Abstract
Biliary atresia (BA) is a neonatal T cell-mediated inflammatory, sclerosing cholangiopathy. In the Rhesus rotavirus (RRV)-induced neonatal mouse model of BA (murine BA), mice lacking B cells do not develop BA, and the lack of B cells is associated with loss of T cell and macrophage activation. The aim of this study was to determine the mechanism of B cell-mediated immune activation (antigen presentation versus cytokine production) in murine BA. Results: Normal neonatal B cells in the liver are predominantly at pro-B and pre-B cellular development. However, BA mice exhibit a significant increase in the number and activation status of mature liver B cells. Adoptively transferred B cells into RRV-infected B cell-deficient mice were able to re-instate T cell and macrophage infiltration and biliary injury. Nonetheless, neonatal liver B cells were incompetent at antigen presentation to T cells. Moreover, 3-83 Ig transgenic mice, in which B cells only present an irrelevant antigen, developed BA, indicating a B cell antigen-independent mechanism. B cells from BA mice produced a variety of innate and adaptive immune cytokines associated with immune activation. In-vitro trans-well studies revealed that BA B cells secreted cytokines that activated T cells based on increased expression of T cell activation marker CD69. Conclusions: Neonatal liver B cells are highly activated in murine BA and contribute to immune activation through production of numerous cytokines involved in innate and adaptive immunity. This work provides increased knowledge on the capacity of neonatal B cells to contribute to an inflammatory disease through cytokine-mediated mechanisms. Future studies should focus on targeting B cells as a therapeutic intervention in human BA.
Keywords: neonatal cholestasis, T cell-mediated immunity, innate immunity, toll-like receptors
Biliary atresia (BA) is a progressive inflammatory biliary disease resulting in fibrosis of the extrahepatic and intrahepatic bile ducts early in life.[1,2] Despite surgical intervention with hepatic portoenterostomy at diagnosis, approximately 80% of BA patients eventually require liver transplantation.[1,3] Although the etiology of BA is unknown, a leading theory of pathogenesis is that disease onset entails a primary perinatal hepatobiliary virus infection followed by exaggerated, progressive inflammatory or autoimmune-mediated bile duct injury. In order to study immune-mediated mechanisms of biliary disease, the Rhesus group A rotavirus (RRV)-induced mouse model of BA (“murine BA”) is utilized. This model mimics the biochemical, immunological and histological abnormalities found early in the human disease.[2,4,5]
The bulk of knowledge regarding immune mechanisms of biliary injury in BA focuses on Th1 and Th17 cell-mediated pathways.[5-11] A substantial increase in infiltrating macrophages also characterizes the liver inflammatory milieu in BA.[5] Far less is known regarding the role of B cells in BA pathogenesis. B cells can promote autoimmunity in many ways, including antigen presentation to T cells, production of pathogenic autoantibodies and generation of cytokines that promote specific T cell activation and differentiation pathways.[12] Our group has shown that Ig-α deficient mice (Ig-α-/-), which bear no B cells because of non-functional B cell receptors, are protected from developing murine BA. Importantly, the absence of functional B cells is associated with a lack of T cell and macrophage activation, suggesting that B cells play a critical role in immune-mediated bile duct injury in BA.[13] Many experimental models of autoimmune diseases have demonstrated that B cells play a crucial role in disease pathogenesis.[14-16] It is suggested that B cells are needed for presenting self-antigens to autoreactive T cells, leading to T cell activation and pathogenic function, independent of antibody production.[17-19] B cells can also play an effector function through cytokine production and a contribution of cytokine-producing B cells in autoimmunity has been proposed.[20-22]
The aim of this study was to determine the mechanism of B cell-mediated immune activation (antigen presentation versus cytokine production) in murine BA. We utilized B cell-deficient mice as well as mice in which all B cells express an irrelevant B cell receptor (BCR) to determine if the primary mechanism of B cell-mediated T cell activation in BA is through antigen presentation or cytokine production.
Methods
RRV-induced mouse model of BA
All animals were housed and handled through the UC Denver Office of Laboratory Animal Medicine. BALB/c mice were purchased from infection-free colonies (Envigo Laboratories, Cambridgeshire, UK) and bred within the UC Denver animal facility. Mice were given a single intraperitoneal (i.p.) injection of RRV (1.8 × 106 pfu/mL) or balanced salt solution (BSS) within 24 hours of life. Ig-α-/- mice[23] and 3-83 Ig knock-in mice[24,25] on the BALB/c background have been previously described. Mice were sacrificed at either day 2, 7, or 14 and pooled sera and tissues from 3-5 mice/ pool were analyzed (minimum 3 pools/ group/ experiment and all experiments performed 3 times). Serum direct bilirubin and alkaline phosphatase levels were determined with kits available from Diagnostic Chemicals Ltd., Charlottetown, Canada. Mouse tissue was formalin fixed, paraffin embedded, and stained with hematoxylin-eosin. Digital photographs were obtained using the Olympus BX41 microscope (Melville, NY). Dr. Orlicky created a pathology scoring system to grade severity of disease: periductal inflammation and parenchymal inflammation: none=0, mild=1, moderate=2, severe=3; necrotic foci: none=0, >0<1 foci/100× field=1, ≥1 foci/100× field=2; ductular proliferation: absent=0, present=1.
Immunohistochemistry of human liver tissue
Human liver tissue was collected through a University of Colorado IRB-approved study (#12-0069; Mack: PI). Frozen human BA livers at time of liver transplant (explants; N=12; age 4.1±4.4 years) and controls [metabolic liver disease at explant (N=3), normal donor liver tissue (N=7); age 10±7 yrs] and paraffin-embedded liver tissue at the time of diagnosis of BA (N=9; age 7.8±3.5 weeks) and controls (N=2 Alagille syndrome; N=7 idiopathic giant cell hepatitis; age: 8.3±2.9 weeks) were analyzed. Frozen tissue was stained with CD20-Cy3, cytokeratin 7-FITC and nuclear DAPI using standard protocols[5] and paraffin-embedded sections were stained with CD20/ DAB. Slides were visualized using the Zeiss Axio Imager. A1 microscope (200× magnification). The number of CD20+ cells per portal tract were counted in a blinded fashion.
