See Aly and Korn (doi:10.1093/brain/awy269) for a scientific commentary on this article.
African Americans with multiple sclerosis demonstrate higher inflammatory disease activity and poorer prognosis than Caucasian Americans. Gonzalez Caldito et al. report that African Americans patients exhibit faster rates of brain and retinal tissue loss, emphasizing the need for future studies involving this group to identify individual differences in treatment responses.
Keywords: multiple sclerosis, race, African American, magnetic resonance imaging (MRI), optical coherence tomography (OCT)
Abstract
On average, African Americans with multiple sclerosis demonstrate higher inflammatory disease activity, faster disability accumulation, greater visual dysfunction, more pronounced brain tissue damage and higher lesion volume loads compared to Caucasian Americans with multiple sclerosis. Neurodegeneration is an important component of multiple sclerosis, which in part accounts for the clinical heterogeneity of the disease. Brain atrophy appears to be widespread, although it is becoming increasingly recognized that regional substructure atrophy may be of greater clinical relevance. Patient race (within the limitations of self-identified ancestry) is regarded as an important contributing factor. However, there is a paucity of studies examining differences in neurodegeneration and brain substructure volumes over time in African Americans relative to Caucasian American patients. Optical coherence tomography is a non-invasive and reliable tool for measuring structural retinal changes. Recent studies support its utility for tracking neurodegeneration and disease progression in vivo in multiple sclerosis. Relative to Caucasian Americans, African American patients have been found to have greater retinal structural injury in the inner retinal layers. Increased thickness of the inner nuclear layer and the presence of microcystoid macular pathology at baseline predict clinical and radiological inflammatory activity, although whether race plays a role in these changes has not been investigated. Similarly, assessment of outer retinal changes according to race in multiple sclerosis remains incompletely characterized. Twenty-two African Americans and 60 matched Caucasian Americans with multiple sclerosis were evaluated with brain MRI, and 116 African Americans and 116 matched Caucasian Americans with multiple sclerosis were monitored with optical coherence tomography over a mean duration of 4.5 years. Mixed-effects linear regression models were used in statistical analyses. Grey matter (−0.9%/year versus −0.5%: P =0.02), white matter (−0.7%/year versus −0.3%: P =0.04) and nuclear thalamic (−1.5%/year versus −0.7%/year: P =0.02) atrophy rates were approximately twice as fast in African Americans. African Americans also exhibited higher proportions of microcystoid macular pathology (12.1% versus 0.9%, P =0.001). Retinal nerve fibre layer (−1.1% versus −0.8%: P =0.02) and ganglion cell+ inner plexiform layer (−0.7%/year versus −0.4%/year: P =0.01) atrophy rates were faster in African versus Caucasian Americans. African Americans on average exhibited more rapid neurodegeneration than Caucasian Americans and had significantly faster brain and retinal tissue loss. These results corroborate the more rapid clinical progression reported to occur, in general, in African Americans with multiple sclerosis and support the need for future studies involving African Americans in order to identify individual differences in treatment responses in multiple sclerosis.
See Aly and Korn (doi:10.1093/brain/awy269) for a scientific commentary on this article.
Introduction
Multiple sclerosis often follows a more aggressive disease course in African American patients, as compared to Caucasian American patients (of self-identified Northern European ancestry) (Naismith et al., 2006; Kister et al., 2010; Wallin et al., 2012; Langer-Gould et al., 2013). Several independent studies have shown that African Americans with multiple sclerosis accumulate disability faster and generally exhibit higher degrees of global disability as estimated by Expanded Disability Status Scale (EDSS) scores, relative to Caucasian Americans (Kaufman et al., 2003; Cree et al., 2004; Naismith et al., 2006). In general, African Americans with multiple sclerosis have been found to exhibit greater inflammatory disease activity, clinically and radiologically (Rinker et al., 2007; Weinstock-Guttman et al., 2010; Howard et al., 2012), experience greater degrees of pyramidal involvement early in the disease course (Marrie et al., 2006; Rinker et al., 2007), and have a higher incidence of spinal cord involvement manifest by more frequent clinical episodes of transverse myelitis (Cree et al., 2004). Furthermore, it has been suggested that African Americans with multiple sclerosis may have a less favourable response to disease-modifying therapies (DMTs), including not only conventional therapies such as glatiramer acetate and the interferons, but also higher-efficacy DMTs such as natalizumab (Cree et al., 2005; Kister et al., 2010; Khan et al., 2015).
