Abstract
Increased expression of adaptor protein p66Shc has been associated with progression of diabetic nephropathy. Afferent arteriolar dilation and glomerular hyperfiltration in diabetes are due to increased KATP channel availability and activity. Hyperglycemia was induced in Dahl salt-sensitive (SS) rats in a model of diabetes induced by streptozotocin (STZ). Renal injury was evaluated in SS rats and genetically modified SS rats either lacking p66Shc (p66Shc knockout [p66ShcKO]) or expressing p66Shc mutant (p66Shc-S36A). Afferent arteriolar diameter responses during STZ-induced hyperfiltration were determined by using the juxtamedullary nephron technique. Albuminuria and glomerular injury were mitigated in p66ShcKO and p66Shc-S36A rats with STZ-induced diabetes. SS rats with STZ-induced diabetes had significantly increased afferent arteriolar diameter, whereas p66ShcKO and p66Shc-S36A rats did not. SS rats with STZ-induced diabetes, but not p66ShcKO or p66Shc-S36A rats with STZ-induced diabetes, had an increased vasodilator response to the KATP channel activator pinacidil. Likewise, the KATP inhibitor glibenclamide resulted in a greater decrease in afferent arteriolar diameter in SS rats with STZ-induced diabetes than in STZ-treated SS p66ShcKO and p66Shc-S36A rats. Using patch-clamp electrophysiology, we demonstrated that p66ShcKO decreases KATP channel activity. These results indicate that inactivation of the adaptor protein p66Shc decreases afferent arteriolar KATP channel activity and decreases renal damage in diabetic SS rats.
Introduction
Patients with poorly controlled diabetes sustain damage to the macrovasculature and microvasculature; this damage is responsible for much of the morbidity and mortality associated with the disease. The risk for myocardial infarction, stroke, and amputation is two to four times greater in patients with diabetes than in patients with normal blood glucose levels (1,2). Risk of blindness among patients with diabetes is 8–16 times greater than that in those without diabetes (3), whereas the occurrence of end-stage renal disease is 10-fold higher (4).
The mechanism by which diabetes damages small and large blood vessels has been investigated intensively. Dysregulation of glomerular blood flow in the kidney has been implicated as one factor in the pathogenesis of diabetic glomerulosclerosis (5). Micropuncture studies found higher glomerular capillary pressures and flow in diabetic animals (6). Alterations in afferent arteriolar resistance primarily drive hyperfiltration in type 1 diabetes (7), and ATP-sensitive potassium (KATP) channels likely regulate that resistance (8). What causes these changes in microvascular function are unresolved at a molecular level.
We recently found that p66Shc, one of three isoforms of SRC homology 2 domain–containing adaptor protein (Shc1), regulates renal vascular tone in a hypertensive model of glomerular disease, the Dahl salt-sensitive (SS) rat (9). We observed increased p66Shc expression in smooth muscle cells (SMC) of renal resistance vessels and showed that the preglomerular afferent renal arterioles have defective autoregulatory vasoconstrictive responses that were restored in rats with deficient p66Shc (p66Shc knockout [p66ShcKO]). We also previously showed that the vasoactive peptide endothelin-1 induces Ser36 phosphorylation of p66Shc, which is required for mitochondrial translocation of cytosolic p66Shc (10). Pro-oxidant properties of p66Shc are usually associated with its mitochondrial location (11,12). In this study we extend observations of hypertensive SS rats to SS rats rendered diabetic through the use of streptozotocin (STZ). In addition to p66ShcKO, we also generated SS rats in which Ser36 in p66Shc is substituted with Ala (p66Shc-S36A), a change that is expected to prevent p66Shc translocation to mitochondria. These models (p66ShcKO and p66Shc-S36A) allow us to assess the role of p66Shc in diabetic nephropathy (DN). In particular, we examined the role of p66Shc in regulating afferent arteriolar dilation through potential regulation of the KATP channel.
