Abstract
Objective
Inorganic polyphosphate (polyP) is known to modulate coagulation, inflammation and metabolic pathways. It also amplifies inflammatory responses of high mobility group box 1 (HMGB1) in endothelial cells. The objective of this study was to evaluate the effect of polyP on von Willebrand factor (VWF) release from endothelial cells with or without HMGB1.
Approach and Results
EA.hy926 endothelial cells were treated with different concentrations of polyP70 alone or in combination with different concentrations of HMGB1. VWF release was measured by an ELISA assay in the absence or presence of pharmacological inhibitors of the receptor for advanced glycation end products (RAGE), P2Y1, and Ca2+. A flow chamber assay was used to monitor polyP70-mediated platelet recruitment and VWF-platelet string formation. PolyP70 and HMGB1 induced VWF release from endothelial cells by a concentration-dependent manner. PolyP70 amplified HMGB1-mediated VWF release from endothelial cells. This was also true if boiled platelet releasate was used as the source of polyP. Gene silencing or pharmacological inhibitors of RAGE, P2Y1 and Ca2+ significantly inhibited VWF release. PolyP70 and HMGB1 synergistically promoted VWF-platelet string formation in the flow chamber assay, which was inhibited by the anti-GPIbα antibody. VWF release by polyP70-HMGB1 complex required phosphorylation of Src and phospholipase C (PLC) since inhibitors of Src, PLC and Ca2+ signaling significantly decreased VWF secretion. The polyP70-HMGB1 complex also increased angiopoietin-2 release, indicating that Weibel-Palade body exocytosis is involved in the VWF release.
Conclusions
PolyP70 can promote thrombotic and inflammatory pathways by inducing VWF release and platelet string formation on endothelial cells.
Keywords: polyphosphate, platelets, von Willebrand factor, HMGB1, endothelial cells
Subject codes: Basic Science Research, Cell Signaling/Signal Transduction, Platelets
Graphical abstract
Introduction
Polyphosphates (polyP) are linear polymers of 3 to over 1,000 inorganic phosphate residues, which are linked together by ATP-like phosphoanhydride bonds.1 It has been shown that polymers of medium size polyP containing ~60-100 phosphate units are stored in dense granules of human platelets at high concentrations, which can be released into circulation upon their activation by thrombin or other physiological stimuli during activation of the blood coagulation cascade.2-4 Microorganisms under different environmental conditions may synthesize longer chain polyP containing over 1000 phosphate units.5,6 Results of several recent studies have established an important role for polyP in the regulation of coagulation, inflammatory and metabolic pathways.3,4,7 In the clotting cascade, polyP functions as a procoagulant factor to promote thrombin generation via both, the intrinsic and extrinsic pathways of coagulation.3,4,6 This function of polyP has been reported to be primarily mediated through the anionic polymer providing a template on which specific coagulation factors from both pathways assemble, thereby promoting coagulation reactions.4,6 In the proinflammatory pathway, we recently demonstrated that polyP can bind to the receptor for advanced glycation end products (RAGE) and P2Y1 purinergic receptor on endothelial cells to mediate proinflammatory responses and phosphorylation-dependent inactivation of the upstream regulatory tuberous sclerosis complex, thereby leading to activation of the mTOR, Wnt/β-catenin and nuclear factor-kB (NF-kB) signaling pathways.7-9 This endothelial cell activation results in the expression/release of a number of pre-synthesized (and stored) and/or newly synthesized effector molecules, which are involved in modulating inflammatory and coagulation reactions.10-13 Among the stored effector molecules that are either readily mobilized to the cell surface or secreted out of the cell, is von Willebrand factor (VWF), which is stored in Weibel-Palade bodies (WPB) and released upon activation of endothelial cells by various physiological stimuli.11-13 VWF plays a key role in regulating coagulation and inflammation under different pathophysiological conditions. The stimulatory effect of polyP on VWF release by endothelial cells has never been studied.
In this study, we treated EA.hy926 endothelial cells with synthetic polyP, either similar to the size found in platelets (polyP70) or found in bacteria (polyP700), and measured the VWF release with an ELISA assay. We discovered that both sizes of polyP stimulate VWF secretion by EA.hy926 cells in a concentration-dependent manner. Boiled activated platelet releasates also induced VWF release by EA.hy926 cells, which was abolished if the releasates were pre-treated with either exopolyphosphatase or alkaline phosphatase. In addition to polyP, platelets are also a rich source of HMGB1 which, like polyP, is stored in dense granules; upon activation of platelets both molecules can be secreted into the circulation.14,15 Low concentrations of polyP or HMGB1 alone were inactive, but when combined, they functioned synergistically to induce VWF release by EA.hy926 cells. Interestingly, in a flow chamber assay using freshly isolated platelets, we discovered that the polyP70-HMGB1 complex effectively promoted VWF-platelet string formation that was inhibited by the anti-GPIbα antibody. The stimulatory effect of polyP70-HMGB1 was mediated through RAGE and P2Y1 receptors since siRNA silencing, soluble RAGE and pharmacological inhibitors of the two receptors inhibited VWF release by EA.hy926 cells. Based on these results we propose an as yet unrecognized function for polyP that links the procoagulant and inflammatory pathways by a novel mechanism through stimulation of VWF release by endothelial cells.
Materials and methods
The authors declare that all supporting data are available within the article and its online supplementary file.
