Abstract
Background
Phase angle (PA) is a bioimpedance measurement that is determined lean body mass and hydration status. Patients with low PA values are more likely to be frail, sarcopenic, or malnourished. Previous work has shown that low PA predicts adverse outcomes after cardiac surgery, but the effect of PA on survival has not previously been assessed in this setting.
Methods and Results
The BICS (Bioimpedance in Cardiac Surgery) study recruited 277 patients undergoing major cardiac surgery at 2 university‐affiliated hospitals in Montreal, QC, Canada. Bioimpedance measurements as well as frailty and nutritional assessments were performed preoperatively. The primary outcome was all‐cause mortality. Secondary outcomes were 30‐day mortality, postoperative morbidity, and hospital length of stay. There were 10 deaths at 1 month of follow‐up and 16 deaths at 12 months of follow‐up. PA was associated with age, sex, body mass index, comorbidities, and frailty, as measured by the Short Physical Performance Battery and Fried scales. After adjusting for Society of Thoracic Surgeons–predicted mortality, lower PA was associated with higher mortality at 1 month (adjusted odds ratio, 3.57 per 1° decrease in PA; 95% confidence interval, 1.35–9.47) and at 12 months (adjusted odds ratio, 3.03 per 1° decrease in PA; 95% confidence interval, 1.30–7.09), a higher risk of overall morbidity (adjusted hazard ratio, 2.51 per 1° decrease in PA; 95% confidence interval, 1.32–4.75), and a longer hospital length of stay (adjusted β, 4.8 days per 1° decrease in PA; 95% confidence interval, 1.3–8.2 days).
Conclusions
Low PA is associated with frailty and is predictive of mortality, morbidity, and length of stay after major cardiac surgery. Further work is needed to determine the responsiveness of PA to interventions aimed at reversing frailty.
Keywords: biompedance, cardiac surgery, frailty, phase angle, sarcopenia
Subject Categories: Mortality/Survival, Complications, Aging
Clinical Perspective
What Is New?
Phase angle is strongly associated with frailty and sarcopenia in older patients, and can rapidly be measured at the bedside using biompedance analysis technology.
Our study shows that phase angle predicts postoperative mortality and morbidity in a prospective cohort of 277 patients undergoing major cardiac surgery.
Our study also shows that phase angle is associated with frailty scores as well as multiple components of the frailty syndrome, including physical performance (grip strength and chair rise time) and muscle mass.
What Are the Clinical Implications?
Phase angle is a potential noninvasive marker of frailty that could help enhance preoperative risk stratification in older patients undergoing cardiac surgery.
Introduction
Frailty is a geriatric syndrome that has demonstrated prognostic significance in older adults undergoing cardiac surgery.1 Although numerous clinical scales exist to evaluate frailty and predict outcomes, many scales are time‐consuming to administer, motivating the search for objective biomarkers that can serve as specific indicators of frailty. Bioimpedance is a noninvasive method for body composition assessment that has been extensively validated for evaluation of body composition2, 3, 4, 5, 6 and hydration status7, 8, 9, 10, 11, 12, 13 in patients undergoing cardiac surgery. Phase angle (PA) is a measurement obtained via bioimpedance analysis that reflects the resistance and reactance of the human body in response to the application of an external current.14 PA is strongly associated with sarcopenia15, 16, 17, 18 and frailty18, 19, 20 in geriatric patients, and represents a promising candidate as a noninvasive tool for frailty assessment in the cardiac population.
PA is determined by lean body mass, total body water (TBW), and the ratio of extracellular water (ECW) to TBW (Figure 1). A decrease in muscle mass or an increase in ECW both tend to decrease PA. In cardiac patients, low PA is associated with low muscle mass and handgrip strength.21, 22 In the setting of heart failure, low PA is associated with increased mortality23, 24, 25 and prolonged length of stay (LOS).26 Thus, PA incorporates lean body mass and hydration status into a noninvasive marker that is well suited to the evaluation of cardiac patients. PA can be obtained in <1 minute at the bedside, and contrary to other bioimpedance markers, such as lean and adipose tissue mass, it is measured directly and avoids errors attributable to regression equations.
Figure 1.
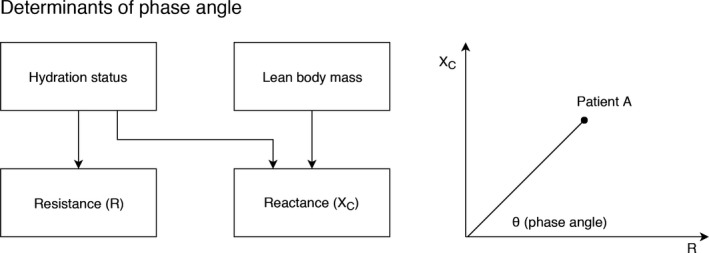
Biological determinants of phase angle. Phase angle is the angle of the vector formed by the body's reactance (XC) and resistance (R). XC (a property of cell membranes) is determined by both lean body mass and hydration status, whereas R is principally determined by the quantity of intracellular and extracellular water found in the body.
Two previous single‐center studies21, 22 have shown that PA is a predictor of postoperative morbidity and prolonged LOS in patients undergoing cardiac surgery. However, one of these studies was limited only to low‐risk patients, and neither reported mortality as an outcome. This study sought to prospectively study the impact of PA on mortality, morbidity, and LOS after cardiac surgery.
Methods
Data Availability
The data, analytic methods, and study materials will be made available on request.