Research Design and Methods
Animals and Induction of Diabetes
All studies were performed on rats generated and maintained on the SS/JrHsdMcwi (SS) genetic background. p66ShcKO and p66Shc-S36A rats have been described previously (9). Male SS and p66Shc mutant rats (9–11 weeks old) were given a single injection of STZ (50 mg/kg i.p.), without insulin treatment, as described previously (13). One week after injection, diabetes was confirmed on the basis of nonfasting blood glucose measured by using a blood glucose meter and test strips (Contour; Bayer HealthCare). Only animals in which hyperglycemia exceeded 300 mg/dL throughout the 12-week induction period were used for analysis. All procedures were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
Measurement of Urinary Albumin Excretion
Rats were housed individually in metabolic cages so urine could be collected overnight every 3 weeks after STZ injection. Water and food were provided ad libitum. Urine was collected after 17–24 h and the volume quantified before being stored at −80°C until used in assays. Urinary albumin was quantified with Albumin Blue 580 dye (Molecular Probes) by using an FL600 Fluorescence Plate Reader (Bio-Tek) and normalized to a 24-h collection period.
Analysis of Glomerular Injury
After STZ treatment, kidneys were processed for periodic acid Schiff (PAS) staining and glomerular injury was scored as previously described (9). The semiquantitative glomerular score of each animal was determined by two investigators (B.S.M. and S.R.B.) blinded to animal genotype and treatment and calculated as the mean of scores from 50 glomeruli per rat.
Mesangial Index Quantification
Mesangial index was determined as the percent area of the glomerular tuft that was positively stained with PAS as described by the Diabetic Complications Consortium (http://www.diacomp.org), with the following modifications: A total of 20 random glomeruli per animal were imaged (original magnification ×40) and analyzed by using a Hamamatsu slide scanner. Images were split into channels to separate PAS pixel data from nuclei through the use of a batch process applying a custom matrix, which was derived from a control slide by using the Color Deconvolution plugin found in the Fiji distribution of ImageJ software (14). Glomerular tuft area (including the capillary lumen) was defined by manual selection, then the PAS channel was segmented manually; we used the global Rényi entropy threshold method to account for stain variability specific to the mesangial matrix.
Afferent Arteriolar Diameter Response
Afferent arteriolar diameter responses were determined 6 weeks after the induction of diabetes by STZ, as described previously (9). Wild-type SS rats, SS p66ShcKO rats, and SS p66Shc-S36A rats with and without STZ-induced diabetes were analyzed for afferent arteriolar contraction response to the KATP channel activator pinacidil (30–300 μmol/L) or the KATP channel blocker glibenclamide (30–300 μmol/L) (15,16).
Patch-Clamp Electrophysiology
Patch-clamp electrophysiology was used to assess the activity of KATP channels in isolated renal vascular SMC. SMC were isolated and cultured in high-glucose (25 mmol/L) DMEM supplemented with 10% FBS, as previously described (9). Passages 15–17 were used for analysis. Single-channel data were acquired and subsequently analyzed with an Axopatch 200B amplifier (Molecular Devices) interfaced via a Digidata 1440A digitizer to a personal computer running pCLAMP software version 10.2 (Molecular Devices). Signals were filtered at 1 kHz with an eight-pole, low-pass Bessel filter (LPF-8; Warner Instruments, Hamden, CT). The single-channel activity of KATP channels in vascular SMC was determined in cell-attached patches made under voltage-clamp conditions. The pipette was pulled with a horizontal Sutter P-97 pipette puller (Sutter Instruments, Novato, CA). The resistance of the pipette in the corresponding bath medium was 7–12 MΩ. Bath solution comprised 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 10 mmol/L HEPES, 2 mmol/L MgCl2, and 5 mmol/L glucose (pH 7.35). Pipette solution contained 140 mmol/L KCl, 10 mmol/L EDTA, and 10 mmol/L HEPES (pH 7.35). The channel events were analyzed by using the Clampfit module in pCLAMP software version 10.2 (a single-channel search in analyze mode). NPo, the product of the number of the channels, was used to measure the channel activity within a patch. The channel open probabilities (NPo) were determined by using the equation NPo = I/i, where I represents the mean channel current (estimated from the time integrals of the currents above the baseline), and i represents the unitary current amplitude (determined from current traces and all-point amplitude histograms).
Statistical Analysis and Data Presentation
Pairwise comparisons between groups over time were performed using two-factor ANOVA for repeated measures with the Holm-Sidak post hoc test (SigmaPlot 11.2.0). Linear least square regression was utilized for unequal sample sizes. Data are reported as the mean ± SEM. Box-and-whisker plot data represent the minimum, first quartile, median, mean, third quartile, and maximum.
