. After 15 h, cytokine production was measured in BALF by ELISA (n = 3). Data are mean ± SE from three independent experiments. (B) Cells from BALF were analyzed by FACScan with the indicated Abs. Live cells were gated (n = 3). *p < 0.05.
STING is essential for CDG-induced DC maturation
DCs play a central role in mediating adjuvant-induced immune responses. Previous studies (1) showed that CDG activates human and mouse DCs, promoting the production of cytokines and chemokines and enhancing DCs’ ability to stimulate T cells. CDG also induces NO production (6). We examined CDG-activated STING−/− BMDCs (Supplemental Fig. 2). CDG stimulation failed to upregulate the costimulatory molecules CD80 and CD86 in STING−/− BMDCs (Supplemental Fig. 2A, 2B). CDG-induced TNF-α, IFN-α, and IL-6 production was absent in STING−/− BMDCs (Supplemental Fig. 2C). STING was needed for CDGinduced inducible NO synthase expression (Supplemental Fig. 2D) and NO production in DCs (Supplemental Fig. 2E). As a control, LPS induces CD80 and CD86 expression (Supplemental Fig. 2A, 2B), as well as TNF-α, IFN-α, IL-6, and NO production in STING−/− BMDCs (Supplemental Fig. 2C, 2E). Notably, NO induced by CDG stimulation is much weaker than that induced by LPS stimulation (Supplemental Fig. 2D, 2E).
Next, we examined the ability of CDG-activated STING−/− BMDCs to stimulate OVA-specific CD4+ T cells. BMDCs were first activated by CDG/OVA or LPS/OVA for 24 h. These activated BMDCs were then cocultured with CFSE-labeled OVA-specific CD4+ T cells from OT II mice for 4 d. IL-2 production was measured to determine T cell activation (Supplemental Fig. 2F). CD4+ T cell proliferation was determined by CFSE dilution (Supplemental Fig. 2G). Compared with WT BMDCs, CDG/ OVA-stimulated STING−/− BMDCs did not induce the production of IL-2 or cell proliferation in OVA-specific CD4+ T cells (Supplemental Fig. 2F, 2G). As a control, LPS/OVA-stimulated STING−/− BMDCs activated OVA-specific CD4+ T cells as efficiently as did the WT BMDCs (Supplemental Fig. 2F, 2G). In summary, STING is critical for CDG-induced DC maturation.
IFN-I signaling is dispensable for the mucosal adjuvant activity of CDG
STING is essential for CDG-induced IFN-I production (15, 16). In fact, the hallmark function of STING is to stimulate IFN-I production (23). The adjuvant activity of a DNA vaccine depends on TBK1-mediated IFN-I production (19). Thus, it is no surprise that STING is required for the adjuvant activity of a DNAvaccine (21). In this study, we asked whether IFN-I signaling is required for STING-mediated adjuvant activity of CDG, as well.
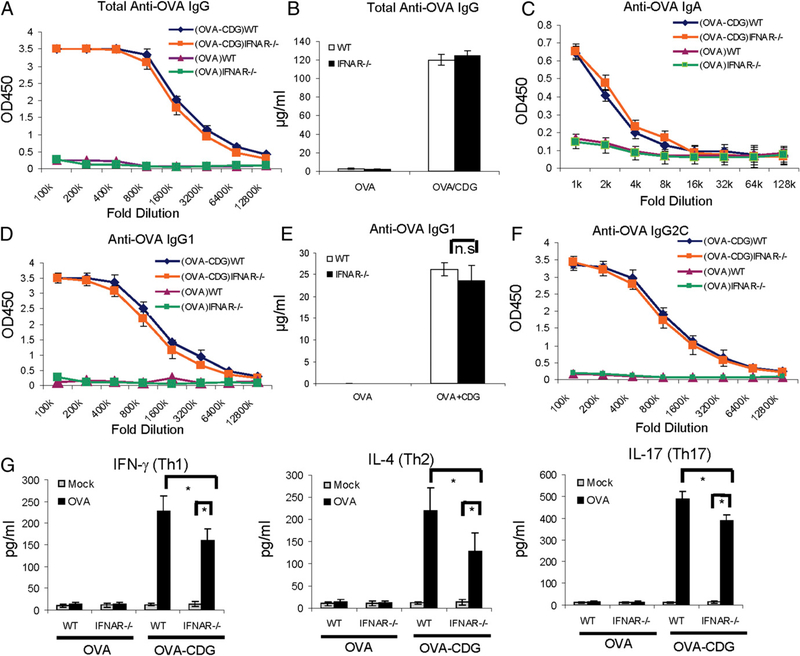
We immunized WT and IFNAR−/− mice with OVA i.n., with or without CDG, as before. OVA-specific Abs were measured by IgG ELISA. To our surprise, CDG/OVA-immunized (i.n.) IFNAR−/− mice showed no difference in OVA-specific Ab responses compared with the CDG/OVA-immunized WT mice (Fig. 3). Both groups of mice have similar total serum IgG, IgG1, IgG2C, and nasal IgA production (Fig. 3A–E). Furthermore, OVA stimulation of ex vivo splenocytes from immunized IFNAR−/− mice showed significant production of Th1, Th2, and Th17 cytokines, although they were slightly decreased compared with splenocytes from immunized WT mice (Fig. 3F). We concluded that CDG-induced IFN-I production is dispensable for the mucosal adjuvant activity of CDG.
FIGURE 3.
