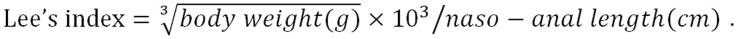Abstract
AIM
To investigate the mechanisms by which Sheng-jiang powder (SJP) ameliorates obesity-induced pancreatic inflammatory injury.
METHODS
Sprague-Dawley rats were randomized into three groups: normal group (NG), obese group (HLG), or SJP treatment group (HSG). Obesity was induced by feeding a high-fat diet in the HLG and HSG, while the NG received standard chow. Rats were euthanized after 12 wk, and blood and pancreatic tissues were collected for histopathological analyses. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor beta (TGF-β) expression, serum triglyceride and adiponectin levels, and apoptosis in pancreatic acinar cells were assessed. A high-fat AR42J acinar cell injury model was established using very low-density lipoprotein (VLDL). AR42J acinar cell culture supernatant, treated with different interventions, was applied to seven groups of pancreatic stellate cells (PSCs). The proliferation of PSCs and the expression of fibronectin and type I collagenase were assessed.
RESULTS
Compared with the NG, we found higher pathological scores for pancreatic tissues, lower serum adiponectin levels, higher expression levels of NF-κB in pancreatic tissues and TGF-β in pancreatic inflammatory cells, and increased apoptosis among pancreatic acinar cells for the HLG (P < 0.05). Compared with the HLG, we found reduced body weight, Lee’s index scores, serum triglyceride levels, and pathological scores for pancreatic tissues; higher serum adiponectin levels; and lower expression levels of NF-κB, in pancreatic tissue and TGF-β in pancreatic inflammatory cells for the HSG (P < 0.05). The in vitro studies showed enhanced PSC activation and increased expression levels of fibronectin and type I collagenase after SJP treatment. An adenosine 5‘-monophosphate-activated protein kinase (AMPK) inhibitor inhibited PSC activation.
CONCLUSION
SJP may ameliorate obesity-induced pancreatic inflammatory injury in rats by regulating key molecules of the adiponectin-AMPK signalling pathway.
Keywords: Obesity, Sheng-jiang powder, Adiponectin, Adenosine 5’-monophosphate-activated protein kinase, Pancreatic inflammatory injury
Core tip: Obesity is a risk factor for non-alcoholic fatty pancreas disease and induces pancreatic inflammatory injury. Sheng-jiang powder (SJP) can ameliorate obesity-induced pancreatic inflammatory injury; however, the specific mechanisms remain unclear. This study demonstrates that SJP may inhibit the inflammatory response, prevent pancreatic fibrosis, promote pancreatic acinar cell repair, and ultimately ameliorate obesity-induced pancreatic inflammatory injury in rats by regulating the key molecules of the AMPK signalling pathway.
INTRODUCTION
Obesity rates have increased sharply over the past 40 years, creating a global public health crisis[1]. According to the results of the Global Burden of Disease Study 2013, the number of overweight and obese individuals increased to 2.1 billion worldwide in 2013, which is 2.28 times more than that in 1980[2]. Obesity or excess weight can lead to high morbidity for many noncommunicable diseases, including 75% of hypertension, 44% of the diabetes burden, 23% of ischaemic heart disease, and 7%-41% of certain cancers[3]. Additionally, the prevalence of non-alcoholic fatty pancreas disease (NAFPD), which is characterized by pancreatic fat infiltration due to obesity, ranges from 16% to 35% in Asian populations[4,5]. In addition, NAFPD may play an important role in the development of type 2 diabetes (T2DM), acute pancreatitis, and even pancreatic cancer[5]. As a result, obesity- and excess weight-related complications have led to a considerable burden on patients and the society. Withrow and Alter (2011)[6] indicated that obesity accounted for between 0.7% and 2.8% of the total healthcare costs of a country. Therefore, due to the side effects of the current treatments for obesity and the lack of specific drugs, people have gradually begun to focus on interventions using traditional Chinese medicine (TCM), such as Sheng-jiang powder (SJP)[3,7].
The pathogenesis of obesity-induced tissue injury is complex and diverse. The most common pathogeneses are endoplasmic reticulum (ER) stress and the inflammatory response[5,8]. According to experimental reports, maternal obesity and postnatal obesogenic diets can result in NAFPD because of an ER imbalance and an alteration in circadian metabolic patterns[9]. Obesity-induced inflammation is a chronic and low-grade form of inflammation, which starts in adipose tissue, with abundant macrophage infiltration, followed by the increased secretion of pro-inflammatory cytokines, such as tumour necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and C-reactive protein, while the production of anti-inflammatory cytokines, such as interleukin 10 (IL-10) and adiponectin, drastically decreases[10]. Gotoh et al[11] found that obesity reduced the production of spleen-derived IL-10, which can protect against the development of NAFPD. In addition, insulin resistance (IR) and β-cell dysfunction also play important roles[12]. IR decreases the inhibitory activity of insulin on peripheral lipolysis, leading to an increase in circulating free fatty acids (FFAs). The chronic exposure of β-cells to elevated FFAs results in β-cell dysfunction and creates a vicious cycle resulting in the continuous deterioration of the glucometabolic state[13]. Although obesity-induced pancreatic injury is known to be related to the inflammatory response[7], the specific and detailed mechanisms involved remain unclear.
According to the TCM theory, obesity belongs to the category of “Turbidity”, which is primarily caused by the “ascending and descending dysfunction” of the spleen[14]. As a classic representative formula for ascending lucidity and descending turbidity, SJP originates from a Nei-Fu-Xian-Fang decoction in Wanbing Huichun, which was compiled by Ting-Xian Gong during the Ming dynasty in China and is composed of Jiangchan (Bombyx Batryticatus), Chantui (Periostracum cicada), Jianghuang (Curcuma longa L.), and Dahuang (Rheum palmatum L.)[15]. Several clinical studies have confirmed that SJP is effective in regulating lipid metabolism and improving IR, and SJP is widely used to treat obesity-related diseases, such as hyperlipidaemia, fatty liver, and diabetes[16-18]. Our previous studies have demonstrated that SJP can ameliorate the inflammatory response and histopathological lesions in the pancreas of obese rats[7]. However, the specific mechanisms underlying the amelioration of obesity-induced pancreatic inflammatory injury by SJP are far from being sufficiently understood. Therefore, we designed this study to further investigate the specific mechanisms of SJP on obesity-induced pancreatic inflammatory injury.
MATERIALS AND METHODS
Preparation of SJP for oral administration to rats
The spray-dried drug particles of SJP ingredients, including Dahuang (batch No. 16110150), Jianghuang (batch No. 16080008), Jiangcan (batch No. 16100147), and Chantui (batch No. 16080020), were purchased from the Affiliated Hospital of Chengdu University of TCM (Chengdu, China) and authenticated by Professor Wang WM (Department of Herbal Pharmacy, West China Hospital, Sichuan University, China), according to the Chinese Pharmacopoeia (The Pharmacopoeia Commission of People’s Republic of China, 2010). Voucher specimens were deposited at our laboratory. The spray-dried drug particles were mixed in the proportions of 4:3:2:1, according to Ting-Xian Gong’s Wanbing Huichun, a famous, classic TCM book from the Ming dynasty[15], and they were completely reconstituted with sterile double-distilled water (concentration: 1 g/mL). This SJP solution was stored at 4 °C until ready for use, and it was administered orally to the rats at a dose of 5 mL/kg of body weight (BW).