MRI of the brain and spinal cord are the most commonly used paraclinical tools in the surveillance of multiple sclerosis (Miller et al., 1998; Napoli and Bakshi, 2005; Filippi, 2015). Through the identification of new/enlarging T2 lesions and/or contrast enhancing lesions, MRI has become established as a sensitive and reliable tool for detecting inflammatory disease activity (Inglese et al., 2005; Sicotte, 2011), which forms the basis for its role in monitoring disease course, as well as response to DMTs that primarily suppress or modulate the immune system (Romeo et al., 2013; Simon, 2014; Wattjes et al., 2015). However, MRI markers of inflammatory activity at baseline, as well as longitudinally, only modestly correlate with disease course and disability progression. In part, this relates to the realization that while multiple sclerosis may be primarily regarded as an inflammatory, demyelinating disorder of the CNS, the principal pathological substrate underlying permanent disability is neurodegeneration (Miller, 2004; Inglese et al., 2011). The immunopathogenic mechanisms underlying axonal and neuronal loss in multiple sclerosis are complex (Buss et al., 2004; Herz et al., 2010; Lassmann, 2010). While inflammatory axonal transection and chronic partial or complete demyelination may represent major contributors to neurodegeneration (Pérez-cerdá et al., 2016), additional pathobiological mechanisms are likely at play, including but not limited to reduction or loss of axonal trophic support, aberrant energy utilization/metabolism (Campbell et al., 2014), and impaired axonal transport (Criste et al., 2014; Pérez-cerdá et al., 2016). Moreover, there appears to be inherent differences among patients with multiple sclerosis with respect to susceptibility to neurodegeneration, which in part accounts for the clinical heterogeneity of the disease (Joy and Johnson, 2001). Over the past 15 years, estimates of neurodegeneration obtained with non-conventional MRI techniques that quantify brain volumes and probe tissue ultrastructure integrity, such as diffusion tensor imaging (DTI) and magnetization transfer ratio (MTR), have been shown to facilitate prediction of disability progression (Agosta et al., 2006; Reich et al., 2009; Inglese et al., 2011; Harrison et al., 2013)
One important factor contributing to disability accumulation in multiple sclerosis may be race (Marrie et al., 2006; Kister et al., 2010). In this paper, we use the term ‘race’ to refer to the socially-defined categorization historically (but not exclusively) applied in the USA, which has sometimes been interpreted as self-identified ancestry with varying degrees of accuracy (Tang et al., 2005; Mersha and Abebe, 2015). Despite its potential clinical relevance, there are few longitudinal studies definitively characterizing neurodegeneration according to race. It has been shown cross-sectionally that African Americans with multiple sclerosis have greater reductions in whole brain volumes, greater aberrations in MTR measures, and higher T2 and T1 lesion volumes as compared to Caucasian Americans (Weinstock-Guttman et al., 2010; Al-Kawaz et al., 2017). However, there is a paucity of longitudinal studies examining MRI volumetrics in African American patients, and the regional or brain substructure atrophy that may be of greater clinical relevance than whole brain atrophy (Bermel and Bakshi, 2006; Horakova et al., 2012; Minagar et al., 2013) has remained largely unexplored with respect to the impact of race. Studying whole brain and brain substructure changes over time is fundamental to elucidate whether neurodegeneration differs by race, thereby helping to advance our understanding of the mechanisms underlying the more aggressive clinical disease course observed in African Americans.
Anterior visual pathway involvement is virtually ubiquitous in multiple sclerosis, with optic neuritis representing the initial manifestation in roughly 25% of cases and occurring in up to 70% of patients during their disease course. Up to 99% of multiple sclerosis cases exhibit demyelinating plaques within their optic nerves at post-mortem, regardless of a clinical history of optic neuritis (Ikuta and Zimmerman, 1976; Toussaint et al., 1983). Although the incidence of optic neuritis in African Americans with multiple sclerosis is not clearly higher than in Caucasian Americans (Cree et al., 2004), the clinical severity is greater, as characterized by lower visual function scores at optic neuritis onset and after 1 year of follow-up (Phillips et al., 1998; Moss et al., 2014; Kimbrough et al., 2015).
