Abstract
Objectives
To investigate the impact that the presence of chronic low back pain with radiculopathy (CLBPR) may have on 1) energy efficiency and 2) energy capacity among community-dwelling older adults.
Design
Matched case-control study.
Setting
Clinical research laboratory.
Participants
38 community-dwelling older adults (60-85 years) with (n=19) and without (n=19) CLBPR were included in this analysis. Participants were matched between-groups on age (± 5 years), sex, and diabetic status.
Interventions
Not applicable.
Main Outcome Measures
Energy cost of walking at self-selected speed (i.e. energy efficiency) and Peak Walking VO2 (i.e. energy capacity).
Results
Older adults with CLBPR had a higher energy cost of walking at self-selected speed (p=.009) and lower Peak Walking VO2 (p=.050), compared to those without pain.
Conclusions
Older adults with CLBPR may benefit from specific rehabilitative interventions that target these potentially modifiable energetic outcomes, thereby reducing the risk of mobility decline. Future studies should identify which mechanisms specifically contribute to diminished energy efficiency and capacity among older adults with CLBPR.
Keywords: Pain, Mobility, Rehabilitation, Metabolism
Chronic low back pain with radiculopathy (CLBPR) (i.e. pain that radiates from the lumbar spine into the leg(s)) is consistent with age-enhanced, degenerative changes of the spine, and is common among older adults.1,2 Walking impairments, which are strongly predictive of disability,3 and mortality,4 are a hallmark of this condition.1,2 Researchers have shown that age-related declines in walking speed may be driven by impaired energetic efficiency and capacity, as measured by the volume of oxygen consumed (VO2).5-7
Prior work has shown that walking is energetically inefficient among individuals with hip8 and knee9 pain. Furthermore, there is evidence to suggest that localized chronic low back pain may be linked to diminished energy capacity,10 with a recent conceptual model theorizing that the pain experience has a unique impact on energy efficiency and capacity, which may drive downstream mobility limitations.11
Along with increased pain while walking, the presence of CLBPR is associated with a higher comorbidity burden.12 For example, those with CLBPR are more likely to have hypertension and arthritis, compared to the general population.12 Age-related comorbidities can contribute to homeostatic imbalance, thereby increasing the energy cost of life (i.e. reduced efficiency).13 It is plausible that the mobility limitations seen among older adults with CLBPR are partly a manifestation of impaired energy metabolism, generated by the multifactorial nature of this condition. Yet our understanding of how the presence of this condition influences these energetic factors among older adults is limited.
The purposes of this study were to investigate whether CLBPR influences the energetic efficiency of walking and energy capacity. We hypothesized that older adults with CLBPR would have a higher energy cost of walking at self-selected speed (i.e. worse energy efficiency), as well as reduced Peak Walking VO2 (i.e. worse energy capacity), compared to age- and sex-matched, pain-free participants. In a secondary, exploratory hypothesis, we theorized that a having CLBPR would be associated with greater odds of having a Peak Walking VO2 <18 mL/kg/min, an established threshold for increased risk of physical function decline.14
Methods
Participants
This study was a comparative analysis of a sample of community-dwelling older adults (60-85 years) with and without CLBPR. Participants with CLBPR met the following pain criteria: low back pain intensity ≥3/10, pain frequency ≥4 days per week, pain duration ≥3 months, and pain that radiated to, or below, the knee during walking. Individuals with unilateral or bilateral leg symptoms due to CLBPR were included. Individuals with pain were excluded if they had any of the following: a non-mechanical low back pain symptom (e.g. unrelenting night pain, lack of sensation in the groin and/or buttocks), severely limited mobility (i.e. needed an assistive device for testing), significant cardiovascular or cardiopulmonary disease (i.e. disease(s) in these systems that could potentially impact normal walking, such as peripheral arterial disease or chronic obstructive pulmonary disease) a progressive neurological disorder, or a terminal illness.
