Abstract
Background
In view of the high incidence of posterior capsule opacification (PCO) and the effects of TGF-β signaling on the epithelial-mesenchymal transition (EMT) of human lens epithelial cells (LECs), our study aimed to explore the mechanism of the function of TGF-β signaling in LECs EMT.
Material/Methods
Human lens epithelial cells (HLEC-h3) were treated with TGF-β, ILK siRNA, ILK inhibitor, and NF-κB inhibitor to study the effects of TGF-β, ILK, and NF-κB on cell migration and EMT. Cell migration assay was used to measure cell migration ability. Western blot was performed to detect the expression of ILK, E-cadherin, and α-SMA at the protein level. QRT-PCR was used to detect the expression of ILK at the mRNA level.
Results
Compared with control cells, TGF-β treatment increased the expression level of ILK HLEC-h3, promoted migration of HLEC-h3 cells, increased the expression level of E-cadherin protein, and decreased the expression level of α-SMA protein. However, treatment with ILK siRNA, ILK inhibitor, and NF-κB inhibitor reversed the effects of TGF-β on HLEC-h3 cells.
Conclusions
TGF-β-stimulated ILK regulates the migration and EMT of human LECs via NF-κB.
MeSH Keywords: Capsule Opacification, Epithelial Cells, Posterior Capsule of the Lens
Background
More than 8 million cataract surgeries are performed in the European Union and the United States every year, and this number is even higher in China [1]. In spite of the application of currently available protective measures, posterior capsule opacification (PCO) is still the most common complication of cataract surgery [2]. It has been reported that about 20–40% of patients with PCO will show visual loss or pupillary distortion with 2–5 years after surgery, seriously affecting patient quality of life [3]. The migration and epithelial-to-mesenchymal transition (EMT) of lens epithelial cells (LECs) are common causes of PCO [4,5]. Transforming growth factor-β (TGF-β) plays pivotal roles in fibrosis and EMT of LECs [6]. However, the mechanism of the function of TGF-β in this process is still unknown.
Integrin-linked kinase (ILK) is a downstream target of TGF-β, and the expression of ILK is regulated by TGF-β in different disease models [7]. ILK is also a key player in EMT of LECs, indicating the importance of signal transduction from TGF-β to ILK in the development of PCO [8]. ILK regulates the expression of E-cadherin and b-catenin [9], which play roles in cell EMT [10]. NF-κB is important in signaling of cell migration and invasion, is closely correlated with the migration of LECs, and can promote PCO [11]. NF-κB performs its biological functions through the interactions with a variety of cellular factors, including TGF-β. Therefore, it seems reasonable to hypothesize that TGF-β also interacts with NF-κB to participate in the development of PCO.
In the present study, the human lens epithelium cell line HLEC-h3 was used as a model to investigate the effect of NF-κB on migration and EMT of human lens epithelial cells, and to explore the possible mechanisms.
Material and Methods
Cells and cell culture
The human lens epithelium cell line HLEC-h3 was purchased from Shanghai Fu Meng Gene Biotechnology Co., Ltd. Cells were cultured in DMEM cell culture medium containing 10% fetal bovine serum in an incubator (37°C, 5% CO2).
Cell treatment
A group of cells were treated with serum-free medium for 24 h and TGF-β was added to make a final concentration of 10 ng/ml, and after incubation for 48 h, ILK siRNA transfection was performed siRNA using Lipofectamine 2000 (11668-019, Invitrogen, Carlsbad, USA). Another group of cells were first treated with 10 nmol/L of ILK inhibitor QLT0267 (QLT, Inc., Vancouver, Canada) or 10 μmol/L of NF-κB inhibitor BAY-11-7082 (Sigma-Aldrich) for 1 h, followed by incubation for 48 h in cell culture medium supplemented with 10 ng/ml of TGF-β.