Preparation of SJP for cell treatment
Our previous study determined that the serum peak concentration of rhein in the plasma of rats that received orally administered SJP (Dahuang, Jianghuang, Jiangcan, and Chantui proportions: 12:9:6:3) was 4388 ± 957 μg/L; thus, for convenience, we used 5000 μg/L for calculations[19]. According to the above concentration and the content of rhein in the SJP compound formula (Dahuang, Jianghuang, Jiangcan, and Chantui proportions: 12:9:6:3; the ratio of rhein to the SJP compound formula was 0.5 mg/g)[20], we calculated the compound dosage for cell treatments as follows: 1 g of SJP compound formula was added to 100 mL of PBS (SH30256.01B, HyClone, Logan, UT, United States) to dilute to a 1 × working concentration. In this study, 1 mL of the above 1 g/mL SJP solution for rat oral administration was diluted to 100 ×, filtered, and sterilized to prepare the highest concentration of the compound for in vitro use.
Preparation of adenosine 5’-monophosphate-activated protein kinase inhibitor Compound C
One gram of Compound C (171260, Merck KGaA, Darmstadt, Hessen, Germany) was dissolved in 1000 mL of phosphate buffer solution (PBS), and the mixture was diluted to a 2.5 mmol/L stock solution (100 ×), sterilized by filtration, and stored at -20 °C. Before use, the appropriate amount of the above stock solution was diluted 100 ×, for a final working concentration of 25 μmol/L.
Induction of obesity, animal treatments, and sample collection
The protocol was reviewed and approved by the Institutional Animal Care and Use Committee of West China Hospital of Sichuan University. Twenty-four male Sprague-Dawley rats, weighing 60-80 g, were purchased from Chengdu Dashuo Experimental Animal Co., Ltd. (Chengdu, China). The protocol was designed to minimize the pain and discomfort of the rats. All rats were acclimatized to laboratory conditions (22 ± 2 °C, 65% ± 10% relative humidity, 12-h light/12-h dark cycle, ad libitum access to water and food) for one week prior to the special feeding. Special feeding meant that the rats had free access to a high-fat diet (HFD; 60% of calories derived from fat; TP23300; Trophic Animal Feed High-tech Co., Ltd., Nantong, China) to induce obesity, or to a control diet (16.7% of calories derived from fat; LAD3001G; Trophic Animal Feed High-tech Co., Ltd., Nantong, China).
All rats were randomly divided into a normal group (NG, control diet), an obese group (HLG, HFD), or an SJP treatment group (HSG, HFD plus SJP), with 8 rats in each group. The whole study lasted for 12 wk. Rats in the HSG were intragastrically administered with SJP (5 g/kg) once daily, beginning in the third week, while the rats in the other two groups were instead administered with equal volumes of normal saline. Food intake was monitored daily. After 12 wk of feeding, the rats were anesthetized (2% sodium pentobarbital, intraperitoneal injection, 40 mg/kg of BW), heart blood samples were taken to test the levels of triglyceride and adiponectin, and the BW and naso-anal length were measured for Lee’s index calculations, using the following formula[21]:
Math 1
Math 1.
Math(A1).
Pancreatic tissue samples were obtained for histopathological analyses, immunohistochemistry tests for nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor-β (TGF-β), and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL). Then, all rats were euthanized with a 2% sodium pentobarbital overdose (intraperitoneal injection, 200 mg/kg of BW).
Measurement of serum triglyceride and adiponectin levels
The blood samples were centrifuged at 1000 r/min for 5 min to collect supernatants for analysis. The levels of triglyceride were measured with a HITACHI automatic biochemical analyser (7170A, HITACHI, Tokyo, Japan), and the levels of adiponectin were measured with ELISA kits (EKT246253, eBio, Wuhan, China). According to the manufacturer’s protocol, absorbance was measured at 450 nm with a High Throughput Universal Microplate Assay. The sample values were then read off the standard curve, and the relative concentrations were calculated.
Histopathological analysis of pancreatic tissues
Fresh pancreatic tissue samples were fixed with 40 g/L paraformaldehyde (AR1068, BOSTER, Wuhan, China), embedded in paraffin, sectioned into 5 μm sections, and stained with haematoxylin and eosin. All histopathological sections were observed and scored in a blinded manner by two independent pathologists using the scoring system described by Kusske et al[22] (0-4 points: oedema, inflammation, haemorrhage, and necrosis). The total histopathology score is the mean of the combined scores for each parameter from both investigators.
Immunohistochemistry
Paraffin-embedded pancreatic samples were deparaffinized and then rehydrated. Endogenous peroxidase was quenched for 10 min with 30 g/L H2O2 and washed three times with distilled water. Sections were immersed in 0.01 mol/L citric acid buffer (pH 6.0), heated in a microwave oven until they were boiled, and then de-energized; the process was repeated 5 min later. After a wash with PBS, the sections were blocked with 5% bovine serum albumin (BSA) confining liquid (AR0004, BOSTER, Wuhan, China) for 10 min, at room temperature, and then excess liquids were removed. Sections were incubated overnight at 4 °C with primary antibody against NF-κB p65 (sc-8008, Santa Cruz Biotechnology, Santa Cruz, CA, United States; 1:200 dilution) or TGF-β (sc-146, Santa Cruz Biotechnology, Santa Cruz, CA, United States; 1:100 dilution). After washing with PBS, the sections were incubated with biotinylated goat-anti-mouse or goat-anti-rabbit IgG (SA2010, BOSTER, Wuhan, China) at 37 °C for 30 min and then incubated with a SABC-POD Kit (SA2010, BOSTER, Wuhan, China) for 30 min at 37 °C. Finally, the sections were stained with a DAB-kit (AR1022, BOSTER, Wuhan, China) for 20 min. The sections were rinsed in tap water and counterstained with haematoxylin. Immunohistochemistry sections were observed and scored in a blinded manner by specialists, using the scoring system described by Xu et al[23]. Briefly, the evaluation of the nuclear or cytoplasmic staining reaction was performed in accordance with the immunoreactive score (IRS): IRS = staining intensity (SI) × percentage of positive cells (PP). SI was determined as follows: 0, colourless; 1, light yellow; 2, brownish yellow; and 3, brown. PP was defined as follows: 0, negative; 1, 10% positive cells; 2, 11%-50% positive cells; 3, 51%-75% positive cells; and 4, 75% positive cells. Ten visual fields from different areas of each pancreatic section were used for the IRS evaluation, using the average for statistical analysis.
TUNEL assay for apoptotic cells in pancreatic tissues
The levels of apoptotic cells in pancreatic tissue samples were analysed using a TUNEL detection kit (14590900, Roche, San Francisco, CA, United States), following the manufacturer’s instructions. Briefly, the pancreatic tissue sections were covered with proteinase K solution (20 μg/mL proteinase K + 0.01 mol/L Tris/HCL, pH 7-8.0) at room temperature for 15 min before the addition of 50 μL of the TUNEL reaction mixture. After incubation in a humid chamber in the dark for 1 h, the sections were incubated with 50 μL of converter-POD solution at 37 °C for 30 min, followed by a final PBS wash. Next, 100 μL of DAB solution (5 μL 20 × DAB + 1 μL 300 g/L H2O2 + 94 μL PBS) was added for 10 min at room temperature to develop the slides, followed by three washes with PBS and haematoxylin counterstaining for 2 min. Images were captured using a fluorescence microscope (AX10 imager A2/AX10 cam HRC, Carl Zeiss Jena, Oberkochen, Germany), and the apoptotic index was calculated as the number of apoptotic cells/total number of cells × 100%.