Results
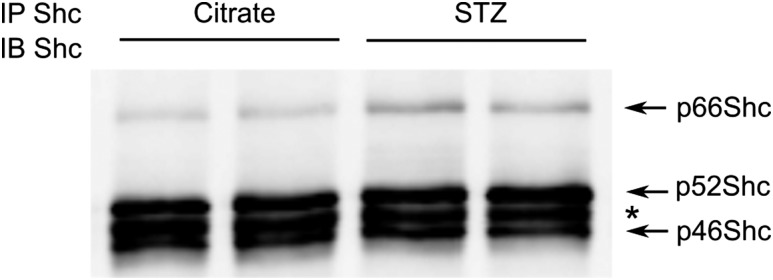
SS rats fed a high-salt diet develop hyperlipidemia and insulin resistance (17). Intravenous glucose tolerance tests established no difference between measured plasma glucose and insulin levels of SS and Sprague-Dawley rats, indicating that SS rats do not have metabolic syndrome (Supplementary Fig. 1). Upon induction of diabetes with STZ injection, we observed an increase in renal p66Shc expression (Fig. 1), a result in agreement with what occurs in human cases of DN (18), suggesting the involvement of p66Shc in the progression of renal DN.
Figure 1.
Renal p66Shc expression is increased in STZ-treated rats. Western blotting reveals an increase of p66Shc expression in STZ-treated rats compared with control (citrate-treated) rats. Renal cortex tissues were subjected to immunoprecipitation (IP) and immunoblotting (IB) with anti-Shc antibodies, which recognize all three Shc isoforms. Each lane corresponds to a separate animal. *Heavy chain (∼50 kDa) of antibody used for IP.
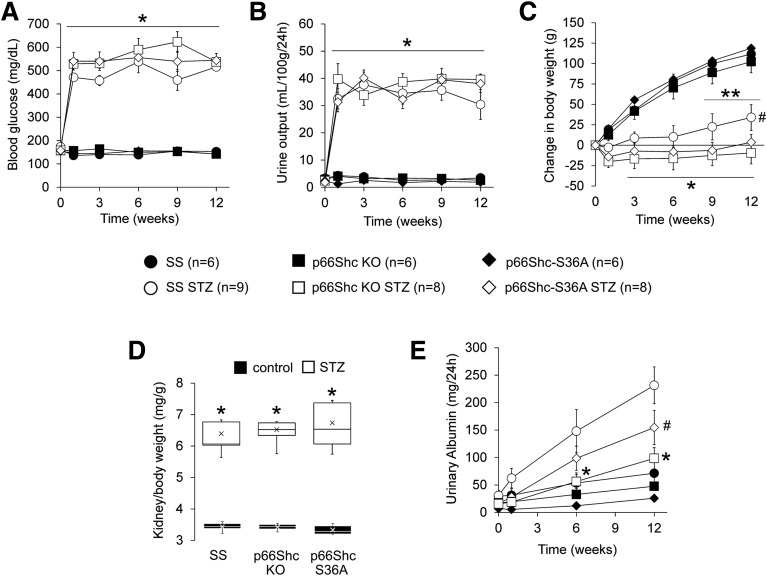
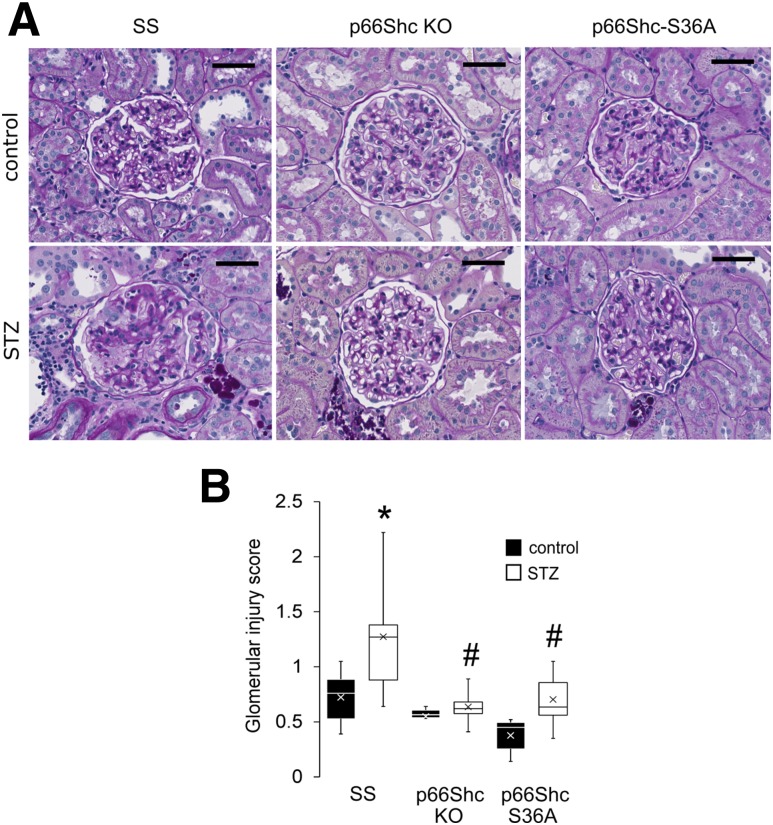
We observed that p66ShcKO did not prevent development of diabetes induced by STZ injection but did prevent the onset of renal damage in our genetically modified SS rats (Fig. 2). Compared with controls, hyperglycemia (Fig. 2A), increased urination (Fig. 2B), and reduced weight gain (Fig. 2C) were observed to the same extent in STZ-treated SS, p66ShcKO, and p66Shc-S36A rats, indicating that loss or modification of p66Shc does not interfere with the establishment of diabetes. Furthermore, we found no significant differences in end-stage renal hypertrophy as measured by kidney weight–to–body weight ratio (Fig. 2D) between STZ-treated groups. In contrast, STZ-treated p66ShcKO and p66Shc-S36A rats exhibited reduced albuminuria 6 and 12 weeks after diabetes induction, respectively, compared with STZ-treated SS rats 6 and 12 weeks after diabetes induction (Fig. 2E). Glomeruli of all nondiabetic control rats displayed mild to moderate levels of mesangial expansion with limited sclerosis (Fig. 3A), features common to Dahl