Pain-free older adults were included as controls if they matched a CLBPR participant already enrolled in the study, based on the following characteristics: age (± 5 years), sex, and diabetic status; matching on these factors was done to reduce potential confounding of these person-level factors on energetic outcomes. Control participants were required to be free from back pain in the year prior to study enrollment and free of any significant pain (≥2/10 intensity) in the 72 hours prior to testing. Exclusion criteria for this group were the same as the CLBPR group. With the exception of diabetes and CLBPR, participants had no other known comorbid factors thought to influence energy efficiency or capacity.
All participants were recruited from local senior centers and health clinics in Northern Delaware; flyers and consent-to-be-contacted forms were distributed to each site for recruitment. Participants were screened for inclusion/exclusion criteria by research personnel via phone, prior to study participation. Study assessments were performed by a trained and licensed physical therapist in a clinical research laboratory, at the University of Delaware. Written informed consent was obtained prior to participation. The flow diagram in Figure 1 illustrates the study overview for the 38 participants included in this study. This study was approved by the University of Delaware Institutional Review Board and was in accordance with the Helsinki Declaration of the World Medical Association.
Figure 1.
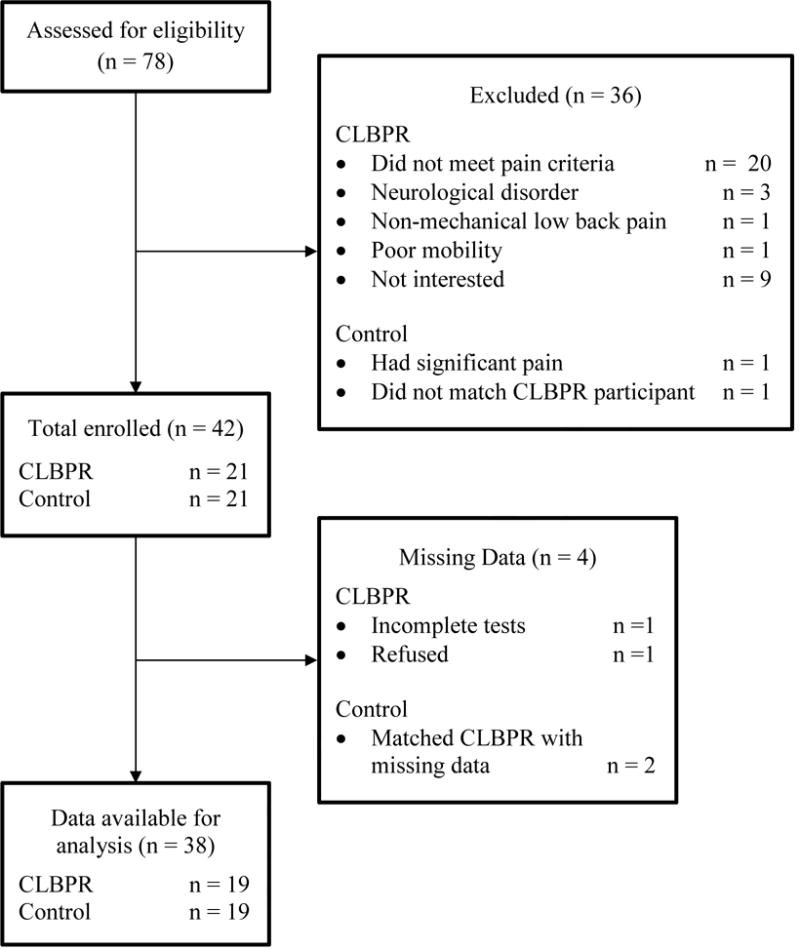
Flow chart for study overview
Descriptive Characteristics
Participants reported their age, sex, race, education level, and diabetic status. In addition, participants estimated the duration of their CLBPR symptoms. A digital scale was used to measure height and weight, from which body mass index (BMI) was calculated. The Quebec Disability Index (QDI) was used to assess low back pain-related disability.15 The numeric pain rating scale16 was used to assess worst pain intensity in the last 24 hours in the leg(s), with anchors from 0 (“no pain”) to 10 (“worst possible pain”).