Study Design
The BICS (Bioimpedance in Cardiac Surgery) study consisted of a prospective cohort of consecutive adult patients undergoing major cardiac surgery at the Jewish General Hospital (Montreal, QC, Canada) and the McGill University Health Centre (Montreal, QC, Canada) between December 2014 and May 2017. The study protocol was approved by the Institutional Review Board, and participants gave informed consent. Inclusion criteria were as follows: (1) patients undergoing coronary artery bypass grafting, aortic, mitral, or tricuspid valve replacement or repair, aortic surgery, or a combination of these procedures; (2) bioimpedance testing performed preoperatively; and (3) consent signed to be enrolled in the longitudinal frailty registry. Exclusion criteria were as follows: (1) cardiovascular implantable electronic device (contraindication to bioimpedance testing); (2) clinical instability requiring emergent surgery; and (3) language barrier or severe neuropsychiatric impairment, leading to inability to cooperate with study procedures.
Data Collection
Before surgery, trained research assistants administered the bioimpedance test in addition to a structured questionnaire and physical performance tests focused on frailty. These tests consisted of 5‐m gait speed, 5 timed chair rises, timed standing balance, and handgrip strength.27 Measurements were used to calculate the Short Physical Performance Battery (SPPB) score, the Fried frailty score, and the Mini Nutritional Assessment Short Form score. Additional variables of interest included age, sex, measured height and weight, comorbid conditions, left ventricular ejection fraction, serum hemoglobin, albumin, and creatinine.
Bioimpedance Measurements
Patients underwent bioimpedance testing with the InBody 770 and InBody S10 bioimpedance machines (InBody, Seoul, Korea) while admitted on the cardiovascular ward, on average 2 days before surgery. The InBody 770 machine acquires measurements with the patient in the standing position, and was used in patients who could ambulate; the portable InBody S10 machine was used in patients who were unable to mobilize or stand. Both machines measure PA at 50 kHz and estimate segmental body composition at multiple frequencies. Telemetry units and electrodes were removed for the test, and patients were asked to empty their bladder and remove their jewelry and clothing (except hospital gowns).
Outcome Measures
The primary outcome was all‐cause mortality at the date of last available follow‐up, ascertained from electronic health records, telephone follow‐up with the patients and their families at 6 and 12 months, and additional interrogation of local obituaries. Secondary outcomes were 30‐day all‐cause mortality, postoperative LOS, and composite major morbidity (defined by the Society of Thoracic Surgeons [STS] as reoperation for any cardiac reason, renal failure, deep sternal wound infection, prolonged ventilation, or stroke). Results were adjusted for the STS‐predicted risk of mortality, morbidity, or prolonged LOS.
In addition to models adjusted for STS‐predicted risk, sensitivity analyses were conducted adjusting for 15 covariates that have been shown to be the most important risk factors for long‐term mortality and morbidity after cardiac surgery. The covariates were as follows: age, sex, body surface area, diabetes mellitus, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral arterial disease, creatinine, left ventricular ejection fraction, heart failure within 2 weeks, myocardial infarction, left main or multivessel coronary artery disease, prior cardiac surgical procedures, surgery status (urgent or elective), and type of cardiac surgery performed.
Statistical Analyses
Descriptive statistics are presented as mean±SD, and were grouped by PA tertiles for tabular display. Distributive histograms were used to assess normality of the predictor and outcome variables. PA was treated as a continuous variable. A Pearson correlation matrix was used to define the association of PA with anthropometric data, frailty markers, and nutritional markers. Cuzick's test for trend was used to compare baseline characteristics and outcomes across tertiles of PA.28
After adjustment for STS predicted mortality, Cox regression was used to assess long‐term survival, and was presented using Kaplan‐Meier curves. Logistic regression analysis was performed to determine the association between PA and early mortality as well as morbidity after adjustment for STS‐predicted risk. Harell's C‐statistics were computed to assess the incremental value of adding PA to prediction models. Cutoffs for PA were obtained on the basis of the optimal balance between sensitivity and specificity in receiver‐operating characteristic curve analysis, by maximization of Youden's statistic.
Multiple linear regression analysis was performed to assess the association between PA and hospital LOS after adjustment for STS‐predicted long LOS. Residuals were assessed for normality using kernel density plots.
Model fit was assessed by comparing the C‐statistic before and after the addition of PA as a predictor. Model generalizability was evaluated using 10‐fold cross‐validation. All statistical analyses were performed using STATA, version 14.0 (StataCorp, College Station, TX).
Results
Of 405 patients enrolled in the longitudinal frailty registry between December 2014 and May 2017, a total of 277 had preoperative bioimpedance data available for analysis, of which 69 (25%) were women and 208 (75%) were men. The median age was 72 years (interquartile range [IQR], 66–83 years), and the median STS‐predicted risk of mortality was 1.3% (IQR, 0.8%–2.4%). The type of procedure was isolated coronary artery bypass grafting in 175 patients, valve surgery in 55 patients, and combined surgeries in 47 patients. Measurements were performed with the InBody 770 machine in 222 patients and with the InBody S10 machine in 55 patients. Patients who were excluded (n=128) on the basis of not having had bioimpedance testing were on average slightly older (median age, 74 years; IQR, 70–78 years), more likely to be women (37.5% women), and had a higher STS‐predicted mortality (2.29%; IQR, 1.25%–4.30%).
PA was normally distributed, as illustrated in Figure 2, with a mean of 5.1±0.9°, a lower tertile of 4.5°, and an upper tertile of 5.4°. Mean PA did not differ significantly according to the type of machine used for measurements. The baseline characteristics of the study population by PA tertile are presented in Table 1. Patients with lower PA were older, with a higher proportion of women and a lower body mass index. Patients with lower PA had higher rates of chronic kidney disease, past or current malignancy, and anemia (P<0.01), but a lower rate of coronary artery disease (P<0.01). Rates of hypertension, diabetes mellitus, stroke, heart failure, chronic pulmonary disease, and cirrhosis did not differ according to PA tertile.
Figure 2.
