Abstract
Background: Oral tongue squamous cell carcinoma (OTSCC) cells are highly proliferative and invasive. Lingonberry contains several polyphenolic compounds similar to curcumin. We hypothesize that fermented lingonberry juice (FLJ) has an anti-invasive and anti-proliferative effect on OTSCC cells similarly to curcumin, which is known to be anti-carcinogenic. Materials and Methods: FLJ, curcumin dissolved in ethanol, or curcumin loaded in Candida extracellular vesicles (EVs) were added to more (HSC-3) and less aggressive (SCC-25) OTSCC cells. Cell proliferation was measured with a 5-bromo-2’-deoxyuridine kit and invasion in the three-dimensional Myogel spheroid assay. Statistical analyses were completed with one-way ANOVA and Bonferroni post-hoc testing. Results: Both FLJ and curcumin significantly reduced the proliferation and invasion of HSC-3 and SCC-25 cells. The effects of curcumin were not improved when cells were treated with curcumin loaded within EVs. Conclusion: Our results suggest that FLJ, like curcumin, has an anti-carcinogenic effect on aggressive OTSCC cells in vitro.
Keywords: Oral cancer cells, anti-invasive, anti-proliferative, 3D spheroid
Oral cancer is the tenth most common cancer in men worldwide, and most of these cancers are oral tongue squamous cell carcinomas (OTSCCs). Bioactive protective anticancer molecules are present in a healthy diet containing high amounts of fruits and vegetables (1). Pavia et al. showed a ~50% reduction in oral cancer risk for each portion of fruit or vegetable consumed per day (2). Similar results for reducing the risk of head and neck cancer by fruits and vegetables was documented by Freedman et al. (3).
Berries from the genus Vaccinium, such as blueberry (V. corymbosum), cranberry (V. macrocarpon), bilberry (V. myrtillus L.) and lingonberry (V. vitis-idaea), have several health benefits. They positively affect gut microbiota and cardiovascular disease outcomes and negatively affect tumour growth (4). When cloudberries (Rubus chamaemorus) or lingonberries were added to a modified high-fat diet in mice, the number and size of colonic adenomas were significantly reduced, demonstrating the tumour-preventative capacities of these berries (5). Similarly, a decrease in lung metastasis of breast cancer by fermented blueberries was documented in a mouse model by Vuong et al. (6). Tsuda et al. analysed five Vaccinium species in vitro and observed that the one with the highest total polyphenol content had the greatest anti-proliferative and pro-apoptotic effects on the pro-myelocytic human leukaemia cell line HL-60 (7). However, to the best of our knowledge, the effects of fermented lingonberry juice (FLJ) on OTSCC proliferation and invasion have not been studied.
Curcumin is the phenolic component of turmeric (Curcuma longa), and although used in Chinese and Indian medicine, it is still mainly applied as a spice. It has antimicrobial, hepatoprotective, cardioprotective, thrombosuppressive, and anti-carcinogenic properties, including inhibitory effects on OTSCC cells (8,9). Recently, curcumin was shown to reduce oral squamous cell carcinoma cell migration at low doses (10), as well as the invasiveness of an OTSCC cell line, SCC-25 (11). A phase I clinical trial reported histological improvement in precancerous lesions after treatment with curcumin for 3 months in 29% of patients who had high risk of cancer transformation (12). The poor bioavailability of curcumin has shifted focus towards loading curcumin into vehicles, such as nanoparticles and liposomes (9). Saengkrit et al. loaded cationic liposomes with curcumin (13), Dhule et al. used a 2-hydroxypropyl-γ-cyclodextrin/curcumin liposome complex (14) and later introduced nanoliposomes containing phospholipids, chitosan and curcumin (15). Instead of using synthetic carriers, Aqil et al. loaded curcumin into extracellular vesicles (EVs) isolated from bovine milk (16).
In this study, we aimed to evaluate the anti-proliferative and anti-invasive properties of FLJ on OTSCC cell lines and compare the effects to those of curcumin. Curcumin was either dissolved in ethanol or combined with EVs isolated from Candida glabrata.