Energy Cost of Walking at Self-Selected Speed (Energy Efficiency)
The energy cost of walking was assessed while participants walked at their self-selected speed around two cones that were 20 meters apart.5-7 Participants were required to walk 2.5 minutes while VO2 in mL/kg/min was measured via an Oxycon Mobile™ Portable VO2 Measurement system (CareFusion™, San Diego, CA). Per manufacturer instruction, this device was calibrated at the beginning of each test using standard calibration gases (16% O2, 4% CO2, balance nitrogen) for gas content, and the auto-calibration function for gas flow.
VO2 was recorded using the single-breath format, and then averaged for each 20-meter interval. Data from the first 1.5 minutes were discarded to ensure the participant reached physiologic steady state; data from the remaining 1-minute window (i.e. 1.5-2.5 minute mark) were used for the analysis. VO2 from each breath in the 1-minute window was averaged. The duration of each length was recorded; gait speed was calculated for each length by dividing 20 meters by the length duration. Gait speed values from the lengths included in this 1-minute window were averaged to arrive at a single value. The energy cost of walking (mL/kg/m) was calculated by dividing average VO2 by average gait speed:
Secondary Outcomes
Average gait speed (m/sec) was considered a secondary outcome. In addition, the metabolic gas analyzer also computed Respiratory Exchange Ratio (RER) for each breath taken. RER was used to determine level of metabolic exertion; values included in the 1-minute window were averaged to arrive at a single value. RER is the ratio of CO2 produced to VO217; high RER values indicate a higher utilization of carbohydrates to lipid ratio. RER increases with exercise intensity, and is often used to help define energy capacity: at a given level of exercise intensity, fit (i.e. higher energy capacity) adults achieve lower RER values, compared to unfit (i.e. lower energy capacity) adults.17 In other words, if a person truly has low energy capacity, they will reach the same RER-level as a person with high energy capacity, but at a significantly lower exercise intensity.
Peak Walking VO2 (Energy Capacity)
Peak Walking VO2 was captured during a 400-meter walk test at peak sustained walking speed, on the course previously described. This test has been validated as a measure of cardiorespiratory fitness in older adults,18 and this protocol replicated that of a previous study on aging and energy expenditure.6 Participants were instructed to walk as fast as they could around the cones for 10 full laps, for a total of 400 meters. Standardized encouragement was given at each lap.
VO2 was measured using the same equipment and parameters as previously described. Data from the first 1.5 minutes were discarded to ensure the participant reached physiologic steady state. To calculate Peak Walking VO2, the remaining VO2 values measured from each breath taken during the duration of the test were averaged.6,19 If participants were unable to complete the 400 meter-walk, the usable data before the walk test ended was averaged for a Peak Walking VO2 measurement; this required a minimum of 30 seconds of usable (i.e. after participants reached physiologic steady state) VO2 measurements. Gait speed was computed as the distance covered during the test divided by the time it took for completion.
Secondary Outcomes
Average gait speed (m/sec) and RER were considered secondary outcomes. RER was measured for each breath in the same time window as Peak Walking VO2, and these values were averaged to arrive at a single measure.
Statistical Analysis
Statistical analyses were performed using SPSS 24 (SPSS, Inc. Armonk, NY). Descriptive analyses were performed for both groups. A mixed-design analysis of covariance (ANCOVA) was used (α=.050). A conservative approach in controlling for BMI was elected, because the between-groups difference on this characteristic approached statistical significance (p=.087). After adjusting for BMI, pairwise between-group comparisons were made for both the energy cost of walking at self-selected speed and Peak Walking VO2. Kolmogorov-Smirnov and Shapiro-Wilk tests were used to determine the normality of residuals of ANCOVA models; p-levels ≥ .050 on these tests indicate a violation of the assumption of normality of residuals. If this occurred, box plots were generated of residual values. Box plot outliers were identified and manually removed in an iterative fashion until normality was achieved. If an outlier was removed in one group, then the corresponding matched participant in the other group was also removed. Partial eta-squared effect size values were calculated to determine the amount of variance group membership explained in each dependent variable. Binary logistic regression was used to determine the odds ratio between the two groups, of having a Peak Walking VO2 of <18 mL/kg/min.