Cell culture
Rat pancreatic acinar AR42J cells (CRL-1492, ATCC, Manassas, VA, United States) were maintained at 37 °C in DMEM/F12 medium (SH30023.01B, HyClone, Logan, UT, United States) supplemented with 10% foetal bovine serum (FBS; 16000044, Gibco, Waltham, MA United States), 100 IU penicillin, and 100 μg/mL streptomycin (SV30010, HyClone, Logan, UT, United States) in a 50 mL/L CO2 atmosphere. Prior to stimulation, cells in the logarithmic growth phase were seeded at 1 × 106 cells/well in 6-well plates and incubated until completely adherent.
Rat pancreatic stellate cells (PSCs; RAT-iCell-g003, Shanghai Deyu Bio-tech Co., Ltd, Shanghai, China) were cultured under the same conditions described above, but the culture medium was changed to 90% RPMI 1640 (SH30809.01B, HyClone, Logan, UT, United States) supplemented with 10% FBS, 100 IU penicillin, and 100 μg/mL streptomycin. PSCs in the logarithmic growth phase were seeded on polylysine-treated slides to perform cell-climbing. After the PSCs covered the slides, they were treated according to the following experimental design.
Induction of a cell model and stimulation
A high-fat AR42J acinar cell injury model was established by stimulation with 0.06 mg/mL of very low-density lipoprotein (VLDL; LP1, Merck-Millipore, Billerica, MA, United States)[24]. AR42J cells were divided into five groups: normal group (AR42J cells + culture medium), model group (AR42J cells + VLDL), SJP group (AR42J cells + VLDL + SJP), VLDL + Compound C group (AR42J cells + VLDL + Compound C), and SJP + Compound C group (AR42J cells + VLDL + SJP + Compound C). After the AR42J cells were completely adherent, 0.5 mL of medium (80% DMEM/F12 + 20% FBS + 100 IU penicillin + 100 μg/mL streptomycin) was added to the normal group and the model group; 0.25 mL of medium and 0.25 mL of SJP were added to the SJP group; 0.25 mL of medium and 0.25 mL of Compound C were added to the VLDL + Compound C group; and 0.25 mL of SJP and 0.25 mL of Compound C were added to the SJP + Compound C group. Thirty minutes later, 30 μL of culture medium was added to the normal group, and 30 μL of VLDL (5 mg/mL) was added to the other groups for model induction. Culture supernatants were collected 24 h after treatment administration to treat the PSCs, according to the following experimental design.
PSCs that covered the slides were divided into seven groups: A, normal PSCs (VLDL-, culture supernatants-); B, PSCs stimulated directly with VLDL (VLDL+, culture supernatant-); C, PSCs stimulated with normal acinar cell culture supernatant (VLDL-, culture supernatant+); D, PSCs stimulated with acinar cell culture supernatant treated with VLDL (VLDL+, culture supernatant+); E, PSCs stimulated with acinar cell culture supernatant treated with SJP (VLDL+, culture supernatant+, SJP+); F, PSCs stimulated with acinar cell culture supernatant treated with Compound C (VLDL+, culture supernatant+, Compound C+); G, PSCs stimulated with acinar cell culture supernatant treated with Compound C and SJP (VLDL+, culture supernatant+, SJP+, Compound C+). The culture medium was added to group A, diluted VLDL (30 μL VLDL + 2.5 mL medium) was added to group B, and the appropriate acinar cell culture supernatants, as described above, were added to each of the remaining five groups for 6 h, according to a ratio of 100 μl/mL. Then, the slides were collected for immunofluorescence analysis of the expression of fibronectin and type I collagenase.
Immunofluorescence
Slides were fixed in 40 g/L paraformaldehyde for 30 min and rinsed with PBS three times (10 min each time). Slides were permeabilized with 1% Triton X-100 (Sigma, Saint Louis, MO, United States) for 30 min and rinsed with PBS three times (10 min each time). Slides were blocked with 10% goat serum (AR0009, BOSTER, Wuhan, China) at 37 °C for 2 h. Anti-collagen I antibody (ab34710, Abcam, Cambridge, MA, United States) and anti-fibronectin antibody (ab6328, Abcam, Cambridge, MA, United States) were added separately and incubated overnight at 4 °C. After washing with PBS, the secondary antibodies, goat anti-rabbit IgG H&L (Alexa Fluor® 488) (ab150077, Abcam, Cambridge, MA, United States) and goat anti-mouse IgG H&L (Alexa Fluor® 488) (ab150113, Abcam, Cambridge, MA, United States), were added separately and protected against light for 2 h at room temperature. The nucleus was stained with DAPI, and the sections were sealed with glycerine. A fluorescence microscope (AX10 imager A2/AX10 cam HRC, Carl Zeiss Jena, Oberkochen, Germany) was used for observation.
Statistical analysis
The statistical methods of this study were reviewed by Dr. Hai Niu from College of Mathematics, Sichuan University. All values are expressed as the mean ± standard deviation. GraphPad Prism 6.01 software (GraphPad Prism 6.01 software Inc., San Diego, CA, United States) was used for statistical analyses. For each test, the experimental unit was an individual animal. Normality was assessed by the Shapiro-Wilk normality test, and homogeneity of variance was assessed by the Bartlett’s test. If data were normally distributed and the variances of three experimental groups were equal, one-way analysis of variance was used for multi-group comparisons, and Dunnett-t test was used for comparisons of two groups. Statistical significance is expressed as aP < 0.05 vs NG or bP < 0.05 vs HLG.
RESULTS
SJP reduces BW, Lee’s index, and serum triglyceride levels of obese rats
After 12 wk of experimental diet consumption, BW, Lee’s index, which is a rapid means of determining obesity, and the levels of serum triglyceride of the rats in the HLG were significantly higher than those of the rats in the NG (P < 0.05; Table 1). Conversely, the above three parameters of the HSG were significantly lower than those of the HLG (P < 0.05; Table 1). However, food intake did not differ significantly among the experimental groups.
Table 1.
Body weight, Lee's index, serum triglyceride levels, and dairy food intake of rats in the three experimental groups
| Parameter | NG | HLG | HSG |
| Initial body weight (g) | 69 ± 4 | 70 ± 5 | 69 ± 8 |
| Final body weight (g) | 461 ± 56 | 537 ± 46a | 467 ± 49b |
| Lee’s index | 3.13 ± 0.07 | 3.43 ± 0.16a | 3.12 ± 0.13b |
| Triglyceride (mmol/L) | 1.57 ± 0.46 | 3.24 ± 1.48a | 1.39 ± 0.41b |
| Food intake (g/d) | 19.57 ± 0.87 | 18.91 ± 1.12 | 18.01 ± 0.77 |
The results are presented as the mean ± SD, n = 8 for each group.
P < 0.05 vs NG;
P < 0.05 vs HLG. NG: Normal group; HLG: High-fat diet group; HSG: High-fat diet group treated with Sheng-jiang powder.
