Abstract
Background
Epidemiological studies demonstrating a relationship between gout and cardiovascular disease are older and predate modern cardiovascular preventive therapy. We assessed the contemporary association between gout and cardiovascular disease in patients with obstructive coronary artery disease.
Methods and Results
Data were from the Duke Databank for Cardiovascular Diseases, which followed up patients undergoing cardiac catheterization with obstructive coronary artery disease at Duke University Medical Center (1998–2013). We assessed the relationship between gout diagnosis at baseline or during follow‐up and the primary composite outcome of cardiovascular death, myocardial infarction, or stroke, adjusting for differences in baseline clinical factors. Secondary end points included cardiovascular death and all‐cause mortality. New, postbaseline, gout diagnosis was included as a time‐dependent covariate. Among 17 201 patients, 1406 (8.2%) had baseline gout and a high burden of cardiovascular risk factors, but high rates of optimal medical therapy. Over a median follow‐up of 6.4 years, gout diagnosis at time of catheterization was not associated with the primary outcome (hazard ratio [95% confidence interval], 1.05 [0.96–1.15]; P=0.31) or cardiovascular death (hazard ratio [95% confidence interval], 1.10 [0.99–1.22]; P=0.08), but was associated with increased all‐cause mortality (hazard ratio [95% confidence interval], 1.13 [1.05–1.23]; P=0.002). After including new, postbaseline, gout diagnosis, the instantaneous risk of the primary outcome was significantly associated with prior gout diagnosis (hazard ratio [95% confidence interval], 1.15 [1.07–1.25]; P=0.0004).
Conclusions
A clinical history of gout is associated with worse outcomes in a contemporary population of patients with obstructive coronary artery disease. This increased risk exists despite high levels of optimal baseline cardiovascular disease medical therapy, suggesting that residual cardiovascular risk is not addressed by standard medical therapy.
Keywords: cardiovascular disease, coronary artery disease, gout
Subject Categories: Mortality/Survival, Coronary Artery Disease, Inflammation
Clinical Perspective
What Is New?
Using retrospective data from the Duke Databank for Cardiovascular Disease, we found that a clinical history of gout is associated with worse outcomes in a contemporary population of patients with obstructive coronary artery disease.
This increased risk exists despite high levels of optimal baseline cardiovascular disease medical therapy.
What Are the Clinical Implications?
Our findings bring into question the possibility of residual risk beyond what can be addressed with standard medical therapy for cardiovascular disease.
As we look to the future, further study is needed to determine whether targeted anti‐inflammatory therapy or better control of gout can improve cardiovascular outcomes.
Gout is a common rheumatologic condition affecting almost 4% of the adult population in the United States, and the prevalence is increasing.1 This increase is particularly concerning given the frequent coexistence of gout and cardiovascular disease (CVD) in the general population.2 Because of many shared risk factors, including hypertension, diabetes mellitus, and obesity,3, 4, 5 the exact nature of the relationship between gout and CVD has remained unclear. Several epidemiologic studies have found an independent relationship between gout and CVD6, 7; however, these were conducted several decades ago, before the advent of modern preventive medical therapy for CVD. Furthermore, many of these studies were conducted among individuals without known coronary artery disease (CAD) or only in men and in the context of a rigorous registry.6, 7 Our goal was to examine the long‐term association between gout and CVD in a contemporary population of men and women with known CAD, a high‐risk population for subsequent cardiovascular events. We addressed this question using the Duke Databank for Cardiovascular Diseases (DDCD), which includes patients who presented for cardiac catheterization at Duke University Medical Center between 1998 and 2013 and were found to have obstructive CAD, with collection of subsequent follow‐up through 2014.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design and Population
The DDCD is a large well‐characterized registry of all patients who underwent cardiac catheterization at Duke University Medical Center between 1969 and 2015.8 Subsequent clinical events are ascertained from Duke Health System records. The DDCD also includes an annual follow‐up telephone call or mailed survey for patients with significant CAD in which patients report non‐Duke hospitalization events and list outpatient medications. For clinical events occurring at Duke, the end points of myocardial infarction (MI), stroke, and cardiovascular death were based on clinical diagnoses assigned by the patient's physician. For outside events reported on follow‐up surveys, an events committee reviewed all information from the patient and, with patient permission, from outside health system records. For patients lost to follow‐up, a query of the National Death Index was obtained to ascertain vital status and cause of death through 2014. Baseline clinical variables for each patient were stored in the DDCD using methods previously described.8 Medication use at baseline (within 6 months before catheterization) was ascertained from data collected in the DDCD (at time of catheterization) and from medication records in the Duke Health System. This retrospective observational study was approved by the Duke Institutional Review Board under a waiver of informed consent and Health Insurance Portability and Accountability Act of 1996 approval.
