Abstract
Background
Experimental evidence indicates that left ventricular (LV) apical pacing is hemodynamically superior to nonapical LV pacing. Some studies have shown that an LV apical lead position is unfavorable in cardiac resynchronization therapy. We sought to determine whether an apical LV lead position influences cardiac mortality after cardiac resynchronization therapy.
Methods and Results
In this retrospective observational study, the primary end point of cardiac mortality was assessed in relation to longitudinal (basal, midventricular, or apical) and circumferential (anterior, lateral, or posterior) LV lead positions, as well as right ventricular (apical or septal), assigned using fluoroscopy. Lead positions were assessed in 1189 patients undergoing cardiac resynchronization therapy implantation over 15 years. After a median follow‐up of 6.0 years (interquartile range: 4.4–7.7 years), an apical LV lead position was associated with lower cardiac mortality than a nonapical position (adjusted hazard ratio: 0.74; 95% confidence interval, 0.56–0.99) after covariate adjustment. There were no differences in total mortality or heart failure hospitalization. Death from pump failure was lower with apical than nonapical positions (adjusted hazard ratio: 0.69; 95% confidence interval, 0.51–0.94). Compared with a basal position, an apical LV position was also associated with lower risk of sudden cardiac death (adjusted hazard ratio: 0.34; 95% confidence interval, 0.13–0.93). No differences emerged between circumferential LV lead positions or right ventricular positions with respect to any end point.
Conclusions
In recipients of cardiac resynchronization therapy, an apical LV lead position was associated with better long‐term cardiac survival than a nonapical position. This effect was due to a lower risk of pump failure and sudden cardiac death.
Keywords: cardiac resynchronization therapy, lead position, mortality
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Heart Failure, Pacemaker
Clinical Perspective
What Is New?
In recipients of cardiac resynchronization therapy, an apical left ventricular lead position was associated with better long‐term cardiac survival than a nonapical position. This effect was due to a lower risk of pump failure and sudden cardiac death.
No difference in outcomes was observed among anterior, posterior, and lateral left ventricular lead positions.
What Are the Clinical Implications?
An apical left ventricular lead position in cardiac resynchronization therapy is more favorable than a nonapical position.
Introduction
Cardiac resynchronization therapy (CRT), with defibrillation (CRT‐D) or without (CRT with pacing), is a standard treatment for selected patients with systolic heart failure (HF) and a wide QRS duration.1 As is the case with any other medical therapy, the response to CRT is variable. The “nonresponder” rate is said to be around 30%, depending on how response is defined.
The position of the left ventricular (LV) lead has been implicated in the variable outcomes of CRT; however, no randomized controlled trial has addressed this issue. A subanalysis of the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy) trial, apical LV lead positions were less favorable than basal or mid‐LV lead positions in terms of total mortality and HF hospitalization.2 Similar findings emerged from a subanalysis of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial.3 These findings are counterintuitive, as previous experimental and clinical evidence shows that an apical LV lead position is more favorable than nonapical positions.4, 5 During normal sinus rhythm in intact myocardium, electrical impulses travel through the rapid conduction system from the His bundle toward the low apex. Thereafter, LV activation proceeds from apex to base as impulses exit the Purkinje system into the slower conducting working myocardium.6 Pacing at the LV apex would thus be expected to provide a near‐physiological sequence of activation. Although an LV apical position may seem anatomically close to a right ventricular (RV) apical lead, the longest electrical delay usually occurs across the interventricular septum. Because the apex is thinner, apical pacing could arguably provide more rapid capture by the endocardium and Purkinje network, which is known to provide the fast apex‐to‐base activation that occurs in the normal heart. We should consider that when CRT is delivered when the atrioventricular delay is shorter than intrinsic PR interval, the ventricular activation sequence is completely determined by the pacing‐induced activation wave fronts. Computer modeling studies support the notion that LV lead positioning should be guided by what is most physiologically close to normal activation rather than targeting the latest activated region.7 In this regard, van Deursen et al used a canine left bundle‐branch block (LBBB) model to show that the highest hemodynamic response during CRT, measured using the rate of rise of LV pressure (LV dP/dt), occurred with LV apical positions rather than with basal and midventricular positions.4 Other pacing studies have shown similar findings.5 Although conduction in intact myocardium is not the same as in scarred myocardium, acute studies in humans show a similar picture. We observed better hemodynamic response from LV apical pacing compared with basal LV pacing in patients with ischemic cardiomyopathy and LBBB.8
Arguably, a link between the physiological effects of LV position and outcomes should perhaps focus on cardiac outcomes rather than total mortality. In this study of real‐world clinical practice, we compared cardiac mortality and total mortality after CRT according to apical and nonapical LV lead positions. We also assessed clinical outcomes according to circumferential LV lead positions and RV lead positions.
Methods
This retrospective study included patients from 2 centers (Good Hope Hospital and Queen Elizabeth Hospital, Birmingham, United Kingdom). The study was approved by the local ethics committee or clinical audit departments in the 2 institutions. The requirement for patient informed consent was waived in the case of clinical audits. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patients
Inclusion criteria were as follows: systolic HF in New York Heart Association (NYHA) classes I to IV; maximum tolerated treatment with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, β‐blockers, and mineralocorticoid receptor antagonists; QRS duration ≥120 ms; LV ejection fraction (LVEF) ≤35%; and use of a unipolar or bipolar LV lead in CRT implantation. Exclusion criteria were as follows: contraindications to cardiac pacing; myocardial infarction or acute coronary syndrome within the previous month; severe structural valvular heart disease; and presence of comorbidities likely to threaten survival for 12 months. The diagnosis of HF was based on echocardiographic evidence of LV systolic dysfunction. The diagnosis of ischemic cardiomyopathy was based on the findings of systolic dysfunction on echocardiography if LV systolic dysfunction was associated with a myocardial infarction or in the background of angiographically significant coronary artery disease. Late gadolinium enhancement cardiovascular magnetic resonance was also used to determine the etiology of HF. The study conforms with the Declaration of Helsinki and was approved by the local clinical audit departments and/or ethics committee.
