There is plenty of evidence that proves the beneficial and reliable effects of rehabilitation therapy, making it the most common treatment for patients with chronic stroke. It is believed that rehabilitation improves functional recovery through neural network remodeling, which is observed as a motor map reorganization or functional connectivity change assessed by intracortical microstimulation or functional magnetic resonance imaging (MRI). This neural network remodeling results from morphological changes, such as dendritic arborization, axonal sprouting, and synapse formation in surviving neurons. Among the various neural networks, descending spinal pathways play a pivotal role in executing voluntary motor tasks since these pathways send motor commands generated from the motor cortex to the spinal cord, which directly controls muscle contraction. Thus, destruction of these pathways causes impairment of motor functions. Descending spinal pathways include direct pathways (e.g., corticospinal pathway) and indirect pathways (e.g., rubrospinal and reticulospinal pathways). While most studies have focused on the corticospinal pathway, both direct and indirect descending spinal pathways are known to be reorganized through axonal remodeling after stroke, to compensate the functional roles of the destroyed neural pathways. Axonal remodeling in these pathways can be promoted by therapeutic intervention including brain stimulation (e.g., direct current stimulation, optogenetic stimulation), blockade of growth inhibiting molecules (e.g., anti-NogoA immunotherapy, chondroitinase ABC), and rehabilitative therapy. Recently, we demonstrated that rehabilitation-induced axonal remodeling in the corticospinal pathway plays a significant role in functional recovery after stroke (Okabe et al., 2016). Nevertheless, our studies also revealed the limitations of rehabilitation in functional recovery and neural network remodeling (Okabe et al., 2017, 2018). In these studies, we investigated the effect of various rehabilitative trainings [skilled forelimb reaching task, constraint induced movement therapy (CIMT), rotarod and treadmill] on functional recovery after partial or total destruction of motor cortex. We also carried out comprehensive histological analysis assessing changes of the descending spinal pathway originating from the cerebral cortex, diencephalon, mesencephalon, cerebellum and brainstem using a retrograde tracer. Our study demonstrated that although animals trained by the skilled forelimb reaching task attained almost complete recovery of motor performance in the trained task (task-specific recovery) after partial destruction of motor cortex (Okabe et al., 2016, 2017), rehabilitation-induced functional recovery was severely impeded when the majority of ipsilesional corticospinal neurons were destroyed by total destruction of the motor cortex (Okabe et al., 2018). Importantly, our severe stroke model destroyed most of the ipsilesional corticospinal neurons but preserved other spinal projection neurons which account for more than 80% of the total descending spinal projection neurons. These results suggest that corticospinal neurons are crucial for rehabilitation-induced functional recovery, and that spinal projections from deep brain areas including rubrospinal and reticulospinal projections cannot compensate the role of corticospinal projections. This finding is consistent with clinical data demonstrating that the integrity of the corticospinal tract assessed by MRI tractography predicts functional potential in chronic stroke patients (Stinear et al., 2007). Our study also revealed that while rehabilitative training restored ipsilesional corticospinal projections through axonal remodeling, rehabilitation could not cause significant changes in any other descending spinal pathways (e.g., rubrospinal and reticulospinal pathways), regardless of the training method applied or severity of stroke (Okabe et al., 2018). Since axonal remodeling after brain injury is activity-dependent, these results suggest rehabilitative training would not significantly affect the neural activity in the brain area other than the ipsilesional cerebral cortex. Otherwise, descending spinal pathways from deep brain area may have limited plasticity compared to the corticospinal pathway. Actually, Bachmann et al. (2014) also reported that the increase of spinal projection neurons caused by axonal remodeling in the brainstem spinal pathway during spontaneous recovery after severe cortical stroke is less significant than that in the corticospinal pathway. Taken together, rehabilitation can promote functional recovery if ipsilesional corticospinal projections are preserved, but it is ineffective when there are insufficient corticospinal projections available, because rehabilitation cannot induce significant reorganization in the contralesional corticospinal pathway or descending spinal pathways from deep brain areas.