Flow cytometry
Tissue was homogenized with Bellco Cellector tissue sieve (Vineland, NJ) and red cells were lysed with ACK buffer. Liver immune cells were enriched by Percoll gradient (40/60). Single cell suspensions were incubated with Fc-block and stained with the following fluorochrome-conjugated antibodies (clone): CD45 (30-F11), CD3 (17A2), CD4 (RM4-5), CD8 (53-6.7), CD11b (M1/70), B220 (RA3-6B2), IgM (II/41), CD19 (6D5), CD49b (DX5), NKG2D (191004), MHC class I (34-1-2S), MHC class II (AMS-32.1), CD80 (16-10A1), CD86 (GL1), CD69 (H1.2F3), CD40 (HM40-3), or isotype matched controls. Cells were visualized with FACS LSR II flow cytometer (Becton-Dickinson, Mountain View, CA) using FlowJo software (Tree Star, Inc., Ashland, OR) for analysis. Intracellular cytokine staining: liver and spleen immune cells were incubated with Brefeldin A, stimulated with PMA and ionomycin and incubated with fluorochrome-conjugated antibodies (B220, CD19, IgM, CD45), followed by permeabilization and incubation with either IFN-γ (XMG1.2), IL-1β (NJTEN3), IL-6 (MP5-20F3), IL-10 (JES5-16E3), IL-2 (JES6-SH4), TNF-α (MP6-XT22), TGF-β (TW7-16B4), or GM-CSF (MPI-22E9P).
In-vitro T cell studies
IFN-γ ELISPOT: Responder T cells (CD3+ cells) and antigen presenting B cells (CD19+ cells) were purified by negative selection with MACS magnetic bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany). Confirmation of >95% purity of selected cell type was performed with flow cytometry. ELISPOT assays were performed on plates coated with murine IFN-γ capture monoclonal antibody. T cells (1×105) and B cells (3×105) were cultured alone, together and with or without RRV (1×104 pfu/ml) or normal mouse cholangiocyte (NMC) homogenate (50 μg/ml) for 24 hours. IFN-γ production was quantified with ELISPOT reader. Co-culture trans-well experiments: Purified T cells were stimulated overnight with plate-bound anti-CD3, washed and plated at 4×106 T cells in the lower compartment. 4×106 purified B cells were added to the top well of the trans-well and co-cultured for 48 hours. T cells were removed from the lower compartment for flow analyses (CD25, CD69).
Adoptive transfer studies
Donor B cells: BALB/c neonates were injected with either BSS or RRV within 24 hours of birth. 14 days after injection, the spleens and livers were homogenized using a Cellector Tissue Sieve and the B cells were purified using a B cell negative selection MACS cell separation kit. Confirmation of >95% purity of B cells was performed with flow cytometry. 1×107 purified B cells (40 μL) were injected intraperitoneally into Ig-α-/- recipient mice, age 12-18 hours of life. For mice receiving both B cells and BSS or RRV, the B cells were resuspended in 40 μL of the BSS or RRV (1.8×106 pfu/mL) solution respectively. 2 weeks after adoptive transfer, tissue was harvested for histology and FACS analysis.
RNA-Seq
Pools of 14 day old mouse livers (20 livers/ pool) were obtained from BALB/c BSS-control and RRV-BA mice (2 pools per group). Liver immune cells were purified by Percoll gradient, followed by ARIA flow cytometric cell sorting on CD19+ and CD3/CD11b/F4-80/NKG2d negative cells, with resultant >98% B cell purity. RNA isolation was performed by standard protocol (Qiagen, Hilden, Germany) and RNA-Seq (Illumina) was performed by the RNA-Seq core facility at National Jewish Health, Denver, CO. RNA-Seq data was analyzed with BaseSpace Illumina software and aligned to the mm10 reference genome using Tophat. R/Bioconductor DeSeq2 was used to detect the differentially expressed genes of samples. Enrichment analysis in Reactome database was performed using genes differentially expressed in RRV mice compared to BSS control mice (Benjamini-corrected p-value ≤0.05).
Statistical analysis
Values are expressed as mean ± standard deviation or standard error of the mean. Two-way analysis of variance (ANOVA) with multiple comparisons were used when more than two groups of mice were compared. The Student t-test was used for comparison between two groups. PRISM Graph Pad software (La Jolla, CA, USA) was employed for statistical analyses. Differences in means were considered significant for p values < 0.05.
Results
B cell development in the normal neonatal liver and spleen
We first sought to clearly define the level of B cell maturation in the liver and spleen of normal mice in the first 14 days of life (time frame of development of murine BA).[26-28] B cell poiesis begins at the pro-B cell stage (rearrangement of Ig heavy chains), followed by the pre-B cell stage (rearrangement of Ig light chains). B cells at the IgM+ immature cell stage are not yet wired for functional responses, requiring first to differentiate into CD23+CD24low, IgMlowIgDhigh mature B cells, a maturation process that occurs via a CD24highCD23+, IgM+IgD+ transitional stage (Figure 1A). While in adult mice B cell poiesis takes place mostly in bone marrow tissue, this process occurs in both liver and spleen in the prenatal, perinatal and early postnatal life[28,29].
Figure 1.
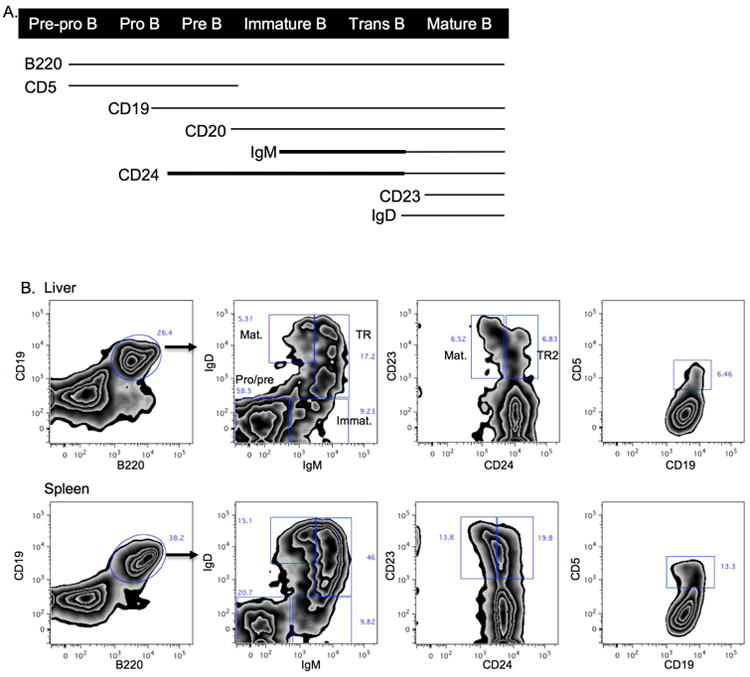
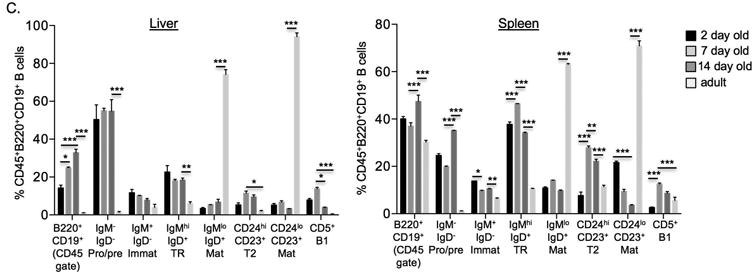
B cell development in the normal neonatal liver and spleen. A. Diagram of B cell surface marker expression during B cell maturation. Thicker lines denote higher levels of expression. B. Representative contour plots of B cell subsets in 7 day old liver and spleen (gated on live cells, FSC/SSC lymphocytes, CD45+cells, B220/ CD19+ cells). C. Summary of B cell subset frequencies in the liver and spleen of 2 day, 7 day and 14 day old mice (gated on live, FSC/SSC, CD45+, B220/ CD19+ cells; note that first set of bar graphs is B220/CD19+ cells gated on live, FSC/SSC and CD45+ cells). Imm: immature, TR: transitional, TR2: transitional-2, Mat: mature. ***p < 0.0005; **p < 0.005; *p < 0.05