Device therapy
Device implantation was undertaken using standard transvenous techniques under local anesthesia and intravenous sedation. Subsequently, patients were followed up in dedicated device clinics. Up to 2013, patients in sinus rhythm underwent transmitral Doppler‐directed optimization of atrioventricular delay using an iterative technique before discharge and at every scheduled visit thereafter. Thereafter, routine echocardiographic optimization was abandoned and undertaken only in symptomatic nonresponders. Backup atrial pacing was set at 60 beats/min, and the pacing mode was set to DDDR with an interventricular delay of 0 to 20 ms (LV first), according to clinician's discretion. In patients with permanent atrial fibrillation, RV and LV leads were implanted and a CRT generator was used, plugging the atrial port and programming to a ventricular triggered mode. Atrioventricular junction ablation was undertaken according to physicians’ decision. Patients underwent a clinical assessment on the day before implantation and at 1, 3, and every 6 months following device implantation.
The United Kingdom National Institute of Clinical Excellence guidelines in 2007 recommended CRT with pacing rather than CRT‐D for patients with nonischemic cardiomyopathy and indications for CRT. With a subsequent guideline change in 2014 recommending CRT‐D in nonischemic cardiomyopathy,9 the proportion of CRT‐D recipients increased thereafter.
Lead position
We adopted the same system for LV lead tip position described by the MADIT‐CRT study group.2 Accordingly, coronary sinus venograms undertaken at the time of implantation were used retrospectively to assign the position of the LV lead tip, as shown in Figure 1. For data analysis, the anterolateral, lateral, and posterolateral sectors were grouped together as the lateral wall and the basal and midventricular sectors were grouped as nonapical. RV lead positions were classified as apical or nonapical, which included low‐, mid‐, and high‐septal positions. All LV lead positions were assessed retrospectively by an experienced implanter (F.L.) who was blinded to clinical outcome data.
Figure 1.
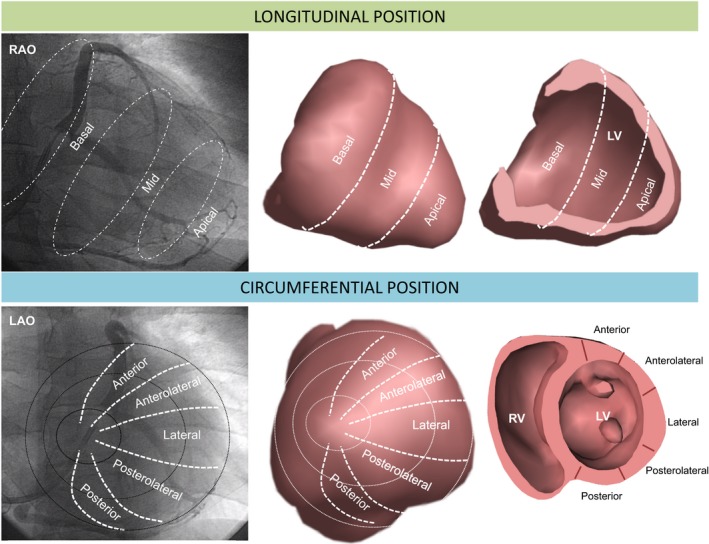
Assessment of LV lead position using fluoroscopy. Longitudinal LV lead positions were assigned using the 30° RAO fluoroscopic view at the time of implantation, into basal, mid and apical. These correspond to the sectors shown in the 3‐dimensional long axis envelope and cross section (upper panel). Circumferential LV lead positions were assigned using the 30° LAO fluoroscopic view into anterior, anterolateral, lateral, posterolateral and posterior sectors, as shown in the short axis envelope and cross‐section (lower panel). LAO indicates left anterior oblique; LV, left ventricular; RV, right ventricular; RAO, right anterior oblique.
End Points
The primary end point was cardiac mortality, which included cardiac transplantation or implantation of a ventricular assist device. The secondary end point was total mortality. Ancilliary end points included the composite end point of total mortality or HF hospitalization; the composite end point of total mortality or unplanned hospitalization for major adverse cardiac events, which included hospitalization for HF, myocardial infarction, acute coronary syndrome, and arrhythmia (ventricular tachycardia, ventricular fibrillation, and atrial fibrillation). Stroke and pulmonary embolism were not considered major adverse cardiac events. For composite end points, the first event was included in statistical analyses. Mortality data were collected through medical records and, as appropriate, from interviews with patients’ caregivers. Clinical outcome data were collected every 6 months by investigators who were blinded to clinical and imaging data.
Mode of death
A “natural, unexpected death due to cardiac causes, heralded by an abrupt loss of consciousness within 1 hour of the onset of acute symptoms”10 was regarded as sudden cardiac death (SCD). Death from pump failure was defined as “death after a period of clinical deterioration in signs and symptoms of HF despite medical treatment.”11 Cause of death was adjudicated on the basis of hospital records or documentation in death certificates or primary care records. Deaths were classified as unknown if no definitive data were found in hospital or primary care records or from interviews with caregivers. When a specific mode of death was considered (pump failure or SCD), deaths from other causes were censored.
Statistical Analysis
Continuous variables are expressed as mean±SD. Normality was tested using the Shapiro–Wilk test. Comparisons between normally distributed continuous variables were made using ANOVA, and categorical variables were analyzed using χ2 tests. To be able to compare our findings with those of MADIT‐CRT,2 we followed statistical analyses. Kaplan–Meier curves and the log‐rank test were used to assess observed cumulative survival. Cox proportional hazards models were used to assess relative risks. Proportionality hypotheses were verified by visual examination of log (survival) graphs to ensure parallel slopes and by examining Schoenfeld residuals. Variables reaching P<0.10 on univariable analyses and variables known to influence clinical outcomes after CRT, such as age, sex, LVEF (<25%), QRS duration (QRS ≥150 ms), QRS morphology (LBBB), HF etiology, NYHA class, diabetes mellitus, and atrial fibrillation, were entered in multivariable models. Statistical analyses were performed using Stata 14 (StataCorp). A 2‐tailed P<0.05 was considered statistically significant.
Results
This study included 1189 patients who underwent CRT device implantation in 2 centers (Queen Elizabeth Hospital and Good Hope Hospital, Birmingham, United Kingdom) over a period of 15 years, from August 2000 to July 2015. Over a follow‐up period of 6.0 years (median; interquartile range: 4.4–7.7 years), 633 of 1189 patients (53.2%) died. The cause of death was unknown for 167 patients. Cardiac mortality affected 357 of 1189 patients (30.0%), and among these, 305 deaths (85%) were due to pump failure and 44 (12%) were SCDs.