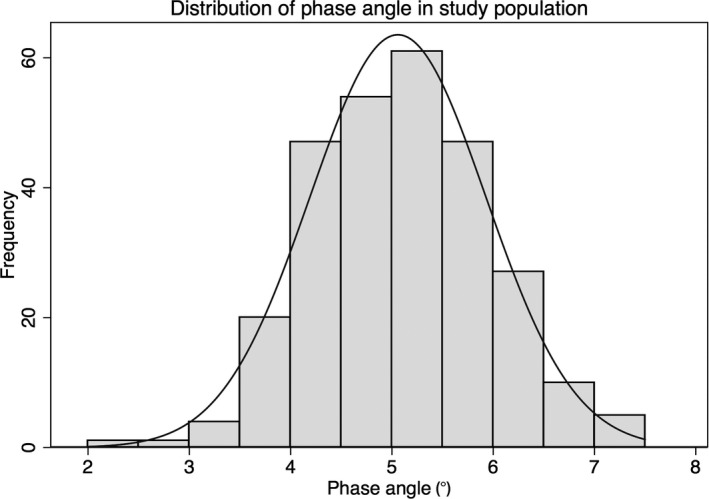
Distribution of phase angle in study population. The mean phase angle in our study population was 5.1±0.9°, with a lower tertile of 4.5° and an upper tertile of 5.4°.
Table 1.
Baseline Characteristics of the Study Population According to PA Tertile
| Characteristics | Total | PA ≤4.5° | PA 4.6°–5.5° | PA ≥5.6° | P Value |
|---|---|---|---|---|---|
| PA, ° | 5.1±0.9 | 4.1±0.41 | 5.0±0.3 | 6.1±0.43 | NA |
| Age, y | 71±8 | 75±7 | 73±6 | 65±7.3 | <0.001 |
| Female sex | 69 (25) | 40 (45) | 25 (24) | 4 (5) | <0.001 |
| Body mass index, kg/m2 | 28±5 | 27±6 | 28±5 | 29±5 | <0.001 |
| Body surface area, m2 | 1.9±0.2 | 1.8±0.2 | 1.9±0.2 | 2±0.2 | <0.001 |
| Comorbid conditions | |||||
| Heart failure | 56 (20) | 20 (22) | 22 (21) | 14 (17) | 0.36 |
| Coronary artery disease | 231 (83) | 64 (72) | 91 (87) | 76 (92) | <0.001 |
| Recent myocardial infarction | 56 (20) | 16 (18) | 25 (24) | 15 (18) | 0.97 |
| Atrial fibrillation | 44 (16) | 19 (21) | 16 (15) | 9 (11) | 0.06 |
| Diabetes mellitus | 94 (34) | 31 (35) | 39 (37) | 24 (29) | 0.42 |
| Hypertension | 204 (74) | 65 (73) | 79 (75) | 60 (72) | 0.92 |
| Stroke | 19 (7) | 8 (9) | 5 (5) | 6 (7) | 0.63 |
| Peripheral arterial disease | 29 (10) | 7 (8) | 14 (13) | 8 (10) | 0.69 |
| Chronic kidney disease | 78 (28) | 39 (44) | 25 (24) | 14 (17) | <0.001 |
| Cirrhosis | 5 (2) | 2 (2) | 1 (1) | 2 (2) | 0.95 |
| Past or current malignancy | 29 (10) | 16 (18) | 10 (10) | 3 (4) | 0.002 |
| Dementia | 4 (1) | 3 (3) | 1 (1) | 0 (0) | 0.06 |
| Echocardiographic parameters | |||||
| Ejection fraction, % | 54±13 | 54±13 | 52±14 | 56±12 | 0.4 |
| Laboratory parameters | |||||
| Hemoglobin, g/L | 130±16 | 122±16 | 132±15.6 | 138±15 | <0.001 |
| Creatinine, μmol/L | 93±40 | 98±60 | 89±26 | 92±29 | 0.6 |
Data are given as mean±SD or number (percentage). NA indicates not applicable; PA, phase angle.
Markers of body composition, frailty, and malnutrition by PA tertile are presented in Table 2, and pairwise correlations are shown in Table 3. Patients with a lower PA had lower fat‐free mass and a higher ECW/TBW ratio (P<0.001). Patients with lower PA had higher degrees of frailty, as measured by the SPPB and Fried scales, and had lower grip strength, slower gait speed, and longer chair rise times (P<0.01). PA was not associated with nutritional status, as assessed by albumin or by the Mini Nutritional Assessment Short Form scale.
Table 2.
Body Composition, Frailty, and Nutritional Markers According to PA Tertile
| Characteeristics | Total | PA ≤4.5° | PA 4.6°–5.5° | PA ≥5.6° | P Value |
|---|---|---|---|---|---|
| Bioimpedance Parameters | |||||
| PA, ° | 5.1±0.9 | 4.1±0.4 | 5.1±0.3 | 6.1±0.4 | <0.001 |
| ECW/TBW ratio | 0.39±0.01 | 0.40±0.01 | 0.39±0.01 | 0.38±0.01 | <0.001 |
| Fat‐free mass, kg | 54.7±10.6 | 49.9±10.8 | 53.6±8.5 | 61.2±9.7 | <0.001 |
| Fat mass, kg | 25.5±11.1 | 25.1±12 | 25.5±10.7 | 25.9±10.8 | 0.68 |
| Frailty markers/scores | |||||
| SPPB score | 8.8±2.3 | 7.8±2.6 | 9±2.1 | 9.7±1.8 | <0.001 |
| Fried score | 1.1±1.1 | 1.6±1.2 | 0.8±0.9 | 0.8±0.9 | <0.001 |
| Grip strength, kg | 33.6±10.6 | 26.6±9.5 | 33.8±8.3 | 40.9±9.5 | <0.001 |
| Gait speed, m/s | 1±0.3 | 0.9±0.3 | 1±0.3 | 1±0.3 | 0.002 |
| Chair rise time, s | 20±14.2 | 25.5±18.1 | 18.5±12 | 15.8±9.4 | <0.001 |
| Nutritional markers | |||||
| MNA‐SF score | 12±2 | 11.6±2.4 | 12.4±1.7 | 11.9±1.8 | 0.65 |
| Albumin, g/L | 39±4 | 38±5 | 39.5±4 | 39±4 | 0.53 |
Data are given as mean±SD. ECW indicates extracellular water; MNA‐SF, Mini Nutritional Assessment Short Form; PA, phase angle; SPPB, Short Physical Performance Battery; TBW, total body water.
