Abstract
We measured the effect of an aerobic exercise session on postprandial glucose control in adolescents with habitually low physical activity. The goal was to determine if the acute or residual response of exercise was altered by overweight/obesity (OW/Ob). Eleven normal weight (NW, BMI = 48 ± 13 percentile) and 12 OW/Ob (BMI = 91 ± 5 percentile) participants completed three trials. In the no exercise (No Ex) trial, participants rested quietly before and after consuming a test meal. In the other 2 trials, a 45-minute aerobic exercise session was performed either 17-hours (Prior Day Ex) or 40 minutes (Same Day Ex) before the test meal. On all trials, the OW/Ob group had higher fasting glucose (~6%) and insulin (~66%), and lower insulin sensitivity (~9%) than the NW group. The Same Day Ex and Prior Day Ex trials resulted in reduced area under the curve for glucose (6% on both trials, p < 0.01) and insulin (15 and 13%, respectively, p < 0.03), and increased insulin sensitivity (8 and 6%, respectively, p < 0.01). The magnitudes of those effects did not differ between the NW and OW/Ob groups. Plasma fatty acids declined and carbohydrate oxidation increased after the meal, but did not differ among trials or groups. The results demonstrate that moderate-intensity aerobic exercise increases insulin sensitivity in NW and OW/Ob adolescents and that the beneficial effects of exercise lasts up to 17 hours. The acute impact of exercise on metabolic health in adolescents is not impaired by overweight/obesity.
Keywords: physical activity, mixed meal, glucose tolerance
INTRODUCTION
Adolescent overweight or obesity elevates the risk for developing diabetes and other cardiometabolic diseases, as shown by reduced insulin sensitivity, reduced rates of whole body fat oxidation, and other signs of metabolic inflexibility (1–3). However, exercise can reverse many of the metabolic consequences of obesity. In several studies glucose tolerance and insulin sensitivity were improved in overweight/obese children and adolescents following 8–13 weeks of structured exercise (3–7), although there are exceptions (8, 9). In many of those studies, insulin sensitivity improvement was independent of weight loss, demonstrating the effect of exercise per se on glycemic control (3–6).
Despite those findings, it is not yet clear whether adolescents who are overweight or obese have similar responses in insulin sensitivity following exercise as their normal weight peers. In one report insulin sensitivity was increased by a similar magnitude in normal weight and obese adolescents following 12 weeks of aerobic exercise training (3). In contrast, when normal weight and overweight girls performed a single aerobic exercise session, the normal weight group had reduced fasting insulin and maintained higher fat oxidation the following morning, but these effects were not observed in the overweight group (10). The latter results raise the possibility that the beneficial effects of exercise on insulin action and fuel metabolism in overweight youth might be blunted or more transient at the beginning of an exercise program. There are, as yet, no other comparisons of insulin sensitivity responses to exercise in normal weight versus overweight/obese adolescents.
We previously reported that a single session of moderate-intensity exercise performed by adolescents with habitually low physical activity resulted in reduced postprandial glucose and insulin compared to a rest-only condition (11). Those responses were evident when the test meal was consumed either 40 minutes or 17 hours after completing the exercise. The participants in that report had a range of body sizes and composition. In the current investigation, we increased the number of participants, with the purpose of determining whether the metabolic responses to the single exercise session would be modified by the presence of overweight/obesity.
METHODS
Participants
The eligibility criteria were age 12–17 years old, maturational development ≥ Tanner Stage 2, habitually low physical activity, and no evidence of medical conditions, or use of medications that could alter the results. Low physical activity was defined as performing less than 30 minutes of moderate-to-vigorous exercise on three or fewer days per week. Body mass index (BMI) was used to classify participants as normal weight (NW; 5 girls, 6 boys) or overweight/obese (OW/Ob; 7 girls, 5 boys). NW was defined as having a BMI > 20th to ≤ 75th percentile on pediatric growth charts from the Centers for Disease Control and Prevention (12). OW/Ob was defined as BMI ≥ 85th percentile. Results from a subset of 4 participants in the NW group and 8 in the OW/Ob group were previously published (11). For the current investigation, we included an additional 7 NW and 4 OW/Ob participants who followed the same protocol.
Study protocol
Each participant completed an initial screening visit and three separate experimental trials, as previously described (11). Children and their parents or guardians provided their informed assent and consent in accordance with the university Institutional Review Board, which approved the study. During the screening visit, a pediatric clinician performed a medical exam to assure that the participant was healthy and met the inclusion criteria. The clinician confirmed that participants had reached puberty but did not determine specific Tanner stage. Whole body and regional composition of fat and lean tissue was measured using dual energy X-ray absorptiometry (Lunar iDXA, GE-Healthcare, Fairfield, CT). Cardiorespiratory fitness was measured during an incremental workload test to volitional exhaustion on a stationary bicycle (Lode Corival, Groningen, The Netherlands). During exercise, HR was recorded with a chest-strap monitor (Polar Electro USA, Lake Success, NY) and oxygen uptake was measured with an indirect calorimetry system (Ultima Cardio2, MedGraphics, St. Paul, MN). Participants were familiarized with the exercise protocol on the first visit (11).