Results
Table 1 displays the descriptive characteristics for both groups. Table 2 displays the results from the between-groups comparison for the energy cost of walking, Peak Walking VO2, and respective secondary outcomes. While walking at their self-selected speed, participants with CLBPR had a higher energy cost of walking (absolute mean difference = .031 mL/kg/m, p=.009) and walked significantly slower (absolute mean difference = .14 m/sec, p=.003), compared to control participants. While walking at their peak speed, participants with CLBPR had a lower Peak Walking VO2 (absolute mean difference = 2.39 mL/kg/min, p=.050) and walked slower (absolute mean difference = .28 m/sec, p=.002), compared to control participants.
Table 1.
Descriptive characteristics
| Control (n=19) | CLBPR (n=19) | |||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Sex (female) | 10 (52.6) | 10 (52.6) | ||
| Diabetes (present) | 4 (21.1) | 4 (21.1) | ||
| Race (Caucasian) | 19 (100) | 17 (89.5) | ||
| Education (college or more) | 14 (73.7) | 11 (57.9) | ||
| Mean (SD) | Min-Max | Mean (SD) | Min-Max | |
| Age (years) | 68.9 (5.8) | (60–80) | 68.8 (4.8) | (60–78) |
| BMI | 27.9 (4.1)* | (18.1–34.7) | 30.8 (8.3)* | (18.3–53.7) |
| Duration of CLBPR (years) | – | – | 12.8 (15.0) | (0.3–50.0) |
| Worst Leg Pain Intensity (0–10) | – | – | 6.0 (3.0) | (2–10) |
| Quebec (0–100%) | – | – | 34.4 (17.5) | (7.0–64.0) |
Abbreviations: CLBPR = Chronic low back pain with radiculopathy; BMI = body mass index; LBP = low back pain; Quebec = Quebec Disability Index
p=.087
Table 2.
Between-group differences in energy expenditure
| Control (n=19) | CLBPR (n=19) | |||
|---|---|---|---|---|
| Adjusted Mean (SE) | Partial Eta Squared | p-value | ||
| Energy Cost of Walking Test | ||||
| Energy Cost of Walking (mL/kg/m) | .186 (.008) | .217 (.008) | .181 | .009* |
| Gait Speed (m/sec) | 1.11 (.03) | .97 (.03) | .195 | .003* |
| Respiratory Exchange Ratio | .784 (.010) | .807 (.010) | .067 | .121 |
| Peak Walking VO2 Test | ||||
| Peak Walking VO2 (mL/kg/min)† | 17.97 (.82) | 15.58 (.82) | .111 | .050* |
| Gait Speed (m/sec) | 1.55 (.06) | 1.27 (.06) | .253 | .002* |
| Respiratory Exchange Ratio | .951 (.027) | .924 (.027) | .013 | .499 |
Abbreviations: CLBPR = Chronic low back pain with radiculopathy
p≤.050
1 outlier removed from each group
RER did not differ significantly between-groups during either walking test, indicating that metabolic exertion was similar for both groups on both tests. Comparisons for Peak Walking VO2 initially violated the assumption of normality of residuals; removal of the greatest outlier (i.e. highest residual value) and the participant matched in the corresponding group, resulted in satisfying this test assumption. Having CLBPR was associated with 2.04 greater odds of having a Peak Walking VO2 <18 mL/kg/min, but this did not reach statistical significance (p=.308).
Discussion
Group membership explained 18.1% of the variance in energy cost of walking, after adjusting for BMI. Schrack et al found normal energy cost values were approximately .170 and .195 mL/kg/m for older adults aged 60 and 80 years, respectively.5 In this study, energy cost values for pain-free older adults were consistent with normative data. However, those with CLBPR had a much higher energy cost compared to reference values for 80 year-old individuals,5 despite an average group-age of 69 years. As energetic inefficiency is a strong risk factor for gait speed decline,7 these data suggest that older adults with CLBPR may have a greater risk for future mobility limitation, and thus, disability3 and mortality.4
To our knowledge, this is the first study to establish a link between energy inefficiency and CLBPR among older adults. CLBPR contributes to gait impairments that may be unique to this condition.20,21 Optimal mechanics contribute to energy efficiency, while gait alterations can reduce efficiency.22 Furthermore, CLBPR is associated with a multitude of comorbid conditions12; comorbidities have been shown to elevate the energy expenditure,13 and thus reduce efficiency. Future studies should investigate the specific mechanisms that contribute to energetic inefficiency among older adults with CLBPR, to identify potentially modifiable targets for rehabilitation.
