Abstract
Background.
Parathyroidectomy improves bone mineral density and decreases risk for fracture in patients with primary hyperparathyroidism. The aim of this study was to determine skeletal consequences of failed parathyroidectomy.
Methods.
A retrospective, cohort study of patients with biochemically confirmed primary hyperparathyroidism within a vertically integrated health system was performed (1995–2014). Failed parathyroidectomy was defined by hypercalcemia within 6 months of initial parathyroidectomy. Time-varying Cox regression was used to estimate the risk for any fracture and hip fracture in 3 comparison groups: observation, successful parathyroidectomy, and failed parathyroidectomy. Bone mineral density changes also were compared.
Results.
The cohort included 7,169 patients, of whom 5,802 (81%) were observed, 1,228 underwent successful parathyroidectomy (17%), and 137 underwent failed parathyroidectomy (2%). The adjusted risk for any fracture (hazard ratio, 1.28; 95% confidence interval, 0.85–1.92) and hip fracture (hazard ratio, 1.63: 95% CI, 0.77–3.45) associated with failed parathyroidectomy was similar to that associated with observation. Successful parathyroidectomy was associated with a decrease in any fracture (hazard ratio, 0.68; 95% confidence interval, 0.57–0.82) and hip fracture (hazard ratio, 0.43; 95% confidence interval, 0.27–0.68) compared with observation. Bone mineral density changes in the failed parathyroidectomy group paralleled those associated with observation.
Conclusion.
Failed parathyroidectomy is associated with a high risk for fracture similar to that seen with observation.
Primary hyperparathyroidism (PHPT) is a common condition that affects 1 in 400 women and 1 in 1,200 men in the United States.1 Consequences of long-standing PHPT include loss of bone mineral density (BMD), increased risk for fracture, nephrolithiasis, neuropsychiatric disturbances, and cognitive impairment.2 We reported previously that parathyroidectomy (PTx) improves BMD and decreases the risk for fracture in patients with PHPT.3 The success rate of PTx is >95% in expert hands and 75% to 90% in community practice.4–8
Patients who have undergone failed PTx (FPTx), defined by hypercalcemia within 6 months of initial PTx, have been difficult to identify historically because the majority do not present for reoperation.9 Hence, the clinical consequences of FPTx remain obscure. We previously have used electronic screening of biochemical follow-up data on patients who have undergone PTx to accurately assess the rate of persistent and recurrent PHPT within a large population.9 The aim of this study was to examine skeletal outcomes in patients who have undergone FPTx in comparison with those who have undergone successful PTx (SPTx), as well as patients who were managed nonoperatively. We hypothesize that patients undergoing FPTx will have outcomes similar to those in the observation group.
Methods
Study participants
Patients with PHPT were identified using the Kaiser Permanente Southern California (KPSC) Laboratory Management System. KPSC is an integrated health system that covers ~20% of the population of the Los Angeles metropolitan area and demographically and socioeconomically reflects the region. Patients meeting criteria for inclusion were those with hypercalcemia (serum total calcium concentration >2.63 mmol/L [> 10.5 mg/dL]) and parathyroid hormone excess (>65 ng/L) during the period between January 1, 1995 and December 31, 2014. We excluded patients who were members for <6 months, those <20 years of age, and those with secondary (renal) hyperparathyroidism (serum creatinine concentration >221 μmol/L ( > 2.5 mg/dL]). We identified patients with tertiary hyperparathyroidism by excluding any patient with ≥2 tests for immunosuppressant levels.
Fracture outcomes
For fracture outcomes, we included those patients who had adequate biochemical follow-up of ≥1 year after PTx. Patients who had a diagnosis of fracture entered on the same day as a diagnosis of PHPT were excluded. Three cohorts were created: (1) observation patients who did not undergo PTx within the study period, (2) SPTx patients who underwent PTx and were either continuously eucalcemic or who at most had 1 increased serum calcium concentration followed by 3 consecutive normal calcium levels within 1 year of surgery, and (3) FPTx patients who had increased serum calcium concentrations within 6 months of PTx. Patients with a fracture before PTx contributed events to the observation group.
Patients in each cohort were followed from the inception date (date of first increased serum calcium concentration) until fracture, disenroilment from KPSC, death, or the end of the follow-up period (September 30, 2016). Fractures were categorized into hip and non-hip by using International Classification of Diseases (ICD)-9 diagnostic codes and an ICD-9 to ICD-10 crosswalk for fractures occurring before and after October 2015, respectively.