Reagents
Recombinant HMGB1 and Src inhibitor, PP2, were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). RAGE antagonist peptide, the P2Y1 inhibitor, MRS 2279, and cell the permeable Ca2+ chelator BAPTA-AM were purchased from Tocris Bioscience (Minneapolis, MN, USA). PLC inhibitor U73122, RAGE and P2Y1 antibodies were from Santa Cruz Biotechnology In. (Santa Cruz, CA, USA). Antibodies against Phospho Src, Phospho PLC were from Cell Signaling Technology (Beverly, MA, USA). PolyP70 was a generous gift from Dr. James Morrissey (University of Michigan, Ann Arbor, MI). Platelet releasates were gifts from Dr. Stephanie Smith (University of Michigan, Ann Arbor, MI) and prepared as described.6 PolyP700 was purchased from Kerafast (Boston, MA, USA). Anti-VWF polyclonal antibody (A0082) was purchased from DAKO (Carpinteria, CA). Biotin-labeled anti-VWF monoclonal antibody was obtained from Cederlane Laboratories (Burlington, NC, USA). Standard VWF protein was from Abcam (Cambridge, MA, USA). GP1bα blocking mouse monoclonal antibody (clone-AK2) was purchased from Bio-Rad (Hercules, CA, USA). Anti-HMGB1 neutralizing monoclonal antibody (clone-3E8) from Biolegend (San Diego, CA). Anti-factor VIII monoclonal antibody (clone-R8B12) (Green Mountain Antibodies, Burlington, VT). Recombinant human factor VIII (FVIII) was a gift from Bayer (Berkeley, CA). Alkaline phosphatase (ALP) was obtained from New England Biolabs Inc. (Beverly, MA, USA) and EcPPXc, the recombinant polyP-binding domain of Escherichia coli exopolyphosphatase fused to maltose-binding protein and a His6 tag, was prepared as described.16 The extracellular domain of RAGE (soluble RAGE, sRAGE) was expressed in Escherichia coli using SUMO expression/purification plasmid system with a His tag and purified using a combination of Ni-Sepharose and Hi-Trap Q HP column chromatography, as described.8
Cell culture experiments
Primary human umbilical vein endothelial cells (HUVECs, Invitrogen) and transformed HUVECs (EA.hy926 cells, ATCC) were maintained as described.8
VWF measurement by Sandwich ELISA
EA.hy926 cells were treated with polyP, HMGB1 or with their combination for the indicated period of time and VWF release was measured using a Sandwich ELISA assay. Briefly, high binding 96-well plates (Greiner bio one, Monroe, NC) were coated with anti-VWF polyclonal antibody (1μg/mL) in bicarbonate coating buffer (pH 9.6) overnight at 4C. After blocking for 1h at room temperature (RT) with 1% bovine serum albumin (BSA), plates were incubated with the diluted endothelial cell culture supernatants for 2h. After washing with TBST (50 mM Tris-Cl, pH 7.4 150mM NaCl containing 0.05% tween 20), the plates were incubated with biotin labeled anti-VWF monoclonal antibody (1μg/mL) for another 2h. Plates were washed again with TBST and incubated with horse radish peroxidase (HRP) conjugated streptavidin for 30 min. After washing, TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution was added, the reaction stopped with 2N sulfuric acid and absorbance measured at 450 nm using a plate reader. A reference standard curve of VWF was generated using standard VWF, and the amount of VWF secretion in the conditioned media was calculated.
Western blotting
Treated endothelial cells were lysed in cell lysis buffer (50mM Tris-HCL, pH 7.4, 150mM NaCl, 1mM EDTA, 1% NP40, 1mM PMSF, 1× protease inhibitor cocktail). Proteins were resolved by (8-10%) SDS-PAGE and then transferred to PVDF membranes. After blocking with 1% BSA in TBST, membranes were incubated with the primary antibody against phospho-PLCγ and phospho-Src (1:1000) for overnight at o4C. After washing with PBST, membranes were incubated in HRP-conjugated secondary antibodies (1:5000) and specific immune-reactive bands were identified using and an Enhanced Chemi-luminescence (ECL) detection system.
VWF-platelet string formation
Blood was collected from healthy adult volunteers (following approved institutional IRB protocol) in acid-citrate dextrose and platelet rich plasma prepared by centrifuging the blood at 150g for 15 min. The platelet rich plasma was again centrifuged at 900g for 5 min to collect platelets. Platelets were resuspended at 10 million/mL in HEPES-Tyrodes buffer (134mM NaCl, 12 mM NaHCO3, 2.9 mM KCl, 0.34 mM NaH2PO4, 5mM HEPES, 5mM glucose) containing 1mM EDTA and 1% HSA. EA.hy926 cells were exposed to different treatments in tissue culture dishes, and the isolated platelets perfused over them in a parallel-plate flow chamber at a shear rate of 8 dyns/cm2, in the absence or presence of a blocking mouse anti-GPIbα monoclonal antibody. In some cases, platelets were fluorescently labeled with 5-chloromethylfluorescein diacetate (CMFDA-2.5μM) before perfusion. Images were acquired with a monochromatic CCD camera (Hamamatsu C11440) in an Axiovert 200 fluorescence microscope (Zeiss) using a 20× objective and videos were captured using NIS-Elements software (Nikon). Video recordings were made between 8-12 min after flow was initiated. 5-10 random fields were selected for recording platelet string formation. Platelet strings were defined as three or more platelets aligning in the direction of the flow. Platelet string lengths were determined as the distance between the first and the last platelet in a single continuous string stretched in the direction of the flow as described.17,18
Factor VIII-VWF binding assay
Factor VIII-VWF binding assay were conducted as described previously.19 Briefly, high binding 96 well plates were coated with anti-VWF polyclonal antibody (1μg/mL) in bicarbonate coating buffer (pH 9.6) overnight at 4 °C. Plates were blocked for 1h with 1% BSA in TBST at RT. After washing plates were incubated with cell culture supernatant for 2h followed by incubation with recombinant human FVIII (200 ng/mL) for 2h. Immobilized VWF-bound FVIII was detected by incubation with anti-FVIII mouse monoclonal antibody (2μg/mL, clone-R8B12) and HRP-conjugated anti-mouse antibody (1:5000) as described.20 After washing, TMB substrate solution was added and reaction stopped with 2N sulfuric acid and absorbance were measured at 450nm. A reference standard curve of VWF-FVIII binding was generated using serial dilutions of standard VWF.
Immunofluorescence microscopy
Confluent EA.hy926 cells were either treated with polyP70 and HMGB1 or left untreated for 16h. Cells were then washed with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min. After blocking with 1% BSA in PBS for 1h at RT, cells were incubated with anti-VWF rabbit polyclonal antibody (10μg/mL) overnight. After washing with PBS, cells were incubated with Alexa Flour-488 labeled goat anti-rabbit IgG as the secondary antibody (1:300). DAPI (4′,6-Diamidino-2-Phenylindole, hydrochloride) were used as nuclear stain. Images were acquired with Nikon C2 confocal microscope.
Statistical analysis
Data were expressed as mean ± standard deviation (unless otherwise specified) from three or more independent experiments. Data were analyzed by the Student t-test and group data were analyzed using analysis of variance (ANOVA) followed by Bonferroni post-hoc test using Graph Pad Prism7 (Graph Pad Prism, CA, USA). A p value of <0.05 was considered statistically significant.