IFN-I signaling is dispensable for the mucosal adjuvant activity of CDG. (A–E) WT and IFNAR−/− mice were vaccinated i.n. as in Fig. 1. Abs in the blood and nasal wash samples were determined as in Fig. 1 (n = 3). (F) Splenocytes from WT and IFNAR−/− mice were collected and activated in vitro with OVA (100 μg/ml) as in Fig. 1. (G) Th1/Th2/Th17 cytokines were determined in supernatants as in Fig. 1 (n = 3). Data are mean ± SE from three independent experiments. *p, 0.05. n.s, Nonsignificant
IFN-I is not required for CDG-induced cytokine and chemokine production in vivo
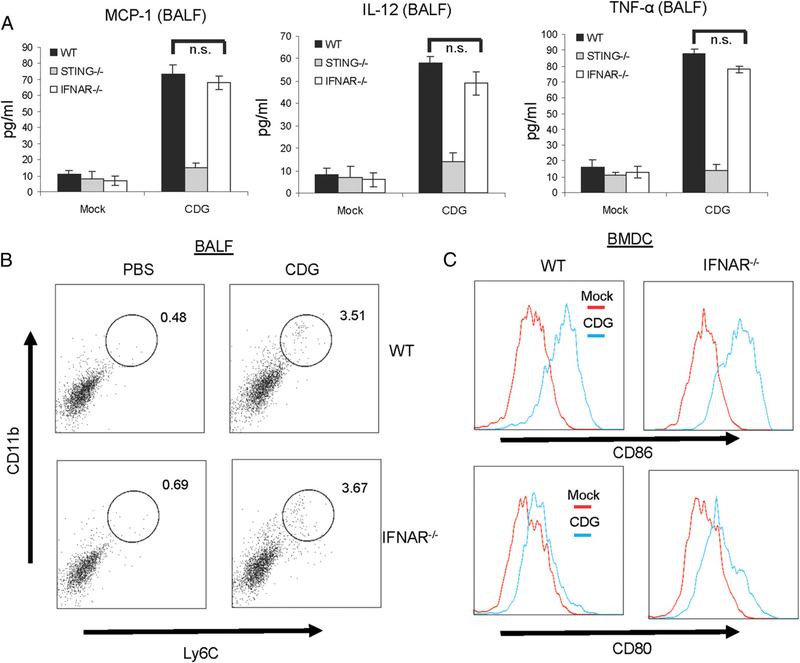
We also examined CDG-induced cytokine and chemokine production in BALF from CDG-treated IFNAR−/− mice. There was no difference in the production of MCP-1, IL-12/p70, or TNF-α between CDG-treated WT and IFNAR−/− mice (Fig. 4A). The recruitment of Ly6ChiCD11b+ inflammatory monocytes to BALF was also similar between WT and IFNAR−/− mice (Fig. 4B). Lastly, CDG-activated IFNAR−/− BMDCs increased surface CD80 and CD86 expression similar to the WT BMDCs (Fig. 4C). These in vivo and in vitro observations are consistent with the notion that IFN-I is dispensable for the mucosal adjuvant activity of CDG.
FIGURE 4.
IFN-I signaling is dispensable for CDG-induced cytokine and chemokine production in vivo. (A) WT, STING−/−, and IFNAR−/− mice were given CDG (10 μg) or PBS i.n. Cytokine production was measured in BALF as in Fig. 2 (n = 3). Data are mean ± SE from three independent experiments. (B) Live cells from BALF were analyzed by FACScan with the indicated Abs as in Fig. 2 (n = 3). (C) BMDCs from WTand IFNAR−/− μiχε were activated by CDG (10 μg/ml) or medium alone (mock) for 18 h. Cells were stained with Abs against CD80 and CD86. Live cells were analyzed by flow cytometry (n = 3). *p, 0.05. n.s., Nonsignificant.
STING differentially regulates CDG- and ISD-induced TNF-α production in vitro
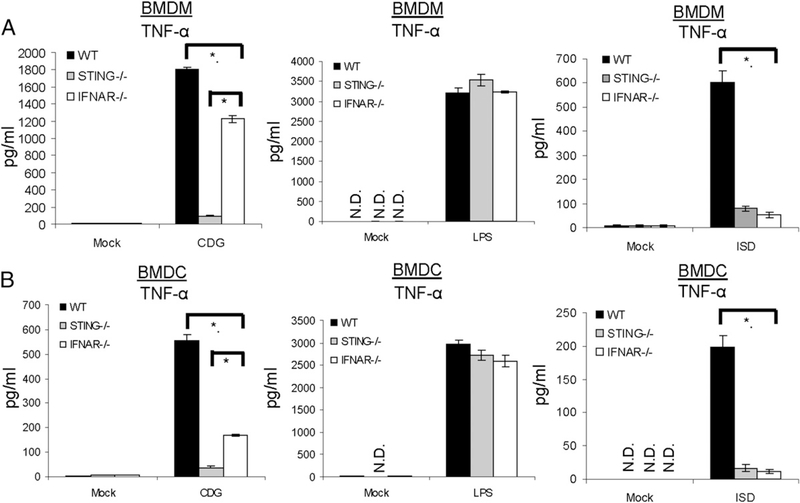
To understand the mechanism by which STING functions in the absence of IFN-I stimulation, we studied CDG activation in BMDMs and BMDCs. Consistent with the in vivo observation, CDG induced a significant amount of TNF-α in BMDMs or BMDCs from IFNAR−/− mice (Fig. 5, left panels). This is especially prominent in BMDMs, in which the CDG-induced TNF-α is largely independent of IFN-I signaling (Fig. 5).
