Abstract
The use of tissue-engineered articular cartilage (TEAC) constructs has the potential to become a powerful treatment option for cartilage lesions resulting from trauma or early stages of pathology. Although fundamental tissue-engineering strategies based on the use of scaffolds, cells, and signals have been developed, techniques that lead to biomimetic AC constructs that can be translated to in vivo use are yet to be fully confirmed. Mechanical stimulation during tissue culture can be an effective strategy to enhance the mechanical, structural, and cellular properties of tissue-engineered constructs toward mimicking those of native AC. This review focuses on the use of mechanical stimulation to attain and enhance the properties of AC constructs needed to translate these implants to the clinic. In vivo, mechanical loading at maximal and supramaximal physiological levels has been shown to be detrimental to AC through the development of degenerative changes. In contrast, multiple studies have revealed that during culture, mechanical stimulation within narrow ranges of magnitude and duration can produce anisotropic, mechanically robust AC constructs with high cellular viability. Significant progress has been made in evaluating a variety of mechanical stimulation techniques on TEAC, either alone or in combination with other stimuli. These advancements include determining and optimizing efficacious loading parameters (e.g., duration and frequency) to yield improvements in construct design criteria, such as collagen II content, compressive stiffness, cell viability, and fiber organization. With the advancement of mechanical stimulation as a potent strategy in AC tissue engineering, a compendium detailing the results achievable by various stimulus regimens would be of great use for researchers in academia and industry. The objective is to list the qualitative and quantitative effects that can be attained when direct compression, hydrostatic pressure, shear, and tensile loading are used to tissue-engineer AC. Our goal is to provide a practical guide to their use and optimization of loading parameters. For each loading condition, we will also present and discuss benefits and limitations of bioreactor configurations that have been used. The intent is for this review to serve as a reference for including mechanical stimulation strategies as part of AC construct culture regimens.
Keywords: : mechanical stimulation, articular cartilage, compression, tension, hydrostatic pressure, shear
Introduction
Degradation of articular cartilage (AC) is caused by trauma or overuse,1,2 which initiates a cascade of pathological events, leading to osteoarthritis (OA). According to the Center for Disease Control, OA affects over 30 million Americans per year. Even before OA is fully manifested, AC injuries can negatively impact the mobility of young patients.3 Currently, there are no substantial, long-term treatment options to repair and halt the progression of AC injuries.
Focal defects (∼5 mm dia.) are generally treated by microfracture and autologous chondrocyte implantation.4 These strategies lead to the development of mechanically inferior fibrocartilage in the treated lesions, which places detrimental stresses on surrounding AC.5 Tissue-engineering strategies show the potential to overcome the drawbacks of current treatment options by designing and developing biomimetic AC tissues for transplantation.
Native, healthy AC consists of a durable, low-friction, mechanically robust tissue, sparsely populated by chondrocytes. Physiologically, AC experiences and endures a myriad of mechanical forces, including compression, shear, hydrostatic pressure (HP), and tension. To achieve translatable, biomimetic AC tissue, the design criteria of engineered AC follow those of native AC in both form and function: high compressive and tensile stiffness, a well-organized matrix rich in collagen type II and sulfated glycosaminoglycans (sGAGs), viable cells of a healthy phenotype, low coefficient of friction, and in vivo durability.
To treat AC injuries, tissue engineering has focused on developing constructs derived from chondrocytes, scaffolds, and signals. Signaling tools used to satisfy the aforementioned design criteria include bioactive and mechanical stimuli. In general, research in the field remains highly centralized on investigating bioactive factors alone because they can be easily applied in culture medium. Transforming growth factor-β1 (TGF-β1), insulin-like growth factor-1 (IGF-1), and bone morphogenetic protein-2 (BMP-2) are all effective in improving extracellular matrix (ECM) content and mechanical properties.4 Mechanical stimulation also increases ECM content and mechanical properties, but, notably, fiber organization in response to mechanical stimulation has also been observed.5–7 This review focuses on the use of mechanical stimuli to address the design criteria that soluble factors have been able to influence and also those that the soluble factors have not shown efficacy toward.
Because articulating joints lack access to blood vessels, chondrocytes dwelling in AC rely on mechanical movement for nutrient delivery, signaling, and cellular waste disposal.5 Mechanical stimulation research on tissue-engineered AC (TEAC) has largely been focused on direct compression (DC) and HP, but, more recently, shear and tension have also shown potential for increasing ECM content, preserving cellular viability, and promoting matrix organization.
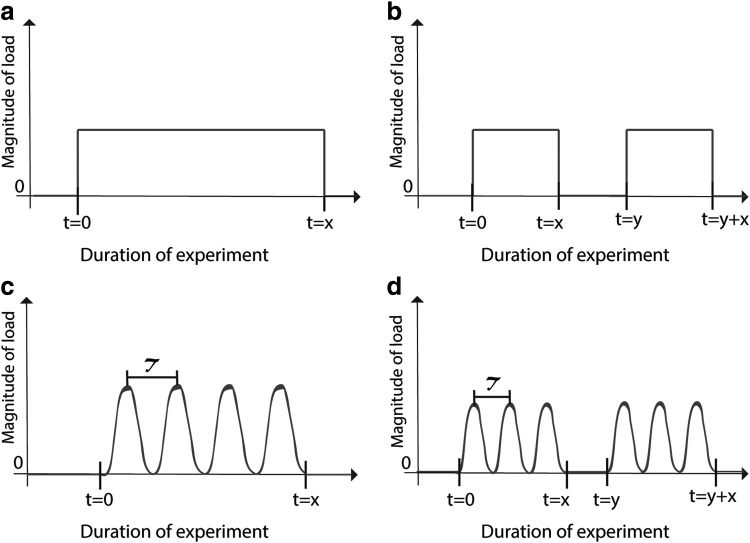
The mechanical stimulation parameters most commonly reported are magnitude and duration.8 The optimal mechanical loading parameters will depend on the matrix or scaffold used for cell culture because of stress shielding on the chondrocytes. Alternatively, studies that use high-density chondrocyte culture and no initial matrix will likely use lower magnitudes of load. For this reason, it is important to report parameters in units that normalize to the matrix, such as percent strain. Frequency, waveform, and time of application are also important parameters (Fig. 1), although less frequently investigated. To date, a description of how specific design criteria can be achieved by manipulating mechanical stimulation parameters is lacking.
FIG. 1.
Waveforms representing common loading patterns used in mechanical stimulation studies. (a) Continuous passive loading, (b) intermittent passive loading, (c) continuous dynamic loading, and (d) intermittent dynamic loading. The x-axis represents the duration of the experiment, where t = 0 represents the commencement of mechanical stimulation, t = x represents the duration of applied stimulation, and τ represents the wave period (frequency = 1/τ). In (b) and (d), t = y − x is the amount of time the tissue is in static culture between mechanical stimulation treatments. The y-axis represents the magnitude of load, which is commonly measured in units of stress, strain, or mass.
The first portion of this review defines specific design criteria, which include mechanical properties and matrix production, content, organization, and integration, as well as criteria specified by the U.S. Food and Drug Administration (FDA), such as durability.9 The second portion introduces different types of mechanical stimulation and their mode of application with bioreactors. Subsections in each type of mechanical stimulation offer integrated discussion on how various design criteria are enhanced by the specified mode of stimulation. Of particular interest are descriptions of how certain design criteria can be addressed more effectively using mechanical stimuli because little or no data exist on how such properties can be manipulated using bioactive factors alone. The limitations of mechanical stimuli are also discussed within the context of how they may be paired with bioactive factors.
Description and Assessment of TEAC Design Criteria
Compressive moduli
Sustaining compressive loads is a critical function of AC. Major weight-bearing joints, such as the hip and knee, experience compressive stress between 0.5 and 7.7 MPa, which typically leads to about 13% strain.1,10 Native AC in healthy people endures thousands of compressive loading cycles per day without suffering injury, and failure to maintain loading on a regular basis leads to cartilage degradation and loss of AC function.11 Thus, TEAC must similarly be capable of withstanding routine compressive cycles without failure.