Western blot
Total protein was extracted using cell lysis solutions (Thermo Fisher Scientific, USA). Nucleoprotein was extracted using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific™ Pierce™). Protein concentration was measured by BCA method. Then, 10% SDS-PAGE gel electrophoresis was performed using 20 μg of protein from each sample, followed by transfer from transmembrane to PVDF membrane (Bio-Rad, Hercules, CA, USA) at 25V for 1 h. Blocking was performed using 5% skimmed milk at room temperature for 1 h. After being washed with TBST, membranes were incubated with primary antibodies of IL-6 (Cell Signaling Technology, USA), NF-κB p65 (Cell Signaling Technology, USA), Histone H2A.X (Cell Signaling Technology, USA), E-cadherin (Santa Cruz Biotechnology, USA), α-SMA (Santa Cruz Biotechnology, USA), and β-actin (Sigma-Aldrich, USA) overnight at 4°C. After washing with TBST, membranes were further incubated with corresponding horseradish peroxidase-labeled IgG secondary antibody (1: 1000, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at room temperature for 1 h. After washing with TBST, signals were detected using ECL (Sigma-Aldrich, USA) method.
Cell migration assay
Cell migration ability was detected by Transwell cell migration assay (BD Biosciences, USA). The upper chamber was filled with cell suspension containing 1×104 cells, while the lower chamber was filled with RPMI-1640 medium (Thermo Fisher Scientific, USA) containing 20% FCS (Sigma-Aldrich, USA). After incubation for 48 h, membranes were collected and stained with 0.5% crystal violet (Sigma-Aldrich, USA) for 30 min. Stained cells were counted under an optical microscope (Olympus, Japan).
Real-time quantitative PCR
Trizol reagent (Invitrogen, USA) was used for total RNA extraction from cells. The quality of RNA samples was tested using NanoDrop™ 2000 Spectrophotometers (Thermo Fisher Scientific, USA). RNA samples with a A260/A280 ratio between 1.8 and 2.0 were used in reverse transcription to synthesize cDNA using Oligo (dT) 15 (Sangon, Shanghai) and AMV reverse transcriptase (GIBCO, USA). SYBR® Green Real-Time PCR Master Mixes (Thermo Fisher Scientific, USA) was used for PCR reaction. The following primers were used in PCR reactions: 5′-TCCACCTGCTCCTCATCC-3′ (forward) and 5′-CCTCATCAATCATTACACTACGG-3′ (reverse) for ILK; 5′-GGCGGCACCACCATGTACCCT-3′ (forward) and 5′-AGGGGCCGGACTCGTCATACT-3′ (reverse) for β-actin. PCR reaction conditions were: 95°C for 15 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. Ct values were processed using 2−ΔΔCT method, and relative expression level of UCA1 was normalized to endogenous control β-actin.
Reporter gene assay
NF-κB transcriptional activity was detected using the Cignal Reporter Assay kit (CCS-013L, Qiagen). Reporter plasmid was transfected using Lipofectamine 2000 (Life Technologies, USA). The Dual-Luciferase Reporter Assay System (Promega, USA) was used to detect the luminescence of luciferase.
Statistical analysis
SPSS19.0 (SPSS, Inc., USA) was used for all statistical analyses. Comparisons between groups were performed by t test or Mann-Whitney rank sum test. p<0.05 was considered to be statistically significant.