Longitudinal Position
Longitudinally, LV lead positions were as follows: 311 patients (26.2%) had leads in basal positions, 604 (50.8%) had them in midventricular positions, and 274 (23.0%) had them in apical positions. A total of 915 (77%) patients had leads in nonapical positions (basal plus midventricular). As shown in Table 1, patients with LV apical and nonapical positions were well matched for age, sex, HF etiology, comorbidities, atrial rhythm, QRS duration, QRS morphology, upgrade from pacemaker, LVEF, and medical therapy. Significant differences emerged with respect to NYHA class and device type between LV apical and nonapical positions, insofar as patients with an apical position were more likely to be in NYHA class III than in NYHA class IV. In addition, patients with apical lead positions were more likely to receive CRT‐D rather than CRT with pacing (P=0.012).
Table 1.
Baseline Characteristics by Longitudinal LV Lead Position
| Basal | Mid | Apical | P Value | Apical | Nonapical | P Valuea | |
|---|---|---|---|---|---|---|---|
| N | 311 | 604 | 274 | 274 | 915 | ||
| Sex (male), n (%) | 226 (72.67) | 453 (75) | 204 (74.45) | 0.744 | 204 (74.45) | 679 (74.21) | 0.935 |
| Age, y | 70.9±10.6 | 72.4±11 | 72.1±10.7 | 0.139 | 72.1±10.7 | 71.9±10.9 | 0.741 |
| ≤59 | 48 (15.43) | 78 (12.91) | 38 (13.87) | 0.541 | 38 (13.87) | 126 (13.77) | 0.997 |
| 60–69 | 88 (28.30) | 149 (24.67) | 70 (25.55) | 70 (25.55) | 237 (25.90) | ||
| 70–79 | 113 (36.33) | 221 (36.59) | 99 (36.13) | 99 (36.13) | 334 (36.50) | ||
| ≥80 | 62 (19.94) | 156 (25.83) | 67 (24.45) | 67 (24.45) | 218 (23.83) | ||
| NYHA class, n (%) | |||||||
| I | 4 (1.32) | 21 (3.60) | 13 (4.87) | <0.001 | 13 (4.87) | 25 (2.82) | 0.012 |
| II | 8 (2.63) | 47 (8.06) | 22 (8.24) | 22 (8.24) | 55 (6.20) | ||
| III | 237 (77.96) | 455 (78.04) | 214 (80.15) | 214 (80.15) | 692 (78.02) | ||
| IV | 55 (18.09) | 60 (10.29) | 18 (6.74) | 18 (6.74) | 115 (12.97) | ||
| Device type, n (%) | |||||||
| CRT‐D | 100 (32.15) | 278 (46.03) | 136 (49.64) | <0.001 | 136 (49.64) | 378 (41.31) | 0.015 |
| CRT‐P | 211 (67.85) | 326 (53.97) | 138 (50.36) | 138 (50.36) | 537 (58.69) | ||
| Upgrade from pacemaker | 40 (12.86) | 119 (19.70) | 44 (16.06) | 0.030 | 44 (16.06) | 159 (17.38) | 0.611 |
| Etiology of cardiomyopathy, n (%) | |||||||
| Ischemic | 184 (59.16) | 321 (53.15) | 151 (55.11) | 0.222 | 151 (55.11) | 505 (55.19) | 0.981 |
| Nonischemic | 127 (40.84) | 283 (46.85) | 123 (44.89) | 123 (44.89) | 410 (44.81) | ||
| Comorbidities, n (%) | |||||||
| Diabetes mellitus | 62 (19.94) | 134 (22.30) | 61 (22.26) | 0.688 | 61 (22.26) | 196 (21.49) | 0.786 |
| Hypertension | 81 (26.05) | 179 (29.78) | 80 (29.20) | 0.484 | 80 (29.20) | 260 (28.51) | 0.825 |
| CABG | 62 (19.94) | 112 (18.64) | 49 (17.88) | 0.809 | 49 (17.88) | 174 (19.08) | 0.657 |
| ECG variables | |||||||
| Sinus rhythm, n (%) | 217 (69.77) | 395 (65.40) | 184 (67.15) | 0.410 | 184 (67.15) | 612 (66.89) | 0.934 |
| Atrial fibrillation, n (%)b | 94 (30.23) | 209 (34.60) | 90 (32.85) | 90 (32.85) | 303 (33.11) | ||
| QRS morphology (LBBB), n (%)c | 224 (73.68) | 487 (83.97) | 220 (83.33) | 0.001 | 220 (83.33) | 711 (80.43) | 0.290 |
| QRS duration (ms)c | 153.9±22.4 | 155.9±22.4 | 155.6±23.2 | 0.463 | 155.6±23.2 | 155.2±22.4 | 0.799 |
| Medication, n (%) | |||||||
| Loop diuretics | 275 (88.42) | 579 (95.86) | 264 (96.35) | <0.001 | 264 (96.35) | 854 (93.33) | 0.064 |
| ACEI/ARB | 279 (89.71) | 515 (85.26) | 235 (85.77) | 0.160 | 235 (85.77) | 794 (86.78) | 0.668 |
| β‐blockers | 196 (63.02) | 412 (68.21) | 190 (69.34) | 0.192 | 190 (69.34) | 608 (66.45) | 0.371 |
| MRA | 117 (37.62) | 258 (42.72) | 128 (46.72) | 0.081 | 128 (46.72) | 375 (40.98) | 0.092 |
| LVEF (%) | 23.4±9.4 | 24.7±9.5 | 24.6±10.2 | 0.224 | 24.6±10.2 | 24.3±9.5 | 0.717 |
Variables are expressed as mean±SD unless indicated otherwise. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy with pacing; LBBB, left bundle‐branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; mid, midventricular; MRA, mineralocorticoid receptor antagonist.
Refers to differences between the groups from ANOVA for continuous variables and from χ2 tests for categorical variables.
Includes permanent, persistent, and paroxysmal atrial fibrillation.
Excludes upgrades to pacemaker.
