Significance
Genomic imprinting is a form of epigenetic regulation causing parent-of-origin differential expression of maternally or paternally inherited alleles. The DNA demethylase DME regulates the imprinting of many genes in the Arabidopsis endosperm. It is not known whether and how other DNA demethylases may also regulate imprinting. Here, we discovered that the DNA demethylase ROS1 negatively regulates DOGL4 imprinting via demethylation of the DOGL4 promoter on the paternal allele. Additionally, we found that DOGL4 negatively regulates seed dormancy and abscisic acid (ABA) response and that ROS1 regulates seed dormancy and ABA response by controlling DOGL4 expression. Our results thus suggest a different mechanism of regulation of gene imprinting and reveal important roles of ROS1 in the regulation of seed dormancy and ABA response.
Keywords: DNA methylation, DNA demethylation, ABA response, DME
Abstract
Genomic imprinting is a form of epigenetic regulation resulting in differential gene expression that reflects the parent of origin. In plants, imprinted gene expression predominantly occurs in the seed endosperm. Maternal-specific DNA demethylation by the DNA demethylase DME frequently underlies genomic imprinting in endosperm. Whether other more ubiquitously expressed DNA demethylases regulate imprinting is unknown. Here, we found that the DNA demethylase ROS1 regulates the imprinting of DOGL4. DOGL4 is expressed from the maternal allele in endosperm and displays preferential methylation and suppression of the paternal allele. We found that ROS1 negatively regulates imprinting by demethylating the paternal allele, preventing its hypermethylation and complete silencing. Furthermore, we found that DOGL4 negatively affects seed dormancy and response to the phytohormone abscisic acid and that ROS1 controls these processes by regulating DOGL4. Our results reveal roles for ROS1 in mitigating imprinted gene expression and regulating seed dormancy.
Genomic imprinting is an epigenetic mechanism that alters gene expression in a parent-of-origin manner whereby some genes display differential expression, depending on whether the allele was inherited from the maternal or paternal parent (1, 2). This epigenetic phenomenon occurs in mammals and seed plants and is considered to have evolved independently in the two lineages (3). Mammals display imprinted gene expression in both the placenta and embryo, whereas imprinting in angiosperms appears to occur predominantly in the seed endosperm (2, 4). The endosperm is a triploid tissue derived after the fertilization of the homodiploid central cell with one sperm cell; the other sperm cell fertilizes the egg cell to form a diploid embryo. The seed endosperm serves to nurture and support the growing embryo and can be considered analogous to the mammalian placenta (5). Although the evolutionary forces for the selection of imprinted expression in the endosperm are still debated, conflicts between maternal and paternal genomes in resource allocation to offspring or dosage control may be important driving forces for this process (6).
Genome-wide RNA sequencing of endosperm has identified hundreds of imprinted genes in Arabidopsis, rice, and maize (7–13). However, different studies in the same species show low overlap in the lists of imprinted genes; therefore, putative imprinted genes identified from genome-wide surveys must be validated. Differential DNA methylation and histone modifications between maternal and paternal alleles are major regulators of imprinted gene expression (2, 14). Active DNA demethylation by the 5-methylcytosine DNA glycosylase/lyase DEMETER (DME) establishes differential DNA methylation at some imprinted loci (15, 16). DME removes methylated cytosines in the central cell of the female gametophyte, leading to DNA hypomethylation on the maternal alleles in the endosperm, which ultimately results in differential expression of parental alleles in the endosperm (17, 18). De novo methylation through the RNA-directed DNA methylation (RdDM) pathway regulates genomic imprinting at some other loci (19). In addition to DNA methylation, trimethylation of histone H3 on lysine 27 (H3K27me3), catalyzed by the Polycomb Repressive Complex 2 (PRC2), is another important epigenetic mark involved in the regulation of some imprinted genes in the endosperm (20, 21).
Whereas DME is preferentially expressed and functions in the central cell and endosperm, other 5-methylcytosine DNA glycosylases/lyases, including ROS1, DEMETER-like 2 (DML2), and DEMETER-like 3 (DML3), are expressed in nearly all tissues of Arabidopsis (22–25). ROS1 dysfunction causes the transcriptional silencing of some transgenes and endogenous genes (22, 25–27). ROS1-mediated DNA demethylation and gene regulation control diverse physiological functions, including antibacterial defense (28), and the production of stomatal stem cells (29). Indeed, genome-wide DNA methylation analyses revealed that ROS1 protects thousands of genomic regions from DNA hypermethylation (25), suggesting that the cellular processes regulated by ROS1 are not fully appreciated. For instance, although ROS1 is expressed in the endosperm (22), whether it regulates imprinted gene expression is still unknown.
Here, we found that the imprinted expression of DOGL4, a paralogous gene of DOG1 that controls seed dormancy (30), depends on differential methylation of its promoter. We show that the paternal allele is methylated and partially repressed in endosperm. ROS1 is specifically required to protect the paternal allele from excessive DNA methylation and complete silencing, whereas the maternal DOGL4 allele remains unmethylated and expressed upon loss of function of ROS1. Thus, ROS1 is a negative regulator of the imprinting of DOGL4. We discovered that ROS1 regulates seed dormancy and the abscisic acid (ABA) response via regulation of DOGL4. These findings reveal roles for ROS1 in the regulation of imprinted gene expression and seed dormancy.
Results
DOGL4 Is an Imprinted Gene in Arabidopsis Endosperm.