Three morning trials were conducted at least one week apart. The trials differed only by the timing of exercise (or lack of exercise) before a test meal was consumed. The first trial for all participants was the No Exercise trial described below. The order of the two trials involving exercise was conducted in randomized order. Participants were instructed to maintain their normal activity pattern and to consume a consistent mixed diet for three days before each trial. Daily physical activity during waking hours was recorded for three to four days before each trial with a step activity monitor worn above the ankle (StepWatch 3, OrthoCare Innovations, Mountlake Terrace, WA). The three trials were:
No Exercise Trial (No Ex). Participants rested quietly throughout the morning. This trial was considered as the control to which the other trials were compared.
-
Prior Day Exercise Trial (Prior Day Ex). A 45-minute bout of moderate intensity aerobic exercise was performed in the afternoon. The exercise session consisted of consecutive 15-minute segments of walking on a treadmill, stationary cycling, and video game boxing (Nintendo Wii Sports, Nintendo of America, Redmond, WA), respectively. Walking and cycling workloads were adjusted to elicit 75% of the peak HR. During boxing the participants were instructed to remain actively engaged in the game. HR during boxing is more variable than steady-state walking or cycling but the average was ~75% of peak HR (11, 13). The exercise session was designed to be feasible and enjoyable for participants who were not habitual exercisers, and to engage multiple muscle groups.
After completing the exercise, the participants went home for the evening and then returned to the research center the following morning, with a time delay of 16.9 ± 0.2 hours between the end of the exercise and the start of the meal. The timing of the exercise and meal were selected for practical reasons; most participants completed the afternoon exercise session after school, and returned the following morning at the same time as the other trials.
Same Day Exercise Trial (Same Day Ex). After completing baseline resting measurements, the same moderate intensity exercise session described above was performed, with a time delay of 38 ± 3 minutes between the end of the exercise and the start of the meal. The time between the end of the exercise and meal allowed participants a short recovery period, for the nurse to assure that the intravenous line was in place, and for preparation of the meal and testing equipment.
Trial protocol
All trials were performed following an overnight fast and began with baseline measurement of resting energy expenditure using an indirect calorimetry system with a flow-through canopy (TrueOne 2400, ParvoMedics, Sandy, UT). Blood pressure and heart rate (HR) were recorded at the end of the energy expenditure measurement. An intravenous catheter was placed in a forearm vein and kept patent with saline infusion for serial blood sampling. Blood samples were collected at 10 and 2 minutes before the start of the meal, and at 10, 20, 30, 40, 60, 90, 120, 150, and 180 minutes after the start of meal consumption. The mixed meal was a chocolate shake made from milk powder, milk cream and chocolate syrup (2803 kJ, 45/40/15% of energy from carbohydrate/fat/protein, respectively). It was consumed within five minutes. Indirect calorimetry was repeated at 40, 110, and 160 minutes following the meal. The last 15 minutes of data collected at each time was used to calculate energy expenditure and carbohydrate oxidation (14).
Blood analyses
Blood samples were separated into plasma or serum at the time of collection and stored at −80°C until analysis. Plasma glucose was measured by the glucose oxidase method (2300STAT Plus, Yellow Springs Instruments, Yellow Springs, OH). Serum insulin and C-peptide were measured using commercial ELISA kits (#EZHIASF-14K and #EZHCP-20K, respectively, Millipore, St. Louis, MO). Serum triglycerides, total cholesterol, and HDL-cholesterol were measured at the Clinical Chemistry Laboratory of the Oklahoma Veterans Administration Hospital (Oklahoma City) using validated enzymatic assays (Synchron Systems, Beckman Coulter, Brea, CA). Serum non-esterified fatty acids (NEFA) were measured in with an enzymatic colorimetric assay (NEFA-HR2, Wako Chemicals, Richmond, VA). All assays were performed with appropriate standards and quality controls according to manufacturer’s directions.
The fasting concentrations of each analyte was calculated as the average of two blood samples collected at 10 and 2 minutes prior to meal ingestion. The concentrations of glucose, insulin, C-peptide, and NEFA from 0–180 minutes after the meal were used to calculate the total area under the curve (AUC) using the trapezoidal method, and the oral glucose insulin sensitivity index (15).
Data Analysis
Descriptive statistics were computed for all variables and presented as mean ± standard deviation unless otherwise specified. An analysis of variance for repeated measures was used to determine the effect of trial, group, and their interaction on the outcome variables. Bonferroni post-hoc tests were used to determine pairwise differences. Student’s t test was used for comparisons between groups for all other variables. Cohen’s d was used to calculate the standardized difference between means (Effect size). Bivariate correlations were used to measure the strength of association among selected variables. P values less than an alpha of 0.05 were considered significant for all tests.
RESULTS
Participant characteristics (Table 1)
Table 1.