In Kaplan–Meier survival analyses (Figure 2), an LV apical position was associated with lower cardiac mortality than a nonapical position (P=0.003). The annualized cardiac mortality death rates were highest with basal positions (8.7%), intermediate with midventricular positions (6.6%), and lowest with apical positions (4.9%; Table 2). Univariable analyses of outcomes according to individual LV lead positions are shown in Table 3. In multivariable analyses including NYHA class and device type as well as other variables, an LV apical position was associated with lower cardiac mortality than a nonapical position (adjusted hazard ratio [HR]: 0.74; 95% confidence interval [CI], 0.56–0.99; Table 4). In a multivariable analysis including only NYHA class (class IV compared with NYHA classes I, II, and III) and device type (CRT‐D or CRT with pacing) as covariates, an LV apical position was associated with lower cardiac mortality than a nonapical position (adjusted HR: 0.72; 95% CI, 0.54–0.96; P=0.026). No differences emerged with respect to total mortality and HF hospitalization or total mortality and major adverse cardiac events (Figure S1).
Figure 2.
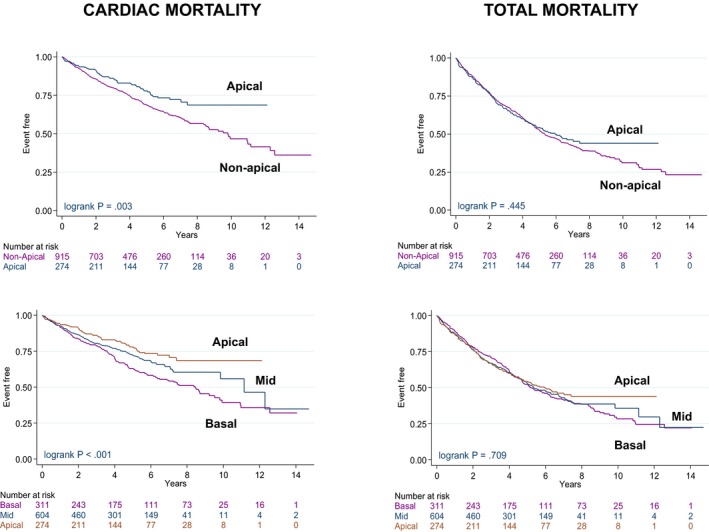
Outcomes according to longitudinal left ventricular lead position. Kaplan–Meier survival curves for cardiac mortality and total mortality according to apical and basal or midventricular (mid; grouped as nonapical) left ventricular lead positions.
Table 2.
Event Rates According to LV Lead Position
| Cardiac Mortality | Total Mortality | HF Hospitalization | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Longitudinal position | |||
| Basal | 137 (8.7) | 192 (12.2) | 61 (4.2) |
| Mid | 163 (6.6) | 306 (12.4) | 106 (4.7) |
| Apical | 57 (4.9) | 135 (11.5) | 43 (4.0) |
| Nonapical | 300 (7.4) | 498 (12.3) | 167 (4.5) |
| Circumferential position | |||
| Anterior | 37 (7.8) | 61 (12.9) | 15 (3.5) |
| Lateral | 303 (6.8) | 542 (12.2) | 184 (4.5) |
| Posterior | 17 (5.9) | 30 (10.3) | 11 (4.1) |
Data are expressed as number of events during the follow‐up period and annualized event rates (%). HF indicates heart failure; LV, left ventricular; mid, midventricular.
Table 3.
Univariable Analyses of LV Lead Positions in Relation to Cardiac Mortality
| LV Lead Position | HR | 95% CI | P Value | |
|---|---|---|---|---|
| Apical vs nonapical | 0.66 | 0.49 | 0.87 | 0.003 |
| Apical vs basal | 0.55 | 0.40 | 0.75 | <0.001 |
| Apical vs mid | 0.75 | 0.55 | 1.01 | 0.060 |
| Mid vs basal | 0.75 | 0.59 | 0.94 | 0.013 |
| Posterior vs anterior | 0.75 | 0.42 | 1.33 | 0.327 |
| Lateral vs anterior | 0.87 | 0.62 | 1.23 | 0.426 |
| Total mortality or HF hospitalization | ||||
| Apical vs nonapical | 0.91 | 0.76 | 1.10 | 0.343 |
| Apical vs basal | 0.90 | 0.73 | 1.12 | 0.347 |
| Apical vs mid | 0.93 | 0.76 | 1.13 | 0.443 |
| Mid vs basal | 0.99 | 0.83 | 1.18 | 0.874 |
| Posterior vs anterior | 0.85 | 0.56 | 1.29 | 0.448 |
| Lateral vs anterior | 0.93 | 0.72 | 1.20 | 0.590 |
| Total mortality | ||||
| Apical vs nonapical | 0.93 | 0.77 | 1.12 | 0.445 |
| Apical vs basal | 0.91 | 0.73 | 1.14 | 0.416 |
| Apical vs mid | 0.95 | 0.77 | 1.16 | 0.586 |
| Mid vs basal | 0.98 | 0.82 | 1.18 | 0.848 |
| Posterior vs anterior | 0.82 | 0.53 | 1.26 | 0.360 |
| Lateral vs anterior | 0.93 | 0.72 | 1.22 | 0.608 |
| HF hospitalization | ||||
| Apical vs nonapical | 0.88 | 0.63 | 1.23 | 0.446 |
| Apical vs basal | 0.87 | 0.58 | 1.28 | 0.469 |
| Apical vs mid | 0.88 | 0.61 | 1.25 | 0.465 |
| Mid vs basal | 1.01 | 0.74 | 1.39 | 0.947 |
| Posterior vs anterior | 1.23 | 0.57 | 2.69 | 0.597 |
| Lateral vs anterior | 1.25 | 0.74 | 2.11 | 0.410 |
Results are presented in terms of HR and 95% CI from Cox proportional hazards analyses. CI indicates confidence interval; HF, heart failure; HR, hazard ratio; LV, left ventricular; mid, midventricular.
Table 4.
Multivariable Analyses of LV Lead Positions in Relation to Cardiac Mortality
| HR | 95% CI | P Value | ||
|---|---|---|---|---|
| Apical vs nonapical | 0.74 | 0.56 | 0.99 | 0.045 |
| Sex, male | 1.62 | 1.24 | 2.13 | <0.001 |
| Age, y | 1.02 | 1.01 | 1.04 | <0.001 |
| NYHA class (IV) | 2.06 | 1.57 | 2.70 | <0.001 |
| Device type (CRT‐D) | 0.67 | 0.53 | 0.86 | 0.002 |
| Etiology (ischemic) | 1.33 | 1.05 | 1.69 | 0.019 |
| Diabetes mellitus | 1.31 | 1.02 | 1.67 | 0.033 |
| QRS morphology (LBBB) | 0.62 | 0.48 | 0.79 | <0.001 |
| Loop diuretics | 2.43 | 1.20 | 4.93 | 0.014 |
| ACEI/ARB | 0.69 | 0.50 | 0.94 | 0.020 |
Variables are expressed as HR and 95% CI. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CRT‐D, cardiac resynchronization therapy with defibrillation; HR, hazard ratio; LBBB, left bundle‐branch block; LV, left ventricular; NYHA, New York Heart Association.
