Abstract
Background
Stroke is the third leading cause of death among US Hispanic and non‐Hispanic black women aged 65 and older. One factor that may protect against stroke is breastfeeding. Few studies have assessed the association between breastfeeding and stroke and whether this association differs by race and ethnicity.
Methods and Results
Data were taken from the Women's Health Initiative Observational Study with follow‐up through 2010; adjusted hazard ratios for stroke subsequent to childbirth were estimated with Cox regression models accounting for left and right censoring, overall and stratified by race/ethnicity. Of the 80 191 parous women in the Women's Health Initiative Observational Study, 2699 (3.4%) had experienced a stroke within a follow‐up period of 12.6 years. The average age was 63.7 years at baseline. Fifty‐eight percent (n=46 699) reported ever breastfeeding; 83% were non‐Hispanic white, 8% were non‐Hispanic black, 4% were Hispanic, and 5% were of other race/ethnicity. After adjustment for nonmodifiable potential confounders, compared with women who had never breastfed, women who reported ever breastfeeding had a 23% lower risk of stroke (adjusted hazard ratio=0.77; 95% confidence interval 0.70‐0.83). This association was strongest for non‐Hispanic black women (adjusted hazard ratio=0.52; 95% confidence interval 0.37‐0.71). Further, breastfeeding for a relatively short duration (1‐6 months) was associated with a 19% lower risk of stroke (adjusted hazard ratios=0.81; 95% confidence interval 0.74‐0.89). This association appeared stronger with longer breastfeeding duration and among non‐Hispanic white and non‐Hispanic black women (test for trend P<0.01).
Conclusions
Study results show an association and dose‐response relationship between breastfeeding and lower risk of stroke among postmenopausal women after adjustment for multiple stroke risk factors and lifestyle variables. Further investigation is warranted.
Keywords: breastfeeding, human lactation; cerebrovascular disease/stroke; epidemiology; health disparities; risk factor; women and minorities
Subject Categories: Cerebrovascular Disease/Stroke, Epidemiology, Race and Ethnicity, Risk Factors, Women
Clinical Perspective
What Is New?
Data from the Women's Health Initiative showed that ever breastfeeding was associated with a lower risk of stroke among postmenopausal women after adjustment for multiple stroke risk factors and lifestyle variables; this association was strongest for non‐Hispanic black women.
Longer duration of breastfeeding was associated with a lower risk of stroke in all women studied and among non‐Hispanic white and non‐Hispanic black women.
Increasing public awareness of the potential impact of breastfeeding on maternal health outcomes later in life may assist in the support for, initiation, and continuation of breastfeeding for those at greatest risk.
What Are the Clinical Implications?
Breastfeeding along with other risk factors or risk markers during women's reproductive years may be associated with stroke risk later in life. Identification of risk factors may help healthcare providers in assessing a woman's risk profile.
The medical and behavioral science communities may be better able to design culturally informed programs that mitigate stroke risk while they promote healthy lifestyle behaviors including breastfeeding among populations that unduly carry the largest health burden of stroke.
Further investigation into the association and dose‐response relationship between breastfeeding and lower risk of stroke among postmenopausal women is warranted.
Introduction
In the United States, cerebrovascular disease, affects 5% to 14% of women aged 60 years or older.1 Stroke is the fourth leading cause of death for women aged 65 and older.2, 3 Stroke is also the third leading cause of death among US Hispanic and non‐Hispanic black women aged 65 and older.2, 4 Stroke costs the nation $34 billion annually including the cost of healthcare services, medications, and lost productivity.1 Approximately 55 000 more US women than men suffer from a stroke annually.1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Compared with elderly men, elderly female stroke survivors are also at increased risk for moderate to severe disability, poorer quality of life, and institutionalization.16, 17
The risk of stroke is distributed disproportionately by race and ethnicity. Stroke is higher among Hispanics and non‐Hispanic blacks compared with non‐Hispanic whites.1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 18 Hispanic and non‐Hispanic black women are at higher risk of stroke, partially due to increased rates of hypertension, obesity, diabetes mellitus, and physical inactivity,1, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 which are a few stroke risk factors. Research is needed to address this health disparity and to reduce disproportionate morbidity from stroke among racially and ethnically diverse populations.
Breastfeeding is 1 factor that may protect women against stroke. Research findings point to the protective effect of breastfeeding on maternal health including a reduced risk of breast cancer and ovarian cancer and improved cardiovascular health.29, 30, 31 Women who report more than 9 cumulative months of breastfeeding over their reproductive lifetime are less likely to develop hypertension, hyperlipidemia, and metabolic syndrome.32, 33, 34, 35, 36, 37 Further, evidence suggests that a longer duration of breastfeeding is associated with a lower risk of developing type 2 diabetes mellitus,38, 39, 40, 41, 42, 43 and reduced maternal postpartum weight.44, 45, 46, 47
Currently, the American Academy of Pediatrics recommends exclusive breastfeeding for 6 months,48 with continuation of breastfeeding for 1 year or longer, which is also recommended by the World Health Organization.49 Compared with the Healthy People 2020 target for exclusive breastfeeding at 6 months,50 exclusivity rates for US women are particularly low, especially for Hispanic and non‐Hispanic black women (Figure).51 Also, whereas 83% of US women initiate breastfeeding soon after giving birth, only 55% of these women report any breastfeeding at 6 months postpartum, and 34% report any breastfeeding at 12 months postpartum.51 More importantly, breastfeeding rates vary by race/ethnicity and are lowest among non‐Hispanic black women with 64% initiating breastfeeding, followed by 35% breastfeeding at 6 months and 17% at 12 months postpartum; duration rates for Hispanic women are slightly higher at 51% at 6 months and 26% at 12 months postpartum but well below those of non‐Hispanic white women (56% and 31%, respectively).52, 53, 54
Figure 1.