Optical coherence tomography (OCT) is a non-invasive, reliable, high resolution, inexpensive, and reproducible imaging tool that allows accurate quantification of tissues such as the discrete retinal layers of the retina (Huang et al., 1991). In recent years, OCT has emerged as a complementary tool to MRI with particular utility for tracking neurodegeneration (and accordingly neuroprotection) in multiple sclerosis (Saidha et al., 2011a; Maldonado et al., 2015; Rebolleda et al., 2015). In particular, OCT-derived measures of peripapillary retinal nerve fibre layer (p-RNFL) and ganglion cell plus inner plexiform layer (GCIP) thickness have been proposed to reflect global aspects of the multiple sclerosis disease process (Saidha et al., 2011b, 2015; Ratchford et al., 2013). GCIP thickness measures may have a number of advantages over p-RNFL thickness measures, including better reliability and reproducibility, less astroglial confound (astrogliosis predominantly occurs in the RNFL), and lower susceptibility to swelling during optic nerve inflammation (González-López et al., 2014; Saidha and Calabresi, 2014). As such, GCIP thickness correlates better with high and low contrast visual function, as well as EDSS scores, than p-RNFL thickness (Saidha et al., 2011b). Rates of GCIP atrophy mirror rates of whole brain and in particular grey matter atrophy over time, are accelerated in patients exhibiting non-ocular inflammatory activity (Ratchford et al., 2013), and are differentially modulated by DMTs (Button et al., 2017). On the other hand, increased volumes of the inner nuclear layer (INL) and the presence of microcystoid macular pathology (MMP), at baseline, have been shown to predict clinical and radiological inflammatory activity, and potentially serve as a biomarker for the anti-inflammatory effects of DMT (Saidha et al., 2012; Knier et al., 2016). The impact of race in this regard remains to be elucidated. In a single longitudinal study to assess the effects of race on rates of retinal atrophy, African Americans exhibited faster rates of thinning in the inner retinal layers, p-RNFL and GCIP, as compared to Caucasian Americans (Kimbrough et al., 2015). That study included 81 African Americans with multiple sclerosis who were tracked with OCT for a median duration of 2 years at three different academic sites.
In the current observational study, we sought to (i) determine whether African Americans exhibit faster rates of whole brain atrophy over time as compared to Caucasian Americans with multiple sclerosis, and to assess whether such differences relate to accelerated atrophy within regional brain areas (substructures) or rather global atrophy across brain compartments in general; and (ii) examine whether African Americans exhibit faster rates of retinal INL and outer nuclear layer (ONL) thinning.
Materials and methods
Participants
Age- and sex-matched healthy controls and participants with multiple sclerosis were recruited by convenience sampling from the Johns Hopkins Multiple Sclerosis Center. Only individuals with at least 1 year of MRI or OCT follow-up were eligible for inclusion in the longitudinal MRI or OCT components of the study, respectively. In the longitudinal OCT component of the study, 36% of the healthy controls and 42% of those with multiple sclerosis overlapped with a previously published study from our group (Kimbrough et al., 2015), which assessed OCT changes in the inner retinal layers in African Americans versus Caucasian Americans with multiple sclerosis.
The study was approved by the Institutional Review Board of Johns Hopkins University, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants. Multiple sclerosis diagnosis was confirmed by the treating neurologist, based on the 2010 revised McDonald criteria (Polman et al., 2011). Disease subtype was classified as relapsing remitting, secondary progressive or primary progressive multiple sclerosis (Lublin and Reingold, 1996). Ancestry was classified as African Americans or Caucasian Americans according to patient self-reporting. Multiple sclerosis cases and healthy controls of other self-identified race were not included in the study. Participants with other known neurological or ophthalmologic disorders, diabetes mellitus, uncontrolled hypertension, glaucoma, or refractive errors exceeding ± 6 dioptres were excluded from study enrolment. For the OCT component of the study, no participants were enrolled within 6 months of an acute optic neuritis episode, and scans of individuals who developed optic neuritis during follow-up were censored from the time of occurrence of the optic neuritis.
Procedures
MRI
Brain MRI was performed with a 3 T Philips Achieva scanner (Philips Medical System). Two axial whole-brain sequences without gaps were used: multi-slice T2-weighted fluid-attenuated inversion recovery (FLAIR; acquired resolution: 0.8 × 0.8 × 2.2 or 0.8 × 0.8 × 4.4 mm; echo time: 68 ms; repetition time: 11 s; inversion time: 2.8 s; SENSE factor: 2; averages: 1); and 3D magnetization-prepared rapid acquisition of gradient echoes (MPRAGE; acquired resolution: 0.8 × 0.8 × 1.2 mm; echo time: 6 ms; repetition time: ∼10 ms; inversion time: 835 ms; flip angle: 8 degrees; SENSE factor: 2; averages: 1).
