Abstract
Background
Atrial fibrillation (AF) and cancer are frequent diseases worldwide. The timewise association between the diagnosis of AF and a subsequent diagnosis of cancer may clarify whether a mutual cause exists, and may also guide clinicians about time windows of high risk of cancer occurrence.
Methods and Results
We conducted a population‐based cohort study among 26 222 men and 28 879 women free of AF and cancer at baseline based on the Danish Diet, Cancer and Health study. The participants were followed for the development of AF (the Danish National Patient Registry) and subsequent cancer (the Danish Cancer Registry) until 2013. We used Cox proportional hazard models with new‐onset AF as time‐dependent exposure. The men (median age 56 years) and women (median age 56 years) were followed for medians of 16.7 and 19.6 years, respectively. AF was associated with higher risks of any type of cancer (men: hazard ratio [HR] 1.41, 95% confidence interval [CI], 1.26–1.58; women: HR 1.15, 95% CI, 1.02–1.32), and for men only, lung (HR 1.66, 95% CI, 1.19–2.30), and colorectal cancer (HR 1.37, 95% CI, 1.02–1.85). Within the initial 90 days following the diagnosis of AF, the risks of any type of cancer (men: HR 2.89, 95% CI, 2.10–3.98; women: HR 3.72, 95% CI, 2.49–5.56), lung (men: HR 7.70, 95% CI, 4.34–13.68; women: HR 7.98, 95% CI, 3.96–16.09), and colorectal cancer (men: HR 3.35, 95% CI, 1.03–10.90; women: HR 5.91, 95% CI, 2.44–14.29) were higher for men and women.
Conclusions
A diagnosis of AF is associated with a higher incidence rate of cancer among men and women. The cancer incidence rate is particularly elevated within 90 days after the diagnosis of AF.
Keywords: atrial fibrillation, cancer and stroke, epidemiology, men, women
Subject Categories: Atrial Fibrillation, Epidemiology, Women, Risk Factors
Clinical Perspective
What Is New?
A new‐onset diagnosis of atrial fibrillation (AF) is associated with a subsequent increased risk of being diagnosed with cancer.
The risk of any type of cancer, colorectal cancer, and lung cancer is particularly high within the initial 90 days following the diagnosis of AF.
Metastatic cancer is likely to be present at the time of diagnosis of AF.
What Are the Clinical Implications?
As shared risk factors may link cardiovascular disease with cancer, it may be worthwhile to be vigilant in patients with AF who have risk factors for cancer such as obesity and smoking.
Our findings do not support recommendations for using new‐onset AF as an indication for systematic cancer screening.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia worldwide, with an increasing incidence rate and prevalence among men and women.1 The lifetime risk is 1 in 4 according to previous studies,2, 3 and more than 1 in 3 based on recent data.4 Several risk factors have been identified, many of which are also risk factors for several types of cancer, such as age, smoking, alcohol consumption, and obesity.5, 6 As shared risk factors link general cardiovascular disease with cancer,6, 7 the overlap between AF and cancer may constitute a mutual cause. However, the identification of a mutual cause is challenging because the association between AF and cancer seems very complex and may include bidirectional interrelations of several factors, many of which are not causal.8
Gaining insight into the relationship between AF and cancer is of public health relevance. The global cancer burden is expected to increase,9 and a recent study from Great Britain has reported the lifetime risk to be 1 in 2 among individuals born since 1960.10 Furthermore, the emotional distress and economic cost of cancers are immense and most likely rising.11 However, only 2 cohort studies have so far examined the association between AF and the risk of subsequent cancer. A Danish nationwide registry‐based cohort study by Ostenfeld et al suggested that occult cancer was likely to be present at the time of the diagnosis of AF.12 Recently, Conen et al reported an association in a population‐based cohort study of female Americans from the Women's Health Study.13 This association needs further substantiation among both men and women because the timewise association between the diagnosis of AF and a subsequent diagnosis of cancer may clarify whether a mutual cause exists and may also guide clinicians about time windows of high risk of cancer occurrence.
Therefore, identifying AF as a potential risk marker for cancer may have important public health and clinical implications. In this study, we examined the association between new‐onset AF and subsequent cancer in a large longitudinal population‐based cohort study of men and women from the Danish Diet, Cancer, and Health cohort.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Design and Population
We conducted a population‐based prospective cohort study based on the Danish Diet, Cancer, and Health study, which has been described in detail elsewhere.14 Briefly, from 1993 to 1997, 80 996 men and 79 729 women born in the Aarhus or Copenhagen areas were invited to participate. A diagnosis of cancer before enrollment in the Danish Diet, Cancer, and Health study led to exclusion. In our study, we defined baseline as the date on which the participants visited a study center and completed a lifestyle questionnaire. Incomplete lifestyle or diet questionnaires or a diagnosis of AF before baseline led to exclusion. Figure 1 shows the flowchart of the exclusion process, and we followed the final study cohort through nationwide registries. In Denmark, all citizens are assigned a unique 10‐digit Civil Registration number that enables cross‐linking between public registers. The Civil Registration System contains individual information on vital statistics and migration that is updated daily.15
Figure 1.