Total B cell frequency (% B220+CD19+) in the liver increased from day 2 to day 14 of life. At 14 days liver B cell frequencies approached those in the spleen, and were significantly higher than adult livers (Figure 1B,C). In the first 14 days the majority of liver B cells are in the pro-B and pre-B stages (IgM-IgD-). This dramatically contrasts with the setting in adult livers where the frequency of pro/ pre-B cells is less than 5%. The frequency of immature (IgM+IgD-), transitional (IgMhighIgD+), transitional 2 (T2; CD24highCD23+) and mature (IgMlowIgDhigh or CD24lowCD23+) liver B cells are relatively constant in the first 14 days. Mature B cells are less than 5% at 14 days, while they are more than 80% of all B cells in the liver of adult mice (Figure 1C). In contrast to the liver, spleen B cells have a lower frequency of pro/pre-B cells in the first 7 days (∼20%), that doubles by 14 days. A higher frequency (∼70%) of immature, transitional and mature B cells collectively are present in the spleen compared to the liver in the first 14 days of life. Similar to the adult liver, the majority of B cells in the adult spleen are mature B cells. CD5 surface expression defines a B1 B cell subset known for its contribution to the pool of natural IgM antibodies. The frequency of this population peaked on day 7 in both the liver and the spleen. Additional analyses of the absolute number of B cell subsets per 0.1 gm of tissue revealed a steady decline in the number of B cells subsets in the liver over time, as expected based on the rapid increase in size of the liver over the first 2 weeks of life (Supplementary Figure 1). The spleen absolute numbers decreased from day 2 to day 7, followed by a substantial increase by day 14. This reflects the previously described effect of a transient influx of high amounts of immature erythroid cells into the spleen at 7 days of life [30], thus decreasing the relative abundance of all other immune cells at that time point. In summary, the neonatal liver is composed of B cells that are in early stages of development (pro-B, pre-B) with a smaller fraction of immature to mature B cells, while the spleen B cells are predominantly at the IgM+ immature to mature B cell stages.
Increase in transitional and mature B cells in murine BA
We next sought to determine if B cell maturation markers were increased in murine BA. Flow cytometric analysis of liver and spleen B cells from 14 day old mice showed significantly increased frequency and absolute numbers of total B cells (CD20+) and of transitional and mature B cells in the liver of BA mice relative to control mice (Figure 2A, B). Interestingly, the frequency of mature B cells in murine BA livers approached those levels found in the spleen, indicating a local effect of disease. In order to determine the degree of B cell infiltration in human BA, we analyzed human liver tissue from BA patients at the time of diagnosis and at liver transplant, compared to age-matched other-liver disease controls. Similar to murine BA, human BA livers had significantly increased numbers of portal tract CD20+ B cells compared to controls (Figure 2C). These data indicate that BA is associated with substantial increase of B cells in the liver in both mice and humans. The dramatic increase of mature B cells in the liver but not the spleen of BA mice suggests the contribution of B cells to disease onset is local.
Figure 2.
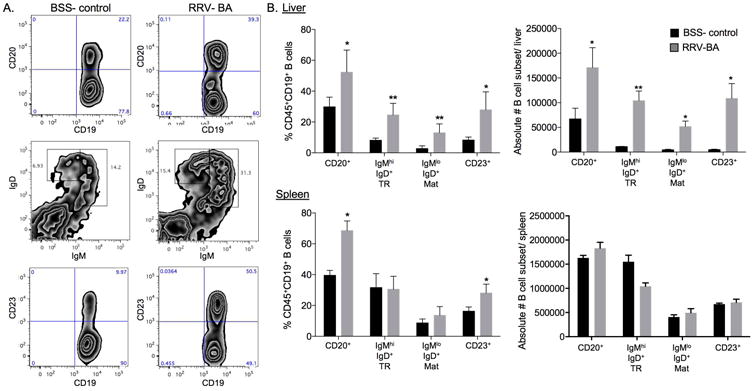
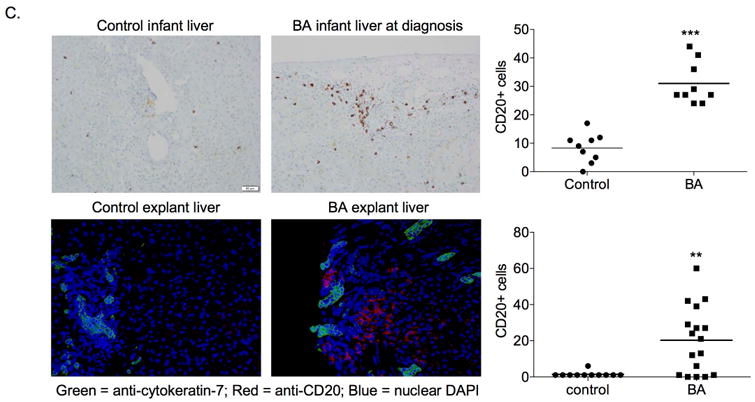
Increase in transitional and mature B cells in murine BA. A. Representative liver B cell contour plots and (B.) summary of frequency and absolute numbers of B cell subsets in liver and spleen of 14 day old BSS- control and RRV- BA mice (gated on FSC/SSC lymphocytes, CD45+cells, B220/ CD19+ cells; note that first set of bar graphs is B220/CD19+ cells gated on FSC/SSC lymphocytes, CD45+cells). C. Immunohistochemistry of CD20+ cellular infiltrates in BA at diagnosis (N=9) and age-matched controls (N=9) and BA (N=12) and control (N=10) livers at time of liver transplant (200× magnification). ***p < 0.0005; **p < 0.005; *p < 0.05
Adoptive transfer of B cells into RRV-infected Ig-α-/- mice results in liver inflammation and biliary injury
The B cell receptor (BCR) is composed of membrane-bound IgM that binds antigen and the signal transduction moiety Ig-α/Ig-β Stimulation of the BCR by antigen leads to the phosphorylation of Ig-α/Ig-β cytoplasmic tyrosine residues, an event that initiates a signaling cascade resulting in activation, proliferation and differentiation of mature B cells.[31-33] Ig-α-/- mice have loss of BCR expression and function, and consequently defective B cell antigen presentation and immunoglobulin and cytokine production.[23] We previously reported that RRV-infected Ig-α-/- mice are protected from developing BA, with no evidence of biliary injury based on high survival rate, normal serum bilirubin levels and bile duct histology and lack of liver T cell and macrophage infiltration and activation.[13] To further assess the contribution of B cells to disease pathology, newborn Ig-α-/- mice were given an intraperitoneal injection of 1×107 B cells from 14 day old BSS-control or RRV-BA mice, with concurrent administration of either BSS or RRV. The adoptive transfer of B cells from either BSS-control or RRV-BA mice into RRV-infected Ig-α-/- mice resulted in significant influxes of periductal T cells, macrophages, and NK cells compared to RRV-infected Ig-α-/- mice without adoptive transfer of B cells (Figure 3, Supplementary Figures 2, 3). There was an increase in serum direct bilirubin levels in the mice that received RRV-BA B cells (0.53±0.65 mg/dL vs. 0.15±0.13 mg/dL in BSS-control group), however the absolute values were within the normal range. Interestingly, adoptive transfer of B cells from BSS-control mice led to the most significant increases in liver T cell and macrophage infiltrates, compared to transfer of B cells from RRV-BA mice. This implied that B cells from naïve mice may become activated upon adoptive transfer into immunodeficient mice. Indeed, this phenomenon of activation of B cells upon adoptive transfer into B-cell deficient mice has been described previously and likely explains the observation of BSS B cell activation upon adoptive transfer into Ig-α-/- mice.[34,35] These results provide further evidence that B cells play a direct role in the activation and proliferation of liver T cells and macrophages in murine BA.