Materials and Methods
Chemicals. Curcumin was obtained from Sigma-Aldrich (St Louis, MO, USA). A stock solution of 5 mg curcumin per ml 70% ethanol was prepared. Locally produced lingonberry juice (0.1 g/ml) was fermented using the yeast Saccharomyces cerevisiae as described by Pärnänen (17).
Isolation and characterization of EVs. C. glabrata G212 (clinical strain; Helsinki University Central Hospital, Finland) was cultivated on Sabouraud agar and incubated overnight at 37˚C before being transferred into Sabouraud broth, which was incubated on a shaker (120 rpm) for 48 h at 37˚C.
EVs were isolated using a two-step ultracentrifugation procedure. Firstly, the broth was filtered (0.22 μm) then centrifuged at 11 000 × g for 30 min at 4˚C. The supernatant was collected and centrifuged again at 100 000 × g for 90 min at 4˚C. The second supernatant was discarded and the pellet containing EVs was resuspended in 300 μl phosphate-buffered saline (PBS) and kept at −80˚C until further use. Purified EVs were analysed by nanoparticle tracking analysis (NTA) using a NanoSight LM14 viewing unit (NanoSight, Salisbury, UK) equipped with a blue (404 nm, 70 mW) laser and scientific CMOS camera. The samples were diluted in Dulbecco’s PBS and three videos of 90 s were recorded using camera level 14. The data were analysed using NTA software version 3.0 (NanoSight) with the detection threshold optimized for each sample and the screen gain at 10 to track as many particles as possible with minimal background. The EV content of C. glabrata G212 was 3.5×106 EVs/μl PBS.
EVs were spiked with 30 μl of curcumin stock solution and incubated for 30 min at room temperature.
Electron microscopy. The samples were prepared for electron microscopy and imaged as described elsewhere (18). Briefly, after fixation with 2% paraformaldehyde, the samples were stained with 2% neutral uranyl acetate and further stained and embedded in uranyl acetate and methyl cellulose mixture (1.8/0.4%). The samples were viewed using a JEOL JEM-1400 (JEOL Ltd, Tokyo, Japan) transmission electron microscope operating at 80 kV. Images were taken with a Gatan Orius SC 1000B CCD-camera (Gatan Inc., Pleasanton, CA, USA) with 4008×2672 pixel image size and no binning.
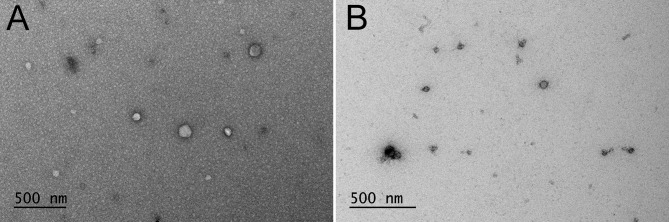
The morphology of the EVs (50-125 nm) before and after adding curcumin was confirmed by electron microscopy (Figure 1).
Figure 1. Morphology and size of extracellular vesicles (EVs). An electron microscope was used to confirm the presence of the EVs before (A) and after (B) adding curcumin .
Cell culture. Two human OTSCC cell lines, HSC-3 (Japan Health Sciences Foundation, Tokyo, Japan) and SCC-25 (American Type Culture Collection, Manassas, VA, USA) were used in this study. HSC-3 is a highly invasive cell line, while SCC-25 is less invasive (19). The cells were cultured in 75 cm2 flasks containing Dulbecco’s modified Eagle’s medium (DMEM)-12 (Gibco, Paisley, UK) supplied with 10% heat-inactivated foetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B, 0.4 μg/ml hydrocortisone and 50 μg/ml ascorbic acid.