Patients were included in this retrospective study if they were at least 18 years old, underwent diagnostic catheterization between January 1998 and December 2013, and had evidence of obstructive CAD, defined as clinically significant occlusion in a major segment in ≥1 coronary arterial systems. Patients were excluded if they did not have at least 1 outpatient encounter at Duke in the 24 months before catheterization (to avoid misclassification of patients who did not receive regular care at Duke), had severe valvular disease, or had high‐risk comorbidities, such as cancer, cardiac transplantation, congenital heart disease, AIDS, or end‐stage renal disease on dialysis. Only the first qualifying diagnostic catheterization for each patient was included in the analysis.
Study Definitions and End Points
A history of gout at baseline was defined as evidence of any of the following, ascertained using electronic medical record data within the Duke Health System: inpatient or outpatient diagnosis of gout before catheterization (International Classification of Diseases, Ninth Revision [ICD‐9], code of 274.XX); or use of gout‐related medications before catheterization, including allopurinol, colchicine, febuxostat, probenecid, or any combination thereof. Diagnosis of gout after date of cardiac catheterization was defined using inpatient or outpatient diagnosis codes. Serum uric acid levels recorded in the Duke Health System were reported using the most recent measurement within 6 months before catheterization.
The primary composite end point for this analysis was time to first event of cardiovascular death, MI, or stroke. Secondary end points included time to all‐cause death, time to cardiovascular death, time to cause‐specific cardiovascular death (ie, fatal MI, sudden death, heart failure death, cardiac procedure death, or other cardiac death), time to noncardiac death, and time to death of unknown cause. “Other cardiac death” was defined as death in which there is evidence of a primary cardiac cause that cannot be classified as definite MI or heart failure. For example, if the death certificate stated that the cause of death was cardiac (eg, MI or heart failure), but no other data were available, then the death would be classified as “other cardiac death.”
Statistical Analyses
A summary of demographic and clinical characteristics of the analysis cohort was stratified by diagnosis of gout at baseline. Continuous characteristics were summarized with median and interquartile range; categorical variables were summarized with frequency count and percentage. Characteristics were compared between individuals with and without a history of gout using a χ2 statistic for categorical variables and a Wilcoxon rank‐sum statistic for continuous variables. Cumulative incidence rates were estimated, accounting for the competing risk of other causes of death where appropriate, and censoring at last date known alive/last follow‐up. Cumulative event rate curves were plotted, stratified by baseline gout status.
The relationship between baseline gout status and each study end point was analyzed using Cox proportional cause‐specific hazards regression. The proportional hazards assumption was evaluated using weighted Schoenfeld residuals. An adjusted model included age, sex, race, medical history (including prior MI), coronary artery bypass graft surgery, congestive heart failure, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, carotid bruits, history of chronic obstructive pulmonary disease, body mass index, congestive heart failure severity class (≤2 weeks precatheterization), history of smoking, ventricular gallop, history of renal disease, baseline estimated glomerular filtration rate, valvular heart disease, hypertension, systolic blood pressure, year of index catheterization, history of liver disease, number of diseased vessels, left main artery stenosis ≥50%, acute coronary syndrome status, and baseline use of aspirin, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, or statins. Multiple imputation (n=25 imputed data sets) was used to simulate missing data for the covariates. The adjusted models were fit within each imputed data set. To provide statistical inference, results were then combined across the data sets, taking into account the uncertainty, attributable to imputation of missing values. Of the 17 201 subjects in the cohort, 14 203 (82.6%) had complete data on all adjustment covariates. Covariates with highest levels of missing data were medications (7.6% missing), systolic blood pressure (5.6% missing), congestive heart failure severity (2.6% missing), and race (1.4% missing). Other covariates were either complete or <1% missing. Natural cubic spline transformations were used for continuous covariates to avoid forcing associations to be linear.
The relationship between gout status at baseline or during follow‐up and each end point was analyzed using Cox proportional cause‐specific hazards regression, with gout status modeled as a time‐dependent covariate that switched a patient into the gout group at the time of postbaseline diagnosis. The same variables listed above were used for adjustment in these models.
Data acquisition and statistical analysis were performed at the Duke Clinical Research Institute using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics of Patient Population by Gout History
Of 85 173 patients who underwent catheterization between January 1998 and December 2013, 17 201 (20.2%) met our inclusion criteria (Figure 1). Of these patients, 1406 (8.2%) had a baseline history of gout and 15 795 (91.8%) did not. Those with a history of gout at the time of catheterization were most commonly identified with a diagnosis of gout in the record (75.3%), but most were also taking at least 1 gout medication at baseline (66.9%) (Table 1). Individuals with gout at baseline were older and more often men and nonwhite, and more commonly had a history of MI, coronary revascularization, congestive heart failure, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, hypertension, and renal disease than those without gout. Compared with patients without gout, those with gout less commonly presented with acute coronary syndrome or underwent coronary revascularization after catheterization, but had a higher number of diseased vessels. This overall population of patients with obstructive CAD had relatively high rates of baseline evidence‐based medication use, including aspirin (≈90%), statin therapy (≈80%), and β blockers (≈90%).
