Abstract
Vector abundance plays a key role in transmission of mosquito-borne disease. In Hawaii, Aedes albopictus (Skuse) (Diptera: Culicidae), the Asian tiger mosquito, has been implicated in locally-transmitted dengue outbreaks, while Culex quinquefasciatus Say (Diptera: Culicidae), the southern house mosquito, is the primary vector of avian malaria, a wildlife disease that has contributed to declines and extinctions of native Hawaiian birds. Despite the importance of these introduced species to human and wildlife health, little is known about the local-scale drivers that shape mosquito abundance across lowland Hawaii, where forest, agricultural, and residential land uses are prevalent. We examined landscape, larval habitat, and climate drivers of Ae. albopictus and Cx. quinquefasciatus abundance in eight lowland wet forest fragments on the Big Island of Hawaii. We found that the abundance of both species increased with the proportion of surrounding developed land and the availability of larval habitat, which were themselves correlated. Our findings suggest that conversion of natural habitats to residential and agricultural land increases mosquito larval habitats, increasing the abundance of Ae. albopictus and Cx. quinquefasciatus and increasing disease risk to humans and wildlife in Hawaii. Our results further indicate that while source reduction of artificial larval habitats—particularly moderately-sized human-made habitats including abandoned cars and tires—could reduce mosquito abundance, eliminating larval habitat will be challenging because both species utilize both natural and human-made larval habitats in lowland Hawaii.
Keywords: Culex, Aedes, larval survey, land use, avian malaria
Mosquito-borne infectious diseases pose major concerns for human health and the conservation of wildlife, as highlighted by the rapid geographic spread and increasing incidence of mosquito-borne viruses in the past two decades including West Nile, dengue, and Zika viruses (Kilpatrick 2011, Bhatt et al. 2013, Musso et al. 2015, Weaver et al. 2015). Transmission of mosquito-borne pathogens is driven by interactions between the environment, hosts, vectors, and the pathogen, with disease emergence often preceded by the invasion of introduced mosquito species (Lounibos 2002, Weaver and Reisen 2010, Kilpatrick and Randolph 2012). Once established, the distribution and abundance of mosquito vectors play a key role in transmission dynamics, and vector abundance is thus the focus of many vector-borne disease control efforts (Dye 1992, Townson et al. 2005). Determining the ecological factors that influence mosquito abundance can enable mapping of higher risk areas and facilitates efforts to reduce transmission for both human and wildlife pathogens.
In Hawaii, two vector-borne pathogens, dengue virus, and avian malaria, have impacted human and wildlife health, respectively, and are transmitted by several introduced mosquito species, including Aedes albopictus (Skuse) (Diptera: Culicidae), the Asian tiger mosquito, and Culex quinquefasciatus Say (Diptera: Culicidae), the southern house mosquito (VanRiper et al. 1986, Effler et al. 2005, LaPointe et al. 2005, Adalja et al. 2012). Ae. albopictus is an invasive mosquito that is widespread throughout Hawaii. It is a known vector for at least 22 arboviruses including dengue, chikungunya, and Zika virus (Gratz 2004, Burt et al. 2012, Musso and Gubler 2016). In 2001, Ae. albopictus was identified as the main vector in the first outbreak of locally-transmitted dengue in Hawaii since World War II on three islands, and again in 2011 on Oahu where Aedes aegypti L. (Diptera: Culicidae) was thought to be absent (Effler et al. 2005, Adalja et al. 2012). It likely also contributed to a recent outbreak on the island of Hawaii in 2015–2016 (http://health.hawaii.gov/docd/dengue-outbreak-2015/). Understanding the drivers of Ae. albopictus abundance is critical given the repeated outbreaks of dengue in Hawaii and the repeated introductions of other pathogens including Zika and Chikungunya (http://www.cdc.gov/zika/reporting/2017-case-counts.html; http://www.cdc.gov/chikungunya/geo/united-states-2017.html).
Cx. quinquefasciatus, the southern house mosquito, is a primary vector of several human diseases including filariasis and West Nile virus encephalitis, as well as wildlife diseases such as avian malaria (Bogh et al. 1998, Turell et al. 2002, LaPointe et al. 2005, Kimura et al. 2010). It was the first of six biting mosquito species introduced to Hawaii, and it spread throughout the main Hawaiian Islands following its introduction in 1826 (Hardy 1960). Cx. quinquefasciatus has contributed to the decline or extinction of many susceptible native Hawaiian birds by vectoring two introduced pathogens, avian pox (Poxvirus avium) and avian malaria (Plasmodium relictum; Warner 1968, VanRiper et al. 1986, Van Riper et al. 2002, LaPointe et al. 2005). Avian malaria transmission limits many native bird species to upper elevation forests where cooler temperatures reduce both Cx. quinquefasciatus population growth and avian malaria replication rates (VanRiper et al. 1986, LaPointe et al. 2010). Cx. quinquefasciatus is also a competent vector for West Nile virus, which could be introduced to Hawaii by multiple pathways and could severely impact both human health and native birds (Kilpatrick et al. 2004, LaPointe et al. 2009, Reisen et al. 2009).
Mosquito abundance is generally thought to be driven by interactions between climate, larval habitat, and host availability. Temperature and precipitation both have strong and sometimes nonlinear effects on multiple aspects of mosquito demography (Ciota et al. 2014). Increasing temperature speeds larval development and shortens the gonotrophic cycle in adults but decreases survival rates (Delatte et al. 2009, Ruybal et al. 2016). Precipitation can increase or decrease mosquito populations, depending on the intensity of rainfall, by creating or flushing larval habitats, and can increase adult mortality (Hayes and Downs 1980, Koenraadt and Harrington 2008, Jones et al. 2012). Ae. albopictus and Cx. quinquefasciatus both use container habitats for larval development, and especially human-made containers (Goff and van Riper 1980, Laird 1988, Yee 2008, Bartlett-Healy et al. 2012). In addition, Ae. albopictus often feeds predominantly on humans (Faraji et al. 2014), whereas Cx. quinquefasciatus feeds on a broader set of hosts (Farajollahi et al. 2011).