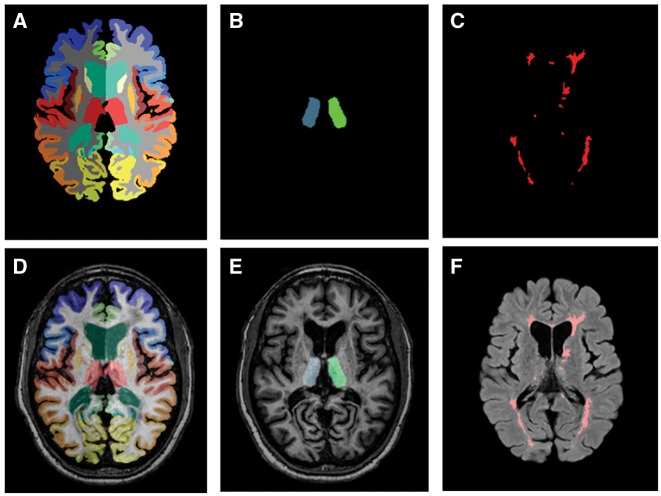
Whole brain segmentation was performed using Multi-Atlas CRUISE (MACRUISE), as described in detail elsewhere (Huo et al., 2016). In brief, this method uses two techniques: Multi-Atlas Label Fusion (MALF) and Cortical Reconstructing Using Implicit Surface Evolution (CRUISE) (Han et al., 2004; Iglesias and Sabuncu, 2015). Lesion Topology-preserving Anatomy-Driven Segmentation (Lesion-TOADS) was run on the skull-stripped T1-weighted and FLAIR images to segment lesions while preserving the topology of other brain structures. This was followed by the MALF step (Bazin and Pham, 2007). For specific lesion segmentation, Subject-Specific Sparse Dictionary Learning (S3DL) was applied to Lesion-TOADS output, which uses a classification technique based on ensembles of decision trees to map multiple sclerosis lesions (Roy et al., 2015). The Random Forest Thalamus Segmentation (RAFTS) algorithm was used for DTI-based thalamic segmentation (Glaister et al., 2016). One advantage of RAFTS is that it excludes white matter tracts from volume determinations, allowing estimation of primarily neuronal content within the thalamus. Collectively, these techniques segment the brain into its component substructures while simultaneously delineating multiple sclerosis lesions, yielding volumes of the cortical grey matter, cerebral white matter, whole and nucleus thalamus (derived from MACRUISE and RAFTS, respectively), and deep grey matter (Fig. 1). Cerebral volume fraction, representative of whole brain volume and analogous to brain parenchymal fraction, was calculated by dividing the summed volume of brain substructures by intracranial volume and expressed as the percentage of intracranial volume occupied by brain matter.
Figure 1.
Representation of the different methods used for brain and lesion segmentation. (A) Whole brain segmentation was performed using MACRUISE (Multi-Atlas CRUISE). (B) MACRUISE segmentation overlapping the counterpart MPRAGE image. (C) Random Forest Thalamus Segmentation (RAFTS) algorithm was used for DTI-based thalamic segmentation (nuclear thalamus). (D) Overlapping of an MPRAGE image and the corresponding RAFTS thalamus segmentation. (E) S3DL was applied to Lesion-TOADS used for specific lesion segmentation. (F) FLAIR image with the corresponding lesion segmentation results overlying.
Optical coherence tomography
Retinal imaging was performed using spectral domain Cirrus HD-OCT (model 4000, software version 6.0; Carl Zeiss Meditec), as described previously (Ratchford et al., 2013). Briefly, peripapillary and macular data were obtained with the Optic Disc Cube 200 × 200 protocol and Macular Cube 512 × 128 protocol, respectively. Scans with signal strength <7/10 or with artefact were excluded, in accordance with OSCAR-IB criteria (Tewarie et al., 2012).
