Abstract
CD8+ induced regulatory T-cells (CD8+ iTregs) have been identified to suppress alloreactive immune responses and expressed Treg ontological markers as similar as CD4+ iTregs. However, adoptive transfer of CD8+ iTreg-based therapy is hampered by the instability of Treg specific-transcription factor, Foxp3. As CD8+ iTregs were previously demonstrated to possess superior tumor-killing ability to CD4+ iTregs, adoptive transfer of stabilized CD8+ iTregs would be a potential therapy to prevent tumor relapse during graft-versus-host disease (GVHD) treatment. In the current study, we generated allo-antigen reactive CD8+ iTregs from JAK2−/− T-cells and adoptively transferred them to MHC-mismatched and haploidentical murine models of allogeneic bone marrow transplantation (allo-BMT). JAK2−/− CD8+ iTregs not only attenuated GVHD but also preserve graft-versus-leukemia (GVL) effect. Mechanistic analysis revealed that JAK2−/− CD8+ iTregs up-regulated nTreg marker (neuropilin-1), and augmented DNA demethylation of CNS2 region within Foxp3 gene. These properties licensed JAK2−/− CD8+ iTregs to retain high Foxp3 expression resulting in less conversion to type 1 CTLs; as a result, JAK2−/− CD8+ iTregs were able to maintain their suppressive and cytolytic function. Thus, our findings provide a strong rationale and means to stabilize CD8+ iTregs by targeting JAK2, and the stabilized CD8+ iTregs exhibit therapeutic potential for alleviating GVHD and preserving the GVL effect.
Introduction
Graft-versus-host-disease (GVHD) remains a major limitation of allogeneic hematopoietic stem cell transplantation (allo-HCT). GVHD develops after activation of donor T-cells by recipient’s antigen presenting cells (APCs) resulting in donor T-cell expansion and differentiation. Activated donor T-cells subsequently release various pro-inflammatory cytokines termed the “cytokine storm”, and differentiate into effector T-cells (Teffs) leading to tissue damage in recipient target organs including gut, liver, lung, and skin (1, 2). Naturally derived regulatory T-cells (nTregs) play a pivotal role in preventing the development of autoimmunity as well as limiting allogeneic responses. nTregs are characterized by the consecutive expression of Foxp3 that is critically required for their suppressive ability (3). Because GVHD can be considered an imbalanced status between Teffs and Tregs that results in inflammation and tissue injury, many efforts have been focused on how to augment Treg reconstitution or adoptively transfer Tregs for GVHD control (4–8). Treg-based immunotherapy for GVHD has been focused on CD4+ nTreg or induced Tregs (iTregs). There is little or no naturally derived CD8+ Tregs (CD8+Foxp3+); although, they can be induced in vitro and in vivo. Theses CD8+ iTregs (CD8+Foxp3+) are a less characterized population because of the instability of Foxp3 under inflammatory milieu (9, 10). Even though CD8+ iTregs are unstable, they possess graft–versus-host-leukemia (GVL) activity, which is essential for preventing tumor relapse during GVHD treatment (11, 12). CD8+ iTregs were previously identified as a major Treg population and markedly induced early after allo-BMT. They have a suppressive function as potent as CD4+ iTregs in vitro and were able to attenuate GVHD severity with moderate efficacy (12–18). Therefore, enhancing the stability of Foxp3 in CD8+ iTregs would be a therapeutic opportunity for control of GVHD in the clinic.
Janus Kinase 2 (JAK2) is one of the non-receptor tyrosine kinases, which transduces cytokine-mediated signals via STAT pathway. During GVHD development, inflammatory cytokines such as IL-6, IL-12, and IL-23 activate donor T-cells through this pathway leading to Teff (Th1 and Th17) differentiation and proliferation (1, 2, 19). Conversely, genetic deletion or pharmacological inhibition of JAK2 or STAT3 on T-cells promotes in vivo induced Treg generation and inhibits Th1 and Th17 polarization both in vitro and in vivo (20–24). Furthermore, Laurence A. et. al (19), have shown that signal transduction through STAT3 was relevant to Foxp3 stability in nTregs, as adoptive transfer of STAT3-deficient nTregs maintained superior Foxp3 expression to WT nTregs in a murine GVHD model. However, transfer of STAT3−/− nTregs could not prevent lethal GVHD due to IL-10 deficiency (JAK1/STAT3 signaling), which is required for Treg suppressive function. In contrast, transfer of STAT3−/− T-cells were able to improve recipient survival by elaborating de novo iTregs from donor T-cells, suggesting that STAT3 signaling might affect iTregs rather than nTregs during GVHD development (19).
In this current study, we attempted to enhance CD8+ iTreg stability by targeting JAK2. Allogeneic JAK2−/− CD8+ iTregs were employed to avoid the interruption of Treg essential cytokine signalings including IL-10 (JAK1/STAT3) (25) and IL-2 (JAK1/JAK3/STAT3) (26–28). Furthermore, targeting JAK2 is more specific to inhibit IL-6 signaling which destabilizes Foxp3 via recruiting DNA methyltransferase 1 (DNMT1) leading to diminishing CpG demethylation in conserved noncoding sequence 2 (CNS2) within Foxp3 gene (29, 30). Here, we demonstrated that adoptive transfer of JAK2−/− CD8+ iTregs attenuated GVHD severity while sparing the GVL activity. Mechanistically, JAK2−/− CD8+ iTregs upregulated Treg suppressive markers, retained high Foxp3 expression, exhibited less plasticity to convert to pathogenic type 1 CTLs, and enhanced in vivo iTreg generation from Teffs. In addition to GVHD alleviation, JAK2−/− CD8+ iTregs maintained the GVL effects because the cytolytic activity of both donor CD8+ Teffs and transferred CD8+ iTregs were preserved. For translational approach by using a potent JAK2 inhibitor (Pacritinib), we demonstrated that transferred WT CD8+ iTregs in combination with Pacritinib administration significantly prolonged mice survival and protected mice from tumor relapse as potently as JAK2−/− CD8+ iTregs.
Material and Methods
Mice
C57BL/6 (B6-Ly5.2; H-2b, CD45.2+), B6-Ly5.1 (H-2b, CD45.1+), BALB/c (H-2d), B6DF1 (B6 × DBA2) F1 (H-2b/d) were purchased from the National Cancer Institute (NCI; Frederick, MD). T-cell conditional JAK2 knock-out mice (JAK2fl/flCD4Cre) were used, where both CD4+ and CD8+ T cells are deficient for JAK2 because CD4 is expressed in all T cells during their development in the thymus at CD4/CD8 double positive stage (31, 32). JAK2fl/flCD4Cre, B6-Foxp3EGFP and Rag1−/− mice were bred and maintained under specific pathogen-free conditions in the American Association for Laboratory Animal Care-accredited Animal Resource Center at Medical University of South Carolina (MUSC). All mice were used at the age of 7–10 weeks, and experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of MUSC.
Flow Cytometry and serum cytokine detection
Cells were isolated from recipient spleen, liver, and mesenteric lymph nodes as previously described (33) and stained for surface molecules and intracellular cytokines using standard flow cytometry protocols. The following antibodies were used for cell-surface staining: anti-CD4 (RM4–5), anti-CD8 (53–6.7), anti-CD11b (M1/70), anti-H-2b (AF6–88.5.5.3), anti-CD45.1 (A20), anti-Nrp1 (3DS304M), anti-CXCR5 (SPRCL5), anti- CD73 (eBioTY/11.8) and anti-PD-1(J43). For intracellular cytokines, cells were stimulated for 4–5 hours at 37°C with PMA (100 ng/ml, Sigma-Aldrich) and ionomycin (100 ng/ml; Calbiochem, EMD) in the presence of GolgiStop (BD Biosciences), permeabilized by using Cytofix/Cytoperm Plus (BD Biosciences), and then stained with the appropriate antibodies including anti-Foxp3 (FJK-16s) and anti-IFNγ (XMG1.2). Live/Dead Fixable Yellow Dead Cell Stain Kit and carboxyfluorescein succinimidyl ester (CFSE) dye were purchased from Invitrogen and stained using the manufacturer’s protocols. Stained cells were analyzed using LSR II (BD Biosciences), and FlowJo (TreeStar). Serum cytokine levels in recipient mice were quantified by using a cytometric bead assay according to the manufacturer’s instructions (BD Biosciences).