Participant characteristics
| NW | OW/Ob | |
|---|---|---|
| Age, y | 15 ± 2 | 14 ± 2 |
| Weight, kg | 55.5 ± 4.9 | 64.8 ± 15.0* |
| Height, m | 1.67 ± 0.07 | 1.60 ± 0.10* |
| BMI, percentile | 48 ± 13 | 91 ± 5* |
| Body fat, kg | 13.0 ± 5.7 | 22.5 ± 6.5* |
| Body fat, % | 23.5 ± 9.9 | 35.1 ± 6.3* |
| Lean mass, kg | 40.3 ± 6.5 | 39.8 ± 10.7 |
| Trunk fat, kg | 5.4 ± 2.8 | 10.3 ± 3.3* |
| Peak bike power, watts | 135 ± 53 | 125 ± 41 |
| Peak VO2, ml/kg LBM/min | 42.6 ± 11.0 | 43.4 ± 6.5 |
| Peak heart rate, b/min | 185 ± 14 | 183 ± 16 |
| Total cholesterol, mmol/l | 3.71 ± 0.82 | 4.36 ± 1.01 |
| HDL cholesterol, mmol/l | 1.10 ± 0.19 | 1.12 ± 0.26 |
| Triglycerides, mmol/l | 0.74 ± 0.31 | 0.99 ± 0.43 |
| NEFA, mEq/l | 0.57 ± 0.23 | 0.58 ± 0.07 |
| Glucose, mmol/l | 4.7 ± 0.3 | 5.0 ± 0.3* |
| Insulin, pmol/l | 38.0 ± 16.6 | 63.2 ± 29.5* |
| C-peptide, nmol/l | 0.48 ± 0.07 | 0.72 ± 0.09* |
| Systolic blood pressure, mmHg | 108 ± 7 | 116 ± 8* |
| Diastolic blood pressure, mmHg | 59 ± 5 | 59 ± 6 |
| Heart rate, beats/min | 64 ± 4 | 68 ± 3 |
Values are mean ± SD for 5 girls and 6 boys in the normal weight (NW) group and 7 girls and 5 boys in the overweight/obese (OW/Ob) group. Body composition was measured using dual X-ray absorptiometry. Peak exercise responses were measured during a bicycle test to volitional exhaustion. Biochemical and blood pressure results are from the fasting period during the No Ex trial.
Higher than the NW group, p <0.05.
The OW/Ob group had higher values for BMI, total body fat, trunk fat, and fasting glucose, insulin, C-peptide, and systolic blood pressure. All other descriptive characteristics were not different between groups. The NW group averaged 7,897 ± 3,104 steps per day and the OW/Ob group averaged 7,733 ± 2,846 steps per day across trials. There were no differences in physical activity across trials or between groups. The NW group spent 88 ± 5% of daily monitoring time (12 hours/day) performing either no activity or low intensity activity (< 30 steps per minute), while the OW/Ob group spent 86 ± 4% of monitoring time (10 hours/day) performing no activity or low intensity activity, with no difference between groups.
Exercise HR and energy expenditure
For the NW group, average HR during the Prior Day Ex and Same Day Ex trials, was 72 ± 4 and 72 ± 5% of peak, respectively. For the OW/Ob group, the corresponding HR values were 73 ± 5 and 73 ± 6% of of peak HR, respectively. Estimated total energy expenditure during exercise was 864 ± 283 kJ for the NW group and 948 ± 305 for the OW/Ob group. There were no differences between exercise trials or between groups for HR or exercise energy expenditure.
Glucose
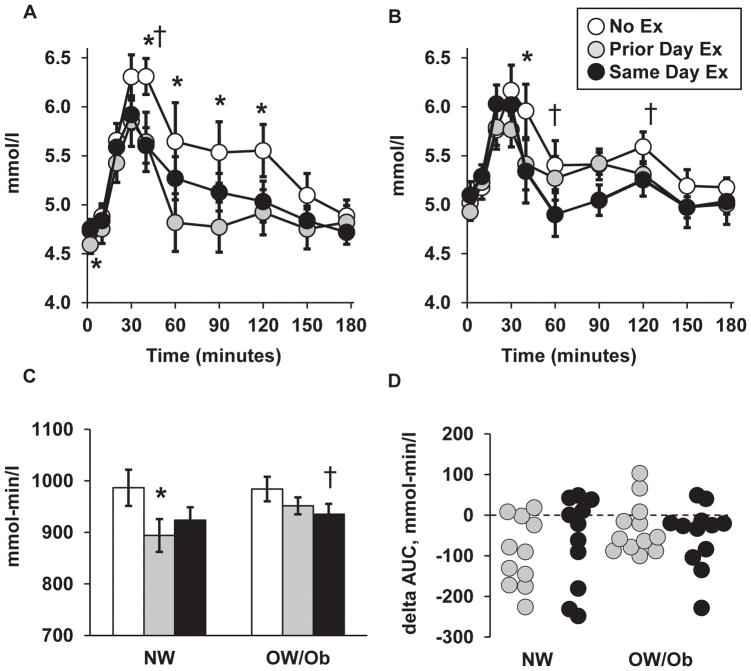
Fasting glucose in the No Ex trial was 6% higher (Effect size = 1.0) for the OW/Ob group than the NW group (Table 1), and this difference was maintained in the other two trials. All participants had fasting glucose within the normal range (NW: 4.4 to 5.1 mmol/l, OW/Ob: 4.3 to 5.3 mmol/l). The glucose excursion curves are shown in Figures 1A and 1B for the NW and OW/Ob groups, respectively. None of the participants had impaired glucose tolerance, defined as glucose concentration greater than 7.8 mmol/l at two hours after the meal (NW: 4.7 to 7.3 mmol/l at 120 minutes, OW/Ob: 5.0 to 6.6 mmol/l). In the No Ex trial, plasma glucose was higher than the fasting baseline from 10 minutes (OW/Ob) or 20 minutes (NW) following the meal, through the 120-minute measurement. In contrast, post meal glucose for the NW group had a shorter period of elevation during the exercise trials; in the Prior Day Ex trial glucose was elevated from 20 to 40 minutes post meal, while in the Same Day Ex trial it was elevated from 20 to 90 minutes post meal. For the OW/Ob group, there was a biphasic elevation in glucose in the Prior Day Ex trial, from 10–40 minutes and again at 90–120 minutes, respectively. In the Same Day Ex trial glucose for the OW/Ob group was elevated only from 10 to 30 minutes post meal.