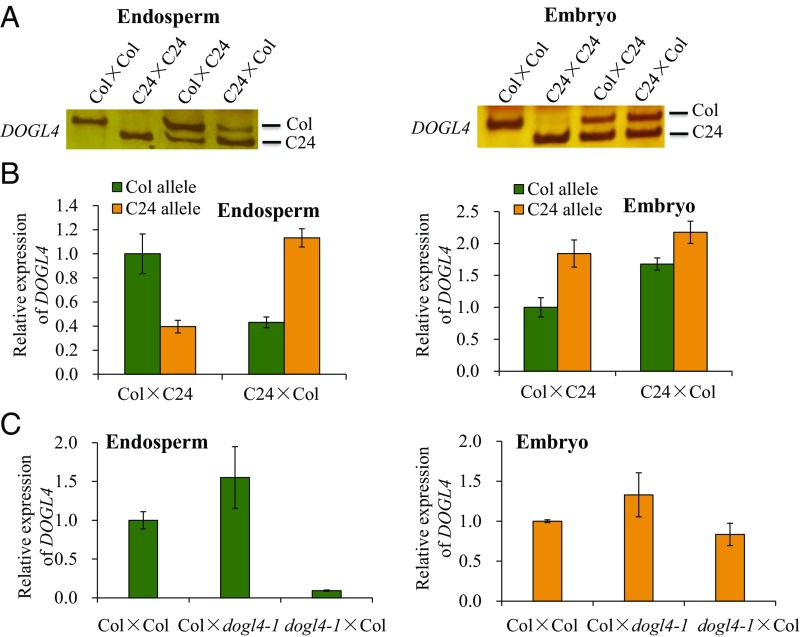
At4g18650 (DOGL4) was previously identified as a putative maternally expressed imprinted gene in the endosperm (9). To confirm the imprinting of DOGL4, we first analyzed reciprocal crosses of Col and C24 accessions. We utilized a G/C polymorphism to discern DOGL4 allele-specific transcripts by RT-PCR-RFLP (Fig. 1A) and qRT-PCR (Fig. 1B). The embryos showed slightly higher levels of the C24 allele, irrespective of the cross (Fig. 1 A and B). Although F1 endosperms derived from reciprocal crosses showed higher expression of the maternal allele compared with the paternal allele, the change was not substantially higher than the expected twofold difference in the absence of imprinting. Therefore, it was unclear if there was imprinting of DOGL4 expression in the endosperm of these crosses. Next, we crossed wild-type Col with a mutant line of DOGL4 (dogl4-1), which has a T-DNA insertion in the intron and does not produce the full gene transcript (SI Appendix, Fig. S1). Consistent with the imprinting of DOGL4 in the endosperm, endosperm showed high DOGL4 expression only when Col WT was the female parent, whereas the embryo showed similar DOGL4 levels in both reciprocal crosses (Fig. 1C). Thus, DOGL4 is imprinted in the endosperm of Col background. The differential expression between the maternal and paternal DOGL4 alleles in endosperm was more obvious within the Col ecotype (Fig. 1C) than in Col/C24 hybrids (Fig. 1A), suggesting that different genetic backgrounds affect the extent of DOGL4 imprinting.
Fig. 1.
Analysis of DOGL4 expression in endosperm and embryo. Endosperms or embryos of 7–9 DAP seeds from various crosses were collected for RNA extraction and expression analysis. UBQ10 was used an internal control. Crosses are indicated as maternal × paternal. (A) Allele-specific RT-PCR of DOGL4 in the endosperm (Left) or embryo (Right) of F1 seeds from reciprocal crosses between Col and C24. (B) Allele-specific qRT-PCR analysis of DOGL4 in the endosperm (Left) or embryo (Right) of F1 seeds from reciprocal crosses between Col and C24. Data are means ± SD of three technical replicates. (C) Quantitative expression analysis of DOGL4 in the endosperm (Left) or embryo (Right) of F1 seeds from reciprocal crosses between Col and dogl4-1 mutant. Data are means ± SD of three technical replicates.
DOGL4 Imprinting Requires DNA Methylation of the Paternal Promoter.
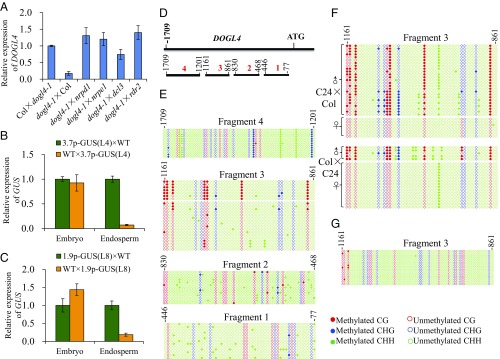
To investigate whether DNA methylation regulates the silencing of the DOGL4 paternal allele in endosperm, we used RdDM-defective mutants as pollen donors in crosses with dogl4-1. We found that the loss of the key RdDM pathway component NRPD1, RDR2, DCL3, or NRPE1 in the male parent released the silencing of paternal DOGL4 in the F1 endosperm (Fig. 2A), suggesting that RdDM-mediated DNA methylation of the paternal allele is required for DOGL4 imprinting in the endosperm.
Fig. 2.
DOGL4 imprinting requires RdDM-mediated methylation of the paternal promoter. (A) Paternal silencing of DOGL4 in the endosperm is dependent on RdDM pathway. RdDM pathway-defective mutants including nrpd1, nrpde1, dcl3, and rdr2 were respectively used as pollen donors to cross with dogl4-1 mutant, and the expression of DOGL4 in these derived endosperms were determined and compared. Data are means ± SD of three technical replicates. (B and C) Expression analysis of GUS reporter gene in embryo and endosperm of F1 seeds from reciprocal crosses between WT and a 3.7-kb DOGL4 promoter-driven GUS transgenic line (L4) (A) and between WT and a transgenic line (L14) with 1.9-kb DOGL4 promoter (B). Data are means ± SD of three technical replicates. (D) Diagram of bisulfite sequencing on four segments covering 1.9-kb promoter of DOGL4. (E) Bisulfite sequencing of the four segments of DOGL4 promoter in 7–9 DAP endosperm of Col wild type and the result showed that DNA methylation of paternal allele in CG sequence context at fragment 3 was increased compared with that of maternal allele. (F) Bisulfite sequencing of fragment 3 in F1 endosperms from reciprocal crosses between Col and C24 ecotypes. (G) Bisulfite sequencing of fragment 3 in F1 endosperm of Col/nrpd1.
To determine if the DOGL4 promoter is sufficient for imprinting in endosperm, we generated transgenic DOGL4 reporter lines in Col that contained 3.7- or 1.9-kb DOGL4 promoter fragments driving β-glucuronidase (GUS) expression (SI Appendix, Fig. S2A). F1 endosperms derived from reciprocal crosses between Col wild-type (WT) and the transgenic lines showed significantly higher GUS expression from the maternal allele than the paternal allele (Fig. 2 B and C and SI Appendix, Fig. S2). In contrast, embryos showed similar expression of GUS from both alleles (Fig. 2 B and C and SI Appendix, Fig. S2). These data indicate that the 1.9-kb DOGL4 promoter is sufficient to confer imprinted expression.