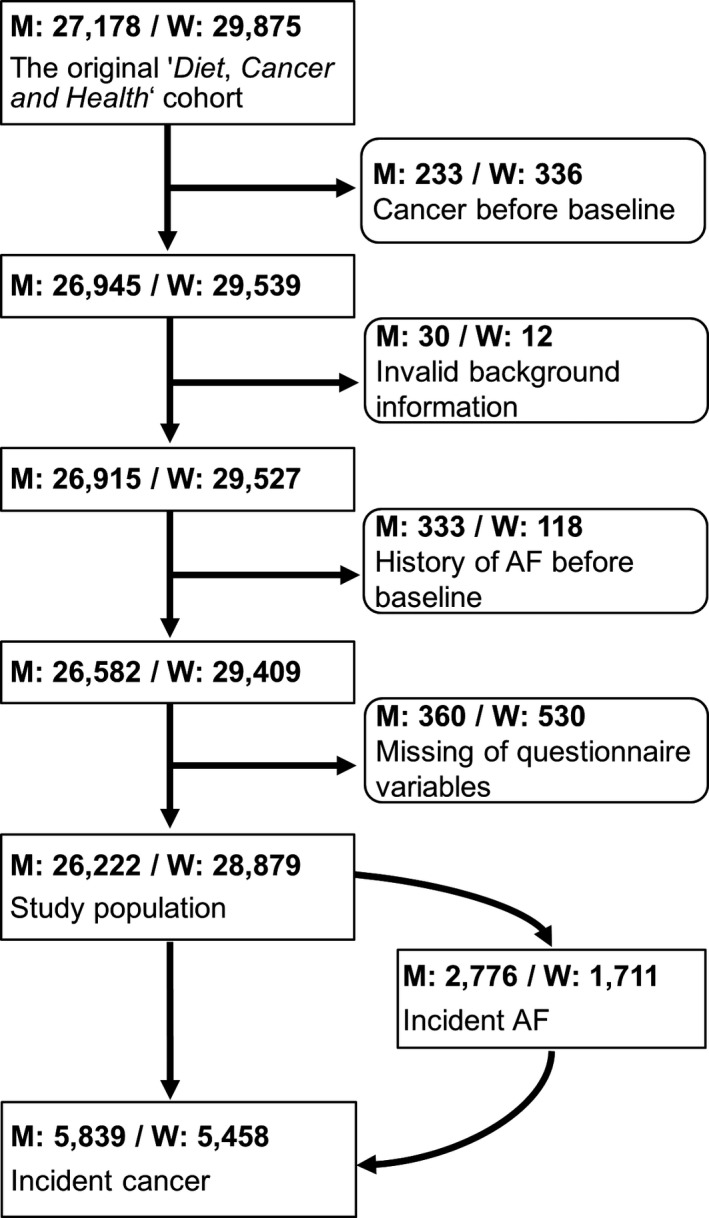
Flowchart. AF indicates atrial fibrillation; M, men; W, women.
Exposure
Our exposure of interest was new‐onset AF, and using the Danish National Patient Registry, we retrieved this information until any diagnosis of cancer was made (except nonmelanoma skin cancer) or until December 31, 2013. The registry was established in 1977 and contains prospectively registered information from Danish hospitals on all inpatients, and after 1995, the registry also began to include outpatients.16 Diagnoses were coded in accordance with the International Classification of Diseases Eighth Revision (ICD‐8) before 1994 and the Tenth Revision (ICD‐10) from 1994 and onwards. Using first hospital contact, we identified all new‐onset AF (and atrial flutter) cases irrespective of the type of AF as ICD‐8: 427.93‐427.94 and ICD‐10: I48. Nearly 5% of ICD‐10 I48 diagnoses correspond to atrial flutter.17 The positive predictive value of AF (and atrial flutter) diagnoses is 92% to 95% in the Danish National Patient Registry.17, 18
Outcomes
Our outcome of interest was cancer, and we followed the participants for cancer diagnoses using the Danish Cancer Registry until December 31, 2013. The registry was established in 1942 and contains information on all incident cancers in the Danish population.19 Our primary outcome was first incident cancer, including any type except nonmelanoma skin cancer (ICD‐10: C00‐C96, except C44). Our secondary outcomes were the most frequent cancers, which were defined as the first diagnosed cancer being prostate cancer (ICD‐10: C61), lung cancer (ICD‐10: C33‐34), colorectal cancer (ICD‐10: C18‐20), or breast cancer (women only, ICD‐10: C50). Moreover, we assessed the stages of the detected cancers based on the reported TNM classification (primary tumor, regional lymph nodes, and distant metastasis) retrieved from the Danish Cancer Registry. The Ann Arbor classification of lymphomas was unavailable beginning in 2004.