BMD outcomes
The KPSC database was queried for data on BMD. For this study, only total hip BMD was examined. Records within 2 years of the index date of biochemical diagnosis for observation patients and within 2 years of initial PTx for the FPTx and SPTx cohorts were defined as the baseline BMD for each patient. When multiple BMD measurements were made within this 4-year time window, we used data from the BMD examination that was closest temporally to the 2 respective time points. Changes in BMD were studied over 4 discrete periods: 0 to 2 years, 2 to 5 years, 5 to 8 years, and >8 years from baseline BMD.
Statistical analysis
Modeling and analyses were carried out using SAS Enterprise Guide 5.1 (SAS Institute, Atlanta, GA). We calculated standardized differences to compare the baseline characteristics among the 3 groups using the SAS macro provided by Yang and Dalton.10 Cohen11 suggested that indices of 0.2, 0.5, and 0.8 can be used to represent small, medium, and large effect sizes, respectively. Fracture rates were compared between the 3 cohorts using a time-varying Cox model in which 2 group indicators, SPTX and FPTX, were coded as binary, time-dependent variables to reflect status change from observation to PTX during study follow-up, with the observation group being the reference. Risk adjustments were made for age, sex, and ethnicity. Percent change in BMD from baseline was compared between the 3 cohorts using linear regression with group being the primary predictor, controlling for age, sex, and race/ethnicity. For all analyses, bisphosphonate use for >1 year was considered a covariate. This study was approved by the institutional review boards of KPSC and the University of California, Los Angeles.
Results
Risk for fracture
Biochemical screening revealed 7,392 patients with PHPT (Fig 1). After exclusion criteria were applied, 7,167 were followed for skeletal outcomes. The study cohort was 78% female and 44% nonwhite (Table 1). The median age was 69 years (interquartile range [IQR] 59–77) for the observation group, 61 years (IQR 52–70) for SPTx group, and 60 years (IQR 52–68) for the FPTx group. Fifty-seven percent of the observation group, 39.4% of the SPTx group, and 38.2% of the FPTx group had Charlson Comorbidity Index scores >0. Baseline BMD was normal in 55% of patients, and 60% of patients were treated with bisphosphonates for >1 year.
Fig 1.
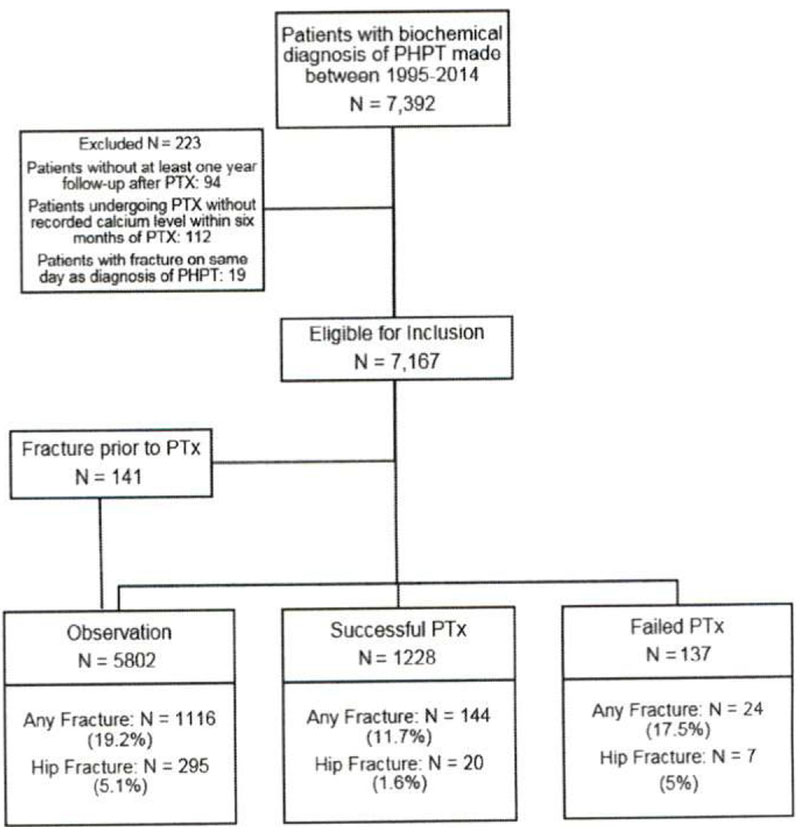
Flow chart depicting observation, successful parathyroidectomy, and failed parathyroidectomy groups and fracture rates.
Table I.