Given that DNA methylation regulates the imprinting of DOGL4, we investigated whether the parental alleles may display differential DNA methylation within the 1.9-kb DOGL4 promoter. We performed bisulfite sequencing of four nonoverlapping promoter fragments in endosperm (Fig. 2D). Fragment 3 (referred to as −1.0-kb region) but not the other fragment methylation profiles revealed two distinct CG methylation patterns, consistent with differential methylation of the alleles (Fig. 2E). To confirm that DNA methylation occurred at the paternal allele, we collected endosperm from reciprocal crosses of Col and C24 and utilized a G/T polymorphism within the −1.0-kb region to distinguish the two alleles. Although there was a bias toward sequenced clones from the Col allele, clones from the maternal and paternal alleles clearly displayed low and high DNA methylation of the −1.0-kb region, respectively (Fig. 2F). Additionally, the higher DNA methylation of the −1.0-kb paternal allele was lost in Col/nrpd1 F1 endosperm (Fig. 2G). Together, these results suggest that paternal DNA methylation at the −1.0-kb region of the DOGL4 promoter is necessary and sufficient for imprinted expression of DOGL4 in endosperm.
Loss of DME and PRC2 Reduces DOGL4 Expression Likely Indirectly Through an Effect on Endosperm Development.
Previous genome-wide analyses had suggested that DME regulates DOGL4 imprinting in the endosperm (9). To clarify the potential role of DME, we examined maternal and paternal DOGL4 expression in dme/C24 F1 endosperm. We found that both alleles were suppressed (SI Appendix, Fig. S3A), consistent with previous data (9). However, reduced DOGL4 expression in dme/C24 endosperm was not associated with substantially increased DNA methylation within the DOGL4 promoter, which even showed reduced CG methylation in fragment 3 of the paternal allele (SI Appendix, Fig. S3B).
To investigate whether the PRC2 complex regulates DOGL4 imprinting, we quantified DOGL4 expression in the endosperm of PRC2-defective mutants, including mea/+, fie/+, msi1/+ and fis2/+. Similar to dme/+, the total expression of DOGL4 was repressed in endosperm from all of the mutants (SI Appendix, Fig. S3C), and CG methylation of fragment 3 was lost from the paternal allele in fie/C24 endosperm (SI Appendix, Fig. S3D).
Given that reduced methylation of DOGL4 correlated with increased expression from the maternal allele in WT plants, it is unlikely that the reduced levels of DOGL4 in dme and PRC2-defective mutants arise from promoter demethylation. PRC2-deficient and dme mutant endosperms display delayed development, and embryos arrest before the heart stage, 4 d after pollination (DAP) (15, 31, 32). We postulated that the delayed development of these mutants may impact DOGL4 expression, independent of the mutant effects on imprinting. We examined DOGL4 levels during the development of WT plants and observed expression in the endosperm starting at 6 DAP and in the embryo by 8 DAP (SI Appendix, Fig. S4 A and B). Similarly, GUS activity in the 3.7-kb promoter pDOGL4:GUS transgenic line was detected in the endosperm by 5–6 DAP and in the embryo by 8–9 DAP (SI Appendix, Fig. S4C). Given that we evaluated the endosperm shortly after DOGL4 expression initiates in the WT, the low expression and altered methylation of DOGL4 in the PRC2-deficient and dme mutants are probably due to the delayed and/or arrested development of endosperm, separate from their defects in imprinting.
ROS1 Negatively Regulates the Imprinting of DOGL4.
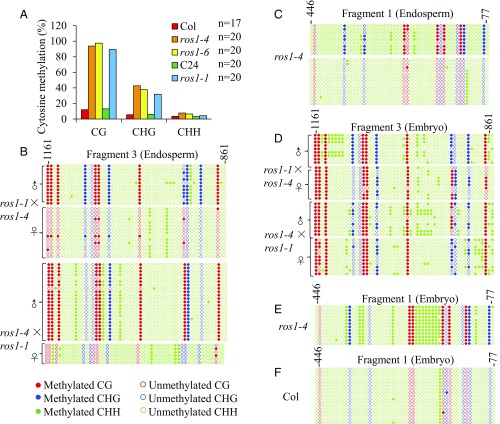
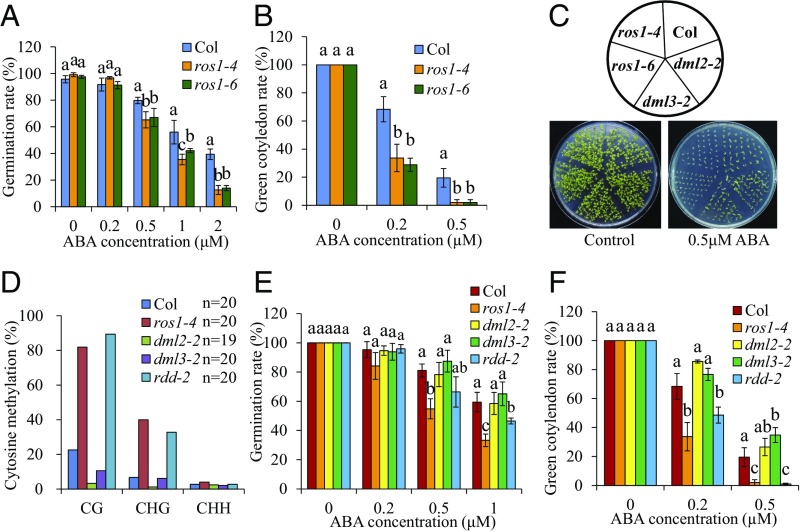
Previous work showed that fragment 1 of the DOGL4 promoter (Fig. 2E, referred to as −0.5-kb region) was hypermethylated in ros1 mutant plants (33). We confirmed increased CG and CHG methylation at the −0.5-kb region in seedlings of both ros1-1 (C24 background) and ros1-4 (Col background) mutants compared with WT controls (SI Appendix, Fig. S5A). Since the −1.0-kb region displayed differential DNA methylation in WT endosperm (Fig. 2 E and F), we investigated whether ROS1 might also regulate DNA methylation of this region. Indeed, the −1.0-kb region displayed increased CG and CHG methylation in seedlings of all ros1 mutants compared with WT controls (Fig. 3A). Thus, ROS1 protects the DOGL4 promoter from hypermethylation in seedlings.