We examined drivers of Ae. albopictus and Cx. quinquefasciatus abundance in eight lowland forest fragments on the Big Island of Hawaii. We conducted a fine-scale larval habitat availability study, quantified surrounding land use, and obtained estimates of temperature and precipitation data for each site. Because both species are considered human commensals, we hypothesized that their abundance would increase with the proportion of developed land in the surrounding area, and with larval habitat availability. We did not have a priori hypotheses for the directional effects of rainfall or temperature since both can both increase and decrease mosquito abundance, as detailed above.
Methods
Study Sites and Mosquito Capture
We captured mosquitoes at six sites ranging from 18 to 349 m in elevation on the Big Island of Hawaii from July to August in 2011, and sampled those six sites and an additional two sites from May to August in both 2012 and 2013. Sites were located on forest fragments embedded in a landscape matrix of residential development, areas covered by lava from recent volcanic activity, and agricultural lands in and near Hilo, Hawaii (Fig. 1A). Each site consisted of 4–6 mosquito trap locations, and trap locations were located at least 150 m apart from each other. Sites and trap locations were accessed and connected by transects consisting of either hand-cut paths or small roads that traversed the forest. All sites had similar vegetative communities and were composed of relatively homogenous habitat. The plant community was dominated by the native ohia tree, Metrosideros polymorpha, in the overstory, with native and non-native shrubs, small trees, and ferns in the understory. All sites contained numerous invasive plant species including strawberry guava (Psidium cattleianum), melastoma (Melastoma septemnervium), and Coster’s curse (Clidemia hirta) (Zimmerman et al. 2008).
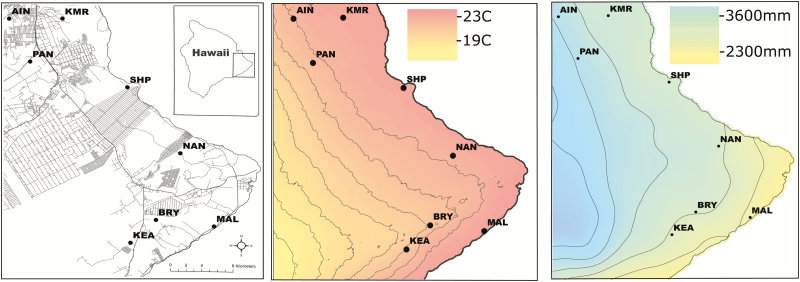
Fig. 1.
Maps of study sites in east Hawaii showing developed land, temperature, and precipitation. (A) Developed land, roads, and residential areas shown. The extent of the study area on the island of Hawaii is depicted in the upper right corner. Full site names are as follows: AIN, Ainako UH-Hilo; KMR, Keaukaha Miltary Reservation; PAN, Panaewa; SHP, W.H. Shipman property; NAN, Nanawale Forest Reserve; MAL, Malama Ki Forest Reserve; BRY, Bryson’s Cinder Cone (Pu’u Kali’u); KEA, Keauohana Forest Reserve. (B) Interpolated mean annual temperature in Celsius. Lines are 100 m elevational contours. (C) Interpolated annual cumulative rainfall. Lines are 500 mm rainfall isoclines; the line west of MAL is 2,500 mm.
Average annual temperatures range from 21.2 to 23.1°C across the study sites, with summer temperatures ranging from 22.5 to 24.2°C (Fick and Hijmans 2017, Fig. 1B). The study area receives substantial rainfall, ranging on average from 2,394 mm to 3,924 mm of precipitation/year (Giambelluca et al. 2013, Fig. 1C). Previous empirical and modeling work suggest that Cx. quinquefasciatus abundance does not exhibit strong seasonality at elevations below ~600 m in Hawaii, and are present and transmitting avian malaria year-round (Ahumada et al. 2004, Woodworth et al. 2005, Samuel et al. 2011). Ae. albopictus is also present throughout the year in the Hawaiian lowlands (Goff and van Riper 1980), though less is known about this species in Hawaii. The spatial variation and relative differences in mosquito abundance among our sites may be consistent across seasons, but we conservatively limit our inferences to the summer season during which the data were collected.
We captured mosquitoes at each trap location for 2–3 nights every 3–4 wk during our sampling periods. Mosquitoes were captured using Center for Disease Control (CDC)-modified Miniature Light Traps (John W. Hock, Gainesville, FL) baited with CO2 (dry ice), which target host-seeking adult female mosquitoes (Sudia and Chamberlain 1967). CO2-baited light traps are frequently used to estimate abundance for Cx. quinquefasciatus, but are known to be inefficient in capturing Ae. albopictus, which are caught more readily with BGS traps (Farajollahi et al. 2009). As a result, we made no comparisons of abundance between species and instead focus on within-species trends; within-species comparisons between sites are unlikely to be biased. Traps were baited with approximately 1 kg of dry ice and were placed in the tree canopy (~2–5 m, depending on the height of the tree). We assumed trapping success at one trap was not significantly influenced by nearby baited mosquito traps because the density of forest birds and rats, among other potential hosts, is likely sufficiently high at these sites (birds occur at ~65 individuals per ha, McClure, unpublished data; rats occur at ~8 individuals per ha, Beard and Pitt 2006) to minimize a potential distraction effect of CO2 gradients emanating from mosquito traps 150 or more meters away. We set traps in late afternoon (before dusk) to sample both diurnal (Ae. albopictus; Hawley 1988, Delatte et al. 2010) and nocturnal mosquitoes (Cx. quinquefasciatus; VanRiper et al. 1986). Mosquitoes were collected the following day, killed by placing them in a −80°C freezer, sorted by species, and counted.