Figure 1.
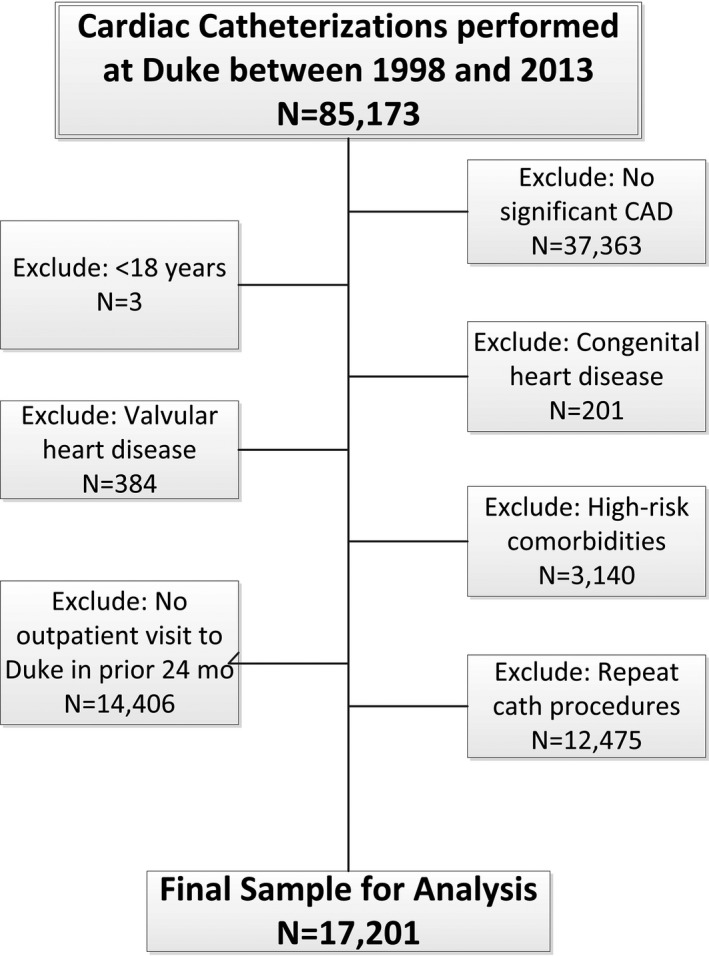
Cohort diagram. This figure displays the final study population, beginning with the initial cohort, through exclusions. CAD indicates coronary artery disease.
Table 1.
Baseline Characteristics by Gout Status at Baseline
| Characteristic | Overall (N=17 201) | No Gout History (N=15 795) | Gout History (N=1406) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 65 (56–73) | 64 (56–73) | 67 (59–75) | <0.0001 |
| Female sex | 5644 (32.8) | 5326 (33.7) | 318 (22.6) | <0.0001 |
| Nonwhite race | 3746 (22.1) | 3391 (21.8) | 355 (25.5) | <0.0001 |
| Medical history | ||||
| MI | 5833 (33.9) | 5290 (33.5) | 543 (38.6) | <0.0001 |
| PCI | 3042 (17.7) | 2763 (17.5) | 279 (19.8) | 0.027 |
| CABG | 4291 (24.9) | 3834 (24.3) | 457 (32.5) | <0.0001 |
| CHF | 4347 (25.8) | 3847 (24.9) | 500 (36.1) | <0.0001 |
| Cerebrovascular disease | 1842 (10.7) | 1644 (10.4) | 198 (14.1) | <0.0001 |
| PVD | 1764 (10.3) | 1608 (10.2) | 156 (11.1) | 0.279 |
| Diabetes mellitus | 5425 (31.5) | 4896 (31.0) | 529 (37.6) | <0.0001 |
| Hypertension | 12 195 (70.9) | 11 053 (70.0) | 1142 (81.2) | <0.0001 |
| Smoking | 8338 (48.5) | 7691 (48.7) | 647 (46.0) | 0.054 |
| Renal disease | 136 (0.8) | 106 (0.7) | 30 (2.1) | <0.0001 |
| Prior medications | ||||
| Aspirin | 14 953 (86.9) | 13 679 (86.6) | 1274 (90.6) | <0.0001 |
| ACE‐I/ARB | 12 084 (76.0) | 11 006 (75.7) | 1078 (79.1) | 0.0047 |
| β Blocker | 13 895 (87.4) | 12 689 (87.3) | 1206 (88.5) | 0.1890 |
| Clopidogrel | 8430 (53.0) | 7759 (53.4) | 671 (49.3) | 0.0036 |
| Statins | 12 644 (79.5) | 11 547 (79.5) | 1097 (80.5) | 0.3404 |
| Warfarin | 1211 (7.6) | 1030 (7.1) | 181 (13.3) | <0.0001 |
| Criteria for gout history | ||||
| Clinical gout diagnosis | 1059 (6.2) | 1059 (75.3) | ||
| Any gout medication before baseline | 940 (5.5) | 940 (66.9) | ||
| Allopurinol | 707 (4.4) | 707 (50.7) | ||
| Colchicine | 379 (2.3) | 379 (27.2) | ||
| Febuxostat | 15 (0.1) | 15 (1.1) | ||
| Probenecid/Colchicine | 2 (0.0) | 2 (0.1) | ||
| Probenecid | 40 (0.2) | 40 (2.9) | ||
| Presentation characteristics | ||||
| Systolic BP, mm Hg | 143 (127–162) | 143 (127–162) | 145 (129–162) | 0.120 |
| Diastolic BP, mm Hg | 80 (71–89) | 80 (71–89) | 81 (72–90) | 0.037 |
| BMI, kg/m2 | 28 (25–32) | 28 (25–32) | 30 (26–34) | <0.0001 |
| eGFR, mL/min per 1.73 m2 | 72.8 (55.7–88.7) | 73.8 (57.2–89.3) | 58.4 (41.5–76.8) | <0.0001 |
| Serum uric acid, mg/dLa | 6.1 (4.9–7.7) | 6.0 (4.8–7.3) | 7.2 (5.6–9.0) | <0.0001 |
| ACS | 8745 (50.8) | 8084 (51.2) | 661 (47.0) | 0.003 |
| No. of diseased vessels | ||||