FIGURE 5.
STING differentially regulates CDG- and ISD-induced TNF-α production in vitro. BMDMs (A) or BMDCs (B) from WT, STING−/−, or IFNAR−/− mice were activated by CDG (10 μg/ml), LPS (200 ng/ml), ISD (10 μg/ml), or medium alone (mock) for 6 h. TNF-α production was measured in the supernatant by ELISA (n = 3). Data are mean ± SE from three independent experiments. *p <, 0.05. N.D., Not detected.
ISD is a 45-nt non-CpG oligomer from the Listeria monocytogenes genome. Distinct from CDG activation, ISD-induced TNF-α production was absent in IFNAR−/− cells (Fig. 5, right panels). Both ISD- and CDG-induced TNF-α required STING (Fig. 5). Thus, ISD and CDG activate the production of TNF-α via distinct STING pathways in vitro. This is in agreement with our in vivo data showing that, although the adjuvant activities of DNA and CDG require STING, they are distinct with regard to IFN-I signaling. As a control, both STING−/− and IFNAR−/− cells respond normally to LPS stimulation (Fig. 5, middle panels).
TNF-α signaling is critical for the mucosal adjuvant activity of CDG
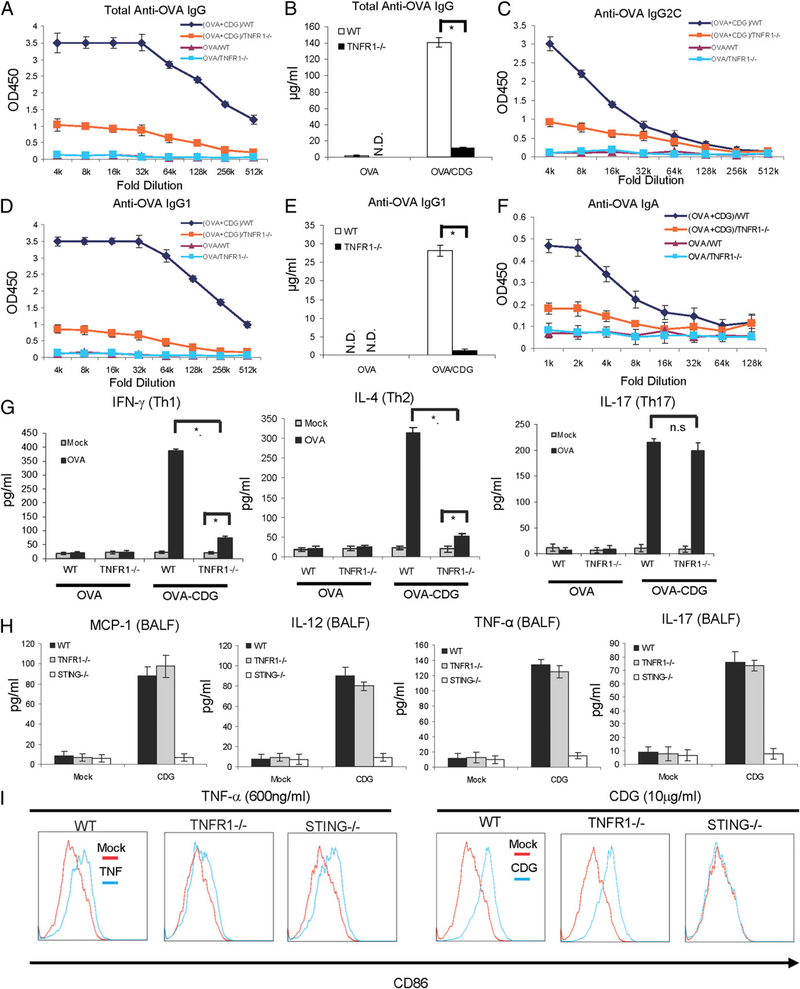
TNF-α has promising mucosal adjuvant activity (24). We next asked whether the IFN-I–independent TNF-α production by CDG is responsible for its mucosal adjuvant activity. We immunized WT and TNFR1−/− mice i.n. with OVA, with or without CDG, as before. Excitingly, CDG/OVA-immunized (i.n.) TNFR1−/− mice showed a dramatic decrease in total OVA-specific IgG responses compared with the CDG/OVA-immunized WT mice (Fig. 6A, 6B). The OVA-specific IgG1, IgG2C, and nasal IgA production were also severely impaired in TNFR1−/− mice (Fig. 6C–F). OVA stimulation of ex vivo splenocytes from immunized TNFR1−/− mice resulted in a significant reduction in Th1 (IFN-γ) and Th2 (IL-4) cytokines (Fig. 6G). Interestingly, OVA-induced IL-17 production was not affected (Fig. 6G).
FIGURE 6.
TNF-α signaling is critical for the mucosal adjuvant activity of CDG. (A–F) WTand TNFR1−/− mice were vaccinated i.n. as in Fig. 1. Abs in the blood and nasal wash samples were determined as in Fig. 1 (n = 3). (G) Splenocytes from WTand TNFR1−/− mice were collected and activated in vitro with OVA (100 μg/ml) as in Fig. 1. Th1/Th2/Th17 cytokines were determined in supernatants as in Fig. 1 (n = 3). Data are mean ± SE from three independent experiments. (H) WT, STING−/−, and TNFR1−/− mice were treated as in Fig. 2. Cytokine production was measured in BALF as in Fig. 2 (n = 3). Data are mean ± SE from three independent experiments. (I) BMDCs from WT, STING−/−, or TNFR1−/− mice were activated by TNF (600 ng/ml), CDG (10 μg/ml), or medium alone (mock) for 18 h. Cells were stained with Abs against CD86. Live cells were analyzed by flow cytometry (n = 2). *p<,0.05. N.D., Not detected; n.s, nonsignificant.