Fig. 3.
Effect of ROS1 mutation on DOGL4 promoter DNA methylation in endosperms and embryos. (A) Bisulfite sequencing of −1.0-kb region of DOGL4 promoter in WT and ros1 mutants. Twelve-day-old seedlings of Col, ros1-4, ros1-6, C24, and ros1-1 were used for DNA isolation and bisulfite sequencing. DNA methylation at CG and CHG contexts was increased in all ros1 mutants. (B–F) DNA methylation analysis of −1.0-kb region (Fragment 3) (B and D) and −0.5-kb region (Fragment 1) (C–E) of DOGL4 promoter. (B and C) DNA methylation analysis in F1 endosperms from reciprocal crosses between ros1-1 and ros1-4 (B) and in ros1-4 endosperms (C). (D–F) DNA methylation analysis in F1 embryos from reciprocal crosses between ros1-1 and ros1-4 (D), ros1-4 embryos (E), and Col embryos (F).
Given that loss of ROS1 led to increased DNA methylation of the −1.0-kb region of the DOGL4 promoter in seedlings, we investigated whether ROS1 may regulate differential DNA methylation of the DOGL4 promoter in endosperm. F1 endosperms from reciprocal crosses between ros1-1 (C24) and ros1-4 (Col) showed increased CG and CHG methylation of the −1.0-kb region only at the paternal allele, but not the maternal allele, compared with WT (compare Fig. 2F with Fig. 3B). Next, we analyzed differential DNA methylation of the −0.5-kb region in ros1 mutants. There are no polymorphisms in the −0.5-kb region between Col and C24 to distinguish the paternal and maternal alleles, therefore we examined ros1-4 mutant endosperm. Our results suggest increased CG and CHG methylation of only one allele, likely the paternal allele, in the −0.5-kb region, as with the −1.0-kb region, in ros1-4 endosperm compared with WT endosperm (Figs. 2E and 3C). Thus, knockout of ROS1 increased the differential DNA methylation at the DOGL4 promoter between parental alleles in endosperm. In contrast, both alleles of the DOGL4 promoter displayed high DNA methylation in the embryos of ros1 mutant (Fig. 3 D and E) but not Col WT (Fig. 3F). Together, these data suggest that ROS1 is required to protect the paternal DOGL4 allele from hypermethylation in endosperm.
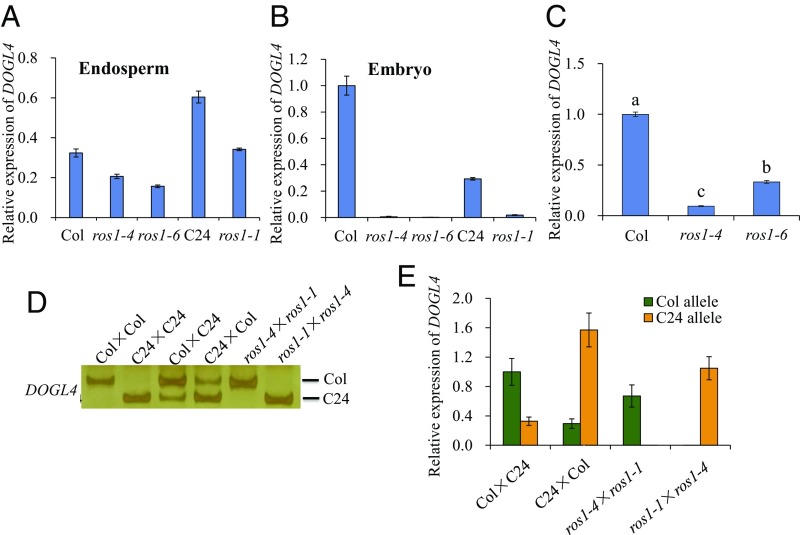
Next, we determined whether ROS1 regulates DOGL4 expression. Relative to WT controls, DOGL4 expression was decreased in ros1 mutant endosperm (Fig. 4A), and even more so in embryos (Fig. 4B) and freshly harvested seeds (Fig. 4C), consistent with DNA methylation levels. We analyzed the expression of maternal and paternal alleles in F1 endosperms from reciprocal crosses between ros1-1 (C24) and ros1-4 (Col) and found that upon knockout of ROS1, DOGL4 expression was reduced from the maternal allele only slightly, whereas paternal DOGL4 expression was completely repressed (Fig. 4 D and E). Therefore, DOGL4 is a maternally expressed, imprinted gene in the ros1 mutant background in ColxC24 crosses (Fig. 4 D and E). Thus, our data suggest that ROS1 negatively regulates gene imprinting by preventing the hypermethylation and complete silencing of the paternal allele.
Fig. 4.
Effect of ROS1 mutation on DOGL4 expression. (A and B) Effect of ROS1 mutation on the expression of DOGL4 in endosperms and embryos. Endosperms (A) or embryos (B) of 7–9 DAP seeds from WT (Col or C24) and ros1 mutants were collected for RNA isolation. The expression of DOGL4 in WT and ros1 mutants was examined and compared. Data are means ± SD of three technical replicates. (C) Effect of ROS1 mutation on the expression of DOGL4 in freshly harvested seeds. Fresh seeds of ros1-4 and ros1-6 and Col wild-type were harvested for RNA extraction and DOGL4 expression analysis. Data are means ± SD of three biological replicates. Means with different letters are significantly different (P < 0.05, Tukey test). (D and E) Allele-specific RT-PCR (D) and qRT-PCR (E) of DOGL4 in the endosperm of F1 seeds from reciprocal crosses between Col and C24 or between ros1-4 and ros1-1.
DOGL4 Regulates Seed Dormancy and ABA Response.