Figure 3.
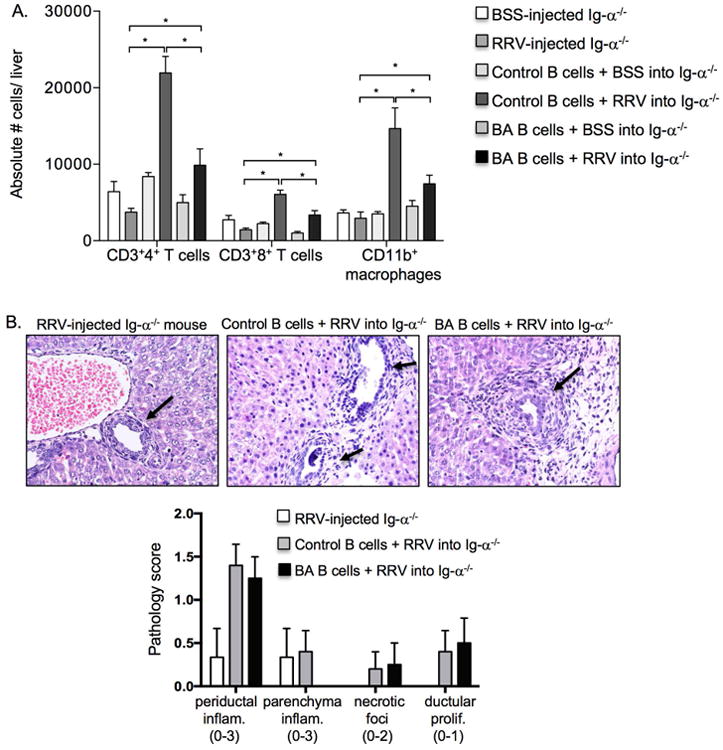
Adoptive transfer of B cells into RRV-infected Ig-α-/- mice results in liver inflammation and biliary injury. A. Summary of absolute numbers of liver T cell and macrophage infiltrates in newborn Ig-α-/- recipient mice that received either BSS or RRV alone, 14 day old control (BSS) B cells (1×107 cells i.p.) with BSS or RRV, or 14 day old BA (RRV) B cells (1×107 cells i.p.) with BSS or RRV. B. Representative liver histology of intrahepatic bile ducts (arrows) showing injury and periductal inflammation in Ig-α-/- recipient mice that received B cells and RRV (200× magnification) and summary of liver injury scores (range of possible scores for an individual injury marker given in parentheses). *p < 0.05
B cells from BA mice express higher levels of surface proteins involved in antigen presentation and activation, but are not competent at presenting antigen to T cells
One function of B cells in T cell-mediated diseases is to present antigens to T cells, resulting in T cell activation and proliferation. We sought to determine if antigen presentation by B cells resulted in liver T cell activation in RRV-BA mice. Liver and spleen RRV-BA B cells exhibited significantly increased levels of MHC-class I compared to BSS-control B cells, while a large fraction of these cells had a mild, yet significant decrease in MHC-class II expression (Figure 4A, B). Moreover, a fraction of RRV-BA liver B cells displayed significantly higher expression of activation and T cell co-stimulation markers, CD69, CD86 (B7-2), and CD40, and both liver and spleen RRV-BA B cells had significantly increased levels of CD80 (B7-1). Therefore, liver RRV-BA B cells had upregulation of markers necessary for antigen presentation to (MHC class I) and cognate collaboration (CD40, CD80, CD86) with T cells.
Figure 4.
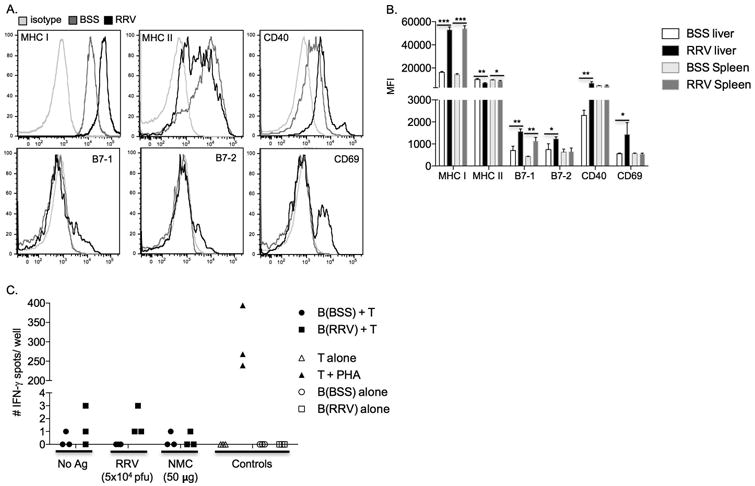
B cells from RRV-BA mice have increased activation and antigen presentation expression but are not competent antigen presenting cells. A. FACS analysis: Representative histograms of markers of activation and antigen presentation on 14 day old liver B cells from BSS-control or RRV-BA mice (gated on live cells, FSC/SSC lymphocytes, CD45+cells, B220/ CD19+ cells) B. Summary of mean fluorescent intensity (MFI) of B cell activation and antigen presentation marker expression on 14 day old BSS-control or RRV-BA mice livers and spleens. C. IFN-γ ELISPOT: 14 day old RRV-BA liver T cells (responders) were cultured alone or with B cells from 14 day old BSS-control mice or RRV-BA mice (stimulators/ antigen presenting cells) and either no antigen, inactivated RRV, normal mouse cholangiocyte (NMC) homogenate, or PHA (positive control). ***p < 0.0005; **p < 0.005; *p < 0.05
To determine if the B cells were efficient at antigen presentation, functional studies of T cell activation were performed. Our group and others have previously shown subsets of activated liver T cells from RRV-BA mice that are RRV-specific memory T cells, as well as autoreactive T cells targeting normal mouse cholangiocytes (NMC) proteins.[6,8] In those studies, adult bulk splenocytes were used for antigen presentation to T cells. We now asked the question as to whether or not neonatal B cells could also be competent antigen presenting cells. 14 day old liver B cells from BSS-control or RRV-BA mice were cultured with liver and spleen T cells from RRV-BA mice, with or without RRV or NMC antigens. IFN-γ-producing T cells were measured by ELISPOT as the readout of T cell activation. Neonatal B cells were not able to present RRV or NMC antigens, based on lack of T cell activation with IFN-γ production (Figure 4C). In summary, neonatal liver and spleen RRV-BA B cells have significantly increased expression of multiple markers of antigen presentation and activation, however they were not capable of presenting antigens to activate T cells.