There were no differences in CDG-induced MCP-1, TNF-α, or IL-12 production in BALF from TNFR1−/− and WT mice (Fig. 6H). IL-17 (Fig. 6H) and GM-CSF (data not shown) production in BALF were also intact in TNFR1−/− mice. CDG also activated TNFR1−/− BMDCs by increasing surface CD86 expression (Fig. 6I). As expected, BMDCs from TNFR1−/− mice had little response to TNF-α stimulation (Fig. 6I, left panels), whereas STING−/− BMDCs had increased CD86 expression after TNF-α treatment. In contrast, STING−/− BMDCs did not respond to CDG activation, whereas TNFR1−/− BMDCs did (Fig. 6I, right panel). We concluded that CDG-induced TNF-α production is critical for the mucosal adjuvant activity of CDG.
TNF-α signaling is critical for the mucosal adjuvant activity of CDA
CDA is one of the most recent cyclic dinucleotide second messengers discovered in bacteria (25). A previous study (9) showed that CDA also has mucosal adjuvant activity. More importantly, we showed previously that, like CDG, CDA signals via STING in vitro (15). We next asked whether CDA used the same STING– TNF-α pathway to elicit its mucosal adjuvant activity.
We immunized WT, STING−/−, and TNFR1−/− mice with OVA i.n., with or without CDA, as before. After two immunizations, blood and nasal wash were collected 14 d after the last boost. OVA-specific Abs were measured by ELISA. Similar to CDG immunization, CDA-induced Ag-specific IgG, IgG1, IgG2C, and IgA production were absent in STING−/− mice and severely impaired in TNFR1−/− mice (Supplemental Fig. 3). We concluded that the STING–TNF-α pathway is also critical for the mucosal adjuvant activity of CDA.
STING is required for CDG-induced NF-𝜅B activation
TNF-α production is mainly a result of canonical NF-κB activation (26, 27). Previous studies (11) indicated that CDG can activate the NF-κB pathway. Because CDG-induced TNF-α production is STING dependent, we hypothesized that STING is required for CDG-induced NF-κB activation, as well.
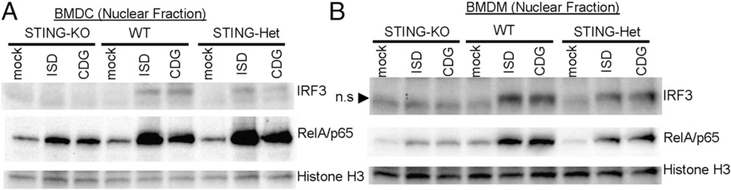
To demonstrate the role of STING in CDG-induced NF-κB signaling, we isolated the nuclear fraction and examined nuclear translocation of RelA/p65 upon CDG stimulation. We used ISD as a positive control because STING is required for cytosolic DNA– induced NF-κB activation (28). As expected, STING−/− BMDMs and BMDCs were defective in CDG-induced nuclear translocation of IRF3 (Fig. 7). Similar to ISD stimulation, CDG-induced RelA/p65 nuclear translocation was dramatically decreased in STING−/− cells (Fig. 7). We concluded that STING is essential for the CDG-induced NF-κB activation.
FIGURE 7.
STING is required for CDG-induced NF-κB activation. BMDCs (A) or BMDMs (B) from C57BL/6, STING2/+, or STING−/− mice were activated by CDG (10 μg/ml) for 4 h. Nuclear fractions were isolated as described in Materials and Methods. Samples were run on SDS-PAGE gel and probed with the indicated Abs (n = 3). n.s, Nonspecific.