SS rats fed a low-salt diet. A semiquantitative assessment of PAS staining determined that STZ-treated SS rats had increased glomerular injury, whereas no significant glomerular injury was found in diabetic p66ShcKO and p66Shc-S36A rats (Fig. 3B). A significant increase in the percentage of glomeruli undergoing sclerosis in STZ-treated SS rats (38.1 ± 6.3% [n = 9] vs. 16.7 ± 3.4% in SS control rats [n = 6]) was absent in either STZ-treated p66ShcKO rats (11.9 ± 1.6% [n = 8] vs. 11.3 ± 2.0% in the control rats [n = 6]) or STZ-treated p66Shc-S36A rats (16.0 ± 3.1% [n = 8] vs. 7.0 ± 1.6% in the control rats [n = 6]). There was no significant difference in the percentage of glomeruli with mesangial matrix expansion among strains or STZ treatment groups, as determined by semiquantitative scoring or by mesangial index determined on the basis of digital image analysis (data not shown). These data suggest that the mitochondrial action of p66Shc plays a role in the renal damage observed in rats with STZ-induced DN.
Figure 2.
Knockout of p66Shc inhibits progression of DN. Type 1 diabetes was induced in control and genetically modified male rats through STZ injection and evaluated for 12 weeks after treatment. A and B: Hyperglycemia (A) and polyuria (B) indicate that diabetes was induced after 1 week and was maintained throughout the protocol. *P < 0.001 vs. controls. C: Induction of diabetes significantly reduced weight gain in all rats. STZ-treated SS rats (SS STZ) gained weight by week 9. *P < 0.001 vs. controls at week 1; **P < 0.01 vs. SS STZ at week 1; #P < 0.05 vs. STZ-treated p66ShcKO rats (p66Shc KO STZ) at week 12. D: Kidney weight–to–body weight ratio increased in STZ-treated rats but did not differ between genotypes. Within the boxes, the × symbols represent the mean values and the horizontal lines represent the medians. *P < 0.001. E: Albuminuria significantly increased by week 6 in SS STZ (P < 0.001), whereas p66ShcKO and p66Shc-S36A rats had significantly reduced albuminuria at 6 and 12 weeks, respectively, when compared with SS STZ. *P < 0.01; #P < 0.05. p66Shc-S36A STZ, STZ-treated p66Shc-S36A rats.