Results
Effects of TGF-β treatment on migration and EMT of HLEC-h3 cells
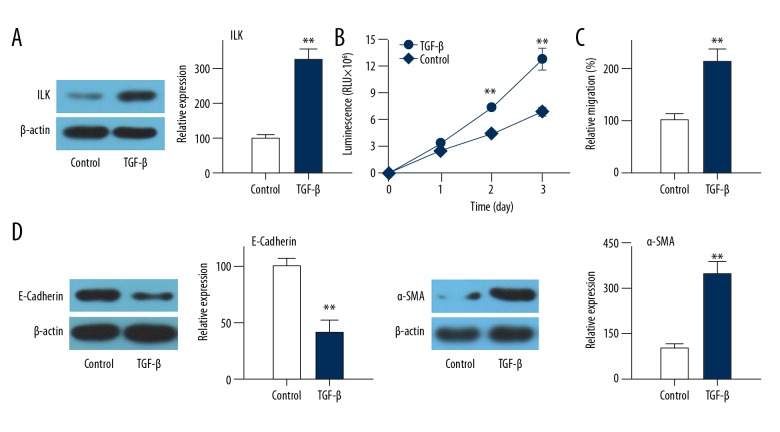
TGF-β regulation of EMT has been suggested to be dependent on integrin-linked kinase (ILK) function during kidney fibrosis [12]. Thus, we examined the effect of TGF-β treatment on ILK expression. Compared with control cells, the expression level of ILK was significantly increased in HLEC-h3 cells after treatment with TGF-β, (Figure 1A). In addition, the migration ability of TGF-β-treated cells was significantly better than that of control cells (Figure 1B, p<0.01). Effects of TGF-β treatment on EMT of HLEC-h3 cells were further compared with control cells, showing that epithelial marker E-cadherin protein expression was significantly down-regulated, while EMT marker α-SMA protein expression was significantly up-regulated after treatment with TGF-β (Figure 1C, 1D, p<0.01). These data suggest that TGF-β treatment can promote migration and EMT of HLEC-h3 cells, as also indicated by other studies [6].
Figure 1.
Effects of TGF-β treatment on migration and EMT of HLEC-h3 cells. (A) Effects of TGF-β treatment on ILK expression. (B) Effects of TGF-β treatment on cell migration. (C) Effects of TGF-β treatment on E-cadherin expression. (D) Effects of TGF-β treatment on α-SMA expression. Each experiment was independently repeated 3 times. ** Compared with control group, p<0.01
Effects of TGF-β treatment on NF-κB
NF-κB is to be closely correlated with the migration of LECs, and it can promote PCO [11], while effects of TGF-β treatment on NF-κB expression and activity need to be confirmed. Compared with control cells, levels of NF-κB-p65 in nucleus of HLEC-h3 cells were significantly increased (Figure 2A). In addition, NF-κB activity was also significantly increased (Figure 2B). These data suggest that TGF-β treatment can promote the expression and activation of NF-κB.
Figure 2.
Effect of TGF-β treatment on NF-κB. (A) Effect of TGF-β treatment on the expression of NF-κB-p65 in the nucleus detected by Western blot. (B) Luciferase reporter system to detect the effect of TGF-β treatment on NF-κB activity. Each experiment was independently repeated 3 times. ** Compared with control group, p<0.01.
Effects of ILK expression downregulation or reduced ILK activity on NF-κB activity
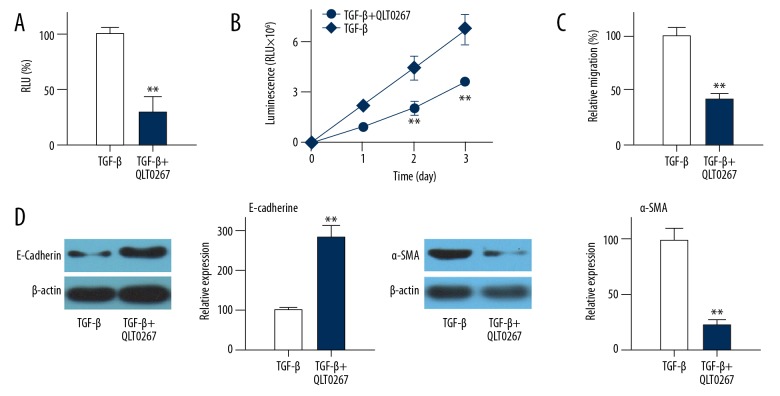
ILK siRNA and small-molecule inhibitor QLT0267 were used to explore the effects of ILK expression downregulation or reduced NF-κB activity on NF-κB activity. Compared with control cells, ILK siRNA transfection significantly reduced the NF-κB activity (Figure 3A, p<0.01) and migration (Figure 3B, p<0.01), promoted the expression of E-cadherin (Figure 3C, p<0.01), and reduced the expression of α-SMA (Figure 3D, p<0.01).