Figure 1.
Plasma glucose responses. Glucose excursion curves for NW group (A) and OW/Ob group (B). Pre-meal baseline = 0 minutes. (C) Total glucose area under the curve (AUC) for each trial. (D) Individual values for the difference in glucose AUC (delta AUC) between each exercise trial and the No Ex trial, respectively. Values for panels A–C shown as mean ± SEM. Legend applies to all panels. *Prior Day Ex versus No Ex within group, p < 0.05. † Same Day Ex versus No Ex within group, p < 0.05.
When the NW and OW/Ob groups were pooled, the glucose AUC was reduced by 6% (Effect size 0.6–0.7, p < 0.01) on both exercise trials relative to the NoEx trial. Within the NW group, glucose AUC (Figure 1C) was reduced by 9% (Effect size 0.8, p = 0.004) in the Prior Day Ex trial and 6% (Effect size 0.6, p = 0.088) in the Same Day Ex trial, respectively. For the OW/Ob group, glucose AUC was reduced by 3% (Effect size 0.5, p = 0.107) and 5% (Effect size 0.7, p = 0.046) in the Prior Day Ex and Same Day Ex, trials, respectively. The glucose AUC did not differ between groups on any of three trials.
Individual values for the difference in glucose AUC on the exercise trials, relative to the No Ex trial, are shown in Figure 1D. The majority of participants in both groups demonstrated a reduction in glucose AUC in one or both exercise trials (78% of participants in the Prior Day Ex, 70% of participants in the Same Day Ex, respectively). There were no differences between groups in the change in glucose AUC on either of the exercise trials.
Insulin
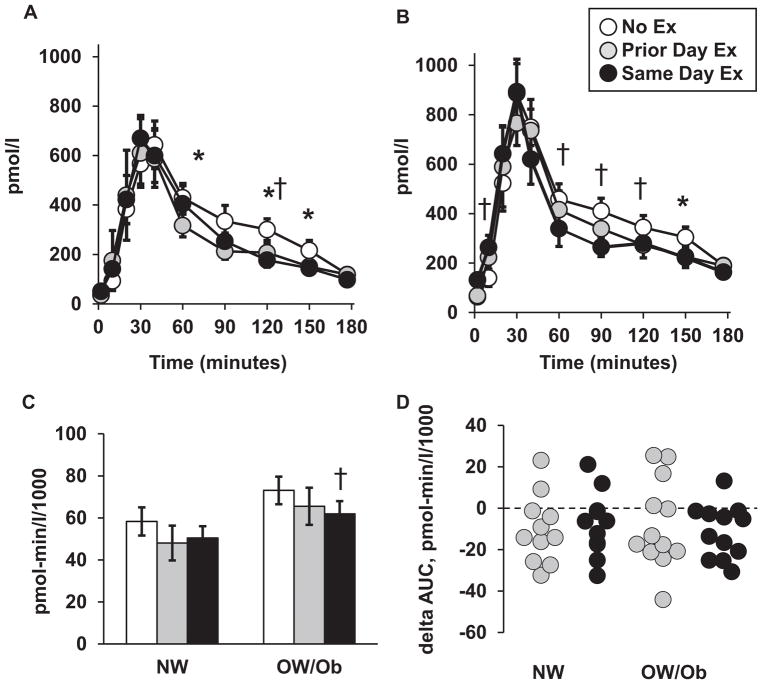
Fasting insulin in the No Ex trial was 66% (Effect size = 1.1) higher for the OW/Ob group than the NW group (Table 1), and this difference was maintained in the other two trials. The insulin excursion curves are shown in Figures 2A and 2B for the NW and OW/Ob groups, respectively. On all three trials, serum insulin remained higher than the fasting baseline from 10 minutes (OW/Ob) or 20 minutes (NW) following the meal through the 180-minute measurement. Compared to the NoEx trial, insulin concentration in the two exercise trials was lower from 60–150 minutes in both groups at the specific times depicted in the figure.
Figure 2.
Serum insulin responses. Insulin excursion curves for NW group (A) and OW/Ob group (B). Pre-meal baseline = 0 minutes. (C) Total insulin area under the curve (AUC) for each trial. (D) Individual values for the difference in insulin AUC (delta AUC) between each exercise trial and the No Ex trial, respectively. Values for panels A–C shown as mean ± SEM. Legend applies to all panels. *Prior Day Ex versus No Ex within group, p < 0.05. † Same Day Ex versus No Ex within group, p < 0.05.
For the pooled NW and OW/Ob groups, the insulin AUC was reduced by 13% (Effect size 0.3, p = 0.034) in the Prior Day Ex and 15% (Effect size = 0.4, p = 0.003) in the Same Day Ex, respectively, relative to the NoEx trial. Within the NW group insulin AUC (Figure 2C) was reduced by 18% (Effect size 0.4, p = 0.065) in the Prior Day Ex trial and 14% (Effect size 0.4, p = 0.122) in the Same Day Ex trial, respectively. For the OW/Ob group, the insulin AUC was reduced by 10% (Effect size 0.3, p = 0.245) and 15% (Effect size 0.5, p = 0.012) in the Prior Day Ex and Same Day Ex trials, respectively. Insulin AUC was 23–36% greater in the OW/Ob group than the NW group on all three trials, though the between-group differences did not reach statistical significance (Effect sizes = 0.6 to 0.7, p = 0.129 to 0.180).