There were no significant differences in total mortality according to longitudinal LV lead position, although survival curves began to separate around year 7 in favor of an LV apical position (Figure 2). No differences emerged with respect to the composite end points of total mortality and HF hospitalization or to total mortality or HF hospitalization separately (Figure S1).
With respect to mode of death, pump failure was lower with an LV apical position than with nonapical positions when SCD was considered censored (adjusted HR: 0.69; 95% CI, 0.51–0.94; Figure 3), and a graded risk was observed for basal, midventricular, and apical LV lead positions (Figure S2). Although no differences in SCD emerged with between LV apical and nonapical lead positions (P=0.089; Figure 3), a significantly lower risk of SCD was observed with an LV apical position compared with a basal LV lead position (adjusted HR: 0.34; 95% CI, 0.13–0.93; Figure 3). In a sensitivity analysis, similar results emerged when deaths from pump failure and SCD were considered as competing risks (Figure S3).
Figure 3.
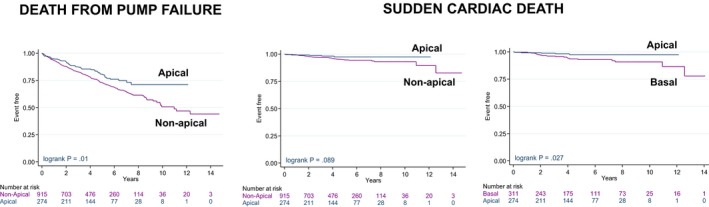
Mode of death according to longitudinal LV lead position. Kaplan–Meier survival curves for death from pump failure and sudden cardiac death to apical and basal or midventricular (mid; grouped as nonapical) LV lead positions. LV indicates left ventricular.
In subgroup analyses, male sex, the age stratum of 60 to 69 years, NYHA class III, CRT‐D, ischemic etiology, no diabetes mellitus, QRS ≥150 ms, and LBBB were associated with lower cardiac mortality with an LV apical position (Figure 4A). With respect to total mortality, an LV apical position was superior to a nonapical position only in the age stratum of 60 to 69 years, not in other subgroups (Figure 4b).
Figure 4.
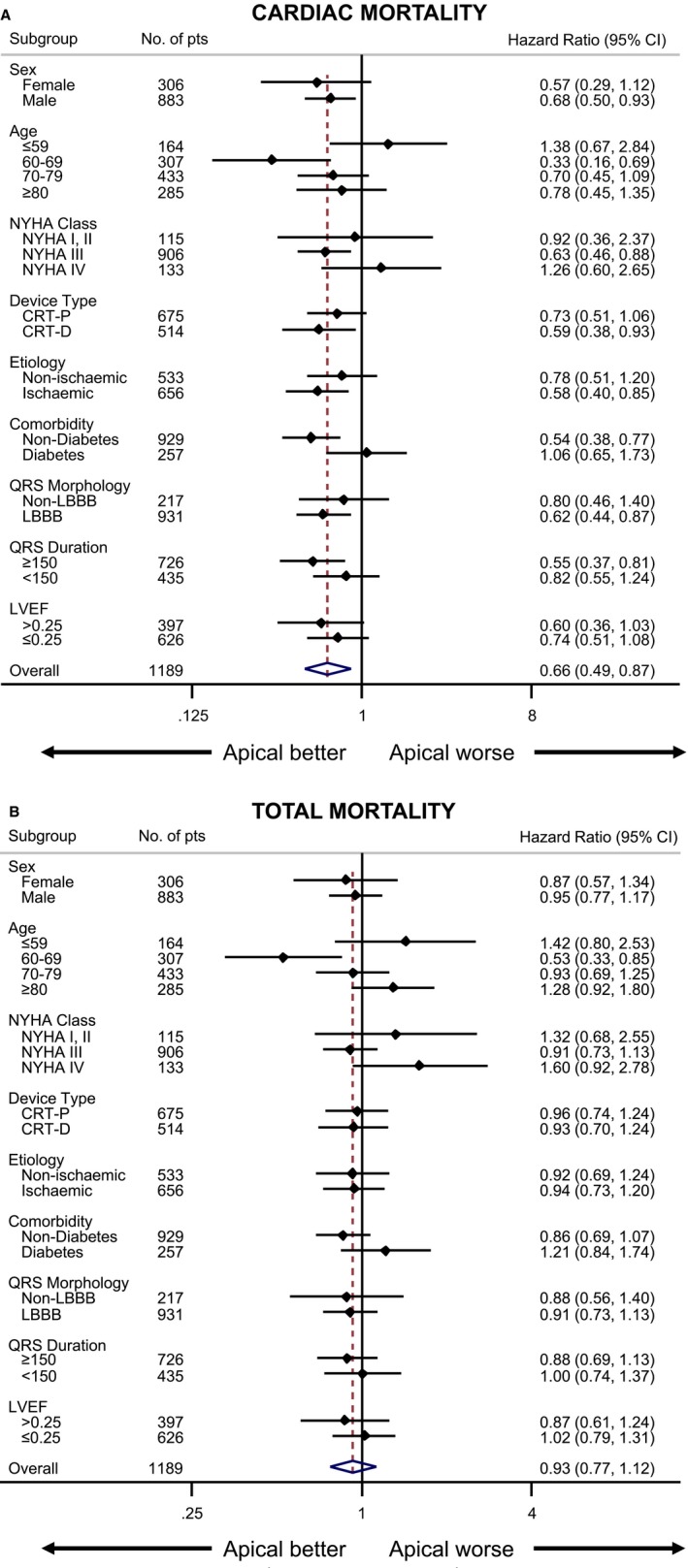
Subgroup analyses of outcomes according to longitudinal LV lead position. Risk of (A) cardiac mortality and (B) total mortality according to longitudinal LV lead position, expressed as hazard ratios and 95% CI (horizontal lines), is shown for various subgroups of patients who received cardiac resynchronization therapy. In the overall analysis, a LV apical lead position was associated with a hazard ratio of 0.66 (95% CI, 0.49–0.87) for cardiac mortality. CI indicates confidence interval; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy with pacing; LBBB, left bundle‐branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; pts, patients.