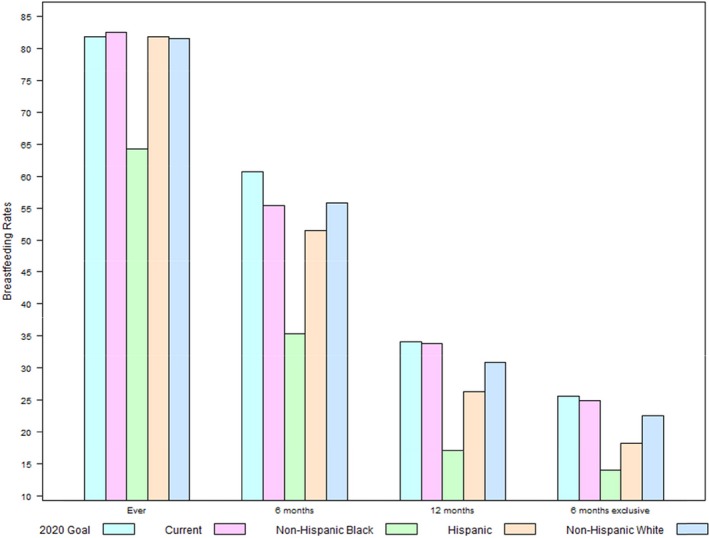
Current breastfeeding rates by race/ethnicity*,†,‡,§,‖,#*US Department of Health and Human Services, Office of Disease Prevention and Promotion, Healthy People 2020, Maternal, Infant, and Child Health Objectives (http://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives). †Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Breastfeeding Report Card, United States, 2016 (http://www.cdc.gov/breastfeeding/data/reportcard.htm). ‡Any breastfeeding at 6 months. Source: Centers for Disease Control and Prevention. Rates of Any and Exclusive Breastfeeding by State among Children Born in 2012 (http://www.cdc.gov/breastfeeding/data/nis_data/rates-any-exclutive-bf-state-2012.htm). §Ever breastfeeding, any breastfeeding at 12 months, exclusive breastfeeding at 6 months. Source: Anstey EH, Chen J, Elam‐Evans LD, Perrine CG. Racial and geographic differences in breastfeeding—United States, 2011‐2015. MMWR Morb Mortal Wkly Rep. 2017;66:723‐727 (DOI: https://doi.org/10.15585/mmwr.mm6627a3). ‖Any breastfeeding is defined as predominant breastfeeding with some supplementation of formula. #Exclusive breastfeeding is defined as breast milk only without any supplementation with the exception of oral rehydration solution or drops/syrups of vitamins, minerals, or medicines (http://www.who.int/elena/titles/exclusive_breastfeeding/en/).
Whereas studies to date have largely focused on the protective effect of breastfeeding on cardiovascular risk factors,32, 33, 34, 35, 36, 37, 55, 56 few studies have assessed whether breastfeeding protects against risk of stroke and whether this association differs by race/ethnicity. Because Hispanic and non‐Hispanic black women experience low breastfeeding rates and are at higher risk for stroke compared with non‐Hispanic white women, there is a need to examine this relationship so that future intervention programming may focus on specific populations that carry the largest health burden of stroke. The current study fills this gap in the literature and contributes to the body of knowledge about reproductive factors and stroke risk, using the Women's Health Initiative Observational Study with follow‐up through 2010. The aim of this study was to examine stroke risk in relation to breastfeeding history at any time between first childbirth and end of follow‐up among postmenopausal women, overall and by race/ethnicity. We hypothesized that lifetime duration of any breastfeeding would be associated with lower risk of stroke among postmenopausal women.
Materials and Methods
Study Population and Procedure
The Women's Health Initiative (WHI), a longitudinal national health study consisting of a set of randomized clinical trials and an observational study, focused on strategies to prevent chronic disease in postmenopausal women.57 Recruitment took place from 1993 to 1998 by 40 clinical centers in 24 states and the District of Columbia.58 Starting in 2005, women who consented were followed up in the WHI Extension Studies (2005‐2010, 2010‐2015, 2015‐present).59, 60 Study design and descriptions of baseline characteristics have been published previously.58, 61, 62 Data collection methods, data management, and verification of data accuracy also are documented elsewhere.61, 63 For the present study, we used data from the WHI Observational Study with follow‐up through 2010. The WHI included community‐dwelling postmenopausal women ranging in age from 50 to 79 years at baseline who completed a self‐administered questionnaire that included demographic information, reproductive history, personal and family medical history, medication use, and lifestyle behaviors. In accord with prevailing ethical principles and guidelines of the Institutional Review Board at the University of Kansas School of Medicine‐Wichita, this study was determined to be exempt from Institutional Review Board review. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.64
Study Outcome, Exposure, and Confounder Measurement
The primary outcome of interest was the occurrence of stroke at any time between the first childbirth and the end of follow‐up for women enrolled in the WHI Observational Study. Stroke during follow‐up was assessed with annual self‐report questionnaires and centrally adjudicated via medical records by vascular neurologists.63 Among women without a history of stroke, stroke was defined as the first postbaseline occurrence of ischemic or hemorrhagic stroke through the first WHI extension period (2010). Women who self‐reported stroke before study entry were included to fully capture the events during the period at risk (from the time of childbearing to the time of stroke or end of follow‐up). However, for those women with stroke before WHI enrollment, stroke was not centrally adjudicated.
All women in the WHI Observational Study who reported having at least 1 live birth and who were not missing information on ever breastfeeding were included. Ever breastfeeding was assessed at enrollment among women who reported at least 1 live birth. Women who responded “No” to “Did you breastfeed or nurse any children for at least 1 month?” were considered to have never breastfed. For women with a history of ever breastfeeding, they were asked to reply to “Thinking about all the children you breastfed, how many months total did you breastfeed? (Your best guess.)” Responses were recorded as a categorical variable indicating a cumulative lifetime duration of breastfeeding33: 1 to 6 months, 7 to 12 months, and 13 months or more. Due to the lack of data on exclusive breastfeeding rates in the Women's Health Initiative data set, we examined breastfeeding history based on the any breastfeeding definition (any breastfeeding is defined as breast milk exclusively or breast milk along with other sources of nutrition). We described “breastfeeding history” as including ever breastfeeding and lifetime duration of breastfeeding (ie, the total number of months of breastfeeding across all children a woman has ever had).
We examined adjustment for nonmodifiable and modifiable participant characteristics from the baseline visit (see Statistical Analysis below). Cardiovascular events and risk factors for stroke at baseline were also described and considered in modeling. These included stroke history, transient ischemic attack, myocardial infarction, heart failure, atrial fibrillation, angina, diabetes mellitus, hypertension treatment, hypercholesterolemia treatment, and family history of diabetes mellitus, stroke, and myocardial infarction.