Table 3.
Correlations Between Body Composition, Frailty, and Nutritional Markers
| Variable | PA | ECW/TBW | Fat‐Free Mass | Fat Mass |
|---|---|---|---|---|
| PA | 1 | ··· | ··· | ··· |
| ECW/TBW | −0.79b | 1 | ··· | ··· |
| Fat‐free mass | 0.39b | −0.12a | 1 | ··· |
| Fat mass | 0.05 | 0.06 | 0.08 | 1 |
| SPPB score | 0.33b | −0.36b | 0.11 | −0.14a |
| Fried score | −0.31b | 0.37b | −0.19b | 0.14a |
| EFT score | −0.32b | 0.38b | −0.07 | −0.02 |
| Grip strength | 0.52b | −0.36b | 0.66b | −0.15a |
| Gait speed | 0.19b | −0.25b | 0.19b | −0.17b |
| Chair rise time | −0.32b | 0.34b | −0.04 | 0.02 |
| MNA‐SF score | 0.13a | −0.13a | 0.1 | 0.14a |
ECW indicates extracellular water; EFT, Essential Frailty Toolset; MNA‐SF, Mini Nutritional Assessment Short Form; PA, phase angle; SPPB, Short Physical Performance Battery; TBW, total body water.
P<0.05.
P<0.01.
Over a median follow‐up period of 369 days (IQR, 198–401 days), a total of 19 patients (7%) died. A total of 70 patients (25%) experienced a STS composite early safety event during the in‐hospital period. Postoperative outcomes by PA tertile are presented in Table 4. Patients with lower PA had a higher incidence of death, major adverse cardiac event, acute kidney injury, bleeding, and delirium, and a longer LOS. Patients with lower PA had higher rates of discharge to a rehabilitation facility or nursing home, as well as higher rates of readmission at 1 year.
Table 4.
Postoperative Outcomes According to PA Tertile
| Outcome | Total | PA ≤4.5° | PA 4.6°–5.5° | PA ≥5.6° | P Value |
|---|---|---|---|---|---|
| STS predicted risk | |||||
| Mortality, % | 1.9±1.9 | 3.0±2.6 | 1.8±1.3 | 1.0±0.7 | <0.001 |
| Morbidity or mortality, % | 14.3±8.1 | 18.6±10.2 | 13.9±6.3 | 10.3±4.5 | <0.001 |
| Long length of stay, % | 6.1±4.7 | 8.7±6.0 | 5.8±3.7 | 3.7±2.2 | <0.001 |
| Postoperative outcomes | |||||
| ICU length of stay, h | 57±119 | 76±171 | 61±111 | 34±35 | <0.001 |
| Hospital length of stay, d | 13±23 | 21±37 | 10±11 | 9±6 | <0.001 |
| Discharge to rehabilitation or nursing home | 64 (23) | 34 (38) | 21 (20) | 9 (11) | <0.001 |
| Death at 1 mo | 10 (4) | 7 (8) | 3 (3) | 0 (0) | 0.006 |
| Death at 12 mo | 16 (9) | 11 (19) | 5 (7) | 0 (0) | <0.001 |
| Readmission at 1 mo | 38 (14) | 15 (18) | 12 (12) | 11 (13) | 0.43 |
| Readmission at 12 mo | 39 (26) | 19 (43) | 10 (17) | 10 (20) | 0.02 |
| STS composite major morbidity | 70 (25) | 33 (37) | 28 (27) | 9 (11) | <0.001 |
| Postoperative complications | |||||
| Stroke | 4 (1) | 1 (1) | 1 (1) | 2 (2) | 0.49 |
| Sepsis | 6 (2) | 4 (4) | 1 (1) | 1 (1) | 0.13 |
| Prolonged ventilation | 34 (12) | 15 (17) | 13 (12) | 6 (7) | 0.06 |
| Acute kidney injury | 58 (21) | 27 (30) | 21 (20) | 10 (12) | 0.003 |
| Dialysis | 3 (1) | 3 (3) | 0 (0) | 0 (0) | 0.03 |
| Myocardial infarction | 4 (1) | 2 (2) | 2 (2) | 0 (0) | 0.22 |
| Bleeding | 59 (21) | 26 (29) | 24 (23) | 9 (11) | 0.003 |
| Vascular complication | 12 (4) | 7 (8) | 3 (3) | 2 (2) | 0.08 |
| Atrial fibrillation | 90 (32) | 30 (34) | 36 (34) | 24 (29) | 0.51 |
| Cardiac arrest | 10 (4) | 5 (6) | 4 (4) | 1 (1) | 0.12 |
| Delirium | 56 (23) | 25 (32) | 20 (21) | 11 (14) | 0.007 |
Data are given as mean±SD or number (percentage). ICU indicates intensive care unit; PA, phase angle; STS, Society of Thoracic Surgeons.