P-RNFL thickness values were generated by conventional Cirrus HD-OCT software, as described elsewhere (Saidha et al., 2015). An automated macular segmentation method described in detail elsewhere (Bhargava et al., 2015) was used to compute thicknesses of the GCIP, INL, ONL, and average macular thickness (Supplementary Fig. 1). This segmentation method uses a validated algorithm (Lang et al., 2013) that generates thickness measurements by averaging the thickness values within a 5 × 5 mm circle centred at the fovea. The foveal region consisting of a 1 × 1 mm circle was excluded from analyses. This reproducible segmentation method has proven capability for detecting differences between multiple sclerosis and healthy control eyes cross-sectionally and longitudinally (Bhargava et al., 2015). Previous studies have shown that OCT segmentation is highly reliable, with inter-visit intra-class correlation coefficients ranging from 0.91 to 0.99 for all thickness measurements (Cettomai et al., 2008; Saidha et al., 2011a). All OCT segmentations were reviewed for quality control purposes.
All macular cube scans were assessed for macular microcystoid changes [referred to by some as either MMP or microcystic macular oedema (MME)], as well as other retinal pathologies that could adversely affect macular segmentation (Saidha et al., 2012; Al-louzi et al., 2017).
Statistical analyses
Statistical analyses were performed using STATA version 13 (StataCorp, College Station, TX). The Shapiro-Wilk test was used to assess the normality of distributions. Comparisons of non-normally distributed variables between groups were performed using the Wilcoxon rank-sum test (age at baseline, disease duration from first symptom onset, duration of follow-up time, DMT treatment duration at baseline). The chi-square (χ2) test and Fisher exact test were used for group comparisons of proportions (sex, race, optic neuritis history, multiple sclerosis subtype and DMT status).
To analyse the course of brain substructure volume and retinal layer thickness changes during the observational follow-up period, time was used as a continuous covariate (starting at the date of the first MRI and first OCT observations, respectively). We applied mixed effects linear regression models utilizing patient-specific and eye-specific random intercepts and slopes, thereby inherently accounting for baseline volumes of brain substructures or thicknesses of OCT measures, which is important since baseline values may have an important impact on rates of future atrophy. We tested for differential rates of atrophy between African Americans and Caucasian Americans using interaction terms. Two models were used in both the MRI and OCT analyses, with the first models being adjusted for matching factors, age at baseline and sex (Rothman et al., 2008), and the second models being adjusted for age at baseline, sex, optic neuritis history, and disease duration. In the MRI component of the study, additional exploratory analyses were also performed adjusting for (i) rates of lesion volume increase/accumulation to examine whether rates of atrophy are independent from overt inflammatory activity; and (ii) the time since prior optic neuritis to account for a potential effect of acute optic neuritis within 6 months of the baseline brain MRI on rates of brain substructure atrophy. In addition, in the OCT analyses, models assessing rates of change of retinal measures also accounted for within-subject inter-eye correlations, since values from both eyes were used in analyses. We did not correct for multiple comparisons as this study was hypothesis-driven (Bender and Lange, 2001). We defined statistical significance as P ≤ 0.05.
Brain volume rates of change are depicted in absolute coefficients and in percentage of volume change per year (approximately estimated by calculating the natural logarithm of each dependent variable). Similarly, with respect to the retinal rates of change, results are presented in absolute coefficients and in percentage of thickness change per year.
Data availability
The data that support the findings of this study are available on request from the corresponding author and approval from the Institutional Review Board. The data are not publicly available due to them containing information that could compromise participants’ privacy and consent.
Results
Study population
Cross-sectional and longitudinal MRI cohorts
For the MRI component of the study, 32 African Americans and 64 Caucasian Americans with multiple sclerosis (1:2 age and sex matching between the African Americans and Caucasian Americans cohorts) were enrolled for the cross-sectional analyses of whole brain and brain substructure volumes at baseline. For the longitudinal analyses, rates of change in 22 of 32 African Americans (69%) and 60 of the 64 Caucasian Americans (94%) were analysed based on at least 1 year of MRI follow-up.
In the cross-sectional MRI cohort, 10 African Americans and 22 Caucasian Americans were enrolled within 6 months of acute optic neuritis; of these, seven African Americans and 20 Caucasian Americans had longitudinal data. The summary of demographics, baseline characteristics, and differences therein, stratified by African and Caucasian American race, are provided in Table 1; demographics for the subset included in the cross-sectional MRI analyses are given in Supplementary Table 1.
Table 1.