Compressive moduli in TEAC are frequently represented by aggregate (HA) and dynamic (ED) moduli. The HA is a measure of the equilibrium resistance of a solid-fluid mixture once fluid has stopped flowing.12 The ED is the ratio of stress to strain under cyclic loading conditions.13 Native human AC tissue has an HA of 0.08–2 MPa.14–16 The ED of native AC has been shown to increase with increasing strain nonlinearly.13,17 Although other compressive properties exist, investigators generally only measure and report one or two compressive moduli. Regardless of which modulus value is measured, the objective of this design criterion is for TEAC to match the compressive properties of native AC.
Tensile properties
Native AC sustains a constant state of static pre-tension caused by negatively charged proteoglycans retaining fluid throughout the ECM.18 Consequently, the collagen in the cartilage matrix imposes tension that allows the tissue to swell without rupturing.10,11 As a result of the Poisson effect, AC is also exposed to tension during compression, as well as shear.19
Tensile properties of AC are quantified using the Young's modulus (EY) and the ultimate tensile strength (UTS). The EY is defined as the slope of the linear portion of the stress–strain curve under conditions of uniaxial loading.20 The UTS is defined as the maximum stress sustained by the material under strain and is considered the stress at failure. To match the tensile properties of native AC, TEAC must have a EY of 5–25 MPa and UTS of 2–8 MPa.15,21,22 Because native AC experiences macroscopic tension indirectly as an effect of compressive and shear loads, tensile properties have not historically been investigated as much as compressive properties, but this characteristic is gaining recognition of its importance.
Collagen content
Two-thirds of the dry mass of AC is collagen. The most abundant collagen type in AC is collagen II, but collagen types III, VI, IX, X, XI, XII, and XIV also contribute to a mature AC matrix.23 Collagen types II, IX, and XI form a reinforcing heteropolymer in the ECM, while collagen type X contributes to regulating ossification of cartilage.24 Collagen X is also found in excess in ECM of OA patients, making it a marker for the disease.25 The goal of this design criterion is to engineer AC constructs with high collagen II content and without collagens that are indicative of fibrocartilage or bone (e.g., collagen I and X).26
Collagen content is recorded in almost all TEAC studies. Generally, a hydroxyproline assay is used when quantifying collagen content in TEAC constructs.27 This assay is not specific to any collagen type and measures total collagen content. Immunohistochemical staining and ELISA assays may be performed to assess collagen type II content in an AC construct.28 Collagen fibril diameter and cross-links, such as pyridinoline,29 are also important aspects of the collagen fibril network found in AC ECM, although they are not often reported in mechanical stimulation studies. Investigating specific collagen type, cross-links, and fibril/fiber dimensions could be helpful for determining what aspects of the collagen network are affected by mechanical loading and if these contribute to mechanical properties.
Glycosaminoglycan content
The highly anionic GAGs found in AC contribute to resisting compressive loads by binding to water molecules.30 GAG takes up about 25% of the dry weight of native AC, of which most are chondroitin and keratan sulfate chains and hyaluronan.31 GAGs have functional roles in tissue remodeling, uptake of proteins, intracellular signaling, and cell migration.15,32 In particular, sGAGs are responsible for withstanding high mechanical loads, and their synthesis is regulated by exposure to compressive forces.33 However, excessive loading, such as strenuous exercise, depletes sGAGs, resulting in reduced HP and compromised compressive properties.33 Some studies only report GAG content instead of sGAG content, but it is crucial to obtain these data because sGAG depletion gives insight to excessive loads in mechanical stimulation studies.
The 1,9-dimethylmethylene blue (DMMB) dye assay is widely used to quantify total sGAG, but it cannot differentiate among sGAGs nor detect nonsulfated hyaluronan.32,34 However, fluorophore-assisted carbohydrate electrophoresis is gaining recognition as a strategy that does differentiate among GAG types.35
Cellular performance
In static cultures, chondrocytes in the inner region of AC constructs have limited access to signals and nutrients causing them to lose function and their chondrocytic phenotype.36 Cell viability and proliferation are measured using metabolic assays, and cellular content may be measured indirectly by quantifying DNA content. TEAC is biomimetic and employable for translation only if it houses viable chondrocytes with high proliferative potential and AC-specific ECM production.37
Investigating mechanical stimulation on AC constructs shows that there is significant increase in chondrocyte viability when AC constructs are cultured under a dynamic regimen.5,11 For example, when DC is induced dynamically it has resulted in fivefold increase of viable cells compared to passive DC cultures.6 Higher chondrocyte viability in dynamic cultures is attributed to higher nutrient and sulfate accessibility compared to passively stimulated or static cultures.11
Fiber organization
Although ECM content has been attributed as the main contributor to AC mechanical properties,38,39 fiber organization has increasingly gained recognition for playing a major role in AC mechanical functionality.40,41 When tested under confined or unconfined compression, as well as tension, zonal architecture and anisotropy have been found to play salient roles in the mechanical properties of native AC.40,42,43 A robust TEAC with zonal architecture has not yet been achieved. The challenge in replicating the zonal architecture of AC comes from the uniqueness of each zone. For example, reconstruction of the superficial zone would require replication of lubricin and superficial zone protein content.44,45
Efforts have been made to study and culture individual zonal subpopulations of chondrocytes under mechanical stimulation.46 For instance, superficial zone chondrocytes have been found to have an increased response to tensile stimulation, whereas deep zone chondrocytes have been found to respond better to HP.47,48 Cross-link content, such as pyridinoline, has also been shown to play a large role in the structure–function relationship of AC.49,50 ECM content, structure, and cross-linking are salient aspects of AC constructs and should be fully characterized.
Investigators may also aim to reconstruct zonal architecture to enhance the functionality and biomimicry of TEAC. Both zonal structure and surface anisotropy are important aspects of fiber organization in AC, but only surface anisotropy has been successfully achieved in TEAC with the use of mechanical stimulation. Surface anisotropy in native AC can be tested using split lines.43,51 Although split lines have never been observed in TEAC, anisotropy may also be assessed using scanning electron microscopy.52
Tribology
Native AC demonstrates exceptionally low friction even under large and repetitive mechanical loads.53 Although the intrinsic nature of AC is not conducive to regeneration, a low coefficient of friction keeps the tissue functional for decades.53 Its low friction and efficient lubrication can be attributed to several mechanisms: lubricin, hyaluronic acid, surface-active phospholipids,54 and interstitial pressurization.55
In AC, the minimum fluid film thickness between articulating surfaces, in conjunction with the surface roughness, loading speed, and magnitude determines the lubrication mode.15,56 In hydrodynamic lubrication, usually a low mechanical load is transmitted at a high speed through a thin layer of fluid lubricant between two articulating surfaces; under this mode, the friction coefficient of native AC may reach 0.001.15,56 In boundary lubrication, a high mechanical load is transmitted directly on the surface of AC at a low speed.15,57 In native AC, the measured friction coefficient at boundary lubrication may be between 0.01 and 0.12.15,58 Tribology properties are usually measured with shear tests and tribometers by sliding a probe with a smooth spherical tip across the tissue surface.58 They are salient in maintaining healthy and functional ECM.