In the logistic regression model adjusted for STS‐predicted risk of mortality, lower PA was associated with increased mortality at 1 month (adjusted odds ratio [OR], 3.57 per 1° decrease in PA; 95% confidence interval [CI], 1.35–9.47) and at 12 months (adjusted OR, 3.03 per 1° decrease in PA; 95% CI, 1.30–7.09). Kaplan‐Meier survival analysis is presented in Figure 3. Adjusting for STS‐predicted risk of major morbidity or mortality, lower PA was associated with an increased risk of experiencing an STS composite major morbidity event (adjusted OR, 1.74 per 1° decrease in PA; 95% CI, 1.19–2.58). In the Cox proportional hazards model, lower PA was associated with increased all‐cause mortality (adjusted hazard ratio, 2.51 per 1° decrease in PA; 95% CI, 1.32–4.75).
Figure 3.
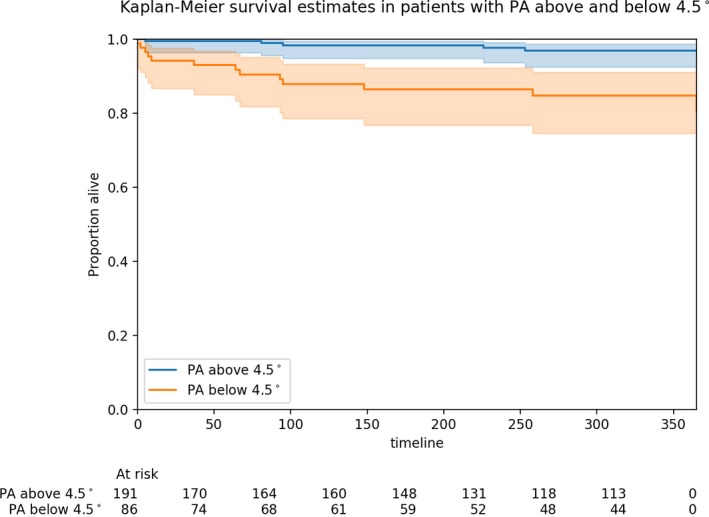
Kaplan‐Meier survival estimates in patients with phase angle (PA) above (blue line) and below (orange line) the lowest tertile (4.5°). Shaded areas depict 95% confidence intervals.
The median hospital LOS was 7 days (IQR, 6–11.5 days). In the linear regression model adjusted for STS‐predicted risk of prolonged LOS, lower PA was associated with longer LOS (adjusted β, 4.8 days per 1° decrease in PA; 95% CI, 1.3–8.2 days).
Receiver‐Operating Characteristic Curve Analysis
The addition of PA to the STS risk score was associated with an increase in the C‐statistic for prediction of mortality at 1 month from 0.78 to 0.84, for mortality at 12 months from 0.77 to 0.84 (Figure 4), and for morbidity from 0.67 to 0.69. Receiver operating curve analysis determined that a PA cutoff of <4.5° was associated with optimal predictive value for mortality at 12 months.
Figure 4.
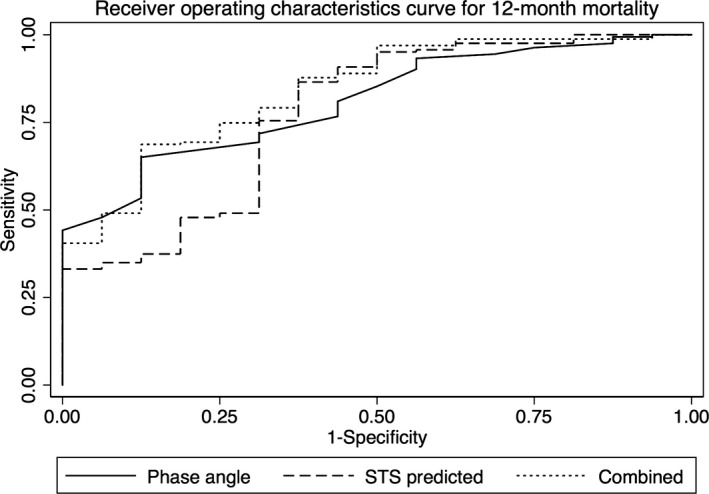
Receiver operating characteristic curve for 12‐month mortality. Receiving operating characteristic curve for prediction of 12‐month mortality using phase angle (solid line), compared with the Society of Thoracic Surgeons (STS) risk score (dashed line) and the combination of both phase angle and the STS risk score (dotted line).
Sensitivity Analyses
In logistic regression models adjusted for a wider panel of individual risk factors, PA remained predictive of our outcomes of interest (Table S1). When adjusting for frailty (as measured by SPPB) in addition to STS‐predicted risk, PA was slightly attenuated but remained an independent predictor of mortality at 1 month (adjusted OR, 2.98; 95% CI, 1.09–8.10) and at 12 months (OR, 2.72; 95% CI, 1.19–6.24). Furthermore, there was no evidence of interaction between PA and the type of cardiac surgery performed.
Discussion
To our knowledge, this is the first study to show that bioimpedance PA predicts early and midterm mortality after major cardiac surgery. Every 1° decrease in PA conferred an ≈3‐fold higher risk of death at 12 months. PA was specifically correlated with muscle mass, muscle strength, and frailty scales, demonstrating its potential utility as a biomarker for frailty. Low PA was predictive of resource use, as evidenced by a higher likelihood of prolonged LOS, discharge to specialized facilities, and need for readmission. Consistent with previous studies, low PA was predictive of postoperative morbidity.21, 22 Notably, PA provided similar predictive value when compared with the STS risk score, provided incremental predictive value when added to it, and remained independently predictive in sensitivity analyses adjusting for 15 known predictors of mortality after cardiac surgery.
In our patient population, a PA cutoff of <4.5° had the highest predictive value for mortality at 12 months. This cutoff coincided with the lowest tertile of PA (<4.5°). These values are in keeping with previous estimates showing that the mean PA in frail geriatric patients is ≈4.5° in women and 4.6° in men,29, 30 and that the optimal PA cutoff for detection of sarcopenia is 4.5°.17 By contrast, the mean PA in healthy geriatric patients has previously been reported as 6.2° in men and 5.6° in women. Thus, a PA cutoff of <4.5° may be used to identify a subgroup of frail older adults at high risk for adverse outcomes after cardiac surgery.