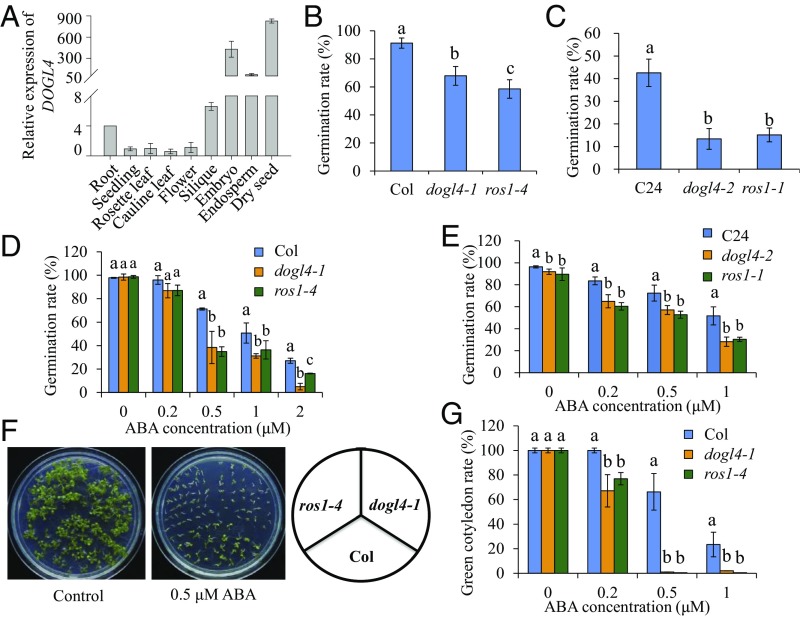
DOGL4 is a member of the plant-specific DOG1 gene family (30). DOG1 is a major regulator of seed dormancy, and knockout of DOG1 renders seeds nondormant. Arabidopsis eFP browser data show that DOGL4 expression is highly enriched in seeds (34). Consistent with these data, our expression analysis also showed that DOGL4 expression in seeds, especially in dry seeds, was much higher than that in other tissues such as roots, leaves, and flowers (Fig. 5A).
Fig. 5.
Mutation of DOGL4 or ROS1 increases seed dormancy and ABA sensitivity. (A) Expression analysis of DOGL4 in various tissues or organs of Col wild-type plants. (B and C) Seed dormancy of wild-type, dogl4, and ros1 mutants. Freshly harvested seeds of Col wild-type, dogl4-1, and ros1-4 (B) or C24 wild-type, dogl4-2, and ros1-1 (C) were sown on water-soaked filter paper for 3 d, and then seed germination based on radicle emergence was scored and compared. (D and E) Germination of mature seeds in response to different concentrations of ABA. Seeds of Col, dogl4-1, and ros1-4 (D) or C24 wild-type, dogl4-2, and ros1-1 (E) were exposed to 0, 0.2, 0.5, 1, or 2 µM ABA for 2 d, and then seed germination rate was calculated and compared. (F and G) Green cotyledon formation of Col, dogl4-1, and ros1-4 in response to ABA. Seeds were grown on an agar plate containing 0, 0.2, 0.5, or 1 µM ABA for 9 d, and then the rate of green cotyledon formation was calculated and compared. Data shown are means ± SD of three biological replicates. Means with different letters are significantly different (P < 0.05, Tukey test).
To investigate whether DOGL4 regulates seed dormancy, we evaluated the germination rates of freshly harvested seeds. We found that the germination rate of dogl4-1 seeds was lower than that of WT (Fig. 5B). To confirm the involvement of DOGL4 in seed dormancy regulation, we used the CRISPR/Cas9 system (35) to generate a mutant allele, dogl4-2, in the highly dormant C24 accession. The dogl4-2 mutant has a frameshift mutation (1-bp insertion at +1154 bp from ATG) in the DOGL4 gene (SI Appendix, Fig. S1). We found that dogl4-2 displayed increased seed dormancy compared with the C24 WT control (Fig. 5C). These data suggest that DOGL4 promotes germination and negatively regulates seed dormancy in WT plants.
The phytohormone ABA is a key factor that induces dormancy (36, 37). We found that DOGL4 expression was induced by ABA treatment in both roots and shoots of Col (SI Appendix, Fig. S5 B and C), suggesting that DOGL4 might attenuate the ABA response. The germination rates of both dogl4-1 and dogl4-2 mutants were less than the WT controls at all ABA concentrations tested (Fig. 5 D and E). To confirm the ABA hypersensitivity phenotype of dogl4-1 mutant, we compared postgermination growth of WT and dogl4-1 mutant on agar media containing different concentrations of ABA. Consistently, dogl4-1 mutants formed green cotyledons at a slower rate than WT at all ABA concentrations (Fig. 5 F and G). We also depleted the expression of DOGL4 in the Col background by RNAi (SI Appendix, Fig. S6A). RNAi lines of DOGL4 were more sensitive to ABA than WT in seed germination and cotyledon greening assays (SI Appendix, Fig. S6 B and C). Together, these results show that DOGL4 negatively regulates seed dormancy and ABA response.
F1 seeds from dogl4×WT should have reduced DOGL4 expression in the endosperm compared with WT×dogl4, but both embryos should have similar DOGL4 expression. We found that F1 seeds of dogl4-2×C24 had stronger seed dormancy than F1 seeds of C24×dogl4-2 (SI Appendix, Fig. S6D), and that F1 seeds of dogl4-1×Col also had higher ABA sensitivity in germination than F1 seeds of Col×dogl4-1 (SI Appendix, Fig. S6E). These results indicate that imprinted DOGL4 expression in the endosperm may contribute to promote seed dormancy and ABA sensitivity.
ROS1 Modulates Seed Dormancy via Regulation of DOGL4 Expression.
As DOGL4 regulates seed dormancy and ABA response (Fig. 5), we hypothesized that ROS1 may also regulate these processes via controlling the expression of DOGL4. We compared seed dormancy phenotypes of ros1-4, dogl4-1, and Col WT. Similar to dogl4-1, ros1-4 showed a lower germination rate than WT (Fig. 5B), indicating that the ros1-4 mutant has increased seed dormancy. We also investigated the seed dormancy phenotype of ros1-1 from the highly dormant C24 accession. Consistently, freshly harvested ros1-1 seeds displayed a lower germination rate than the C24 WT control (Fig. 5C). These results indicate that ROS1 regulates seed dormancy.