Integration and durability
Toward clinical translation, integration and durability are salient properties in TEAC and are crucial design criteria for functionality and success in translation to the clinic. Unfortunately, only a few mechanical stimulation studies on TEAC have assessed construct durability and integration in vivo or in vitro. Instead, studies have typically been focused on immobilization to show the effects of how durability decreases in the absence of mechanical stimuli.59–62
In preclinical studies with animal models, the FDA recommends “a minimum of 1 year in length to provide an adequate period for completion of healing…allowing assessment of durability of the therapeutic response and of the integrity of the product.” For clinical studies, the FDA recommends “a minimum of 2-year follow-up clinical information” for phase 2 and “a minimum of 5-year follow-up” in phase 3.9 These guidelines demonstrate the necessity for further investigation on the durability properties of TEAC cultured under mechanical stimulation. Routine mechanical stimulation is important for AC maintenance, but how mechanical stimulation during culture affects implant durability and integration remains an area that lacks sufficient data.9
Types of Mechanical Stimulation and Their Effects on TEAC Design Criteria
Direct compression
DC is the most abundantly investigated mechanical stimulation strategy in TEAC. DC is applied by directly loading the surface of an AC construct (Fig. 2a). Studies show that both passive and dynamic DC at less than 10% strain is beneficial for mechanical and biochemical properties.6,63–65 Similarly, studies using stress as a measurement of load show that properties benefit only up to a peak stress.66,67 For example, it was found that in a self-assembling culture system, TEAC properties were improved only between 3.3 kPa and 5 kPa of stress.67 Stress, and correspondingly deformation, is a critical parameter of DC stimulation for obtaining beneficial responses in TEAC.
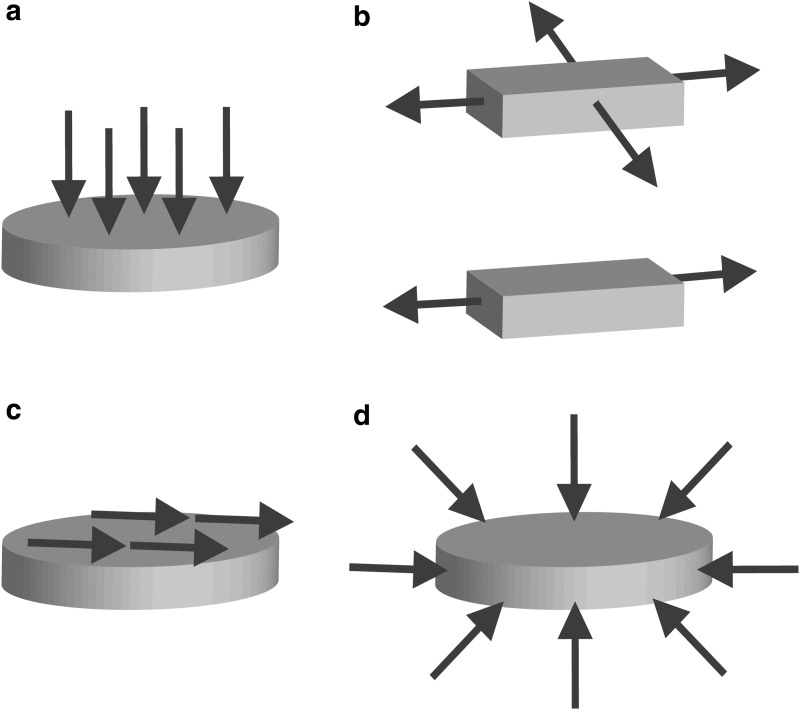
FIG. 2.
Arrows indicate the direction of mechanical loads acting on tissue-engineered articular cartilage during mechanical stimulation. (a) Direct compression, (b) biaxial tension (top), uniaxial tension (bottom), (c) shear, and (d) hydrostatic pressure.
DC bioreactors
Bioreactors used to apply passive DC are simple in design; weights coated with agarose are placed on top of AC constructs.66,67 These weights produce low compressive stresses, with corresponding strains under 10%. The weights rest on top of constructs during culture and are removed during media change. The duration of loading and the magnitude of stress are determined based on cell type and scaffold material properties.
Bioreactors used for dynamic DC stimulation use pistons or springs to load and unload the platen cyclically on the constructs. Dynamic DC alleviates the diffusion limitations of waste and nutrients that are experienced in passive DC and static culture. This mass transport is produced by pressure gradients within the matrix in addition to the physical mixing of the surrounding media.11,68 Commercial bioreactors allow for exchanging media through a reservoir and may also allow the investigator to assess mechanical properties throughout culture.69 Alternatively, dynamic DC bioreactors have been developed in-house and have achieved frequency ranges between 0.01 and 10 Hz and displacements of 0.1–15 mm.70,71 The decision to use either a commercial or in-house dynamic DC bioreactor is made by considering study needs.
DC improvements of TEAC compressive moduli
Dynamic DC loading has typically been applied at 1 Hz14,63,71 because it is similar to the pace of human gait, although it remains to be seen if other frequencies can also be efficacious in engineering cartilage. In particular, at 1 Hz of 20% strain for 21 and 28 days yielded threefold and sixfold increases in aggregate modulus, respectively.71 These studies demonstrate that adding dynamic DC stimulation to culture regimes improves compressive moduli in TEAC.
Passive DC has also improved compressive moduli in self-assembled AC constructs. The instantaneous and relaxation moduli of passively compressed, self-assembled cartilage constructs under 5 kPa of stress were increased significantly to about 700 and 275 kPa, respectively.66 Stress magnitude studies of compressive loading on self-assembling costal chondrocytes showed that compressive properties improve only up to a peak load of 5 kPa.67 Passive loads at higher stresses were found to yield insignificant and even detrimental results in HA and Er, demonstrating the importance of identifying a range of beneficial loading parameters.67
DC increases of TEAC collagen and GAG content
A compressive stress of 0.5 kPa significantly increased collagen content in self-assembling AC to 1.5-fold of free-swelling controls.66 A separate study found that collagen content was enhanced by 61% when AC derived from costal chondrocytes was cultured with a combination of passive DC at 5 kPa and bioactive stimuli.67 This study showed that collagen content trended lower in constructs stimulated at higher loads, suggesting that an excess of compressive loads may indeed lead to degeneration of salient ECM proteins (Fig. 3). Fibrocartilage derived from meniscus and articular chondrocyte cocultures also yielded a 27% increase in collagen content when stimulated passively with a 0.1 N DC load.72 Although collagen II content specifically needs to be investigated more thoroughly, these studies show that DC is a potent regimen for increasing total collagen content.
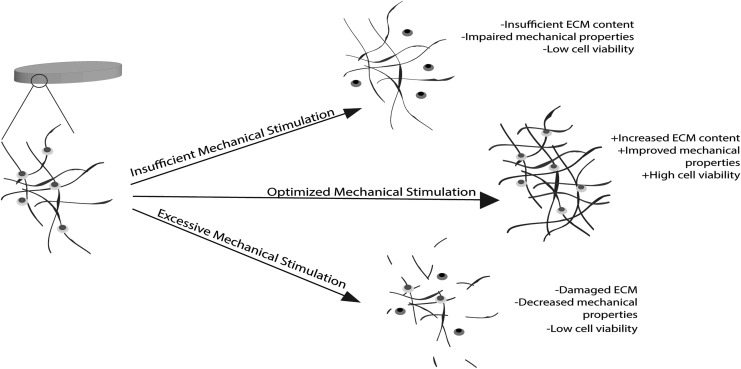
FIG. 3.
The importance of optimizing mechanical stimulation parameters: Insufficient mechanical stimulation results in low levels of signaling and nutrient diffusion causing low cell viability, ECM content, and mechanical properties. Excessive mechanical stimulation impairs mechanotransduction pathways by physically damaging ECM and sending chondrocytes to apoptosis, which leads to low mechanical properties. Optimized mechanical stimulation yields high cell viability, robust ECM, and improved mechanical properties by delivering nutrients and signaling cells to produce robust ECM components. ECM, extracellular matrix.
Dynamic DC has also been shown to increase GAG content by 60% in chondrocyte-seeded agarose gels.14,71 When combined with bioactive factor IGF-1, dynamic DC stimulation on these AC constructs yielded GAG content at 50% that of native AC.72 In addition, chondrocytes in monolayer stimulated with 20% compressive strain at 2 Hz exhibited an estimated 45% upregulation of GAG production.36 These studies along with others shown in Table 1 confirm the benefits that DC stimulation has on GAG content in AC constructs.8,11
Table 1.