Figure 3.
Effects of ILK expression downregulation on NF-κB activity. (A) Effects of ILK siRNA treatment on NF-κB activity in EMT cell model. (B) Effects of ILK siRNA treatment on cell migration. (C) Effects of ILK siRNA treatment on E-cadherin expression. (D) Effects of ILK siRNA treatment on α-SMA expression. Each experiment was independently repeated 3 times. Control group was TGF-β-induced transdifferentiated cells. ** Compared with control group, p<0.01; RLU, relative light unit. Similar results were found after treatment with QLT0267. Compared with control cells, treatment with QLT0267 significantly reduced the NF-κB activity (Figure 4A, p<0.01), inhibited cell migration (Figure 4B, p<0.01), promoted the expression of E-cadherin (Figure 4C, p<0.01), and reduced the expression of α-SMA (Figure 4D, p<0.01). Those data suggest that expression and activity of ILK are essential for the activity of NF-κB as well as migration and EMT of HLEC-h3 cells. Each experiment was independently repeated 3 times.
Effects of NF-κB specific inhibitor BAY-11-7082 on migration and EMT of HLEC-h3 cells
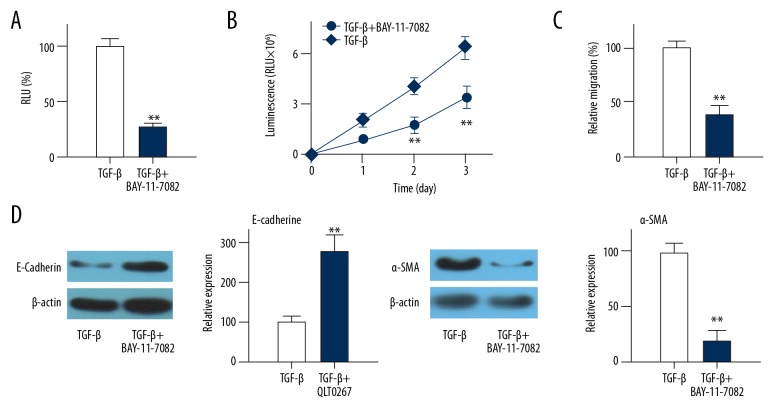
Compared with control group, treatment with BAY-11-7082 significantly reduced the NF-κB activity (Figure 5A, p<0.01) and migration (Figure 5B, p<0.01), increased the expression level of E-cadherin (Figure 5C, p<0.01), and reduced the expression of α-SMA (Figure 5D, p<0.01). These data suggest that NF-κB activity is important for migration and EMT of HLEC-h3 cells.
Figure 5.
Effects of NF-κB specific inhibitor BAY-11-7082 on migration and EMT of HLEC-h3 cells. (A) Effects of BAY-11-7082 treatment on NF-κB activity in EMT cell model. (B) Effects of BAY-11-7082 treatment on cell migration. (C) Effects of BAY-11-7082 treatment on E-cadherin expression. (D) Effects of BAY-11-7082 treatment on α-SMA expression. Control group was TGF-β-induced transdifferentiated cells. Each experiment was independently repeated 3 times. ** Compared with control group, p<0.01; RLU – relative light unit.