Low PA was associated with higher odds of physical frailty, as assessed by the SPPB scale, as well as low skeletal muscle mass and decreased handgrip strength. This finding reinforces the notion that a low PA is associated with sarcopenia, a key biological substrate of the frailty syndrome that results from accelerated age‐related loss of skeletal muscle mass and strength.31 PA has previously been shown to correlate with biomarkers of muscle degeneration32 and to decrease after muscle injury,33, 34, 35 supporting the biological plausibility of a link between decreased PA and not only low muscle mass, but also impaired muscle function. More important, the addition of low PA to risk models that included the SPPB demonstrated incremental prognostic value, highlighting the complementary relationship between PA and existing frailty scales. In patients unable to complete performance‐based frailty scales, such as those who are acutely ill or nonmobile, PA can still be used to gain information about the patient's level of frailty.
Although PA is commonly purported to be a marker of nutritional status, there was no significant association between PA and nutritional screening tools or hypoalbuminemia in our study. Despite being more prevalent in malnourished patients, a low PA has been shown to have poor specificity for predicting malnutrition.36, 37 Previous studies showed only a borderline significant correlation between PA and Mini Nutritional Assessment Short Form score and no correlation with the Malnutrition Universal Screening Tool score.29 The low prevalence of malnutrition in our population (3% as defined by Mini Nutritional Assessment Short Form ≤7) and the short‐form screening tool used may have limited the power of this study to detect a significant association with PA.
Consistent with radioisotope dilution experiments, a strong correlation was identified between PA and bioimpedance estimates of the ECW/TBW ratio (R=−0.79), with a higher ECW/TBW ratio corresponding to a lower PA.38 However, rates of heart failure were not different according to PA tertile, and although a trend toward higher rates of chronic kidney disease was observed in the lowest PA tertile group, the difference in mean creatinine clearance between the lowest and highest tertiles was marginal (98±60 versus 92±29 mL/min). Thus, the prognostic significance of PA in our population is unlikely to be solely reflective of renal or heart failure, reiterating the added value of capturing lean body mass in addition to hydration status in a single risk marker.
Beyond the prognostic value of PA in patients undergoing cardiac surgery, PA has the potential to be actionable and responsive to therapeutic interventions that target frailty and sarcopenia. Preliminary studies suggest that PA may decrease in response to undernutrition,39 and that oral nutritional supplementation can improve PA.40 Similarly, it has been shown that PA decreases after physical inactivity, and that physical training can restore PA toward normal values.41 In patients with decompensated heart failure, PA increases in response to diuretic treatment.42 Taken together, these findings suggest that PA is a dynamic marker that can potentially be targeted using interventions to restore adequate nutritional status, increase physical activity, and optimize fluid status.
The results of our study must be interpreted considering the following limitations. First, the total number of deaths in our cohort was small. Nevertheless, the effect on mortality persisted at all time points of the study and after adjusting for predicted mortality using the STS risk score. Sensitivity analyses adjusting for malnutrition and frailty scales as potential confounders did not negate results for primary or secondary outcomes. Second, our cohort consisted of a mostly male population, and although PA is known to be lower in women than in men,43 it is not known whether there is effect modification by sex. Third, patients who were clinically unstable could not undergo bioelectrical impedance analysis testing, limiting the generalizability to this group (as reflected by the slightly higher age and STS‐predicted risk of excluded patients). However, a portable bioimpedance machine was used to enable testing on patients who could not stand. Because sicker patients are expected to have a lower PA, exclusion of these patients would result in conservative estimates of the effect of PA on outcomes. Fourth, although the multifrequency bioelectrical impedance analysis machine used was fairly costly (and hence less accessible), less costly single‐frequency bioelectrical impedance analysis machines can be used to measure PA with equal accuracy. Sensitivity analyses performed using single‐frequency upper extremity PA in our data set yielded similar results to whole body PA. Finally, bioimpedance machines are validated for measurement of body composition at steady state, and the validity of results may be affected by changes in water distribution resulting from acute illness. To mitigate this potential source of bias, bioimpedance measurements were performed after initial stabilization of patients. In addition, because PA is directly measured rather than predicted from regression equations, the validity of measurements does not depend on assumptions about the constancy of body composition.44
Conclusion
The BICS study has identified bioimpedance PA as a novel biomarker for frailty in patients undergoing cardiac surgery. PA is potentially applicable at the point of care to supplement the assessment of frailty in older adults. Low PA (<4.5°) is incrementally predictive of mortality, morbidity, and LOS after major cardiac surgery. Further work is needed to better delineate the physiological determinants of PA in cardiac patients, and to investigate the responsiveness of this marker to interventions aimed at reversing frailty.
Sources of Funding
Dr Afilalo is supported by the Fonds de Recherche du Québec en Santé, Heart and Stroke Foundation of Canada, and Canadian Institutes of Health Research.
Disclosures
None.
Supporting information
Table S1. Odds of Mortality, Morbidity and Prolonged LOS According to Phase Angle and Known Predictors of Mortality Following Cardiac Surgery
Acknowledgments
We thank the members of the Division of Cardiac Surgery at the Jewish General Hospital (Dr Jean‐Francois Morin, Dr Yves Langlois, Dr Felix Ma, and Dr Moss) and the Royal Victoria Hospital (Dr Renzo Cecere, Dr Benoit DeVarennes, Dr Patrick Ergina, Dr Kevin Lachapelle, Dr Dominique Shum‐Tim, and Dr Christo Tchervenkov) for their respective contributions to patient recruitment.
(J Am Heart Assoc. 2018;7:e008721 DOI: 10.1161/JAHA.118.008721.)