Circumferential Position
Circumferentially, LV lead positions were as follows: 100 of 1189 patients (8.41%) had leads in anterior positions; 1027 of 1189 (86.4%) had them in lateral positions, and 62 of 1189 (5.21%) had them in posterior positions (Table 5). Patients with the different LV circumferential positions were well matched for age, sex, NYHA class, device type, upgrade from pacemaker, HF etiology, atrial rhythm, QRS duration, QRS morphology, LVEF, and medical therapy, except mineralocorticoid receptor antagonists, the uptake of which was higher in lateral positions (P=0.007). There was a lower proportion of hypertensive patients among those with LV posterior positions.
Table 5.
Baseline Characteristics by Circumferential LV Lead Position
| Anterior | Lateral | Posterior | P Value | |
|---|---|---|---|---|
| Patients, n | 100 | 1027 | 62 | |
| Sex (male), n (%) | 79 (79) | 755 (73.52) | 49 (79.03) | 0.331 |
| Age, y | 70±11.6 | 72.2±10.7 | 71.6±11.6 | 0.162 |
| ≤59 | 18 (18) | 136 (13.24) | 10 (16.13) | 0.705 |
| 60–69 | 26 (26) | 265 (25.80) | 16 (25.81) | |
| 70–79 | 38 (38) | 375 (36.51) | 20 (32.26) | |
| ≥80 | 18 (18) | 251 (24.44) | 16 (25.81) | |
| NYHA class, n (%) | ||||
| I | 1 (1.04) | 36 (3.61) | 1 (1.61) | 0.613 |
| II | 5 (5.21) | 66 (6.63) | 6 (9.68) | |
| III | 81 (84.38) | 778 (78.11) | 47 (75.81) | |
| IV | 9 (9.38) | 116 (11.65) | 8 (12.90) | |
| Device type, n (%) | ||||
| CRT‐D | 42 (42) | 451 (43.91) | 21 (33.87) | 0.291 |
| CRT‐P | 58 (58) | 576 (56.09) | 41 (66.13) | |
| Upgrade from pacemaker | 19 (19) | 180 (17.53) | 4 (6.45) | 0.069 |
| Etiology of cardiomyopathy, n (%) | ||||
| Ischemic | 61 (61) | 561 (54.63) | 34 (54.84) | 0.472 |
| Nonischemic | 39 (39) | 466 (45.37) | 28 (45.16) | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 20 (20) | 230 (22.46) | 7 (11.29) | 0.107 |
| Hypertension | 25 (25) | 306 (29.88) | 9 (14.52) | 0.024 |
| CABG | 17 (17) | 194 (18.95) | 12 (19.35) | 0.887 |
| ECG variables | ||||
| Sinus rhythm, n (%) | 67 (67) | 687 (66.89) | 42 (67.74) | 0.990 |
| Atrial fibrillation, n (%)a | 33 (33) | 340 (33.11) | 20 (32.26) | |
| QRS morphology (LBBB), n (%)b | 77 (81.05) | 808 (81.37) | 46 (76.67) | 0.665 |
| QRS duration (ms)b | 153±20.2 | 155.6±22.8 | 153.2±22.3 | 0.412 |
| Medication, n (%) | ||||
| Loop diuretics | 91 (91) | 971 (94.55) | 56 (90.32) | 0.162 |
| ACEI/ARB | 86 (86) | 890 (86.66) | 53 (85.48) | 0.953 |
| β‐blocker | 66 (66) | 686 (66.80) | 46 (74.19) | 0.470 |
| MRA | 28 (28) | 451 (43.91) | 24 (38.71) | 0.007 |
| LVEF (%) | 23.5±8.8 | 24.5±9.8 | 23±9 | 0.376 |
Variables are expressed as mean±SD unless indicated otherwise. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy with pacing; LBBB, left bundle‐branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
*Refers to differences between the groups from ANOVA for continuous variables and from χ2 tests for categorical variables.
Includes permanent, persistent and paroxysmal atrial fibrillation.
Excludes upgrades to pacemaker.
In univariable analyses, no differences emerged between circumferential positions with respect to cardiac mortality or total mortality (Figure 5) or the composite end points (Tables 2 and 3, and Figure S1).
Figure 5.
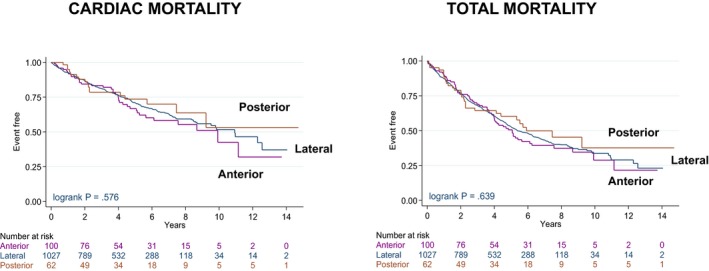
Outcomes according to circumferential left ventricular lead position. Kaplan–Meier survival curves for cardiac mortality and total mortality according to circumferential LV lead positions. CI indicates confidence interval; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy with pacing; LBBB, left bundle‐branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; pts, patients.
RV Lead Positions
Cardiac mortality (univariate HR: 1.19; 95% CI, 0.83–1.70) and total mortality (univariate HR: 1.03; 95% CI, 0.80–1.33) in patients with an apical RV lead position were no different from those in patients with a nonapical RV lead position.
Discussion
This study of LV lead positions in CRT provides the highest number of patients and the longest follow‐up compared with subanalyses of randomized controlled trials or observational studies to date. Several findings have emerged. First, an LV apical lead position was associated with lower cardiac mortality than nonapical positions over a median follow‐up period of 6 years. This effect was mediated by both pump failure and SCD. Second, total mortality and HF hospitalization were similar in LV apical and nonapical lead positions. Third, no difference in these outcomes was observed among anterior, posterior, and lateral LV lead positions. Fourth, no difference in outcomes emerged between apical and septal RV lead positions.