What strategies could be used to enhance rehabilitation-induced functional recovery in severe stroke patients in which most corticospinal projections are destroyed? To discuss this problem, we divided the restoration of corticospinal projections into three types. Although each type of restoration could be enhanced by rehabilitative training, all of them had limitations. The first type of restoration is via a rerouting of neural pathways (Figure 1b). Previous studies reported that corticospinal neurons in the contralesional motor cortex, ipsilesional hindlimb area and ipsilesional secondary motor area form new connections to the spinal area which control the movement of the affected forelimb. As described above, rehabilitative training can promote this type of restoration depending on the ipsilesional corticospinal projection. Thus, the effect of rehabilitation is limited by the integrity of the corticospinal tract. The second type of restoration is a relay of neural pathways (Figure 1c), in which corticospinal neurons with injured spinal projections form new connections to the deep brain nucleus, such as the red nucleus and reticular formation, and make a bypass route to the spinal cord. While complete destruction of corticospinal neurons severely impedes rehabilitation-induced functional recovery, it has been demonstrated that rehabilitative training significantly improves functional recovery even after complete transection of the corticospinal pathway (Mosberger et al., 2018). Therefore, it seems possible that rubrospinal or reticulospinal projections can take over the role of corticospinal projections if neural projections from the motor cortex to the deep brain area are available. However, ipsilesional corticospinal neurons are also necessary for restoration by the relay process, since animals with severe cortical stroke which destroys most ipsilesional corticospinal neurons show almost no recovery. Furthermore, it may be less effective in patients because the rubrospinal pathway is small and rudimentary in humans compared to rodents. The third type of restoration is via regeneration of corticospinal neurons (Figure 1d). Although regeneration of corticospinal neurons after stroke has not been investigated, Chen et al. (2004) reported neurogenesis of corticospinal neurons extending spinal projections in adult mice using selective elimination of corticospinal neurons induced by chlorin e6 photoactivation. Although rehabilitative training has been reported to promote neurogenesis after brain injury, regeneration of corticospinal neurons seems very difficult. The study of Chen et al. (2004) reported that it takes at least 12 weeks for newborn neurons to extend axons to the cervical spinal cord and the number of newborn neurons decreases to one eighth during this period. Moreover, only 14% of the surviving newborn neurons extended axons to the cervical spinal cord.
Figure 1.
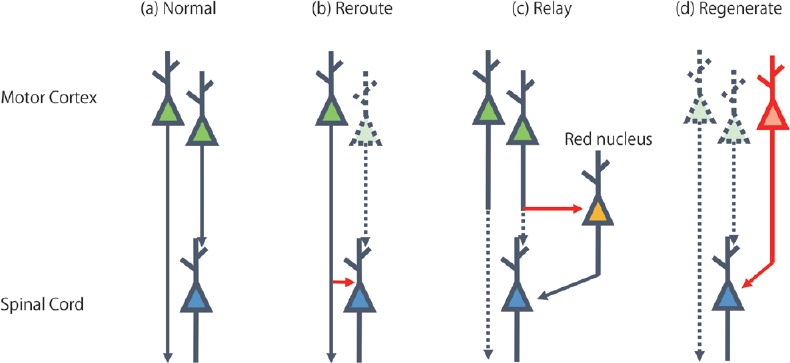
Restoration of corticospinal projections.
Restoration of corticospinal projections can be divided into three types. The scheme shows (a) the normal state of corticospinal pathway, restoration by (b) reroute, (c) relay and (d) regeneration. Green and blue neurons indicate corticospinal neurons in the motor cortex and neurons in the spinal cord, respectively. Yellow neuron indicates rubrospinal neurons in the red nucleus. Destroyed neuron and axon are indicated with a dashed line. Newly formed axon and neuron are indicated with a red line.
A lack of projection neurons limits the effect of rehabilitation after severe stroke, thus an increase of available projection neurons should improve the effectiveness of rehabilitation. The most possible solution is combination therapy of rehabilitation and plasticity-enhancing therapy. While rehabilitation promotes axonal remodeling only in the ipsilesional cortex, most of plasticity-enhancing therapies promotes axonal remodeling bilaterally and even in brainstem spinal pathways. Thus, combination therapy with these approaches enable the restoration of spinal projections by promoting rerouting or relay of neural projections from the contralesional cortex and deep brain areas. Recent studies also demonstrated that rehabilitative training and plasticity-enhancing therapy, such as chondroitinase ABC, can synergistically enhance axonal remodeling and functional recovery. Furthermore, plasticity-enhancing therapy can also reopen a therapeutic window for effective motor training, even weeks after stroke (Wiersma et al., 2017). However, Wahl et al. (2014) demonstrated that combination therapy of rehabilitation and anti-NogoA immunotherapy induced deleterious effects if rehabilitative training was carried out at the same time with anti-NogoA immunotherapy while sequential therapy induced almost full recovery. Although the mechanism underlying the time-dependent effect of combination therapy is not yet known, since plasticity-enhancing therapies are basically intended to be used together with rehabilitative training, understanding the mechanism of the time-dependent effect of rehabilitation and interaction with combined therapy is essential to maximize their efficacy without inducing side effects. In addition, assessment of both structural and functional connectivity using reliable methods, such as MRI tractography or transcranial magnetic stimulation, in clinical trials is essential for elucidating the contribution of neural plasticity in humans.
Regeneration of corticospinal neurons is the most fundamental solution to restoring corticospinal projections. While regenerative therapy including both endogenous neurogenesis and exogenous cell transplantation is being enthusiastically investigated to supply specific type of neurons (e.g., dopaminergic neuron for Parkinson's disease), little is known about regeneration of corticospinal neurons. However, since adult neurogenesis of corticospinal neurons is possible (Chen et al., 2004), it seems that the molecular mechanisms which guide axons of newly generated neurons to the spinal cord exist in the adult animal. Thus, poor generation or survival of newborn neurons, inefficient differentiation and long distance to spinal cord would be the main obstacles for regeneration of corticospinal neurons. Previous studies have substantial evidence about therapies which promote proliferation of neural stem cells (e.g., growth factors, erythropoietin and aerobic exercise) and axon growth (blockade of growth inhibiting molecules and brain stimulation). Furthermore, recent studies demonstrated that postmitotic neurons like callosal projection neurons of layer II/III can be lineage reprogrammed into layer-V/VI corticofugal projection neurons following expression of the transcription factor encoded by Fezf2 (Rouaux and Arlotta, 2013). Thus, transduction of corticospinal neuron-specific transcription factor would increase the efficiency of differentiation. Perhaps, combination of these methods could increase productivity of corticospinal neurons.