Direct Compression
| Reference | Cell+scaffold type | Loading parameters | Waveform | Bioactive factors (Y/N) | Enhanced design criteria |
|---|---|---|---|---|---|
| Nebelung et al.64 | Human chondrocytes+col I hydrogel | 10% strain 0.3 Hz 28 days |
Dynamic Continuous |
N | 5.9-fold increase in col II |
| Kisiday et al.63 | Juvenile bovine chondrocytes+peptide hydrogel | 2.5% strain 1 Hz 39 days |
Dynamic Continuous |
N | 18% increase in HA 53% increase in sGAG 20% increase in cell viability |
| El-Ayoubi et al.6 | Canine chondrocytes+PLLA scaffold | 10% strain 1 Hz 14 days |
Dynamic Continuous |
N | “cell number was significantly higher in stimulated groups” |
| Mauck et al.7 | Juvenile bovine chondrocytes+agarose | 10% strain 1 Hz 5 days |
Dynamic Intermittent |
Y | 90% increase in HA 105% increase in col II 35% increase in GAG |
| Mauck et al.71 | Juvenile bovine chondrocytes+agarose hydrogel | 10% strain 1 Hz 28 days |
Dynamic Intermittent |
N | Sixfold increase in HA 60% increase in sGAG |
| Elder et al.66 | Juvenile bovine chondrocytes+scaffold free | 0.5 kPa 4 days |
Passive Continuous |
N | 40% increase in EY 52% increase in total col |
| Huwe et al.67 | Bovine costal chondrocytes+scaffold free | 5 kPa 4 days |
Passive Continuous |
Y | 39% increase in Er 1.62-fold increase in EY 61% increase in total col |
| MacBarb et al.72 | Juvenile ovine chondrocytes and meniscus cells+scaffold free | 10 g 4 days |
Passive Continuous |
N | 96% increase in Er 1.5-fold increase in Ei 2.5-fold increase in Ey 2.7-fold increase in UTS 27% increase in total col 67% increase in GAG |
Table of research articles on direct compression stimulation on AC constructs. The quantitative results reported in the column labeled “enhanced design criteria” were either taken directly from the referenced article or calculated from their reported data. Quotations directly from the referenced article were used when quantitative data were not available.
AC, articular cartilage; HA, aggregate; Ei, instantaneous; Er, relaxation; EY, Young's modulus; UTS, ultimate tensile strength; col, collagen; GAG, glycosaminoglycan; sGAG, sulfated glycosaminoglycan.
Shear
AC experiences shear stresses during normal physiological movement and loading.11 Shear stress is thought to be detrimental to native AC because it causes wear, tear, and degradation over time. However, in vitro, at low frequencies (<1 Hz) and magnitudes of stress (<0.5 Pa), shear stimulation is suggested to yield enhanced AC construct properties.73–75 Shear stress is applied along the horizontal plane of the tissue (Fig. 2c), causing ECM and chondrocytes to slide upon each other in an antiparallel manner. Shear stress is applied by flowing fluid across TEAC, or as direct shear by sliding a solid sphere or platen along the surface of the AC construct. A common loading pattern of shear stress used in both fluid and direct shear bioreactors is oscillatory shear stress because of its similarities to physiological joint movement.76–78
Although upregulation in ECM proteins, such as collagen II, has been found with shear stimulation, it is still unclear whether it is caused by shear forces exerted on the constructs or by increased nutrient perfusion.79,80 There have been no studies to uncouple the response to shear stimulation and perfusion. Further study is needed to determine the cause of positive responses in TEAC cultured under shear stress, but multiple studies have shown that it is an effective tactic for the enhancement of mechanical properties (Table 2) and 40–140% increases in collagen II content.73–75,80
Table 2.
Shear
| Reference | Cell+scaffold type | Loading parameters | Waveform | Bioactive factors (Y/N) | Enhanced design criteria |
|---|---|---|---|---|---|
| Freyria et al.80 | Juvenile bovine chondrocytes+col I sponge | 30 RPM 30 days |
Fluid oscillatory | N | Twofold increase in cell proliferation |
| Pei et al.89 | Caprine bone marrow MSCs+β-TCP scaffold | 300 RPM 14 days |
Fluid continuous | N | “Significant increase in col 2” |
| Gemmiti et al.73 | Juvenile bovine chondrocytes+scaffold free | 0.1 Pa 7 days |
Fluid continuous | N | 79% increase in EY 86% increase in UTS 100% increase in col II |
| Gemmiti et al.74 | Juvenile bovine chondrocytes+scaffold free | 0.15 Pa 3 days |
Fluid continuous | N | 2.5-fold increase in EY 42% increase in UTS 1.4-fold increase in col II |
| Waldman et al.85 | Juvenile bovine chondrocytes+porous calcium phosphate | 2% strain 1 Hz 7 days |
Direct oscillatory | N | 40% increase in col II 35% increase in GAG |
| Grad et al.75 | Juvenile bovine chondrocytes+polyurethane scaffold | 15% strain 1 Hz 21 days |
Direct oscillatory | N | 37% decrease in friction coefficient “More pronounced staining for col 2” |
Table of research articles studying shear stress stimulation on AC constructs. The quantitative increases reported in the column labeled “enhanced design criteria” refer to comparisons between nonstimulated controls with no bioactive factors and stimulated controls. The results were either taken directly from the referenced article or calculated from their reported data. Quotations directly from the referenced article were used when quantitative data were not available.
Shear bioreactors
Fluid-induced shear stimulation requires fluid flow across the surface of TEAC. Many of the bioreactors used for fluid-induced shear stimulation are known as perfusion bioreactors because, in many configurations, medium moves through the pores of the tissue as well. Mechanically stirred bioreactors include spinner flasks, which produce a high-shear and turbulent environment.11 Positive outcomes include improved ECM retention, but the high-shear environment results in increased levels of apoptosis and cell lysis.11,81 In contrast, low-shear bioreactors use rotating walls or parallel plates. The mechanical force applied in low-shear bioreactors is usually below 0.5 Pa and conducive to increased ECM content and chondrogenic phenotypes without being harmful to cells.11
To ensure sustained contact for shear application, direct-shear bioreactors typically compress constructs while applying shear stress to mimic the compressive rolling action of articulating joints.82 Thus, TEAC in direct-shear bioreactors often experience 2–10% compressive strain and 0.1–1 Pa shear stress.65,76,77,82,83 However, direct shear has shown conflicting results, ranging from no significant differences in ECM content to a 35% increase in GAG content and 40% increase in collagen II content.65,84,85
Shear stress improvements of TEAC tensile properties
Fluid-induced shear stress has yielded increases in tensile properties of scaffold-free TEAC. A parallel plate bioreactor was used to induce a shear stress of 0.15 Pa on TEAC. The EY of the stimulated tissue increased to 2.28 MPa compared to the 1.55 MPa of statically cultured controls.73 A second study using the same methods tested shear stimulation at 0.1 Pa and showed EY and UTS improve to 5 MPa and 1.3 MPa, respectively.74 These studies show that shear is an effective tactic for increasing tensile properties and that the benefits may be optimized within a narrow range of stress.
Shear stress increases of TEAC collagen content
Enhancement in collagen deposition may be attained with shear stimulation.73,74,85 Using a solid sphere, at 2% strain, shear elicited a 40% increase in collagen II content compared to nonstimulated controls. Conversely, groups stimulated at 6% and 12% shear strain exhibited deleterious effects on collagen II content.85 Studies investigating the effects of fluid-induced shear also found that 0.1 Pa yielded the highest percentage of collagen II (7.5%) compared to nonstimulated controls (3.7%).74 These studies show that collagen II content increases significantly in AC constructs when cultured under low magnitudes of shear.