Uncoupling STING-mediated NF-𝜅B and IRF3 activation
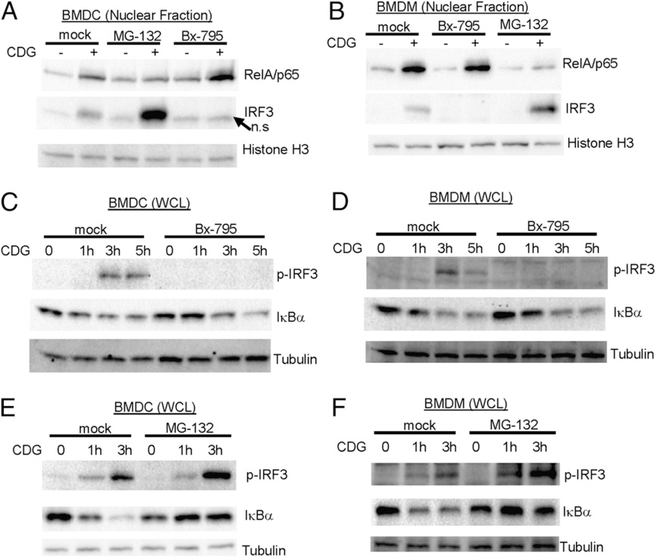
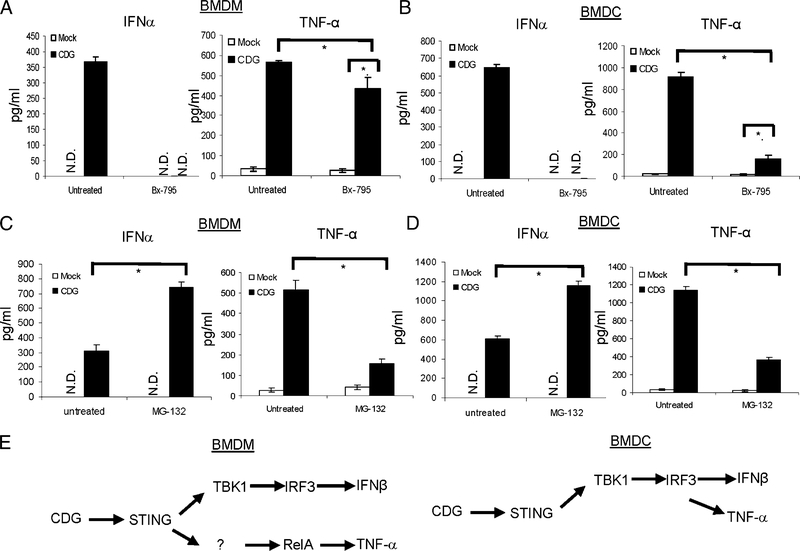
CDG simultaneously activates STING-dependent IRF3–IFN-I and RelA–TNF-α pathways. The fact that STING-mediated TNF-α, but not IFN-I, production is critical for the adjuvant activity of CDG suggested that IRF3 and RelA may be activated via different STING pathways. To determine whether these STING-mediated events are indeed uncoupled, we used chemical inhibitors Bx-795 (29) to inhibit IRF3 activation and MG-132 (30) to inhibit NF-κB activation. Nuclear-translocation analysis showed that, although Bx-795 inhibits CDG-induced IRF3 translocation, it did not affect CDG-induced RelA nuclear translocation in BMDMs and BMDCs (Fig. 8A, 8B). In contrast, MG-132 inhibits CDG-induced RelA, but not IRF3, nuclear translocation in BMDMs and BMDCs (Fig. 8A, 8B). In fact, MG-132 treatment increased CDG-induced IRF3 nuclear translocation (Fig. 8A, 8B).
FIGURE 8.
Uncoupling STING-mediated NFkB activation and IRF3 activation. BMDCs (A) or BMDMs (B) from C57BL/6 mice were treated with Bx-795 (1 μM), MG-132 (4 μg/ml), or DMSO (mock) for 2 h. Cells were then activated by CDG (10 μg/ml) for 4 h. Nuclear fractions were isolated and processed as in Fig. 7 (n = 3). BMDCs (C) or BMDMs (D) were treated with Bx-795 (1 μM) or DMSO (mock) as in (A) and (B). Cells were then activated by CDG (10 μg/ml) for the indicated time. WCLs were run on SDS-PAGE gel and probed with the indicated Abs (n = 3). BMDCs (E) or BMDMs (F) were treated with MG-132 (4 μg/ml) or DMSO (mock) as in (A) and (B). Cells were then activated by CDG (10 μg/ml) for the indicated time. WCLs were run on SDS-PAGE gel and probed with the indicated Abs (n = 3). n.s, Nonspecific.
To further demonstrate that STING-mediated NF-κB and IRF3 activation can be uncoupled from each other, we examined IRF3 phosphorylation and IκBα degradation in WCL upon Bx-795 or MG-132 treatment. Bx-795 efficiently inhibited CDG-induced IRF3 phosphorylation, but it did not affect CDG-induced IκBα degradation in both BMDMs and BMDCs (Fig. 8C, 8D). Similarly, MG-132 inhibited CDG-induced IκBα degradation and did not reduce, but rather increased, IRF3 phosphorylation (Fig. 8E, 8F). We concluded that STING mediated NF-κB and IRF3 activation via two separate signaling pathways.
Uncoupling STING-mediated TNF-α and IFN-I production
We next examined whether NF-κB–mediated TNF-α production and IRF3-mediated IFN-I production can also be uncoupled. Treating WT BMDMs with Bx-795 abolished CDG-induced IFN-α production (Fig. 9A). However, CDG-induced TNF-α production in BMDMs decreased only slightly (Fig. 9A). In contrast, MG-132 inhibited CDG-induced TNF-α production but did not inhibit, but rather increased, CDG-induced IFN-a production (Fig. 9C). Thus, CDG-induced STING-mediated TNF-α and IFN-I production can be uncoupled in BMDMs (Fig. 9E, left panel).
FIGURE 9.
Uncoupling STING-mediated TNF-α and IFN-I production. BMDMs (A) or BMDCs (B) from C57BL/6 mice were treated with Bx-795 (1 mM) or DMSO (untreated) for 2 h. Cells were then activated by CDG (10 μg/ml) for 5 h. TNF-α and IFN-α were measured in supernatant (n = 3). Data are mean ± SE from three independent experiments. BMDMs (C) or BMDCs (D) were treated with MG-132 (4 μg) or DMSO (untreated) for 2 h. Cells were then activated by CDG (10 μg/ml) for 5 h. TNF-α and IFN-α were measured in supernatant (n = 3). Data are mean ± SE from three independent experiments. (E) Uncoupling of CDG–STING–TBK1–IRF3–IFN-I with CDG–STING–NF-κB–TNF-α in BMDMs and BMDCs. *p<, 0.05. N.D., Not detected.