Covariates
Self‐administered and interviewer‐checked questionnaires and a physical examination provided information on covariates. The lifestyle and comorbidity questionnaire included questions on hypertension, hypercholesterolemia, diabetes mellitus, education, daily physical activity, alcohol consumption, smoking habits, and for women, hormone treatment in relation to menopause. Based on the detailed food frequency questionnaire,20, 21 we computed the Alternative Healthy Eating Index 2010,22 and by sex, the Mediterranean Diet Score23 and the Healthy Nordic Food Index.24 The physical examination provided information on height and weight to calculate body mass index.
Statistical Analysis
All analyses were sex specific. With respect to baseline characteristics, we calculated medians with interquartile ranges (IQR) for continuous variables, and percentages and numbers for categorical variables.
The Aalen‐Johansen estimator was used to compute stacked cumulative incidences of cancers until 12 years after the diagnosis of AF for the specified subtypes and other cancers. The follow‐up of 12 years after a diagnosis of AF was chosen because it was the most appropriate period based on assessment of the available data. The time from the diagnosis of AF was applied as underlying timescale. The cumulative incidence of each cancer outcome was adjusted for the competing risk of death and the other aforementioned cancer types.
We used Cox proportional hazards models with 95% confidence intervals (95% CIs) in which we applied age as underlying timescale, because of its strong associations with AF and cancer, and delayed entry at the recruitment day. As a Cox analysis is based on comparisons between individuals with event and individuals without event at the same time on the timescale, using age as the underlying timescale gives the best possible correction for age.25 Age was considered the most important time parameter because the time of inclusion into this population‐based study was not given by a health‐related event. Death and emigration led to censoring, and in the analyses of cancer subtypes, a first‐time cancer diagnosis different from the subtypes of interest led to censoring as well. We applied 2 models. Model 1 was adjusted for age (timescale). Model 2 was adjusted for age (timescale); body mass index, cumulated alcohol consumption, smoking duration, and tobacco consumption as continuous variables; hypertension, hypercholesterolemia, diabetes mellitus, physical activity, and hormone treatment (women only) as dichotomous variables; and educational level, education length, smoking status, and Healthy Nordic Food Index as categorical variables.
In our primary analysis, we examined the associations between AF and risk of any cancer and cancer subtypes until end of follow‐up by using AF as a time‐dependent exposure. In the secondary analyses, we estimated the associations between time from the diagnosis of AF and risk of any cancer and subtypes compared with participants without AF. The cancer risk in the periods 0 to 90 days and 0 to 365 days after the diagnosis of AF was examined using AF as a time‐dependent indicator variable. In sensitivity analyses, we evaluated the importance of diet with respect to development of colorectal cancer in the periods after AF, 0 to 90 days, 0 to 365 days, and 0 days until end of follow‐up, by replacing the Healthy Nordic Food Index with the Mediterranean Diet Score (categorical variable) or Alternative Healthy Eating Index 2010 (continuous variable) in Model 2. In the tertiary analyses, the associations between AF and detection of localized, regional, and metastatic cancer were examined in the aforementioned periods. We applied AF as a time‐dependent indicator variable and adjusted as in Model 2. In these analyses, we excluded all individuals with lymphomas beginning in 2004 since staging information was unavailable, and if stages were missing afterwards.
Based on Schoenfeld residuals, we tested the proportional‐hazards assumption in all the Cox models.
All analyses were performed in Stata version 14.2 (College Station, TX).
Ethics
The Regional Committee on Health Research Ethics in Copenhagen and Aarhus, Denmark, and the Danish Data Protection Agency approved The Diet, Cancer and Health study and this present study (1‐16‐02‐559‐16). Written informed consent was obtained from all the participants.
Results
Participants
The final study cohort consisted of 26 222 men and 28 879 women (Figure 1), and Table 1 presents their baseline characteristics. The median age of the men was 56 years at baseline, and they were followed for a median time of 16.7 years (IQR, 14.3–17.6). During follow‐up, 2776 had a diagnosis of AF. The median age at diagnosis was 68.7 years (IQR, 64.5–73.0). Among the women, the median age was 56 years at baseline, and they were followed for a median time of 16.9 years (IQR, 16.2–17.7). During follow‐up, 1711 had a diagnosis of AF. The median age at diagnosis was 70.4 years (IQR, 66.3–74.9). Approximately 22% of the men and 19% of the women had a cancer diagnosis during follow‐up, and prostate and breast cancers were the most frequent (Table 2).
Table 1.