Apical Versus Nonapical LV Lead Position
The cause and mode of death were not considered in either MADIT‐CRT2 or REVERSE.3 Our finding of lower cardiac mortality as well as death from pump failure with an apical LV lead position is in keeping with experimental and clinical studies showing that LV apical pacing is physiologically superior to nonapical pacing.4, 5
The observed beneficial effect of LV apical pacing in CRT, in terms of total mortality or HF hospitalization, is at odds with a subanalysis of MADIT‐CRT, in which an apical LV lead position, compared with a nonapical position (basal or mid), was associated with an increased risk of total mortality (HR: 2.91) as well as the combined end point of total mortality or HF hospitalization (HR: 1.72) among a subset of 799 patients followed up over 2.4 years.2 In the REVERSE trial,3 comprising a subset of 346 patients and a follow‐up of 1.05 years, an LV nonapical lead position was associated a lower risk of total mortality or HF hospitalization compared with an LV apical position (HR 0.27). We should consider, however, that in the subanalyses of these randomized controlled trials, outcomes were reported in relation to other CRT patients with other LV lead positions. It follows that such subanalyses were not subject to the intended randomization rule of the trial. Consequently, these subanalyses should be regarded as “observational,” as is the present study.
In MADIT‐CRT, worse outcomes from an LV apical position were restricted to patients with LBBB.2 In contrast, we found that, in terms of cardiac mortality, an LV apical position is better in the context of LBBB or QRS ≥150 ms. In this context, evidence suggests that not all LV apical lead positions are unfavorable. Addressing whether effects of apical LV pacing should vary according to level of conduction block in a study that followed the MADIT‐CRT subanalysis, Kandala et al12 showed that a longer LV electrical delay in apically positioned LV leads was associated with more favorable LV reverse remodeling and lower risk of total mortality or HF hospitalization compared with apically positioned LV leads with shorter LV electrical delays. Although this study did not compare outcomes with those of nonapically positioned leads, it nevertheless shows that not all apical lead positions have the same effect on outcomes. In the present study, Q‐LV was not systematic in choosing LV lead positions.
Among the notable differences between the MADIT‐CRT patient population and the present cohort is the fact that all patients in MADIT‐CRT were in NYHA class I or II,2 which compares with 13.1% in the present study. We observed significantly lower cardiac mortality with apical LV lead positions in patients in NYHA class III (HR: 0.63; 95% CI, 0.46–0.88) but not in NYHA classes I and II. This suggests an interaction between LV lead position and the severity of HF, according to which an apical lead position is only neutral or “detrimental” in mild HF. Clearly, however, this interaction can be explored only in a study that includes patients with mild and severe HF.
In MADIT‐CRT, the extent of CRT benefit was similar among all lead positions in patients with nonischemic cardiomyopathy and patients with ischemic cardiomyopathy in NYHA class II.2 We found that an apical LV lead position had no significant effect on cardiac mortality in nonischemic cardiomyopathy, but lower cardiac mortality was found in ischemic cardiomyopathy. In this respect, we have previously shown that an LV lead positioned over a myocardial scar was associated with >6‐fold increase in the risk of cardiovascular death.13 In the present study, we did not explore LV position in relation to myocardial scar. It is likely, however, that LV lead position is more crucial in patients with ischemic cardiomyopathy, which is typically more heterogeneous than nonischemic cardiomyopathy with respect to the distribution of myocardial scar.
Circumferential Position
Early CRT studies showed that a lateral or posterolateral LV lead position was associated with a superior hemodynamic benefit than anterior positions.14 Other clinical studies, however, have not been consistent. Gasparini et al found no difference in NYHA class, 6‐minute walking distance, or LVEF between anterior and posterolateral LV lead positions.15 Similar survival was observed for anterior and posterolateral LV lead positions in other observational studies16 and in the COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure) study.17 These findings are consistent with our observation that circumferential position has no impact on long‐term clinical outcomes.
RV Lead Position
The meta‐analysis of Zografos et al18 found similar changes in LV end‐systolic volume and functional status in RV apical versus nonapical lead positions in CRT. Importantly, however, hard end points were not addressed, given unacceptable study heterogeneity. In the REVERSE trial, no differences in a composite clinical score were observed between RV apical and nonapical lead positions.3 In the SEPTAL‐CRT (SEPTAL‐cardiac resynchronization therapy) study,19 the LV reverse remodeling response as well as total mortality or HF hospitalization were similar with RV apical and nonapical lead positions. The present study adds further evidence for a lack of influence on outcomes of RV pacing site (apical or septal) in CRT.
Clinical Implications
The findings from the subanalyses of the MADIT‐CRT2 and REVERSE3 studies indicating that an LV apical lead position is less favorable than a nonapical position in CRT has been noted in consensus statements.20 In MADIT‐CRT, however, an apical LV lead position was only “detrimental” in men, LBBB, and nonischemic cardiomyopathy.2 Our findings, suggest that LV apical pacing may be more favorable than nonapical pacing, at least with respect to cardiac mortality. With respect to total mortality, the effect was neutral rather than detrimental. Although the findings of the present study are limited by its observational nature, the findings from MADIT‐CRT and REVERSE are also limited by the study design of subanalyses, which do not strictly equate with an intention‐to ‐treat analysis within the intended randomization. In the absence of a randomized controlled trial for LV lead positions, the decision of which LV position is most appropriate in CRT currently rests on whether the patient in question is better represented by subanalyses of randomized controlled trials or by a study of “real‐world” patients, as described in this study.
Limitations
This article reports a 2‐center, nonrandomized, retrospective, observational study. In the absence of randomization, we cannot exclude the possibility that unobserved variables may have contributed to outcomes. As in randomized controlled studies, the position of the LV lead was left to the discretion of the implanter, and the reasons for choosing LV pacing sites were not assessed. Our findings from fluoroscopy do not necessarily exclude the possibility that targeting LV pacing sites using Q‐LV or imaging techniques might have led to different outcomes. We should also consider, however, that these techniques were not used in MADIT‐CRT2 or REVERSE.3 Although fluoroscopy may not permit precise localization of lead position, it has nevertheless been used in randomized controlled trials2, 3, 17 and is routinely used in clinical practice worldwide.15, 16
Conclusions
In this study of real‐world clinical practice, an LV apical position was associated with lower cardiac mortality than nonapical positions, and a neutral effect was observed with respect to total mortality. We found no difference in outcomes among LV anterior, posterior, or lateral lead positions or between RV septal and apical lead positions.