Statistical Analysis
Descriptive analyses compared baseline characteristics by breastfeeding history. Comparisons between categorical characteristics were made by chi‐squared tests of association and for continuous characteristics through a 2‐sample test, assuming unequal variances. We examined the association between breastfeeding history and risk of stroke; those with a history of stroke at WHI entry were included, and their stroke event time was considered left censored at the time of entry to WHI. For participants without a history of stroke, the time until stroke was observed until the end of the first extension period (2010). Adjusted hazard ratios were estimated through the Cox regression model framework accounting for left and right censoring that allowed a flexible spline fit to the baseline hazard.65 Models used age as the time scale and accounted for left censoring of participants who reported stroke before WHI enrollment and right censoring for those who did not have a stroke event. These models further allowed adjustment for baseline demographic characteristics and confounders. We modeled exposure to breastfeeding as ever breastfeeding and as lifetime duration of breastfeeding to investigate the relationship between exposure dose and stroke risk. Adjusted models (ie, models 1 and 2) first focused on a subset of nonmodifiable characteristics at the baseline visit including age, regional center, extension study inclusion, race/ethnicity, educational attainment, parity, age at menarche, and family history. Then, a subsequent model (model 3) included characteristics from models 1 and 2 plus modifiable characteristics/behaviors at the baseline visit (ie, exercise as measured by metabolic equivalent task–hours per week, Healthy Eating Index 2005,66 smoking history, body mass index [BMI, calculated from height and weight measured at baseline], multivitamin use). Finally, the effect of ever breastfeeding and lifetime duration of breastfeeding on stroke risk was estimated by racial and ethnic groups through fully stratified Cox models, using the previously described framework. Separate models were fit for each racial/ethnic group, allowing the baseline hazards to be unique to each subgroup. As a measure of potential unmeasured confounding, we also report the e‐value for the primary models.67 All reported P‐values and confidence intervals (CIs) were 2‐sided and reported at the nominal level. Analyses were conducted in STATA (version 13, StataCorp, College Station, TX) and SAS version 9.4, SAS/STAT 13.2 (SAS Institute, Inc, Cary, NC). All analyses used WHI follow‐up data sets that were disseminated in November 2016.
Results
Of the 93 676 women in the WHI Observational Study, 80 951 women reported at least 1 previous live birth, 80 191 of whom reported information on their breastfeeding history. Of these 80 191 women, a total of 1244 had a history of stroke at study entry, and 2699 had incident stroke during a median follow‐up of 12.6 years. At screening, participants’ average age was 63.7 years (95% CI 63.6‐63.7 years). Except for age and race/ethnicity, the demographic and health history characteristics of 760 women who did not report on breastfeeding were not substantially different from those who did report on breastfeeding. Those who did not report on breastfeeding were a few years older (median [minimum, maximum] age at entry 67 [50, 79] years) as compared with those who did report breastfeeding history (64 [49, 81] years) and were more often identified as non‐Hispanic black (14% versus 8%, respectively).
Of the 2699 women with incident stroke during follow‐up, 86.2% were non‐Hispanic white, 7.5% were non‐Hispanic black, 1.9% were Hispanic, and 4.4% self‐identified as another race/ethnicity. Of the 1244 women with a history of stroke at WHI baseline, 71.8% were non‐Hispanic white, 17% were non‐Hispanic black, 5.2% were Hispanic, and 6% self‐identified as another race/ethnicity. Women with a history of stroke at baseline tended to be younger at study entry, had higher BMIs, and had a history of smoking as compared with women with incident stroke during follow‐up (mean [SD] age at entry was 66.9 [7.0] years versus 68.3 [6.4] years, P<0.001; mean [SD] BMI was 28.5 [6.1] versus 27.5 [5.9], P<0.001; 53.6% versus 49.8% ever smoking, P=0.029).
Approximately three‐fifths (58%) of parous women in the sample reported ever breastfeeding (Table 1). Among these women, 51% breastfed for 1 to 6 months, 22% for 7 to 12 months, and 27% for 13 or more months. Duration of breastfeeding was missing for 281 (0.4%) women; these women were not included in the breastfeeding duration analysis, but they were included in the analysis for ever breastfeeding. Compared with women who ever breastfed, women who never breastfed were more likely to be younger at baseline (63.3 years versus 63.9 years), to be a high school graduate or less (37.1% versus 29.5%), to reside in the Northeast (28.4% versus 18.8%), to have lower parity (2.7 versus 3.1), to have a slightly higher BMI (27.5 versus 27.2), to exercise less (13.3 versus 14.0), and to have ever smoked (52.6% versus 46.6%). Women who never breastfed were less likely to use multivitamins (40.9% versus 42.2%), eat healthy foods (68.5 versus 69.8), and use hormone therapy (47.6% versus 49.5%) or aspirin (20.6% versus 21.3%). Also, women who never breastfed were more likely to have a family history of diabetes mellitus (33.0% versus 30.7%) or myocardial infarction (53.8% versus 51.6%) but were less likely to have a family history of stroke (35.9% versus 36.9%).
Table 1.
Participant Characteristics by Breastfeeding History Among Postmenopausal Women
| Never Breastfed (n=33 492) % | Ever Breastfed (n=46 699) % | Breastfed 1 to 6 Months (n=23 666) % | Breastfed 7 to 12 Months (n=10 177) % | Breastfed 13 or More Months (n=12 575) % | |
|---|---|---|---|---|---|
| Race/ethnicitya , b | |||||