We also examined TNF-α and IFN-I uncoupling in BMDCs. We found that Bx-795 inhibited CDG-induced TNF-α production greatly (Fig. 9B). This is different from the response in BMDMs (Fig. 9A) and suggested that TBK1 activation and the subsequent IFN-I signaling play major roles in CDG-induced TNF-α activation in BMDCs (Fig. 9E, right panel). This is consistent with the observation in BMDCs from IFNAR−/− mice (Fig. 5B).
Nevertheless, Bx-795–resistant TNF-α production was still seen in CDG-activated BMDCs (Fig. 9B). Similar to in BMDMs, MG132 treatment inhibited CDG-induced TNF-α production and did not inhibit, but rather increased, CDG-induced IFN-α production (Fig. 9D). Thus, CDG-induced TNF-α and IFN-I production can also be uncoupled in BMDCs but to a much lesser extent.
Bx-795 inhibits TBK1 activation (29). We next examined TNF-α and IFN-I uncoupling in TBK1-knockdown cells. We used SMARTpool small interfering RNA from Dharmacon to knock down TBK1 in BMDCs and BMDMs. The knockdown efficiency was confirmed by Western blot (Supplemental Fig. 4) and the lack of IFN-I production by CDG (Supplemental Fig. 4). Similar to the observation with Bx-795 treatment, decreasing TBK1 expression reduced, but did not eliminate, CDG-induced TNF-α production in BMDCs and BMDMs (Supplemental Fig. 4). We concluded that STING mediates CDG stimulation by simultaneously activating parallel NF-κB–TNF-α and TBK1–IRF3–IFN-I pathways.
Discussion
Cytosolic sensing of DNA or bacterial second messenger CDG activates a common STING–TBK1–IRF3–signaling axis and leads to IFN-I production (31). Both DNA and cyclic dinucleotides have promising adjuvant activity (32). Previous studies (19, 21) showed that the adjuvant activity of DNA requires STING and IFN-I. In this study, we demonstrated, surprisingly, that the adjuvant activity of CDG requires STING-mediated TNF-α, but not IFN-I, production. Therefore, STING-mediated DNA and CDG sensing can be dissociated in vivo.
STING is required for both CDG- and DNA-induced TNF-α production. However, CDG-induced TNF-α is largely independent of IFN-I signaling, whereas DNA-induced TNF-α production requires intact IFN-I signaling. We hypothesize that CDG and DNA adopt distinct STING pathways to activate TNF-α. Recently, cyclic GMP-AMP synthase was identified as a DNA sensor (33). It catalyzes the synthesis of a metazoan second messenger, cGAMP, in the presence of DNA (34). This novel metazoan second messenger contains G(2ʹ,5ʹ)pA and A(3ʹ,5ʹ)pA phosphodiester linkages and binds to STING with high affinity (Kd = 4 nM) (14). This is ~300-fold lower than the Kd for CDG–STING binding (Kd = 1.2 μM) (14). This association difference with STING may explain why STING mediates the adjuvant effect of DNA, through the generation of 2ʹ5ʹ−3ʹ5ʹ cGAMP, and CDG differently. Alternatively, STING may use different coreceptors for sensing DNA and CDG, leading to different outcomes in vivo.
It was shown that TNF-α (24), and its family member TL1A (35), have mucosal adjuvant activity. However, like many cytokine mucosal adjuvants, they are costly and prone to degradation when administrated via the i.n. route (24). Furthermore, soluble TNF-α, as a mucosal adjuvant, primarily elicits Th2 responses (24). CDGinduced local production of TNF-α in lung likely has different kinetics and potency than exogenous soluble TNF-α. In this study, we found that TNF-α is required for CDG-induced Th2 responses, as well as Th1 responses, which supports this notion. Interestingly, a previous study (36) showed that particulate TNF-α can elicit both Th1 and Th2 responses and protects against a lethal flu challenge. Thus, although CDG adjuvant activity is mediated by TNF-α, it has many advantages over soluble TNF-α as a mucosal adjuvant.
It is not clear which cell type(s) makes TNF-α in response to CDG immunization in vivo. Alveolar macrophages, bronchial epithelial cells, pulmonary mast cells, lung T cells, and B cells can all make TNF-α (37–39). Inflammatory DCs (Ly6ChiCD11b+) also make TNF-α in lung (40). However, the type of stimuli likely determines the source of TNF-α. Systemic TNF-α in response to LPS is produced mainly by macrophages (38), whereas TNF-α is produced largely by Ly6C+CD11b+ inflammatory DCs during fungal infection (40). Importantly, TNF-α produced by different types of cells could have distinct and nonredundant in vivo functions (38). Additional studies are required to determine the source of TNF-α, as well as effector cells, during CDG immunization.
Of note, TNFR1−/− mice have some residual Ag-specific Ab response during CDG immunization, which is different from STING−/− mice. TNFR1 is one of the two TNF-α receptors (TNFR1 and TNFR2) found in mice and humans. TNFR1 is the major mediator of TNF-α signaling and is ubiquitously expressed on almost all cell types (41). In contrast, TNFR2 expression is limited to specific cell types, such as endothelial cells and CD4+ T and CD8+ T cells (42). Moreover, TNFR1 is activated by both membrane-bound and soluble TNF-α, whereas TNFR2 is preferentially activated by membrane-integrated TNF-α (43). Currently, we are pursuing the hypothesis that the residual CDG adjuvant activity seen in TNFR1−/− mice comes from the recognition of TNF-α by TNFR2.