Direct shear improvements of TEAC tribology
The application of direct shear on chondrocyte-seeded polyurethane scaffolds yielded a significant decrease in the boundary lubrication friction coefficient from 0.681 to 0.427.75 Certain bioactive factors, such as interleukin-1β (IL-1β), TGF-β1, and oncostatin M, have also been found to alter the frictional properties of AC constructs, but they have not been tested in combination with mechanical stimulation.86 It was also found that TEAC that underwent shear forces in two directions had an even lower friction coefficient (0.251) than those loaded in only one direction.75 This was shown to validate that gliding motions on the articulating surface of TEAC during culture significantly decreases friction coefficients.87
Shear stress-aided integration
There is some evidence that suggests that the use of fluid shear bioreactors, such as spinner flasks, promotes integration of TEAC with native AC.88,89 It has been shown that shear-stimulated TEAC was better integrated with surrounding tissues in an in vivo model than TEAC that was cultured statically. It was also found that collagen matrix organization was better in the shear-stimulated groups.89 A separate study used a spinner flask to enhance integration in an in vitro model. This study created defects in native AC explants, press-fitted them with TEAC, and cultured the pair in a spinner flask set to 90 RPM, showing better integration with the surrounding native AC tissue.88 Although the mechanisms behind the results of these studies are unclear, the potential for using mechanical stimulation, such as continuous passive motion (CPM), early on in postoperative physical therapies to promote integration has been elucidated.88 To investigate further, mechanical stimulation studies that include in vivo phases may consider the use of CPM to enhance integration and functionality of the implant.
Hydrostatic pressure
Under HP, tissues and cells experience uniform and normal compression on all surfaces (Fig. 2d). HP has been a popular form of mechanical stimulation in the field for over 15 years because it is experienced by native AC in every aspect of joint movement.18 Native AC encounters HP when negatively charged proteoglycans trap fluid within the cartilage matrix during joint loading.18 Physiologically, AC typically experiences 3–10 MPa of HP.90,91 Because HP does not shear or deform the essentially incompressible tissues, damage to the ECM is minimized during in vitro stimulation.11
HP bioreactors
HP bioreactors have a fluid-filled chamber and a piston that applies pressure to the chamber and subsequently the tissue.92–95 Research has focused on stimulating TEAC with HP at magnitudes ranging from 3 to 18 MPa and in general should not exceed 30 MPa because it alters chondrocyte proteoglycan synthesis.90,92,96 Both passive and dynamic (up to 1 Hz) HPs have been investigated, yielding improved mechanical properties, ECM protein expression, and ECM content.90,91,97,98 As an example, dynamic HP stimulation (0.5 MPa at 0.5 Hz) is used commercially to enhance sGAG production in constructs.99,100 These applications suggest that HP can be a necessary accessory toward increasing matrix synthesis in TEAC.
HP enhancements of TEAC compressive moduli
A few studies have found that passive HP culturing regimes result in an enhancement of compressive moduli in self-assembling cartilage tissues. For example, HA peaked at 238 kPa in self-assembling cartilage constructs stimulated at stresses under 10 MPa for 1 h a day for 14 days. HP stimulation for longer than 14 days was deleterious to compressive moduli.101 The combination of TGF-β1 and passive HP of 10 MPa increased the HA by nearly twofold compared to either stimulus alone.102 Although the effects of HP stimulation on TEAC mechanical properties have not been heavily investigated, these studies suggest that short-term HP is a potent stimulus for enhancing compressive moduli.
HP increases of TEAC sGAG content
A 1.3-fold increase in sGAG was found in AC constructs derived from deep zone chondrocytes when exposed to HP compared to static controls.48 Self-aggregating suspension cultures stimulated with passive HP yielded a significant increase of 64% more GAG per chondrocyte.103 When stimulated between 7 and 10 MPa, GAG content was significantly increased in tissues derived from juvenile chondrocytes.48,97,102 These studies, along with others shown in Table 3, show that HP stimulation enhances GAG content in TEAC.
Table 3.
Hydrostatic Pressure
| Reference | Cell+scaffold type | Loading parameters | Waveform | Bioactive factors (Y/N) | Enhanced design criteria |
|---|---|---|---|---|---|
| Kraft et al.103 | Porcine chondrocytes+scaffold free | 5 MPa 0.1 Hz 21 days |
Dynamic Intermittent |
N | 12% increase in total col 64% increase in GAG |
| Correia et al.70 | Human adipose SCs+gellan gum hydrogels | 5 MPa 0.5 Hz 28 days |
Dynamic Intermittent |
N | 57% increase in GAG |
| Elder et al.102 | Juvenile bovine chondrocytes+scaffold free | 10 MPa 4 days |
Passive Intermittent |
Y | 1.6-fold increase in HA 2.3-fold increase in EY 1.7-fold increase in total col 84% increase in GAG |
| Gunja et al.94 | Mature leporine meniscus cells+PLLA scaffold | 10 MPa 28 days |
Passive Intermittent |
Y | 100% increase in Ei 100% increase in Er 2.75-fold increase in total col |
| Chen et al.95 | Porcine chondrocytes+PGA scaffold | 5 MPa 56 days |
Passive Intermittent |
N | Fivefold increase in EY |
| Gunja et al.98 | Mature leporine meniscus cells+PLLA scaffold | 10 MPa 28 days |
Passive Intermittent |
N | 60% increase in Ei 100% increase in Er Twofold increase in total col Twofold increase in GAG |
| Heyland et al.93 | Porcine chondrocytes+alginate beads | 0.3 MPa 7 days |
Passive Intermittent |
N | “65% increase in col2/col1 ratio” |
| Elder et al.101 | Juvenile bovine chondrocytes+scaffold free | 10 MPa 4 days |
Passive Continuous |
N | 1.6-fold increase in HA 63% increase in EY |
Table of research articles on hydrostatic pressure stimulation on AC constructs. The quantitative increases reported in the column labeled “enhanced design criteria” refer to comparisons between nonstimulated controls with no bioactive factors and stimulated controls. The results were either taken directly from the referenced article or calculated from their reported data. Quotations directly from the referenced article were used when quantitative data were not available.
Tension
Tensile forces are applied on engineered tissues by directly pulling the tissue outward along the edges (Fig. 2b) resulting in axial strain. Tension may be delivered to TEAC in a uniaxial or biaxial manner (Fig. 2b). Very few studies have explored the effects of tensile stimulation on TEAC. However, the potential for developing robust AC constructs using passive uniaxial tension to stimulate mechanosensitive ion channels has been elucidated and has yielded TEAC at 90% native AC tensile properties and collagen content.104
Tension bioreactors
In the most common uniaxial tension bioreactor, the tissue is draped over hooks, or clamped, along the opposing edges and pulled away.105 Biaxial tension bioreactors stimulate mechanically using equidistant rakes attached along all edges of the tissue that move apart and remain equidistant during loading to attain uniform deformation across the tissue.106,107 Both uniaxial and biaxial tension are applied passively or dynamically usually within 2–15% strain, but the most promising outcomes thus far have followed passive uniaxial tension in combination with bioactive factors such as TGF-β1.104
Passive uniaxial tension enhancements of TEAC tensile properties
One recent study produced tensile stiffness reaching 94% and 60% of native AC EY and UTS with application of continuous passive tension stimulation. The constructs were strained to 12–15% on the first day of stimulation and an additional 4–5% per day for 5 days.104 In this study, a bioactive regimen of TGF-β1, chondroitinase-ABC (C-ABC), and lysyl oxiolase-like 2 (LOX-L2) was combined with passive tensile stimulation on self-assembling AC constructs derived from human chondrocytes.104 Compared to nonstimulated controls, the addition of passive tensile stimulation and bioactive stimuli elicited a sixfold increase in both EY and UTS. Uniaxial tension is seldom investigated for enhancing mechanical properties in TEAC, but the results presented in this study suggest that it is a potent regimen for improving tensile properties.