SJP relieves the pathological damage to pancreatic tissues in obese rats
The histopathological evaluation results showed significantly higher pathological scores for pancreatic tissues from rats in the HLG than for those in the NG (P < 0.05; Figure 1A). Conversely, SJP treatment distinctly lowered the pathological scores of the pancreas, with reduced inflammatory cell infiltration, mild tissue oedema, and reduced cell necrosis (Figure 1A and B).
Figure 1.
Histological images and pathologic scores of pancreatic tissues from the three experimental groups. A: Pathological images of the pancreatic tissues (× 200); B: Histological scores of the pancreatic tissues. The results are presented as the mean ± SD. aP < 0.05 vs NG; bP < 0.05 vs HLG. NG: Normal group; HLG: High-fat diet group; HSG: High-fat diet group treated with Sheng-jiang powder.
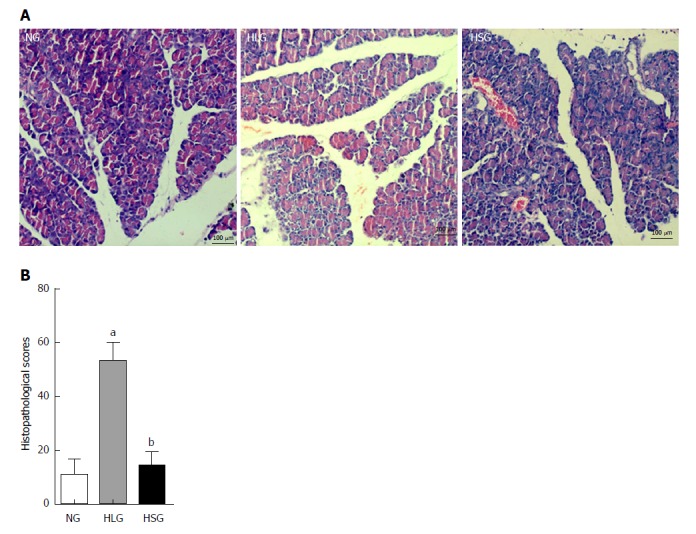
SJP stimulates the expression of serum adiponectin in obese rats
Adiponectin is an adipokine with anti-inflammatory, anti-oxidant, anti-atherogenic, pro-angiogenic, vasoprotective, and insulin-sensitizing properties, which is markedly decreased in obesity[25]. Thus, we determined the levels of adiponectin in serum after SJP administration. Adiponectin levels were significantly reduced in the HLG (P < 0.05; Figure 2), whereas they were absent in the NG. After SJP administration, adiponectin levels were much higher in rats in the HSG than in rats in the HLG (P < 0.05; Figure 2).
Figure 2.
Levels of serum adiponectin. The results are presented as the mean ± SD. aP < 0.05 vs NG; bP < 0.05 vs HLG. NG: Normal group; HLG: High-fat diet group; HSG: High-fat diet group treated with Sheng-jiang powder.
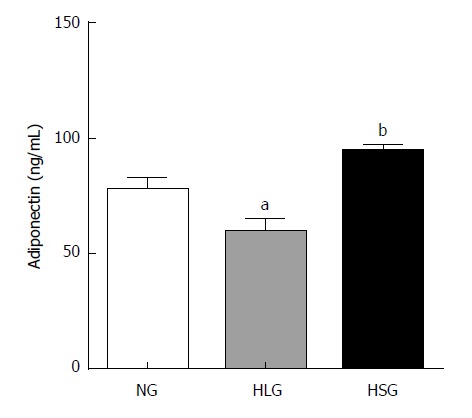
Effect of SJP on the expression levels of NF-κB and TGF-β in pancreatic acinar cells and inflammatory cells from obese rats
As shown in Figure 3A, the expression levels of NF-κB in both pancreatic acinar cells and inflammatory cells from rats were higher in the HLG than in the NG (P < 0.05). After SJP administration, the expression of NF-κB in both types of cells was inhibited. Although no differences were found in TGF-β expression levels among the groups in the pancreatic acinar cells, an expression pattern similar to that of NF-κB was observed in inflammatory cells, where TGF-β expression was stimulated by HFD and inhibited by SJP administration (Figure 3A).
Figure 3.
Nuclear factor kappa-light-chain-enhancer of activated B cells and transforming growth factor beta expression in pancreatic tissues. A: Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor beta (TGF-β) expression in pancreatic acinar cells and inflammatory cells; B: Immunohistochemistry assay for NF-κB expression; C: Immunohistochemistry assay for TGF-β expression. Negative: Blue; Positive: Yellow-brown. The results are presented as the mean ± SD. aP < 0.05 vs NG; bP < 0.05 vs HLG. NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; TGF-β: Transforming growth factor beta; NG: Normal group; HLG: High-fat diet group; HSG: High-fat diet group treated with Sheng-jiang powder; N/P ratio: Nuclear/cytoplasmic ratio; AC: Acinar cells; IC: Inflammatory cells.
Effects of SJP on apoptosis of pancreatic acinar cells in obese rats
Apoptosis of pancreatic acinar cells was significantly higher in the HLG than in the NG (P < 0.05; Figure 4). Although we found no significant differences in apoptosis following treatment with SJP, we found a downward trend in the HSG (Figure 4).
Figure 4.
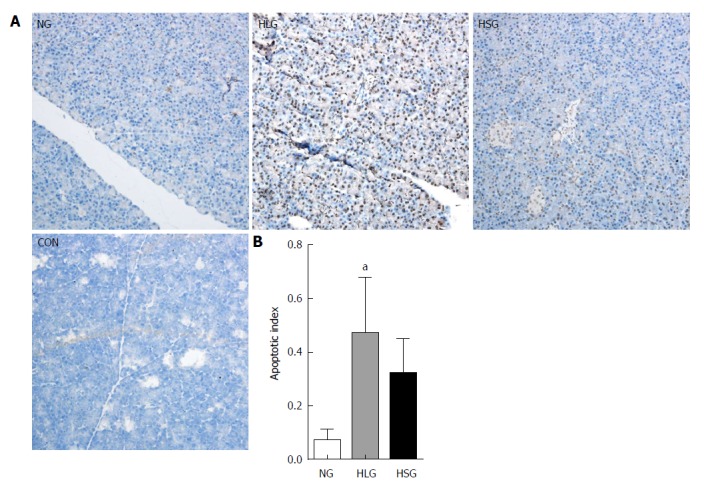
Results of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling staining of pancreatic acinar cells. A: TUNEL staining images of pancreatic acinar cells; B: Apoptotic index of pancreatic acinar cells. Negative: blue; positive: yellow-brown. The results are presented as the mean ± SD. aP < 0.05 vs NG. TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; NG: Normal group; HLG: High-fat diet group; HSG: High-fat diet group treated with Sheng-jiang powder group; CON: Negative staining control group.
SJP promotes PSC activation
To explore whether the repair effects of SJP on pancreatic acinar cell damage occur through adenosine 5‘-monophosphate-activated protein kinase (AMPK) signalling, we performed cellular tests of AR42J cells and PSCs. The results showed that the expression of fibronectin and type I collagenase was weak in normal PSCs (Figure 5A). After stimulation with VLDL or normal acinar cell culture supernatant, the expression of fibronectin and type I collagenase increased (Figure 5B and C). The supernatant of VLDL-treated AR42J cells stimulated PSCs to trigger increased expression levels of fibronectin and type I collagenase (Figure 5D), and the expression of these two proteins was enhanced after stimulation with acinar cell culture supernatant treated with SJP (Figure 5E). The fibronectin and type I collagenase expression levels were reduced (Figure 5F and G) in the two groups of PSCs treated with the AMPK inhibitor.