In vitro generation of allogeneic-CD8+ iTregs and stability assay
Generation and CD25+ enrichment of CD8+ iTregs in vitro was done as previously described (11). Briefly, purified CD8+T-cells were co-cultured with enriched splenic DCs (10:1 ratio) in the presence of IL-2, TGF-β, and retinoic acid (RA). On day 5, allogeneic-CD8+ iTregs were enriched from the bulk culture by positive selection with CD25hi cells. To measure Foxp3 stability in vitro, enriched CD8+ iTregs (2 × 105) were co-cultured with 6 × 105 allogeneic-APCs at 1:3 ratio (iTregs: APCs) in Foxp3 stability favored condition (IL-2, 5ng/mL) and Th1 favored condition (IL-2, 5ng/mL and IL-12, 2ng/mL). On day 3 and day 6, iTregs were harvested and analyzed for Foxp3 expression by using flow cytometry.
Adoptive transfer of CD8+ iTregs in GVHD and GVL models
To study the ability of CD8+ iTregs in preventing GVHD, recipient mice were lethally irradiated at 700 cGy for BALB/c or 1,100 cGy for BDF1 (split dose). Irradiated mice were adoptively transferred with CD8+ iTregs 1 × 106 /mouse for BALB/c or 2 × 106 /mouse for BDF1 together with 5×106 TCD-BM from C57BL/6 (WT). Three days later, CD25-depleted T-cells (Teffs) from WT were injected to induced GVHD; 0.7×106 for BALB/c or 3×106 for BDF1. Recipient survival rate, GVHD clinical score, and body weight were followed for 80 days. To study in GVL model, MLL-AF9-GFP leukemic cells 2×104 /mouse or P815 mastocytoma 2.5–5 ×103/mouse were infused on the day of BMT. MLL-AF9 is a mixed lineage leukemia including myeloid leukemia expressing CD11b; therefore, MLL tumor growth was monitored in peripheral blood of recipient mice and determined for CD11b+GFP+ cells by flow cytometry (34, 35). P815 growth was measured with bioluminescent imaging (BLI) using Xenogen-IVIS®200 in vivo Imaging System (Perkin-Elmer). Pacritinib (PAC) was administered at 100 mg/kg/day/mouse by oral gavage for 4 weeks. The mice were monitored for survival rate and tumor mortality.
In vitro killing assay
To determine T-cell cytolytic activity, various numbers of T cells were co-cultured with 1 ×104 allogeneic tumor target cells, luciferase-expressing P815 mastocytoma, to achieve effector to target (E:T) ratios to 20:1, 10:1, 5:1, 2.5:1 and 1.25:1 at 37°C for 4 hrs. Target-cells incubated alone without effector cells were used to measure spontaneous death as a negative control. For positive control, target-cells were treated with cell lysis reagent as a measurement of maximum killing. After incubation, luciferin was added to the culture. Bioluminescent imaging data were analyzed and quantified using Living Imager Software. Triplicate wells were averaged and percent lysis was calculated from the data with the following equation: %cytotoxicity = (maximal-experimental)/(maximal-spontaneous)×100.
DNA methylation assay
Genomic DNA was isolated from purified T cells through FACS sorting by using Blood & Tissue Genomic DNA Extraction kit (Qiagen). Extracted genomic DNAs were converted by the EZ DNA methylation kit (Zymo Research). Anti-sense (30) strands of bisulfite-treated DNA were then subjected to PCR for amplification of CNS2. The PCR products obtained were cloned into the pCR™2.1-TOPO® vector (Thermo Fisher Scientific), and 10–15 individual clones from each sample were sequenced with M13-reverse primers. Sequencing results for methylation status were analyzed for 12 CpG position in CNS2 region by using quantification tool for methylation analysis (QUMA) at http://quma.cdb.riken.jp/.
Statistics
For comparison of recipient survival, body weight, clinical score and tumor mortality among groups in GVHD and GVL experiments, the log-rank test by GraphPad Prism 6 was used to determine statistical significance. To compare cell frequency, marker expression, pathology scores and cytokine levels, a One-way ANOVA or a Student t-test was performed.
Results
JAK2 deficiency enhances allogeneic CD8+ iTregs generation and maintains their Foxp3 stability under an inflammatory environment
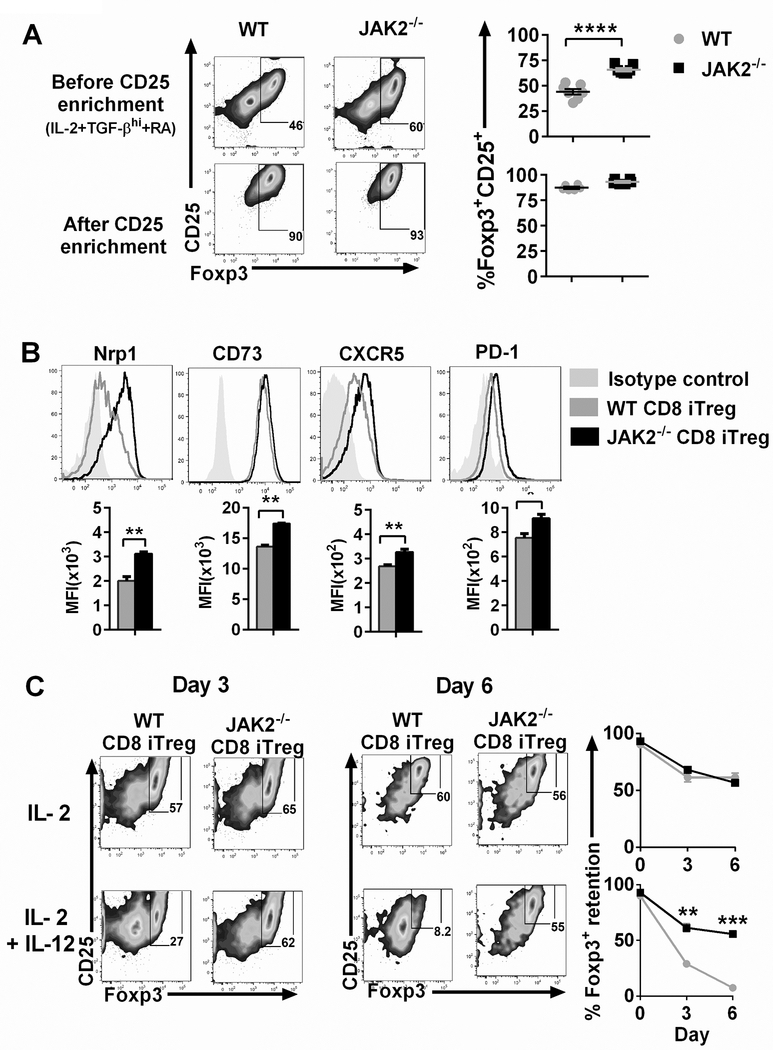
Our recent study illustrated that in the absence of JAK2 in T-cells, the generation of CD4+ iTregs from donor T-cells was significantly increased in recipients after allo-BMT (20). How JAK2 signaling affects the generation and function of CD8+ iTregs have not been delineated. To address this question, we generated allogeneic CD8+ iTregs in vitro by using conditional JAK2 knockout mice (JAK2flox/flox on CD4-Cre). Consistent with JAK2−/− CD4+ T-cells, we observed that JAK2−/− CD8+ T-cells significantly enhanced Foxp3 expression compared to WT CD8+ T cells in the presence of low or high concentrations of TGF-β (Supplemental Fig. 1A). We subsequently supplied additional retinoic acid (RA) to the cell culture and found that RA further pronounced Foxp3 expression (Fig. 1A); therefore, the culture condition with IL-2+TGF-βhi+ RA was conducted for CD8+ iTreg generation hereafter. As we sought to obtain comparable levels of Foxp3+, CD8+ iTregs that expressed a high level of CD25+ were enriched from a bulk culture of WT and JAK2−/− CD8+ iTregs and used for the entire study.
FIGURE 1. Effects of JAK2 on Foxp3 expression and stability of CD8+ iTregs.
(A) Allogeneic CD8+ iTregs were generated in vitro by co-culturing naïve CD8+ T-cells isolated from C57BL/6 mice with allogeneic DCs isolated from BALB/c mice in the presence of IL-2 (5 ng/ml), TGF-β 5 ng/ml, and retinoic acid (RA) 40 nM for 5 days. Expression of Foxp3 was analyzed by flow cytometry, and CD8+ iTregs were enriched from the bulk culture using positive selection with CD25+ microbeads. FACS plots and graphs show Foxp3 expression (n=7/group). (B) Nrp1, CD73, CXCR5 and PD-1 expressions are shown on gated CD8+Foxp3+ iTregs (n=3/group). (C) Foxp3 expression is shown among WT and JAK2−/− CD8+ iTregs under IL-2 and IL-2+IL-12 at day 3 and day 6 (n=3/group). Student’s t-test used to compare the data. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and, ****p ≤ 0.0001. Data represent the mean ± SEM.