Tension increases of TEAC GAG content
Uniaxial tensile loading has been found to enhance GAG content in self-assembling AC constructs by an estimated 33%.104 The effect of tension stimulation has also been investigated on AC constructs derived from chondrocytes of the deep zone, middle zone, and superficial zone of bovine AC. In particular, superficial zone chondrocytes are significantly more responsive to tensile loading, leading to a 20.6% increase in sGAG production.47 Although studies in tensile stimulation are limited, current research shows encouraging results toward increased GAG production (Table 4).
Table 4.
Tension
| Reference | Cell+scaffold type | Loading parameters | Waveform | Bioactive factors (Y/N) | Enhanced design criteria |
|---|---|---|---|---|---|
| Vanderploeg et al.47 | Juvenile bovine chondrocytes+fibrin gel | 10% strain 1 Hz 2 days |
Uniaxial Dynamic Continuous |
N | “Further increased DNA content ….and cell viability” |
| Vanderploeg et al.108 | Juvenile bovine chondrocytes+fibrin hydrogel | 5% strain 1 Hz 3 days |
Uniaxial Dynamic Intermittent |
N | 12.3% increase in total col 12.9% increase in sGAG |
| Connelly et al.109 | Juvenile bovine BMSCs+Fibrin gel | 10% strain 1 Hz 14 days |
Uniaxial Dynamic Intermittent |
N | 27% increase in total col 12.5% increase in sGAG |
| Lee et al.104 | Human chondrocytes+scaffold free | 4–15% strain 5 days |
Uniaxial Passive Continuous |
Y | Threefold increase in HA Fourfold increase in EY 4.3-fold increase in UTS |
| Fan et al.19 | Juvenile bovine chondrocytes+scaffold free | 16% strain 28 days |
Biaxial Passive Intermittent |
N | 1.2-fold increase in total col |
Table of research articles on tensile stimulation on AC constructs. The quantitative increases reported in the column labeled “enhanced design criteria” refer to comparisons between nonstimulated controls with no bioactive factors and stimulated controls. The results were either taken directly from the referenced article or calculated from their reported data. Quotations directly from the referenced article were used when quantitative data were not available.
Uniaxial tension-aided organization of TEAC ECM
Uniaxial tension develops surface anisotropy that is similar to that of native AC.104 Fiber organization in TEAC is not improved upon the addition of bioactive factors alone. However, when used together, uniaxial tension and C-ABC lead to a dramatic change in anisotropy.104 Fiber organization is achieved because the catabolic enzyme chondroitinase-ABC cleaves and removes excess GAGs, while uniaxial tension provides physical reorganization of the ECM.104
Perspectives
The role of mechanical stimulation has been experimentally confirmed in vitro as a way to enhance design criteria in TEAC. It has been shown that TEAC properties benefit from a narrow range of loading magnitudes and durations in DC and HP stimulation because both excessive and insufficient loading can lead to deleterious consequences. For example, compressive moduli increase with the application of either DC or HP, and they both show that excessive stress (>10 MPa) and strain (>20%) can be detrimental (Fig. 3).
Although dynamic DC is beneficial for long durations, studies in HP have shown short-term passive stimulation to work best for improving compressive moduli. This suggests that different types of mechanical stimulation may be applied in tandem to further improve multiple design criteria. The different mechanotransduction mechanisms through which DC and HP affect the engineered tissue should be elucidated to clarify this difference in optimal loading regimens.
TEAC research in tension and shear stimulation is not as extensive as in DC and HP. This may be due to the association of shear and tensile loads to cartilage damage in vivo. However, shear stimulation has produced up to a 257% increase in EY, and tension stimulation has produced AC with nearly biomimetic EY and UTS. Furthermore, both shear stress and tension have led to enhancements in TEAC properties, such as fiber organization and integration, which have been elusive under static cultures or DC and HP. Beneficial loading parameters for tension and shear stimulation should be further investigated and expanded upon by assessing all design criteria.
There is also a stark unevenness in the amount of research and literature among TEAC design criteria. For example, attaining biomimetic tensile properties in TEAC has proven to be a challenging feat, yet the effects of mechanical stimulation on tensile properties have been scarcely investigated. In contrast, an extensive number of studies show that TEAC cultured with DC, tension, and HP stimulation yields increases in GAG composition. For example, a comparison of HP and tension stimulation studies suggests that GAG production is partially regulated by tensile stimulation in superficial zone chondrocytes and by HP in deep zone chondrocytes. To engineer AC to translatability, salient design criteria (e.g., compressive and tensile properties, ECM content, cellular viability, ECM organization, tribology, integration, and durability) must be investigated fully in mechanical stimulation studies.
To identify ranges of beneficial loading for each type of mechanical stimulation technique, investigators must adequately report loading parameters and TEAC characteristics. Adequately reporting loading parameters includes specifying the mechanical loads in units that normalize to the characteristics of the TEAC scaffold or matrix. For example, when reporting deformation, units of strain should be used instead of length. Furthermore, not all mechanical stimulation studies report construct characteristics fully. In particular, mechanical and tribology properties should be reported, collagen fibril dimensions, organization, and specific type should be included with total content, and in vivo durability must be assessed whenever possible. Thus, this information must be included in all studies where mechanical stimulation is investigated to avoid reaching incomplete conclusions.
Mechanical stimulation has proven itself a powerful addition to AC engineering procedures. Static cultures are inadequate in AC engineering because the lack of inherent vascularization and mechanical loading leads to limited nutrient and waste transport. The studies shown in this study confirm that, compared to static AC tissue cultures, mechanical stimulation is a valuable promoter of ECM synthesis and concomitant mechanical property enhancement. Furthermore, the addition of mechanical stimulation has also yielded characteristics, such as matrix organization, that were not previously attainable with bioactive factors alone.
From the physical pressure gradients that lead to mass nutrient transport in dynamic DC and shear to the tension-driven activation of TRPV 4 ion channels, mechanical stimulation strategies drive the increase of important ECM components and enhance mechanical properties. Toward expanding translatability, the vast potential of mechanical stimulation needs to be explored to aid integration of TEAC within the diarthrodial joint. The routine inclusion of mechanical stimulation in culture regimes may lead to additive and synergistic enhancements in design criteria necessary for the successful tissue engineering of AC.
Disclosure Statement
No competing financial interests exist.