Figure 5.
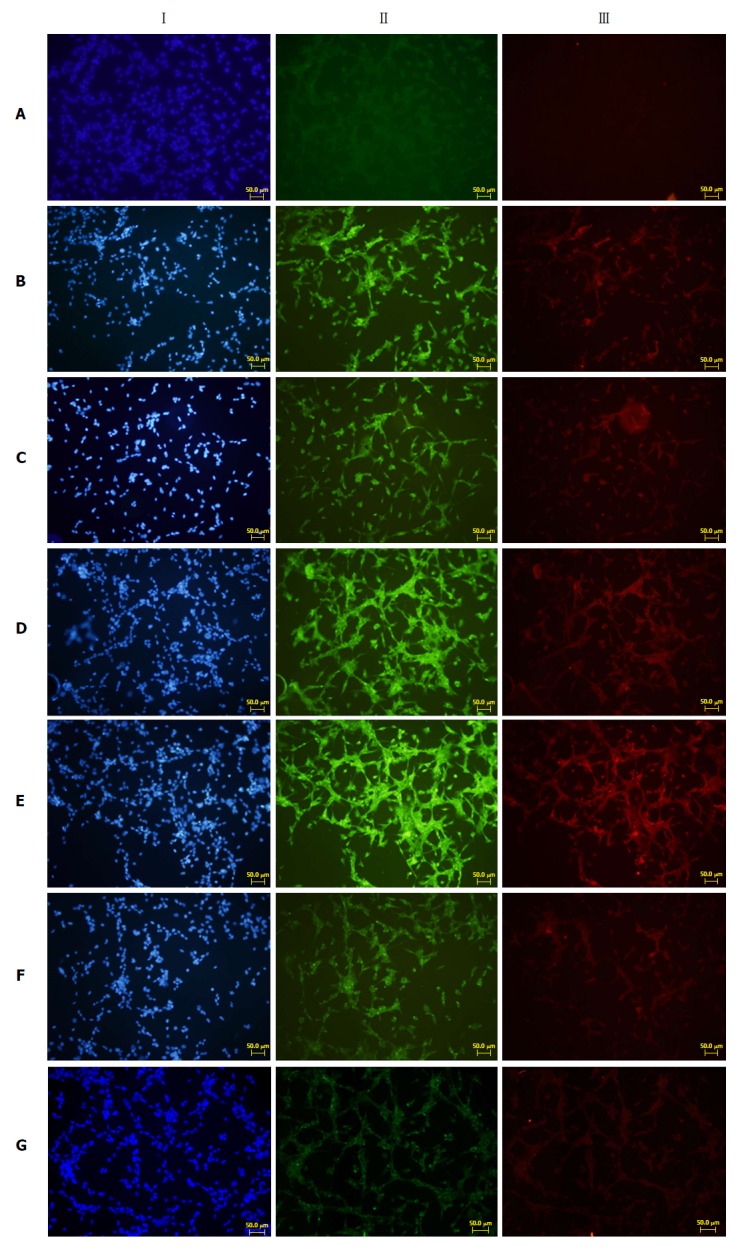
Immunofluorescence results of fibronectin and type I collagenase expression in cells growing on glass coverslips from each group. I: Nuclear staining of rat pancreatic stellate cells (blue fluorescence); II: Fibronectin staining (green fluorescence); III: Type I collagenase staining (red fluorescence). A: Normal PSC (VLDL-, culture supernatant-); B: PSC stimulated directly with VLDL (VLDL+, culture supernatant-); C: PSC stimulated with normal acinar cell culture supernatants (VLDL-, culture supernatant+); D: PSC stimulated with acinar cell culture supernatant treated with VLDL (VLDL+, culture supernatant+); E: PSC stimulated with acinar cell culture supernatant treated with SJP (VLDL+, culture supernatant+, SJP+); F: PSC stimulated with acinar cell culture supernatant treated with Compound C (VLDL+, culture supernatant+, Compound C+); G: PSC stimulated with acinar cell culture supernatant treated with Compound C and SJP (VLDL+, culture supernatant+, SJP+, Compound C+). PSC: Pancreatic stellate cell; VLDL: Very low-density lipoprotein; SJP: Sheng-jiang powder.
DISCUSSION
In the present study, HFD successfully induced an obese rat model, as in our previous study[7]. Our results showed significantly higher BW, Lee’s index scores, and serum triglyceride levels, lower serum adiponectin levels, higher expression levels of NF-κB in pancreatic tissues, and increased apoptosis of pancreatic acinar cells in obese rats, while SJP effectively reduced BW, Lee’s index scores, and serum triglyceride levels, stimulated the expression of serum adiponectin, and inhibited the expression of NF-κB in pancreatic tissues. In addition, the expression levels of TGF-β in inflammatory cells of the pancreas were significantly higher in obese rats, while SJP could reduce the expression of TGF-β in inflammatory cells but had no influence in acinar cells. The in vitro studies have shown that the culture supernatant from AR42J acinar cells that were incubated with VLDL stimulated the proliferation and matrix synthesis of PSCs. After SJP treatment, PSC activation was enhanced, and the expression of fibronectin and type I collagenase was further increased. Interestingly, AMPK inhibitors inhibited the PSC activation process described above.
Adipose tissue is considered to be an endocrine organ with an important role in local and systemic homeostasis. It has been demonstrated that adipose is responsible for the production and release of many potent signalling molecules, including adipokines, lipokines, and inflammatory mediators[25]. Adiponectin is a well-known adipokine that promotes insulin sensitivity and has an anti-inflammatory effect, and its production becomes blunted as adiposity increases[26]. Generally, obesity in humans is a symptom of energy imbalance, where energy intake exceeds energy output, while AMPK plays a key role in controlling energy homeostasis[27]. Importantly, adiponectin can regulate energy intake and consumption by stimulating the phosphorylation of AMPK; adiponectin phosphorylates and subsequently inhibits acetyl-CoA carboxylase and inhibits malonyl-CoA synthesis, thereby decreasing the inhibitory effect of carnitine acyltransferase 1 (the key enzyme required for activated fatty acid entry into the mitochondria) and leading to increased fatty acid oxidation and glucose uptake[28]. Therefore, with the long-term intake of HFD, the decrease in adiponectin observed in obese rats may affect the energy imbalance and promote fat infiltration or accumulation; in turn, fat accumulation may affect the expression of adiponectin, which leads to a vicious cycle.