Results
PolyP and HMGB1 induce VWF release by endothelial cells
PolyP70 (polyP concentration is expressed in terms of phosphate monomer) induced VWF secretion by transformed HUVECs (EA.hy926 endothelial cells) in a concentration-dependent (Figure 1A) and time-dependent manner (Figure 1B). The minimum concentration of polyP70 required to induce a significant amount of VWF secretion is 25 μM (Figure 1A). The VWF concentration in the supernatant increased significantly within 1h of incubation and reached its maximum level after 8h of polyP treatment (Figure 1B). Extracellular HMGB1 is known to induce platelet aggregation and endothelial cell activation. Thus, we explored the effect of HMGB1 on VWF release from EA.hy926 cells. Similar to results described above for polyP70, HMGB1 induced VWF release from EA.hy926 cells in a concentration- (Figure 1C) and time-dependent manner (Figure 1D). The HMGB1-induced VWF release started with late kinetics of 3h of stimulation and reached its maximum level after 16h of treatment with 40 nM HMGB1 (Figure 1D). We previously demonstrated that polyP70 could bind HMGB1 with high affinity to augment the proinflammatory effect of the nuclear protein.8 To determine whether polyP70 can amplify the HMGB1-mediated VWF release, cells were treated with sub-threshold concentrations of HMGB1 together with a low concentration of polyP70 (2.5 μM) which by itself exhibits no VWF releasing function. Analysis of the concentration-dependence of the HMGB1 effect indicated that 2.5 μM polyP70 was sufficient to significantly amplify the effect of a low concentration of HMGB1 (5 nM, inactive by itself) on VWF release from endothelial cells (Figure 1E). Similar results were obtained if the concentration of HMGB1 (5 nM) was kept constant in the presence of increasing concentrations (2.5-10 nM) of polyP70 (Figure 1F). The released VWF in the cell culture supernatant was functional as evidenced by its ability to interact with the purified human factor VIII in the FVIII-VWF binding assay (Figure 1G). To determine whether any endogenous HMGB1, possibly secreted into the cell culture supernatant during the experiments, contributes to VWF release, the ability of polyP70 to induce VWF release was evaluated in the absence and presence of a HMGB1 neutralizing antibody. The results indicated that a significant amount of endogenous HMGB1 is not secreted into the supernatant to influence the assay (Figure I in the online-only Data Supplement). This is evidenced by the similar extent of VWF release by polyP70 in either the absence or presence of the HMGB1 neutralizing antibody (Figure IB in the online-only Data Supplement).
Figure 1.
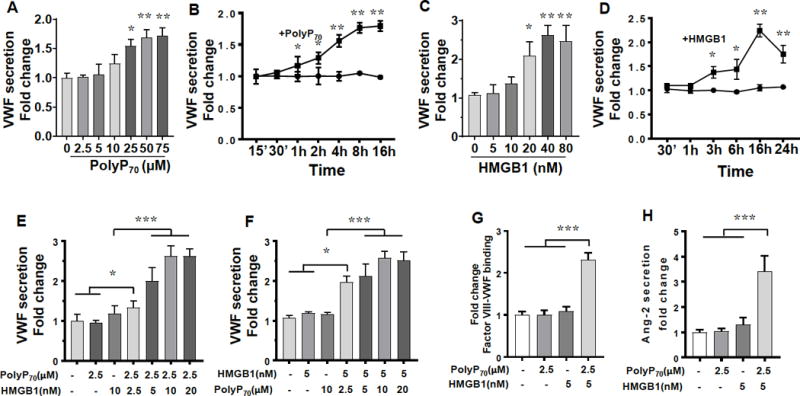
Effect of HMGB1 and polyP70 on VWF release from EA.hy926 cells. (A) EA.hy926 cells were incubated with increasing concentration of polyP70 for 16h and VWF release was measured by a Sandwich ELISA. (B) The same as A except that the time dependent effect of polyP70 (50 μM) on VWF release was measured. The symbols are: •, buffer control; and ■, polyP70. (C) The same as A except that the HMGB1-mediated (16 h) release of VWF by EA.hy926 cells was measured. (D) The same as B except that HMGB1 (40 nM) was used to measure VWF release. The symbols are: •, buffer control; and ■, HMGB1 (E) The same as above except that cells were incubated with indicated concentration of HMGB1 in presence of a fixed and low concentration of polyP70 (2.5 μM) for 16h. (F) The same as E except that endothelial cells were incubated with indicated concentration of polyP70 in the presence of a fixed and low concentration of HMGB1 (5 nM) for 16h. (G) Analysis of the interaction of the cell culture supernatant VWF with purified human factor VIII by Factor VIII-VWF binding assay as described under Materials and methods. (H) Cells were incubated with 2.5 μM polyP70 or 5 nM HMGB1 alone or in combination of both followed by measuring Ang-2 release by a Sandwich ELISA. All results are shown as means ± SD of three to five independent experiments (n=3-5). Statistical significance was analyzed by student t-test (A-D) and one way ANOVA (E-H) followed by Bonferroni post hoc test. *p<0.05, **p<0.01, ***p<0.005.
To explore the possible mechanism through which polyP70 and HMGB1 induced VWF release from EA.hy926 cells, the effect of both polyP70 and HMGB1, alone or in combination, on the secretion of angiopoietin -2 (Ang-2), which is localized with VWF in WPB granules of endothelial cells,21 was evaluated. Results showed that, similar to VWF release, low concentrations of both stimuli, which were individually inactive in the assay, but in combination dramatically induced Ang-2 release by EA.hy926 cells (Figure 1H). These results strongly suggest that the polyP70-HMGB1 complex induces VWF release through exocytosis of the WPB storage granules in endothelial cells. The efficiency of polyP70-HMGB1 (2.5 μM polyP and 5 nM HMGB1 as in panel F of Figure 1) to induce VWF release from primary HUVECs was essentially identical to that observed in transformed HUVECs (EA.hy926 cells) (Figure II in the online-only Data Supplement). Thus, due to ease of manipulation, all of the studies of the manuscript were conducted using EA.hy926 cells.
HMGB1 and PolyP70 induced VWF-dependent platelet string formation
To delineate the functional consequences of polyP70 and HMGB1-mediated VWF secretion by EA.hy926 cells, the capacity of the treated cells to recruit platelets to their surfaces was analyzed in a flow chamber-based assay system. Thus, freshly isolated human platelets were perfused over EA.hy926 cells, treated with polyP70, HMGB1 or with their combination, at the laminar shear rate of 8 dyns/cm2 as described.17,18 The results showed that cells treated with either polyP70 or HMGB1 alone did not significantly recruit platelets, however, the combination of the two stimuli effectively induced the recruitment of the platelets as evidenced by the platelet string formation on the surface of the cells (Figure 2A-D). The synergistic polyP70-HMGB1-mediated platelet string formation was inhibited by the anti-GPIbα antibody (Figure 2C). Treatment with polyP70 alone did not cause any observable platelet string formation, however, HMGB1 by itself mediated a small amount of shorter length VWF strings (<10 μm) on cells. However, the combination of polyP70 and HMGB1 lead to formation of much longer length VWF-platelet strings (~80-100μm) (Figure 2D). In a separate experiment, immunofluorescent staining of VWF on EA.hy926 cells indicated that a fraction of VWF in polyP70-HMGB1-treated cells is not secreted into the supernatant but remains on the surface of endothelial cells (Figure 2E).
Figure 2.