| 1 | 6379 (37.1) | 5949 (37.7) | 430 (30.6) | <0.0001 |
| 2 | 4530 (26.3) | 4167 (26.4) | 363 (25.8) | |
| >2 | 6292 (36.6) | 5679 (36.0) | 613 (43.6) | |
| Management after catheterization | ||||
| PCI | 7832 (45.5) | 7255 (45.9) | 577 (41.0) | 0.0002 |
| CABG | 2877 (16.7) | 2650 (16.8) | 227 (16.1) | |
| Medical management | 6492 (37.7) | 5890 (37.3) | 602 (42.8) | |
| Duration of follow‐upb | 6.4 (3.0–10.7) | 6.6 (3.1–10.8) | 4.6 (2.1–8.4) | |
Continuous variables are presented as median (25th–75th percentile). Categorical variables are presented as number (percentage). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; and PVD, peripheral vascular disease.
Serum uric acid measurement is most recent ≤6 months before catheterization, and only available in a minority of patients (in 1396 overall, in 1158 patients without gout history, and in 238 patients with gout history).
Duration of follow‐up reported as years from baseline catheterization to death or last known alive date.
Association Between Gout History and Cardiovascular Outcomes
Over the total follow‐up period (median [interquartile range] follow‐up, 6.4 [3.0–10.7] years; maximum follow‐up, 17.7 years), the primary composite event occurred in 6177 patients overall, with 10‐year cumulative incidence of 46.0% in those with gout compared with 37.2% in those without gout history at baseline. All‐cause death occurred in 7679 individuals (10‐year cumulative incidence of 58.9% versus 44.2% in those with or without gout, respectively) (Table 2). The cumulative incidence curves for the composite end point of cardiovascular death, MI, or stroke by baseline gout status are shown in Figure 2. In unadjusted analysis, individuals with gout were 40% more likely to experience the primary composite end point compared with those without gout (hazard ratio [HR] [95% confidence interval {CI}], 1.40 [1.29–1.53]; P<0.0001; Table 2). This estimate was not modified by patient sex (interaction P=0.66). However, after adjustment for possible confounding factors, gout status at baseline was no longer associated with the primary composite end point (HR [95% CI], 1.05 [0.96–1.15]; P=0.31) or with cardiovascular death (HR [95% CI], 1.10 [0.99–1.22]; P=0.08). In comparison, a history of gout was related to all‐cause mortality both before and after adjustment (adjusted HR [95% CI], 1.13 [1.05–1.23]; P=0.002; Figures S1 and S2). Similarly, a history of gout was related to other cardiac death (adjusted HR [95% CI], 1.23 [1.07–1.42]; P=0.003) and noncardiac death (adjusted HR [95% CI], 1.14 [1.02–1.28]; P=0.021) both before and after adjustment (Table S1).
Table 2.