Figure 3.
p66ShcKO reduces diabetes-induced glomerular injury. A: Representative images of PAS-stained kidneys comparing glomeruli of STZ-treated animals to those in control animals. Scale bars = 50 µm (original magnification ×40). Glomeruli of STZ-treated SS rats exhibit increased sclerosis compared to STZ-treated p66ShcKO and p66Shc-S36A rats. B: Quantification of glomerular injury. At least six rats per group and 50 glomeruli per rat were scored. Within the boxes, the × symbols represent the mean values and the horizontal lines represent the medians. *P < 0.001 vs. SS rats; #P < 0.001 vs. STZ-treated SS rats (two-factor ANOVA with Bonferroni correction, α = 0.01).
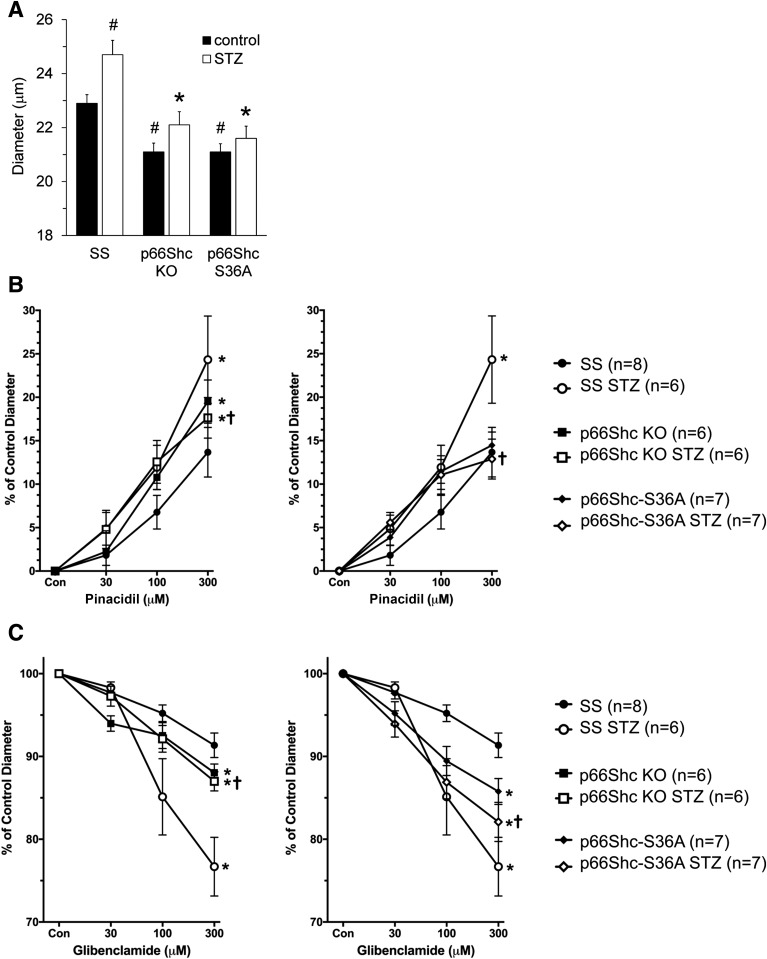
Because we found that p66ShcKO restores microvascular responses in isolated perfused afferent arterioles from SS rats with hypertension-induced nephropathy (9), we tested the role of p66Shc in renal vascular dysfunction in rats with STZ-induced DN. Afferent arteriolar diameters of control rats at renal perfusion pressure 100 mmHg averaged 22.9 ± 0.9 μm in SS rats (n = 8), 21.1 ± 0.8 μm in control p66Shc-S36A rats (n = 7), and 21.1 ± 0.8 μm in p66ShcKO rats (n = 6). SS rats with STZ-induced diabetes had significantly increased afferent arteriolar diameter (24.7 ± 1.3 μm [n = 6]; P < 0.05), whereas p66ShcKO and p66Shc-S36A rats with STZ-induced diabetes did not (22.1 ± 1.2 μm [n = 6] and 21.6 ± 1.2 μm [n = 7], respectively) (Fig. 4A). Afferent arteriolar dilation and glomerular hyperfiltration in diabetes is due to increased KATP channel availability and activity (19). Afferent arteriolar dilator responses to the pharmacological vasodilator pinacidil, which targets KATP channels, were not different between controls of SS rats, p66ShcKO rats, or p66Shc-S36A rats. However, SS rats with STZ-induced diabetes, but not p66ShcKO or p66Shc-S36A rats, had an increased vasodilator response to pinacidil (Fig. 4B), suggesting that p66Shc increases afferent arteriolar KATP channel activity in diabetes. Likewise, the KATP inhibitor glibenclamide resulted in a greater decrease in afferent arteriolar diameter in SS rats with STZ-induced diabetes (23 ± 4% [n = 6]) than that which occurred in p66ShcKO rats with STZ-induced diabetes (13 ± 2% [n = 6]) (Fig. 4C), suggesting that diabetic SS rats have increased KATP channel activity. Nitroprusside, whose vasodilating effects are based on the release of nitric oxide, had similar vasodilator responses in the SS, p66Shc-S36A, and p66ShcKO groups (Supplementary Fig. 2), indicating that targeted editing of the Shc1 gene does not affect the ability of vessels to dilate.