| Non‐Hispanic white | 84.8 | 82.8 | 82.3 | 83.3 | 83.9 |
| Non‐Hispanic black | 8.3 | 7.6 | 8.1 | 7.7 | 6.2 |
| Hispanic | 3.4 | 4.2 | 4.0 | 3.9 | 4.5 |
| Other race/ethnicity | 3.6 | 5.4 | 5.5 | 5.2 | 5.4 |
| Age at screening, mean (SD)a , b | 63.3 (7.1) | 63.9 (7.4) | 64.1 (7.3) | 63.7 (7.5) | 63.8 (7.4) |
| 50 to 59a , b | 32.4 | 30.1 | 28.9 | 32.4 | 30.8 |
| 60 to 69 | 46.0 | 44.1 | 44.9 | 42.2 | 44.2 |
| 70 to 79 | 21.6 | 25.8 | 26.2 | 25.4 | 25.1 |
| Educationa , b | |||||
| High school or less | 37.1 | 29.5 | 32.2 | 27.7 | 25.7 |
| Some college | 39.8 | 38.6 | 40.2 | 37.4 | 36.6 |
| Postcollege | 23.1 | 31.9 | 27.7 | 34.9 | 37.6 |
| Regiona , b | |||||
| Northeast | 28.4 | 18.8 | 19.4 | 18.5 | 18.0 |
| South | 25.8 | 25.9 | 26.9 | 26.2 | 23.5 |
| Midwest | 20.8 | 23.1 | 22.4 | 22.5 | 24.7 |
| West | 25.0 | 32.3 | 31.3 | 32.8 | 33.8 |
| Extension cohorta , b | 66.2 | 69.0 | 67.3 | 70.1 | 71.5 |
| Age at menarche, mean (SD)a , b | 12.6 (1.5) | 12.6 (1.5) | 12.6 (1.5) | 12.6 (1.5) | 12.7 (1.5) |
| Parity, mean (SD)a , b | 2.7 (1.2) | 3.1 (1.2) | 2.9 (1.2) | 3.0 (1.1) | 3.6 (1.1) |
| Body mass index at baseline, mean (SD)a , b | 27.5 (5.9) | 27.2 (5.8) | 27.3 (5.8) | 27.0 (5.7) | 27.2 (5.7) |
| Under/normal (≤24.9)a , b | 39.2 | 41.0 | 40.3 | 42.4 | 41.2 |
| Overweight (25‐29.9) | 34.5 | 34.2 | 34.4 | 34.3 | 33.6 |
| Obese (≥30) | 26.3 | 24.8 | 25.3 | 23.4 | 25.2 |
| Physical activity (metabolic equivalent task/wk), mean (SD)a , b | 13.3 (14.2) | 14.0 (14.4) | 13.6 (14.3) | 14.3 (14.3) | 14.6 (14.8) |
| Ever smokera , b | 52.6 | 46.6 | 49.7 | 45.9 | 41.5 |
| Multivitamin usea , b | 40.9 | 42.2 | 42.4 | 42.1 | 41.9 |
| Healthy eating index 2005 mean (SD) | 68.5 (10.7) | 69.8 (10.5) | 69.3 (10.5) | 70.1 (10.2) | 70.3 (10.2) |
| Hormone therapyWomen's Health Initiative baselinea , b | |||||
| Never | 31.3 | 29.2 | 27.9 | 28.1 | 32.1 |
| Past | 21.1 | 21.4 | 21.9 | 21.6 | 20.1 |
| Current | 47.6 | 49.5 | 50.1 | 50.3 | 47.8 |
| Aspirin use | 20.6 | 21.3 | 21.6 | 20.4 | 21.3 |
| Family history | |||||
| Diabetes mellitusa , b | |||||
| No | 62.4 | 64.9 | 63.7 | 66.0 | 66.1 |
| Yes | 33.0 | 30.7 | 31.5 | 29.7 | 30.1 |
| Unknownc | 4.6 | 4.5 | 4.8 | 4.3 | 3.8 |
| Strokea | |||||
| No | 59.3 | 58.2 | 58.0 | 58.1 | 58.6 |
| Yes | 35.9 | 36.9 | 37.2 | 36.8 | 36.5 |
| Unknownc | 4.9 | 4.9 | 4.9 | 5.1 | 4.9 |
| Myocardial Infarction a , b | 53.8 | 51.6 | 52.6 | 50.0 | 51.2 |
| Cardiovascular disease and risk factors at baseline | |||||
| Stroke | 1.5 | 1.6 | 1.6 | 1.6 | 1.4 |
| Transient ischemic attack | 2.4 | 2.5 | 2.6 | 2.3 | 2.6 |
| Myocardial infarction | 2.5 | 2.5 | 2.7 | 2.2 | 2.3 |
| Heart failure | 1.0 | 1.0 | 0.9 | 1.0 | 0.9 |
| Atrial fibrillationb | 4.7 | 4.9 | 5.2 | 4.6 | 4.4 |
| Anginab | 5.8 | 6.3 | 6.7 | 5.9 | 5.7 |
| Diabetes mellitus | 5.7 | 5.6 | 5.8 | 5.2 | 5.7 |
| Hypertension treatmenta , b | |||||
| Never | 65.6 | 66.8 | 65.8 | 67.5 | 68.1 |
| Untreatedd | 8.0 | 7.8 | 7.8 | 8.0 | 7.7 |
| On treatment | 26.4 | 25.4 | 26.4 | 24.5 | 24.2 |
| Hypercholesterolemia treatmenta , b | 16.1 | 14.4 | 15.2 | 13.7 | 13.2 |
Data are presented as n (%) or mean (SD).
Comparison between participant characteristics and (ever and never) breastfeeding chi‐squared test or t test, P<0.01.
Comparison between participant characteristics and breastfeeding duration chi‐squared test or ANOVA, F‐test P<0.01.
Unlike other listed variables, this variable includes “unknown” values.
Unlike other listed variables, this variable includes “untreated” values.
Women who reported ever breastfeeding (for at least 1 month or more) had a lower risk of stroke in adjusted regression models (Table 2). There was little change in the hazard ratio from model 1 (minimally adjusted), model 2 (adjusted for nonmodifiable potential confounders), and model 3 (further adjusted for modifiable lifestyle factors). We further examined adjustment for medical therapies that may have occurred following breastfeeding exposure (ie, hormone therapy use at baseline, aspirin use at baseline, hypertension treatment at baseline, and hypercholesterolemia treatment at baseline); these additions did not change model estimates substantially. Our summary of inferential results focuses on estimates from model 3, which adjusts for characteristics associated with both breastfeeding and stroke that are not considered in the causal pathway. Compared with parous women who never breastfed, women who reported ever breastfeeding (1 month or longer) had a 23% lower risk of stroke (adjusted for nonmodifiable risk factors, adjusted hazard ratio=0.77; 95% CI 0.70‐0.84). For this hazard ratio to be explained away by any additional unmeasured confounder, the unmeasured confounder would require a moderately strong relationship, a risk ratio of 1.7 or more, with both breastfeeding and stroke (the e‐value). Compared with parous women who never breastfed, breastfeeding for 1 to 6 months was associated with 19% lower risk of stroke (adjusted hazard ratio=0.81; 95% CI 0.74‐0.90). This association was stronger with longer breastfeeding duration (test for trend P<0.01).
Table 2.
Risk of Stroke in Relation to Breastfeeding History Among Postmenopausal Women
| Number of Strokes | Model 1Adjusted HRa (95% CI) | Model 2Adjusted HRb (95% CI) | Model 3Adjusted HRc (95% CI) | |
|---|---|---|---|---|
| Breastfed ever | ||||
| No (n=33 492) | 1555 | (Reference) | (Reference) | (Reference) |
| Yes (n=46 699) | 2389 | 0.79 (0.73, 0.85) | 0.77 (0.70, 0.83) | 0.77 (0.70, 0.84) |
| Duration of breastfeeding | ||||
| Never (n=33 492) | 1555 | (Reference) | (Reference) | (Reference) |
| 1‐6 mo (n=23 666) | 1234 | 0.83 (0.76, 0.91) | 0.81 (0.74, 0.89) | 0.81 (0.74, 0.90) |
| 7‐12 mo (n=10 177) | 522 | 0.74 (0.66, 0.84) | 0.71 (0.62, 0.80) | 0.75 (0.66, 0.85) |
| 13 mo or more (n=12 575) | 612 | 0.75 (0.67, 0.84)d | 0.72 (0.64, 0.81)d | 0.74 (0.65, 0.83)d |
CI indicates confidence interval; HR, hazard ratio.