Baseline Characteristics
| Characteristics | Men (n=26 222) | Women (n=28 879) |
|---|---|---|
| Age, median (IQR), y | 56.0 (52.7–60.2) | 56.2 (52.8–60.4) |
| BMI, median (IQR), kg/m2 | 26.2 (24.1–28.5) | 24.8 (22.5–27.8) |
| Hypertension, % (n) | 14.9 (3894) | 17.1 (4951) |
| Hypercholesterolemia, % (n) | 8.6 (2243) | 6.2 (1799) |
| Diabetes mellitus, % (n) | 2.7 (703) | 1.5 (433) |
| Educational level, % (n) | ||
| No vocational education | 19.2 (5542) | 10.0 (2621) |
| Low level of higher education | 31.5 (9104) | 13.6 (2570) |
| Medium level of higher education | 38.0 (10 967) | 42.3 (11 078) |
| High level of higher education | 11.3 (3266) | 34.1 (8953) |
| Education length, % (n) | ||
| ≤7 y | 31.1 (8983) | 34.6 (9075) |
| 8–10 y | 50.3 (13 528) | 41.6 (10 918) |
| >10 y | 18.6 (5368) | 23.8 (6229) |
| Physical activity, % (n) | ||
| <30 min/d | 59.1 (16 983) | 62.1 (16 210) |
| ≥30 min/d | 40.9 (11 770) | 37.9 (9909) |
| Cumulative alcohol, median (IQR), alcohol units | 16 328 (9152–27 612) | 5876 (2600–10 660) |
| Smoking status, % (n) | ||
| Current | 32.7 (9437) | 39.6 (10 393) |
| Former | 23.5 (6773) | 34.6 (9062) |
| Never | 43.9 (12 669) | 25.8 (6767) |
| Smoking duration, median (IQR), y | 26 (0–37) | 10 (0–33) |
| Tobacco consumption, median (IQR), g/d | 0 (0–15) | 0 (0–10) |
| Mediterranean diet score, % (n) | ||
| 0 | 0.3 (87) | 0.3 (66) |
| 1 | 2.5 (726) | 2.0 (533) |
| 2 | 8.8 (2545) | 6.8 (178) |
| 3 | 16.4 (4732) | 13.9 (3647) |
| 4 | 21.1 (6099) | 19.7 (5169) |
| 5 | 21.7 (6271) | 22.5 (5905) |
| 6 | 17.3 (5000) | 19.1 (5017) |
| 7 | 9.0 (2601) | 11.5 (3015) |
| 8 | 2.5 (721) | 3.7 (982) |
| 9 | 0.3 (97) | 0.4 (107) |
| Healthy Nordic Food Index, % (n) | ||
| 0 | 3.0 (877) | 2.2 (587) |
| 1 | 13.2 (3818) | 12.7 (3336) |
| 2 | 20.9 (6042) | 20.5 (5365) |
| 3 | 23.2 (6698) | 22.7 (5955) |
| 4 | 21.1 (6081) | 21.3 (5580) |
| 5 | 14.1 (4076) | 15.1 (3955) |
| 6 | 4.5 (1287) | 5.5 (1444) |
| Alternative healthy eating index, median (IQR) | 45.4 (39.2–51.7) | 54.3 (47.8–60.8) |
| Receiving hormone treatment, % (n) | ··· | 43.9 (12 683) |
BMI indicates body mass index; IQR, interquartile range.
Table 2.
Numbers of Incident Cancers During Follow‐Up
| Men | Women | |
|---|---|---|
| Any cancer, % (n) | 22.3 (5839) | 18.9 (5.458) |
| Lung cancer, % (n) | 2.7 (697) | 2.4 (685) |
| Colorectal cancer, % (n) | 3.1 (800) | 2.2 (642) |
| Prostate cancer, % (n) | 7.7 (2014) | ··· |
| Breast cancer, % (n) | ··· | 6.9 (1990) |
AF and Risk of Cancer
Among the men with new‐onset AF, 15.0% (416/2776) had a subsequent diagnosis of cancer, whereas 23.1% (5423/23 446) of the men without a new‐onset AF had a diagnosis of cancer. Among the women with new‐onset AF, 11.1% (190/1711) had a diagnosis of cancer, whereas 19.4% (5268/27 168) of the women without new‐onset AF had a diagnosis of cancer. Figure 2 shows the stacked cumulative incidence of cancer among participants with new‐onset AF adjusted for the competing risk of death. During 12 years after the diagnosis of AF, 23.3% (95% CI, 21.1–25.7) of men and 19.1% (95% CI, 16.2–22.1) of women had a cancer diagnosis. The median time to cancer after new‐onset AF was 4.5 years for men and 4.0 years for women. For all cancers, we noted a higher incidence rate during the initial months after the diagnosis of AF for both sexes (Figure 2).