Larval Habitat Survey
We conducted a mosquito larval habitat survey once at each site between May and July in 2013. We counted all water-holding containers or natural cavities along 4–7 transects 33–603 m in length immediately adjacent to mosquito trap locations using modified standard protocols (Reiter and LaPointe 2009). Along each transect, we examined a 5-m-wide band from ground level to approximately 1.5 m high. We used this relatively small width because thick vegetation and unstable volcanic substrate at several sites made a wider search infeasible or unsafe. We noted whether potential habitats were wet or dry, and tested the water-holding capacity of each potential larval habitat by pouring water in it. We estimated the volume of the habitat by measuring the length, width, and depth of the cavity in cm, and converted the volume to liters (1,000 cm3). If larvae were present, we collected a sample and identified them with a dissecting microscope either as larvae or after allowing them to develop into adults using Darsie et al. (2005). We classified all potential larval habitats as either naturally occurring (e.g., rock holes, downed tree ferns, and water-holding vegetation) or anthropogenic (e.g., artificial containers, discarded car tires, and the wheel arch of an upturned, abandoned car). We estimated the overall and artificial larval habitat count density as the total number of observed natural and human-made habitats/ha surveyed and the total number of human-made habitats/ha surveyed, respectively. Similarly, we estimated the overall and artificial volumetric larval habitat density as the total observed volume of natural and human-made habitats/ha surveyed and the total observed volume of human-made habitats/ha surveyed, respectively. Field constraints limited our ability to conduct multiple surveys, and we thus consider the larval habitat patterns observed as a snapshot in time rather than a dynamic process.
Landscape Analysis and Environmental Data
We used ArcGIS (v. 10.3.1) to quantify the proportion of developed land surrounding each site. We used 3m resolution raster land cover data obtained from the National Oceanic and Atmospheric Administration’s C-Coastal Change Analysis Program (http://coast.noaa.gov/ccapftp/#/). These land cover data were derived from high-resolution Quickbird satellite images of coastal Hawaii from 2002 to 2010, with anthropogenic landcover that characterizes land use current as of 2010. We grouped three landcover classes associated with human development—impervious cover, developed open space, and agricultural land, and converted the raster data into a vector file. We assumed that there were no seasonality effects in the land cover data (e.g., cultivated land classified as bare ground during winter) because of Hawaii’s tropical climate and year-round growing season. We created 250 m, 500 m, 1 km, and 2 km spatial buffer polygons surrounding each of the 4–6 mosquito-trapping stations per site. We quantified the proportion of developed land within each spatial buffer by calculating the spatial intersection of the buffer and the landcover vector files using the Tabulate Intersection tool in ArcGIS. We averaged the proportion of developed land surrounding each mosquito trap per site and used this measure as a site-level estimate of surrounding land use for further analyses.
To determine the spatial scale of developed land most predictive of mosquito abundance, we fit generalized linear mixed models of mosquito abundance with a negative binomial distribution (function glmer.nb in package lme4 in Program R, v.3.4) for each species at each of four spatial scales, with proportion of developed land and year as fixed effects and site as a random effect. We explored four spatial scales because of differences in flight distances of the two mosquito species; Ae. albopictus generally disperse several hundred meters while Cx. quinquefasciatus disperse distances ~1–2 kilometers (Niebylski and Craig 1994, LaPointe 2008, Medeiros et al. 2017). We selected the best fitting spatial scale model—the 250 m scale for both Ae. albopictus and Cx. quinquefasciatus—using Aikaike’s information criterion (AIC, Akaike 1973; Supp. Table S1). We used the proportion of surrounding developed land at the 250 m scale as a predictor variable in further statistical analyses.
We extracted interpolated estimates of average monthly temperature for each site from WorldClim GIS raster files at a spatial resolution of 1 km2 (www.worldclim.org; Fick and Hijmans 2017). These temperature data were created from compiled climate data collected from weather stations around the world from 1970 to 2000. Weather station data, satellite-derived meteorological data, and covariate data were interpolated using a thin-plate smoothing spline algorithm to create global climate surfaces (Fick and Hijmans 2017). We calculated a mean summer temperature estimate (in °C) for each site by averaging mean temperatures from the months of June–August. We extracted average monthly rainfall estimates for each site for the months of June-August from the Hawaii Rainfall Atlas, which was based on precipitation data from 1976 to 2007 drawn from rain gauges, PRISM data, radar rainfall, among other data sources (Giambelluca et al. 2013).
Statistical Analyses
We used generalized linear mixed models with a negative binomial distribution to explore the role of several fixed effects on the relative abundance of Cx. quinquefasciatus and Ae. albopictus. The response variable was the number of mosquitoes caught in each trap over one trapping night. Fixed effects included mean summer temperature (°C), mean summer precipitation (mm), density of available larval habitat (liters/ha), proportion of developed land within 250 m surrounding each site, year, and linear and quadratic terms for Julian date. Site was included as a random effect to account for repeated sampling at sites within and across years. The quadratic term for Julian date was included to account for nonlinear temporal variation in mosquito abundance, and year was included to account for unmeasured inter-annual variation in environmental conditions. We examined mixed effects models with a single fixed effect to avoid overfitting and because several predictors were moderately correlated with one another (r > 0.5; Supp. Fig. S3). We fit mixed effects models with a quadratic term for temperature, precipitation, larval habitat density, and developed land in separate mixed-effects models to explore nonlinear relationships of these predictors with mosquito abundance. Finally, we examined the effect of overall volumetric larval habitat (liters/ha), larval habitat count density (number of habitats/ha), and the contribution of human-made habitats to both count and volumetric density on mosquito abundance.