Proliferation assay. Cell proliferation assay was performed using a Cell Proliferation enzyme-linked immunosorbent assay (ELISA), 5-bromo-2’-deoxyuridine BrdU (colorimetric) Kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, the cells were cultured in 96-well plates at a density of 5×103 cells/200 μl and exposed to FLJ (500, 2,500, or 5,000 μg/ml), curcumin (25, 125, or 250 μg), or curcumin-loaded EVs (25, 125, or 250 μg); phosphate-buffered saline was used as a control. After 48 h, the cells were labelled using 10 μM BrdU per well and re-incubated for 2 h at 37˚C in a humidified atmosphere. The culture medium was removed, the cells were fixed, and the DNA was denatured in one step by adding FixDenat. Next, the cells were incubated with anti-BrdU-POD for 90 min at room temperature. After the removal of the antibody conjugate, the cells were washed, and the substrate solution was added. The reaction product was quantified by measuring the absorbance using a scanning multi-well spectrophotometer (Thermo Scientific Multiskan EX, Thermo Fisher Scientific, MA, USA) at 450 nm with a reference wavelength of 690 nm. The results are expressed as mean absorbance values.
Three-dimensional (3D) tumour Myogel spheroid invasion assay. Cells were added (1000 cells per well) into a Corning™ ultra-low attachment 96-well round bottom plate (Costar® Corning Inc., Kennebunk, ME, USA). The plate was transferred to an incubator (37˚C, 5% carbon dioxide, 95% humidity). After 4 days, tumour spheroid formation was visually confirmed.
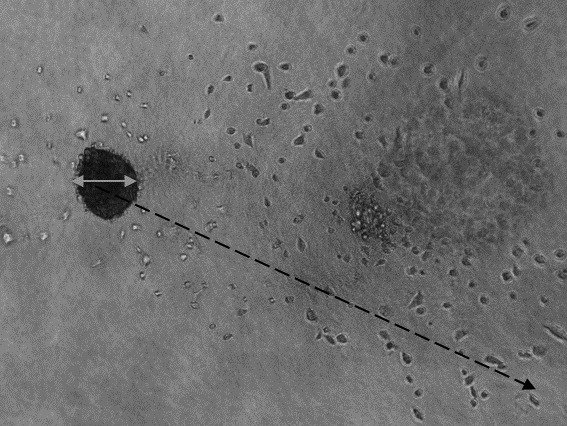
One hundred microlitres of growth medium was removed from each well and replaced by 100 μl of a mixture of Myogel (0.5 mg/ml) and collagen I (0.5 mg/ml; BD Biosciences, Bedford, MA, USA) cFLJ (500, 2500, or 5,000 μg/ml), curcumin (25, 125, or 250 μg), or curcumin-loaded EVs (25, 125, or 250 μg). The plate was transferred to an incubator at 37˚C and the Myogel–collagen mixture was allowed to solidify. After 1 h, 100 μl/well of complete growth medium plus FLJ, curcumin or EVs loaded with curcumin at the same concentration was added. Images were taken at 0, 24, 48, 72 and 96 h using a Nikon DS-Fi2 camera (Nikon, Tokyo, Japan) with 4× magnification (light microscope, no phase contrast). The spheroid diameter (Figure 2, indicated by the solid arrow) and cell invasion (Figure 2, indicated by the dashed arrow) were measured using ImageJ 1.50i (Wayne Rasband, National Institute of Mental Health, Bethesda, MD, USA).
Figure 2. The three-dimensional Myogel spheroid assay. Spheroid diameter (solid arrow) and cell invasion (dashed arrow) were measured using the ImageJ 1.50i software .
Statistical analysis. All experiments were repeated three times independently, each in triplicate. Values are given as mean±standard deviation. To determine the statistical significance, a one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test was performed. Statistical significance was set at p<0.05. All statistical analyses were performed using IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA).