Figure 2.
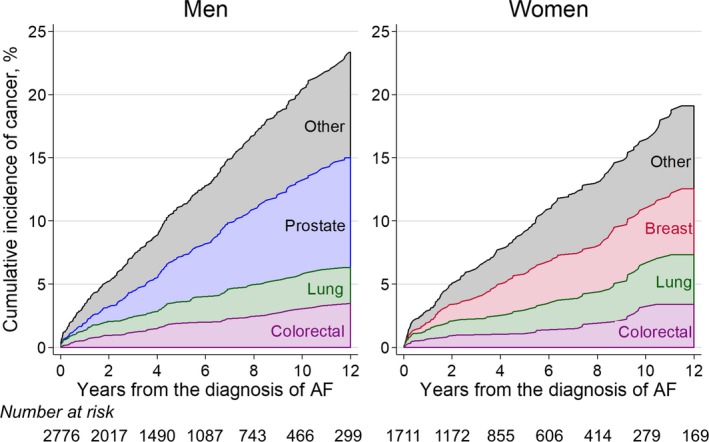
Stacked cumulative incidence of cancer after the diagnosis of AF by sex. The individual cumulative incidence is adjusted for the competing risk of death and other cancer subtypes. AF indicates atrial fibrillation.
Figure 3 shows the risks of any type of cancer and cancer subtypes until the end of follow‐up. Among the men, AF was associated with any type (hazard ratio [HR] 1.41, 95% CI, 1.26–1.58), lung (HR 1.66, 95% CI, 1.19–2.30), and colorectal cancer (HR 1.37, 95% CI, 1.02–1.85). Among the women, AF was associated with any cancer type (HR 1.15, 95% CI, 1.02–1.31), but not colorectal or lung cancer.
Figure 3.
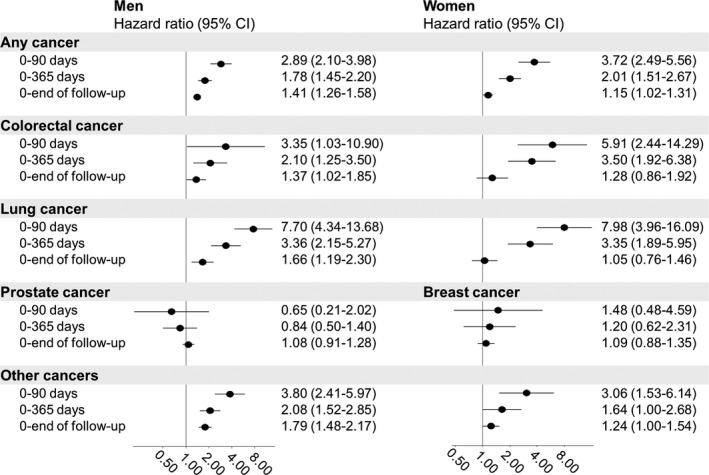
Risk of cancer by days after a new‐onset diagnosis of atrial fibrillation. The estimates are adjusted as in Model 2: age (timescale), body mass index, cumulative alcohol consumption, smoking duration, tobacco consumption, hypertension, hypercholesterolemia, diabetes mellitus, physical activity, hormone treatment (women only), educational level, education length, smoking status, and Healthy Nordic Food Index.
Time After the Diagnosis of AF and Risk of Cancer
Figure 3 also shows the risk of cancer in the time following the diagnosis of AF. During the initial 90 days, the HR for any type of cancer was 2.89 (95% CI, 2.10–4.98) in men and 3.72 (95% CI, 2.49–5.56) in women. During this period, AF was also associated with lung (men: HR 7.70, 95% CI, 4.34–13.68; women: HR 7.98, 95% CI, 3.96–16.09) and colorectal cancer (men: HR 3.35, 95% CI, 1.03–10.90; women: HR 5.91, 95% CI, 2.44–14.29) for both sexes.
During the first year, the association with any type of cancer (men: HR 1.78, 95% CI, 1.45–2.20; women: HR 2.01, 95% CI, 1.51–2.67) was weaker than the association during the initial 90 days following the diagnosis of AF for both sexes. We also noted a lower risk of lung (men: HR 3.36, 95% CI, 2.15–5.27; women: HR 3.35, 95% CI, 1.89–5.95), colorectal (men: HR 2.10, 95% CI, 1.25–3.50; women: HR 3.50, 95% CI, 1.92–6.38), and other cancers (men: HR 2.08, 95% CI, 1.52–2.85; women: HR 1.64, 95% CI, 1.00–2.68) during the first year than during the initial 90 days.
Diet Scores and Colorectal Cancer
We adjusted for the Healthy Nordic Food Index in our main analyses and examined the influence of adjustment for the other diet scores in Model 2. Adjusting for different diet scores did not change the magnitude of the HRs substantially.