Sources of Funding
This study was supported by an unrestricted educational grant from Boston Scientific. The sponsors had no input in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the article.
Disclosures
Leyva has held consultancies with and has received research funding from Medtronic Inc, Boston Scientific, St Jude Medical and LivaNova. Patel has received speaker honoraria from Medtronic Inc. Prinzen has received research grants from Medtronic, Abbott, Biotronik, LivaNova, EBR Systems, Biosense Webster. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Clinical outcomes after cardiac resynchronization therapy according to lead position.
Figure S2. Mode of death according to left ventricular lead position.
Figure S3. Mode of death according to left ventricular lead position.
(J Am Heart Assoc. 2018;7:e008508 DOI: 10.1161/JAHA.117.008508.)
References
- 1. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047–1058. [DOI] [PubMed] [Google Scholar]
- 2. Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP, Zareba W, Moss AJ. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT) trial. Circulation. 2011;123:1159–1166. [DOI] [PubMed] [Google Scholar]
- 3. Thebault C, Donal E, Meunier C, Gervais R, Gerritse B, Gold MR, Abraham WT, Linde C, Daubert JC; group Rs . Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the reverse trial. Eur Heart J. 2012;33:2662–2671. [DOI] [PubMed] [Google Scholar]
- 4. van Deursen C, van Geldorp IE, Rademakers LM, van Hunnik A, Kuiper M, Klersy C, Auricchio A, Prinzen FW. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle‐branch hearts. Circ Arrhythm Electrophysiol. 2009;2:580–587. [DOI] [PubMed] [Google Scholar]
- 5. Peschar M, de Swart H, Michels KJ, Reneman RS, Prinzen FW. Left ventricular septal and apex pacing for optimal pump function in canine hearts. J Am Coll Cardiol. 2003;41:1218–1226. [DOI] [PubMed] [Google Scholar]
- 6. Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation. 1970;41:899–912. [DOI] [PubMed] [Google Scholar]
- 7. Pluijmert M, Bovendeerd PH, Lumens J, Vernooy K, Prinzen FW, Delhaas T. New insights from a computational model on the relation between pacing site and CRT response. Europace. 2016;18:iv94–iv103. [DOI] [PubMed] [Google Scholar]
- 8. Umar F, Taylor RJ, Stegemann B, Marshall H, Flannigan S, Lencioni M, De Bono J, Griffith M, Leyva F. Haemodynamic effects of cardiac resynchronization therapy using single‐vein, three‐pole, multipoint left ventricular pacing in patients with ischaemic cardiomyopathy and a left ventricular free wall scar: the Maestro study. Europace. 2016;18:1227–1234. [DOI] [PubMed] [Google Scholar]
- 9. National Institute of Health and Care Excellence . Nice technology appraisal [ta 314]: implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of ta95 and ta120). 2014.
- 10. Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. New York: WB Saunders Publishing Co; 1997:742–779. [Google Scholar]
- 11. Rockman HA, Juneau C, Chatterjee K, Rouleau JL. Long‐term predictors of sudden and low output death in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1989;64:1344–1348. [DOI] [PubMed] [Google Scholar]
- 12. Kandala J, Upadhyay GA, Altman RK, Bose A, Heist EK, Mela T, Singh JP. Electrical delay in apically positioned left ventricular leads and clinical outcome after cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2013;24:182–187. [DOI] [PubMed] [Google Scholar]
- 13. Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, Auricchio A. Cardiac resynchronization therapy guided by late gadolinium‐enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butter C, Auricchio A, Stellbrink C, Fleck E, Ding J, Yu Y, Huvelle E, Spinelli J. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation. 2001;104:3026–3029. [DOI] [PubMed] [Google Scholar]
- 15. Gasparini M, Mantica M, Galimberti P, Bocciolone M, Genovese L, Mangiavacchi M, Marchesina UL, Faletra F, Klersy C, Coates R, Gronda E. Is the left ventricular lateral wall the best lead implantation site for cardiac resynchronization therapy? Pacing Clin Electrophysiol. 2003;26:162–168. [DOI] [PubMed] [Google Scholar]
- 16. Kronborg MB, Albertsen AE, Nielsen JC, Mortensen PT. Long‐term clinical outcome and left ventricular lead position in cardiac resynchronization therapy. Europace. 2009;11:1177–1182. [DOI] [PubMed] [Google Scholar]
- 17. Saxon LA, Olshansky B, Volosin K, Steinberg JS, Lee BK, Tomassoni G, Guarnieri T, Rao A, Yong P, Galle E, Leigh J, Ecklund F, Bristow MR. Influence of left ventricular lead location on outcomes in the companion study. J Cardiovasc Electrophysiol. 2009;20:764–768. [DOI] [PubMed] [Google Scholar]
- 18. Zografos TA, Siontis KC, Jastrzebski M, Kutyifa V, Klein HU, Zareba W, Katritsis DG. Apical vs. non‐apical right ventricular pacing in cardiac resynchronization therapy: a meta‐analysis. Europace. 2015;17:1259–1266. [DOI] [PubMed] [Google Scholar]
- 19. Leclercq C, Sadoul N, Mont L, Defaye P, Osca J, Mouton E, Isnard R, Habib G, Zamorano J, Derumeaux G, Fernandez‐Lozano I. Comparison of right ventricular septal pacing and right ventricular apical pacing in patients receiving cardiac resynchronization therapy defibrillators: the septal CRT study. Eur Heart J. 2016;37:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF, Breithard O, Brignole M, Cleland J, DeLurgio DB, Dickstein K, Exner DV, Gold M, Grimm RA, Hayes DL, Israel C, Leclercq C, Linde C, Lindenfeld J, Merkely B, Mont L, Murgatroyd F, Prinzen F, Saba SF, Shinbane JS, Singh J, Tang AS, Vardas PE, Wilkoff BL, Zamorano JL, Anand I, Blomstrom‐Lundqvist C, Boehmer JP, Calkins H, Cazeau S, Delgado V, Estes NA, Haines D, Kusumoto F, Leyva P, Ruschitzka F, Stevenson LW, Torp‐Pedersen CT. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow‐up recommendations and management. Europace. 2012;14:1236–1286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Clinical outcomes after cardiac resynchronization therapy according to lead position.
Figure S2. Mode of death according to left ventricular lead position.
Figure S3. Mode of death according to left ventricular lead position.