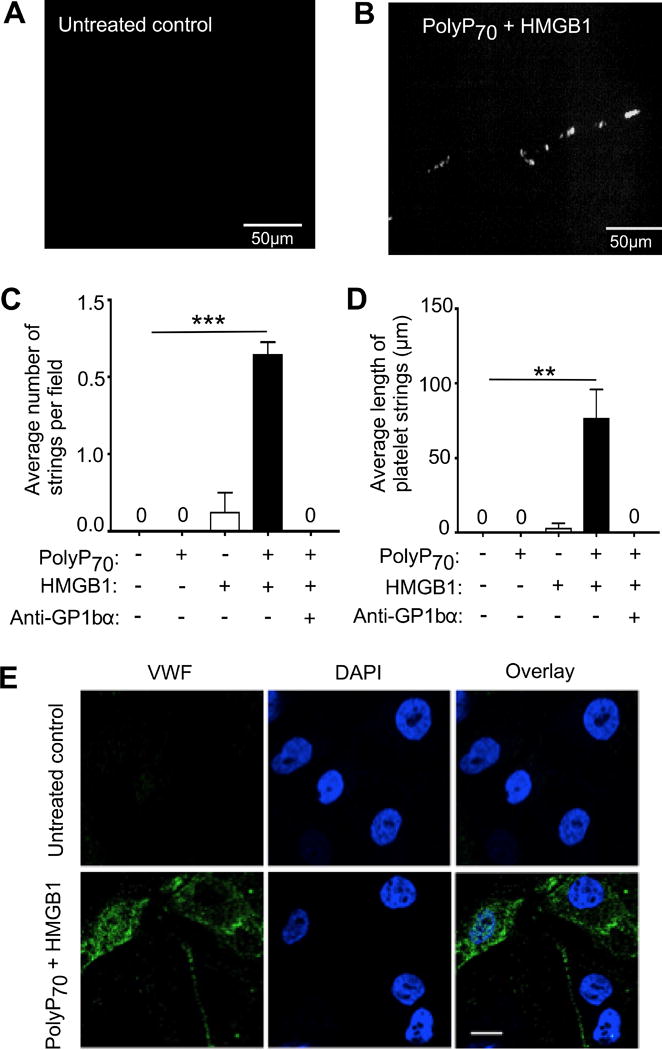
PolyP70-HMGB1 induced VWF-platelet string formation. EA.hy926 cells were incubated with polyP70 (10 μM) or HMGB1 (20 nM) alone or in combination for 16h. Platelets were perfused on different treatment groups with or without pre-incubation with the GP1bα monoclonal antibody. 5-10 random fields were selected for recording platelet string formation. Average number of platelet strings per field were determined as three or more platelets aligned in the direction of the flow. (A) Untreated control. (B) Fluorescent image of CMFDA-labeled platelet string formation on polyP70-HMGB1 treated cells. (C) Average number of strings per field was counted in different treatment groups. (D) Average length of platelet strings was measured in different treatment groups and expressed as mean ± SEM. **p<0.01, ***p<0.005. (E) Evaluation of the cell surface associated VWF by immunocytochemistry. VWF on cell surface was detected using specific anti-VWF rabbit antibody followed by secondary staining with Alexa Flour-488 conjugated anti-rabbit IgG as described in Materials and methods. Scale bar = 10μm. Statistical significance was analyzed by one way ANOVA followed by Bonferroni post hoc test (panels C and D).
Boiled platelet releasate induces VWF release
Activated platelets are known to secrete soluble polyP polymers of ~60-100 phosphate units long into circulation.2,4,22 To demonstrate that the platelet-derived polyP can similarly induce VWF release, we treated EA.hy926 cells with the platelet releasates obtained from thrombin-receptor agonist peptide-activated platelets as described.6,23 To ensure that only the effect of polyP is measured in the assay, platelet releasates were boiled for 30 min, which deactivates all other activators that may be present in the platelet releasates except polyP.6 Results showed that boiled platelet releasates induced VWF release in a concentration-dependent manner (Figure 3A). Thus, a platelet releasate ratio of 0.04-0.08 (platelet releasate volume/total volume) effectively induced VWF release by EA.hy926 cells and the stimulatory effect was not inhibited by boiling (Figure 3A), but it was abolished by the specific polyP inhibitor, EcPPXc, or alkaline phosphatase (ALP) which cleaves polyP (Figure 3B). Similarly, platelet releasate-derived polyP was capable of amplifying the VWF releasing effect of a low concentration of HMGB1. Thus, a platelet releasate ratio of 0.02, which does not have any effect on VWF release by itself, significantly promoted VWF release in the presence of a low concentration of HMGB1 (5 nM, inactive by itself), and the stimulatory effect was abolished by prior treatment of the platelet releasates with either EcPPXc or ALP (Figure 3C), suggesting that the effect is specifically mediated through the platelet polyP.
Figure 3.
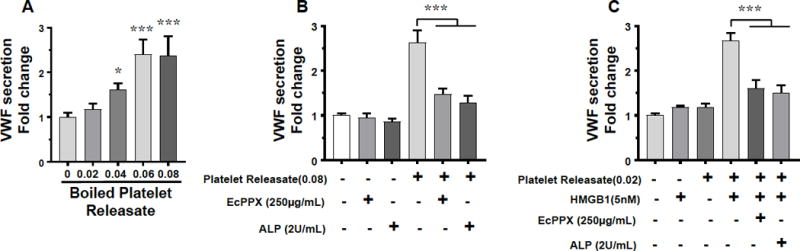
Effect of boiled platelet releasates on VWF release from EA.hy926 cells. (A) Cells were incubated with increasing concentrations of boiled platelet releasates (ratios of 0.02-0.08). The VWF release was measured by an ELISA as describe above. (B) The same as A except that cells were incubated with boiled platelet releasate in the presence of either EcPPXc (250 μg/mL) or ALP (2 U/mL). (C) The same as B except that a low concentration of platelet releasate (0.02 ratio) was incubated with a low concentration of HMGB1 (5 nM). All results are shown as means ± SD of three independent experiments (n=3). Statistical significance was analyzed by one way ANOVA followed by Bonferroni post hoc test. *p<0.05, ***p<0.005.
PolyP700 is more effective in inducing VWF release
Previous results have indicated that longer chain polyP polymers have higher procoagulant and proinflammatory activity.6,8 In agreement with those results, we found that polyP700 is more efficient in inducing VWF release from EA.hy926 cells. Thus, in contrast to 25 μM polyP70, which was required to induce a significant amount of VWF release (Figure 1A), a polyP700 concentration of 2.5 μM (~10 fold lower) was sufficient to induce the same amount of VWF release (Figure 4A). Similarly, the amplification effect of polyP700 on HMGB1 required a much lower concentration (250 nM) of the polymer to induce VWF release by endothelial cells (Figure 4B).