Next, we investigated whether ros1 mutants show an altered ABA response. Like dogl4 mutants, the germination rates of ros1-1, ros1-4, and ros1-6 were less than their WT controls at all ABA concentrations tested (Figs. 5 D and E and 6A). Further, ros1 mutants formed green cotyledons at a slower rate than their respective WT controls at all ABA concentrations tested (Figs. 5 F and G and 6 B and C), indicating an increased sensitivity of ros1 mutants to ABA. Loss-of-function of either DML2 or DML3, two paralogues of ROS1, did not elevate the DNA methylation level of DOGL4 promoter (Fig. 6D). The sensitivity to ABA in dml2-2 or dml3-2 mutants was also not altered (Fig. 6 E and F). Furthermore, the triple mutant rdd-2 (ros1-4 dml2-2 dml2-3) showed a similar DNA methylation level of DOGL4 promoter and ABA sensitivity to the ros1-4 single mutant (Fig. 6 D–F). Therefore, of the three ubiquitously expressed DNA demethylases, ROS1 is significantly involved in the regulation of seed dormancy and ABA response.
Fig. 6.
Mutation of ROS1 but not DML2 and DML3 affects ABA sensitivity. (A) Seed germination of Col WT, ros1-4, and ros1-6 under different concentrations of ABA. Seeds were exposed to 0, 0.2, 0.5, 1, or 2 µM ABA for 3 d, and then seed germination rate was calculated and compared. (B) Green cotyledon formation of Col, ros1-4, and ros1-6 in response to ABA. Seeds were exposed to 0, 0.2, or 0.5 µM ABA for 7 d, and then the rate of green cotyledon formation was calculated and compared. (C) Picture of green cotyledon formation of Col, ros1-4, ros1-6, dml2-2, and dml3-2 in response to ABA. Seeds were grown on an agar plate containing 0 or 0.5 µM ABA for 9 d. (D) DNA methylation analysis of −1.0 kb region of DOGL4 promoter. Twelve-day-old seedlings of Col wild-type, ros1-4, dml2-2, dml3-2, and rdd-2 (ros1-4 dml2-2 dml3-2) was sampled for bisulfite sequencing. (E) Seed germination of Col, ros1-4, dml2-2, and dml3-2 under different concentrations of ABA. Seeds were exposed to 0, 0.2, 0.5, and 1 µM ABA for 3 d, and then seed germination rate was calculated and compared. (F) Green cotyledon formation of Col, ros1-4, dml2-2, dml3-2, and rdd-2 under different concentrations of ABA. Seeds were exposed to 0, 0.2, or 0.5 µM ABA for 9 d, and then the rate of green cotyledon formation was calculated and compared. Data shown are means ± SD of three biological replicates. Means with different letters are significantly different (P < 0.05, Tukey test).
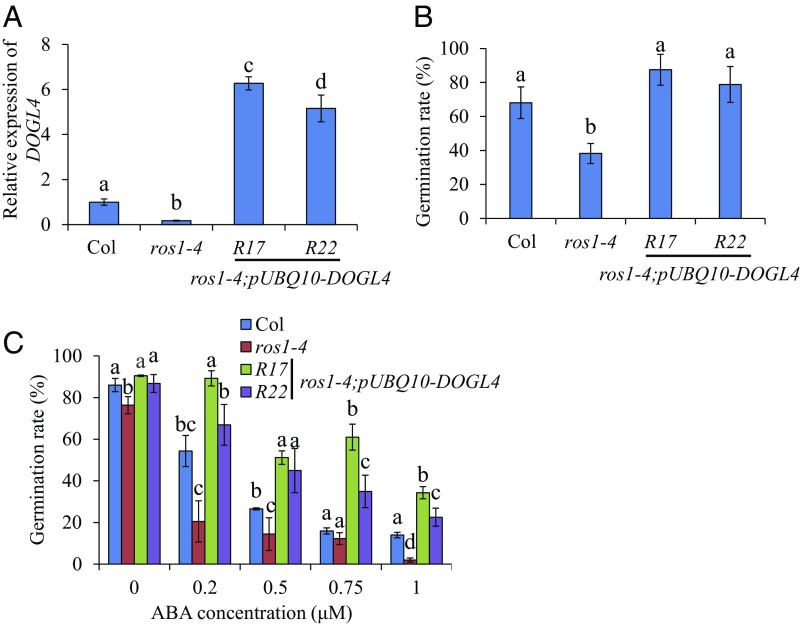
To confirm that the decreased expression of DOGL4 is responsible for the increased seed dormancy and ABA sensitivity in ros1 mutants, we overexpressed DOGL4 in a ros1-4 mutant background (Fig. 7A). Phenotypic analysis in the transgenic lines revealed that overexpression of DOGL4 could rescue the defective seed dormancy and ABA sensitivity phenotypes of ros1-4 (Fig. 7 B and C). Thus, ROS1 negatively regulates seed dormancy and ABA response via DOGL4.
Fig. 7.
Overexpression of DOGL4 rescues the seed dormancy and ABA sensitivity phenotypes of ros1-4 mutant plants. (A) Expression analysis of DOGL4 in freshly harvested seeds of Col, ros1-4, and two independent DOGL4 overexpression lines with ros1-4 mutant background. (B) Seed dormancy of Col, ros1-4, and two DOGL4 overexpression lines with ros1-4 mutant background. Freshly harvested seeds were sown on water-soaked filter paper for 3 d, and then seed germination based on radicle emergence was scored and compared. (C) Seed germination of Col, ros1-4, and two DOGL4 overexpression lines under different concentrations of ABA. Seeds were exposed to 0, 0.2, 0.5, 0.75, or 1 µM ABA for 2 d, and then seed germination rate was calculated and compared. Data shown are means ± SD of three biological replicates. Means with different letters are significantly different (P < 0.05, Tukey test).