Summary of demographics and baseline characteristics
| African Americans | Caucasian Americans | P-value | |
|---|---|---|---|
| MRI cohort | n = 22 | n = 60 | |
| Average age, years (SD) | 36.7 (10.7) | 38.0 (11.1) | 0.601a |
| Female, n (%) | 16 (72.7) | 47 (78.3) | 0.594b |
| Multiple sclerosis subtype: | 0.186c | ||
| RRMS, n (%) | 21 (95.5) | 49 (81.7) | |
| PPMS, n (%) | 1 (4.6) | 5 (8.3) | |
| SPMS, n (%) | 0 (0.0) | 6 (10.0) | |
| History of optic neuritis, n (%) | 14 (63.6) | 35 (58.3) | 0.704b |
| Disease duration, years, mean (SD) | 5.46 (4.9) | 6.17 (6.5) | 0.878a |
| On DMT (at baseline), n (%) | 16 (72.7) | 35 (58.3) | 0.234b |
| Acute optic neuritis within 6 months of baseline MRI, n (%) | 7 (31.8) | 19 (31.7) | 0.990b |
| Duration follow-up, years, mean (SD) | 3.1 (2.6) | 5.1 (2.9) | 0.016a |
| EDSS, median (Q1–Q3) | 2.8 (1.5–3.5) | 2 (1.5–3.5) | 0.590a |
| Optical coherence tomography cohort | n = 116 | n = 116 | |
| Baseline age, years, mean (SD) | 37.5 (9.6) | 37.6 (10.2) | 0.956a |
| Female, n (%) | 90 (77.6) | 94 (81.0) | 0.517b |
| Multiple sclerosis subtype: | 0.826c | ||
| RRMS, n (%) | 105 (90.5) | 107 (92.2) | |
| PPMS, n (%) | 5 (4.3) | 3 (2.6) | |
| SPMS, n (%) | 6 (5.2) | 6 (5.2) | |
| History of optic neuritis, n (%) | 51 (44.0) | 52 (44.8) | 0.895b |
| Disease duration, years, mean (SD) | 6.24 (5.8) | 5.79 (5.8) | 0.509a |
| On DMT (at baseline), n (%) | 75 (64.7) | 83 (71.6) | 0.260b |
| MMP, n (%) | 14 (12.1) | 1 (0.9) | 0.001b |
| Duration follow-up, years, mean (SD) | 4.0 (2.4) | 5.03 (2.3) | 0.001a |
| EDSS, median (Q1–Q3) | 2 (1–3) | 1.5 (1–2) | 0.016a |
aWilcoxon rank-sum test.
bχ2 test.
cFisher’s exact test.
PPMS = primary progressive multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis; Q1 = first quartile, Q3 = third quartile. Bold values indicate statistical significance (P < 0.05).
In the multiple sclerosis cross-sectional and longitudinal MRI cohorts, there were no significant differences between African Americans and Caucasian Americans with respect to clinical phenotype, history of optic neuritis, mean disease duration, median EDSS, and proportion of patients receiving DMTs. The mean duration of follow-up was higher in Caucasian Americans (5.1 years) as compared to African Americans (3.1 years; P = 0.016).
Optical coherence tomography cohorts
For the OCT study, 31 African American and 61 Caucasian American healthy controls (1:2 age and sex matched cohorts; n = 92) and 116 African Americans and 116 Caucasian Americans with multiple sclerosis (age and sex matched cohorts; n = 232) were enrolled. All the patients from the MRI cohorts were also included in the OCT component of the study. For the longitudinal OCT analyses, rates of change in 17 of 31 African American healthy controls (55%) and 33 of 61 Caucasian American healthy controls (54%) could be analysed longitudinally based on at least 1 year of follow-up. In the multiple sclerosis cohorts, all recruited participants were included in the longitudinal OCT analyses (116 in the African Americans and Caucasian Americans cohorts). The summary of demographics, baseline characteristics, and differences therein, stratified by African and Caucasian American race, are provided in Table 1. The baseline demographics of the healthy control cohorts stratified by race are given in Supplementary Table 2.
Similar to the MRI cohorts, there were no significant differences in the majority of baseline characteristics between the African American and Caucasian American multiple sclerosis OCT cohorts, except that the mean duration of follow-up was higher in Caucasian Americans (5.0 years) relative to African Americans (4.0 years; P = 0.001). In addition, a larger proportion of African American patients exhibited MMP at baseline (12.1% versus 0.9% in Caucasian Americans, P = 0.001). Furthermore, median EDSS was higher in African Americans (African versus Caucasian Americans: 2 versus 1.5; P < 0.001).