AF and Detected Cancer Stages
Information on cancer stage was retrieved in 92.2% (n=5050) of male cancer patients and 90.3% (n=4667) of female cancer patients after excluding 364 male patients and 290 female patients with lymphomas beginning in 2004. Table 3 shows the sex‐specific distributions of the cancer stages stratified by new‐onset AF, and Table 4 gives the adjusted HRs for the associations between time after new‐onset AF and detected cancer stages.
Table 3.
Distribution of Incident Cancer Stages During Follow‐Upa
| New‐Onset Atrial Fibrillation | No Atrial Fibrillation | |
|---|---|---|
| Men (n=5050) | ||
| Localized, % (N) | 27.1 (98) | 32.9 (1542) |
| Regional, % (N) | 13.3 (48) | 20.5 (959) |
| Metastatic, % (N) | 59.7 (216) | 46.7 (2187) |
| Women (n=4667) | ||
| Localized, % (N) | 36.8 (63) | 43.4 (1954) |
| Regional, % (N) | 25.7 (44) | 30.0 (1347) |
| Metastatic, % (N) | 37.4 (64) | 26.6 (1195) |
After excluding lymphomas beginning in 2004, information on cancer stage was retrieved for 5050 males and 4667 females with cancer.
Table 4.
Time After Atrial Fibrillation and Risk of Cancer Stages During Follow‐Up
| Men (HR, 95% CI) | Women (HR, 95% CI) | |
|---|---|---|
| Metastatic cancer | ||
| 0–90 d | 3.54 (2.30–5.45) | 6.23 (3.43–11.29) |
| 0–365 d | 2.19 (1.65–2.90) | 2.48 (1.40–4.39) |
| 0–end of follow‐up | 1.65 (1.37–1.99) | 1.68 (1.21–2.32) |
| Regional cancer | ||
| 0–90 d | 0.90 (0.22–3.60) | 3.58 (1.60–7.98) |
| 0–365 d | 1.05 (0.55–2.03) | 1.45 (0.75–2.80) |
| 0–end of follow‐up | 1.50 (1.15–1.97) | 1.03 (0.81–1.31) |
| Localized cancer | ||
| 0–90 d | 2.03 (0.97–4.28) | 2.18 (0.90–5.25) |
| 0–365 d | 1.06 (0.63–1.80) | 1.30 (0.72–2.35) |
| 0–end of follow‐up | 1.41 (1.15–1.73) | 1.16 (0.93–1.44) |
The estimates are adjusted as in Model 2: age (timescale), body mass index, cumulative alcohol consumption, smoking duration, tobacco consumption, hypertension, hypercholesterolemia, diabetes mellitus, physical activity, hormone treatment (women only), educational level, education length, smoking status, and Healthy Nordic Food Index. CI indicates confidence interval; HR, hazard ratio.
Among men with new‐onset AF and subsequent cancer (n=362), 59.7% of cancers were metastatic, and 27.1% were localized. Among men without AF (n=4688) before cancer, 46.7% of cancers were metastatic, and 32.9% were localized. A new‐onset diagnosis of AF was strongly associated with metastatic cancer within 90 days after AF (HR 3.54, 95% CI, 2.30–5.45) and somewhat weaker within 365 days after AF (HR 2.19, 95% CI, 1.65–2.90) but persisted until the end of follow‐up (HR 1.65, 95% CI, 1.37–1.99). The tendency was more inconsistent with respect to localized cancer. A new‐onset diagnosis of AF was not significantly associated with localized cancer within 90 days or within 365 days after AF, but a diagnosis of AF was significantly associated with an elevated incidence of localized cancer until the end of follow‐up (HR 1.41, 95% CI, 1.15–1.73).
Among women with new‐onset AF (n=171) and subsequent cancer, 37.4% of cancers were metastatic and 36.8% of cancers were localized. Among women without AF (n=4496) before cancer, 26.6% of cancers were metastatic, and 43.5% of cancers were localized. A new‐onset diagnosis of AF was strongly associated with metastatic cancer within 90 days after AF (HR 6.23, 95% CI, 3.43–11.29) and was somewhat weaker within 365 days (HR 2.48, 95% CI, 1.40–4.39) and until the end of follow‐up (HR 1.68, 95% CI, 1.21–2.32). An AF diagnosis was not statistically associated with localized cancer within 90 days, within 365 days, or until end of follow‐up.
Discussion
In this long‐term prospective cohort study, we demonstrated an association between a diagnosis of new‐onset AF and a subsequent diagnosis of any type of cancer in both sexes. The relative cancer risk was highest within the initial 90 days after the diagnosis of AF and remained elevated during the initial 365 days after AF for both sexes. AF was strongly associated with metastatic cancer, particularly within 90 days after AF. AF was also associated with a higher risk of localized cancer during full follow‐up in both sexes, although not statistically significant in women. The risks of colorectal and lung cancer were highest within the initial 90 days after AF and remained elevated during the initial 365 days for both sexes. AF was not associated with prostate or breast cancer.