Figure 4.
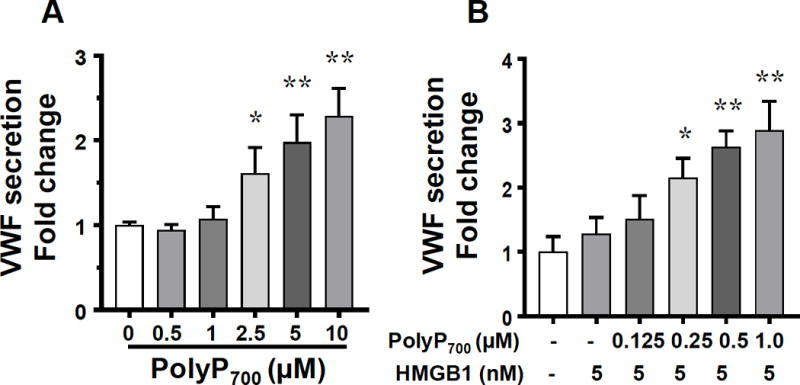
Effect of long chain polyP700 on VWF release from EA.hy926 cells. (A) Cells were incubated with increasing concentrations of polyP700 and VWF release was measured as described above. (B) The same as A except that low concentrations of polyP700 (0.125-1.0 nM) were incubated with a low concentration of HMGB1 (5 nM). The results are shown as means ± SD of three independent experiments. Results are shown as means ± SD of three independent experiments (n=3). Statistical significance was analyzed by one way ANOVA followed by Bonferroni post hoc test. *p<0.05, **p<0.01.
PolyP-HMGB1 mediated VWF release is dependent on RAGE and P2Y1 receptor signaling
Previous results have indicated that the synergistic signaling effect of polyP and HMGB1 in inducing a proinflammatory phenotype in endothelial cells is mediated through two receptors, RAGE and P2Y1.8 The siRNA silencing of either RAGE (Figure 5A) or P2Y1 (Figure 5B) significantly reduced the polyP70-HMGB1-mediated secretion of VWF from EA.hy926 cells. These results suggest that a similar signaling mechanism accounts for the synergistic effect of polyP70 and HMGB1 in inducing VWF release from endothelial cells. In agreement with these results, MRS2279 as an inhibitor of P2Y1 signaling (Figure 5C) and soluble RAGE (sRAGE) as a competitive inhibitor of RAGE signaling (Figure 5D), both significantly inhibited the VWF releasing function of polyP70-HMGB1 in EA.hy926 cells.
Figure 5.
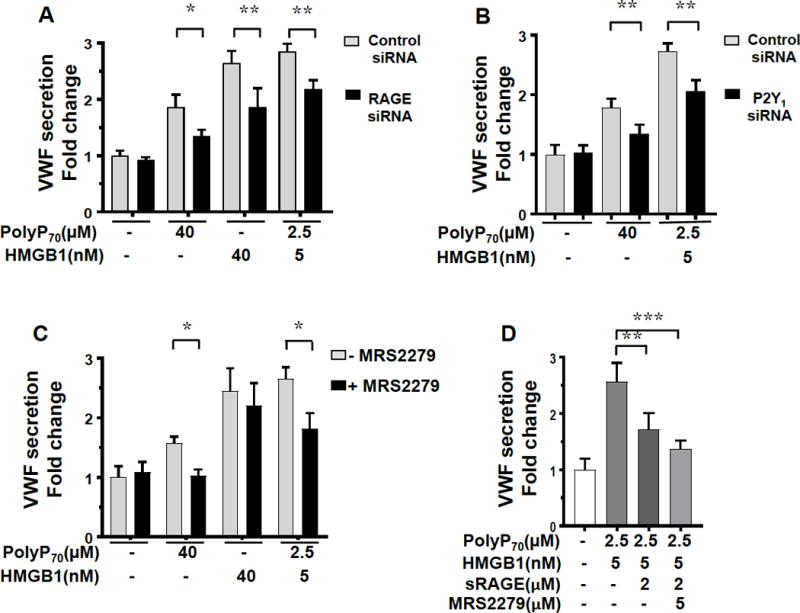
PolyP70 and HMGB1 induced VWF release is mediated by RAGE and P2Y1 signaling. (A) Confluent EA.hy926 cells were transfected with control siRNA (5 μg for 2 days) or specific RAGE siRNA (5μg for 2 days) followed by treating them with polyP70 (40 μM) or HMGB1 (40 nM) or with a combination of polyP70 (2.5 μM) and HMGB1 (5 nM) followed by measuring VWF release as described above. (B) The same as A except that VWF release by polyP70-HMGB1 was measured following transfection of cells with control or specific P2Y1 siRNAs. (C) The same as above expect that VWF release by polyP70-HMGB1 was measured following treatment of cells with the P2Y1 inhibitor, MRS2279 (5 μM). (D) The same as other panels listed above except that VWF release by the combination of low concentrations of polyP70-HMGB1 was measured following treatment of cells with either soluble RAGE (sRAGE) or the P2Y1 inhibitor, MRS2279. Results are shown as means ± SD of three independent experiments (n=3). Statistical significance was analyzed by one way ANOVA followed by Bonferroni post hoc test. *p<0.05, **p<0.01, ***p<0.005.
PolyP and HMGB1 induce VWF release through PLC and Src phosphorylation
Immunoblotting studies presented in Figure 6A demonstrate that the polyP70-HMGB1 complex increases the phosphorylation of both Src and PLC in EA.hy926 cells. RAGE signaling was involved in the activation of both signaling molecules since a RAGE antagonist peptide (Figure 6B) and sRAGE (not presented) both significantly downregulated polyP70-HMGB1-induced Src and PLC phosphorylation. Results with pharmacological inhibitors of the Src family of tyrosine kinases (PP2) and PLC phosphorylation (U73122) confirmed the Src- and PLC-dependence of VWF release by polyP70-HMGB1 since both inhibitors significantly decreased polyP70-HMGB1-induced VWF release from endothelial cells (Figure 6C). The intracellular Ca2+ chelator (BAPTA-AM) also inhibited VWF release, suggesting the involvement of Ca2+ signaling by polyP70-HMGB1 in mediating VWF release from endothelial cells.
Figure 6.