Individual values for the difference in insulin AUC on the exercise trials, relative to the No Ex trial, is shown in Figure 2D. When results from both groups were pooled, insulin AUC was reduced in 74% of participants in the Prior Day Ex trial, and 87% of participants in the Same Day Ex trial, respectively. There were no differences between groups for the change in insulin AUC on either of the exercise trials.
C-peptide
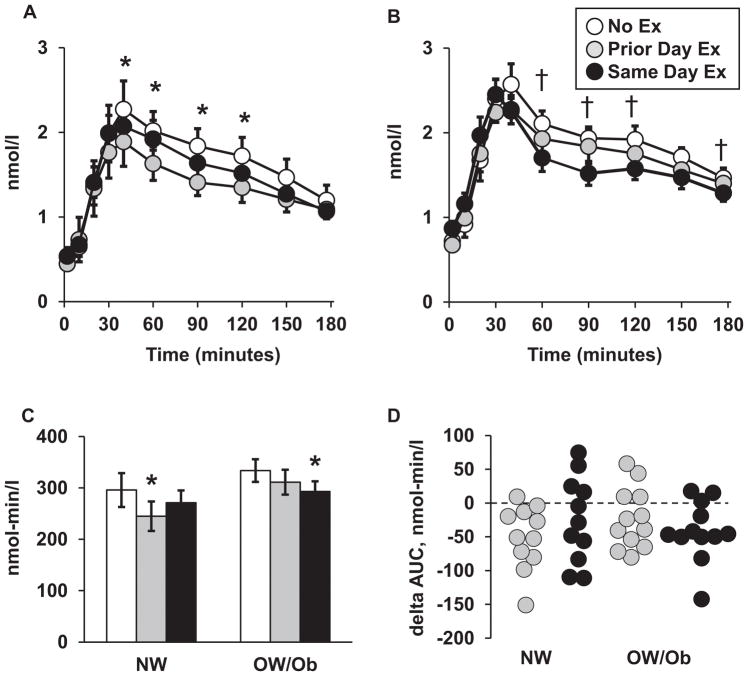
Fasting C-peptide in the No Ex trial was 50% higher (Effect size 0.9) for the OW/Ob group (Table 1), and this difference was maintained in the other two trials. The C-peptide excursion curves are shown in Figures 3A and 3B for the NW and OW/Ob groups, respectively. Like insulin, on all three trials, C-peptide remained significantly higher than the fasting baseline from 10 minutes (OW/Ob) or 20 minutes (NW) following the meal, through the 180-minute measurement. For the NW group, C-peptide in the Prior Day Ex trial was reduced from 30–120 minutes compared to the NoEx trial, but did differ from NoEx in the Same Day Ex trial. For the OW/Ob, the Prior Day Ex trial did not result in a significant change in C-peptide, but in the Same Day Ex trial, it was reduced from 60–120 and at 180 minutes, as depicted in the figure.
Figure 3.
Serum C-peptide responses. C-peptide excursion curves for NW group (A) and OW/Ob group (B). Pre-meal baseline = 0 minutes. (C) Total C-peptide area under the curve (AUC) for each trial. (D) Individual values for the difference in C-peptide AUC (delta AUC) between each exercise trial and the No Ex trial, respectively. Values for panels A–C shown as mean ± SEM. The legend applies to all panels. *Prior Day Ex versus No Ex within group, p < 0.05. † Same Day Ex versus No Ex within group, p < 0.05.
When the NW and OW/Ob groups were pooled, C-peptide AUC was reduced by 10–11% (Effect size 0.4, p < 0.008) on both exercise trials relative to the NoEx trial. Within the NW group, C-peptide AUC (Figure 3C) was reduced by 17% (Effect size 0.5, p = 0.005) in the Prior Day Ex trial and 8% (Effect size 0.3, p = 0.087) in the Same Day Ex trial, respectively. For the OW/Ob group, the C-peptide AUC was reduced by 7% (Effect size 0.3, p = 0.108) and 12% (Effect size 0.6, p = 0.008) in the Prior Day Ex and Same Day Ex, trials, respectively. C-peptide AUC did not differ between groups on any of the three trials.
Individual values for the difference in C-peptide AUC on the exercise trials, relative to the No Ex trial, is shown in Figure 3D. Like the insulin response, the majority of participants in both groups (78% in the Prior Day Ex trial, 70% in the Same Day Ex trial, respectively) had a reduction in C-peptide AUC on one or both exercise trials. There were no differences between groups in the change in AUC on either of the exercise trials.