We next determined other canonical markers expressed by Tregs. Interestingly, neuropilin-1 (Nrp-1) was significantly up-regulated in JAK2−/− CD8+ iTregs compared to WT controls (Fig. 1B). Moreover, the kinetics of Nrp1+ expression was increased in parallel with Foxp3+ expression (Supplemental Fig. 1B). Nrp-1 has been reported as one of the markers that used to distinguish nTregs from iTregs and previously shown to be associated with Treg stability (36–38). Other markers including CD73, CXCR5, and PD-1 were also highly expressed among JAK2−/− CD8+ iTregs (Fig. 1B).
Given that JAK2 signaling mediates various inflammatory cytokines, we hypothesized that CD8+ iTregs deficient for JAK2 would be more stable than their WT counterparts. To address this question, we initially performed in vitro Treg stability assay. In Treg-friendly condition with IL-2 cytokine alone, WT and JAK2−/− CD8+ iTregs retained Foxp3 levels similarly. In contrast, JAK2−/− CD8+ iTregs were able to maintain their Foxp3 expression under pro-inflammatory condition whereas WT CD8+ iTregs substantially lost their Foxp3 expression in the presence of IL-12 (Fig. 1C).
JAK2−/− CD8+ iTregs augment DNA demethylation in CNS2 region of Foxp3
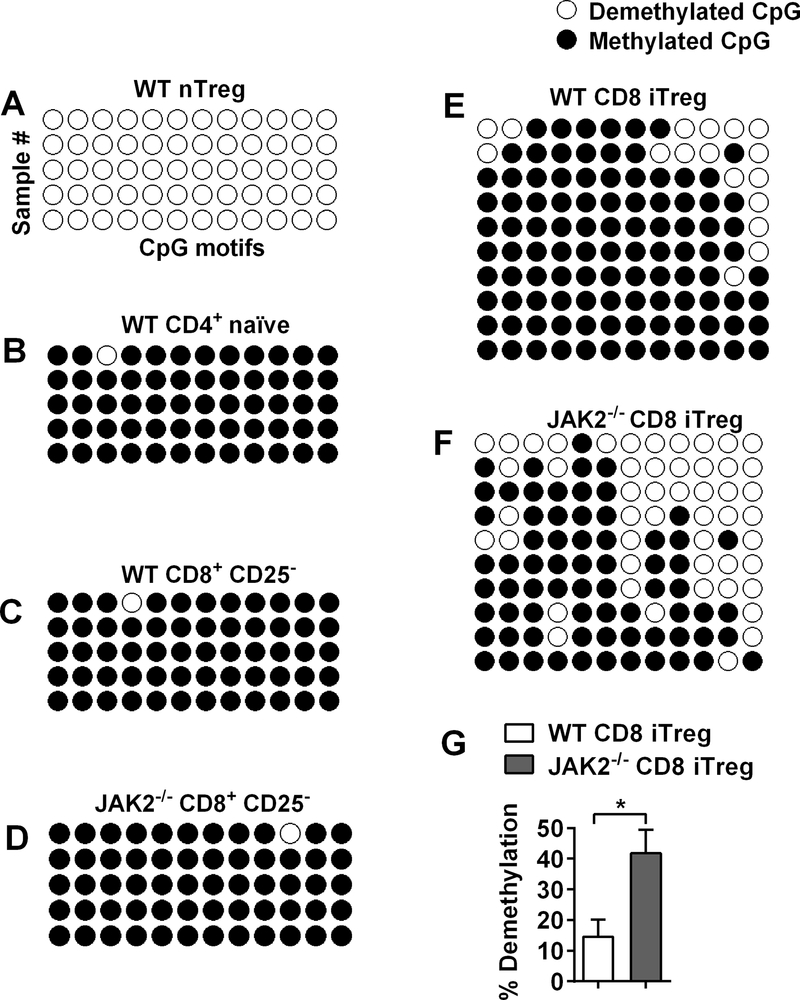
Prior studies have shown that DNA demethylation status of the CNS2 region within Foxp3 gene is a critical indicator for a stable Foxp3 expression in Tregs (39–41). We therefore evaluated the demethylation status in JAK2−/− CD8+ iTregs. nTregs (CD4+Foxp3+) and CD4+ naïve T-cells (CD4+Foxp3−) were sorted from Foxp3 GFP male mice and used as a positive and negative control, respectively. As expected, nTregs (GFP+) as a positive control were completely demethylated at CNS2 region (Fig. 2A), whereas demethylation was not observed from CD4+GFP− cells (Fig. 2B). To determine methylation status between WT and JAK2−/− CD8+ iTregs, CD8+CD25+ and CD8+CD25− were sorted from a bulk culture of WT and JAK2−/− at day 5 after the generation. As shown in Fig. 2C-D, CD8+CD25− of WT or JAK2−/− cells were densely methylated (~0% demethylation) in CNS2 region. While WT CD8+CD25+ showed only~15% demethylation (Fig. 2E, 2G), JAK2−/− CD8+ iTregs exhibited ~40% demethylation (Fig. 2F, 2G), significantly higher than WT counterparts. Collectively, these data indicate that the absence of JAK2 on CD8+ iTregs facilitates DNA demethylation in CNS2 of Foxp3 gene, which likely contributes to their superior Foxp3 stability.
FIGURE 2. DNA demethylation status in CNS2 region of Foxp3.
(A, B) CD4+ T cells were isolated by sorting from splenocytes of male C57BL/6-Foxp3+EGFP mice and subjected to bisulfite sequencing to analyze the demethylation patterns of CpG motifs in CNS2 region. Methylation status of CD4+ GFP+ cells (nTregs) and naïve CD4+ GFP− cells are shown by white (demethylated CpGs) or black (methylated CpGs) colors. (C and F) CD8+ T-cells isolated from male WT or JAK2−/− were cultured under iTreg condition. At day 5, CD8+CD25+ (WT CD8+ iTregs), CD8+CD25− (WT CD8+), JAK2−/− CD8+ iTregs and JAK2−/−CD8+CD25− (JAK2−/− CD8+) were sorted and subjected to bisulfite sequencing to analyze. Methylation status of WT CD8+, JAK2−/− CD8+, WT CD8+ iTreg, and JAK2−/− CD8+ iTreg are shown in C, D, E, and F respectively. (G) Bar graph shows demethylation of WT CD8+ iTreg and JAK2−/− CD8+ iTreg (n=10/group). Student’s t-test was used to compare the data. *p ≤ 0.05. Data represent the mean ± SEM.
Adoptive transfer of JAK2−/− CD8+ iTregs attenuates aGVHD
Given that Foxp3 of JAK2−/− CD8+ iTregs is more stable under in vitro Th1 condition (Fig. 1C), which is a dominant pro-inflammatory condition during GVHD development, we further hypothesized that adoptive transfer of JAK2−/− CD8+ iTregs would be superior to WT counterparts in mitigating GVHD. By using a MHC-mismatched B6→BALB/c BMT model, we found that adoptive transfer of WT CD8+ iTregs could reduce GVHD severity and prolonged recipient survival. However, JAK2−/− CD8+ iTregs were significantly more effective reflected by ~70% long-term recipient survival, lower body weight loss and lower clinical scores (Fig. 3A-C).
FIGURE 3. Effects of CD8+ iTregs in GVHD development.
(A-C) B6→BALB/c: lethally irradiated BALB/c mice were adoptively transferred with 1×106 CD8+ iTregs and 5×106 WT-TCD BM. Three days later, 0.7×106 CD25-depleted T-cells were i.v injected to induce GVHD. Recipients were monitored for survival rate (A), body weight loss (B), and GVHD clinical signs (C) until day 80 (n=10/group). (D-F) B6→BDF1: lethally irradiated BDF1 mice were adoptively transferred with 2×106 CD8+ iTregs and 5×106 WT-TCD BM. Three days later, 3 ×106 CD25-depleted T-cells were i.v injected to induced aGVHD. Recipients were monitored for survival rate (D), body weight loss (E), and GVHD clinical signs (F) until day 80 (n=8–10/group). (G) Cytokine levels in BALB/c recipient serum at 14 days after BMT were quantified (n=4/group). Log-rank (Mantel-Cox) test was used to compare the survival data, the body weight loss, GVHD clinical sign, and Student’s t-test was used to compare cytokine data. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001. Data represent the mean ± SEM.