In addition to its effect on controlling glucose and lipid metabolism, adiponectin can also inhibit lipopolysaccharide (LPS)-primed inflammasome activation in macrophages via AMPK signalling-dependent mechanisms[29], while adiponectin-AMPK signalling can be inhibited during chronic low-grade inflammatory responses, including obesity, non-alcoholic fatty liver disease, atherosclerosis, IR, and T2DM[30]. Therefore, the decrease in adiponectin observed in obese rats may reduce its inhibitory effect on inflammasome activation and promote the inflammatory response. In our study, SJP ameliorated the expression of adiponectin in rats with obesity induced with an HFD. Similarly, some studies showed that SJP could significantly increase serum adiponectin levels in obesity-related glomerulopathy patients and T2DM patients with dyslipidaemia[18,31]. As a component of Curcuma longa, curcumin could attenuate HFD-induced hepatic steatosis by regulating hepatic lipid metabolism via AMPK activation[32]. Thus, combined with the anti-inflammatory effect of SJP, we speculate that SJP may reduce the suppressive effect of obesity on the adiponectin-AMPK signalling pathway, which may contribute to energy consumption and further inhibit the inflammatory response, eventually regulating lipid metabolism in obese rats.
In addition to the aforementioned decrease in adiponectin levels associated with the inflammatory response in obese rats, the NF-κB signalling pathway and ER stress are two other important mechanisms of the obesity-induced inflammatory response. As adiposity increases, the balance between pro-inflammatory and anti-inflammatory cytokines secreted by adipocytes gradually becomes deregulated. In addition, those unbalanced inflammatory cytokines can activate macrophages via the Toll-like receptor 4 signalling pathways, whereas the binding of TNF-α released by macrophages to TNF-α receptors on adipocytes activates the NF-κB signalling pathway[33] and promotes the amplification of inflammatory responses. In addition, ER stress is currently recognized to be a mechanism of the obesity-induced inflammatory response. Obesity-induced ER stress primarily manifests itself in the activation of two classic signalling pathways: The nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor (IκB)/NF-κB signalling pathway and the c-Jun N-terminal kinase (JNK) signalling pathway[34,35]. The phosphorylation of IκB and JNK triggers the activation of transcription factors, such as NF-κB, that are closely related to the inflammatory response downstream, thereby promoting the development of inflammatory responses[36]. We know that SJP was effective for anti-inflammation and could significantly downregulate the expression of NF-κB in inflammatory diseases, such as acute lung injury and glomerulonephritis[37,38]. This study also confirmed that SJP reduced the expression of NF-κB in pancreatic tissues. Therefore, SJP may reduce the inflammatory response in the pancreas of obese rats via the NF-κB signalling pathway.
TGF-β is a regulatory molecule with pleiotropic effects on cell proliferation, differentiation, migration, and survival and affects multiple biological processes, including development, carcinogenesis, fibrosis, wound healing, and immune responses[39]. Although TGF-β is an important cytokine that regulates tissue inflammation and repair, its overexpression induces fibrosis, and the inhibition of TGF-β improves fibrotic disorder[40,41]. Matsuda et al[42] found that, in Zucker diabetic fatty rats fed a chronic HFD, fat could accumulate in pancreatic acinar cells, which was related to subsequent pancreatic fibrosis and acinar cell injury. Similarly, Yoshikawa et al[43] demonstrated that TGF-β1 could extend from peri-islets to the exocrine pancreas to become involved in pancreatic fibrosis in Otsuka Long-Evans Tokushima fatty rats, a model of naturally occurring obesity-related diabetes.
In our study, the expression of TGF-β in pancreatic acinar cells was rarely increased after 12 wk of HFD intake, but it was highly expressed in pancreatic inflammatory cells. Therefore, we speculate that obesity, a persistent chronic injury with an accompanying inflammatory response, may result in pancreatic fibrosis. Interestingly, after SJP treatment, the expression of TGF-β in pancreatic inflammatory cells was significantly decreased with a dramatic reduction in NF-κB and an increase in adiponectin. Thus, given the common use of anti-inflammatory drugs in the treatment of fibrosis[40] and the anti-inflammatory effect of SJP, we speculate that SJP may prevent pancreatic fibrosis by inhibiting the inflammatory response in the pancreas of obese rats.
It has been well established that brain, liver, and heart cells undergo apoptosis under obesity conditions[44-46]. To confirm whether obesity induces pancreatic acinar cell apoptosis, we performed TUNEL staining. The results showed that obesity could induce apoptosis of pancreatic acinar cells. Moreover, one study has shown that the phosphorylation of AMPK induced by adiponectin could block interleukin 8-mediated endothelial cell death and exert an anti-apoptotic effect[47]. Considering the fact that SJP increased the levels of serum adiponectin in our study and that curcumin effectively reduced apoptosis of pancreatic acinar cells caused by the long-term intake of alcohol and different amounts of proteins[48], we speculated that SJP might play a role in the obesity-induced apoptosis of pancreatic acinar cells. However, there was no significant difference in the amount of apoptosis in acinar cells after SJP treatment in this study. A possible explanation for this finding might be that a single dose or the dosing concentration was insufficient. To the best of our knowledge, only one study has demonstrated that SJP inhibited the apoptosis of brain cells in rats with vascular dementia, and its administration method was intravenous drip at 10 mL/kg of BW SJP[49].
Almost immediately, from the start of injury, multiple types of cells participate in the process of exocrine pancreas repair and regeneration. These cells include not only acinar cells, which are both villains and victims in pancreatic injury, but also ductal epithelial cells, inflammatory cells of the immune system, and PSCs[50]. Given the importance of epithelial-mesenchymal interactions during pancreas development[51], interactions between parenchymal cells and PSCs are almost certain to be important for proper pancreatic repair. Under physiological conditions, PSCs are at rest. In the presence of profibrogenic mediators, such as inflammatory cytokines and oxidative stress, PSCs are activated[52]. Activated PSCs produce large amounts of a-smooth muscle actin and extracellular matrix proteins, particularly fibronectin and type I collagenase, to achieve the replacement of inflammatory infiltrates and the repair or regeneration of tissue injuries[53]. On the basis of the in vivo data, under obesity conditions, inflammation occurred in pancreatic tissues, and subsequently, acinar cell injury arose. Furthermore, culture media treated with fat could stimulate acinar cells to produced profibrogenic mediators. In addition, the supernatants collected from acinar cells stimulated the proliferation of PSCs and the synthesis of extracellular matrix proteins, particularly fibronectin and type I collagenase. After treatment with the AMPK inhibitor, the expression levels of fibronectin and type I collagenase were reduced. For the first time, we found that the inhibition of the AMPK signalling pathway could impair the therapeutic effects of SJP by diminishing the expression of fibronectin and type I collagenase. Therefore, we boldly speculate that SJP may promote acinar cell injury repair through the activation of the AMPK signalling pathway.
This study expanded on the research from our previous study[7]. However, some limitations exist. First, in the in vivo experiment, no critical upstream or downstream factors were detected in the adiponectin-AMPK pathway in pancreatic tissue other than serum adiponectin. Second, the relationship between dose or dose frequency and the concentration effect requires further study. Finally, the specific effective monomer components of SJP should be taken under consideration.
In conclusion, we demonstrated that obesity exacerbates pancreatic inflammatory injury in rats and promotes the apoptosis of pancreatic acinar cells. SJP can inhibit the inflammatory response, prevent pancreatic fibrosis, and promote pancreatic acinar cell repair, through the regulation of key molecules of the adiponectin-AMPK signalling pathway, and eventually ameliorate obesity-induced pancreatic inflammatory injury in rats.
ARTICLE HIGHLIGHTS
Research background
Obesity is a risk factor for non-alcoholic fatty pancreas disease and induces pancreatic inflammatory injury. Sheng-jiang powder (SJP) can ameliorate obesity-induced pancreatic inflammatory injury, but the specific mechanisms remain unclear. Therefore, the investigation of the specific mechanisms underlying the SJP amelioration of obesity-induced pancreatic inflammatory injury is urgently required.