Results
Larval Habitat Survey
We found a total of 279 potential larval habitats across our sites, with an average site-level density ranging from 13.9 habitats/ha to 150.3 habitats/ha. The larval habitat volumetric density ranged from 2.9 to 163 liters/ha across our sites (Supp. Fig. S4). Individual larval habitats ranged from 0.003 to 37.4 l (mean volume = 0.795 ± 0.18 SE), consisting of small (e.g., discarded plastic bag holding ~10 ml, tree hole cavity holding ~25 ml) to medium-sized habitats (e.g., industrial-sized buckets holding ~19 liters, ephemeral ground pools holding ~34 liters). Our sites lacked large (>22,000 liters), persistent habitats like ponds, streams, or cattle tanks. Across all study sites, 16% of the potentially available habitats were human-created, while 84% were naturally-occurring. Human-made larval habitats were larger than naturally-occurring sites (mean human-made habitat volume = 1.92 liters ± 0.54 SE; mean naturally-occurring habitat volume = 0.58 liters ± 0.19 SE; t = 2.36, P = 0.02). Approximately 0.7% of the observed potential larval habitats contained Cx. quinquefasciatus larvae, while 3% contained Ae. albopictus. Ae. albopictus larvae were found in seven human-made habitats and in three naturally-created habitats (Supp. Table S2). Cx. quinquefasciatus larvae were found in two human-made larval habitats, in both of which Ae. albopictus larvae were also present. Ae. albopictus larvae were significantly more likely to be found in human-created habitat than expected given the relative availability of natural- and human-created larval habitats (Fisher’s exact test; Ae. albopictus: odds ratio = 13.2, P = 0.049). The volumetric density of human-made larval habitats increased with developed land cover within 250 m (Supp. Fig. S5).
Mosquito Abundance
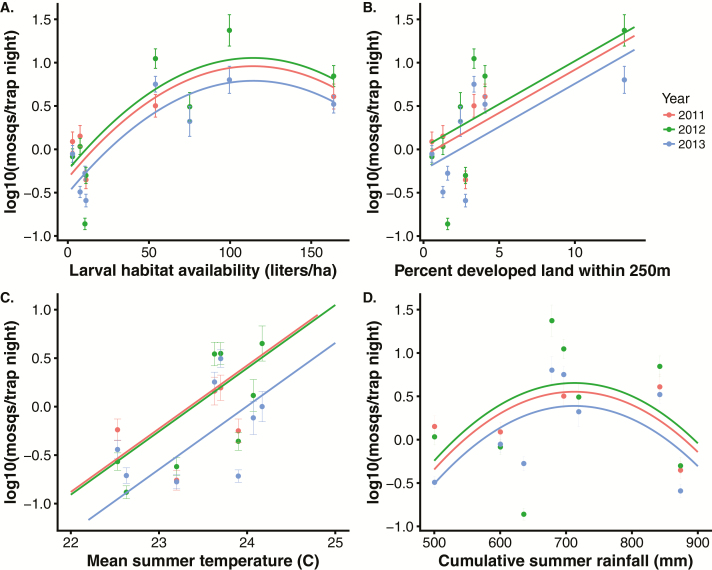
We captured a total of 20,502 mosquitoes in CDC light traps baited with CO2 over 770 trap-nights from 2011 to 2013 representing six mosquito species, Cx. quinquefasciatus, Ae. albopictus, Ae. Aegypti, Aedes japonicus Theobald, Aedes vexans Meigen, and Wyeomia mitchellii Theobald. Ae. albopictus abundance increased with volumetric larval habitat density, proportion of developed land within 250 m, mean summer temperature, and showed a unimodal relationship with summer rainfall (Fig. 2 and Supp. Fig. S1). Ae. albopictus abundance varied significantly among years (Fig. 2) and varied over the summer months with a minimum in late June (Supp. Fig. S6). Ae. albopictus abundance was more tightly correlated with measures of volumetric density of larval habitats (liters/ha) than with either total count density or human-contributed count density (number/ha) (Supp. Fig. S7). The correlations between Ae. albopictus abundance and either total volumetric density or artificial volumetric density were similarly high (Supp. Fig. S7).
Fig. 2.
Aedes albopictus abundance, developed land, larval habitat availability, temperature and rainfall. All fitted lines include site as a random effect. (A) Mosquito abundance plotted against larval habitat availability (liters/ha). Points are site-level log-transformed Aedes albopictus abundance (± 1 SE) from 2011 to 2013. Fitted lines: Abundance = −0.78 + 0.5 (±SE = 0.004) * larval habitat availability (P = 1.22 × 10−5) − 2.3 × 10−4 (±SE = 7.4 × 10−5) * larval habitat availability2, P = 0.002; year 2012 coefficient compared to 2011 = 0.22 ± 0.16, P = 0.17; year 2013 coefficient compared to 2011 = −0.39 ± 0.16, P = 0.02; N trapping nights = 770). (B) Mosquito abundance plotted against percent developed land within 250 m of sites. Fitted lines: Abundance = −0.18 + 0.23 (±SE = 0.08) * percent developed land within 250 m, P = 0.004; year 2012 coefficient compared to 2011 = 0.23 ± 0.16, P = 0.15; year 2013 coefficient compared to 2011 = −0.37 ± 0.16, P = 0.02. Excluding the right-most points increases the land use slope to 0.59 and the regression remains significant (P = 0.03). (C) Mosquito abundance plotted against mean summer temperature (°C). Fitted lines: Abundance = −35.4 + 1.5 (±SE = 0.5) * mean summer temperature, P = 0.003; year 2012 coefficient compared to 2011 = 0.24 ± 0.17, P = 0.15; year 2013 coefficient compared to 2011 = −0.37 ± 0.17, P = 0.03. (D) Mosquito abundance plotted against cumulative summer rainfall (mm). Fitted lines: Abundance = −7.92 + 0.019 (±SE = 0.01) * summer rainfall (P = 0.07) − 4.6 × 10−5 (±SE = 2.6 × 10−5) * summer rainfall2; year 2012 coefficient compared to 2011 = 0.23 ± 0.16, P = 0.15; year 2013 coefficient compared to 2011 = −0.38 ± 0.16, P = 0.02.