To exclude model specific observation, we employed a haploidentical B6→BDF1 BMT model as it is more clinically relevant. We observed that JAK2−/− CD8+ iTregs were also significantly more effective than WT counterparts in alleviating GVHD (Fig. 3D-F). Consistent with GVHD severity, serum cytokines detected 14 days post-transplant revealed that IFN-γ, TNF-α and IL-6 were significantly decreased in recipient mice receiving JAK2−/− CD8+ iTregs compared to WT iTregs (Fig. 3G). Taken together, adoptive transferred of JAK2−/− CD8+ iTregs results in enhanced protection from GVHD lethality.
JAK2−/− CD8+ iTregs retain higher Foxp3 and produce less IFN-γ during GVHD development
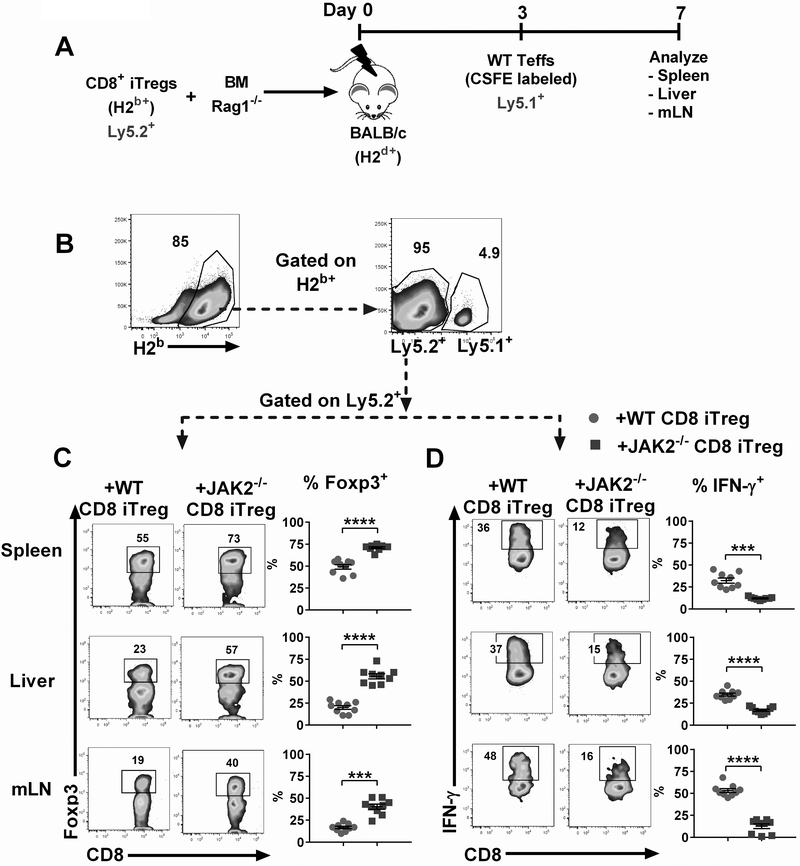
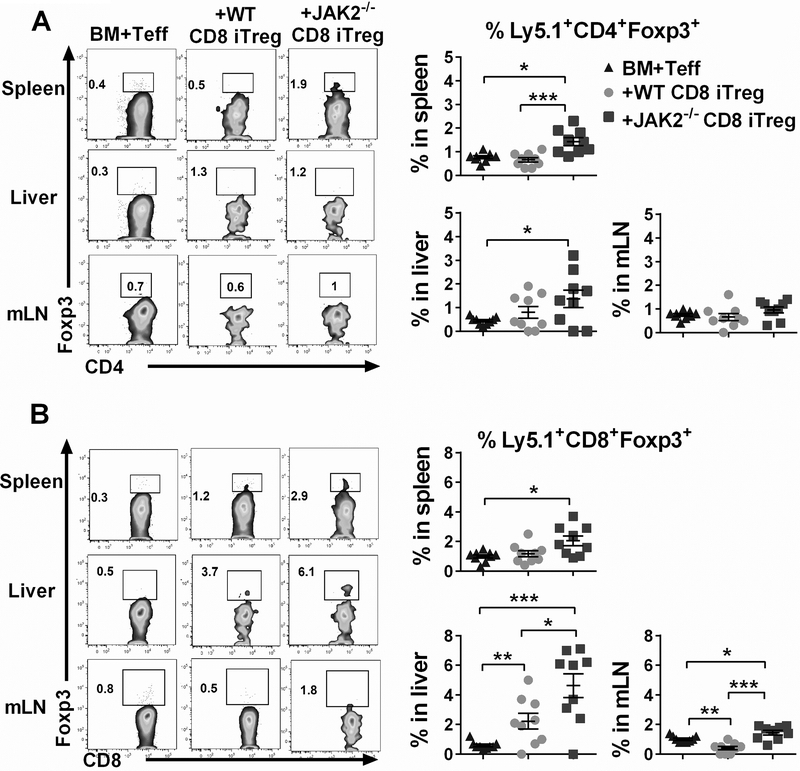
To determine whether the stability of transferred CD8+ iTregs in vivo was correlated with the results from GVHD models, lethally irradiated BALB/c mice were adoptively transferred with Ly5.2+ JAK2−/− or WT CD8+ iTregs together with BM from Rag1−/− mice to exclude any Tregs derived from donor BM (Fig. 4A). Three days later, Ly5.1+ Teffs were injected to induce GVHD among recipient mice. Cohorts of mice were sacrificed on day 7 post-BMT and transferred CD8+ iTregs were analyzed by flow cytometry gating on H-2b+Ly5.2+ population (Fig. 4B). We observed that JAK2−/− CD8+ iTregs retained higher Foxp3 levels compared to WT CD8+ iTregs in both lymphoid and target organs during GVHD development (Fig. 4C), confirming that JAK2−/− CD8+ iTregs were more stable in maintaining Foxp3 than WT counterparts under the inflammatory environment in vivo.
FIGURE 4. Stability and function of CD8+ iTregs in vivo.
(A) Experimental scheme: Lethally irradiated BALB/c mice were adoptively transferred with 5×106 Rag1−/− BM and 1×106 CD8+ iTregs (Ly5.2+). Three days later, 2×106 CD25-depleted T-cells (CFSE labeled) from C57BL/6 (Ly5.1+) congenic mice were i.v injected to induce GVHD. At day 7 after allo-BMT, spleen, liver and mesenteric lymph nodes (mLNs) were collected and analyzed. (B) Representative gating strategies: H2b+ Ly5.2+ cells were analyzed as transferred CD8+ iTregs. (C) Foxp3 retention of transferred WT and JAK2−/− CD8+ iTregs is shown. (D) IFN-γ expression of transferred CD8+ iTregs is shown. Data are combined from 2 independent experiments (n=9/group). Student’s t-test was used to compare the data. ***p ≤ 0.001 and ****p ≤ 0.0001. Data represent the mean ± SEM.
Given that IFN-γ cytokine is a major contributor to GVHD pathology (42, 43) and previous studies have shown that WT CD8+ iTregs tend to lose Foxp3 and convert to Tc1 cells with increased IFN-γ production(9, 44). Thus, we reasoned that JAK2−/− CD8+ iTregs would produce less IFN-γ than WT CD8+ iTregs since their Foxp3 expression is more stable. As expected, JAK2−/− CD8+ iTregs secreted significantly less IFN-γ compared to WT counterparts in spleen, liver, and mLN (Fig. 4D). Collectively, we demonstrated JAK2−/− CD8+ iTregs had greater Foxp3 stability, and secreted less IFN-γ than WT iTregs during GVHD development.