Research motivation
Our previous studies have demonstrated that SJP can ameliorate the inflammatory response and histopathological lesions in the pancreas of obese rats. However, the specific mechanisms underlying ameliorating effects of SJP on obesity-induced pancreatic inflammatory injury are far from sufficiently understood. Therefore, this study aimed to further explore the specific mechanisms of SJP on obesity-induced pancreatic inflammatory injury, to provide evidence for its clinical application in the future.
Research objectives
This study aimed to investigate the specific mechanisms by which SJP can ameliorate obesity-induced pancreatic inflammatory injury.
Research methods
In the in vivo study, an obese rat model was induced by high-fat diet feeding, which is widely accepted and used for the induction of obesity in rats. The serum adiponectin levels were measured by enzyme-linked immunosorbent assay (ELISA), which is a simple, rapid, accurate, and sensitive method. The expression levels of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor beta (TGF-β) in pancreatic tissues were measured by immunohistochemistry. The levels of apoptotic cells in pancreatic tissue samples were analysed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay.
In the in vitro study, a high-fat AR42J acinar cell injury model was established with very low-density lipoprotein (VLDL), and the AR42J acinar cell culture supernatants, treated with different interventions, were applied to pancreatic stellate cells (PSCs). The proliferation of PSCs and the expression of fibronectin and type I collagenase were measured by immunofluorescence analysis.
All statistical analyses were performed with GraphPad Prism 6.01 software. Quantitative data are expressed as the mean ± standard deviation when normally distributed. One-way analysis of variance followed by multiple pair-wise comparisons using Dunnett-t test was used to detect differences among the above parameters.
Research results
In the in vivo study, compared to the obese group (HLG), we found reduced body weight, Lee’s index scores, serum triglyceride levels, and pathological scores of pancreatic tissues; higher serum adiponectin levels; and lower expression levels of NF-κB in pancreatic tissue and TGF-β in the inflammatory cells of the pancreas in the SJP treatment group (HSG) (P < 0.05). In the in vitro study, PSC activation was enhanced after SJP treatment, and the expression levels of fibronectin and type I collagenase were increased after SJP treatment. An adenosine 5‘-monophosphate-activated protein kinase (AMPK) inhibitor inhibited the PSC activation process described above.
What remains to be determined is the relationship between dose or dose frequency and the concentration effect. Furthermore, the specific effective monomer components of SJP should be taken under consideration to provide more systematic and comprehensive evidence for the clinical application of this Chinese decoction.
Research conclusions
This study demonstrates, for the first time, that obesity exacerbates pancreatic inflammatory injury in rats and promotes apoptosis in pancreatic acinar cells. In addition, SJP can inhibit the inflammatory response, prevent pancreatic fibrosis, promote pancreatic acinar cell repair, through the regulation of key molecules of the adiponectin-AMPK signalling pathway, and eventually ameliorate obesity-induced pancreatic inflammatory injury in rats. Therefore, our study provides molecular mechanisms as evidence for the clinical application of SJP.
Research perspectives
As we have found that SJP may ameliorate obesity-induced pancreatic inflammatory injury in rats by regulating key molecules of the adiponectin-AMPK signalling pathway, further investigation regarding the potential active components of SJP and the interactions among these components is urgently required to provide evidence for wider clinical usage and to optimize and simplify the formula.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Institutional Animal Care and Use Committee of West China Hospital of Sichuan University.
Institutional animal care and use committee statement: All rats were handled according to the University Guidelines and the Animal Care Committee Guidelines of West China Hospital (Chengdu, China) (protocol number, 2017052A).
Conflict-of-interest statement: None of the authors have any conflicts of interest to declare.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Peer-review started: July 9, 2018
First decision: August 25, 2018
Article in press: October 5, 2018
P- Reviewer: Bramhall S, Neri V S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Yi-Fan Miao, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Juan Li, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Yu-Mei Zhang, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Lv Zhu, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Huan Chen, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Ling Yuan, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Jing Hu, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Xiao-Lin Yi, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Qiu-Ting Wu, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Mei-Hua Wan, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Wen-Fu Tang, Department of Integrative Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China. tangwf@scu.edu.cn.
References
- 1.World Health Organization. 2018. Obesity and overweight. October 18, 2017. Accessed July 2. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, Hu FB, Raz I, Van Gaal L, Wolfe BM, et al. Advances in the Science, Treatment, and Prevention of the Disease of Obesity: Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2015;38:1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J Clin Biochem Nutr. 2010;46:212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catanzaro R, Cuffari B, Italia A, Marotta F. Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol. 2016;22:7660–7675. doi: 10.3748/wjg.v22.i34.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Zhang YM, Li JY, Zhu L, Kang HX, Ren HY, Chen H, Yuan L, Miao YF, Wan MH, et al. Effect of Sheng-Jiang Powder on Obesity-Induced Multiple Organ Injuries in Rats. Evid Based Complement Alternat Med. 2017;2017:6575276. doi: 10.1155/2017/6575276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Ciaula A, Portincasa P. Fat, epigenome and pancreatic diseases. Interplay and common pathways from a toxic and obesogenic environment. Eur J Intern Med. 2014;25:865–873. doi: 10.1016/j.ejim.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Soeda J, Mouralidarane A, Cordero P, Li J, Nguyen V, Carter R, Kapur SR, Pombo J, Poston L, Taylor PD, et al. Maternal obesity alters endoplasmic reticulum homeostasis in offspring pancreas. J Physiol Biochem. 2016;72:281–291. doi: 10.1007/s13105-016-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debnath M, Agrawal S, Agrawal A, Dubey GP. Metaflammatory responses during obesity: Pathomechanism and treatment. Obes Res Clin Pract. 2016;10:103–113. doi: 10.1016/j.orcp.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh K, Inoue M, Shiraishi K, Masaki T, Chiba S, Mitsutomi K, Shimasaki T, Ando H, Fujiwara K, Katsuragi I, et al. Spleen-derived interleukin-10 downregulates the severity of high-fat diet-induced non-alcoholic fatty pancreas disease. PLoS One. 2012;7:e53154. doi: 10.1371/journal.pone.0053154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu TY, Wang CY. Impact of non-alcoholic fatty pancreas disease on glucose metabolism. J Diabetes Investig. 2017;8:735–747. doi: 10.1111/jdi.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XM, Tong XL, Wang PQ. Discussion on the theory of turbidity pathopoiesis. Shijie Zhongxiyi Jiehe Zazhi. 2009;4:839–842. [Google Scholar]