Insulin sensitivity
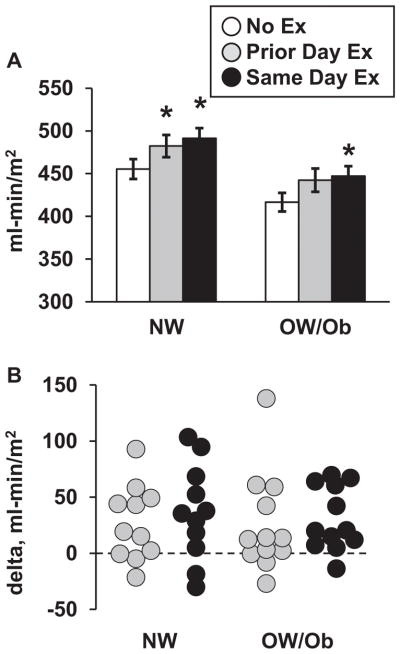
In each trial, insulin sensitivity was 8–9% lower (Effect size 1.0, p < 0.05,) in the OW/Ob group versus the NW group. For the pooled NW and OW/Ob groups, insulin sensitivity was increased 6% and 8% on the Prior Day Ex and Same Day Ex trials compared to the No Ex trial (Effect size = 0.6 and 0.8, respectively; both p < 0.01). Within the NW group, insulin sensitivity (Figure 4A) was increased by 6% (Effect size 0.7, p = 0.024) in the Prior Day Ex trial and 8% (Effect size 0.9, p = 0.019) in the Same Day Ex trial, respectively. For the OW/Ob group, insulin sensitivity was increased by 6% (Effect size 0.6, p = 0.068) and 7% (Effect size 0.8, p = 0.003) in the Prior Day Ex and Same Day Ex trials, respectively.
Figure 4.
Insulin sensitivity. (A) Insulin sensitivity values (mean ± SEM) for each trial. (B) Individual values for the difference (delta) in insulin sensitivity between the exercise trials and the No Ex trial, respectively. The legend applies to both panels. *Greater than No Ex trial within group, p < 0.05. Insulin sensitivity was ~9% lower (p < 0.05) in the OW/Ob group versus the NW group during each trial.
Individual values for the difference in insulin sensitivity on the exercise trials, relative to the No Ex trial, are shown in Figure 4B. A majority of participants in both groups demonstrated an increase on one or both exercise trials, with 74% improving in the Prior Day Ex trial and 87% improving in the Same Day Ex trial. The change in insulin sensitivity did not differ between groups for either Prior Day Ex or Same Day Ex.
The lower insulin sensitivity in the OW/Ob group versus the NW group may be at least partly attributable to trunk fat since the OW/Ob group had nearly twice as much trunk fat than the NW group, and trunk fat was inversely correlated with insulin sensitivity (r = −0.695 for both groups combined). Variation in trunk fat, however, was not associated with the change in insulin sensitivity in response to exercise.
NEFA
Fasting NEFA did not differ between groups in the NoEx trial (Table 1) or on either of the exercise trials. The NEFA excursion curves are shown in Support Figures 1A and 2B for the NW and OW/Ob groups, respectively. On all three trials, NEFA significantly declined from the fasting baseline value following the meal. For both groups, the decline became statistically significant (p < 0.005) at 30 minutes in the No Ex trial and at 60 minutes and 10 minutes in the Prior Day Ex and Same Day Ex trials, respectively. For both groups, baseline NEFA was ~28% lower at the the start of the Prior-Day Ex trial compared to the No Ex trial (Effect size 0.8, p = 0.004 for the pooled groups). However, as depicted in Support Figures 1C and 1D, there were no differences between trials or between groups for the NEFA AUC.
Indirect calorimetry
The energy expenditure rate while fasting in the No Ex trial was 24% higher for the OW/Ob group (4.52 ± 0.12 kJ/min) than the NW group (3.63 ± 0.17 kJ/minute, Effect size = 1.4, p = 0.005) and remained 10–24% higher in the OW/Ob group throughout each trial (Support Figure 2A & 2B). Postprandial energy expenditure was elevated above baseline at all measurement times for both the NW group (21–53% above baseline) and the OW/Ob group (14–20% increased), with no differences among trials within either group. The energy expenditure AUC did not differ between trials or between groups on any trial (not shown).
Pre-meal carbohydrate oxidation accounted for 36 ± 5% of energy expenditure for the NW group and 50 ± 4% for the OW/Ob group in the NoEx trial (Effect size = 1.0, p = 0.032). Following the meal, carbohydrate oxidation increased on both the NoEx and Prior Day Ex trials and remained higher than baseline at all measurement times in both groups (NW: 52–64% of energy expenditure, OW/Ob: 62–77% of energy expenditure from 45–180 minutes postprandial). In the Same Day Ex trial, for both groups, carbohydrate oxidation was increased above baseline only at the first postprandial measurement, (45–60 minutes), and returned toward baseline thereafter. The resulting net carbohydrate oxidation was thus lowest in the Same Day Ex trial in the NW group (Same Day Ex: 7.6 ± 1.4 g, No Ex: 11.2 ± 0.9 g, Effect size for difference = 0.9, p = 0.055) and the OW/Ob group (Same Day Ex: 6.5 ± 2.0 g, No Ex: 12.2 ± 2.3 g; Effect size for difference = 0.8, p = 0.010). Rates of carbohydrate oxidation in the Prior Day Ex trial were intermediate and not different from the No Ex trial (NW: 8.4 ± 1.7 g, Effect size versus NoEx = 0.6, p = 0.153; OW/Ob: 10.7 ± 1.6 g, Effect size versus NoEx = 0.2, p = 0.408).