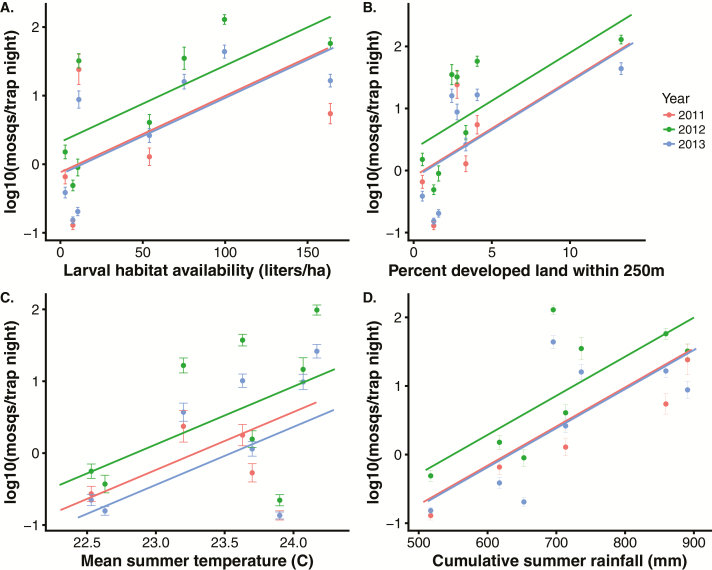
Cx. quinquefasciatus abundance increased significantly with summer cumulative rainfall, mean summer temperature, volumetric larval habitat density, and proportion of developed land within 250 m (Fig. 3 and Supp. Fig. S2). There was little support for Cx. quinquefasciatus abundance varying over time within the summer months, but abundance did vary significantly among years (Fig. 3). Cx. quinquefasciatus was more tightly correlated with measures of volumetric density of larval habitats (liters/ha) than with count density (number/ha), artificial larval habitat volumetric, or count density (Supp. Fig. S8). Correlations among predictor variables ranged from 6 to 55% (Supp. Fig. S3). Results were similar for separate analyses in which Ae. albopictus and Cx. quinquefasciatus count data were aggregated across three levels of decreasing spatial and temporal resolution (see Supp. Methods and Supp. Tables S3 and S4).
Fig. 3.
Cx. quinquefasciatus abundance, developed land, larval habitat availability, temperature and rainfall. All fitted lines include site as a random effect. (A) Mosquito abundance plotted against larval habitat availability (liters/ha). Points are site-level log-transformed means of Cx. quinquefasciatus abundance (± 1 SE) from 2011 to 2013. Fitted lines: Abundance = −0.31 + 0.026 (± SE = 0.009) * larval habitat availability, P = 0.008; year 2012 coefficient compared to 2011 = 1.06 ± 0.15, P < 0.001; year 2013 coefficient compared to 2011 = 0.014 ± 0.16, P = 0.92; N trapping nights = 770). (B) Mosquito abundance plotted against percent developed land within 250 m of sites. Fitted lines: Abundance = 0.36 + 0.14 (±SE = 0.14) * percent developed land within 250 m, P = 0.01; year 2012 coefficient compared to 2011 = 1.06 ± 0.15, P < 0.001; year 2013 coefficient compared to 2011 = 0.012 ± 0.15, P = 0.91. Excluding the right-most points increases the land use slope to 1.21 and the regression remains significant (P = 0.003). (C) Mosquito abundance plotted against mean summer temperature (°C). Fitted lines: Abundance = −43.45 + 1.85 (±SE = 0.8) * mean summer temperature, P = 0.02; year 2012 coefficient compared to 2011 = 1.1 ± 0.15, P < 0.001; year 2013 coefficient compared to 2011 = 0.02 ± 0.16, P = 0.91. (D) Mosquito abundance plotted against cumulative summer rainfall (mm). Fitted lines: Abundance = −8.29 + 0.013 (±SE = 0.004) * cumulative summer rainfall, P = 0.002; year 2012 coefficient compared to 2011 = 1.1 ± 0.15, P < 0.001; year 2013 coefficient compared to 2011 = 0.03 ± 0.16, P = 0.85.
Discussion
Invasive mosquitoes in Hawaii threaten wildlife and human health, underscoring the need for insight into drivers of mosquito abundance in lowland Hawaii. We found that both Ae. albopictus and Cx. quinquefasciatus abundance increased with larval habitat density, and developed land, which were themselves positively correlated. This suggests that in Hawaii, land use change associated with development of natural habitats increases mosquito larval habitats, which increases the abundance of both Ae. albopictus and Cx. quinquefasciatus. Increases in the abundance of disease vectors associated with the development of natural habitats in Hawaii, like other locations, raises the disease risk for both dengue virus and avian malaria.
Our results support a growing consensus that the conversion of natural systems to developed land increases the abundance of some invasive vector species. Other studies have documented positive associations between Cx. quinquefasciatus abundance and urbanization, suburban, or agricultural land use, with associations often attributed to increased larval habitats in more developed areas in both temperate and tropical habitats (Kamdem et al. 2012, Landau and van Leeuwen 2012, Samson et al. 2015, Zahouli et al. 2016, Cardo et al. 2018). Our findings are also consistent with previous work conducted on the Big Island that found that Cx. quinquefasciatus capture probability increased with agricultural land cover and forest fragmentation in a higher elevation, cooler, mixed residential-agricultural community (Reiter and LaPointe 2007). Similarly, Ae. albopictus abundance has been shown to increase in urban, suburban, and agricultural land use types, but not always relative to rural land use, underscoring the broad ecological plasticity of this species (Rey et al. 2006, Tsuda et al. 2006, Paupy et al. 2009, Li et al. 2014).