Standardized differences: effect size indices of 0.2, 0.5, and 0.8 can be used to represent small, medium, and large effect sizes, respectively12,13
| Baseline demographic data | ||||
|---|---|---|---|---|
| Observation |
Failed PTx |
Successful PTx |
Standardized difference | |
| n = 5,802 | n = 137 | n = 1,228 | ||
| Age, mean y ± SD | 67.7 ± 13 | 60.1 ± 13.1 | 59.8 ± 11.6 | −0.59 |
| Age, median (Q1–Q3)* | 69(59–77) | 61(52–70) | 60(52–68) | |
| Sex, n (%) | −0.017 | |||
| Female | 4533(78.1) | 108(78.8) | 949(77.3) | |
| Male | 1269(21.9) | 29(21.2) | 279(22.7) | |
| Race/ethnicity, n (%) | 0.25 | |||
| White | 3261 (56.2) | 82(59.9) | 686(55.9) | |
| Asian | 255(4.4) | 4(2.9) | 70(5.7) | |
| Black | 1090(18.8) | 25(18.2) | 208(16.9) | |
| Hispanic | 873(15) | 24(17.5) | 250(20.4) | |
| Other | 323 (5.6) | 2(1.5) | 14(1.1) | |
| Charlson Comorbidity Index, n (%) | 0.41 | |||
| 0 | 2473 (42.6) | 83 (60.6) | 759 (61.8) | |
| 1–2 | 1605(27.7) | 33 (24.1) | 313(25.5) | |
| > = 3 | 1724(29.7) | 21(15.3) | 156(12.7) | |
| Bisphosphonate use, n (%) | 0.19 | |||
| None | 689(11.9) | 12(8.8) | 113(9.2) | |
| ≤1 y | 1641 (28.3) | 50(36.5) | 345(28.1) | |
| >1 y | 3472(59.8) | 75(54.7) | 770(62.7) | |
| Bone density, n (%) | 0.14 | |||
| Normal | 1,394(53.3) | 44 (56.4) | 427(60.6) | |
| Osteopenia | 792(30.3) | 25(32.1) | 183(26) | |
| Osteoporosis | 427(16.3) | 9(11.5) | 95(13.5) | |
| Not measured | 3189 | 59 | 523 | |
| Serum calcium concentration | ||||
| Mean (SD) | 11 (0.59) | 13.4(25.19) | 11.2(0.55) | 0.13 |
| Median (Q1–Q3) | 10.9(10.7–11.2) | 11.2(10.8–11.6) | 11.1 (10.8–11.4) | |
| Serum PTH | ||||
| Mean (SD) | 123.5(74) | 165.2(127) | 150.0(90) | 0.40 |
| Median (Q1–Q3) | 101(80–138) | 120(92–174) | 125(96–169) | |
Q1–Q3: Interquartile range.
PTH. parathyroid hormone.
PTx was undertaken in 1,506 patients (21%). Of these patients, 141 had a fracture before PTx and thus contributed their outcomes to the observation group. For these 141 patients, the median time from biochemical diagnosis of PHPT to fracture was 1.88 years (IQR 0.44–3.53 years). For fracture analyses, 5,802 patients were in the observation group, 1,228 in the SPTx group, and 137 in the FPTx group (Fig 1). The median postoperative parathyroid hormone was 92 ng/L (IQR 55–143 ng/L) for the FPTx group and 55 ng/L (IQR 35–78 ng/L) for the SPTx group. The rate of any fracture was 36 events/1,000 person-years in the observation group, 14/1,000 person-years in the SPTx group, and 26.6/1000 person-years in the FPTx group. The rate of hip fracture was 8.8 events/1,000 person-years in the observation group, 1.7/1,000 person-years in the SPTx group, and 7.1/1,000 person-years in the FPTx group. Using time-varying survival analysis, the hazard ratio (HR) for total risk for fracture after SPTX was 0.56 (95% confidence interval [CI], 0.47–0.66) and FPTX was 1.18 (95% CI, 0.79–1.77) compared with observation. After adjusting for age, sex, and race/ethnicity, the HR for SPTX was 0.68 (95% CI, 0.57–0.82) and FPTX was 1.28 (95% CI, 0.85–1.92). Further adjusting for bisphosphonate use, the HR for SPTX was 0.69 (95% CI. 0.58–0.82) and FPTX was 1.31 (95% CI. 0.8795% CI. 1.96; Table II).