Results
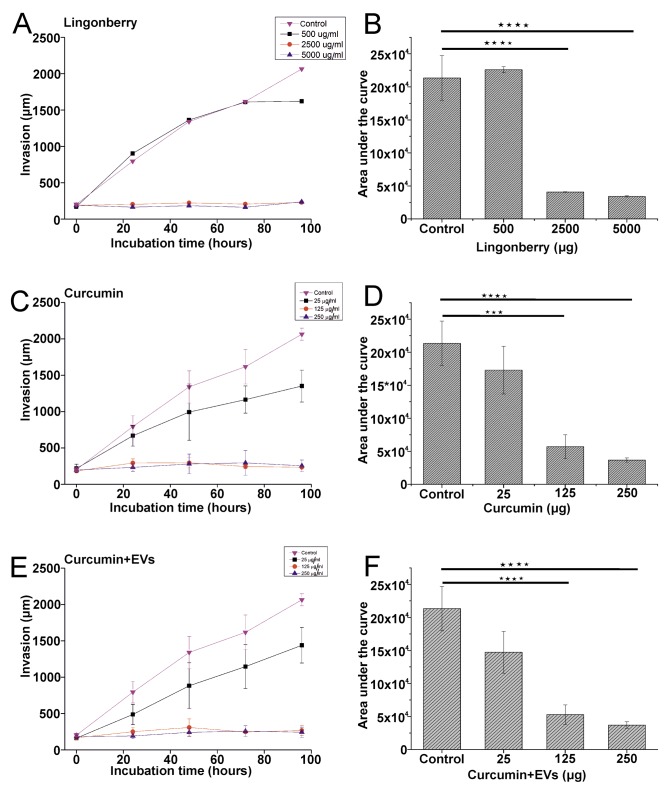
FLJ and curcumin inhibited proliferation of OTSCC cells. Exposing the HSC-3 cells to FLJ inhibited cell proliferation at concentrations of 2.5 and 5.0 mg/ml (Figure 3A). Curcumin with and without Candida EVs acted similarly but at lower concentrations (125 and 250 μg/ml; Figure 3B and C). The half maximal-inhibitory concentration (IC50) for FLJ was 1162 μg/ml, and was 35 μg/ml for curcumin.
Figure 3. Inhibition of HSC-3 and SCC-25 cell proliferation after 48 h ofexposure to fermented lingonberry juice (FLJ), curcumin and curcuminloadedextracellular vesicles (EVs). A colorimetric proliferation assaywas used to study the effects of FLJ (A), curcumin (B) and curcuminloadedEVs (C) on oral tongue squamous cell carcinoma. PBS was usedas control. Statistical significance was determined using one-way ANOVAfollowed by a Bonferroni post-hoc test. All experiments were repeatedindependently three times and each in triplicate. Results are the means ofthe three replicate experiments±standard error. Significantly different at*p≤0.05, **p≤0.01, ***p≤0.001, and ****p≤0.0001.
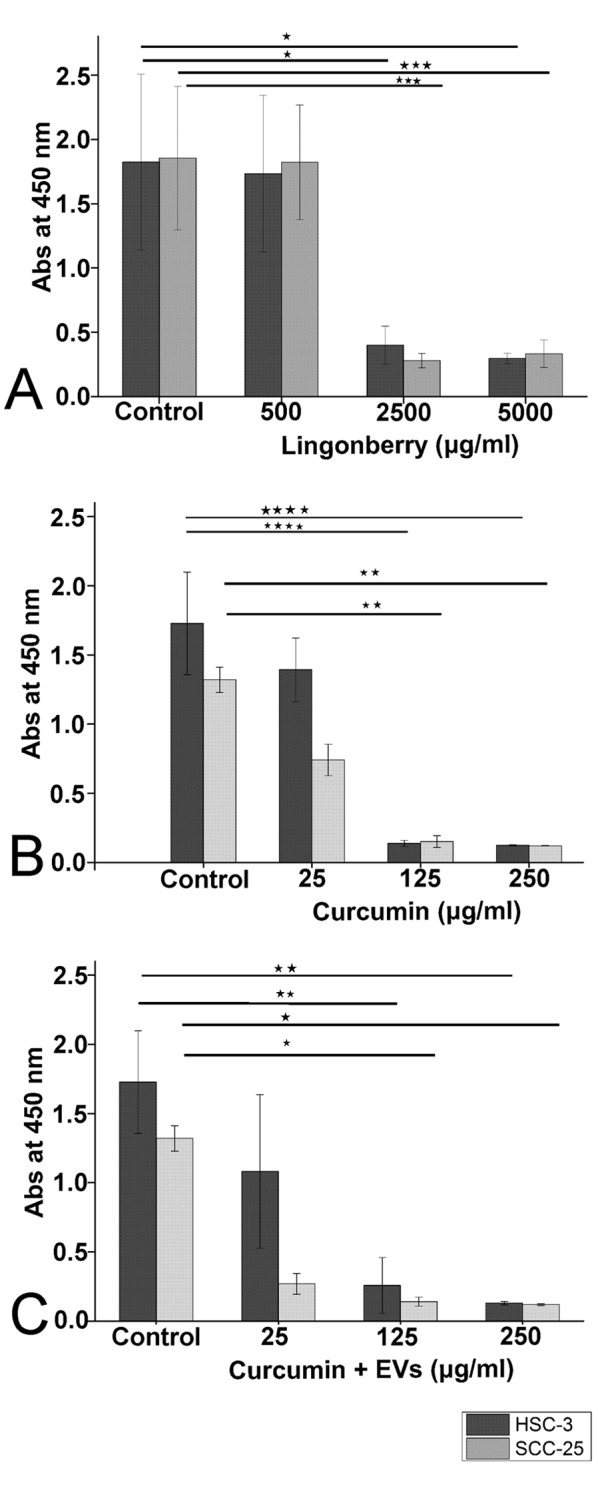