Association Between Gout at Baseline and Clinical End Points
| End Point | Cumulative Incidence (Unadjusted), No. (%) With Event | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|---|
| Follow‐Up Time Point, y | No Gout History | Gout History | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Cardiovascular death/MI/stroke | 1 | 1736 (11.0) | 204 (14.6) | 1.40 (1.29–1.53) | <0.0001 | 1.05 (0.96–1.15) | 0.306 |
| 5 | 3796 (25.3) | 443 (33.5) | |||||
| 10 | 5062 (37.2) | 546 (46.0) | |||||
| 15 | 5554 (46.0) | 578 (53.8) | |||||
| Cardiovascular death | 1 | 828 (5.3) | 114 (8.1) | 1.62 (1.47–1.80) | <0.0001 | 1.10 (0.99–1.22) | 0.081 |
| 5 | 2069 (13.8) | 283 (21.5) | |||||
| 10 | 3072 (23.2) | 375 (32.5) | |||||
| 15 | 3588 (32.3) | 417 (42.8) | |||||
| All‐cause death | 1 | 1307 (8.3) | 170 (12.1) | 1.55 (1.44–1.67) | <0.0001 | 1.13 (1.05–1.23) | 0.002 |
| 5 | 3765 (25.3) | 475 (36.6) | |||||
| 10 | 5790 (44.2) | 660 (58.9) | |||||
| 15 | 6818 (62.0) | 746 (79.3) | |||||
CI indicates confidence interval; HR, hazard ratio; MI, myocardial infarction.
Adjusted for age, sex, race, medical history (including prior MI, coronary artery bypass grafting, congestive heart failure, congestive heart failure severity class, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, carotid bruits, history of chronic obstructive pulmonary disease, body mass index, congestive heart failure severity class [≤2 weeks precatheterization], history of smoking, ventricular gallup, history of renal disease, baseline estimated glomerular filtration rate [Chronic Kidney Disease Epidemiology Collaboration], any valvular heart disease, hypertension, systolic blood pressure, year of index catheterization, history of liver disease, number of diseased vessels, left main stenosis ≥50%, acute coronary syndrome status, and baseline use of aspirin, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, or statin therapy).
Figure 2.
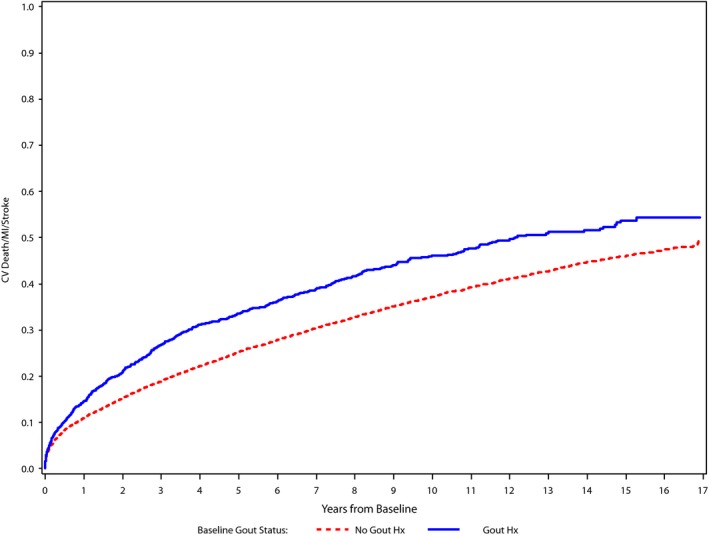
Cumulative incidence curves. Cumulative incidence curves for cardiovascular (CV) death, myocardial infarction, or stroke incidence by baseline gout status. Hx indicates history; MI, myocardial infarction.
Of the 15 795 patients who did not have gout at baseline, 917 had a subsequent gout diagnosis reported over the course of the study (cumulative incidence of 6.1% at 10 years). In unadjusted analysis with gout diagnosis modeled as a time‐varying covariate, individuals with gout at baseline or during study follow‐up were 50% more likely to experience the primary composite end point compared with those without gout at any point during the study (HR [95% CI], 1.50 [1.39–1.61]; P<0.0001; Table 3). After adjustment for baseline factors, the association was attenuated, but still significant (HR [95% CI], 1.15 [1.07–1.25]; P=0.0004). The adjusted relationships between gout at baseline or during the study and the outcomes of cardiovascular death and all‐cause mortality were also significant (HR [95% CI], 1.19 [1.09–1.31] [P=0.0002] and 1.21 [1.13–1.30] [P<0.0001], respectively). In addition, the adjusted relationships between gout at baseline or during the study and the outcomes of heart failure death, other cardiac death, and noncardiac death were also significant (Table S2).
Table 3.
Association Between Gout at Baseline or During Follow‐Up and Clinical Outcomes
| End Point | Unadjusted HR (95% CI) | Unadjusted P Value | Adjusted HR (95% CI)a | Adjusted P Valuea |
|---|---|---|---|---|
| Cardiovascular death/MI/stroke | 1.50 (1.39–1.61) | <0.0001 | 1.15 (1.07–1.25) | 0.0004 |
| Cardiovascular death | 1.73 (1.59–1.88) | <0.0001 | 1.19 (1.09–1.31) | 0.0002 |
| All‐cause death | 1.65 (1.55–1.76) | <0.0001 | 1.21 (1.13–1.30) | <0.0001 |
CI indicates confidence interval; HR, hazard ratio; MI, myocardial infarction.