Adjusted for age, regional center, and extension study inclusion.
Adjusted for age, regional center, extension study inclusion, race/ethnicity, education, parity, age at menarche, and family history.
Adjusted for age, regional center, extension study inclusion, race/ethnicity, education, parity, age at menarche, family history, exercise at baseline, Healthy Eating Index at baseline, smoking history, body mass index at baseline, and multivitamin use at baseline.
Test for trend significant at the P<0.01 level.
The association between ever breastfeeding and all categories of breastfeeding duration was statistically significant for non‐Hispanic white and non‐Hispanic black women (Table 3). The association between ever breastfeeding and risk of stroke was strongest for non‐Hispanic black women (adjusted hazard ratio=0.52; 95% CI 0.37‐0.71). When minimally adjusted, the association of a reduced risk of stroke increased with breastfeeding duration in non‐Hispanic white and non‐Hispanic black women (test for trend P<0.01); this relationship changed little when further adjusted.
Table 3.
Risk of Stroke and Breastfeeding History by Race/Ethnicity
| Number of Strokes | Model 1 Adjusted HRa (95% CI) | Model 2 Adjusted HRb (95% CI) | |
|---|---|---|---|
| Breastfed ever | |||
| Non‐Hispanic white (n=67 075) | |||
| No (n=28 394) | 1300 | 1.0 | 1.0 |
| Yes (n=38 681) | 1919 | 0.80 (0.74, 0.88) | 0.79 (0.73, 0.87) |
| Non‐Hispanic black (n=6319) | |||
| No (n=2772) | 163 | 1.0 | 1.0 |
| Yes (n=3547) | 251 | 0.54 (0.40, 0.72) | 0.52 (0.37, 0.71) |
| Hispanic (n=3073) | |||
| No (n=1129) | 34 | 1.0 | 1.0 |
| Yes (n=1944) | 83 | 0.45 (0.23, 0.88) | 0.68 (0.30, 1.54) |
| Other race/ethnicity (n=3724) | |||
| No (n=1197) | 58 | 1.0 | 1.0 |
| Yes (n=2527) | 136 | 0.77 (0.52, 1.14) | 0.76 (0.48, 1.21) |
| Duration of breastfeeding | |||
| Non‐Hispanic white (n=66 900) | |||
| Never (n=28 394) | 1300 | 1.0 | 1.0 |
| 1‐6 mo (n=19 480) | 996 | 0.84 (0.76, 0.93) | 0.84 (0.76, 0.93) |
| 7‐12 mo (n=8473) | 413 | 0.76 (0.67, 0.87) | 0.74 (0.65, 0.85) |
| 13 mo or more (n=10 553) | 495 | 0.77 (0.68, 0.87)d | 0.75 (0.66, 0.85)d |
| Non‐Hispanic black (n=6258) | |||
| Never (n=2772) | 163 | 1.0 | 1.0 |
| 1‐6 mo (n=1918) | 126 | 0.66 (0.46, 0.94) | 0.71 (0.49, 1.04) |
| 7‐12 mo (n=786) | 69 | 0.46 (0.31, 0.70) | 0.41 (0.26, 0.64) |
| 13 mo or more (n=782) | 50 | 0.46 (0.29, 0.73)d | 0.34 (0.20, 0.58)d |
| Hispanic (n=3044) | |||
| Never (n=1129) | 34 | 1.0 | … |
| 1‐6 mo (n=956) | 37 | 0.41 (0.19, 0.86) | … |
| 7‐12 mo (n=394) | 14 | 0.91 (0.27, 3.15) | … |
| 13 mo or more (n=565) | 32 | 0.42 (0.18, 0.96) | … |
| Other race/ethnicityc (n=3708) | |||
| Never (n=1197) | 58 | 1.0 | … |
| 1‐6 mo (n=1312) | 75 | 0.80 (0.52, 1.24) | … |
| 7‐12 mo (n=524) | 26 | 0.77 (0.44, 1.38) | … |
| 13 mo or more (n=675) | 35 | 0.68 (0.37, 1.24) | … |
CI indicates confidence interval; HR, hazard ratio.
Adjusted for age, regional center, and extension study inclusion.
Adjusted for age, regional center, extension study inclusion, race/ethnicity, education, parity, age at menarche, and family history.
Due to the limited number of observed events, further adjustment for modifiable risk factors was not possible.
Test for trend significant at the P<0.01 level.
Discussion
This study sample was drawn from a large racially and ethnically diverse cohort of women who participated in the WHI, a longitudinal national health study that focused on strategies to prevent chronic disease in postmenopausal women. Participating women received long‐term follow‐up (median 12.6 years) for incident stroke. Study findings suggest that women who reported ever breastfeeding appeared to have a lower risk of stroke than parous women who never breastfed; this association seemed particularly strong for non‐Hispanic black women. Additionally, lifetime duration of any breastfeeding was associated with lower risk of stroke among postmenopausal women, especially among non‐Hispanic black women.
Many studies have reported that longer duration of breastfeeding may reduce long‐term risk of cardiovascular markers including hypertension, hyperlipidemia, and metabolic syndrome.32, 33, 34, 35, 36, 37, 47 Few studies have measured cardiovascular markers longitudinally from time of breastfeeding to menopause, and those that have are observational studies that are limited to self‐reported disease outcomes. In their review of the literature, Binns and colleagues state that current evidence suggests the beneficial effects of breastfeeding on chronic disease among mothers who breastfeed and infants who are breastfed.44 Without knowing the exact biological pathways of breastfeeding's protective effect, we do know that breastfeeding has few contraindications and that breastfeeding women tend to live healthier lives, so it should continue to be promoted for mothers and their infants. Meanwhile, as the links among breastfeeding, maternal biological changes, and future disease risk have not yet been firmly established, we do need longitudinal biochemical measurements to corroborate epidemiologic evidence that lactation positively influences chronic disease etiology.