SCC-25 cell proliferation, similarIy to HSC-3, was inhibited by FLJ and curcumin with and without EVs (Figure 3A-C) at concentrations of 2.5 mg/ml or more for FLJ and 125 μg/ml or more for curcumin. The IC50 was 773 μg/ml for FLJ and 25 μg/ml for curcumin. This demonstrates that the less aggressive SCC-25 cells were more sensitive to FLJ and curcumin than the more aggressive HSC-3 cells.
The anti-proliferative effects on HSC-3 and SCC-25 cells were statistical significant for FLJ (p=0.023 and p=0.001, respectively) and curcumin (p=0.00003 and p=0.002, respectively).
FLJ and curcumin significantly reduced the invasion of OTSCC cells in the 3D Myogel spheroid assay without affecting spheroid size. Exposing the 3D Myogel spheroids of OTSCC cells to curcumin with and without EVs and FLJ for 96 h did not change the spheroid size (data not shown). For HSC-3 cells, the spheroid diameter was 200-250 μm and for SCC-25 cells 150-200 μm.
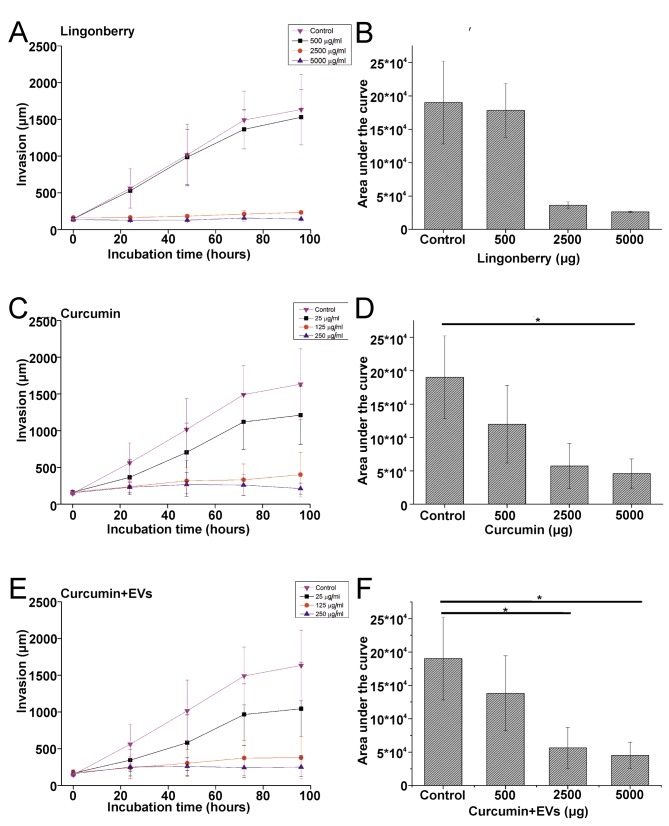
The invasion of HSC-3 cells was significantly inhibited by FLJ (p=0.000009; Figure 4A and B), curcumin (p=0.001; Figure 4C and D), and curcumin-loaded EVs (p=0.000125; Figure 4E and F). FLJ (Figure 5A and B), curcumin (p=0.034; Figure 5C and D), curcumin-loaded EVs (p=0.028; Figure 5E and F) inhibited SCC-25 cell invasion at concentrations similar to those against HSC-3, but for FLJ the effect was not statistically significant. The statistical significance was calculated based on the area under the curve of the invading cancer cells over time.