References
- 1.Afoke N., Byers P., and Hutton W. Contact pressures in the human hip joint. J Bone Joint Surg Br 69, 536, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Eisenhart R., Adam C., Steinlechner M., and Eckstein F. Quantitative determination of joint incongruity and pressure distribution during simulated gait and cartilage thickness in the human hip joint. J Orthop Res 17, 532, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Kalichman L., Li L., Kim D., et al. . Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila. Pa. 1976) 33, 2560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makris E., Gomoll A., Malizos K., Hu J., and Athanasiou K. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11, 21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K., Zhang C., Qiu L., Gao L., and Zhang X. Advances in application of mechanical stimuli in bioreactors for cartilage tissue engineering. Tissue Eng Part B Rev 23, 399, 2017 [DOI] [PubMed] [Google Scholar]
- 6.El-Ayoubi R., DeGrandpre C., DiRaddo R., and Yousefi A. Design and dynamic culture of 3D-scaffolds for cartilage tissue engineering. J Biomater Appl 25, 429, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Mauck R., Nicoll S., Seyhan S., Ateshian G., and Hung C. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng 9, 597, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Natenstedt J., Kok A., Dankelman J., and Tuijthof G. What quantitative mechanical loading stimulates in vitro cultivation best? J Exp Orhtop 2, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA. Guidance for industry: preparation of IDEs and INDs for products intended to repair or replace knee cartilage. U.S. Food and Drug Administration, 2011 [Google Scholar]
- 10.Grad S., Eglin D., Alini M., and Stoddart M. Physical stimulation of chondrogenic cells In vitro: a review. Clin Orthop Relat Res 469, 2764, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darling E., and Athanasiou K. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 9, 9, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Williamson A., Chen A., and Sah R. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res 19, 1113, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Park S., Hung C., and Ateshian G. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage 12, 65, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Athanasiou K., Responte D., Brown W., and Hu J. Harnessing biomechanics to develop cartilage regeneration strategies. J Biomech Eng 137, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athanasiou K., Darling E., Hu J., Durain G., and Reddi H. Articular Cartilage. Boca Raton, FL: CRC Press, LLC, 2017. [Google Scholar]
- 16.Athanasiou K., Agarwal A., and Dzida F. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res 12, 340, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Desrochers J., Amrein M., and Matyas J. Viscoelasticity of the articular cartilage surface in early osteoarthritis. Osteoarthrithis Cartilage 20, 413, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Narmoneva D., Wang J., and Setton L. Nonuniform swelling-induced residual strains in articular cartilage. J Biomech 32, 401, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Fan J., and Waldman S. The effect of intermittent static biaxial tensile strains on tissue engineered cartilage. Ann Biomed Eng 38, 1672, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Wortman J., and Evans R. Young's modulus, shear modulus, and poisson's ratio in silicon and germanium. J Appl Phys 36, 153, 1965 [Google Scholar]
- 21.Mansour J. Biomechanics of Cartilage. In Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore, MD: Wolters Kluwer Health, 2013, pp. 66–79 [Google Scholar]
- 22.Williamson A., Chen A., Masuda K., and Sah R. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orhtop Res 21, 872, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Eyre D. Collagen of articular cartilage. Arthritis Res 4, 30, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieminen J. Effect of functional loading on remodelling in canine, and normal and collagen type II transgenic murine bone [PhD dissertation thesis]. Department of Anatomy and Institute of Clinical Medicine, University of Kuopio, Kuopio, Finland, 2009 [Google Scholar]
- 25.Gelse K., Po E., and Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55, 1531, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Viguet-carrin S., Garnero P., and Delmas P. The role of collagen in bone strength. Osteoporos Int 17, 319, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Cissell D., Link J., Hu J., and Athanasiou K. A modified hydroxyproline assay based on hydrochloric acid in ehrlich's solution accurately measures tissue. Tissue Eng Part C Methods 23, 243, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts S., Menage J., Sandell L., Evans E., and Richardson J. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 5, 398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyre D., Weis M., and Wu J. Advances in collagen cross-link analysis. Methods 45, 65, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckwalter J., and Mankin H. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect 47, 477–486, 1998 [PubMed] [Google Scholar]
- 31.Bayliss M., Osborne D., Woodhouse S., and Davidson C. Sulfation of chondroitin sulfate in human articular cartilage: the effect of age, topographical position, and zone of cartilage on tissue composition. J Bio Chem 274, 15892, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Kuiper N., and Sharma A. A detailed quantitative outcome measure of glycosaminoglycans in human articular cartilage for cell therapy and tissue engineering strategies. Osteoarthritis Cartilage 23, 2233, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Siebelt M., Groen H., Koelewijn S., et al. . Increased physical activity severely induces osteoarthritic changes in knee joints with papain induced sulfate-glycosaminoglycan depleted cartilage. Arthritis Res Ther 16, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farndale R., Buttle D., and Barrett A. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochem Biophys Acta 2, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Calabro A., Midura R., Wang A., West L., Plaas A., and Hascall V. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthrithis Cartilage 9, 16, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Lin W., Chang Y., Wang H., et al. . The study of the frequency effect of dynamic compressive loading on primary articular chondrocyte functions using a microcell culture system. Biomed Res Int 2014, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Susante L., Pieper J., Buma P., et al. . Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 22, 2359, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Sophia Fox A., Bedi A., and Rodeo S. The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverberg J., Barrett A., Das M., Petersen P., Bonassar L., and Cohen I. Structure-function relations and rigidity percolation in the shear properties of articular cartilage. Biophys J 7, 1721, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stolz M., Raiteri R., Daniels A., Vanlandingham M., and Baschong W. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J 86, 3269, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazaeli S., Ghazanfari S., Everts V., Smit T., and Koolstra J. The contribution of collagen fibers to the mechanical compressive properties of the temporomandibular joint disc. Osteoarthritis Cartilage 24, 1292, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Jurvelin J., Buschmann M., and Hunziker E. Mechanical anisotropy of the human knee articular cartilage in compression. Proc Inst Mech Eng H 217, 215, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Julkunen P., Jurvelin J., and Isaksson H. Contribution of tissue composition and structure to mechanical response of articular cartilage under different loading geometries and strain rates. Biomech Model Mechanobiol 9, 237, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Peng G., McNary S., Athanasiou K., and Reddi H. Surface zone articular chondrocytes modulate the bulk and surface mechanical properties of the tissue-engineered cartilage. Tissue Eng Part A 20, 3332, 2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jay G., and Waller K. The biology of lubricin: near frictionless joint motion. Matrix Biol 39, 17, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Schuurman W., Klein J., Dhert W., Van Weeren P., Hutmacher D., and Malda J. Cartilage regeneration using zonal chondrocyte subpopulations: a promising approach or an overcomplicated strategy? J Tissue Eng Regen Med 9, 669, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Vanderploeg E., Wilson C., and Levenston M. Articular chondrocytes derived from distinct tissue zones differentially respond to in vitro oscillatory tensile loading. Osteoarthritis Cartilage 16, 1228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizuno S., and Ogawa R. Using changes in hydrostatic and osmotic pressure to manipulate metabolic function in chondrocytes. Am J Physiol Cell Physiol 300, 1234, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Robins S., and Duncan A. Cross-linking of collagen: location of pyridinoline in bovine articular cartilage at two sites of the molecule. Biochem J 1, 175, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNerny E., Gardinier J., and Kohn D. Exercise increases pyridinoline cross-linking and counters the mechanical effects of concurrent lathyrogenic treatment. Bone 81, 327, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Below S., Arnoczky S., Dodds J., Kooima C., and Walter N. The split-line pattern of the distal femur: a consideration in the orientation of autologous cartilage grafts. Arthroscopy 18, 613, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Gruber H., and Wiggins W. Methods for transmission and scanning electron microscopy of bone and cartilage. In: An Y.H., Martin K.L., eds. Handbook of Histology Methods for Bone and Cartilage. Humana Press, 2003, pp. 497–504 [Google Scholar]