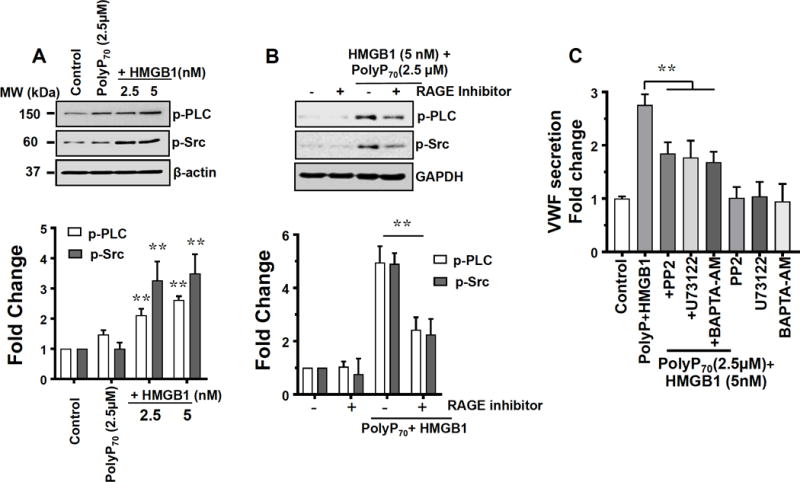
RAGE-dependent VWF release by polyP70-HMGB1 is mediated through PLC and Src phosphorylation. (A) Immunoblot analysis of PLC and Src phosphorylation following treatment of EA.hy926 cells with polyP70-HMGB1. B-actin was used as a loading control. (B) RAGE antagonist peptide inhibits polyP70-HMGB1 induced PLC and Src phosphorylation in EA.hy926 cells. GAPDH was used as a loading control. Densitometry analysis of blots were performed through image J software and represented as fold change. (C) Effect of Src inhibitor, PLC inhibitor and intracellular calcium chelator on polyP70-HMGB1 induced VWF release. Cells were pretreated with Src inhibitor (PP2, 10 μM), PLC inhibitor (U73122, 10 μM), and intracellular calcium chelator (BAPTA-AM, 10 μM) before measuring polyP70-HMGB1 mediated VWF release. Inhibitors by themselves had no effect on VWF release (last three bars). Results are shown as means ± SD of three independent experiments (n=3). Statistical significance was analyzed by one way ANOVA followed by Bonferroni post hoc test **p<0.01.
Discussion
We recently demonstrated that polyP70 binds chromatin-associated nuclear proteins, HMGB1 and histone 4, to amplify their proinflammatory signaling functions in both cellular and animal models.8 We discovered that the synergistic effect of polyP in promoting the proinflammatory effect of the nuclear proteins is mediated through polyP interacting with two cell surface receptors, RAGE and P2Y1 on endothelial cells.8 In this study, for the first time, we have discovered that polyP by a similar mechanism promotes HMGB1-mediated VWF release from endothelial cells. This hypothesis is supported by the observation that siRNA silencing of either RAGE or P2Y1 significantly reduced polyP70-HMGB1-mediated VWF release from endothelial cells. Further support for this hypothesis was provided by the results from two competitive inhibitors of RAGE and a chemical inhibitor of P2Y1 (MRS2279), all of which significantly decreased the polyP70-HMGB1-mediated secretion of VWF. Both polyP and HMGB1 were also individually capable of inducing VWF release, nevertheless, their combination exhibited a synergistic effect, thereby dramatically reducing the concentration of each stimulus required to induce VWF release. The RAGE- and P2Y1-dependent VWF releasing function of polyP70-HMGB1 required phosphorylation of Src, PLC and Ca2+ signaling since the inhibitors of these signaling molecules all significantly reduced VWF release from endothelial cells. It is worth noting that in an in vivo mouse model, it has been demonstrated that HMGB1 facilitates hypoxia-mediated VWF upregulation through TLR2 signaling.24 This is consistent with previous findings that, in addition to signaling via TLR4 and RAGE,8,25 HMGB1 can also signal through TLR2, however, the combination of polyP and HMGB1 has been found to signal primarily through the RAGE receptor.8
Endothelial cells store VWF multimers primarily in the WPB storage granules, which are released by exocytosis when cells are stimulated with thrombin, histamine, terminal complement components, epinephrine and a variety of other calcium-mediated agonists.11-13 Agonist-mediated release of VWF is composed of high molecular weight VWF multimers that upon release from endothelial cells can recruit platelets and form long string-like structures that play critical roles in regulating primary hemostasis at vascular injury sites.11-13 The results of the flow chamber assay with freshly isolated human platelets, perfused over polyP70-HMGB1-treated EA.hy926 endothelial cells, suggest that a combination of the two stimuli effectively induced the recruitment of the platelets to cell surfaces as evidenced by platelet string formation on the surface of endothelial cells (Figure 2B). The synergistic polyP70-HMGB1-mediated platelet string formation was mediated through the interaction of the GPIbα receptor present on platelets with the endothelial cell surface VWF since it was effectively abolished by prior incubation of platelets with an anti-GPIbα antibody (Figure 2C,D). Analysis of the length of VWF-platelet strings on EA.hy926 cells indicated that polyP70 alone did not cause any observable platelet string formation, and HMGB1 mediated only a small number of shorter length VWF strings (<10 μm). By contrast, the combination of polyP70 and HMGB1 led to formation of a significant amount of longer VWF-platelet strings (~80-100μm) (Figure 2D). The stimulated secretion of VWF has been hypothesized to result from exocytosis of larger size VWF multimers stored in WPB storage granules, although shorter size VWF multimers may be released continuously through a constitutive process that does not require stimulation.11-13 The circulating plasma type of VWF that functions as a carrier protein for factor VIII may constitute this latter form of the protein. Analysis of both the time course of VWF release by polyP70 (Figure 1B) and the size of the VWF-platelet strings indicate that polyP70-HMGB1 induced a regulated exocytosis of WPB storage granules. Further support for this hypothesis was provided by the observation that the combination of polyP70 and HMGB1 also released Ang-2, which is known to be co-localized with VWF in WPB storage granules. These observations further suggest that the ELISA assay used to measure the VWF release may underestimate the amount of polyP-mediated VWF release from endothelial cells since it only detects VWF secreted to the cell culture supernatant, but not the VWF species that remain on the surface of endothelial cells. Immunofluorescence staining of VWF in polyP70-HMGB1-treated cells by confocal microscopy (Figure 2E) further supports our hypothesis that a fraction of VWF remains associated with endothelial cells.