Our findings offer insight into the larval habitats used by both species, which provide some guidance for control efforts in Hawaii. First, similar to other studies, we found that Ae. albopictus and Cx. quinquefasciatus utilize both artificial and natural-holding oviposition sites, including water-holding vegetation, tires, and discarded water-holding trash (Goff and van Riper 1980, Laird 1988, Yee 2008, Bartlett-Healy et al. 2012). Second, we found that abundance of both Ae. albopictus and Cx. quinquefasciatus were more strongly correlated with the volumetric density of available larval habitat than simply the count density. This suggests that moderately-sized habitats (e.g., the grill or wheel wells of abandoned cars, tires, etc.) are more important, potentially because of a lower surface-to-volume ratio, making them less prone to desiccation in warm temperatures (Kingsolver 1979). Source reduction efforts could focus first on these habitats. Finally, we found that abundance of Cx. quinquefasciatus was more tightly correlated with total larval habitat than just artificial or human-made larval habitat volume, while the correlation for Ae. albopictus was equally high for both total and human-made larval habitat. This suggests that even though both species might be more likely to use human-made habitat, natural habitats (e.g., tree hole cavities, large fallen leaves, puddles) also appear to be important. Control efforts that focus on removing human-made objects will likely help reduce abundance, but are not likely to be sufficient to eliminate either species.
Vector-borne diseases are a resurgent threat to wildlife and public health, and pathogens continue to be introduced into new regions, with Zika, chikungunya, and West Nile virus being recent examples. Hawaii has yet to have local transmission of these viruses, but has had imported cases of all three, and a recent outbreak of dengue virus on the Big Island in 2015–2016 occurred with 264 cases that lasted over 6 mo (http://health.hawaii.gov/docd/dengue-outbreak-2015/). Our results indicate that in Hawaii, as in other regions of the world, populations of two introduced mosquitoes, Ae. albopictus and Cx. quinquefasciatus, increase with land development, due to an increase in larval habitat. This suggests that development in forested areas is likely to increase mosquito abundance of both species. Controlling these species in Hawaii by eliminating larval habitat will be challenging due to their use of both human-made and natural habitats. Preventing the introduction of new pathogens should be a top priority to protect the people and wildlife of Hawaii.
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
Acknowledgments
We gratefully acknowledge Kamehameha Schools, Keaukaha Military Reservation, W.H. Shipman Ltd., Hawaii Department of Land & Natural Resources, and University of Hawaii at Hilo for providing access to land to sample. We thank all the hardworking interns and volunteers who spent many hours carrying stinky water and counting mosquitoes, including Marie Russell, Mike McFarlin, Katy Ward, Kacie Jonasen, Tony Castro, Kristi Masuhara, and Chris Davis. Thanks to Pat Hart for supporting field efforts, and to Dennis LaPointe for guidance and comments on an earlier draft of this manuscript. Funding was provided by a University of California Cota-Robles Fellowship, an American Ornithologists’ Union grant, NSF GAANN Fellowships A16-0061-002 and P200A030188. NSF grants EF-0914866 and DEB-1717498, and NIH grant 1R01AI090159.
References Cited
- Adalja A. A., T. K. Sell N. Bouri, and Franco C.. 2012. Lessons learned during dengue outbreaks in the United States, 2001-2011. Emerg. Infect. Dis. 18: 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahumada J. A., D. LaPointe, and Samuel M. D.. 2004. Modeling the population dynamics of Culex quinquefasciatus (Diptera: Culicidae), along an elevational gradient in Hawaii. J. Med. Entomol. 41: 1157–1170. [DOI] [PubMed] [Google Scholar]
- Akaike H. 1973. Information theory and an extension of the maximum likelihood principle, pp. 267–281. InPetran B. N., F. Csaki (eds.), Second International Symposium on Information Theory 2–8 September 1971, Budapest, Hungary. [Google Scholar]
- Bartlett-Healy K., I., Unlu P., Obenauer T., Hughes S., Healy T., Crepeau A., Farajollahi B., Kesavaraju D., Fonseca G., Schoeler, et al. 2012. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J. Med. Entomol. 49: 813–824. [DOI] [PubMed] [Google Scholar]
- Beadell J. S., F., Ishtiaq R., Covas M., Melo B. H., Warren C. T., Atkinson S., Bensch G. R., Graves Y. V., Jhala M. A., Peirce, et al. 2006. Global phylogeographic limits of Hawaii’s avian malaria. Proc. Biol. Sci. 273: 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard K. H., and Pitt W. C.. 2006. Potential predators of an invasive frog (Eleutherodactylus coqui) in Hawaiian forests. J. Trop. Ecol. 22: 345–347. [Google Scholar]
- Bhatt S., P. W., Gething O. J., Brady J. P., Messina A. W., Farlow C. L., Moyes J. M., Drake J. S., Brownstein A. G., Hoen O., Sankoh, et al. 2013. The global distribution and burden of dengue. Nature. 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøgh C., E. M. Pedersen D. A. Mukoko, and Ouma J. H.. 1998. Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med. Vet. Entomol. 12: 52–59. [DOI] [PubMed] [Google Scholar]
- Burt F. J., M. S. Rolph N. E. Rulli S. Mahalingam, and Heise M. T.. 2012. Chikungunya: a re-emerging virus. Lancet. 379: 662–671. [DOI] [PubMed] [Google Scholar]