References
- 1. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 2. Boban M, Peršić V, Zulj M, Petricević M, Kramarić RP, Vcev A. Bioelectrical impedance analysis offers clinically relevant appraisal of body composition, but fails to recognize nutritional risk or differences between surgery and percutaneous coronary intervention treatments: a non‐randomized cohort. Coll Antropol. 2014;38:979–985. [PubMed] [Google Scholar]
- 3. Visser M, van Venrooij LM, Vulperhorst L, de Vos R, Wisselink W, van Leeuwen PA, de Mol BA. Sarcopenic obesity is associated with adverse clinical outcome after cardiac surgery. Nutr Metab Cardiovasc Dis. 2013;23:511–518. [DOI] [PubMed] [Google Scholar]
- 4. Van venrooij LM, De Vos R, Zijlstra E, Borgmeijer‐hoelen MM, Van leeuwen PA, De Mol BA. The impact of low preoperative fat‐free body mass on infections and length of stay after cardiac surgery: a prospective cohort study. J Thorac Cardiovasc Surg. 2011;142:1263–1269. [DOI] [PubMed] [Google Scholar]
- 5. Boban M, Barisic M, Persic V, Zekanovic D, Medved I, Zulj M, Vcev A. Muscle strength differ between patients with diabetes and controls following heart surgery. J Diabetes Complications. 2016;30:1287–1292. [DOI] [PubMed] [Google Scholar]
- 6. Nakajima M, Morishita S, Yuguchi S, Saito K, Matsuo T, Yoshimura K, Ujikawa T, Otsuka S, Hojo Y, Ishihara K, Kochi Y, Harada K, Sakaguchi T, Yoshitaka H. Relationships between changes in the skeletal muscle mass index and number of days needed for independent gait after cardiac surgery. Physiotherapy. 2015;101:1069. [Google Scholar]
- 7. Patel RV, Peterson EL, Silverman N, Zarowitz BJ. Estimation of total body and extracellular water in post‐coronary artery bypass graft surgical patients using single and multiple frequency bioimpedance. Crit Care Med. 1996;24:1824–1828. [DOI] [PubMed] [Google Scholar]
- 8. Bracco D, Revelly JP, Berger MM, Chioléro RL. Bedside determination of fluid accumulation after cardiac surgery using segmental bioelectrical impedance. Crit Care Med. 1998;26:1065–1070. [DOI] [PubMed] [Google Scholar]
- 9. Yamaguchi H, Yamauchi H, Hazama S, Hamamoto H. Evaluation of body fluid status after cardiac surgery using bioelectrical impedance analysis. J Cardiovasc Surg (Torino). 2000;41:559–566. [PubMed] [Google Scholar]
- 10. Stellato D, Cirillo M, De Santo LS, Anastasio P, Frangiosa A, Cotrufo M, De Santo NG, Di Iorio B. Bioelectrical impedance analysis in heart transplantation: early and late changes. Semin Nephrol. 2001;21:282–285. [DOI] [PubMed] [Google Scholar]
- 11. Perko MJ, Jarnvig IL, Højgaard‐rasmussen N, Eliasen K, Arendrup H. Electric impedance for evaluation of body fluid balance in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001;15:44–48. [DOI] [PubMed] [Google Scholar]
- 12. Bottoni A, Marco D, Oliveira GP, Bottoni A, Da silva Mde L, Waitzberg DL. Resistance and reactance in patients undergoing coronary artery bypass. Nutr Hosp. 2003;18:147–152. [PubMed] [Google Scholar]
- 13. Slight RD, Demosthenous N, Nzewi OC, Soliman AR, McClelland DB, Mankad PS. The effect of gain in total body water on haemoglobin concentration and body weight following cardiac surgery. Heart Lung Circ. 2006;15:256–260. [DOI] [PubMed] [Google Scholar]
- 14. Valle R, Aspromonte N, Milani L, Peacock FW, Maisel AS, Santini M, Ronco C. Optimizing fluid management in patients with acute decompensated heart failure (ADHF): the emerging role of combined measurement of body hydration status and brain natriuretic peptide (BNP) levels. Heart Fail Rev. 2011;16:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marini E, Buffa R, Saragat B, Coin A, Toffanello ED, Berton L, Manzato E, Sergi G. The potential of classic and specific bioelectrical impedance vector analysis for the assessment of sarcopenia and sarcopenic obesity. Clin Interv Aging. 2012;7:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basile C, Della‐Morte D, Cacciatore F, Gargiulo G, Galizia G, Roselli M, Curcio F, Bonaduce D, Abete P. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp Gerontol. 2014;58:43–46. [DOI] [PubMed] [Google Scholar]
- 17. Kilic MK, Kizilarslanoglu MC, Arik G, Bolayir B, Kara O, Dogan Varan H, Sumer F, Kuyumcu ME, Halil M, Ulger Z. Association of bioelectrical impedance analysis‐derived phase angle and sarcopenia in older adults. Nutr Clin Pract. 2017;32:103–109. [DOI] [PubMed] [Google Scholar]
- 18. Navigante A, Morgado PC, Casbarien O, Delgado NL, Giglio R, Perman M. Relationship between weakness and phase angle in advanced cancer patients with fatigue. Support Care Cancer. 2013;21:1685–1690. [DOI] [PubMed] [Google Scholar]
- 19. Wilhelm‐leen ER, Hall YN, Horwitz RI, Chertow GM. Phase angle, frailty and mortality in older adults. J Gen Intern Med. 2014;29:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delgado C, Doyle JW, Johansen KL. Association of frailty with body composition among patients on hemodialysis. J Ren Nutr. 2013;23:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ringaitiene D, Gineityte D, Vicka V, Zvirblis T, Norkiene I, Sipylaite J, Irnius A, Ivaskevicius J. Malnutrition assessed by phase angle determines outcomes in low‐risk cardiac surgery patients. Clin Nutr. 2016;35:1328–1332. [DOI] [PubMed] [Google Scholar]