Figure 4.
p66ShcKO restored renal microvascular responses in diabetic rats. A: Comparison of afferent arteriole diameters in SS, p66ShcKO, and p66Shc-S36A rats before and after induction of STZ-induced DN. #P < 0.05 vs. SS rats; *P < 0.01 vs. STZ-treated SS rats. B: Effect of pinacidil. Pinacidil resulted in a greater increase in afferent arteriolar diameter in SS rats with STZ-induced DN than in p66ShcKO rats with STZ-induced diabetes and p66Shc-S36A rats with STZ-induced diabetes. Values in untreated SS rats, p66ShcKO rats, and p66Shc-S36A rats are also shown. C: Effect of glibenclamide. Glibenclamide resulted in a greater decrease in afferent arteriolar diameter in SS rats with STZ-induced DN than in p66ShcKO rats with STZ-induced diabetes and p66Shc-S36A rats with STZ-induced diabetes. Values in untreated SS rats, p66ShcKO rats, and p66Shc-S36A rats are also shown. For panels B and C, *P < 0.01 vs. SS rats; †P < 0.05 vs. STZ-treated SS rats.
The difference in afferent arteriole diameters between SS rats and SS rats with STZ-induced DN is likely due to increased KATP activity (P < 0.05) (Fig. 4A). The difference in afferent arteriole diameter between untreated SS rats and p66ShcKO rats is due to the increased vascular tone of afferent arterioles in the absence of p66Shc (P < 0.05) (Fig. 4A). Finally, the differences in the diameter of afferent arterioles between SS rats treated with STZ and p66ShcKO rats treated with STZ is likely to be the consequence of increased vascular tone and decreased KATP influence (P < 0.01).
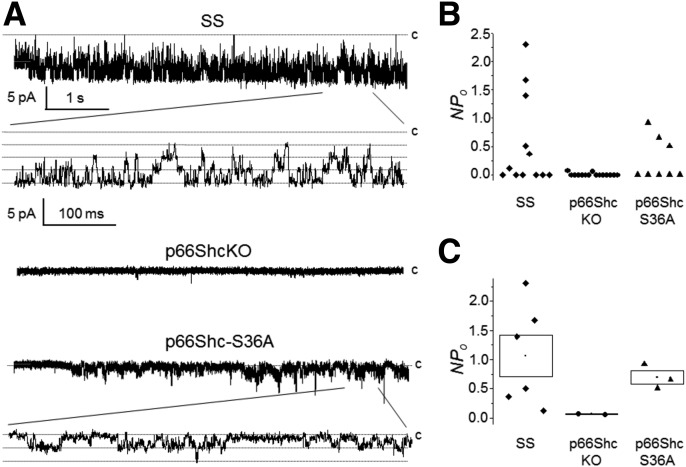
To assess the possible functional implications of the inactivation of p66Shc in control KATP channels, we assessed endogenous channel activity in renal SMC isolated from wild-type (SS), p66ShcKO, and p66Shc-S36A mutant rats. The basal level of activity of KATP channels was measured through a cell-attached configuration. Representative current trace recordings are displayed in Fig. 5B. Channel activity was observed in 6 of 12, 2 of 15, and 3 of 8 experiments for SS, p66ShcKO, and p66Shc-S36A SMC, respectively (Fig. 5B; each point represents an individual experiment). Figure 5C summarizes NPo for those experiments where KATP channels were active. As shown in this figure, inactivation of p66Shc results in downregulation of the frequency of observed channels and the activity of KATP channels. The two recordings from SMC isolated from p66ShcKO rats in which channel activity was detected demonstrated very low NPo. KATP activity was also attenuated in p66Shc-S36A SMC, albeit to a lesser extent.
Figure 5.