Mice with B cells specific for irrelevant antigen develop murine BA
In order to further analyze the role of neonatal B cells as possible antigen presenting cells, we utilized the 3-83 Ig knock-in mouse, whereby all B cells bear the same antigen receptor that is specific for an irrelevant antigen (3-83).[24,25] In 3-83 Ig mice, 95% of all B cells express only the 3-83 (Ig heavy and kappa) BCR, as shown by staining B cells for the 3-83 Ig, Igκ and Igλ (Supplementary Figure 4). We hypothesized that if B cell antigen presentation function was essential for disease, then 3-83 Ig mice would be protected from disease. However, RRV-infected 3-83 Ig neonatal mice developed murine BA at a similar rate and severity to wildtype (WT) mice, including the degree of bile duct injury based on jaundice, elevated serum direct bilirubin and alkaline phosphatase levels and histologic evidence of bile duct inflammation and injury (Figure 5A-C). In addition, the intrahepatic inflammatory profile observed in RRV-infected 3-83 Ig mice paralleled that found in WT RRV-BA mice, with a significant influx of CD4+ and CD8+ T cells and CD11b+ macrophages (Figure 5D). In summary, the lack of competent antigen presentation in WT mice and the presence of murine BA in RRV-infected 3-83 Ig transgenic mice indicates that B cells participate in disease in a manner independent of antigen presentation, and points toward an alternative B cell mechanism of action.
Figure 5.
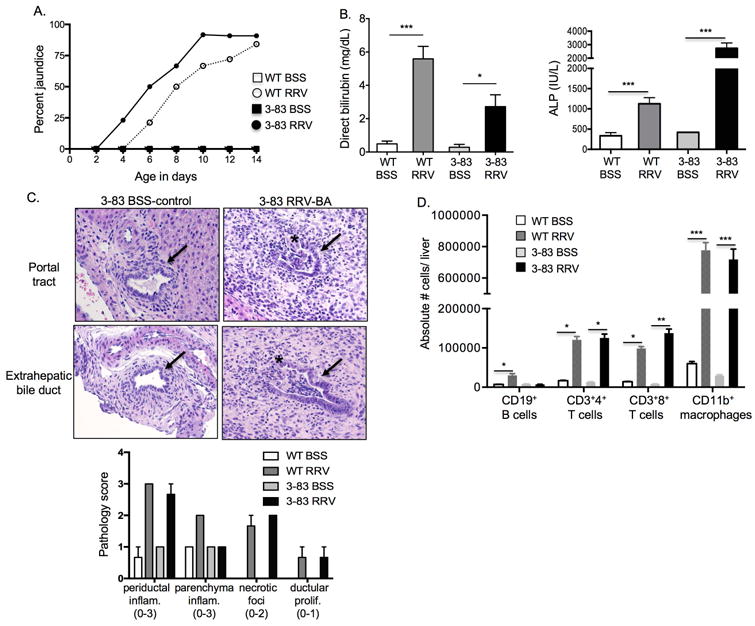
3-83 Ig transgenic mice with B cells specific for irrelevant antigen (3-83) develop murine BA. Newborn wildtype (WT) or 3-83 Ig knock-in mice (3-83) were injected with BSS (control) or RRV (BA) and analyses performed at 14 days of age. A. Percent incidence of jaundice in non-fur bearing regions. B. Serum direct bilirubin and alkaline phosphatase levels. C. Representative H&E of intrahepatic and extrahepatic bile ducts (arrows) showing bile duct inflammation and injury with disruption of lining of ducts (*) and summary of liver injury scores (range of possible scores for an individual injury marker given in parentheses). D. Absolute number of liver immune cells (gated on FSC/SSC, CD45+ cells). ***p < 0.0005; **p < 0.005; *p < 0.05
RRV-BA B cells produce pro-inflammatory molecules associated with adaptive and innate immune activation
Intracellular cytokine staining was employed to assess those cytokines previously described in the literature to be produced by B cells. RNA-Seq technology was utilized to discover novel B cell cytokines and signaling molecules associated with immune activation in BA. It has been previously reported that B cells are capable of producing potent inflammatory cytokines that modulate T cell as well as innate immune responses.[21,22,36] We found that liver and/ or spleen RRV-BA B cells produced increased amounts of IFN-γ, IL-2, TNF-α, IL-1, IL-6 and IL-10 compared to BSS-control B cells, both in ex-vivo analysis (Supplementary Figure 5) and after in-vitro activation with PMA/ionomycin (Figure 6A). In the liver, B cells able to secrete TNF-α were particularly increased in murine BA. There was no increase in TGF-β or GM-CSF in the livers or spleens of RRV-BA mice compared to controls (data not shown).
Figure 6.