Our results indicated that group membership explained 11.1% of the variance in Peak Walking VO2, after adjusting for BMI. This analysis required the removal of one outlier from each group. Outlier removal was necessary to satisfy the assumption of normality, which improves the confidence in the results. However, with small sample sizes, outlier removal can have a profound effect on the analyses. In this study, had the outlier not been removed, the comparison would have yielded a p=.064. While not statistically significant, p=.064 clearly demonstrates a similar pattern to the analyses with outlier removal (p=.050).
To reach an RER of .924, older adults with CLBPR walked at 1.27 m/sec; those without pain, however, walked at a much faster speed (1.55 m/sec) to reach a similar RER. This indicates that both groups were working equally hard metabolically, but their speeds were vastly different. The reason that RER did not differ between groups, despite vastly different speeds, is likely because older adults with CLBPR had a significantly lower energy capacity. As a result, older adults with CLBPR likely are incapable of achieving the same mobility level (i.e. walking speed and distance) as their pain-free counterparts. Prior work suggests that a Peak Walking VO2 <18 mL/kg/min is predictive of developing difficulty in aspects of self-reported physical function (e.g. pushing/pulling objects; stooping, crouching, kneeling).14 Our results indicate that the having CLBPR was associated with 2× greater odds of having a Peak Walking VO2 <18, but this was not statistically significant. It is possible that we were underpowered to detect this relationship, given that this was a secondary hypothesis.
Of note, older adults with CLBPR walked at significantly slower self-selected and fast speeds compared to control participants, and those differences exceeded clinical relevance (>.10 m/sec).23 The slowness of gait may play a role in the energetic inefficiency observed.
Overall, this work has important clinical implications. Given their link to mobility decline, energy efficiency5,7 and capacity6,14 may be important outcomes in the rehabilitation of older adults with CLBPR. It is important to note that these outcomes require specific tools (i.e. metabolic gas analysis equipment). While these tools may not yet be commonplace for physical rehabilitation, they are commonly used in specific clinical settings (e.g. cardiac rehabilitation). It is possible that interdisciplinary collaboration could lead to improved management of mobility among older adults with CLBPR. Nevertheless, when collaborations are not feasible, clinical tests exist to estimate energy capacity. Simonsick et al found that the Long Distance Corridor Walk test,18 which requires little resources and training, can provide a valid estimate of Peak VO2 in older adults.
Regarding treatment, our work also has specific implications. As mentioned, we suspect that the poor energy efficiency seen among older adults with CLBPR, may be due to two potentially modifiable factors: gait alterations and high comorbidity burden. We hypothesize that both mechanisms contribute to the poor energy efficiency seen among older adults with CLBPR. Our future work will examine these pathways.
Regardless, there is no known harm in targeting both factors. First, if pain drives gait impairments, then clinicians should focus on optimal pain management during walking. There are a variety of modalities that can be used to manage pain (e.g. transcutaneous electrical stimulation), and clinicians should trial modalities to see which is most effective. However, mobility deficits often persist even after pain is effectively managed. Fortunately, prior work has shown that there are effective rehabilitation strategies, such as exercises that focus on the timing and coordination of walking, that improve the energy efficiency of walking among older adults.24 Second, clinicians should consider optimizing chronic disease management to help reduce, or prevent rise in, the energy cost of walking. To accomplish this, clinicians should take a more holistic approach, collecting a detailed medical history and orchestrating interdisciplinary care (e.g. contacting primary care physicians).