One other possible explanation is that the observed association of breastfeeding with lower risk of stroke may be due to unmeasured confounding or systematic error in assessing measured risk factors. Women who never breastfed generally had less‐healthy lifestyles evidenced by higher rates of smoking, higher BMI, less exercise, and less healthy food consumption, and these factors contribute to a higher risk of stroke. However, adjusting for these covariates had little effect on the association between breastfeeding and risk of stroke, which remained statistically significant. Moreover, the estimated effect of an unmeasured confounder with stroke and breastfeeding would need a risk ratio of 1.7 or greater to impact the comparison of primary interest as measured by the e‐value.
Health Disparities in Stroke and Breastfeeding
Although this study's findings could not establish a causal relationship between breastfeeding and risk of stroke, health disparities surrounding stroke1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and breastfeeding rates52, 53, 54 persist. Stroke is 1 of the leading causes of death among Hispanic and non‐Hispanic black women over 65.2, 4 These same groups of women also experience low breastfeeding rates.52, 53, 54
According to a recent report by the Robert Wood Johnson Foundation, health disparities are rooted in inequities among available resources associated with healthier outcomes including living and working conditions, education, income, residence, geographic location, social support, and medical care.68 In other words these social determinants of health are components of an individual's life that can impact their health and thus could be a cause of health inequities. Documentation of these social determinants and resulting health inequities can help to identify the obstacles that need to be overcome and to place greater emphasis on groups previously marginalized.68 For example, sufficient scientific evidence exists that, compared with non‐Hispanic white women, Hispanic and non‐Hispanic black women more often develop stroke risk factors including hypertension, obesity, and diabetes mellitus and frequently report physical inactivity.1, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Additionally, evidence exists that compared with non‐Hispanic whites, breastfeeding that is less than the recommended duration of 6 months is associated with a greater burden of disease among Hispanic and non‐Hispanic black women and their children.69, 70 There are many other documented reasons for the unequal distribution of stroke and nonadherence to the recommended length of breastfeeding including the fact that Hispanic and non‐Hispanic black women tend to work in jobs that are less supportive of breastfeeding, earn less, attain less education, have less social support, and have limited access to healthcare services.53, 71, 72, 73
In summary, these social determinants of health hinder breastfeeding and the ability to make healthy choices to help minimize stroke risk factors such as the ones mentioned above. This study provides data to inform interventions that aim to reduce stroke risk factors by promoting healthy lifestyle behaviors including appropriate and supportive breastfeeding interventions, thereby reducing the overall health burden of stroke among parous women. These study data suggest that non‐Hispanic black women may especially benefit from longer breastfeeding duration as 1 of many factors to help guard against stroke.
Study Limitations and Future Direction
This study is limited by the total number of strokes that occurred in the sample despite WHI's large sample size and extended duration of follow‐up as well as WHI's exclusion of enrolling postmenopausal women who had experienced severe or fatal strokes.58 Approximately one‐third of the strokes were self‐reported at WHI baseline and were not adjudicated by medical record review, limiting classification by stroke subtype (eg, ischemic versus hemorrhagic). The inclusion of self‐reported strokes also provides potential for recall bias if women who had strokes later were less likely to report breastfeeding. Additionally, in a small number of women, stroke may have preceded childbearing and prevented those women from breastfeeding. Further, source data lacked information on pregnancy‐related complications (ie, gestational diabetes mellitus, pregnancy‐induced hypertension, pre‐eclampsia), gestational weight gain, physiological conditions that prevented women from breastfeeding, exclusive breastfeeding behavior, and whether women breastfed for less than 1 month. Also, the average age at baseline was 63.7 years, thereby indicating that women were subject to recall bias and inability to remember the total number of months they breastfed. However, previous research has shown that recalled breastfeeding practices including total duration of breastfeeding are quite accurate, including recalls up to 20 years later.74, 75 Further, a small number of events limited the ability to fully adjust the model for Hispanic women and women who self‐identified as another race/ethnicity. Additionally, women who were overweight or obese before pregnancy, gained excessive weight during pregnancy, or experienced pregnancy‐related complications may have had trouble breastfeeding76, 77, 78, 79, 80, 81 and may have an increased risk of stroke later in life, and thus, breastfeeding for longer durations could be a marker for better health in general rather than specifically a risk factor for stroke. Last, it is important to note that this article examined the relationship between stroke risk and breastfeeding and did not address whether racial/ethnic differences in breastfeeding contribute to disparities in stroke risk. Future research should consider the degree to which breastfeeding attenuates racial/ethnic differences in stroke risk.
Conclusion
Breastfeeding in this study was associated with a lower risk of stroke among postmenopausal women, particularly non‐Hispanic black women. This association remained statistically significant even after adjustment for other stroke risk factors potentially modified by or associated with lactation. Study findings could partially be explained by the fact that breastfeeding women tend to live healthier lives than nonbreastfeeding women, indicating that breastfeeding is more of a risk marker than risk factor. One may still conclude that breastfeeding along with other risk factors or risk markers during women's early reproductive years may be associated with stroke risk later in life, and their identification may help healthcare providers to assess a woman's risk profile. Further, by understanding these risk factors and risk markers, the medical and behavioral science communities may be better able to design, implement, and administer culturally informed programs that may mitigate stroke risk while promoting healthy lifestyle behaviors including breastfeeding among populations that carry the largest health burden of stroke. The effectiveness of these types of interventions should be investigated in future studies. Increasing public awareness of the potential impact of breastfeeding on maternal health outcomes later in life may assist in the initiation and continuation of breastfeeding for those at greatest risk.
Sources of Funding
This study is funded by Frontiers: The Heartland Institute for Clinical and Translational Research and the Wichita Center for Graduate Medical Education‐Kansas Bioscience Authority. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Disclosures
None.
Acknowledgments
The Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD) included Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. The Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA) included Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and academic centers included (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski‐Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston‐Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis. Women's Health Initiative Memory Study (Wake Forest University School of Medicine, Winston‐Salem, NC) Mark Espeland. For a list of all the investigators who have contributed to WHI science, please visit https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
(J Am Heart Assoc. 2018;7:e008739 DOI: 10.1161/JAHA.118.008739.)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Center for Health Statistics. Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death in Selected Age Groups, by Race and Sex. United States, 2014. Available at: http://www.cdc.gov/nchs/nvss/mortality/lcwk3.htm. Updated May 17, 2017. Accessed June 21, 2017.