Adjusted for age, sex, race, medical history (including prior MI, coronary artery bypass grafting, congestive heart failure, congestive heart failure severity class, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, carotid bruits, history of chronic obstructive pulmonary disease, body mass index, congestive heart failure severity class [≤2 weeks precatheterization], history of smoking, ventricular gallup, history of renal disease, baseline estimated glomerular filtration rate [Chronic Kidney Disease Epidemiology Collaboration], any valvular heart disease, hypertension, systolic blood pressure, year of index catheterization, history of liver disease, number of diseased vessels, left main stenosis ≥50%, acute coronary syndrome status, and baseline use of aspirin, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, or statin therapy).
Discussion
In a contemporary population of men and women with obstructive CAD treated with high rates of optimal medical therapy, we found that a minority of patients had a diagnosis of gout at the time of catheterization (8.2%), but those with gout were more likely to have other cardiovascular comorbidities. Over a median follow‐up of 6.4 years, a diagnosis of gout at the time of catheterization was not associated with the primary composite outcome of cardiovascular death, MI, or stroke after adjustment. Nevertheless, baseline gout was significantly associated with a higher rate of all‐cause mortality and related to cardiac and noncardiac causes of death. Furthermore, when including new gout diagnosis postbaseline in the analysis as a time‐dependent covariate, we found that instantaneous risks of all 3 outcomes (primary composite, cardiovascular death, and all‐cause mortality) were significantly associated with gout diagnosis. In particular, postbaseline gout diagnosis was associated with a >2‐fold increase in risk of heart failure death. Overall, these data suggest that, despite aggressive medical therapy, a clinical history of gout is associated with worse long‐term cardiovascular clinical outcomes and all‐cause mortality in patients with CAD.
These findings are in line with some prior literature on this topic. Several studies have previously demonstrated an increased risk of CVD death associated with gout,6, 7 and a meta‐analysis of 6 studies revealed a 29% increase in CVD mortality (95% CI, 1.14–1.44).9 However, these analyses examined individuals without known CAD. A single prior analysis focused on patients with gout and known baseline coronary heart disease participating in a rigorous clinical registry. In that study of >50 000 male participants in the Health Professionals Follow‐Up Study (1986–1998), there was a 26% increased risk (95% CI, 1.07–1.50) of CVD death compared with those without gout.7
Our study adds to this prior literature. We examined long‐term outcomes among a contemporary population of both men and women treated in clinical practice. We also focused on patients with baseline obstructive CAD to assess the impact of gout on cardiovascular outcomes in this higher‐risk population. More important, our patient population was relatively well treated for CVD with guideline‐recommended medications for CVD, including aspirin, statin therapy, and β blockers. This suggests that the cardiovascular risk associated with gout may not be fully treated with traditional medical therapies, although whether better control of gout itself improves cardiovascular outcomes is uncertain. Our data also support aggressive treatment of comorbid conditions, such as hypertension and diabetes mellitus, among patients with gout and CAD, as well other secondary prevention efforts, such as smoking cessation, in this population.
We further explored mechanisms of death in our study population. Overall, relatively few deaths were categorized as fatal MI, sudden cardiac death, or heart failure death, and most cardiovascular deaths were classified as “other cardiac death.” This finding likely reflects DDCD conventions in event adjudication: although there may have been sufficient evidence for treating providers to determine a cardiac cause of death, if the death certificate listed a specific type of cardiac death (eg, MI, heart failure, or arrhythmia), but was provided without supporting hospital records, DDCD registrars would classify the event as other cardiac death. Consequently, our findings demonstrate independent relationships between baseline and postbaseline gout with cardiovascular mortality. Furthermore, we found that postbaseline gout was strongly associated with heart failure death. This is consistent with prior literature on the topic.10 For example, in the Framingham Offspring Study, individuals with gout were significantly more likely to develop clinical heart failure and have higher mortality than those without gout.11
Possible mechanisms accounting for residual risk associated with gout include oxidative stress12 and inflammation.13, 14, 15 Oxidative stress is generated by xanthine oxidase, an enzyme that catalyzes the formation of urate.16 Allopurinol, a xanthine oxidase inhibitor, has been shown to improve oxidative stress and endothelial dysfunction in patients with stable CAD.17, 18 Considerable observational data suggest that allopurinol is associated with cardiovascular benefit.10, 19, 20 Yet, febuxostat, a more potent xanthine oxidase inhibitor and hypouricemic agent, has recently been shown in a randomized clinical trial to lead to increased cardiovascular and all‐cause mortality compared with allopurinol.21 The ongoing ALL‐HEART (Allopurinol and Cardiovascular Outcomes in Patients With Ischemic Heart Disease) randomized trial will assess whether patients with ischemic heart disease derive cardiovascular benefit from allopurinol therapy versus standard of care.22