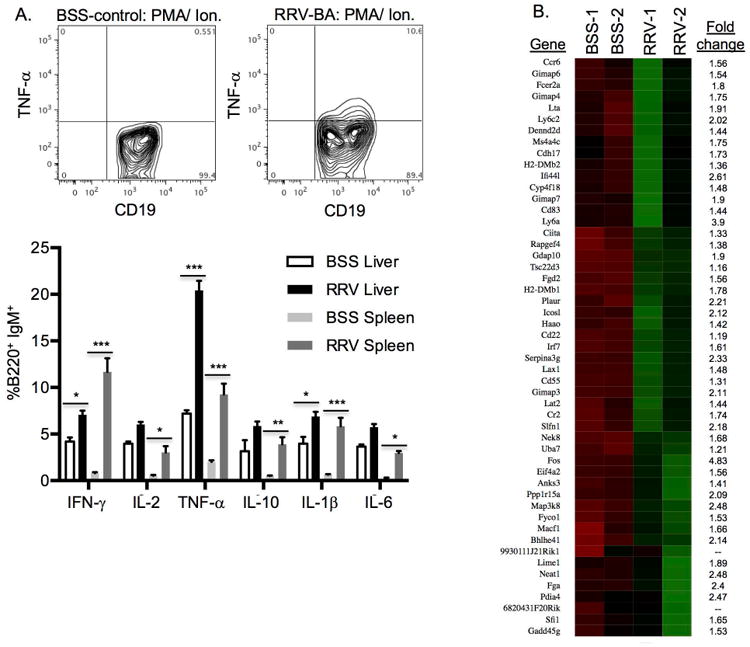
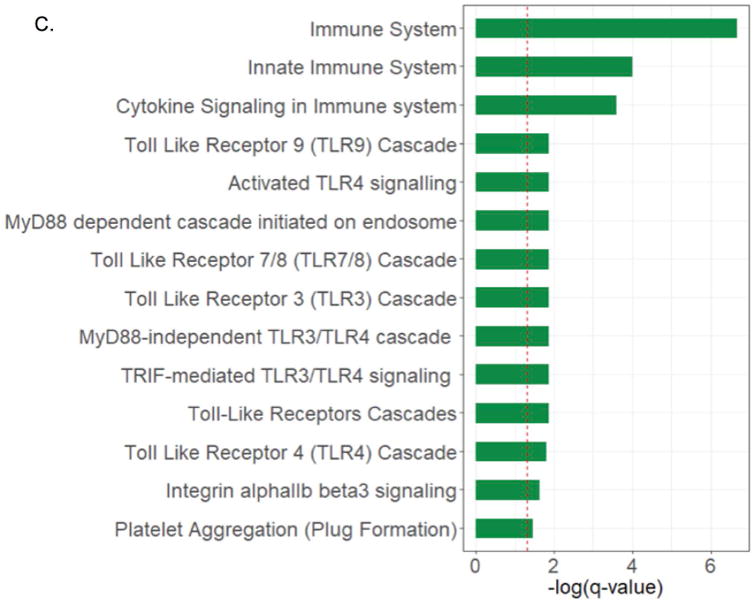
RRV-BA B cells produce pro-inflammatory molecules associated with adaptive and innate immune activation. A. Representative contour plot of spleen TNF-α-producing B cell and summary of intracellular cytokine staining of liver and spleen B cells from 14 day old BSS-control and RRV-BA mice after PMA/ ionomycin activation (gated on FSC/SSC lymphocytes, CD45+cells, CD19+IgM+ cells). (***p < 0.0005; **p < 0.005; *p < 0.05) B. RNA-Seq heat map of genes from BSS-control and RRV-BA liver B cells [low expression (red) to high expression (green)]. Shown are the up-regulated genes and fold-change increase in RRV-BA liver B cells compared to BSS-controls. C. Bar graph showing gene category scores in negative log value; “threshold” indicates the minimum significance level [scored as −log(p-value) from Fisher's exact test, set to 1.3] (red dotted line).
RNA-Seq technology was employed in order to identify additional novel molecules associated with B cell activation and function in RRV-BA mice. Liver B cells from 14 day old BSS-control and RRV-BA mice were isolated in high purity (Supplementary Figure 6) and analyzed by RNA-Seq technology. Approximately 350 genes were significantly down-regulated and 50 genes were significantly up-regulated in liver B cells from RRV-BA mice compared to controls (Figure 6B. and Supplementary Figure 7). The majority of down-regulated genes were associated with cellular metabolism. The most significantly up-regulated genes in RRV-BA liver B cells were FOS (transcription factor AP-1) (4.83-fold increase), lymphocyte 6 antigen (Ly6a) (3.9-fold increase) and interferon induced protein 44-like (Ifi44l) (minor histocompatibility antigen) (2.61-fold increase). FOS, together with mitogen activated protein3 kinase 8 (MAP3k8) (2.48-fold increase), are signal transduction molecules necessary for full B cell activation.[KEGG pathway; 37] Ly6a is involved in B cell development and maturation.[38,39] Further evidence of B cell-driven T cell activation, in addition to the cytokines discovered by intracellular staining, includes significantly increased liver RRV-BA B cell production of inducible co-stimulator ligand (ICOSL) (2.12-fold increase), an important co-receptor for T cell activation [40], and of lymphotoxin-α (LTA) (1.91-fold increase), a critical mediator of lymphoid architecture and a requirement for T cell activation.[41,42] Analysis of pathways associated with the highly expressed genes in RRV-BA liver B cells revealed numerous pathways involved in B cell activation and innate immune activation, including TLR pathways, cytokine signaling and MyD88 signaling (Figure 6C.). Taken together, B cells from BA mice produce a multitude of pro-inflammatory molecules that promote activation of both adaptive and innate immunity.
Cytokine-producing RRV-BA B cells activate T cells in-vitro
In order to confirm that B cell cytokines were directly leading to T cell activation, trans-well in-vitro studies were performed. Naïve T cells were stimulated overnight with plate-bound anti-CD3 and the next day were co-cultured in a trans-well with either purified liver/spleen B cells from 2 week old BSS-control or RRV-BA mice. RRV-BA B cells, and not BSS-control B cells, produced cytokines that crossed the well membrane, entering the T cell culture well, leading to significant up-regulation of the T cell activation marker CD69. This was seen both with analysis of CD69 MFI, as well as the percent of total T cells expressing CD69. (Figure 7) There was no change in expression of the activation marker CD25 (data not shown). These in-vitro analyses provided further evidence for B cell-mediated T cell activation in RRV-BA mice.
Figure 7.
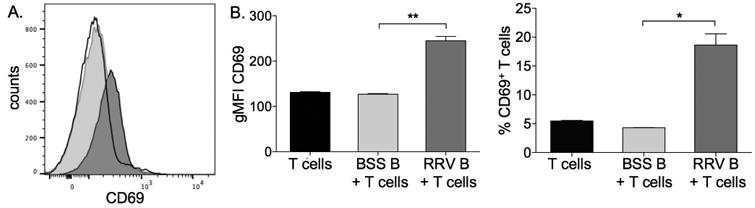
RRV-BA B cells produce cytokines that activate T cells in-vitro. A. Representative histogram of CD69 expression on purified T cells alone (black line), or co-cultured in trans-well with BSS-control B cells (light grey) or RRV-BA B cells (dark grey). B. Geometric mean fluorescent intensity (gMFI) of CD69 on T cells (left panel) and the % of T cells expressing CD69 (right panel). **p < 0.005; *p < 0.05 B cells.