Table II:
Fracture risk of three groups
| Risk for fracture | ||||
|---|---|---|---|---|
|
| ||||
| Any fracture |
||||
| Unadjusted HR(95% CI) | Adjusted HR* (95% CI) | Adjusted HR† (95% CI) | Adjusted HR‡ (95% CI) | |
| Observation | 1 | 1 | 1 | 1 |
| Failed PTX | 1.18 (0.79–1.77) | 1.28(0.85–1.92) | 1.31 (0.87–1.96) | 1.37 (0.91–2.06) |
| Successful PTX | 0.56 (0.47–0.66) | 0.68 (0.57–0.82) | 0.69 (0.58–0.82) | 0.72(0.60–086) |
|
| ||||
| Hip fracture | ||||
|
| ||||
| Unadjusted HR (95% CI) | Adjusted HR* (95% CI) | Adjusted HR† (95% CI) | Adjusted HR‡ (95% CI) | |
|
| ||||
| Observation | 1 | 1 | 1 | 1 |
| Failed PTX | 1.33(0.63–2.82) | 1.63(0.77–3.45) | 1.70 (0.80–3.61) | 1.77 (0.83–3.77) |
| Successful PTX | 0.29 (018–0.46) | 0.43 [0.27–0.68) | 0.44 (0.28–0.69) | 0.45 (0.29–0.72) |
|
| ||||
| Non-hip fracture | ||||
|
| ||||
| Unadjusted HR(95% CI) | Adjusted HR* (95% CI) | Adjusted HR† (95% CI) | Adjusted HR‡ (95% CI) | |
|
| ||||
| Observation | 1 | 1 | 1 | 1 |
| Failed PTX | 1.21 (0.79–1.85) | 1.29(0.84–1.97) | 1.31 (0.86–1.99) | 1.37(0.90–2.09) |
| Successful PTX | 0.62 (0.52–074) | 0.74 (0.62–0.89) | 0.74 (0.62–0.89) | 0.78 (0.65–0.93) |
Adjusted for age, sex, and race/ethnicity.
Adjusted for age, sex, race/ethnicity, and bisphosphonate use.
Adjusted for age, sex, race/ethnicity, bisphosphonate use, and Charlson Comorbidity Index.
Changes in BMD
For 745 patients, at least 2 measurements of BMD in the femoral neck were reported during the follow-up period. In the observation group, adjusted BMD in the femoral neck BMD decreased by 3% (95% CI. −4.2% to −1.9%) at 2 to 5 years, by 4.6% (95% CI. −6.2% to −2.9%) at 5 to 8 years, and by 6.8% (95% CI. −9.7% to −4%) >8 years (Fig 2). SPTx was associated with an increase in BMD of 2.5% at 2 to 5 years (95% CI. 0.7%–4.4%), 1.3% at 5 to 8 years (95% CI. −1.0 to 3.8%), and 1.1 % (95% CI, −2.8% to 4.9%) >8 years. In the FPTx group, BMD changes closely mirrored those of observed patients (Fig 2), and no significant differences were found between the BMD changes in any period of time (P = .9, .09, .5, and .5 for 0–2 years, 2–5 years, 5–8 years and >8 years, respectively). In the SPTx group, the trajectory of BMD was better than that of observed patients for 0 to 2 years, 2 to 5 years, 5 to 8 years, and >8 years (P< .0001 each).
Fig 2.
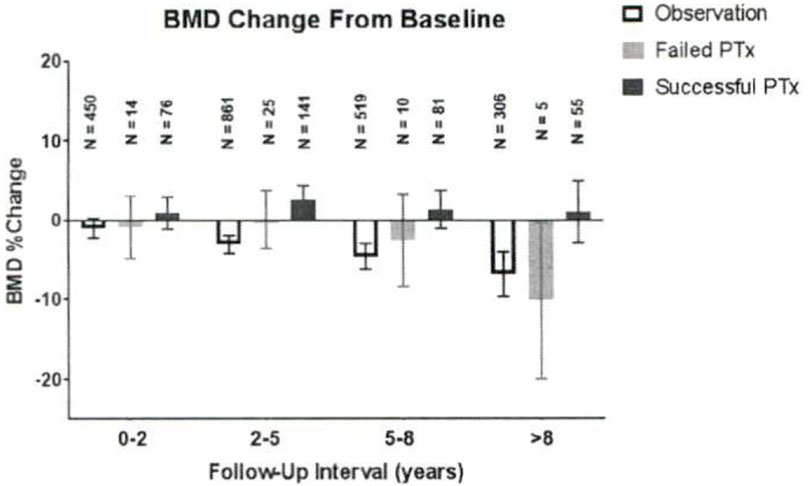
Bone mineral density changes over baseline. Error bars represent 95% confidence intervals.