Energy capacity can be improved through a variety of exercise interventions (e.g. cycling, ergometer, walking).25 For example, as previously mentioned, older adults with CLBPR have difficulty walking, due to increases in leg pain. For these patients, a clinician may choose an exercise mode that is less provocative, such as recumbent cycling. Alternatively, clinicians may also consider selecting mobility aids that may attenuate pain provocation during walking, such as a single point cane. Regardless, clinicians should still emphasize that the overarching goal of treatment is to be more physically active.
Study Limitations
In addition to being small, the sample included high-functioning individuals. The testing protocol required participants to walk for long periods without the use of an assistive walking device, which potentially excluded more debilitated participants. To isolate the impact that CLBPR had on these measures, individuals with significant comorbidities were excluded. Furthermore, the CLBPR group was heterogeneous regarding clinical presentation (e.g. symptom history ranged from 3 months to 50 years; unilateral and bilateral presentations were included). With longstanding pain, it can be difficult for people to estimate the exact duration of their symptoms (i.e. recall bias). The specific exclusion criteria on potentially confounding factors, as well as the matching criteria, limited our ability to focus on specific pain and diagnostic characteristics. However, it was necessary to include such criteria for this study, to examine the hypotheses adequately. In future studies, different criteria (e.g. diagnostic imaging confirmation of stenosis, duration of symptoms, unilateral versus bilateral symptom distribution) could be used, to elucidate the potential impact of different CLBPR-related clinical characteristics (e.g. symptom duration, sensation, pain intensity) on energetic outcomes. Finally, one may argue that conducting the energy efficiency test on a treadmill would allow for the fixation of walking speed, making the energy cost results easier to interpret; however, treadmill walking artificially inflates energy cost,26,27 therefore an over ground protocol was selected.
Conclusion
Older adults with CLBPR are energetically inefficient, have a diminished energy capacity, and have a clinically slower gait speed than older adults without pain. Although our study design prevents us from concluding that these individuals are at a higher risk for mobility decline, these two energetic factors have been shown to be linked to mobility limitation and are potentially modifiable. Clinicians may focus on them as treatment outcomes, which may potentially reduce the risk of onset and/or progression of disability in this patient population, but future studies are needed.
Highlights.
Older adults with CLBPR have poor energy efficiency and capacity
Prior work has shown that poor efficiency and capacity contribute to worse mobility
Future research should identify the underlying mechanisms to these energy deficits
Acknowledgments
The authors are grateful to Dr. Jennifer Brach for proofreading this manuscript and offering feedback to improve its quality.
FUNDING: This work was supported by the National Institutes of Health (grant numbers R01AG0412202, R21HD057274, K12HD055931, and T32HD007490); and the American Physical Therapy Association’s Academy on Geriatric Physical Therapy (Adopt-A-Doc Award).
Abbreviations
- ANCOVA
Analysis of Covariance
- BMI
body mass index
- CLBPR
chronic low back pain with radiculopathy
- VO2
volume of oxygen consumed
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PRIOR PRESENTATION OF MATERIAL: None.
DISCLSOURES: None.