- Cardo M. V., A. Rubio M. T. Junges D. Vezzani, and Carbajo A. E.. 2018. Heterogeneous distribution of Culex pipiens, Culex quinquefasciatus and their hybrids along the urbanisation gradient. Acta Trop. 178: 229–235. [DOI] [PubMed] [Google Scholar]
- Ciota A. T., A. C. Matacchiero A. M. Kilpatrick, and Kramer L. D.. 2014. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 51: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie R. F. J., and Ward R. A.. 2005. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. University Press of Florida, Gainsville, FL. [Google Scholar]
- Delatte H., G. Gimonneau A. Triboire, and Fontenille D.. 2009. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 46: 33–41. [DOI] [PubMed] [Google Scholar]
- Delatte H., A. Desvars A. Bouétard S. Bord G. Gimonneau G. Vourc’h, and Fontenille D.. 2010. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector Borne Zoonotic Dis. 10: 249–258. [DOI] [PubMed] [Google Scholar]
- Dye C. 1992. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 37: 1–19. [DOI] [PubMed] [Google Scholar]
- Effler P. V., L., Pang P., Kitsutani V., Vorndam M., Nakata T., Ayers J., Elm T., Tom P., Reiter J. G., Rigau-Perez, et al. ; Hawaii Dengue Outbreak Investigation Team. 2005. Dengue fever, Hawaii, 2001-2002. Emerg. Infect. Dis. 11: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraji A., A. Egizi D. M. Fonseca I. Unlu T. Crepeau S. P. Healy, and Gaugler R.. 2014. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission. Plos Negl. Trop. Dis. 8: e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A., B. Kesavaraju D. C. Price G. M. Williams S. P. Healy R. Gaugler, and Nelder M. P.. 2009. Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J. Med. Entomol. 46: 919–925. [DOI] [PubMed] [Google Scholar]
- Farajollahi A., D. M. Fonseca L. D. Kramer, and Marm Kilpatrick A.. 2011. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 11: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick S., and Hijmans R.. 2017. Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37: 4302–4315. [Google Scholar]
- Giambelluca T., Chen Q., Frazier A., Price J., Chen Y-L., Chu P-S., Eischeid J., and Delparte D.. 2013. Online Rainfall Atlas of Hawai’i. Bull. Am. Meteorol. Soc. 94: 313–316. [Google Scholar]
- Goff M. L., and van Riper C.. 1980. Distribution of mosquitoes (Diptera: Culicidae) on the east flank of Mauna Loa Volcano, Hawaii. Pacific Insects. 22: 178–188. [Google Scholar]
- Gratz N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Hardy D. 1960. Insects of Hawaii, Diptera: Nematocera-Brachycera. University of Hawaii Press, Honolulu, Hawaii: 10:368. [Google Scholar]
- Hawley W. A. 1988. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1: 1–39. [PubMed] [Google Scholar]
- Hayes J., and Downs T. D.. 1980. Seasonal changes in an isolated population of Culex pipiens quinquefasciatus (Diptera: Culicidae): a time series analysis. J. Med. Entomol. 17: 63–69. [DOI] [PubMed] [Google Scholar]
- Jones C. E., Lounibos L. P., Marra P. P., and Kilpatrick A. M.. 2012. Rainfall influences survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the mid-Atlantic United States. J. Med. Entomol. 49: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdem C., B., Tene Fossog F., Simard J., Etouna C., Ndo P., Kengne P., Boussès F. X., Etoa P., Awono-Ambene D., Fontenille, et al. 2012. Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae. PLoS One. 7: e39453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M. 2011. Globalization, land use, and the invasion of West Nile virus. Science. 334: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M. and Randolph S. E.. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 380: 1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M., Gluzberg Y., Burgett J., and Daszak P.. 2004. Quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. Ecohealth. 1: 205–209. [Google Scholar]
- Kimura M., J. M. Darbro, and Harrington L. C.. 2010. Avian malaria parasites share congeneric mosquito vectors. J. Parasitol. 96: 144–151. [DOI] [PubMed] [Google Scholar]
- Kingsolver J. G. 1979. Thermal and hydric aspects of environmental heterogeneity in the pitcher plant mosquito. Ecol. Monogr. 49: 357–376. [Google Scholar]
- Koenraadt C. J. M., and Harrington L. C.. 2008. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 45: 28–35. [DOI] [PubMed] [Google Scholar]
- Laird M. 1988. The natural history of larval mosquito habitats. Academic Press, London, United Kingdom. [Google Scholar]
- Landau K. I. and van Leeuwen W. J.. 2012. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J. Vector Ecol. 37: 407–418. [DOI] [PubMed] [Google Scholar]
- Lapointe D. A. 2008. Dispersal of Culex quinquefasciatus (Diptera: Culicidae) in a Hawaiian rain forest. J. Med. Entomol. 45: 600–609. [DOI] [PubMed] [Google Scholar]
- LaPointe D. A., M. L. Goff, and Atkinson C. T.. 2005. Comparative susceptibility of introduced forest-dwelling mosquitoes in Hawai’i to avian malaria, Plasmodium relictum. J. Parasitol. 91: 843–849. [DOI] [PubMed] [Google Scholar]
- LaPointe D. A., E. K. Hofmeister C. T. Atkinson R. E. Porter, and Dusek R. J.. 2009. Experimental infection of Hawai’i ‘Amakihi (Hemignathus virens) with West Nile virus and competence of a co-occurring vector, Culex quinquefasciatus: potential impacts on endemic Hawaiian avifauna. J. Wildl. Dis. 45: 257–271. [DOI] [PubMed] [Google Scholar]
- LaPointe D. A., M. L. Goff, and Atkinson C. T.. 2010. Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai’i. J. Parasitol. 96: 318–324. [DOI] [PubMed] [Google Scholar]