Discussion
In this study, we validated DOGL4 as a maternally imprinted gene in endosperm. Our results revealed that DOGL4 imprinting depends on DNA methylation of the paternal allele in the −1.0-kb region of DOGL4 promoter. The increased DOGL4 promoter DNA methylation and reduced DOGL4 expression in the paternal allele were suppressed in F1 endosperms when RdDM-pathway defective mutants were used as male parents, suggesting that differential DNA methylation might be established before fertilization. In plants, DME and PRC2 are the key regulators of imprinted gene expression in the endosperm (2, 14). PRC2-deficient and dme mutant plants displayed defective endosperm development; this developmental defect likely contributes to the low expression of DOGL4 in these mutants since DOGL4 is highly expressed only later during endosperm development. Intriguingly, the paternally derived DNA methylation in the −1.0-kb DOGL4 promoter in WT endosperms was lost in dme/C24 and fie/C24 endosperms (SI Appendix, Fig. S3). The underlying mechanism of this effect on paternal DOGL4 methylation remains to be elucidated.
Among the family of four 5-methylcytosine DNA glycosylases/lyases, we found that only ROS1 negatively regulates DNA methylation of the DOGL4 promoter. We found that multiple regions within the DOGL4 promoter were hypermethylated on the paternal allele in ros1 endosperms, which might underlie the full suppression of paternal DOGL4 expression. By contrast, mutation of ROS1 did not affect DOGL4 promoter DNA methylation and gene expression from the maternal allele in the endosperm. Consequently, the differential expression between parental alleles was increased in ros1 mutants. Our results thus revealed a role of ROS1 in mitigating imprinting effects by demethylating the otherwise highly methylated and suppressed paternal allele. Further, our findings suggest that ROS1 and the RdDM pathway antagonize each other to control the DNA methylation of DOGL4 promoter of the paternal allele in the endosperm and, in turn, DOGL4 imprinting.
Whether ROS1 regulates imprinted gene expression more broadly remains to be determined. Since mutation of ROS1 did not influence DNA methylation of the DOGL4 maternal allele in the endosperm, we speculate that ROS1 regulates DOGL4 promoter methylation of the paternal allele in pollen before fertilization. The maternal allele in the endosperm is derived from central cells where DME is preferentially expressed and the CG maintenance methyltransferase MET1 is repressed (15, 38, 39). We found that DME does not regulate maternal DOGL4 promoter DNA methylation; however, MET1 repression might contribute to the relatively low DNA methylation of the maternal DOGL4 allele in both WT and ros1 endosperms. Further work is required to test this hypothesis.
DOGL4 is a member of the plant-specific DOG1 gene family, which regulates seed dormancy. Mutation of DOG1 renders seeds nondormant (30). We found that DOGL4 also controls seed dormancy but, in contrast to DOG1 mutations, mutations of DOGL4 enhanced seed dormancy (Fig. 5). Like DOG1, DOGL4 is highly and preferentially expressed in seeds (Fig. 5A). dogl4 mutants did not show pleiotropic phenotypes, which suggests that DOGL4 is specifically involved in the regulation of seed dormancy. We found that DOGL4 expression in the endosperm may contribute to regulation of seed dormancy and ABA response (SI Appendix, Fig. S6 D and E). These observations support a recent view that expression of maternally imprinted genes in the endosperm plays important roles in modulating seed dormancy (40). DOG1 regulates seed germination through inhibiting the expression of gibberellin-regulated genes encoding cell-wall remodeling proteins (41). Whether DOGL4 has an opposite role in regulating the expression of these genes, and whether DOGL4 antagonizes DOG1 to regulate seed dormancy, remain to be investigated in the future.
ROS1 positively regulates DOGL4 expression through demethylation of the DOGL4 promoter region. We found that seed dormancy and ABA sensitivity were increased in ros1 mutants compared with their WT controls, phenocopying dogl4 mutants. Furthermore, overexpression of DOGL4 in a ros1 mutant background suppressed the enhanced seed dormancy and ABA hypersensitivity phenotypes of ros1 mutant plants. These results indicate that the higher seed dormancy and ABA sensitivity germinating seeds in ros1 mutants are at least partly caused by reduced expression of DOGL4. In summary, we have discovered a role for ROS1 in mitigating the paternal-specific silencing of DOGL4 in Arabidopsis endosperm and found that ROS1 negatively regulates seed dormancy and ABA sensitivity by promoting the expression of DOGL4 (SI Appendix, Fig. S7).
Materials and Methods
Plant Materials and Growth Conditions.
Arabidopsis thaliana ecotypes Columbia-0 (Col-0) and C24 were obtained from the Arabidopsis Stock Centre (https://www.arabidopsis.org). The mutants were either ordered from ABRC or generated in our laboratory (SI Appendix, Table S1). Plants were grown in a growth room with 16 h of light and 8 h of darkness at 20–24 °C.
RNA and DNA Isolation from Dissected Seed Embryo and Endosperm.
Seeds of 7–9 d after pollination were dissected with a fine forceps on a slide under a dissecting microscope, and RNA or DNA was then isolated from the dissected seed embryo and endosperm. See SI Appendix, SI Materials and Methods.
RT-PCR and Quantitative PCR.
Various tissues of control or treated plants were collected for RNA isolation and RT-PCR analysis. See SI Appendix, SI Materials and Methods.
Allele-Specific Expression Analysis of DOGL4.
A dCAPS marker and allele-specific primers were designed based on a G/C polymorphism between Col and C24 at DOGL4 and were used for allele-specific expression analysis of DOGL4. See SI Appendix, SI Materials and Methods.
DNA Methylation Analysis.
Extracted DNA was sodium-bisulfite converted and purified and then amplified using two rounds of nested primers. The PCR products were cloned and sequenced, and DNA methylation was analyzed using Kismeth tool. See SI Appendix, SI Materials and Methods.
Seed Dormancy and ABA Response.
Radicle emergence of freshly harvested seeds was scored for seed dormancy determination. For ABA response tests, after-ripened seeds were grown on plates containing different concentrations of ABA, and radicle emergence and green cotyledon formation were recorded for seed germination rate and postgermination growth, respectively. See SI Appendix, SI Materials and Methods.
GUS Analysis.
Vector construct and GUS analysis for pDOGL4:GUS transgenic lines were described in detail in SI Appendix, SI Materials and Methods.
Vector Constructs for DOGL4 and Phenotypic Analysis of Transgenic Lines.