JAK2−/− CD8+ iTregs possess suppressive function as potent as WT CD8+ iTregs
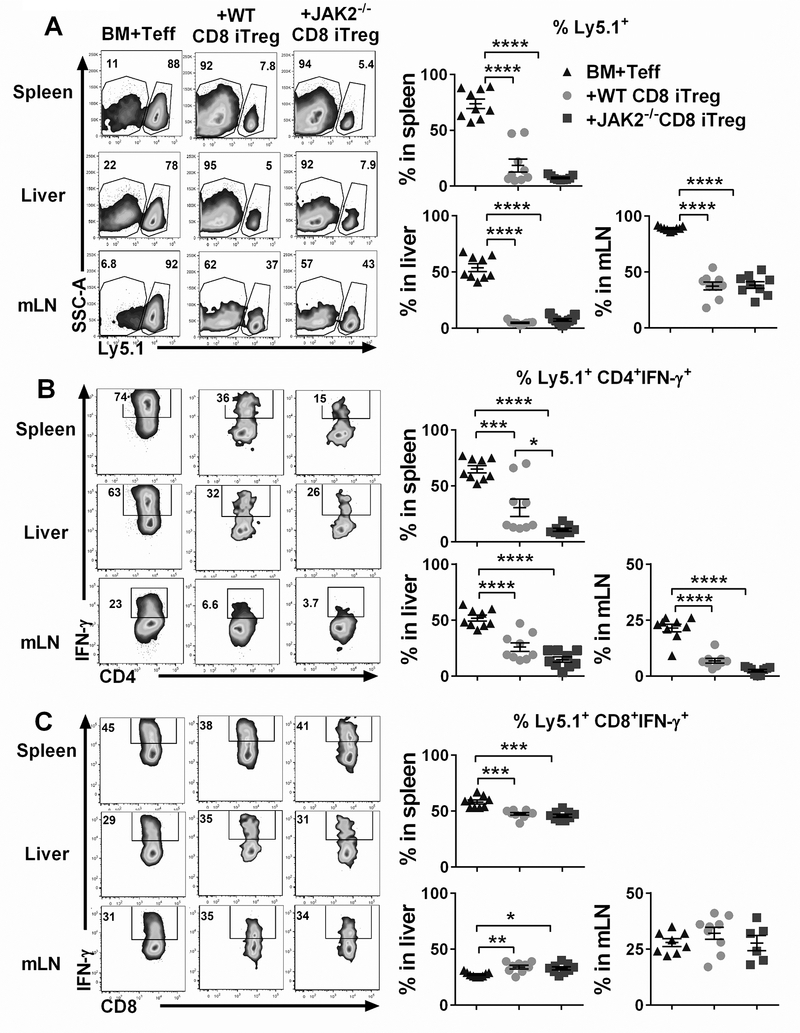
We next sought to compare the functionality of CD8+ iTregs in vivo by using the same experimental setting as shown in Fig. 4A. Therefore, the frequency of Ly5.1+ cells in lymphoid and target organs was analyzed to determine the ability of CD8+ iTregs in suppressing Teff expansion. Strikingly, we observed that both WT CD8+ iTregs and JAK2−/− CD8+ iTregs could significantly suppress Teff expansion in recipient’s spleen, liver and mLNs reflected by the percentages of Ly5.1+ cells (Fig. 5A) and their CSFE dilution (Supplemental Fig. 2A-B). Furthermore, both WT and JAK2−/− CD8+ iTregs efficiently inhibited IFN-γ production by donor CD4+ Teffs (Fig. 5B).
FIGURE 5. Suppressive activity of CD8+ iTregs in vivo.
Lethally irradiated BALB/c mice were adoptively transferred as in figure 4A. H2b+ Ly5.1+ cell was analyzed as Teffs. (A) The ability of transferred CD8+ iTregs (Ly5.2+) to suppress Teffs (Ly5.1+) expansion is shown. (B, C) IFN-γ expression of CD4+ and CD8+ Teffs are shown respectively. Data are combined from 2 independent experiments (n=9/group). A one-way ANOVA was used to compare the data. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and, ****p ≤ 0.0001. Data represent the mean ± SEM.
In comparison between WT and JAK2−/− CD8+ iTregs, we found that JAK2−/− CD8+ iTregs were superior to WT counterparts in inhibiting IFN-γ production by CD4+ Teffs in recipient spleens, not in livers or mLNs (Fig. 5B). However, there was no difference in IFN-γ production from CD8+ Teffs between WT and JAK2−/− CD8+ iTregs (Fig. 5C). Thus, the absence of JAK on CD8+ iTregs does not affect the ability of Treg to suppress pathogenic T cell expansion, and transfer of JAK2−/− CD8+ iTregs had greater capability to inhibit IFN-γ production from CD4+ Teffs, which is a main inflammatory cytokine produced during GVHD induction.
JAK2−/− CD8+ iTregs enhance de novo iTreg generation
Prior studies have demonstrated that de novo iTreg generated from donor T cell suppressed Teff expansion and was sufficient to limit lethal GVHD (14, 19, 24), we further asked whether transferred CD8+ iTregs would affect the de novo iTreg generation. To address this question, we measured Foxp3+ expression derived from donor CD4+ (Ly5.1+CD4+) and CD8+ (Ly5.1+CD8+). As shown in Fig. 6A-B, transferred JAK2−/− CD8+ iTreg resulted in the enhancement of de novo iTregs, particularly among donor CD8+ T cells. Thus, we interpret that superior functional activity of JAK2−/− CD8+ iTregs in the alleviation of GVHD was likely attributed to their enhanced abilities in suppressing Teffs expansion and simultaneously promoting de novo iTreg generation from donor T cells.
FIGURE 6. De novo iTreg generation from donor T cells in GVHD recipients.
Lethally irradiated BALB/c mice were adoptively transferred as in figure 4A. Seven days after BMT, H2b+Ly5.1+ (Teffs) were analyzed for de novo generated (iTregs). Representative FACS plots and graphs show %Foxp3+ expression on gated (A) CD4+ and (B) CD8+ Teffs. Data are from 2 independent experiments (n=9/group). A One-way ANOVA was used to compare the data. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001. Data represent the mean ± SEM.
JAK2−/− CD8+ iTregs significantly alleviate GVHD severity while preserving GVL activity
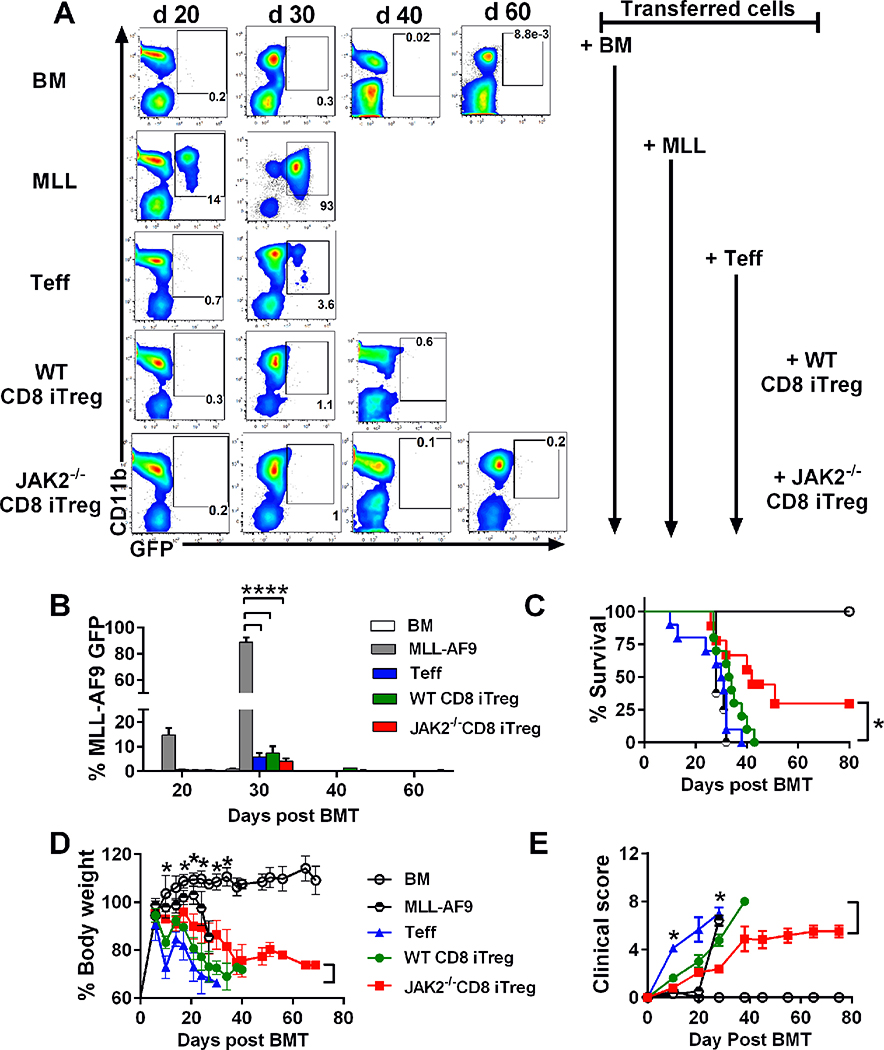
We next addressed a critical question whether adoptive transfer of JAK2−/−CD8+ iTreg maintains the GVL effect. To assess this, we initially performed in vitro cytotoxic assay to evaluate the killing activity against allogeneic tumors of iTregs. Consistent with our previous study (11), WT CD8+ iTregs display cytotoxic activity superior to CD4+ Teffs and CD4+ iTregs, but lower than WT CD8+ Teffs (Supplemental Fig. 3A-B). Furthermore, JAK2−/− CD8+ iTregs had a similar killing activity to WT counterparts (Supplemental Fig. 3C-D). We extended our study to evaluate how CD8+ iTregs affect the GVL effect by using a clinical relevant GVL model with mixed lineage leukemia (MLL-AF9-GFP). We observed that the recipients without T-cell infusion succumbed to tumor relapse within 30 days after allo-BMT whereas the recipients received Teffs alone died from GVHD as reflected by the low frequency of MLL-AF9-GFP in peripheral blood (Fig. 7A-B), excessive body weight loss (Fig. 7D) and clinical scores (Fig. 7E). Similarly, the recipients with additional WT CD8+ iTregs also developed GVHD without any signs of tumor relapse. In contrast, the recipients with additional JAK2−/− CD8+ iTregs had reduced GVHD reflected by less body weight loss and lower clinical scores. More importantly, none of the recipients had tumor relapse and ~30% of the recipients had a long-term survival with no signs of tumor (Fig. 7A-E). We also extended our observation using a haploidentical B6→BDF1 model with p815 mastocytoma. Consistent with MLL-AF9 model, we found that additional of JAK2−/− CD8+ iTregs significantly prolonged recipient survival while maintaining the GVL effect (Supplemental Fig. 4A-C). These results provide evidence that JAK2−/− CD8+ iTregs were more effective than WT CD8+ iTregs in controlling GVHD while still preserving the GVL effect.