Discussion
We previously reported that patients with PHPT who undergo PTx have a decreased risk for fracture. These findings persist in this updated cohort. Our new finding that patients who undergo FPx carry a risk for fracture that is similar to that of observed patients may be intuitive; however, to our knowledge these are the first published data that support this hypothesis. Our findings indicate that the cohort of patients who have undergone FPTx have been subjected to the risks for PTx without accruing any durable benefit with respect to their skeletal outcomes.
KPSC is a vertically integrated health care system serving 4.2 million people, within which we reported previously a success rate of 92% for initial PTx. This rate is less than reported success rates from expert centers staffed by high-volume surgeons.5·8 In KPSC, >120 surgeons perform PTx with presumably different techniques and outcomes. This overall success rate is likely reflective of those found in general community practice.9 As enrollees in an integrated medical system, Kaiser patients stay predominantly within Kaiser facilities for all of their health care needs and referral outside of the system is rare. As an alternative strategy, it may be beneficial to identify high-volume parathyroid surgeons within the Kaiser network. Additionally, reoperation should be offered to patients with well-localized persistent or recurrent disease because cure rates in specialized centers are as high as 95%.14
Limitations of this study include its retrospective, nonrandomized design. Additionally, the transition from ICD-9 to ICD-10 during the study period may have led to differential capture of fractures as a result of changes in the coding. A small number of patients was excluded from the study due to lack of biochemical follow-up data. This study is strengthened the racial, ethnic, and socioeconomic profile of the KPSC population, which closely mirrors that of the state of California. Furthermore, long-term follow-up was possible in a large fraction of patients because two-thirds of subscribers maintain membership for ≥5 years.9
Osteoporotic fractures, especially hip fractures, constitute a serious public health burden and are associated with increased delayed mortality in the elderly.15,16 It has been estimated that 10 parathyroidectomies must be performed to prevent 1 major fracture.17 The recommendation that PTx is the preferred treatment for patients with PHPT is predicated on high cure rates for PTx. The recent finding that bisphosphonates are ineffective in decreasing the risk for fracture in patients with PHPT further underscores the importance of maintaining high quality in endocrine surgical care.3
Acknowledgments
Supported by National Institutes of Health/National Institute on Aging RFA-AG-11-007.
Footnotes
Presented at the 38th Annual Meeting of the Amencan AssoCiation of Endocrine Surgeons. Orlando. Florida, April 2–4 2017
References
- 1.Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 2013;98:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016:151:959–68. [DOI] [PubMed] [Google Scholar]
- 3.Yeh MW, Zhou H, Adams AL, et al. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med 2016:164:715–23. [DOI] [PubMed] [Google Scholar]
- 4.Abdulla AG, Ituarte PH, Harari A, Wu JX, Yeh MW, Trends in the frequency and quality of parathyroid surgery: analysis of 17,082 cases over 10 years. Ann Surg 2015:261:746–50. [DOI] [PubMed] [Google Scholar]
- 5.Udelsman R Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg 2002;235:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siperstein A, Berber E, Barbosa GF, et al. Predicting the success of limited exploration for primary hyperparathyrodisim using ultrasound, sestamibi. and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg 2008:248:420–8. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Wang TS, Yen TW, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg 2010:252:691–5. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell J, Milas M, Barbosa G, Sutton J, Berber E, Siperstein A, Avoidable reoperations for thyroid and parathyroid surgery: effect of hospital volume. Surgery 2008;144:899–906. [DOI] [PubMed] [Google Scholar]
- 9.Yeh MW. Wiseman JE, Chu SD, et al. Population-level predictors of persistent hyperparathyroidism. Surgery 2011:150:1113–9. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Dalton JE, A unified approach to measuring the effect size between two groups using. SAS Statistics and Data Analysis. SAS Global Forum 2012:1–6. [Google Scholar]
- 11.Cohen J, Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, Nj: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 12.Sullivan GM, Feinn R, Using effect size—or why the P value is not enough. J Grad Med Ed 2012;4:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasserstein RL, Lazar NA, The ASA’s statement on p-values: context, process, and purpose. Am Stat 2016:70:129–33. [Google Scholar]
- 14.Shen W, Düren M, Morita E, et al. Reoperation for persistent or recurrent primary hyperparathyroidism. Arch Surg 1996; 131:861–7. discussion 867–9. [DOI] [PubMed] [Google Scholar]
- 15.Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 2008:93:3462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Internal Med 2010:152:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh MW, Editorial: primary hyperparathyroidism: consequences of non-surgical management. Surgery 2017;161:51–3. [DOI] [PubMed] [Google Scholar]