Figure 4. Effects of fermented lingonberry juice (FLJ) and curcumin on the invasiveness of HSC-3 cells after 96 h of exposure. The three-dimensionaltumour spheroid invasion assay was used to study the effects of FLJ (A), curcumin (B) and curcumin-loaded extracellular vesicles (C) on oraltongue squamous cell carcinoma invasion. Phosphate-buffered solution was used as control. Invasion was measured using the ImageJ software. Allexperiments were repeated independently three times and each in triplicate, except for FLJ, which was performed twice in triplicate. Results arethe means±standard error. Statistical significance was determined by calculating the area under the curve of the invading cancer cells over time,and using one-way ANOVA followed by a Bonferroni post-hoc test. Significantly different at ***p≤0.001 and ****p≤0.0001.
Figure 5. Effects of fermented lingonberry juice (FLJ) and curcumin on the invasiveness of SCC-25 cells after 96 h of exposure. The threedimensionaltumour spheroid invasion assay was used to study the effects of FLJ (A), curcumin (B) and curcumin-loaded extracellular vesicles (C)on OTSCC invasion. Invasion was measured using the ImageJ software. All experiments were repeated independently three times and each intriplicate. Results are the means±standard error. Statistical significance was determined by calculating the area under the curve and using onewayANOVA followed by a Bonferroni post-hoc test. Significantly different at *p≤0.05.
Discussion
Our in vitro study showed anti-proliferative and anti-invasive effects of FLJ on more (HSC-3) and less (SCC-25) aggressive OTSCC cell lines similarly to curcumin. However, the IC50 of curcumin was approximately 30-times lower than that of FLJ in both cell lines tested.
The inhibition of OTSCC cell proliferation by FLJ is similar to the results obtained by Brown et al. (20) using a highly invasive human colorectal adenocarcinoma cell line, HT115. The anti-proliferative effects of lingonberry were also demonstrated in human cervical (HeLa) and colon (Caco-2) cancer cells (21).
Curcumin inhibited OTSCC proliferation and invasion in both cell lines similarly to the study by Lee et al. (11) in which they also analysed SCC-25 cells. In that study, proliferation was measured by trypan blue and invasion in the transwell assay, while in the present study a BrdU Kit and 3D spheroid assay, respectively, were used. In addition, in their experiments, a concentration as low as 10 μM curcumin reduced cell invasion by 95%, and 15 μM curcumin reduced cell viability by 50%. In our experiments, the IC50 of curcumin for SCC-25 cell proliferation was 25 μM.