Analysis of responders versus non-responders
As shown in Figures 1–4, insulin sensitivity and glucose, insulin, and C-peptide AUCs, demonstrated a broad range in their magnitudes of response to exercise. As an exploratory analysis, we assessed whether that variability was associated with the participants’ characteristics: age, sex, anthropometry, body composition, aerobic fitness, and physical activity level as steps per day. However, none of the bivariate correlations between the participant characteristics and the change scores for glucose, insulin, and c-peptide AUC, or insulin sensitivity, on either exercise trial, reached statistical significance. Thus, there are likely other unexplored factors that account for the variable responses in glycemic control to exercise.
DISCUSSION
The results demonstrate that, despite having higher body fat, fasting glucose, insulin, and carbohydrate oxidation, and lower insulin sensitivity, overweight/obese adolescents derive the same acute benefits on glycemic control from a single exercise session as their normal weight peers. In response to exercise concluded 40 minutes before the test meal (Same Day Ex trial), postprandial glucose, insulin, and C-peptide AUCs were reduced, and insulin sensitivity was increased compared to a trial without exercise. These responses were largely maintained when measurements were performed 17 hours after exercise (Prior Day Ex trial). Moreover, the magnitude of the responses did not differ between normal weight and overweight/obese participants.
The current study extends the results from a subset of participants that we previously reported (11). There is now general agreement that a single exercise session completed up to 24 hours before a carbohydrate-containing meal results in reduced glycemic and/or insulin excursion in adolescents (10, 16, 17). Cockcroft et al. (16, 17) reported that this effect was independent of exercise intensity. In their studies, normal weight boys, 14–15 y old, had similar reduction in glucose AUC and improvement in insulin sensitivity following either moderate- or higher-intensity exercise bouts. The improvement in insulin sensitivity was observed during oral glucose tolerance tests performed 10–40 minutes after exercise (16, 17) and persisted at 24 hours (16), but not 48 hours after exercise (16). Zackrzewski and Tolfrey (10) did not measure the acute effect of exercise, but found that fasting insulin and fat oxidation were lower in NW, but not OW girls during a high glycemic index meal consumed ~15 hours after the exercise was completed. MacEneaney et al. (18) also examined the effects of prior day exercise in adolescents but measured responses to a high fat meal. They found that both NW and OW boys had lower fasting and postprandial serum triglycerides compared to rest-only trial, but glucose and insulin were unaffected (18). Collectively, the current and prior studies highlight the short-term benefits of exercise on the regulation of glucose, lipids, and fuel metabolism in NW and OW/Ob adolescents.
A potential reason that the NW and OW/Ob groups had similar metabolic responses to the exercise sessions is that they had similar amounts of lean mass that could be activated during exercise, and similar, low levels of habitual physical activity and cardiorespiratory fitness. None of the participants were engaged in regular exercise or sports, which is reflected in their low daily ambulation of less than 8,000 steps per day. That level of movement falls below the estimated 11,000–12,000 steps/day that are required to meet current recommendations for adolescents to achieve 60 minutes per day of moderate-to-vigorous physical activity (19, 20). Not surprisingly then, cardiorespiratory fitness for both groups were below thresholds for healthy fitness established in studies of American (21) and European (22) adolescents. Results from the National Health and Nutrition Examination Survey have shown that cardiorespiratory fitness in American adolescents declined from 1999 to 2012, so less than half of American adolescents now have a healthy level of fitness (23). Thus, the findings from the current study are generalizable to a majority of American adolescents who are not habitually active and have low cardiorespiratory fitness. The implication is that many of the adolescents who are not meeting current guidelines for physical activity can derive immediate metabolic benefit from a moderate intensity aerobic exercise session. A remaining challenge, however, is to promote daily exercise behavior so that long-term metabolic health is maintained or improved in both normal weight and overweight or obese young people.
A potential limitation of the study is the relatively small sample size. Although we identified several within- and between-group differences, a larger sample size might have allowed for additional comparisons to reach statistical significance. However, as shown by the distribution of individual results, the effects of exercise (i.e., differences relative to the No Ex trial) on all of the metabolic outcomes had considerable overlap between the NW and OW/Ob participants. Thus, the sample size would need to be several-fold higher to reveal between-group differences in those variables, if they exist. It is also possible that differences between the NW and OW/Ob groups may have emerged under different exercise or meal conditions, but we chose the current protocol to increase generalizability. The type and duration of exercise activated several muscle groups, is feasible for someone starting an exercise program, and demonstrated improvement in glycemic control in the majority of participants. We acknowledge that the specific Tanner stage of each participant was not determined so we cannot assess whether the effect of exercise on insulin sensitivity varies with the stage of pubertal development. Insulin resistance increases during puberty (24, 25), but it has not yet been demonstrated that the acute metabolic responses to an exercise session vary across the stages of puberty.
In conclusion, the current findings demonstrate that both NW and OW/Ob adolescents with low aerobic fitness and habitual physical activity respond to a single moderate intensity bout of exercise with improvement in insulin sensitivity that lasts at least 17 hours. These results support the need to promote daily physical activity in adolescents to improve insulin sensitivity and reduce the risk for cardiometabolic disease.
Supplementary Material
Acknowledgments
We are grateful for the excellent nursing and dietary support, and the clinical assistance several colleagues contributed to these studies.