Another exciting finding is the STING–TBK1–IRF3–independent activation of STING–NF-κB–TNF-α production. It is known that CDG can induce the production of TNF-α and NO (1), which are indicators of NF-κB activation. Indeed, McWhirter et al. (11) found that CDG activates RelA/p65. However, it is not clear how this NF-κB signaling is related to the well-established STING– TBK1–IRF3–signaling axis; also, the biological significance of CDG-induced NF-κB activation is unknown. In this study, we showed that STING–NF-κB–TNF-α signaling is critical for the mucosal vaccine adjuvant activity of CDG in vivo and demonstrated that this arm of STING signaling is independent of its well-known TBK1–IRF3–signaling axis. For the future implementation of CDG or CDA as mucosal adjuvants in human trials, it will be important to determine how the human STING variants that we identified previously (44) affect this unique STING–NFκB–TNF-α pathway.
Cyclic dinucleotides are promising adjuvant candidates (32). In light of our current finding, we suggest that 2ʹ5ʹ−3ʹ5ʹ cGAMP, generated during DNA sensing, requires IFN-I for its adjuvant activity, whereas the 3ʹ5ʹ−3ʹ5ʹ cyclic dinucleotide, similar to CDG and CDA, uses a TNF-α–dependent mechanism to mediate its adjuvant activity. Indeed, while this manuscript was in review, Li et al. (45) showed that 2ʹ5ʹ−3ʹ5ʹ cGAMP exhibits adjuvant activity that is dependent on IFN-I signaling. Thus, potential chemical engineering on various cyclic dinucleotides can pave the way for the development of a new group of adjuvants with different underlying mechanisms: IFN-I or TNF-α dependent.
Supplementary Material
Acknowledgments
We thank Dr. John Cambier (National Jewish Health, Denver, CO) for generous support of the project and Dr. Dennis Metzger and Dr. Katherine MacNamara (Albany Medical College, Albany, NY) for IFNAR−/− mice.
This work was supported by Albany Medical College New Faculty Startup funds (to L.J.).
Abbreviations
- BALF
bronchoalveolar lavage fluid
- BMDC
bone marrow–derived dendritic cell
- BMDM
bone marrow–derived macrophage
- CDA
(3ʹ–5ʹ)-cyclic-di-adenosine-monophosphate
- CDG
(3ʹ–5ʹ)-cyclic-di-guanosine-monophosphate
- cGAMP
cyclic-GMP–AMP
- DC
dendritic cell
- ISD
IFN-stimulating DNA
- i.n.
intranasal(ly)
- STING
stimulator of IFN genes
- WCL
whole cell lysate
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E,Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, et al. 2007. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol 178: 2171–2181. [DOI] [PubMed] [Google Scholar]
- 2.Ebensen T, Schulze K, Riese P, Link C, Morr M, and Guzma CA´n. 2007. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 25: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 3.Madhun AS, Haaheim LR, Nøstbakken JK, Ebensen T, Chichester J,Yusibov V, Guzman CA, and Cox RJ 2011. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 29: 4973–4982. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, KuoLee R, Harris G, Tram K, Yan H, and Chen W 2011. c-di-GMPprotects against intranasal Acinetobacter baumannii infection in mice by chemokine induction and enhanced neutrophil recruitment. Int. Immunopharmacol 11: 1378–1383. [DOI] [PubMed] [Google Scholar]
- 5.Hu DL, Narita K, Hyodo M, Hayakawa Y, Nakane A, and Karaolis DK 2009. c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine 27: 4867–4873. [DOI] [PubMed] [Google Scholar]
- 6.Karaolis DK, Newstead MW, Zeng X, Hyodo M, Hayakawa Y, Bhan U,Liang H, and Standiford TJ 2007. Cyclic di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect. Immun 75: 4942–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogunniyi AD, Paton JC, Kirby AC, McCullers JA, Cook J, Hyodo M,Hayakawa Y, and Karaolis DK 2008. c-di-GMP is an effective immunomodulator and vaccine adjuvant against pneumococcal infection. Vaccine 26:4676–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan H, KuoLee R, Tram K, Qiu H, Zhang J, Patel GB, and Chen W 2009. 39,59-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem. Biophys. Res. Commun 387: 581–584. [DOI] [PubMed] [Google Scholar]
- 9.Ebensen T, Libanova R, Schulze K, Yevsa T, Morr M, and Guzma´n CA 2011. Bis-(39,59)-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 29: 5210–5220. [DOI] [PubMed] [Google Scholar]
- 10.Gray PM, Forrest G, Wisniewski T, Porter G, Freed DC, DeMartino JA,Zaller DM, Guo Z, Leone J, Fu TM, and Vora KA 2012. Evidence for cyclic diguanylate as a vaccine adjuvant with novel immunostimulatory activities. Cell. Immunol 278: 113–119. [DOI] [PubMed] [Google Scholar]
- 11.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M,Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, et al. 2009. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med 206: 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, and Barber GN 2008. STING is an endoplasmic reticulumadaptor that facilitates innate immune signalling. Nature 455: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, and Cambier JC 2008. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol. Cell. Biol 28: 5014–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, and Chen ZJ 2013. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL,Cambier JC, and Lenz LL 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol 187: 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, and Vance RE 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun 79: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang S, Song X, Wang Y, Ru H, Shaw N, Jiang Y, Niu F, Zhu Y, Qiu W,Parvatiyar K, et al. 2012. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity 36: 1073–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin Q, Tian Y, Kabaleeswaran V, Jiang X, Tu D, Eck MJ, Chen ZJ, and Wu H 2012. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell 46: 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T,Uematsu S, Takeuchi O, Takeshita F, Coban C, and Akira S 2008. TANKbinding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451: 725–729. [DOI] [PubMed] [Google Scholar]