Discussion
Cataract is a common disease, especially for women older than 40 years [13]. As the most common complication of cataract surgery, PCO affects more than 10% of patients within the first year after surgery, and the proportion increases to 20% 1 year later [2,3]. Development of PCO is affected by various factors, such as surgical methods [2]. Although a considerable number of genetic factors have been identified to be responsible for the progression of PCO, the pathogenesis of this disease still has not been fully elucidated. EMT of LECs is a common cause of PCO [4,5]. TGF-β is the major cytokine that promotes EMT-driven fibrosis in many systems and diseases [14]. For example, TGF-β has been proven to compete with BMP signaling to regulate the EMT level of both type II to type I alveolar epithelial cells [15]. In addition, TGF-β can also regulate the CTGF and gremlin to induce fibrosis and EMT of human LECs, indicating the potential role of TGF-β in the development of PCO. ILK is a serine/threonine protein kinase, as well as a focal adhesion adaptor regulate proliferation, survival, and epithelial-to-mesenchymal transition of different types of cells, including LECs [8,16]. NF-κB is also reported to be closely correlated with the migration and proliferation of LECs [11]. Consistent with previous studies, in our study, TGF-β treatment significantly increased migration of HLEC-h3 cells. In addition, expression of E-cadherin protein (an epithelial marker) was significantly down-regulated, while α-SMA protein (a cell EMT maker) expression was significantly up-regulated after treatment with TGF-β, indicating that TGF-β can promote migration and EMT of HLEC-h3 cells. However, ILK siRNA silencing and treatment with ILK and NF-κB inhibitors significantly inhibited migration and EMT of HLEC-h3 cells. These data suggest that TGF-β, ILK, and NF-κB play important roles in migration and EMT of HLEC-h3 cells.
ILK is a mediator regulating transdifferentiation in PCO-related EMT [8]. In a melanoma study, Janji et al. reported that TGF-β can regulate the expression of ILK, so as to determine tumor metastasis [7]. TGF-β also regulates ILK glomerular cells to participate in the development of glomerular damage [17]. ILK overexpression down-regulates the expression of E-cadherin through different pathways in different models so as to promote cell EMT [17]. In addition, the function of ILK has been reported to be critical for the role of TGF-β in regulating cell EMT [16]. All these previous studies suggest that ILK also serves as a downstream target of TGF-β to participate in the regulation of LECs EMT. Consistent with previous studies, in our study, treatment with TGF-β significantly increased the expression level of ILK in human LECs. In addition, treatment with ILK siRNA and inhibitor significantly inhibited the migration of LECs, increased the expression level of E-cadherin, and decreased the expression level of α-SMA. These data suggest that ILK serves as a downstream target of TGF-β signaling to participate in the development of PCO.
The cross-talk between TGF-β signaling and NF-κB is critical for various biological processes, including the development of different human diseases. In a study of lung cancer, suppression of NF-κB-mediated Snail activation was found to inhibit epithelial–mesenchymal transition induced by TGF b [18]. In the development of glioblastoma, NF-κB can induce the expression of miR-148a, which in turn sustains activation of TGF-β/Smad signaling [19]. In our study, treatment with TGF-β significantly increased the expression and activity of NF-κB. However, ILK siRNA silencing and treatment with ILK inhibitor significantly reduced the expression and activity of NF-κB. Inhibition of NF-κB itself also significantly inhibited the migration and EMT of HLEC-h3 cells. All these data suggest that NF-κB serves as a downstream target of TGF-β/ILK signaling to participate in PCO.
Conclusions
TGF-β treatment increased the expression level of ILK HLEC-h3. It promoted migration of HLEC-h3 cells, increased the expression level of E-Cadherin protein, and decreased the expression level of α-SMA protein. Treatment with ILK siRNA, ILK inhibitor, and NF-κB inhibitor reversed the effects of TGF-β on HLEC-h3 cells. Therefore, we concluded that ILK mediates EMT of human LECs by interacting with NF-κB.
Figure 4.
Effects of QLT0267 treatment on NF-κB activity. (A) Effects of QLT0267 treatment on NF-κB activity in EMT cell mode. (B) Effects of QLT0267 treatment on cell migration. (C) Effects of QLT0267 treatment on E-cadherin expression. (D) Effects of QLT0267 treatment on α-SMA expression. Control group was TGF-β-induced transdifferentiated cells. Each experiment was independently repeated 3 times. ** Compared with control group, p<0.01; RLU – relative light unit.