- 15.Tian SX, Li SM. Clinical application on the effect of Sheng-jiang powder. Hebei Zhongxiyi Xuebao. 1994;9:40–44. [Google Scholar]
- 16.Zheng WL, Su HS. Treatment of 60 cases of hyperlipidemia by modified Sheng-jiang powder. Shaanxi Zhongyi. 2010;31:1486–1487. [Google Scholar]
- 17.Han JH, Su HS. Clinical observation on the effect of Sheng-jiang powder combined with metformin on nonalcoholic fatty liver disease with metabolic syndrome. Shaanxi Zhongyi. 2013;34:989–991. [Google Scholar]
- 18.Yang J, Ni HG, Guo XY, Li L. Effect of modified Sheng-jiang powder on the pancreatic function of patients with T2DM and dyslipidemia. Zhongxiyi JIehe Yanjiu. 2016;8:1–4. [Google Scholar]
- 19.Zhu L, Li JY, Zhang YM, Kang HX, Chen H, Su H, Li J, Tang WF. Pharmacokinetics and pharmacodynamics of Shengjiang decoction in rats with acute pancreatitis for protecting against multiple organ injury. World J Gastroenterol. 2017;23:8169–8181. doi: 10.3748/wjg.v23.i46.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi MM, Ma J, He LW. Simultaneous determination of four components in sheng-jiang powder by HPLC. Zhongyaocai. 2015;38:2418–2420. [Google Scholar]
- 21.Li M, Ye T, Wang XX, Li X, Qiang O, Yu T, Tang CW, Liu R. Effect of Octreotide on Hepatic Steatosis in Diet-Induced Obesity in Rats. PLoS One. 2016;11:e0152085. doi: 10.1371/journal.pone.0152085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284–288; discussion 289. doi: 10.1016/s0039-6060(96)80299-6. [DOI] [PubMed] [Google Scholar]
- 23.Xu LZ, Yang WT. Judging criteria for the results of immunohistochemical reactions. Zhongguo Aizheng Zazhi. 1996;6:229–231. [Google Scholar]
- 24.Siech M, Zhou ZF, Zhou SX, Bair B, Alt A, Hamm S, Gross H, Mayer J, Beger HG, Tian XD, et al. Stimulation of stellate cells by injured acinar cells: A model of acute pancreatitis induced by alcohol and fat (VLDL) Am J Physiol-Gastr L. 2009;297:G1163–G1171. doi: 10.1152/ajpgi.90468.2008. [DOI] [PubMed] [Google Scholar]
- 25.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425:560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–37. doi: 10.1016/j.ceb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: focus on adiponectin. Curr Opin Pharmacol. 2006;6:580–585. doi: 10.1016/j.coph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim MJ, Kim EH, Pun NT, Chang JH, Kim JA, Jeong JH, Choi DY, Kim SH, Park PH. Globular Adiponectin Inhibits Lipopolysaccharide-Primed Inflammasomes Activation in Macrophages via Autophagy Induction: The Critical Role of AMPK Signaling. Int J Mol Sci. 2017;18:pii: E1275. doi: 10.3390/ijms18061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day EA, Ford RJ, Steinberg GR. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol Metab. 2017;28:545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Hao YJ, Dong YF, Wang GY, Tian LM, Yin XW, Wang YH, Zhang PX. Effect of Sheng-jiang powder combined acupuncture on serum leptin and adiponectin in patients with obesity-related glomerulopathy. Jichu Zhangyi Zazhi. 2015;21:1438–1440. [Google Scholar]
- 32.Um MY, Hwang KH, Ahn J, Ha TY. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 2013;113:152–157. doi: 10.1111/bcpt.12076. [DOI] [PubMed] [Google Scholar]
- 33.Razolli DS, Moraes JC, Morari J, Moura RF, Vinolo MA, Velloso LA. TLR4 expression in bone marrow-derived cells is both necessary and sufficient to produce the insulin resistance phenotype in diet-induced obesity. Endocrinology. 2015;156:103–113. doi: 10.1210/en.2014-1552. [DOI] [PubMed] [Google Scholar]
- 34.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 35.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 36.Yin J, Gu L, Wang Y, Fan N, Ma Y, Peng Y. Rapamycin improves palmitate-induced ER stress/NF κB pathways associated with stimulating autophagy in adipocytes. Mediators Inflamm. 2015;2015:272313. doi: 10.1155/2015/272313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei KF, Geng YH, Chang JS, Pan Y, Zhang QH, Zhang MY. Effects of Sheng-jiang powder on expression of NF-κB in pulmonary microvascular endothelial cells of rats with acute lung injury. Nanjing Zhongyiyao Daxue Xuebao. 2008;24:341–342. [Google Scholar]
- 38.Yu JS, Wang Q, Yu HQ. Effects of Shengjiangsan on expression of NF-κB in rats with mesangial proliferative glomerulonephritis. Zhongguo Yanfangxue Xuebao. 2011;17:190–193. [Google Scholar]
- 39.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 40.Gerarduzzi C, Di Battista JA. Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res. 2017;66:451–465. doi: 10.1007/s00011-016-1019-x. [DOI] [PubMed] [Google Scholar]
- 41.Nagashio Y, Ueno H, Imamura M, Asaumi H, Watanabe S, Yamaguchi T, Taguchi M, Tashiro M, Otsuki M. Inhibition of transforming growth factor beta decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice. Lab Invest. 2004;84:1610–1618. doi: 10.1038/labinvest.3700191. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda A, Makino N, Tozawa T, Shirahata N, Honda T, Ikeda Y, Sato H, Ito M, Kakizaki Y, Akamatsu M, et al. Pancreatic fat accumulation, fibrosis, and acinar cell injury in the Zucker diabetic fatty rat fed a chronic high-fat diet. Pancreas. 2014;43:735–743. doi: 10.1097/MPA.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa H, Kihara Y, Taguchi M, Yamaguchi T, Nakamura H, Otsuki M. Role of TGF-beta1 in the development of pancreatic fibrosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G549–G558. doi: 10.1152/ajpgi.00323.2001. [DOI] [PubMed] [Google Scholar]
- 44.Kang DH, Heo RW, Yi CO, Kim H, Choi CH, Roh GS. High-fat diet-induced obesity exacerbates kainic acid-induced hippocampal cell death. BMC Neurosci. 2015;16:72. doi: 10.1186/s12868-015-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long W, Hui Ju Z, Fan Z, Jing W, Qiong L. The effect of recombinant adeno-associated virus-adiponectin (rAAV2/1-Acrp30) on glycolipid dysmetabolism and liver morphology in diabetic rats. Gen Comp Endocrinol. 2014;206:1–7. doi: 10.1016/j.ygcen.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Lekli I, Szabo G, Juhasz B, Das S, Das M, Varga E, Szendrei L, Gesztelyi R, Varadi J, Bak I, et al. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol. 2008;294:H859–H866. doi: 10.1152/ajpheart.01048.2007. [DOI] [PubMed] [Google Scholar]
- 47.Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J Biol Chem. 2008;283:24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou XC. Effects of Curcumin on Pancreatic Acinar Cell Injury in Rats with Long-term Alcohol Intake and Different Amount of Protein. Zhongguo Yaofang. 2011;22:4041–4043. [Google Scholar]
- 49.Shi JF, Ma J, Guo CH. Efficacy and mechanism of Sheng-jiang powder on vascular dementia in rats. Zhongguo Laonianxue Zazhi. 2017;37:1341–1344. [Google Scholar]
- 50.Murtaugh LC, Keefe MD. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol. 2015;77:229–249. doi: 10.1146/annurev-physiol-021014-071727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landsman L, Nijagal A, Whitchurch TJ, Vanderlaan RL, Zimmer WE, Mackenzie TC, Hebrok M. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. 2011;9:e1001143. doi: 10.1371/journal.pbio.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011;40:557–566. doi: 10.1097/MPA.0b013e318214efaf. [DOI] [PMC free article] [PubMed] [Google Scholar]