FIGURE 7. Effects of CD8+ iTregs on GVH and GVL responses.
Lethally irradiated BALB/c mice were adoptively transferred with 1×106 CD8+ iTregs, 5×106 WT-TCD BM, and 2×104 MLL-AF9. Three days later, 0.7×106 CD25-depleted T-cells were i.v injected to induced aGVHD. (A) % MLL-AF9 in recipient peripheral blood were analyzed with CD11b+GFP+, and representative FACS plots on day 20, 30, 40 and 60 posts BMT were shown. (B) Bar graph showed quantified %GFP+ MLL-AF9 in blood at the indicated time point. Survival rate (C), body weight loss (D), and GVHD clinical signs (E) of recipient mice were monitored until day 60. Data are combined from 2 independent experiments (n=8–10/group). Log-rank (Mantel-Cox) test was used to compare the survival data. Student’s t-test was used to compare % MLL, body weight loss and GVHD clinical sign data. *p ≤ 0.05, **p ≤ 0.01 and, ****p ≤ 0.0001. Data represent the mean ± SEM.
Targeting JAK2 in CD8+ iTregs with Pacritinib delays survival and prevents tumor relapse
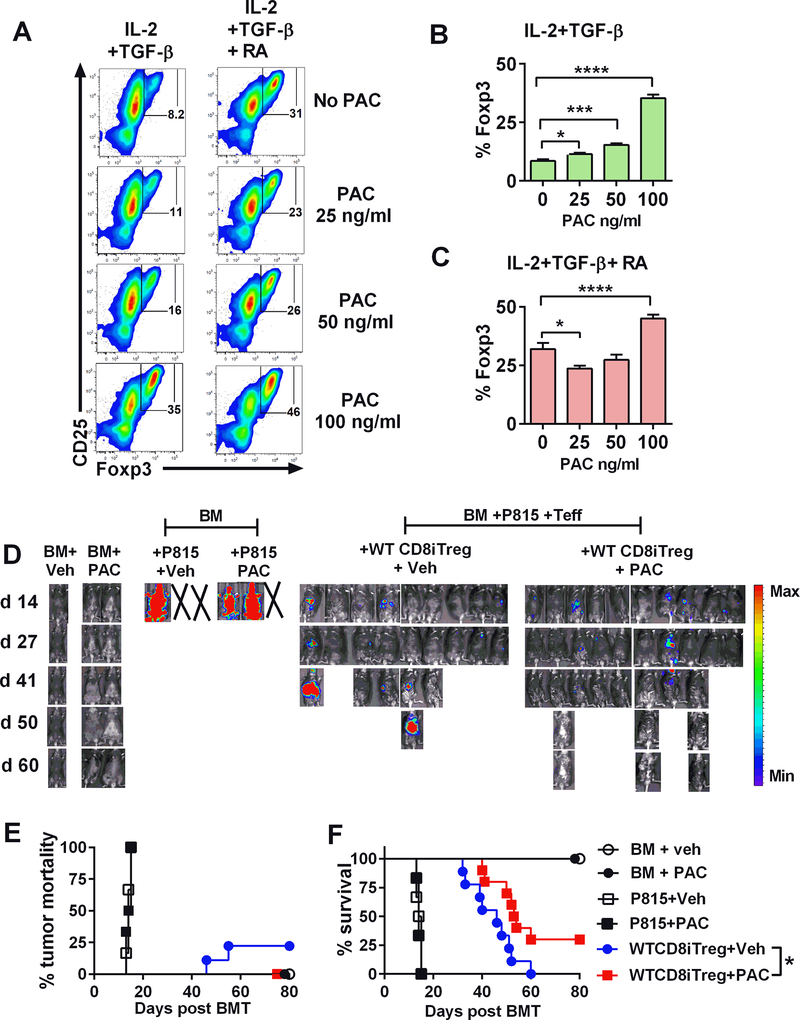
To enhance the translational potential of our findings in clinical application, we employed Pacritinib, potent JAK2 inhibitor in the next set of experiments. We initially tested the effect of Pacritinib in CD8+ iTreg generation, and observed that the addition of Pacritinib in the Treg-culture promoted Foxp3 expression in both TGF-β alone or plus RA condition in a dose-dependent manner (Fig. 8A-C). However, JAK2 inhibition with Pacritinib in vitro per se did not have a significant effect on the stability or activity of these iTregs in vivo (data not shown), presumably because JAK2 activity was recovered due to transient inhibition. We next evaluated the effects of CD8+ iTregs plus Pacritinib in vivo on the GVH and GVL responses using a haploidentical B6→BDF1 model with P815 tumors. The recipients of BM plus tumor quickly died from tumor relapse regardless of the treatment (Fig. 8D), indicating that Pacritinib treatment did not have a direct effect on tumor growth. Treatment of Pacritinib alone without iTregs transfer failed to alleviate GVHD induced by Teffs under the experimental setting (data not shown). However, treatment of Pacritinib together with CD8+ iTregs transfer significantly enhanced the ability of CD8+ iTregs in the prevention of GVHD while maintaining the GVL effect compared to vehicle control (Fig. 8D-F). These data suggest that pharmacological inhibition of JAK2 with Pacritinib promotes the efficacy of CD8+ iTreg therapy.
FIGURE 8. Impact of Pacritinib and iTregs in GVHD and tumor relapse.
Allogeneic WT CD8+ iTregs were generated in vitro in the presence or absence of Pacritinib (PAC) at the indicated concentration. After 5 days incubation, Foxp3 expression was analyzed by flow cytometry. (A-C) FACS plots and graphs show %Foxp3 expression on CD8+ cells (n=3/group) under indicated conditions. Student’s t-test was used to compare the data. *p ≤ 0.05, ***p ≤ 0.001 and, ****p ≤ 0.0001. Data represent the mean ± SEM. (D-F) Lethally irradiated BDF1 mice were adoptively transferred with 2×106 WT CD8+ iTregs, 5×106 WT-TCD BM, and 2–5 ×103 P815 mastocytoma. Three days later, 3 ×106 CD25-depleted T-cells were i.v injected. Pacritinib 100 mg/kg/day or methylcellulose vehicle was given by oral gavage daily for 4 weeks starting on day 0 of BMT. Recipients were monitored for tumor burden (D) tumor mortality (E) and survival (F) until day 80. Data are combined from 2 independent experiments (n=9–10/group). *p ≤ 0.05. Log-rank (Mantel-Cox) test was used to compare the tumor mortality and survival.