Finally, the importance of neuroprotective therapy should be noted. Previous studies have revealed many cellular and molecular events mediating neuronal cell death, including failure of energy metabolism, glutamate release, elevation of intracellular Ca2+, and activation of pro-apoptotic proteins. Based on these pathophysiologies, many pharmacological agents (e.g., glutamate receptor agonists, calcium channel blockers, and free radical scavengers) have been tested and have demonstrated significant neuroprotection in preclinical trials. Nevertheless, due to failure of translation from medical research into clinical practice, use of neuroprotective agents has not been approved in the clinical setting. However, neuroprotection of corticospinal neurons seems more efficient compared to regenerative therapy which has many obstacles as described above. In our previous study, rehabilitation with CIMT improved functional recovery after severe cortical stroke associated with increase of corticospinal neurons in the peri-infarct area, although the magnitude of the increase accounted for a small proportion of total corticospinal projection neurons (only 3.8% of corticospinal projection neurons in normal animals). This result indicates that small changes in ipsilesional corticospinal projections could cause significant differences in the functional recovery induced by rehabilitative training following severe stroke (Okabe et al., 2018). Reduction of damage in the corticospinal pathway would not only attenuate the initial disability but would also provide neuronal projections which can be reorganized to compensate lost functions. Therefore, development and re-examination of neuroprotective agents should be encouraged. One reason for the failure of translation from medical research to clinical practice may be that experimental stroke does not mimic human stoke. Although young adult inbred strain animals are used for most preclinical stroke research, human patients are usually older and have comorbid diagnoses, such as hypertension and diabetes. Furthermore, human stroke is highly heterogeneous. Thus, re-examination using several stroke models in aged animals with comorbidities may provide valuable information for clinical trials.
In conclusion, although rehabilitation can promote reorganization of surviving neural networks by changing axonal targets (rerouting) or making a bypass route (relay), rehabilitation-induced functional recovery depends on the integrity of corticospinal projections. Thus, restoration of corticospinal projections by enhancing survival, axonal remodeling, or regeneration of corticospinal neurons is critical to improve the efficacy of rehabilitation in severely affected stroke patients. Understanding of biological mechanisms underlying rehabilitation-induced recovery would also be necessary to establish optimal combination therapy.
This work was supported by a Grant-in-Aid for Scientific Research (grant No. 17K01493; to NO) from the Japan Society for the Promotion of Science (https://www.jsps.go.jp/english/index.html).
Additional file: Open peer review report 1 (91.4KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ramon Navarro, Cleveland Clinic Abu Dhabi, Neurological Institute, United Arab Emirates.
P-Reviewer: Navarro R; C-Editor: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Bachmann LC, Lindau NT, Felder P, Schwab ME. Sprouting of brainstem-spinal tracts in response to unilateral motor cortex stroke in mice. J Neurosci. 2014;34:3378–3389. doi: 10.1523/JNEUROSCI.4384-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosberger AC, Miehlbradt JC, Bjelopoljak N, Schneider MP, Wahl AS, Ineichen BV, Gullo M, Schwab ME. Axotomized corticospinal neurons increase supra-lesional innervation and remain crucial for skilled reaching after bilateral pyramidotomy. Cereb Cortex. 2018;28:625–643. doi: 10.1093/cercor/bhw405. [DOI] [PubMed] [Google Scholar]
- 4.Okabe N, Himi N, Maruyama-Nakamura E, Hayashi N, Narita K, Miyamoto O. Rehabilitative skilled forelimb training enhances axonal remodeling in the corticospinal pathway but not the brainstem-spinal pathways after photothrombotic stroke in the primary motor cortex. PLoS One. 2017;12:e0187413. doi: 10.1371/journal.pone.0187413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okabe N, Himi N, Nakamura-Maruyama E, Hayashi N, Sakamoto I, Narita K, Hasegawa T, Miyamoto O. Constraint-induced movement therapy improves efficacy of task-specific training after severe cortical stroke depending on the ipsilesional corticospinal projections. Exp Neurol. 2018;305:108–120. doi: 10.1016/j.expneurol.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Okabe N, Shiromoto T, Himi N, Lu F, Maruyama-Nakamura E, Narita K, Iwachidou N, Yagita Y, Miyamoto O. Neural network remodeling underlying motor map reorganization induced by rehabilitative training after ischemic stroke. Neuroscience. 2016;339:338–362. doi: 10.1016/j.neuroscience.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 9.Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–1255. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- 10.Wiersma AM, Fouad K, Winship IR. Enhancing spinal plasticity amplifies the benefits of rehabilitative training and improves recovery from stroke. J Neurosci. 2017;37:10983–10997. doi: 10.1523/JNEUROSCI.0770-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