- 3. Heron M. Deaths: Leading causes for 2013. National Vital Statistics Reports. Vol 65. Hyattsville, MD; National Center for Health Statistics; 2016. [Google Scholar]
- 4. Centers for Disease Control and Prevention . National Center for Health Statistics. Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death in Selected Age Groups, by Hispanic Origin, Race for non‐Hispanic Population and Sex. United States, 2014. Available at: http://www.cdc.gov/nchs/nvss/mortality/lcwk6.htm. Updated May 17, 2017. Accessed June 21, 2017
- 5. Sacco RL, Boden‐Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race‐ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–1731. [DOI] [PubMed] [Google Scholar]
- 6. Ayanian JZ, Landon BE, Newhouse JP, Zaslavsky AM. Racial and ethnic disparities among enrollees in Medicare Advantage plans. N Engl J Med. 2014;371:2288–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonnell MN, Hillier SL, Hooker SP, Le A, Judd SE, Howard VJ. Physical activity frequency and risk of incident stroke in a national US study of blacks and whites. Stroke. 2013;44:2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kisseal BM. Stroke incidence is decreasing in whites but not in blacks: a population‐based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al‐Wabil A, Al‐Senani F, Brown DL, Moye LA. Excess stroke in Mexican Americans compared with non‐Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgenstern LB, Smith MA, Sanchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Baek J, Lisabeth LD. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgenstern LB, Kissela BM. Stroke disparities: large global problem that must be addressed. Stroke. 2015;46:3560–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, Moomaw CJ, Schneider A, Miller R, Shukla R, Kissela B. The unchanging incidence and case‐fatality of stroke in the 1990s: a population‐based study. Stroke. 2006;37:2473–2478. [DOI] [PubMed] [Google Scholar]
- 13. Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kessela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R, Broderick J. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. [DOI] [PubMed] [Google Scholar]
- 15. Petrea RE, Beiser AS, Seshadri S, Kelly‐Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly‐Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. [DOI] [PubMed] [Google Scholar]
- 18. Gutierrez J, Williams OA. A decade of racial and ethnic stroke disparities in the United States. Neurology. 2014;82:1080–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011‐2014. NCHS Data Brief. 2015: 1–8. [PubMed]
- 20. Hayes DK, Denny CH, Keenan NL, Croft JB, Sundaram AA, Greenlund KJ. Racial/ethnic and socioeconomic differences in multiple risk factors for heart disease and stroke in women: behavioral risk factor surveillance system, 2003. J Womens Health. 2006;15:1000–1008. [DOI] [PubMed] [Google Scholar]
- 21. Sundaram AA, Ayala C, Greenlund KJ, Keenan NL. Differences in the prevalence of self‐reported risk factors for coronary heart disease among American women by race/ethnicity and age: Behavioral Risk Factor Surveillance System, 2001. Am J Prev Med. 2005;29:25–30. [DOI] [PubMed] [Google Scholar]
- 22. Willey JZ, Paik MC, Sacco R, Elkind MS, Boden‐Albala B. Social determinants of physical inactivity in the Northern Manhattan Study (NOMAS). J Community Health. 2010;35:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . Diabetes Report Card 2014. Atlanta, GA: Centers for Disease Control and Prevention, US Departmentof Health and Human Services; 2015. [Google Scholar]
- 24. Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Natl Health Stat. 2012;10:1–207. [PubMed] [Google Scholar]
- 25. Mathieu RA, Powell‐Wiley TM, Ayers CR, McGuire DK, Khera A, Das SR, Lakoski SG. Physical activity participation, health perceptions, and cardiovascular disease mortality in a multiethnic population: the Dallas Heart Study. Am Heart J. 2012;163:1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crespo CJ, Smit E, Andersen RE, Carter‐Pokras O, Ainsworth BE. Race/ethnicity, social class and their relation to physical inactivity during leisure time: results from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Prev Med. 2000;18:46–53. [DOI] [PubMed] [Google Scholar]
- 27. Ickes MJ, Sharma M. A systematic review of physical activity interventions in Hispanic adults. J Environ Public Health. 2012;2012:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44:S126–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernier MO, Plu‐Bureau G, Bossard N, Ayzac L, Thalabard JC. Breastfeeding and risk of breast cancer: a meta‐analysis of published studies. Hum Reprod Update. 2000;6:374–386. [DOI] [PubMed] [Google Scholar]
- 30. Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes Control. 2007;18:517–523. [DOI] [PubMed] [Google Scholar]
- 31. Jordan SJ, Cushing‐Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Breast‐feeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McClure CK, Catov JM, Ness RB, Schwarz EB. Lactation and maternal subclinical cardiovascular disease among premenopausal women. Am J Obstet Gynecol. 2012;207:46.e1–46.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwarz EB, McClure CK, Tepper PG, Thurston R, Janssen I, Matthews KA, Sutton‐Tyrrell K. Lactation and maternal measures of subclinical cardiovascular disease. Obstet Gynecol. 2010;115:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich‐Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1–138.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stuebe AM, Schwarz EB, Grewen K, Rich‐Edwards JW, Michels KB, Foster EM, Curhan G, Forman J. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunderson EP, Jacobs DR Jr, Chiang V, Lewis CE, Feng J, Quesenberry CP, Sidney S. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20‐year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults)+++. Diabetes. 2010;59:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stuebe AM, Rich‐Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. [DOI] [PubMed] [Google Scholar]
- 39. Schwarz EB, Brown JS, Creasman JM, Stuebe A, McClure CK, Van Den Eeden SK, Thom D. Lactation and maternal risk of type 2 diabetes: a population‐based study. Am J Med. 2010;123:863.e1–863.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gunderson EP, Hurston SR, Ning X, Lo JC, Crites Y, Walton D, Dewey KG, Azevedo RA, Young S, Fox G, Elmasian CC, Salvador N, Lum M, Sternfeld B, Quesenberry CP Jr. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med. 2015;163:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N, Lum M, Quesenberry CP, Lo JC, Sternfeld B, Ferrara A, Selby JV. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chouinard‐Castonguay S, Weisnagel SJ, Tchernof A, Robitaille J. Relationship between lactation duration and insulin and glucose response among women with prior gestational diabetes. Eur J Endocrinol. 2013;168:515–523. [DOI] [PubMed] [Google Scholar]
- 43. O'Reilly M, Avalos G, Dennedy MC, O'Sullivan EP, Dunne FP. Breast‐feeding is associated with reduced postpartum maternal glucose intolerance after gestational diabetes. Ir Med J. 2012;105:31–36. [PubMed] [Google Scholar]
- 44. Binns C, Lee M, Low WY. The long‐term public health benefits of breastfeeding. Asia Pac J Public Health. 2016;28:7–14. [DOI] [PubMed] [Google Scholar]
- 45. Kirkegaard H, Stovring H, Rasmussen KM, Abrams B, Sorensen TI, Nohr EA. How do pregnancy‐related weight changes and breastfeeding relate to maternal weight and BMI‐adjusted waist circumference 7 y after delivery? Results from a path analysis. Am J Clin Nutr. 2014;99:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin J, MacDonald‐Wicks L, Hure A, Smith R, Collins CE. Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: a pilot randomised controlled trial. Nutrients. 2015;7:1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stuebe AM, Rich‐Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Academy of Pediatrics . Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. [DOI] [PubMed] [Google Scholar]
- 49. Saadeh MR. A new global strategy for infant and young child feeding. Forum Nutr. 2003;56:236–238. [PubMed] [Google Scholar]
- 50. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion . Healthy People 2020. Maternal, infant, and child health objectives. Available at: http://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives. Accessed May 12, 2016.