Discussion
In this current study, we demonstrated that JAK2 deficiency enhanced CD8+ iTreg generation via promoting Foxp3 expression in vitro. JAK2-deficient CD8+ iTregs maintained greater Foxp3 stability than WT iTregs under the inflammatory condition, which is likely attributed to the increased expression of Nrp1+ and demethylated DNA in CNS2 region of their Foxp3 gene. Consistent with the increased stability, adoptively transfer of JAK2−/− CD8+ iTregs significantly attenuated GVHD, presumably due to their stable Foxp3 and insensitivity to be converted into Teffs under inflammatory condition. Importantly, with higher stability and superior suppressive activity in vivo, JAK2−/− CD8+ iTreg did not compromise their cytolytic function and had potential capacity to maintain the GVL effect.
CD8+ iTregs have similar suppressive activity to their CD4+ iTregs in vitro, and more importantly CD8+ iTregs preserve the GVL effect in sharp contrast to their CD4+ counterparts; however, their inferior stability limits their therapeutic potential (9–12, 14). Thus, stabilizing CD8+ iTregs becomes an important step to augment their therapeutic potential. This study revealed that JAK2−/− CD8+ iTregs resembled nTreg phenotype, as they expressed high level of a canonical nTreg marker, Neuropilin 1 (Nrp1) (36, 37). Many reports have shown that Nrp1 was positively associated with Foxp3 stability and suppressive capacity of Tregs (37, 38, 45). Nrp1hi CD4+ Tregs were more efficient at attenuating EAE severity compared with Nrp1low counterparts after adoptive transfer (45, 46). Nrp1-deficient CD4+ T-cells tended to differentiate into Th17 phenotype and were more proliferative than WT counterparts, whereas Nrp1-deficient CD4+ Tregs had impaired ability to suppress Teffs proliferation and cytokine production (37, 45). Consistent with the previous study (36), we observed that Nrp1hi Tregs (JAK2−/− CD8+ iTregs) significantly improved demethylation in CNS2 region of Foxp3, which is known to correlate with enhanced Foxp3 stability (39). Additionally, IL-6 exposure was previously shown to negatively regulate Nrp1 expression and stability in Treg cells (36); therefore, enhanced Nrp1 expression on JAK2−/− CD8+ iTregs could be a result of IL-6 inhibition via blocking JAK2/STAT3 signaling. Moreover, Nrp1 also serve as a receptor for TGF-β (47–49). Thus, up-regulation of Nrp1 on JAK2−/− CD8+ iTregs would recruit more TGF-β and promote Treg function. Continuous high retention of TGF-β in JAK2−/− CD8+ iTregs could also prevent the loss of Foxp3 expression during inflammatory environment as previously described by Floess, S. et al(39).
There may be multiple mechanisms that are employed by JAK2−/− CD8+ iTregs for the superior GVHD attenuation to WT counterparts. Retaining stable Foxp3 during inflammatory environment after transfer could be a main mechanism of JAK2−/− CD8+ iTregs to control GVHD. Given higher plasticity of WT CD8+ iTregs in the presence of inflammatory cytokines, they rapidly converted into Tc1/Tc17 phenotypes and produced a significant amount of IFN-γ and IL-17, which would subsequently contribute to cytokine storm leading to more GVHD severity. In contrast, JAK2−/− CD8+ iTregs were less prone to switch to Tc1/Tc17 cells resulting in less IFN-γ and IL-17 production. This notion was supported by even higher levels of IFN-γ and IL-17 in the serum of the recipients with Teff plus WT CD8+ iTregs than those with Teffs alone (Fig. 3G). Conversely, the levels of IFN-γ were significantly lower in the serum of the recipients with Teffs plus JAK2−/− CD8+ iTregs than those with Teffs alone, whereas the levels of IL-17 were comparable. Second possible mechanism, stabilized CD8+ iTregs markedly suppressed Th1 cells (CD4+ IFN-γ+) from donor T-cells (Fig. 5B). In addition to IFN-γ, TNF-α in recipient serum were dramatically reduced after transfer JAK2−/− CD8+ iTregs (Fig. 3G). Therefore, decreased quantities of Th1-associated cytokines (IFN-γ and TNF-α) in GVHD are directly associated with less severe GVHD after allo-BMT as consistent with previous reports (50–52). Third, the stabilized CD8+ iTregs promoted iTregs-derived donor T-cells (de novo iTregs) (Fig. 6A-B). These de novo iTregs, in particular CD8+ iTreg, would also contribute to suppress T-cell proliferation and cytokine secretion of pathogenic T cells in the early stage of allo-BMT as previously demonstrated by others (10, 14).
Given that GVL effect is crucial for preventing tumor relapse after allo-BMT, we found that transfer of additional CD8+ iTregs, regardless of WT or JAK2−/−, did not impair donor CD8+ T-cell cytotoxic activity, as they still produced comparable levels of IFN-γ as without iTreg transfer. In addition to cytotoxic activity of donor CD8+ Teffs, transferred CD8+ iTregs also possess their own cytotoxic function as shown in our previous study that CD8+ iTregs expressed various cytolytic molecules including granzyme B, granzyme C, Fas-L and perforin (11). As a result, synergistic tumor-killing effect of donor CD8+ Teffs and transferred CD8+ iTregs could engage in tumor regression. The current study indicated that stabilized iTregs did not lose their cytolytic activity (Supplemental Fig. 3) or potential to preserve the GVL effect mediated by donor Teffs (Fig. 7 and Supplemental Fig. 4).
Recently, two studies from independent groups successfully expanded ex-vivo human CD8+ iTregs, and adoptively transfer these iTregs into an established human allogeneic GVHD in humanized murine models (12, 53). They found that ex-vivo induced human CD8+ iTregs alleviated GVHD in allo-specific manner by reducing alloreactive T-cell proliferation as well as decreasing inflammatory cytokines within target organs. These reports are in support to our current study in regard to the therapeutic potential of CD8+ iTregs. For a translational approach, we demonstrated that CD8+ iTregs generation in the presence of potent JAK2 inhibitor (Pacritinib) also increased Foxp3 expression as similar as observed in JAK2 genetic-deletion. Given Pacritinib has been tested in controlling GVHD in the preclinical model (20) and is also being tested in clinical trial (NCT02891603), our study provides a strong rationale and translational feasibility to stabilize CD8+ iTregs by targeting JAK2, and the stabilized CD8+ iTregs could be a therapeutic potential in controlling GVHD in clinic.
Supplementary Material
Acknowledgments
The authors would like to thank the technical support provided by the Department of Laboratory Animal Research (DLAR) and Flow Cytometry Core and Imaging Core at Medical University of South Carolina (Hollings Cancer Center). We also would like to thank Dr. Sophie Paczesny, Indiana University School of Medicine, and CTI BioPharma Corp., for generously providing the MLL-AF9-GFP leukemic cells and Pacritinib, respectively.
This work was supported in part by National Institutes of Health Grants R01s CA118116, CA169116, AI118305 and HL137373, and SmartState Endowment in Cancer Stem Cell Biology & Therapy Program to X.-Z.Y.