- 20.Jin L, Getahun A, Knowles HM, Mogan J, Akerlund LJ, Packard TA,Perraud AL, and Cambier JC 2013. STING/MPYS mediates host defense against Listeria monocytogenes infection by regulating Ly6C(hi) monocyte migration. J. Immunol 190: 2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa H, Ma Z, and Barber GN 2009. STING regulates intracellular DNAmediated, type I interferon-dependent innate immunity. Nature 461: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee AS, Burchill MA, Munks MW, Jin L, Kappler JW,Friedman RS, Jacobelli J, and Marrack P 2013. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc. Natl. Acad. Sci. USA 110: E1122–E1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdette DL, and Vance RE 2013. STING and the innate immune responseto nucleic acids in the cytosol. Nat. Immunol 14: 19–26. [DOI] [PubMed] [Google Scholar]
- 24.Kayamuro H, Abe Y, Yoshioka Y, Katayama K, Nomura T, Yoshida T,Yamashita K, Yoshikawa T, Kawai Y, Mayumi T, et al. 2009. The use of a mutant TNF-alpha as a vaccine adjuvant for the induction of mucosal immune responses. Biomaterials 30: 5869–5876. [DOI] [PubMed] [Google Scholar]
- 25.Corrigan RM, and Gru A¨ndling. 2013. Cyclic di-AMP: another second messenger enters the fray. Nat. Rev. Microbiol 11: 513–524. [DOI] [PubMed] [Google Scholar]
- 26.Yao J, Mackman N, Edgington TS, and Fan ST 1997. Lipopolysaccharideinduction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J. Biol. Chem 272: 17795–17801. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Dziarski R, Wang Q, Swartz K, Sakamoto KM, and Gupta D 2001. Bacterial peptidoglycan-induced tnf-alpha transcription is mediated through the transcription factors Egr-1, Elk-1, and NF-kappaB. J. Immunol 167: 6975–6982. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Yuan B, Bao M, Lu N, Kim T, and Liu YJ 2011. The helicaseDDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol 12: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark K, Plater L, Peggie M, and Cohen P 2009. Use of the pharmacologicalinhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J. Biol. Chem 284: 14136–14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palombella VJ, Rando OJ, Goldberg AL, and Maniatis T 1994. Theubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78: 773–785. [DOI] [PubMed] [Google Scholar]
- 31.Danilchanka O, and Mekalanos JJ 2013. Cyclic dinucleotides and the innateimmune response. Cell 154: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libanova R, Becker PD, and Guzmán CA 2012. Cyclic di-nucleotides: new era for small molecules as adjuvants. Microb. Biotechnol 5: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L, Wu J, Du F, Chen X, and Chen ZJ 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, and Chen ZJ 2013. CyclicGMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339: 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayamuro H, Yoshioka Y, Abe Y, Katayama K, Yoshida T, Yamashita K,Yoshikawa T, Hiroi T, Itoh N, Kawai Y, et al. 2009. TNF superfamily member, TL1A, is a potential mucosal vaccine adjuvant. Biochem. Biophys. Res. Commun 384: 296–300. [DOI] [PubMed] [Google Scholar]
- 36.St John AL, Chan CY, Staats HF, Leong KW, and Abraham SN 2012. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater 11: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Togbe D, Grivennikov SI, Noulin N, Couillin I, Maillet I, Jacobs M,Maret M, Fick L, Nedospasov SA, Quesniaux VF, et al. 2007. T cell-derived TNF down-regulates acute airway response to endotoxin. Eur. J. Immunol 37: 768–779. [DOI] [PubMed] [Google Scholar]
- 38.Grivennikov SI, Tumanov AV, Liepinsh DJ, A Kruglov A, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Fo¨rster I, Clausen BE, et al. 2005. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22: 93–104. [DOI] [PubMed] [Google Scholar]
- 39.Tumanov AV, Grivennikov SI, Kruglov AA, Shebzukhov YV,Koroleva EP, Piao Y, Cui CY, Kuprash DV, and Nedospasov SA 2010. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood 116: 3456–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S,Iwakura Y, Fallert Junecko BA, Reinhart TA, et al. 2011. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. USA 108: 5360–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tartaglia LA, and Goeddel DV 1992. Two TNF receptors. Immunol. Today 13: 151–153. [DOI] [PubMed] [Google Scholar]
- 42.Faustman D, and Davis M 2010. TNF receptor 2 pathway: drug target forautoimmune diseases. Nat. Rev. Drug Discov 9: 482–493. [DOI] [PubMed] [Google Scholar]
- 43.Grell M, Douni E, Wajant H, Lo¨hden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, and Scheurich P 1995. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83: 793–802. [DOI] [PubMed] [Google Scholar]
- 44.Jin L, Xu LG, Yang IV, Davidson EJ, Schwartz DA, Wurfel MM, and Cambier JC 2011. Identification and characterization of a loss-of-function human MPYS variant. Genes Immun. 12: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XD, Wu J, Gao D, Wang H, Sun L, and Chen ZJ 2013. Pivotal roles ofcGAS-cGAMP signaling in antiviral defense and immune adjuvant effects.Science 341: 1390–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.