Funding: This project was supported by National Center for Research Resources grants P20-RR024215 and M01-RR14467. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Aucouturier J, Duché P, Timmons BW. Metabolic flexibility and obesity in children and youth. Obesity Reviews. 2011;12:e44–e53. doi: 10.1111/j.1467-789X.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 2.Twig G, Tirosh A, Leiba A, Levine H, Ben-Ami Shor D, Derazne E, et al. BMI at age 17 years and diabetes mortality in midlife: A nationwide cohort of 2.3 million adolescents. Diabetes Care. 2016;39:1996–2003. doi: 10.2337/dc16-1203. [DOI] [PubMed] [Google Scholar]
- 3.van der Heijden G-J, Toffolo G, Manesso E, Sauer PJJ, Sunehag AL. Aerobic exercise increases peripheral and hepatic insulin sensitivity in sedentary adolescents. J Clin Endocrinol Metab. 2009;94:4292–9. doi: 10.1210/jc.2009-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Salem GJ, Crespo NC, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38:1208. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 5.Bell LM, Watts K, Siafarikas A, Thompson A, Ratnam N, Bulsara M, et al. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab. 2007;92:4230–5. doi: 10.1210/jc.2007-0779. [DOI] [PubMed] [Google Scholar]
- 6.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–9. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, et al. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA. 2012;308:1103–12. doi: 10.1001/2012.jama.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigal RJ, Alberga AS, Goldfield GS, Prud’homme D, Hadjiyannakis S, Gougeon R, et al. Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents: The healthy eating aerobic and resistance training in youth randomized clinical trial. JAMA Pediatrics. 2014;168:1006–14. doi: 10.1001/jamapediatrics.2014.1392. [DOI] [PubMed] [Google Scholar]
- 9.Davis JN, Tung AMY, Chak SS, Ventura EE, Byrd-Williams CE, Alexander KE, et al. Aerobic and strength training reduces adiposity in overweight Latina adolescents. Med Sci Sports Exerc. 2009;41:1494–503. doi: 10.249/MSS.0b013e31819b6aea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakrzewski JK, Tolfrey K. Acute effect of fatmax exercise on metabolism in overweight and nonoverweight girls. Med Sci Sports Exerc. 2012;44:1698–705. doi: 10.1249/MSS.0b013e31825804cf. [DOI] [PubMed] [Google Scholar]
- 11.Short KR, Pratt LV, Teague AM, Cobelli C, Dalla-Man C. Postprandial improvement in insulin action after a single session of exercise in sedentary adolescents. Pediatr Diabetes. 2013;14:129–37. doi: 10.1111/j.1399-5448.2012.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 2002;11:1–203. [PubMed] [Google Scholar]
- 13.Graf DL, Pratt LV, Hester CN, Short KR. Playing active video games increases energy expenditure in children. Pediatrics. 2009;124:534–40. doi: 10.1542/peds.2008-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1996;55:628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 15.Mari APHD, Pacini GDSC, Murphy EMD, Ludvik BMD, Nolan JJMD. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001:24. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft EJ, Williams CA, Weaver H, O’Connor A, Jackman SR, Armstrong N, et al. Acute exercise and insulin sensitivity in boys: a time-course study. Int J Sports Med. 2017;38:967–74. doi: 10.1055/s-0043-118007. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft EJ, Williams CA, Tolmliinson OW, Vlachopoulos D, Jackman SR, Armstrong N, et al. High intensity interval exercise is an effective alternative to moderate intensity exercise for improving glucose tolerance and insulin sensitivity in adolescent boys. J Sci Med Sport. 2015;18:720–4. doi: 10.1016/j.jsams.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.MacEneaney OJ, Harrison M, O’Gorman DJ, Pankratieva EV, O’Conner PL, Moyna NM. Effect of prior exercise on postprandial lipemia and markers of inflammation and endothelial activation in normal weight and overweight adolescent boys. Eur J Appl Physiol. 2010;106:721–9. doi: 10.1007/s00421-009-1073-y. [DOI] [PubMed] [Google Scholar]
- 19.Colley RC, Janssen I, Tremblay MS. Daily step target to measure adherence to physical activity guidelines in children. Med Sci Sports Exerc. 2012;44:977–82. doi: 10.1249/MSS.0b013e31823f23b1. [DOI] [PubMed] [Google Scholar]
- 20.Tudor-Locke C, Craig CL, Beets MW, Belton S, Cardon GM, Duncan S, et al. How many steps/day are enough? for children and adolescents. Int J Behav Nutr Phys Act. 2011;8:1–14. doi: 10.1186/1479-5868-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welk GJ, Laurson KR, Eisenmann JC, Cureton KJ. Development of youth aerobic-capacity standards using receiver operating characteristic curves. Am J Prev Med. 2011;41:S111–S6. doi: 10.1016/j.amepre.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Adegboye ARA, Anderssen SA, Froberg K, Sardinha LB, Heitmann BL, Steene-Johannessen J, et al. Recommended aerobic fitness level for metabolic health in children and adolescents: a study of diagnostic accuracy. Br Med J. 2011;45:722–8. doi: 10.1136/bjsm.2009.068346. [DOI] [PubMed] [Google Scholar]
- 23.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang CY, Fulton JE. Cardiorespiratory fitness levels among U.S. youth aged 12–15 years: United States, 1999–2004 and 2012. NCHS Data Brief. 2014;153:1–8. [PubMed] [Google Scholar]
- 24.Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery SC, Hosking J, Jeffery AN, Murphy MJ, Voss LD, Wilkin TJ, et al. Insulin resistance is higher in prepubertal girls but switches to become higher in boys at age 16: A Cohort Study (EarlyBird 57) Pediatr Diab. 2018;19:223–30. doi: 10.1111/pedi.12571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.