Abbreviations:
- Treg
Regulatory T-cell
- JAK2
Janus Kinase 2
- GVHD
graft-versus-host disease
- GVL
graft-versus-leukemia
- allo-BMT
allogeneic bone marrow transplantation
References
- 1.Markey KA, MacDonald KP, and Hill GR. 2014. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood 124: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeiser R, and Blazar BR. 2017. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. The New England journal of medicine 377: 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101: 455–458. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PA, Lees CJ, and Blazar BR. 2002. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99: 3493–3499. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann P, Ermann J, Edinger M, Fathman CG, and Strober S. 2002. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. The Journal of experimental medicine 196: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, and Negrin RS. 2003. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nature medicine 9: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 7.Beres AJ, and Drobyski WR. 2013. The role of regulatory T cells in the biology of graft versus host disease. Frontiers in immunology 4: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazar BR, MacDonald KPA, and Hill GR. 2018. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beres A, Komorowski R, Mihara M, and Drobyski WR. 2011. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clinical cancer research : an official journal of the American Association for Cancer Research 17: 3969–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ, Williams CB, and Drobyski WR. 2012. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. Journal of immunology (Baltimore, Md. : 1950) 189: 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrichs J, Li J, Nguyen H, Wu Y, Bastian D, Daethanasanmak A, Sofi MH, Schutt S, Liu C, Jin J, Betts B, Anasetti C, and Yu XZ. 2016. CD8(+) Tregs promote GVHD prevention and overcome the impaired GVL effect mediated by CD4(+) Tregs in mice. Oncoimmunology 5: e1146842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Liu Y, Liu Y, Liu M, Xiang Z, Lam KT, Lewis DB, Lau YL, and Tu W. 2013. Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Science translational medicine 5: 168–169. [DOI] [PubMed] [Google Scholar]
- 13.Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, and Luo X. 2012. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 12: 2335–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, Hammerling GJ, Markey KA, Koyama M, Clouston AD, Engwerda CR, Hill GR, and MacDonald KP. 2012. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood 119: 5898–5908. [DOI] [PubMed] [Google Scholar]
- 15.Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y, and Kambayashi T. 2012. Cell-autonomous role of TGFbeta and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood 119: 5575–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, and Weyand CM. 2012. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. Journal of immunology (Baltimore, Md. : 1950) 189: 2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong H, Liu Y, Xu Z, Liang P, Yang H, Zhang X, Zhao J, Chen J, Fu S, Tang Y, Lv J, Wang J, Olsen N, Xu A, and Zheng SG. 2018. TGF-beta-Induced CD8(+)CD103(+) Regulatory T Cells Show Potent Therapeutic Effect on Chronic Graft-versus-Host Disease Lupus by Suppressing B Cells. Frontiers in immunology 9: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agle K, Vincent BG, Piper C, Belle L, Zhou V, Shlomchik W, Serody JS, and Drobyski WR. 2018. Bim regulates the survival and suppressive capability of CD8(+) FOXP3(+) regulatory T cells during murine GVHD. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O’Shea JJ, and Fowler DH. 2012. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 37: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts BC, Bastian D, Iamsawat S, Nguyen H, Heinrichs JL, Wu Y, Daenthanasanmak A, Veerapathran A, O’Mahony A, Walton K, Reff J, Horna P, Sagatys EM, Lee MC, Singer J, Chang YJ, Liu C, Pidala J, Anasetti C, and Yu XZ. 2018. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proceedings of the National Academy of Sciences of the United States of America 115: 1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betts BC, Abdel-Wahab O, Curran SA, St Angelo ET, Koppikar P, Heller G, Levine RL, and Young JW. 2011. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood 118: 5330–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betts BC, Sagatys EM, Veerapathran A, Lloyd MC, Beato F, Lawrence HR, Yue B, Kim J, Sebti SM, Anasetti C, and Pidala J. 2015. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. Journal of leukocyte biology 97: 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betts BC, Veerapathran A, Pidala J, Yu XZ, and Anasetti C. 2014. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. Journal of leukocyte biology 95: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radojcic V, Pletneva MA, Yen HR, Ivcevic S, Panoskaltsis-Mortari A, Gilliam AC, Drake CG, Blazar BR, and Luznik L. 2010. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. Journal of immunology (Baltimore, Md. : 1950) 184: 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tawara I, Sun Y, Liu C, Toubai T, Nieves E, Evers R, Alrubaie M, Mathewson N, Tamaki H, and Reddy P. 2012. Donor- but not host-derived interleukin-10 contributes to the regulation of experimental graft-versus-host disease. Journal of leukocyte biology 91: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q, Kim YC, Laurence A, Punkosdy GA, and Shevach EM. 2011. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. Journal of immunology (Baltimore, Md. : 1950) 186: 6329–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton AM, Donovan EE, Piccirillo CA, and Shevach EM. 2004. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. Journal of immunology (Baltimore, Md. : 1950) 172: 6519–6523. [DOI] [PubMed] [Google Scholar]
- 28.Frank DA, Robertson MJ, Bonni A, Ritz J, and Greenberg ME. 1995. Interleukin 2 signaling involves the phosphorylation of Stat proteins. Proceedings of the National Academy of Sciences of the United States of America 92: 7779–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, and Bromberg JS. 2009. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. Journal of immunology (Baltimore, Md. : 1950) 182: 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasidharan Nair V, Song MH, and Oh KI. 2016. Vitamin C Facilitates Demethylation of the Foxp3 Enhancer in a Tet-Dependent Manner. Journal of immunology (Baltimore, Md. : 1950) 196: 2119–2131. [DOI] [PubMed] [Google Scholar]
- 31.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, and Wilson CB. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15: 763–774. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, and Zhu J. 2014. Immunologic applications of conditional gene modification technology in the mouse. Curr Protoc Immunol 105: 10 34 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HD, Chatterjee S, Haarberg KM, Wu Y, Bastian D, Heinrichs J, Fu J, Daenthanasanmak A, Schutt S, Shrestha S, Liu C, Wang H, Chi H, Mehrotra S, and Yu XZ. 2016. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. The Journal of clinical investigation 126: 1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somervaille TC, and Cleary ML. 2006. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 10: 257–268. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Ramadan AM, Griesenauer B, Li W, Turner MJ, Liu C, Kapur R, Hanenberg H, Blazar BR, Tawara I, and Paczesny S. 2015. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Science translational medicine 7: 308–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, and Lafaille JJ. 2012. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. The Journal of experimental medicine 209: 1723–1742, S1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, and Bluestone JA. 2012. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. The Journal of experimental medicine 209: 1713–1722, S1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, and Vignali DA. 2013. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, and Huehn J. 2007. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS biology 5: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huehn J, Polansky JK, and Hamann A. 2009. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nature reviews. Immunology 9: 83–89. [DOI] [PubMed] [Google Scholar]
- 41.Yue X, Trifari S, Aijo T, Tsagaratou A, Pastor WA, Zepeda-Martinez JA, Lio CW, Li X, Huang Y, Vijayanand P, Lahdesmaki H, and Rao A. 2016. Control of Foxp3 stability through modulation of TET activity. The Journal of experimental medicine 213: 377–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, and Drobyski WR. 2007. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood 110: 3804–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, and Yang YG. 2014. The complex and central role of interferon-gamma in graft-versus-host disease and graft-versus-tumor activity. Immunological reviews 258: 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasahara H, Kondo T, Nakatsukasa H, Chikuma S, Ito M, Ando M, Kurebayashi Y, Sekiya T, Yamada T, Okamoto S, and Yoshimura A. 2017. Generation of allo-antigen-specific induced Treg stabilized by vitamin C treatment and its application for prevention of acute graft versus host disease model. International immunology 29: 457–469. [DOI] [PubMed] [Google Scholar]
- 45.Solomon BD, Mueller C, Chae WJ, Alabanza LM, and Bynoe MS. 2011. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America 108: 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campos-Mora M, Morales RA, Gajardo T, Catalan D, and Pino-Lagos K. 2013. Neuropilin-1 in transplantation tolerance. Frontiers in immunology 4: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glinka Y, and Prud’homme GJ. 2008. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. Journal of leukocyte biology 84: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glinka Y, Stoilova S, Mohammed N, and Prud’homme GJ. 2011. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis 32: 613–621. [DOI] [PubMed] [Google Scholar]
- 49.Cao Y, Szabolcs A, Dutta SK, Yaqoob U, Jagavelu K, Wang L, Leof EB, Urrutia RA, Shah VH, and Mukhopadhyay D. 2010. Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. The Journal of biological chemistry 285: 31840–31848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, and Ferrara JL. 1997. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 90: 3204–3213. [PubMed] [Google Scholar]
- 51.Robb RJ, Kreijveld E, Kuns RD, Wilson YA, Olver SD, Don AL, Raffelt NC, De Weerd NA, Lineburg KE, Varelias A, Markey KA, Koyama M, Clouston AD, Hertzog PJ, Macdonald KP, and Hill GR. 2011. Type I-IFNs control GVHD and GVL responses after transplantation. Blood 118: 3399–3409. [DOI] [PubMed] [Google Scholar]
- 52.Lu Y, Sakamaki S, Kuroda H, Kusakabe T, Konuma Y, Akiyama T, Fujimi A, Takemoto N, Nishiie K, Matsunaga T, Hirayama Y, Kato J, Kon S, Kogawa K, and Niitsu Y. 2001. Prevention of lethal acute graft-versus-host disease in mice by oral administration of T helper 1 inhibitor, TAK-603. Blood 97: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 53.Bézie S, Meistermann D, Boucault L, Kilens S, Zoppi J, Autrusseau E, Donnart A, Nerrière-Daguin V, Bellier-Waast F, Charpentier E, Duteille F, David L, Anegon I, and Guillonneau C. 2018. Ex Vivo Expanded Human Non-Cytotoxic CD8+CD45RClow/− Tregs Efficiently Delay Skin Graft Rejection and GVHD in Humanized Mice. Frontiers in immunology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.