Discussion
We have previously shown that RRV-infected B cell-deficient mice are protected from developing BA, with associated lack of T cell and macrophage liver infiltrates.[13] In our current study, we show that wildtype RRV-BA mice have increased mature and activated B cell subsets in the liver, and have discovered that the predominant B cell mechanism of action in disease pathogenesis is cytokine production. The importance of B cell pro-inflammatory molecules was highlighted by the findings that RRV-infected 3-83 Ig knock-in mice, which have greatly restricted B cell antigen presentation capabilities, still developed BA that mimicked wildtype mice. This implies an antigen-independent B cell mechanism of action and we discovered that murine BA B cells are potent producers of cytokines that affect both adaptive and innate immune responses. Importantly, the BA B cells producing IFN-γ, IL-2, and TNF-α may work in concert to promote CD4+ Th1 cellular differentiation and CD8+ T cell activation[21,22,35,43], key pathways that are upregulated in murine and human BA.[5,7-10] In addition, B cell production of IFN-γ or IFN-γ plus TNF-α leads to activation of macrophages and dendritic cells, respectively.[36] RNA-Seq data revealed additional B cell molecules associated with T cell differentiation and activation, specifically ICOSL and LTA. B cell ICOSL binds to T cell ICOS, which is essential for T helper cell development.[40] The LTA cytokine is part of a TNF-family LT heterodimer (LT-α/ LT-β) on B cells that is essential for maintaining the microarchitecture of secondary lymphoid organs.[41] In addition, LTA can also form a soluble complex that is inducible and can impact T cell activation.[42] Important to our findings, recent work in multiple sclerosis (MS) showed that miRNA-132 enhanced B cell production of LTA, and in concert with TNF-α was associated with T cell predominant relapses in MS.[42]
RNA-Seq technology also led to the discovery that liver B cells from neonatal RRV-BA mice have activation of multiple innate immune pathways, including upregulation of TLR4 and TLR 7/9. It was recently shown that TLR4 activation plays a key role in promoting the maturation of immature and transitional B cells.[44] Recognition of nucleic acids (NA) by TLRs 7 and 9 on B cells results in aberrant B cell activation, with subsequent production of pro-inflammatory cytokines.[21,45] Potential NAs stimulating B cells in murine BA may include microbial-derived NAs (i.e. RRV) or endogenous NAs from damaged bile duct epithelial cells. There is a paucity of data on the role of the B cell in innate immune activation and this area warrants further investigation in BA.
There were some limitations to our study. First, adoptive transfer of RRV and B cells into Ig-α-/- mice resulted in bile duct injury and liver inflammation, however these mice did not develop complete biliary obstruction. This suggests that either B cells alone are not sufficient to recapitulate full penetrance of disease or the Ig-α-/- mice have defects in other immune pathways due to the lack of B cell development in-utero. Second, adoptive transfer of BSS control B cells into Ig-α-/- mice resulted in greater amounts of liver T cell and macrophage infiltrates compared to adoptive transfer of RRV-BA B cells. This finding is likely due to the previously described phenomenon of naïve B cells becoming activated after transfer into B-cell deficient mice.[34,35] The finding that activated, previously naïve BSS B cells were more potent than chronically activated RRV-BA B cells at stimulating the immune response is likely due to a degree of exhaustion in a subset of the persistently activated RRV-BA B cells.[46] Third, the role of B cell production of antibodies was not assessed in this study. This would entail antigen-specific activation of the BCR with subsequent immunoglobulin production. Our findings of lack of antigen presentation capabilities in the neonatal mice suggests that the B cell activation occurring in BA is antigen-independent and therefore we would not predict that antibodies contribute to disease pathogenesis; however, further work in this area is warranted.
To date, published work on the contribution of B cells to disease pathogenesis in human BA have been limited to characterization studies, such as those describing periductal immunoglobulin deposits[7,8,47] and circulating non-disease specific autoantibodies.[48] Recently, analysis of hilar lymph nodes of BA patients at the time of diagnosis revealed that 53% of BA infants (and none of controls) had mature germinal centers, suggesting recent B cell stimulation.[49] Future research focusing on the role of B cells in human BA is necessary, including the analysis of B cell production of pro-inflammatory molecules through laser capture microscopy techniques. Understanding the role of the B cell in the immunopathogenesis of human BA has the potential to lead to new B cell-targeted therapies aimed at halting the injury to intrahepatic bile ducts and preserving liver function lifelong.
Supplementary Material
Acknowledgments
NIH NIDDK R01 DK094937-01A1 (Mack CL), 2T32 DK067009-11 (Mack CL, Bringham D), NIH NIAID R01 AI052310 (Pelanda, R)
Abbreviations
- BA
biliary atresia
- BCR
B cell receptor
- BSS
balanced salt solution
- CD
cluster of differentiation
- CTCF
corrected total cellular fluorescence
- IFN
interferon
- IL
interleukin
- RRV
Rhesus rotavirus
- TLR
toll-like receptor
- TNF
tumor necrosis factor
References
- 1.Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl. 2017;23(1):96–109. doi: 10.1002/lt.24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkade HJ, Bezerra JA, Davenport M, Schreiber RA, Mieli-Vergani G, Hulscher JB, et al. Biliary atresia and other cholestatic childhood diseases: Advances and future challenges. J Hepatol. 2016;65(3):631–42. doi: 10.1016/j.jhep.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Bucuvalas JC, Zeng L, Anand R Studies of Pediatric Liver Transplantation Research Group. Predictors of cost of liver transplantation in children: a single center study. J Pediatr. 2001;139(1):66–74. doi: 10.1067/mpd.2001.115068. [DOI] [PubMed] [Google Scholar]
- 4.Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol. 2015;12(6):342–52. doi: 10.1038/nrgastro.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115(2):200–9. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivakumar P, Sabla G, Mohanty S, McNEal M, Ward R, Stringer K, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology. 2007;133(1):268–77. doi: 10.1053/j.gastro.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114(3):322–9. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack CL, Tucker RM, Lu BR, Sokol RJ, Fontenot AP, Ueno Y, Gill RG. Cellular and Humoral Autoimmunity Directed at Bile Duct Epithelia in Murine Biliary Atresia. Hepatology. 2006;44:1231–1239. doi: 10.1002/hep.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mack CL, Falta MT, Sullivan AK, Karrer F, Sokol RJ, Fontenot AP. Oligoclonal Expansions of CD4+ and CD8+ T Cells in the Target Organ of Patients with Biliary Atresia. Gastroenterology. 2007;133(1):278–287. doi: 10.1053/j.gastro.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen J, Zhou Y, Wang J, Chen J, Yan W, Wu J, et al. Interactions between Th1 cells and Tregs affect regulation of hepatic fibrosis in biliary atresia through the IFN-γ/STAT1 pathway. Cell Death Differ. 2017;24(6):997–1006. doi: 10.1038/cdd.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Lages CS, Simmons J, Maddox A, Jones K, Karns R, Sheridan R, et al. The dendritic cell- T helper 17-macrophage axis controls cholangiocyte injury and disease progression in murine and human biliary atresia. Hepatology. 2017;65(1):174–88. doi: 10.1002/hep.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper N, Arnold DM. The effect of rituximab on humoral and cell mediated immunity and infection in the treatment of autoimmune diseases. Brit J Haemat. 2010;149:3–13. doi: 10.1111/j.1365-2141.2010.08076.x. [DOI] [PubMed] [Google Scholar]