Only 2 studies have examined the association between AF and cancer thus far.12, 13 In these studies, restricted time intervals from AF were explored, which is a methodology different from the methods used in our study, as we explored extended time intervals from the time of AF. The advantage of our approach is prevention of possible selection bias because of the most susceptible participants developing cancer early, leaving a more resistant exposed cohort over time. Therefore, not all of our results are directly comparable to the previous studies. Based on the Woman's Health Study, Conen et al reported HRs of 1.48 (95% CI, 1.25–1.75) until the end of follow‐up, 3.54 (95% CI, 2.05–6.10) for the initial 90 days after the diagnosis of AF, and 1.42 (95% CI, 1.18–1.71) beyond 1 year after the diagnosis of AF.13 The fact that we also found an association substantiates the relationship between AF and cancer among women. Our association within 90 days after diagnosis of AF is in accordance with the findings of Conen et al and suggests that cancer is mainly detected in connection with the diagnosis of AF. Conen et al also reported a HR of 2.11 (95% CI, 1.33–3.36) for colon cancer until the end of follow‐up, but our study found that this association was not significant. In contrast to Conen et al, we retrieved AF and cancer diagnoses from nationwide registries independent of patient reporting. In the Danish registries, the positive predictive value of AF diagnoses is 92% to 95% and of cancer diagnoses 98%.17, 18 However, in general, our results among women are largely consistent with the Women's Health Study; therefore, our study extends this association to comprise female Europeans. A Danish nationwide cohort study by Ostenfeld et al that involved both sexes supported an increased risk of cancer following the diagnosis of AF. This association was particularly strong within the initial 90 days after the diagnosis of AF.12 However, the study lacked information on lifestyle markers, such as smoking and dietary habits, which may have caused residual confounding.
Cancer screening programs could have influenced our study. Organized breast cancer screening programs were introduced in Copenhagen in 1991 and became nationalized in late 2007 but were still incomplete in 2014.26 In Denmark, unorganized and opportunistic individual‐based prostate cancer screening initiated by the patient or the doctor was frequently performed using prostate‐specific antigen.27, 28 Interestingly, AF was not associated with breast or prostate cancer in our study. Organized colorectal cancer screening could not have influenced our study as it was introduced in 2014.
Multiple reasons may account for the timewise association between a diagnosis of AF and a subsequent cancer diagnosis. First, diagnostic evaluation of AF symptoms may reveal occult cancer. If the cancer remains undetected, AF‐related antithrombotic treatment may increase the risk of bleeding, followed by cancer detection. Anticoagulant‐related gastrointestinal bleeding has been shown to unmask cancer during the first month of treatment,29 which is consistent with the increased risk of a colorectal cancer diagnosis within the initial 90 days after AF for both sexes that was found in our study. Antithrombotic treatment may also cause hematuria; however, only a small percentage of patients with macroscopic hematuria are diagnosed with prostate cancer.30 Second, other AF‐related treatments, such as amiodarone or digoxin, may also be associated with an increased risk of cancer,31, 32 and a causal relationship between AF per se and cancer may exist. However, both seem unlikely because of the strong association with metastatic cancer within 90 days, the initial sharp increase in the cumulative incidence of cancer, and the attenuation of the HRs over time, all of which suggest that cancer is already present at the time of the diagnosis of AF. Our results showed an increased risk of localized cancer during full follow‐up, which may suggest a common underlying mechanism. Chronic inflammation is an important part of the initiation and maintenance of AF, in which inflammatory pathways contribute to electrical and structural atrial remodeling.33 In cancer, chronic inflammation promotes carcinogenesis and tumor progression.34 Several common risk factors, such as alcohol and obesity, may share biology with AF and cancer because of proinflammatory and inflammatory causal pathways.6 Third, undiagnosed AF is common,5 and cancer symptoms may lead to diagnostic evaluations that detect both AF and cancer. This is supported by the finding of the high risk of metastatic cancer that is expected to be symptomatic. Fourth, the association may be a result of residual confounding, which may explain the long‐term association, although we adjusted for the most relevant confounders.
Our findings may have clinical implications. In our study, a new‐onset diagnosis of AF was associated with coexistent cancer that was probably already metastatic. Our findings do not support recommendations for using new‐onset AF as an indication for systematic cancer screening. As shared risk factors may link cardiovascular disease with cancer, it may be worthwhile to be vigilant in patients with AF who have risk factors for cancer, such as obesity and smoking. Future studies are needed to clarify the prognostic impact of whether a new‐onset diagnosis of AF should be an impetus to secure adherence to nationally recommended systematic screening programs for colorectal cancer. In addition, symptom‐driven opportunistic screening for any cancer should be considered.