Knockout of p66Shc inhibits KATP channels. A: Representative cell-attached patch-clamp recording of vascular SMC isolated from wild-type, p66ShcKO, and p66Shc-S36A mutant rats. Membrane potential (Vp) = 0 mV. Scales are the same for all three recordings. Activity is also shown at an expanded scale. The closed state (c) is upward. B: Scatter plot demonstrate individual channel activity (NPo) for KATP channels recorded in cell-attached patches from SS, p66ShcKO, and p66Shc-S36A SMC cells at a 0 mV holding potential. C: NPo for experiments with observed channel activity (shown in B). Data are the mean ± SE.
Discussion
p66Shc plays a significant role in the progression of a number of renal pathologies (20). We developed zinc finger nuclease–mediated targeted knockouts of p66Shc in SS rats and used this rat strain to evaluate the role of p66Shc in the development of DN. All modifications of the Shc1 gene were made on the genetic background of SS rats. The SS rat is a well-established model for the study of salt-sensitive hypertension (21) but it has only recently been used to investigate DN (13,22). Unlike Sprague-Dawley rats, which are resistant to STZ-induced type 1 diabetes, SS rats are susceptible to renal injury induced by STZ. SS rats develop progressive proteinuria and hyperfiltration and display renal histological lesions similar to those seen in human patients with DN (13,22). Our data clearly indicate that loss of p66Shc does not prevent the development of STZ-induced diabetes but does mitigate the renal damage that ensues after the onset of diabetes. One mechanism by which p66ShcKO mitigates renal damage could be a restoration of vascular function of renal microvessels.
KATP channels are present in vascular SMC, and they contribute to physiological regulation of vascular tone by modulating vascular responses to diverse stimuli (23). Our analysis of renal microvascular reactivity using in vitro juxtamedullary nephron segments revealed that increased afferent arteriolar KATP channel activity contributes to dilation of the afferent arteriole, an increase in arteriolar diameter, and hyperfiltration injury in SS rats with STZ-induced diabetes. Elevated KATP function and decreased calcium influx in diabetes may contribute to preglomerular vasodilation and hyperfiltration (16,24). p66Shc expressed in renal vascular SMC inhibits transient receptor potential cation channel–dependent calcium influx (9), and as we show in this study, promotes KATP channel function. Conversely, inactivation of the adaptor protein p66Shc decreases afferent arteriolar KATP channel activity, presumably restores transient receptor potential cation channel function, and decreases renal damage in diabetic Dahl SS rats.
Increased oxidative stress within the kidney contributes to progression of DN (11,18,25,26). p66Shc is capable of acting to regulate sensitivity to oxidative stress and was reported to be overexpressed in proximal tubule cells and podocytes in STZ-induced diabetic mice and other genetic models of diabetes (27,28). We analyzed the progression of STZ-induced DN in p66Shc-S36A rats in which p66Shc is expressed but is devoid of its mitochondrial action. Supporting the role of p66Shc as an inducer of mitochondrial reactive oxygen species in DN, p66Shc-S36A rats with STZ-induced diabetes displayed decreased albuminuria and glomerular damage. It is interesting that both restoration of vascular function and a decrease in albuminuria in diabetic p66Shc-S36A rats occurred to a lesser extent than in p66ShcKO rats, implying that nonmitochondrial action of p66Shc, presumably related to KATP activity, is also important. It was recently reported that Sirt1-regulated lysine acetylation of p66Shc contributes to vascular oxidative stress and diabetic vascular pathophysiology (29).
In summary, our data suggest a crucial role of p66Shc in renal and microvascular damage associated with DN.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. J. Lazar (HudsonAlpha Institute for Biotechnology, Huntsville, AL) for carrying out intravenous glucose tolerance tests. The authors also thank Dr. O. Palygin (Medical College of Wisconsin, Milwaukee, WI) for discussing the results.
Funding. This work was supported by the National Heart, Lung, and Blood Institute (grant no. R35 HL135749 to A.St.), the National Institute of Diabetes and Digestive and Kidney Diseases (grant nos. R01 DK103616 to J.D.I. and R01 DK098159 to A.So. ), and the Medical College of Wisconsin Cancer Center (a grant from the Wisconsin Breast Cancer Showhouse to A.So.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.S.M., S.R.B., A.Sh., K.D.W., A.St., J.D.I., and A.So. analyzed and interpreted the data, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work. B.S.M., S.R.B., A.Sh., K.D.W., and J.D.I. conducted the investigation. B.S.M., S.R.B., K.D.W., A.St., and A.So. wrote the manuscript. A.St. designed the experiments dealing with direct measurement of KATP channel activity. A.So. conceived and supervised the study. A.So. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the American Society of Nephrology Kidney Week 2017, New Orleans, LA, 31 October–5 November 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0308/-/DC1.