Inflammation may also provide a link between gout and CVDs. Gout is characterized by short‐term periods of clinically apparent inflammation, separated by periods of subclinical inflammation.5, 23 Low‐grade inflammation also has a major role in the development of CVDs, and elevated levels of C‐reactive protein and interleukin‐6 have been found to be associated with increased cardiovascular risk.24 Recently, canakinumab, an anti‐inflammatory agent, was found to confer cardiovascular benefit among individuals with prior MI and elevated C‐reactive protein.25 Whether individuals with gout would derive cardiovascular benefit from this therapy remains to be seen. In addition, the effect of colchicine, a relatively inexpensive anti‐inflammatory agent, on cardiovascular events in patients after acute MI is currently being evaluated in the COLCOT (Colchicine Cardiovascular Outcomes Trial; URL: http://www.clinicaltrials.gov. Unique identifier: 02551094); results are anticipated in 2019.26
Limitations
Our results should be interpreted in the context of several caveats. First, our database only included individuals who underwent cardiac catheterization and who were found to have obstructive CAD, so these results cannot be generalized to patients without catheterization or those with nonobstructive CAD. Second, these data are reflective of a single academic health center. However, the large sample size and long duration of follow‐up increase the likelihood that our findings are not because of chance. Third, our definition of gout was based on clinical diagnoses and medication information ascertained from electronic medical records. Previous studies suggest that such algorithms may have limited accuracy compared with how such patients would be prospectively diagnosed by a rheumatologist following established criteria.27, 28 Fourth, we performed a retrospective analysis in which we adjusted for multiple potential confounding variables, yet our results could be limited by residual confounding. In particular, use of medications may be underestimated because of limitations inherent in extracting such data from electronic medical record sources. Fifth, we only accounted for clinical factors and medication use at the time of index catheterization; our analysis did not account for changes in clinical risk factors or medication use that might potentially confound the associations seen with incident gout diagnosis during the study follow‐up. Sixth, we did not have access to data on over‐the‐counter medications, such as nonsteroidal anti‐inflammatory medications, or on medications prescribed by other institutions; therefore, we were unable to assess the effect of gout medication on the relationship between baseline gout and clinical outcomes. Finally, our database had limited data on serum uric acid, so we were unable to assess the impact that uric acid has on the relationship between gout and cardiovascular outcomes.
Summary
A clinical history of gout, at either the time of catheterization or any point thereafter, is associated with worse long‐term cardiovascular outcomes in a contemporary population of patients with obstructive CAD. This increased risk exists despite relatively high levels of optimal medical therapy, raising the possibility of residual risk beyond what can be addressed with standard medical therapy for CVD. Whether targeted anti‐inflammatory therapy or better control of gout can improve cardiovascular outcomes could be topics of future investigation.
Sources of Funding
This work was supported by Ardea Biosciences, Inc, and Ironwood Pharmaceuticals, Inc.
Disclosures
Pagidipati reports having ownership interest in Freedom Health, Inc, Physician Partners, LLC, RXAdvance, LLC, Florida Medical Associates, LLC; and research grants to Duke from Amgen, Novartis, Sanofi, Regeneron, Alexion, and Verily. Keenan reports being on the advisory board of AstraZeneca, Takeda, and Horizon Pharmaceuticals. Roe reports research funding from AstraZeneca, Eli Lilly & Company, Janssen Pharmaceuticals, Sanofi‐Aventis, Daiichi‐Sankyo, Familial Hypercholesterolemia Foundation, and Ferring Pharmaceuticals; educational activities (generates revenue for Duke) for Amgen and Bristol Myers Squibb; and consulting (including continuing medical education) for AstraZeneca, Eli Lilly & Company, Merck & Co, Daiichi‐Sankyo, Elsevier Publishers, Amgen, Boehringer‐Ingelheim, PriMed, and Myokardia. Hess reports receiving research grants to CPC Clinical Research from Merck and Bayer Pharmaceuticals. All other authors report no relevant disclosures.
Supporting information
Table S1. Association Between Gout at Baseline and Cause‐Specific Mortality
Table S2. Association Between Gout at Baseline OR during Follow‐Up and Cause‐Specific Mortality
Figure S1. Cumulative incidence curves for CV death by baseline gout status.
Figure S2. Cumulative incidence curves for all‐cause mortality by baseline gout status.
(J Am Heart Assoc. 2018;7:e009328 DOI: 10.1161/JAHA.118.009328.)