Vector constructs and phenotypic analysis for DOGL4 RNAi, knockout, and overexpression transgenic lines were described in detail in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Life Science Editors for editorial assistance. This work was supported by the Shanghai Center for Plant Stress Biology, Jiangsu Science Fund for Distinguished Young Scholars Grant BK20150027, Chinese Academy of Sciences, and Strategic Priority Research Program Grant XDB27040000 of the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812847115/-/DCSupplemental.
References
- 1.Reik W, Walter J. Genomic imprinting: Parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 2.Köhler C, Wolff P, Spillane C. Epigenetic mechanisms underlying genomic imprinting in plants. Annu Rev Plant Biol. 2012;63:331–352. doi: 10.1146/annurev-arplant-042811-105514. [DOI] [PubMed] [Google Scholar]
- 3.Feil R, Berger F. Convergent evolution of genomic imprinting in plants and mammals. Trends Genet. 2007;23:192–199. doi: 10.1016/j.tig.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MJ, Fischer RL. Genome demethylation and imprinting in the endosperm. Curr Opin Plant Biol. 2011;14:162–167. doi: 10.1016/j.pbi.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger F. Endosperm: The crossroad of seed development. Curr Opin Plant Biol. 2003;6:42–50. doi: 10.1016/s1369526602000043. [DOI] [PubMed] [Google Scholar]
- 6.Haig D. Kin conflict in seed development: An interdependent but fractious collective. Annu Rev Cell Dev Biol. 2013;29:189–211. doi: 10.1146/annurev-cellbio-101512-122324. [DOI] [PubMed] [Google Scholar]
- 7.Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo M, et al. A genome-wide survey of imprinted genes in rice seeds reveals imprinting primarily occurs in the endosperm. PLoS Genet. 2011;7:e1002125. doi: 10.1371/journal.pgen.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh TF, et al. Regulation of imprinted gene expression in Arabidopsis endosperm. Proc Natl Acad Sci USA. 2011;108:1755–1762. doi: 10.1073/pnas.1019273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeown PC, et al. Identification of imprinted genes subject to parent-of-origin specific expression in Arabidopsis thaliana seeds. BMC Plant Biol. 2011;11:113. doi: 10.1186/1471-2229-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters AJ, et al. Parent-of-origin effects on gene expression and DNA methylation in the maize endosperm. Plant Cell. 2011;23:4221–4233. doi: 10.1105/tpc.111.092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff P, et al. High-resolution analysis of parent-of-origin allelic expression in the Arabidopsis endosperm. PLoS Genet. 2011;7:e1002126. doi: 10.1371/journal.pgen.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, et al. Extensive, clustered parental imprinting of protein-coding and noncoding RNAs in developing maize endosperm. Proc Natl Acad Sci USA. 2011;108:20042–20047. doi: 10.1073/pnas.1112186108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehring M. Genomic imprinting: Insights from plants. Annu Rev Genet. 2013;47:187–208. doi: 10.1146/annurev-genet-110711-155527. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y, et al. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in arabidopsis. Cell. 2002;110:33–42. doi: 10.1016/s0092-8674(02)00807-3. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh TF, et al. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehring M, et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita T, et al. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science. 2004;303:521–523. doi: 10.1126/science.1089835. [DOI] [PubMed] [Google Scholar]
- 19.Vu TM, et al. RNA-directed DNA methylation regulates parental genomic imprinting at several loci in Arabidopsis. Development. 2013;140:2953–2960. doi: 10.1242/dev.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuettengruber B, Cavalli G. Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development. 2009;136:3531–3542. doi: 10.1242/dev.033902. [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, Köhler C. Evolution, function, and regulation of genomic imprinting in plant seed development. J Exp Bot. 2012;63:4713–4722. doi: 10.1093/jxb/ers145. [DOI] [PubMed] [Google Scholar]
- 22.Gong Z, et al. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111:803–814. doi: 10.1016/s0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 23.Agius F, Kapoor A, Zhu JK. Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc Natl Acad Sci USA. 2006;103:11796–11801. doi: 10.1073/pnas.0603563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lister R, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian W, et al. A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science. 2012;336:1445–1448. doi: 10.1126/science.1219416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Kapoor A, Sridhar VV, Agius F, Zhu JK. The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Curr Biol. 2007;17:54–59. doi: 10.1016/j.cub.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 27.Lei M, et al. Regulatory link between DNA methylation and active demethylation in Arabidopsis. Proc Natl Acad Sci USA. 2015;112:3553–3557. doi: 10.1073/pnas.1502279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu A, et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc Natl Acad Sci USA. 2013;110:2389–2394. doi: 10.1073/pnas.1211757110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamuro C, et al. Overproduction of stomatal lineage cells in Arabidopsis mutants defective in active DNA demethylation. Nat Commun. 2014;5:4062. doi: 10.1038/ncomms5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentsink L, Jowett J, Hanhart CJ, Koornneef M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:17042–17047. doi: 10.1073/pnas.0607877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiyosue T, et al. Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:4186–4191. doi: 10.1073/pnas.96.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köhler C, et al. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J. 2003;22:4804–4814. doi: 10.1093/emboj/cdg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CF, et al. A Pre-mRNA-splicing factor is required for RNA-directed DNA methylation in Arabidopsis. PLoS Genet. 2013;9:e1003779. doi: 10.1371/journal.pgen.1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter D, et al. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Z, et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013;23:1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- 37.Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 38.Jullien PE, et al. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008;6:e194. doi: 10.1371/journal.pbio.0060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jullien PE, Susaki D, Yelagandula R, Higashiyama T, Berger F. DNA methylation dynamics during sexual reproduction in Arabidopsis thaliana. Curr Biol. 2012;22:1825–1830. doi: 10.1016/j.cub.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 40.Piskurewicz U, et al. Dormancy-specific imprinting underlies maternal inheritance of seed dormancy in Arabidopsis thaliana. eLife. 2016;5:e19573. doi: 10.7554/eLife.19573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graeber K, et al. DELAY OF GERMINATION 1 mediates a conserved coat-dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proc Natl Acad Sci USA. 2014;111:E3571–E3580. doi: 10.1073/pnas.1403851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.