- 51. Centers for Disease Control and Prevention . Breastfeeding Report Card United States. 2016. Available at: http://www.cdc.gov/breastfeeding/data/reportcard.htm. Updated December 1, 2017. Accessed October 1, 2016.
- 52. Centers for Disease Control and Prevention . Rates of Any and Exclusive Breastfeeding by Socio‐demographics Among Children Born in 2012. Available at: http://www.cdc.gov/breastfeeding/data/nis_data/rates-any-exclusive-bf-socio-dem-2012.htm. Accessed April 18, 2016.
- 53. Jones KM, Power ML, Queenan JT, Schulkin J. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McKinney CO, Hahn‐Holbrook J, Chase‐Lansdale PL, Ramey SL, Krohn J, Reed‐Vance M, Raju TN, Shalowitz MU. Racial and ethnic differences in breastfeeding. Pediatrics. 2016;138:e20152388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McClure CK, Schwarz EB, Conroy MB, Tepper PG, Janssen I, Sutton‐Tyrrell KC. Breastfeeding and subsequent maternal visceral adiposity. Obesity. 2011;19:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McClure CK, Catov J, Ness R, Schwarz EB. Maternal visceral adiposity by consistency of lactation. Matern Child Health J. 2012;16:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. WHI Study Group . Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 58. Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. [DOI] [PubMed] [Google Scholar]
- 59. Baird J, Jarman M, Lawrence W, Black C, Davies J, Tinati T, Begum R, Mortimore A, Robinson S, Margetts B, Cooper C, Barker M, Inskip H. The effect of a behaviour change intervention on the diets and physical activity levels of women attending Sure Start Children's Centres: results from a complex public health intervention. BMJ Open. 2014;4:e005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tong V, Dietz P, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, England LJ. Trends in smoking before, during, and after pregnancy—pregnancy risk assessment monitoring system, United States, 40 Sites, 2000–2010. MMWR Morb Mortal Wkly Rep. 2013;62:1–19. [PubMed] [Google Scholar]
- 61. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. [DOI] [PubMed] [Google Scholar]
- 62. Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–S86. [DOI] [PubMed] [Google Scholar]
- 63. Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx‐Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. [DOI] [PubMed] [Google Scholar]
- 64. Women's Health Initiative . Researchers. Available at: https://www.whi.org/researchers/SitePages/Home.aspx. Accessed June 1, 2017.
- 65. Royston P, Parmar MK. Flexible parametric proportional‐hazards and proportional‐odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. [DOI] [PubMed] [Google Scholar]
- 66. US Department of Health and Human Services, US Department of Agriculture . Dietary Guidelines for Americans, 2005. 6th ed Washington, DC: US Government Printing Office; 2005. [Google Scholar]
- 67. VanderWeele TJ. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268. [DOI] [PubMed] [Google Scholar]
- 68. Braveman P, Arkin E, Orleans T, Proctor D, Plough A. What is Health Equity? And What Difference Does a Definition Make? Princeton, NJ: Robert Wood Johnson Foundation; 2017. [Google Scholar]
- 69. Bartick MC, Schwarz EB, Green BD, Jegier BJ, Reinhold AG, Colaizy TT, Bogen DL, Schaefer AJ, Stuebe AM. Suboptimal breastfeeding in the United States: maternal and pediatric health outcomes and costs. Matern Child Nutr. 2017;13:e12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bartick MC, Jegier BJ, Green BD, Schwarz EB, Reinhold AG, Stuebe AM. Disparities in breastfeeding: impact on maternal and child health outcomes and costs. J Pediatr. 2017;181:49–55.e6. [DOI] [PubMed] [Google Scholar]
- 71. Johnson AM, Kirk R, Muzik M. Overcoming workplace barriers: a focus group study exploring African American mothers’ needs for workplace breastfeeding support. J Hum Lact. 2015;31:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lind JN, Perrine CG, Li R, Scanlon KS, Grummer‐Strawn LM. Racial disparities in access to maternity care practices that support breastfeeding—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:725–728. [PMC free article] [PubMed] [Google Scholar]
- 73. McCarter‐Spaulding D, Lucas J, Gore R. Employment and breastfeeding outcomes in a sample of black women in the United States. J Natl Black Nurses Assoc. 2011;22:38–45. [PubMed] [Google Scholar]
- 74. Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63:103–110. [DOI] [PubMed] [Google Scholar]
- 75. Natland ST, Andersen LF, Nilsen TI, Forsmo S, Jacobsen GW. Maternal recall of breastfeeding duration twenty years after delivery. BMC Med Res Methodol. 2012;12:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Winkvist A, Brantsaeter AL, Brandhagen M, Haugen M, Meltzer HM, Lissner L. Maternal prepregnant body mass index and gestational weight gain are associated with initiation and duration of breastfeeding among Norwegian mothers. J Nutr. 2015;145:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bever Babendure J, Reifsnider E, Mendias E, Moramarco MW, Davila YR. Reduced breastfeeding rates among obese mothers: a review of contributing factors, clinical considerations and future directions. Int Breastfeed J. 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kachoria R, Moreland JJ, Cordero L, Oza‐Frank R. Trends in breastfeeding initiation, continuation, and exclusivity by maternal prepregnancy weight: 2004–2011. Obesity. 2015;23:1895–1902. [DOI] [PubMed] [Google Scholar]
- 79. Kair LR, Colaizy TT. When breast milk alone is not enough: barriers to breastfeeding continuation among overweight and obese mothers. J Hum Lact. 2016;32:250–257. [DOI] [PubMed] [Google Scholar]
- 80. Kendall‐Tackett K. Weighing in on obesity and breastfeeding: factors possibly related to lower breastfeeding rates in women with higher BMIs. Breastfeed Rev. 2015;23:7–12. [PubMed] [Google Scholar]
- 81. Nommsen‐Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first‐time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92:574–584. [DOI] [PubMed] [Google Scholar]