Our attempt to increase curcumin bioavailability by mixing curcumin with Candida EVs did not show similar additive effects to those in the study by Aqil et al. (16), which used EVs from raw bovine milk. The EVs isolated from milk were mixed with curcumin and those curcumin-loaded EVs were able to inhibit the proliferation of lung (A549 and H1299), cervical (CaSki and HeLa) and breast cancer (MDA-MB-231 and T47D) cell lines more efficiently than curcumin itself. In our study, the EVs isolated from C. glabrata G212 did not affect cell proliferation; after the EVs were loaded with curcumin, the effects were similar to those of curcumin alone on OTSCC cell proliferation and invasion. One explanation could be that bovine milk EVs may take up more curcumin compared with Candida EVs. The amount of curcumin in different EV isolations may vary and should be compared. It would be interesting to analyse whether raw cow milk EVs mixed with curcumin would have different effects on the invasion and proliferation of OTSCC cells than EVs from other sources, such as Candida or saliva (22).
Berries from the genus Vaccinium have been tested in some anticancer studies as described in a review article by Neto (23). However, so far, only the effect of blueberry powder has been analysed on oral carcinogenesis in a hamster model, where it inhibited both cancer invasion and angiogenesis (24). The bioactive compounds of lingonberry in vitro were shown to be similar before and after fermentation, and degradation (20). The variety of anthocyanin metabolites after ingesting another Vaccinium, chokeberry, included at least 10 individual anthocyanin metabolites found in urine and serum (25). The concentration of anthocyanins detected in plasma was 138 nmol/l derived from 1435 μmol ingested from bilberries and lingonberries (26). The concentration of curcumin detected in serum after ingesting 4 g per day was on average 0.51±0.11 μM, after 6 g it was 0.63±0.06 μM and after 8 g it was 1.77±1.87 μM (12). The concentration of curcumin which inhibited the proliferation and invasion of OTSCC cells was 30 times lower than that required for FLJ. However, a person is undoubtedly able to consume high amounts (more than 100 g) of berries daily, whereas consuming the required daily amount of curcumin is less noticeable. Therefore, the anti-carcinogenic effects for both compounds could easily, at least in theory, be ingested on a daily basis.
In conclusion, our study showed that FLJ significantly inhibited the invasion and proliferation of OTSCC cells, but the concentration needs to be higher than that of curcumin. Loading curcumin into Candida EVs improved its poor bioavailability, but the anti-carcinogenic effect on OTSCC cells was unchanged. In vivo animal studies of FLJ compounds should be conducted to see whether it is able to prevent or reduce the progression of OTSCC.
Acknowledgements
The Authors thank the EV Core at the University of Helsinki for performing the nanoparticle tracking analysis as well as the Electron Microscopy Unit of the Institute of Biotechnology at the University of Helsinki for providing the facilities. This work was supported by the Sigrid Juselius Foundation, the Cancer Society of Finland, and Helsinki University Central Hospital
References
- 1.McClements DJ, Xiao H. Designing food structure and composition to enhance nutraceutical bioactivity to support cancer inhibition. Semin Cancer Biol. 2017;46:215–226. doi: 10.1016/j.semcancer.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: A meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 3.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330–2336. doi: 10.1002/ijc.23319. [DOI] [PubMed] [Google Scholar]
- 4.Seeram NP. Berries and human health: research highlights from the Fifth Biennial Berry Health Benefits Symposium. J Agric Food Chem. 2014;62(18):3839–3841. doi: 10.1021/jf404349f. [DOI] [PubMed] [Google Scholar]
- 5.Mutanen M, Pajari AM, Paivarinta E, Misikangas M, Rajakangas J, Marttinen M, Oikarinen S. Berries as chemopreventive dietary constituents – a mechanistic approach with the ApcMin/+ mouse. Asia Pac J Clin Nutr 17. 2008;1:123–125. [PubMed] [Google Scholar]