References
- 1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. [DOI] [PubMed] [Google Scholar]
- 2. Stack AG, Hanley A, Casserly LF, Cronin CJ, Abdalla AA, Kiernan TJ, Murthy BV, Hegarty A, Hannigan A, Nguyen HT. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM. 2013;106:647–658. [DOI] [PubMed] [Google Scholar]
- 3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow‐up study. Arch Intern Med. 2005;165:742–748. [DOI] [PubMed] [Google Scholar]
- 4. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–687.e1. [DOI] [PubMed] [Google Scholar]
- 5. Richette P, Perez‐Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, Punzi L, So AK, Bardin T. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10:654–661. [DOI] [PubMed] [Google Scholar]
- 6. Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH; MRFIT Research Group. Long‐term cardiovascular mortality among middle‐aged men with gout. Arch Intern Med. 2008;168:1104–1110. [DOI] [PubMed] [Google Scholar]
- 7. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 8. Harris PJ, Lee KL, Harrell FE Jr, Behar VS, Rosati RA. Outcome in medically treated coronary artery disease: ischemic events: nonfatal infarction and death. Circulation. 1980;62:718–726. [DOI] [PubMed] [Google Scholar]
- 9. Clarson LE, Chandratre P, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased cardiovascular mortality associated with gout: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2015;22:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thanassoulis G, Brophy JM, Richard H, Pilote L. Gout, allopurinol use, and heart failure outcomes. Arch Intern Med. 2010;170:1358–1364. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open. 2012;2:e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez‐Ruiz F, Taylor W, Liote F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR, Robbins M, Wenger N, Terkeltaub R; American College of Rheumatology . 2012 American College of Rheumatology guidelines for management of gout, part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyngdoh T, Marques‐Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, Vollenweider P. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population‐based colaus study. PLoS One. 2011;6:e19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richette P, Frazier A, Bardin T. Impact of anti‐inflammatory therapies, xanthine oxidase inhibitors and other urate‐lowering therapies on cardiovascular diseases in gout. Curr Opin Rheumatol. 2015;27:170–174. [DOI] [PubMed] [Google Scholar]
- 15. Perez‐Ruiz F, Martinez‐Indart L, Carmona L, Herrero‐Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73:177–182. [DOI] [PubMed] [Google Scholar]
- 16. Richette P, Bardin T. Gout. Lancet. 2010;375:318–328. [DOI] [PubMed] [Google Scholar]
- 17. Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta‐analysis. Cardiovasc Ther. 2012;30:217–226. [DOI] [PubMed] [Google Scholar]
- 18. Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high‐dose allopurinol in angina pectoris. J Am Coll Cardiol. 2011;58:820–828. [DOI] [PubMed] [Google Scholar]
- 19. Kelkar A, Kuo A, Frishman WH. Allopurinol as a cardiovascular drug. Cardiol Rev. 2011;19:265–271. [DOI] [PubMed] [Google Scholar]
- 20. Grimaldi‐Bensouda L, Alpérovitch A, Aubrun E, Danchin N, Rossignol M, Abenhaim L, Richette P; PGRx MI Group. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. 2015;74:836–842. [DOI] [PubMed] [Google Scholar]
- 21. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, Hunt B, Castillo M, Gunawardhana L; CARES Investigators. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–1210. [DOI] [PubMed] [Google Scholar]
- 22. Mackenzie IS, Ford I, Walker A, Hawkey C, Begg A, Avery A, Taggar J, Wei L, Struthers AD, MacDonald TM; ALL‐HEART study group . Multicentre, prospective, randomised, open‐label, blinded end point trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: protocol of the ALL‐HEART study. BMJ Open. 2016;6:e013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, Krammer G, Murphy V, Richard D, So AK. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active‐controlled, double‐blind trials and their initial extensions. Ann Rheum Dis. 2012;71:1839–1848. [DOI] [PubMed] [Google Scholar]
- 24. Emerging Risk Factors Collaboration , Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 26. Colchicine Cardiovascular Outcomes Trial (COLCOT) . ClinicalTrials.gov web site. https://clinicaltrials.gov/ct2/show/NCT02551094. Updated June 14, 2018. Accessed July 6, 2018.
- 27. Malik A, Dinnella JE, Kwoh CK, Schumacher HR. Poor validation of medical record ICD‐9 diagnoses of gout in a veterans affairs database. J Rheumatol. 2009;36:1283–1286. [DOI] [PubMed] [Google Scholar]
- 28. Harrold LR, Saag KG, Yood RA, Mikuls TR, Andrade SE, Fouayzi H, Davis J, Chan KA, Raebel MA, Von Worley A, Platt R. Validity of gout diagnoses in administrative data. Arthritis Rheum. 2007;57:103–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association Between Gout at Baseline and Cause‐Specific Mortality
Table S2. Association Between Gout at Baseline OR during Follow‐Up and Cause‐Specific Mortality
Figure S1. Cumulative incidence curves for CV death by baseline gout status.
Figure S2. Cumulative incidence curves for all‐cause mortality by baseline gout status.