- 53.Kienle S., Boettcher K., Wiegleb L., et al. . Comparison of friction and wear of articular cartilage on different length scales. J Biomech 48, 3052, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Chang D., Guilak F., Jay G., and Zauscher S. Interaction of lubricin with type II collagen surfaces: adsorption, friction, and normal forces. J Biomech 47, 659, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Setton L., Zhu W., and Mow V. The biphasic poroviscoelasic behavior of articular cartilage role of the surface zone in governing the compressive behavior. J Biomech 26, 581, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Gleghorn J.P., and Bonassar L. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech 41, 1910, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Atarod M., Ludwig T., Frank C., Schmidt T., and Shrive N. Cartilage boundary lubrication of ovine synovial fluid following anterior cruciate ligament transection: a longitudinal study. Osteoarthritis Cartilage 23, 640, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Blum M., and Ovaert T. Low friction hydrogel for articular cartilage repair: evaluation of mechanical and tribological properties in comparison with natural cartilage tissue. Mater Sci Eng C Mater Biol Appl 33, 4377, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Responte D., Lee J., Hu J., and Athanasiou K. Biomechanics-driven chondrogenesis: from embryo to adult. FASEB J 26, 3614, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roddy K., Prendergast P., and Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS One 6, e17526, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall B., and Herring S. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol 206, 45, 1990 [DOI] [PubMed] [Google Scholar]
- 62.Nowlan N., Sharpe J., Roddy K., Prendergast P., and Murphy P. Mechanobiology of embryonic skeletal development: insights from animal models. Birth Defects Res C Embryo Today 90, 203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kisiday J., Jin M., Dimicco M., Kurz B., and Grodzinsky A. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech 37, 595, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Nebelung S., Gavenis K., Lüring C., et al. . Simultaneous anabolic and catabolic responses of human chondrocytes seeded in collagen hydrogels to long-term continuous dynamic compression. Ann Anat 194, 351, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Shahin K., and Doran P. Tissue engineering of cartilage using a mechanobioreactor exerting simultaneous mechanical shear and compression to simulate the rolling action of articular joints. Biotechnol Bioeng 109, 1060, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Elder B., and Athanasiou K. Effects of confinement on the mechanical properties of self-assembeled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res 26, 238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huwe L., Sullan G., Hu J.C., and Athanasiou K. Using costal chondrocytes to engineer articular cartilage with applications of passive axial compression and bioactive stimuli. Tissue Eng Part A 24(5–6), 516, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suh J. Dynamic unconfined compression of articular cartilage under a cyclic compressive load. Biorheology 33, 289304, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Tran S., Cooley A., and Elder S. Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotechnol Bioeng 108, 1421, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Correia C., Pereira A., Duarte A., Frias A., Pedro A., and Oliveira T. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A 18, 1979, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mauck R., Soltz M., Wang C., Wong D., Chao P., and Ateshian G. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122, 252, 2000 [DOI] [PubMed] [Google Scholar]
- 72.MacBarb R., Paschos N., Abeug R., Makris E., Hu J., and Athanasiou K. Passive strain-induced matrix synthesis and organization in shape-specific, cartilaginous neotissues. Tissue Eng Part A 20, 3290, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gemmiti C., and Guldberg R. Fluid flow increases type II collagen deposition and tissue-engineered cartilage. Tissue Eng 12, 7, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Gemmiti C., and Guldberg R. Shear stress magnitude and duration modulates matrix composition and tensile mechanical properties in engineered cartilaginous tissue. Biotechnol Bioeng 104, 809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grad S., Loparic M., Peter R., Stolz M., Aebi U., and Alini M. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthritis Cartilage 20, 288, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Stoddart M., Ettinger L., Jo H., and Zu C. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng 95, 1043, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Yusoff N., Azuan N., Osman A., and Pingguan-murphy B. Design and validation of a bi-axial loading bioreactor for mechanical stimulation of engineered cartilage. Med Eng Phys 33, 782, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Sun M., Lv D., Zhang C., and Zhu L. Culturing functional cartilage tissue under a novel bionic mechanical condition. Med Hypotheses 75, 657, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Zhu G., Mayer-wagner S., Schröder C., et al. . Comparing effects of perfusion and hydrostatic pressure on gene profiles of human chondrocyte. J Biotechnol 210, 59, 2015 [DOI] [PubMed] [Google Scholar]
- 80.Freyria A., Cortial D., Ronziere M., Guerret S., and Herbage D. Influence of medium composition, static and stirred conditions on the proliferation of and matrix protein expression of bovine articular chondrocytes cultured in a 3-D collagen scaffold. Biomaterials 25, 687, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Zhao J., Griffin M., Cai J., Li S., Bulter P., and Kalaskar D. Bioreactors for tissue engineering: an update. Biochem Eng J 109, 268, 2016 [Google Scholar]
- 82.Gharravi A., Orazizadeh M., Ansari-asl K., and Banoni S. Design and fabrication of anatomical bioreactor systems containing alginate scaffolds for cartilage tissue engineering. Avicenna J Med Biotechnol 4, 65, 2012 [PMC free article] [PubMed] [Google Scholar]
- 83.Di Federico E., Bader D., and Shelton J. Design and validation of an in vitro loading system for the combined application of cyclic compression and shear to 3D chondrocytes-seeded agarose constructs. Med Eng Phys 36, 534, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Bian L., Fong J., Lima E., Stoker A., Ateshian G., and Cook J. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A 16, 1781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waldman S., Spiteri C., Grynpas M., Pilliar R., and Kandel R. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res 21, 590, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Gleghorn J., Jones A., Flannery C., and Bonassar L. Alteration of articular cartilage frictional properties by transforming growth factor-beta, interleukin-1Beta, and oncostatin M. Arthritis Rheum 60, 440, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Wimmer M., Alini M., and Grad S. The effect of sliding velocity on chondrocytes activity in 3D scaffolds. J Biomech 42, 424, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Theodoropoulos J., DeCroos A., Petrera M., Park S., and Kandel R. Mechanical stimulation enhances integration in an in-vitro model of cartilage repair. Knee Surg Sport Traumatol Arthosc 24, 2055, 2016 [DOI] [PubMed] [Google Scholar]
- 89.Pei Y., Fan J., Zhang X., Zhang Z., and Yu M. Repairing the osteochondral defect in goat with the tissue-engineered osteochondral graft preconstructed in a double-chamber stirring bioreactor. Biomed Res Int 2014, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elder B., and Athanasiou K. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev 15, 43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith L., Lin J., Trindade M., Shida J., and Kajiyama G. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev 37, 153, 2000 [PubMed] [Google Scholar]
- 92.Smith L., Rusk S., Ellison B., Wessels P., Tsuchiya K., and Carter D. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res 14, 53, 1996 [DOI] [PubMed] [Google Scholar]
- 93.Heyland J., Wiegandt K., Goepfert C., Scumacher U., and Portner R. Redifferentiation of chondrocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol Lett 28, 1641, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Gunja N., and Athanasiou K. Effects of hydrostatic pressure on leporine meniscus cell-seeded PLLA scaffolds. J Biomed Mater Res A. 92A, 896, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Chen J., Yuan Z., Liu Y., Zheng R., Dai Y., and Tao R. Improvement of in vitro three-dimensional cartilage regeneration by a novel hydrostatic pressure bioreactor. Stem Cells Transl Med 6, 982, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parkkinen J., Lammi M., Pelttari A., Helminen H., Tammi M., and Virtanen I. Altered golgi apparatus in hydrostatically loaded articular cartilage chondrocytes. Ann Rheum Dis 52, 192, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu J., and Athanasiou K. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng 12, 1337, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Gunja N., Uthamathil R., and Athanasiou K. Effects of TGF-beta1 and hydrostatic pressure on mensicus cell-seeded scaffolds. Biomaterials 30, 565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang B., Hu J., and Athanasiou K. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizuno S., Kusanagi A., Tarrant L., Tokuno T., and Smith R. System useful for repairing cartilage, comprises lyophilized acellular collagen matrix containing pores and bioactive agent disposed within the pores patent. United States patent US20130273121A1. 2013
- 101.Elder B., and Athanasiou K. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A Part A 15, 1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elder B., and Athanasiou K. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One 3, e2341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kraft J., Jeong C., Novotny J., et al. . Effects of hydrostatic loading on a self-aggregating, suspension culture-derived cartilage tissue analog. Cartilage 2, 254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J., Huwe L., Paschos N., et al. . Tension stimulation drives tissue formation in scaffold-free systems. Nat Mater 16, 864, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu S., Wang Y., Streubel P., and Duan B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell triculture and mechanical stimulation. Acta Biomater 62, 102, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bieler F., Ott C., Thompson M., et al. . Biaxial cell stimulation: a mechanical validation. J Biomech 42, 1692, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Wartella K., and Wayne J. Bioreactor for biaxial mechanical stimulation to tissue engineered constructs. J Biomech Eng 131, 1, 2017 [DOI] [PubMed] [Google Scholar]
- 108.Vanderploeg E.J., Imler S.M., Brodkin K.R., Garcia A.J., et al. . Oscillitory tension differentially modulates matrix metabolism and cytoskeletal organization in chondroctyes and fibrochondrocytes. J Biomech 37, 1913, 2004 [DOI] [PubMed] [Google Scholar]
- 109.Connelly J.T., Wilson C.G., and Levenston M.E. Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthr Cartil 16, 1228, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]