Footnotes
Source of support: We thank the financial support from the National Natural Science Foundation of China (No. 81500705)
References
- 1.Wang SY, Stem MS, Oren G, et al. Patient-centered and visual quality outcomes of premium cataract surgery: A systematic review. Eur J Ophthalmol. 2017;27(4):387–401. doi: 10.5301/ejo.5000978. [DOI] [PubMed] [Google Scholar]
- 2.Maedel S, Buehl W, Findl O. Intraocular lens optic edge design for the prevention of posterior capsule opacification after cataract surgery. The Cochrane Library. :2017. doi: 10.1002/14651858.CD012516.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification. Arch Ophthalmol. 2009;127:555–62. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- 4.Cobo LM, Ohsawa E, Chandler D, et al. Pathogenesis of capsular opacification after extracapsular cataract extraction: An animal model. Ophthalmology. 1984;91:857–63. doi: 10.1016/s0161-6420(84)34225-7. [DOI] [PubMed] [Google Scholar]
- 5.Wormstone IM. Posterior capsule opacification: A cell biological perspective. Exp Eye Res. 2002;74:337–47. doi: 10.1006/exer.2001.1153. [DOI] [PubMed] [Google Scholar]
- 6.Lee EH, Joo CK. Role of transforming growth factor-β in EMT and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci. 1999;40(9):2025–32. [PubMed] [Google Scholar]
- 7.Janji B, Melchior C, Gouon V, et al. Autocrine TGF-β-regulated expression of adhesion receptors and integrin-linked kinase in HT-144 melanoma cells correlates with their metastatic phenotype. Int J Cancer. 1999;83(2):255–62. doi: 10.1002/(sici)1097-0215(19991008)83:2<255::aid-ijc18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Huet-Calderwood C, Brahme NN, Kumar N, et al. Differences in binding to the ILK complex determines kindlin isoform adhesion localization and integrin activation. J Cell Sci. 2014;127(Ptl9):4308–21. doi: 10.1242/jcs.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan C, Costello P, Sanghera J, et al. Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene. 2001;20(1):133–40. doi: 10.1038/sj.onc.1204052. [DOI] [PubMed] [Google Scholar]
- 10.Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal EMT in gastric epithelial cells. Oncogene. 2007;26(32):4617–26. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Bae S, Seomun Y, et al. The role of nuclear factor kappa B in lens epithelial cell proliferation using a capsular bag model. Ophthalmic Res. 2008;40(5):273–78. doi: 10.1159/000128162. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Tan X, Dai C, et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:1907–18. doi: 10.1681/ASN.2008090930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer RN, Rosenthal G. Comparison of cataract incidence in normal and glaucomatous population. Am J Ophthalmol. 1970;69(3):368–70. doi: 10.1016/0002-9394(70)92266-x. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Yee M, O’Reilly MA. EMT of alveolar epithelial type II to type I cells is controlled by opposing TGF-β and BMP signaling. Am J Physiol Lung Cell Mol Physiol. 2013;305(6):L409–18. doi: 10.1152/ajplung.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serrano I, McDonald PC, Lock FE, et al. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFβ-1-induced epithelial-mesenchymal transition (EMT) Oncogene. 2013;32(1):50–60. doi: 10.1038/onc.2012.30. [DOI] [PubMed] [Google Scholar]
- 17.Jung KY, Chen K, Kretzler M, et al. TGF-β1 regulates the PINCH-1-integrin-linked kinase – α-parvin complex in glomerular cells. J Am Soc Nephrol. 2007;18(1):66–73. doi: 10.1681/ASN.2006050421. [DOI] [PubMed] [Google Scholar]
- 18.Feng H, Lu JJ, Wang Y, et al. Osthole inhibited TGF β-induced epithelial-mesenchymal transition (EMT) by suppressing NF-κB mediated Snail activation in lung cancer A549 cells. Cell Adhesion & Migration. 2017:1–12. doi: 10.1080/19336918.2016.1259058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Pan JQ, Luo L, et al. NF-κB induces miR-148a to sustain TGF-β/Smad signaling activation in glioblastoma. Mol Cancer. 2015;14(1):2. doi: 10.1186/1476-4598-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]