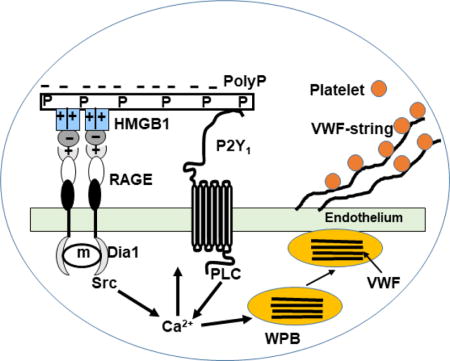
Previous results have indicated that polyP modulates procoagulant, fibrinolytic and complement pathways through providing an ionic surface on which different protease-activation complexes assemble. This function of polyP has been demonstrated to be of high physiological importance for the activation of the contact pathway in which factors XII, XI and prekallikrein are activated to initiate the intrinsic pathway of the coagulation cascade.3,4,6,15 It was recently demonstrated that polyP could also bind VWF through a similar mechanism to enhance its interaction with platelet GPIbα, thereby promoting the binding of platelets to VWF and as such contributing to hemostasis.26 Interestingly, this previous study evaluated the platelet polyP levels of a cohort of type 1 von Willebrand disease (VWD) and noted that the platelet polyP levels of these individuals are ~40% lower than the controls, leading the authors to hypothesize that there is a relationship between platelet polyP levels and VWF activity, which may be of high physiological relevance.26 In the context of these previous findings, we envision a possible hemostatic role for the platelet polyP-mediated VWF release (by itself or in combination with basal HMGB1) through this mechanism, which may be compromised in individuals with type 1 VWD, who reportedly have lower platelet polyP levels.26 This hypothesis warrants further investigation, however, if true, it would support a therapeutic role for polyP in type 1 VWD. It is of interest to note that the capacity of polyP to amplify the HMGB1-mediated release of VWF from endothelial cells can also promote thrombosis by recruiting platelets under certain pathological conditions. Moreover, by amplifying HMGB1 (or histone H4) signaling through presenting the nuclear protein to its receptor, RAGE, polyP can contribute to the pathogenesis of various acute and chronic proinflammatory disorders including severe sepsis, diabetes, atherosclerosis, and cancer.27-29 In support of this, RAGE null mice (or wild-type mice treated with sRAGE) and inhibitors of HMGB1 have been shown to improve survival in various animal models of acute inflammation and severe sepsis in response to endotoxin challenge.30-33 Elevated levels of VWF and HMGB1 have been found to be associated with disease severity and clinical out come in infectious diseases.28,34 Based on our results, we propose the following model for polyP-HMGB1-mediated VWF release and platelet string formation (Figure 7). We hypothesize that the interaction of the polyP-loaded HMGB1 with RAGE and P2Y1 receptors results in the activation of a number of proinflammatory signaling molecules including phosphorylation of Src and PLC, thereby leading to an elevation of intracellular Ca2+ levels, which facilitates the fusion of WPB storage granules with the endothelial cell membrane and the exocytosis of the stored VWF and other molecules. A fraction of VWF, which is released into the cell culture supernatant, is detected by ELISA, but a fraction of the released VWF (possibly larger molecular weight species) remains bound to the cell surface to recruit platelets and form platelet strings. The polyP-mediated VWF release or RAGE signaling occurs much more efficiently with longer chain polyP700 that may be released during bacterial infection, thus the VWF-platelet string formation is expected to occur with a much higher efficiency with low nM concentrations of longer chain polyPs.8 In this context, complement-mediated bacterial lysis can result in the release of such longer chain polyP molecules that can bind HMGB1 (or histones), thereby dramatically amplifying thrombotic and proinflammatory responses by RAGE signaling in general and by VWF-platelet string formation in particular as demonstrated in this study. Thus, in addition to its potential therapeutic utility in different types of bleeding disorders, polyP may also constitute an ideal target for developing inhibitors capable of neutralizing the function of polyP in thrombotic and inflammatory disorders.
Figure 7.
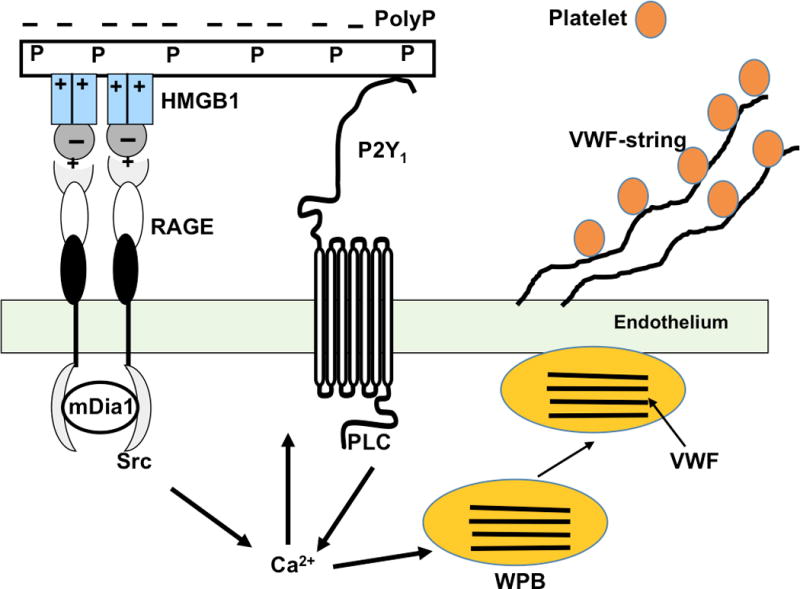
Hypothetical model of VWF-platelet string formation by polyP70-HMGB1. The interaction of the highly negatively charged polyP70 with the positively charged residues of HMGB1 eliminates their repulsive interactions with the similarly positively charged residues of the ligand-binding N-terminal domain of RAGE, thereby promoting the interaction of HMGB1 with RAGE via the acidic domain of the ligand. PolyP binds to multiple HMGB1 molecules and presents them to multiple receptors, thereby inducing receptor dimerization (oligomerization) and activation by facilitating the cooperative interaction of the cytoplasmic tails of the receptor with the adaptor molecule mDia1, which is responsible for initiating downstream signaling. Since polyP can also bind P2Y1, the interaction between RAGE and HMGB1-loaded polyP is further enhanced by a bridging mechanism. This dual receptor interaction mechanism results in the activation of a number of proinflammatory signaling molecules (i.e., activation of PI3K/Akt, Erk, p38, JNK, NF-kB, ROS generation; not shown) including phosphorylation of Src and PLC, thereby leading to an elevation of intracellular Ca2+ levels, which facilitates the fusion of WPB storage granules with the endothelial cell membrane and the exocytosis of the stored VWF. A fraction of VWF is released into the cell culture supernatant, which is detected by ELISA, but a fraction of the high molecular weight VWF polymers remains bound to the cell surfaces, which recruits platelets and forms platelet strings (see the text for more details).
Supplementary Material
Highlights.
PolyP and HMGB1 synergistically induce VWF release from WPB granules of endothelial cells through RAGE and P2Y1 signaling.
VWF released by polyP and HMGB1 recruits platelets and forms platelet strings on endothelial cells.
PolyP and HMGB1 mediated platelet string formation on endothelial cells is inhibited by anti-GPIbα antibody.
PolyP and HMGB1 mediated VWF release by endothelial cells is inhibited by inhibitors of Src, PLC and Ca2+ signaling.