- Li Y., F. Kamara G. Zhou S. Puthiyakunnon C. Li Y. Liu Y. Zhou L. Yao G. Yan, and Chen X. G.. 2014. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. Plos Negl. Trop. Dis. 8: e3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L. P. 2002. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 47: 233–266. [DOI] [PubMed] [Google Scholar]
- Medeiros M. C., E. C. Boothe E. B. Roark, and Hamer G. L.. 2017. Dispersal of male and female Culex quinquefasciatus and Aedes albopictus mosquitoes using stable isotope enrichment. Plos Negl. Trop. Dis. 11: e0005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D. and Gubler D. J.. 2016. Zika Virus. Clin. Microbiol. Rev. 29: 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D., V. M. Cao-Lormeau, and Gubler D. J.. 2015. Zika virus: following the path of dengue and chikungunya?Lancet. 386: 243–244. [DOI] [PubMed] [Google Scholar]
- Niebylski M. L. and Craig G. B. Jr. 1994. Dispersal and survival of Aedes albopictus at a scrap tire yard in Missouri. J. Am. Mosq. Control Assoc. 10: 339–343. [PubMed] [Google Scholar]
- Paupy C., H. Delatte L. Bagny V. Corbel, and Fontenille D.. 2009. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 11: 1177–1185. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., B. D. Carroll R. Takahashi Y. Fang S. Garcia V. M. Martinez, and Quiring R.. 2009. Repeated West Nile virus epidemic transmission in Kern County, California, 2004-2007. J. Med. Entomol. 46: 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter M. E. and LaPointe D. A.. 2007. Landscape factors influencing the spatial distribution and abundance of mosquito vector Culex quinquefasciatus (Diptera: Culicidae) in a mixed residential-agricultural community in Hawai’i. J. Med. Entomol. 44: 861–868. [DOI] [PubMed] [Google Scholar]
- Reiter M. E. and LaPointe D. A.. 2009. Larval habitat for the avian malaria vector Culex quinquefasciatus (Diptera: Culicidae) in altered mid-elevation mesic-dry forests in Hawai’i. J. Vector Ecol. 34: 208–216. [DOI] [PubMed] [Google Scholar]
- Rey J. R., N. Nishimura B. Wagner M. A. Braks S. M. O’Connell, and Lounibos L. P.. 2006. Habitat segregation of mosquito arbovirus vectors in south Florida. J. Med. Entomol. 43: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruybal J. E., Kramer L. D., and Kilpatrick A. M.. 2016. Geographic variation in the response of Culex pipiens life history traits to temperature. Parasit. Vectors. 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D. M., R. S. Archer T. O. Alimi K. L. Arheart D. E. Impoinvil R. Oscar D. O. Fuller, and Qualls W. A.. 2015. New baseline environmental assessment of mosquito ecology in northern Haiti during increased urbanization. J. Vector Ecol. 40: 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M. D., Hobbelen P. H. F., DeCastro F., Ahumada J. A., LaPointe D. A., Atkinson C. T., Woodworth B. L., Hart P. J., and Duffy D. C.. 2011. The dynamics, transmission, and population impacts of avian malaria in native Hawaiian birds: a modeling approach. Ecol. Appl. 21: 2960–2973. [Google Scholar]
- Sudia W. D., and Chamberlain R. W.. 1967. Collection and processing of medically important arthropods for arbovirus isolation. Center for Disease Control, Atlanta, GA. [Google Scholar]
- Townson H., M. B. Nathan M. Zaim P. Guillet L. Manga R. Bos, and Kindhauser M.. 2005. Exploiting the potential of vector control for disease prevention. Bull. World Health Organ. 83: 942–947. [PMC free article] [PubMed] [Google Scholar]
- Tsuda Y., W. Suwonkerd S. Chawprom S. Prajakwong, and Takagi M.. 2006. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Control Assoc. 22: 222–228. [DOI] [PubMed] [Google Scholar]
- Turell M., Sardelis M., O’Guinn M., and Dohm D.. 2002. Potential vectors of West Nile virus in North America, pp. 241–252. InMackenzie J., Barrett A., Deubel V. (eds.), Japanese Encephalitis and West Nile Viruses. Current Topics in Microbiology and Immunology. Springer, Berlin, Heidelberg, Germany. [DOI] [PubMed] [Google Scholar]
- VanRiper C., VanRiper S. G., Goff L. M., Laird M., Goff M. L., and Laird M.. 1986. The epizootiology and ecological significance of malaria in Hawaiian Land birds. Ecol. Monogr. 56: 327–344. [Google Scholar]
- Van Riper C., Van Riper S., and Hansen W.. 2002. Epizootiology and effect of avian pox on Hawaiian forest birds. Auk. 119: 929–942. [Google Scholar]
- Warner R. E. 1968. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor. 70: 101–120. [Google Scholar]
- Weaver S. C. and Lecuit M.. 2015. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 372: 1231–1239. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., and Reisen W. K.. 2010. Present and future arboviral threats. Antiviral Res. 85: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth B. L., C. T., Atkinson D. A., Lapointe P. J., Hart C. S., Spiegel E. J., Tweed C., Henneman J., Lebrun T., Denette R., Demots, et al. 2005. Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc. Natl. Acad. Sci. U. S. A. 102: 1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee D. A. 2008. Tires as habitats for mosquitoes: a review of studies within the eastern United States. J. Med. Entomol. 45: 581–593. [DOI] [PubMed] [Google Scholar]
- Zahouli J. B., J. Utzinger M. A. Adja P. Müller D. Malone Y. Tano, and Koudou B. G.. 2016. Oviposition ecology and species composition of Aedes spp. and Aedes aegypti dynamics in variously urbanized settings in arbovirus foci in southeastern Côte d’Ivoire. Parasit. Vectors. 9: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman N., Hughes R. F., Cordell S., Hart P., Chang H. K., and Perez D.. 2008. Patterns of primary succession of native and introduced plants in lowland wet forests in Eastern Hawai’i. Biotropica. 4: 277–284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.