- 22. Visser M, van Venrooij LM, Wanders DC, de Vos R, Wisselink W, van Leeuwen PA, de Mol BA. The bioelectrical impedance phase angle as an indicator of undernutrition and adverse clinical outcome in cardiac surgical patients. Clin Nutr. 2012;31:981–986. [DOI] [PubMed] [Google Scholar]
- 23. Alves FD, Souza GC, Clausell N, Biolo A. Prognostic role of phase angle in hospitalized patients with acute decompensated heart failure. Clin Nutr. 2016;35:1530–1534. [DOI] [PubMed] [Google Scholar]
- 24. Colín‐Rramírez E, Castillo‐Martínez L, Orea‐Tejeda A, Vázquez‐Durán M, Rodríguez AE, Keirns‐davis C. Bioelectrical impedance phase angle as a prognostic marker in chronic heart failure. Nutrition. 2012;28:901–905. [DOI] [PubMed] [Google Scholar]
- 25. Kastyro JV, Hassib AA. Predictive value of bioelectrical impedance analysis for prognosis of survival rate in arterial hypertension patients with heart failure. Eur J Prev Cardiol. 2016;23:S17. [Google Scholar]
- 26. Doesch C, Suselbeck T, Leweling H, Fluechter S, Haghi D, Schoenberg SO, Borggrefe M, Papavassiliu T. Bioimpedance analysis parameters and epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure. Obesity (Silver Spring). 2010;18:2326–2332. [DOI] [PubMed] [Google Scholar]
- 27. Ling CH, Taekema D, De craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85‐plus study. CMAJ. 2010;182:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cuzick J. A Wilcoxon‐type test for trend. Stat Med. 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 29. Slee A, Birc D, Stokoe D. Bioelectrical impedance vector analysis, phase‐angle assessment and relationship with malnutrition risk in a cohort of frail older hospital patients in the United Kingdom. Nutrition. 2015;31:132–137. [DOI] [PubMed] [Google Scholar]
- 30. Barbosa‐silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. [DOI] [PubMed] [Google Scholar]
- 31. Afilalo J. Conceptual models of frailty: the sarcopenia phenotype. Can J Cardiol. 2016;32:1051–1055. [DOI] [PubMed] [Google Scholar]
- 32. Scherbakov N, Knops M, Ebner N, Valentova M, Sandek A, Grittner U, Dahinden P, Hettwer S, Schefold JC, von Haehling S, Anker SD, Joebges M, Doehner W. Evaluation of C‐terminal Agrin Fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J Cachexia Sarcopenia Muscle. 2016;7:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell‐ferrer J, Rodas G. Localized bioimpedance to assess muscle injury. Physiol Meas. 2013;34:237–245. [DOI] [PubMed] [Google Scholar]
- 34. Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell‐ferrer J, Rodas G. Effects of muscle injury severity on localized bioimpedance measurements. Physiol Meas. 2015;36:27–42. [DOI] [PubMed] [Google Scholar]
- 35. Nescolarde L, Yanguas J, Terricabras J, Lukaski H, Alomar X, Rosell‐Ferrer J, Rodas G. Detection of muscle gap by L‐BIA in muscle injuries: clinical prognosis. Physiol Meas. 2017;38:L1–L9. [DOI] [PubMed] [Google Scholar]
- 36. Varan HD, Bolayir B, Kara O, Arik G, Kizilarslanoglu MC, Kilic MK, Sumer F, Kuyumcu ME, Yesil Y, Yavuz BB, Halil M, Cankurtaran M. Phase angle assessment by bioelectrical impedance analysis and its predictive value for malnutrition risk in hospitalized geriatric patients. Aging Clin Exp Res. 2016;28:1121–1126. [DOI] [PubMed] [Google Scholar]
- 37. Scheunemann L, Wazlawik E, Bastos JL, Ristow Cardinal T, Mayumi Nakazora L. Agreement and association between the phase angle and parameters of nutritional status assessment in surgical patients. Nutr Hosp. 2011;26:480–487. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez MC, Barbosa‐silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016;103:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caccialanza R, Cereda E, Klersy C, Bonardi C, Cappello S, Quarleri L, Turri A, Montagna E, Iacona I, Valentino F, Pedrazzoli P. Phase angle and handgrip strength are sensitive early markers of energy intake in hypophagic, non‐surgical patients at nutritional risk, with contraindications to enteral nutrition. Nutrients. 2015;7:1828–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Norman K, Kirchner H, Freudenreich M, Ockenga J, Lochs H, Pirlich M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non‐neoplastic gastrointestinal disease: a randomized controlled trial. Clin Nutr. 2008;27:48–56. [DOI] [PubMed] [Google Scholar]
- 41. Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Changes in phase angle and body composition induced by resistance training in older women. Eur J Clin Nutr. 2016;70:1408–1413. [DOI] [PubMed] [Google Scholar]
- 42. Alves FD, Souza GC, Aliti GB, Rabelo‐silva ER, Clausell N, Biolo A. Dynamic changes in bioelectrical impedance vector analysis and phase angle in acute decompensated heart failure. Nutrition. 2015;31:84–89. [DOI] [PubMed] [Google Scholar]
- 43. Kumar S, Dutt A, Hemraj S, Bhat S, Manipadybhima B. Phase angle measurement in healthy human subjects through bio‐impedance analysis. Iran J Basic Med Sci. 2012;15:1180–1184. [PMC free article] [PubMed] [Google Scholar]
- 44. Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20:330–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Odds of Mortality, Morbidity and Prolonged LOS According to Phase Angle and Known Predictors of Mortality Following Cardiac Surgery
Data Availability Statement
The data, analytic methods, and study materials will be made available on request.