Limitations
This study was based on the Danish Diet, Cancer, and Health study in which 35% of the invited individuals agreed to participate. Selection bias is unlikely to explain the observed associations because it is doubtful that the association between AF and cancer after enrollment depended on study participation, and we had no loss to follow‐up. Generalizability may be an issue since invitation was restricted to individuals born in Denmark; therefore, the vast majority of the participants were white. We were unable to classify AF according to duration; therefore, paroxysmal AF may have been underreported. The number of missed AF diagnoses in the Danish National Patient Registry is unknown. As with all other registers, the Danish National Patient Registry is not expected to be complete.
The exact time of the diagnosis of AF or cancer is difficult to establish because both diseases may be asymptomatic for a long time. Residual confounding and potential changes of baseline information during follow‐up are also possible limitations.
Conclusions
In this large prospective cohort study, we found that a new‐onset diagnosis of AF was associated with a subsequent increased risk of being diagnosed with cancer. The risk of any type of cancer, colorectal cancer, and lung cancer were particularly high within the initial 90 days following the diagnosis of AF. AF was not associated with breast or prostate cancer. Metastatic cancer was more likely to be present at the time of diagnosis of AF, and the detection of cancer may have been a result of increased medical attention. An increased association between AF and localized cancer during full follow‐up may suggest a common causal pathway. Our findings confirm and extend the previous findings in women to involve men as well.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009543 DOI: 10.1161/JAHA.118.009543.)
References
- 1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 4. Weng LC, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B, Trinquart L, McManus DD, Staerk L, Lin H, Lunetta KL, Ellinor PT, Benjamin EJ, Lubitz SA. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation. 2018;137:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 6. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32:900–907. [DOI] [PubMed] [Google Scholar]
- 8. Rahman F, Ko D, Benjamin EJ. Association of atrial fibrillation and cancer. JAMA Cardiol. 2016;1:384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 10. Ahmad AS, Ormiston‐Smith N, Sasieni PD. Trends in the lifetime risk of developing cancer in great Britain: comparison of risk for those born from 1930 to 1960. Br J Cancer. 2015;112:943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014;9:e102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM. Risk of malignant cancer among women with new‐onset atrial fibrillation. JAMA Cardiol. 2016;1:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in diet, cancer and health: a population‐based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35:432–441. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sundboll J, Adelborg K, Munch T, Froslev T, Sorensen HT, Botker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open. 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39:42–45. [DOI] [PubMed] [Google Scholar]
- 20. Overvad K, Tjonneland A, Haraldsdottir J, Ewertz M, Jensen OM. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol. 1991;20:900–905. [DOI] [PubMed] [Google Scholar]
- 21. Tjonneland A, Overvad K, Haraldsdottir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20:906–912. [DOI] [PubMed] [Google Scholar]
- 22. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 24. Olsen A, Egeberg R, Halkjaer J, Christensen J, Overvad K, Tjonneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr. 2011;141:639–644. [DOI] [PubMed] [Google Scholar]
- 25. Korn EL, Graubard BI, Midthune D. Time‐to‐event analysis of longitudinal follow‐up of a survey: choice of the time‐scale. Am J Epidemiol. 1997;145:72–80. [DOI] [PubMed] [Google Scholar]
- 26. Jorgensen KJ, Gotzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017;166:313–323. [DOI] [PubMed] [Google Scholar]
- 27. Jessen K, Sondergaard J, Larsen PV, Thomsen JL. Danish general practitioners’ use of prostate‐specific antigen in opportunistic screening for prostate cancer: a survey comprising 174 Gps. Int J Family Med. 2013;2013:540707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen‐Nielsen M, Borre M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med. 2016;46:484–490. [DOI] [PubMed] [Google Scholar]
- 29. Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant‐related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med. 2014;46:672–678. [DOI] [PubMed] [Google Scholar]
- 30. Mladenov BS, Mariyanovski V, Hadzhiyska V. Macroscopic hematuria in patients on anticoagulation therapy. Cent European J Urol. 2015;68:330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su VY, Hu YW, Chou KT, Ou SM, Lee YC, Lin EY, Chen TJ, Tzeng CH, Liu CJ. Amiodarone and the risk of cancer: a nationwide population‐based study. Cancer. 2013;119:1699–1705. [DOI] [PubMed] [Google Scholar]
- 32. Boursi B, Haynes K, Mamtani R, Yang YX. Digoxin use and the risk for colorectal cancer. Pharmacoepidemiol Drug Saf. 2014;23:1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. [DOI] [PubMed] [Google Scholar]
- 34. Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park). 2011;25:400–410, 413. [PubMed] [Google Scholar]