Acknowledgments
We thank Cindy Carter for technical assistance and Audrey Rezaie for proofreading the manuscript.
Source of funding
This work was supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of HL 101917 and HL 62565 to ARR.
Nonstandard Abbreviations and Acronyms
- PolyP
Polyphosphate
- HMGB1
High mobility group box 1
- VWF
von Willebrand factor
- WPB
Weibel-Palade body
- RAGE
Receptor for advanced glycation end products
Footnotes
Disclosure
The authors declare no conflict of interests.
Authorship contributions
I.B. designed experiments, conducted VWF measurements, signaling assays, analyzed data and contributed to manuscript preparation; S.R.P designed and conduced flow chamber assays and analyzed the data; X.C conducted immunofluorescence imaging of the cell surface associated VWF using confocal microscopy; P.M.D. prepared human platelets and A.R.R. designed experiments, analyzed data and wrote the paper. All authors approved the final version of the manuscript.
References
- 1.Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 3.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassanian SM, Dinarvand P, Smith SA, Rezaie AR. Inorganic polyphosphate elicits pro-inflammatory responses through activation of the mammalian target of rapamycin complexes 1 and 2 in vascular endothelial cells. J Thromb Haemost. 2015;13:860–871. doi: 10.1111/jth.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarvand P, Hassanian SM, Qureshi SH, Manithody C, Eissenberg JC, Yang L, Rezaie AR. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood. 2014;123:935–945. doi: 10.1182/blood-2013-09-529602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassanian SM, Ardeshirylajimi A, Dinarvand P, Rezaie AR. norganic polyphosphate promotes cyclin D1 synthesis through activation of mTOR/Wnt/β-catenin signaling in endothelial cells. J Thromb Haemost. 2016;14:2261–2273. doi: 10.1111/jth.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami M. Signaling required for blood vessel maintenance: molecular basis and pathological manifestations. Int J Vasc Med. 2012;2012:293641. doi: 10.1155/2012/293641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019–2028. doi: 10.1182/blood-2014-06-528406. [DOI] [PubMed] [Google Scholar]
- 12.Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- 13.Nightingale T, Cutler D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J Thromb Haemost. 2013;11(Suppl 1):192–201. doi: 10.1111/jth.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maugeri N, Franchini S, Campana L, Baldini M, Ramirez GA, Sabbadini MG, Rovere-Querini P, Manfredi AA. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity. 2012;45:584–587. doi: 10.3109/08916934.2012.719946. [DOI] [PubMed] [Google Scholar]
- 15.Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119:5972–5979. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner TP, Amrhein N, Freimoser FM. Specific localization of inorganic polyphosphate (poly P) in fungal cell walls by selective extraction and immunohistochemistry. Fungal Genet Biol. 2007;44:845–852. doi: 10.1016/j.fgb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Gogia S, Kelkar A, Zhang C, Dayananda KM, Neelamegham S. Role of calcium in regulating the intra- and extracellular cleavage of von Willebrand factor by the protease ADAMTS13. Blood Adv. 2017;1:2063–2074. doi: 10.1182/bloodadvances.2017009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michels A, Albánez S, Mewburn J, Nesbitt K, Gould TJ, Liaw PC, James PD, Swystun LL, Lillicrap D. Histones link inflammation and thrombosis through the induction of Weibel-Palade body exocytosis. J Thromb Haemost. 2016;14:2274–2286. doi: 10.1111/jth.13493. [DOI] [PubMed] [Google Scholar]
- 19.Bendetowicz AV, Morris JA, Wise RJ, Gilbert GE, Kaufman RJ. Binding of factor VIII to von willebrand factor is enabled by cleavage of the von Willebrand factor propeptide and enhanced by formation of disulfide-linked multimers. Blood. 1998;92:529–538. [PubMed] [Google Scholar]
- 20.Manithody C, Fay PJ, Rezaie AR. Exosite-dependent regulation of factor VIIIa by activated protein C. Blood. 2003;101:4802–4807. doi: 10.1182/blood-2003-01-0126. [DOI] [PubMed] [Google Scholar]
- 21.Fiedler U, Augustin HG. Angiopoietin: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Verhoef JJ, Barendrecht AD, Nickel KF, Dijkxhoorn K, Kenne E, Labberton L, McCarty OJ, Schiffelers R, Heijnen HF, Hendrickx AP, Schellekens H, Fens MH, de Maat S, Renné T, Maas C. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129:1707–1717. doi: 10.1182/blood-2016-08-734988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassanian SM, Dinarvand P, Smith SA, Rezaie AR. Inorganic polyphosphate elicits proinflammatory responses through activation of mTOR complexes 1 and 2 in vascular endothelial cells. J Thromb Haemost. 2015;13:860–871. doi: 10.1111/jth.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh B, Biswas I, Bhagat S, Surya Kumari S, Khan GA. HMGB1 facilitates hypoxia-induced vWF upregulation through TLR2-MYD88-SP1 pathway. Eur J Immunol. 2016;46:2388–2400. doi: 10.1002/eji.201646386. [DOI] [PubMed] [Google Scholar]
- 25.Skrha J, Jr, Kalousová M, Svarcová J, Muravská A, Kvasnička J, Landová L, Zima T, Skrha J. Relationship of soluble RAGE and RAGE ligands HMGB1 and EN-RAGE to endothelial dysfunction in type 1 and type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2012;120:277–281. doi: 10.1055/s-0031-1283161. [DOI] [PubMed] [Google Scholar]
- 26.Montilla M, Hernández-Ruiz L, García-Cozar FJ, Alvarez-Laderas I, Rodríguez-Martorell J, Ruiz FA. Polyphosphate binds to human von Willebrand factor in vivo and modulates its interaction with glycoprotein Ib. J Thromb Haemost. 2012;10:2315–2323. doi: 10.1111/jth.12004. [DOI] [PubMed] [Google Scholar]
- 27.Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36:625–632. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 29.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 30.Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 32.Sama AE, D’Amore J, Ward MF, Chen G, Wang H. Bench to bedside: HMGB1-a novel proinflammatory cytokine and potential therapeutic target for septic patients in the emergency department. Acad Emerg Med. 2004;11:867–873. doi: 10.1197/j.aem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Ward MF, Sama AE, Wang H. Extracellular HMGB1 as a proinflammatory cytokine. J Interferon Cytokine Res. 2004;24:329–333. doi: 10.1089/107999004323142187. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Ning Z, Qiu Y, Liao Y, Chang H, Ai Y, Wei Y, Deng Y, Shen Y. Elevated levels of von Willebrand factor and high mobility group box 1 (HMGB1) are associated with disease severity and clinical outcome of scrub typhus. Int J Infect Dis. 2017;61:114–120. doi: 10.1016/j.ijid.2017.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