References
- 1.Al-Delaimy WK, Merchant AT, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med 2004;116:236–240 [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet 2010;376:958]. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camacho P, Pitale S, Abraira C. Beneficial and detrimental effects of intensive glycaemic control, with emphasis on type 2 diabetes mellitus. Drugs Aging 2000;17:463–476 [DOI] [PubMed] [Google Scholar]
- 4.Prischl FC, Auinger M, Säemann M, et al.; Austrian Dialysis and Transplant Registry . Diabetes-related end-stage renal disease in Austria 1965-2013. Nephrol Dial Transplant 2015;30:1920–1927 [DOI] [PubMed] [Google Scholar]
- 5.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 2015;95:405–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 1986;77:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang GK, Maahs DM, Perkins BA, Cherney DZ. Renal hyperfiltration and systemic blood pressure in patients with uncomplicated type 1 diabetes mellitus. PLoS One 2013;8:e68908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens 2010;19:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller B, Palygin O, Rufanova VA, et al. . p66Shc regulates renal vascular tone in hypertension-induced nephropathy. J Clin Invest 2016;126:2533–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foschi M, Franchi F, Han J, La Villa G, Sorokin A. Endothelin-1 induces serine phosphorylation of the adaptor protein p66Shc and its association with 14-3-3 protein in glomerular mesangial cells. J Biol Chem 2001;276:26640–26647 [DOI] [PubMed] [Google Scholar]
- 11.Francia P, Cosentino F, Schiavoni M, et al. . p66(Shc) protein, oxidative stress, and cardiovascular complications of diabetes: the missing link. J Mol Med (Berl) 2009;87:885–891 [DOI] [PubMed] [Google Scholar]
- 12.Giorgio M, Migliaccio E, Orsini F, et al. . Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005;122:221–233 [DOI] [PubMed] [Google Scholar]
- 13.Slaughter TN, Paige A, Spires D, et al. . Characterization of the development of renal injury in Type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2013;305:R727–R734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, et al. . Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmines PK, Fujiwara K. Altered electromechanical coupling in the renal microvasculature during the early stage of diabetes mellitus. Clin Exp Pharmacol Physiol 2002;29:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol 2008;295:F171–F178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz A, Kreutz R. Mapping genetic determinants of kidney damage in rat models. Hypertens Res 2012;35:675–694 [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Zhu X, Ma M, et al. . p66Shc: a novel biomarker of tubular oxidative injury in patients with diabetic nephropathy. Sci Rep 2016;6:29302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallon V, Albinus M, Blach D. Effect of KATP channel blocker U37883A on renal function in experimental diabetes mellitus in rats. J Pharmacol Exp Ther 1998;286:1215–1221 [PubMed] [Google Scholar]
- 20.Wright KD, Staruschenko A, Sorokin A. Role of adaptor protein p66Shc in renal pathologies. Am J Physiol Renal Physiol 2018;314:F143–F153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotchen TA, Cowley AW Jr., Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med 2013;368:1229–1237 [DOI] [PubMed] [Google Scholar]
- 22.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep 2015;5:17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 2002;29:312–316 [DOI] [PubMed] [Google Scholar]
- 24.Salomonsson M, Brasen JC, Sorensen CM. Role of renal vascular potassium channels in physiology and pathophysiology. Acta Physiol (Oxf) 2017;221:14–31 [DOI] [PubMed] [Google Scholar]
- 25.Menini S, Amadio L, Oddi G, et al. . Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 2006;55:1642–1650 [DOI] [PubMed] [Google Scholar]
- 26.Yang SK, Xiao L, Li J, Liu F, Sun L. Oxidative stress, a common molecular pathway for kidney disease: role of the redox enzyme p66Shc. Ren Fail 2014;36:313–320 [DOI] [PubMed] [Google Scholar]
- 27.Bock F, Shahzad K, Wang H, et al. . Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci U S A 2013;110:648–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Xiao L, Nie J, et al. . p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Renal Physiol 2010;299:F1014–F1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Kim YR, Vikram A, et al. . Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci U S A 2017;114:1714–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.