References
- 1.Hicks GE, Gaines JM, Shardell M, Simonsick EM. Associations of back and leg pain with health status and functional capacity of older adults: findings from the retirement community back pain study. Arthritis Rheum. 2008;59(9):1306–1313. doi: 10.1002/art.24006. [DOI] [PubMed] [Google Scholar]
- 2.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi: 10.1136/bmj.h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera S, Patel KV, Rosano C, et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. J Gerontol A Biol Sci Med Sci. 2016;71(1):63–71. doi: 10.1093/gerona/glv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60(10):1811–1816. doi: 10.1111/j.1532-5415.2012.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrack JA, Simonsick EM, Ferrucci L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehabil. 2013;92(1):28–35. doi: 10.1097/PHM.0b013e3182644165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrack JA, Zipunnikov V, Simonsick EM, Studenski S, Ferrucci L. Rising Energetic Cost of Walking Predicts Gait Speed Decline With Aging. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gussoni M, Margonato V, Ventura R, Veicsteinas A. Energy cost of walking with hip joint impairment. Phys Ther. 1990;70(5):295–301. doi: 10.1093/ptj/70.5.295. [DOI] [PubMed] [Google Scholar]
- 9.Ko SU, Simonsick EM, Ferrucci L. Gait energetic efficiency in older adults with and without knee pain: results from the Baltimore Longitudinal Study of Aging. Age (Dordr) 2015;37(1):9754. doi: 10.1007/s11357-015-9754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smeets RJ, Wittink H, Hidding A, Knottnerus JA. Do patients with chronic low back pain have a lower level of aerobic fitness than healthy controls?: are pain, disability, fear of injury, working status, or level of leisure time activity associated with the difference in aerobic fitness level? Spine (Phila Pa 1976) 2006;31(1):90–97. doi: 10.1097/01.brs.0000192641.22003.83. discussion 98. [DOI] [PubMed] [Google Scholar]
- 11.Coyle PC, Schrack JA, Hicks GE. Pain Energy Model of Mobility Limitation in the Older Adult. Pain Med. 2017 doi: 10.1093/pm/pnx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battié MC, Jones CA, Schopflocher DP, Hu RW. Health-related quality of life and comorbidities associated with lumbar spinal stenosis. Spine J. 2012;12(3):189–195. doi: 10.1016/j.spinee.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. “IDEAL” aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62(4):667–672. doi: 10.1111/jgs.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morey MC, Pieper CF, Cornoni-Huntley J. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults? Med Sci Sports Exerc. 1998;30(8):1223–1229. doi: 10.1097/00005768-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hicks GE, Manal TJ. Psychometric properties of commonly used low back disability questionnaires: are they useful for older adults with low back pain? Pain Med. 2009;10(1):85–94. doi: 10.1111/j.1526-4637.2008.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor LJ, Harris J, Epps CD, Herr K. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabil Nurs. 2005;30(2):55–61. doi: 10.1002/j.2048-7940.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramos-Jiménez A, Hernández-Torres RP, Torres-Durán PV, et al. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin Med Circ Respirat Pulm Med. 2008;2:1–9. doi: 10.4137/ccrpm.s449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 19.Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(Suppl 2):S329–336. doi: 10.1111/j.1532-5415.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part B. Preoperative versus postoperative gait variability. Physiol Meas. 2009;30(11):1187–1195. doi: 10.1088/0967-3334/30/11/004. [DOI] [PubMed] [Google Scholar]
- 21.Suda Y, Saitou M, Shibasaki K, Yamazaki N, Chiba K, Toyama Y. Gait analysis of patients with neurogenic intermittent claudication. Spine (Phila Pa 1976) 2002;27(22):2509–2513. doi: 10.1097/00007632-200211150-00016. [DOI] [PubMed] [Google Scholar]
- 22.Larish DD, Martin PE, Mungiole M. Characteristic patterns of gait in the healthy old. Ann N Y Acad Sci. 1988;515:18–32. doi: 10.1111/j.1749-6632.1988.tb32960.x. [DOI] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Brach JS, Lowry K, Perera S, et al. Improving motor control in walking: a randomized clinical trial in older adults with subclinical walking difficulty. Arch Phys Med Rehabil. 2015;96(3):388–394. doi: 10.1016/j.apmr.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang G, Gibson CA, Tran ZV, Osness WH. Controlled endurance exercise training and VO2max changes in older adults: a meta-analysis. Prev Cardiol. 2005;8(4):217–225. doi: 10.1111/j.0197-3118.2005.04324.x. [DOI] [PubMed] [Google Scholar]
- 26.Berryman N, Gayda M, Nigam A, Juneau M, Bherer L, Bosquet L. Comparison of the metabolic energy cost of overground and treadmill walking in older adults. Eur J Appl Physiol. 2012;112(5):1613–1620. doi: 10.1007/s00421-011-2102-1. [DOI] [PubMed] [Google Scholar]
- 27.Parvataneni K, Ploeg L, Olney SJ, Brouwer B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin Biomech (Bristol, Avon) 2009;24(1):95–100. doi: 10.1016/j.clinbiomech.2008.07.002. [DOI] [PubMed] [Google Scholar]