- 6.Vuong T, Mallet JF, Ouzounova M, Rahbar S, Hernandez-Vargas H, Herceg Z, Matar C. Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J Transl Med. 2016;14:13. doi: 10.1186/s12967-016-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuda H, Kunitake H, Kawasaki-Takaki R, Nishiyama K, Yamasaki M, Komatsu H, Yukizaki C. Antioxidant activities and anti-cancer cell proliferation properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and blueberry cultivars. Plants. 2013;2(1):57–71. doi: 10.3390/plants2010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med. 2017;7:339–346. doi: 10.1016/j.jtcme.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G. New insights into therapeutic activity and anticancer properties of curcumin. J Exp Pharmacol. 2017;9:31–45. doi: 10.2147/JEP.S70568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Campos PS, Matte BF, Diel LF, Jesus LH, Bernardi L, Alves AM, Rados PV, Lamers ML. Low doses of curcuma longa modulates cell migration and cell-cell adhesion. Phytother Res. 2017;31(9):1433–1440. doi: 10.1002/ptr.5872. [DOI] [PubMed] [Google Scholar]
- 11.Lee AY, Fan CC, Chen YA, Cheng CW, Sung YJ, Hsu CP, Kao TY. curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-E-cadherin pathway. Integr Cancer Ther. 2015;14(5):484–490. doi: 10.1177/1534735415588930. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 13.Saengkrit N, Saesoo S, Srinuanchai W, Phunpee S, Ruktanonchai UR. Influence of curcumin-loaded cationic liposomes on anticancer activity for cervical cancer therapy. Colloids Surf B Biointerfaces. 2014;114(10):349–356. doi: 10.1016/j.colsurfb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, He J, Alb A, John V, Pochampally R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8(4):440–451. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhule SS, Penfornis P, He J, Harris MR, Terry T, John V, Pochampally R. The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm. 2014;11(2):417–427. doi: 10.1021/mp400366r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017;19(6):1691–1702. doi: 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- 17.Pärnänen P. [Google Scholar]
- 18.Puhka M, Nordberg ME, Valkonen S, Rannikko A, Kallioniemi O, Siljander P Af, Hällström TM. KeepEX, a simple dilution protocol for improving extracellular vesicle yields from urine. Eur J Pharm Sci. 2017;98:30–39. doi: 10.1016/j.ejps.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Ramos DM, Chen BL, Boylen K, Stern M, Kramer RH, Sheppard D, Nishimura SL, Greenspan D, Zardi L, Pytela R. Stromal fibroblasts influence oral squamous-cell carcinoma cell interactions with tenascin-C: Int J Cancer. 1997;72:369–376. doi: 10.1002/(sici)1097-0215(19970717)72:2<369::aid-ijc28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Brown EM, Nitecki S, Pereira-Caro G, McDougall GJ, Stewart D, Rowland I, Crozier A, Gill CI. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: potential impact on colonic health. Biofactors. 2014;40(6):611–623. doi: 10.1002/biof.1173. [DOI] [PubMed] [Google Scholar]
- 21.McDougall GJ, Ross HA, Ikeji M, Stewart D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J Agric Food Chem. 2008;56(9):3016–3023. doi: 10.1021/jf073469n. [DOI] [PubMed] [Google Scholar]
- 22.Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R, Vered M. Human saliva-derived exosomes: comparing methods of isolation. J Histochem Cytochem. 2015;63(3):181–189. doi: 10.1369/0022155414564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51(6):652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 24.Baba AB, Kowshik J, Krishnaraj J, Sophia J, Dixit M, Nagini S. Blueberry inhibits invasion and angiogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters via suppression of TGF-β and NF-ĸB signaling pathways. J Nutr Biochem. 2016;35:37–47. doi: 10.1016/j.jnutbio.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Kay CD, Mazza G, Holub BJ, Wang J. Anthocyanin metabolites in human urine and serum. Br J Nutr. 2004;91(6):933–942. doi: 10.1079/bjn20041126. [DOI] [PubMed] [Google Scholar]
- 26.Nurmi T, Mursu J, Heinonen M, Nurmi A, Hiltunen R, Voutilainen S. Metabolism of berry anthocyanins to phenolic acids in humans. J Agric Food Chem. 2009;57(6):2274–2281. doi: 10.1021/jf8035116. [DOI] [PubMed] [Google Scholar]