- 13.Feldman AG, Tucker RM, Fenner EK, Pelanda R, Mack CL. B cell deficient mice are protected from biliary obstruction in the rotavirus-induced mouse model of biliary atresia. PLoS One. 2013;8(8):e73644. doi: 10.1371/journal.pone.0073644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ig mu null mice J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan O, Shlomchik M. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51. [PubMed] [Google Scholar]
- 16.O'Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 17.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 18.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SA, Schlachterman A, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical inovercoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 19.Wong SF, Wen L, Tnage M, Ramanathan M, Visintin I, Daugherty J, et al. Investigation of the role of B cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 20.Anolik JH, Looney RJ, Lund FE, Randall TD, Sanz I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity and use as therapeutic targets. Immunol Res. 2009;45:144–158. doi: 10.1007/s12026-009-8096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao Y, Cao X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. J Autoimmun. 2014;55:10–23. doi: 10.1016/j.jaut.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Lund FE. Cytokine-producing B lymphocytes- key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelanda R, Braun U, Hobeika E, Nussenzweig MC, Reth M. B cell progenitors are arrested in maturation but have intact VDJ recombination in the absence of Ig-α and Ig-β. Jrl Immunol. 2002;169(2):865–72. doi: 10.4049/jimmunol.169.2.865. [DOI] [PubMed] [Google Scholar]
- 24.Braun U, Rajewsky K, Pelanda R. Different sensitivity to receptor editing of B cells from mice hemizygous or homozygous for targeted Ig transgenes. PNAS. 2000;97(13):7429–34. doi: 10.1073/pnas.050578497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 26.Calvert JE, Kim MF, Gathings WE, Cooper MD. Differentiation of B lineage cells from liver of neonatal mice: generation of immunoglobulin isotype diversity in vitro. J Immunol. 1983;131(4):1693–7. [PubMed] [Google Scholar]
- 27.Kamps WA, Cooper MD. Microenvironmental studies of pre-B and B cell development in human and mouse fetuses. J Immunol. 1982;129(2):526–31. [PubMed] [Google Scholar]
- 28.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immuol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 29.Tsuneto M, Kajikhina E, Seiler K, Reimer A, Tornack J, Bouquet C, et al. Environments of B cell development. Immun Letters. 2014;160:109–112. doi: 10.1016/j.imlet.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Rincon MR, Oppenheimer K, Bonney EA. Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen. INt J Biol Sci. 2012;8:719–30. doi: 10.7150/ijbs.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immun. 2010:S33–S40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig-a. Science. 1996;272:411. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 33.Minegishi Y, Coustan-Smith E, Rapalus L, Ersoy F, Campana D, Conley ME. Mutations in Iga (CD79a) result in a complete block in B-cell development. J Clin Invest. 1999;104(8):1115–21. doi: 10.1172/JCI7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agenes F, Freitas AA. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J Exp Med. 1999;189(2):319–29. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anson M, Amado I, Mailhe MP, Donnadieu E, Garcia S, Huetz F, et al. Regulation and maintenance of an adoptive T cell dependent memory B cell pool. PLOS One. 2016;11(11):e0167003. doi: 10.1371/journal.pone.0167003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74(2):318–26. doi: 10.1016/j.cyto.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol. 2002;169(4):1922–9. doi: 10.4049/jimmunol.169.4.1922. [DOI] [PubMed] [Google Scholar]
- 38.Upadhyay G, Yin Y, Yuan H, Li X, Derynch R, Glazer RI. Stem cell antigen-1 enhances tumorigenicity by disruption of growth differentiation factor-10-dependent TGF-beta signaling. PNAS. 2011;108(19):7820–25. doi: 10.1073/pnas.1103441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MA, et al. Investigating B Cell Development, Natural and Primary Antibody Responses in Ly-6A/Sca-1 Deficient Mice. PLoS One. 2016 doi: 10.1371/journal.pone.0157271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lownik JC, Luker AJ, Damle SR, Cooley LF, El Sayed R, et al. ADAM10-mediated ICOS ligand shedding on B cells is necessary for proper T cell ICOS regulation and T follicular helper responses. J Immunol. 2017;199:2305–15. doi: 10.4049/jimmunol.1700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumanov AV, Kuprash DV, Lagarkova M, Grivennikov SI, Abe K, Shakhov AN, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissue. Immunity. 17:239–50. doi: 10.1016/s1074-7613(02)00397-7. 2--2. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki Y, Li R, Rezk A, Misirliyan H, Moore C, Farooqi N, et al. A novel microRNA-132-surtuin-1 axis underlies aberrant B cell cytokine regulation in patients with relapsing-remitting multiple sclerosis. PLOS One. 2104;9(8):e105421. doi: 10.1371/journal.pone.0105421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luu VP, Vazquez MI, Zlotnik B cells participate in tolerance and autoimmunity through cytokine production. Auotimmun. 2014;47(1):1–12. doi: 10.3109/08916934.2013.856006. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Wang H, Zhou H. The role of TLR4 on B cell activation and anti-beta2 GPI antibody production in the antiphospholipid syndrome. Jrl Immun Res. 2016 doi: 10.1155/2016/1719720. 101155/1719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suthers AN, Sarantopoulos S. TLR7/TLR9 and B cell receptor signaling crosstalk: promotion of potentially dangerous B cells. Frontiers Immun. 2017 doi: 10.3389/fimmu.2017.00775. 10.3389/immu.2017.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu XY, Zhang HY, Liu YF, Zhao D, Shan YX, Jiang YF. Peripheral B cell activation and exhaustion markers in patients with ankylosing spondylitis. Life Sciences. 2013;18(4):687–92. doi: 10.1016/j.lfs.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Hadchouel M, Hugon RN, Odievre M. Immunoglobulin deposits in the biliary remnants of extrahepatic biliary atresia: a study by immunoperoxidase staining in 128 infants. Histopathology. 1981;5:217–221. doi: 10.1111/j.1365-2559.1981.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 48.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. Alpha-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139(5):1753–61. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bove KE, Sheridan R, Fei L, Anders R, Chung CT, Cummings OW, et al. Hepatic Hilar Lymph Node Reactivity at Kasai Portoenterostomy for Biliary Atresia. Pediatr Dev Pathol. 2017 Jan 1; doi: 10.1177/1093526617707851. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