Differences in brain substructure volumes and brain atrophy rates between African and Caucasian Americans
At baseline (Table 2), adjusting for age, sex, optic neuritis history, and disease duration, African Americans had higher T2 lesion volumes (P = 0.007), as well as borderline lower whole and nuclear thalamic volumes (P = 0.056 and P = 0.059, respectively). Percentage change per year of brain substructures are summarized in Table 3 and Supplementary Table 3. Additionally, absolute volume changes per year of brain substructures are summarized in Supplementary Table 4. Over the course of study follow-up, adjusting for age at baseline, sex, optic neuritis history, and disease duration, both African Americans and Caucasian Americans exhibited significant rates of cerebral volume fraction (whole brain), cortical grey matter, cerebral white matter, nuclear thalamic, whole thalamic, and deep grey matter atrophy (P < 0.001 for all), as well as increases in T2 lesion volumes (P < 0.001).
Table 2.
Comparisons of regional (substructure) brain volume fractions (normalized to intracranial volume) at baseline
| Brain substructure volumes at baseline (cerebral volume fractions) | ||||
|---|---|---|---|---|
| African Americans | Caucasian Americans | African Americans versus Caucasian Americans | ||
| (n = 32) | (n = 64) | P-valuea | P-valueb | |
| Mean (SD) | Mean (SD) | |||
| Whole brain | 0.8304 (0.0307) | 0.8301 (0.0296) | 0.867 | 0.905 |
| Cortical grey matter | 0.3857 (0.0212) | 0.3883 (0.0188) | 0.303 | 0.319 |
| Cerebral white matter | 0.2867 (0.0200) | 0.2867 (0.0187) | 0.952 | 0.996 |
| Whole thalamus | 0.0079 (0.0012) | 0.0082 (0.0011) | 0.064 | 0.059 |
| Nucleus thalamus | 0.0102 (0.0018) | 0.01073 (0.0014) | 0.058 | 0.056 |
| Deep grey matter | 0.0307 (0.0038) | 0.0310 (0.0032) | 0.558 | 0.572 |
| T2 lesions | 0.0056 (0.0063) | 0.0032 (0.0035) | 0.007 | 0.007 |
aDerived from models adjusted for age and sex.
bDerived from models adjusted by sex, age at baseline, disease duration and history optic neuritis. Bold values indicate statistical significance (P < 0.05).
Table 3.
Comparisons of regional (substructure) atrophy (percentage change/year)a of the brain
| Substructure atrophy (percentage of change/year) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| African Americans | Caucasian Americans | African Americans versus Caucasian Americans | |||||||
| (n = 22) | (n = 60) | P-value | |||||||
| %/year | 95% CI | %/year | 95% CI | Model 1b | Model 2c | Model 3d | Model 4e | ||
| Whole brain | −0.53 | (−0.76 to −0.30) | −0.30 | (−0.39 to −0.21) | 0.086 | 0.083 | 0.080 | 0.084 | |
| Cortical grey matter | −0.87 | (−1.16 to −0.58) | −0.46 | (−0.58 to −0.34) | 0.013 | 0.012 | 0.012 | 0.012 | |
| Cerebral white matter | −0.69 | (−0.96 to −0.41) | −0.34 | (−0.46 to −0.22) | 0.039 | 0.038 | 0.039 | 0.038 | |
| Whole thalamus | −1.08 | (−1.59 to −0.57) | −0.71 | (0.96 to −0.45) | 0.293 | 0.292 | 0.283 | 0.292 | |
| Nucleus thalamus | −1.50 | (−2.03 to −0.97) | −0.66 | (−0.91 to −0.41) | 0.021 | 0.021 | 0.020 | 0.021 | |
| Deep grey matter | −0.95 | (−1.27 to −0.63) | −0.62 | (−0.77 to −0.48) | 0.104 | 0.104 | 0.120 | 0.104 | |
| T2 lesion | 10.97 | (4.04 to 18.37) | 4.65 | (1.64 to 7.75) | 0.006 | 0.006 | NA | 0.006 | |
aPercentage of change derived by the natural logarithm of each variable obtained in Model 1.
bModels adjusted for sex and baseline age.
cModels adjusted for sex, age at baseline, disease duration and history of optic neuritis.
dModels adjusted for sex, age at baseline, disease duration, history optic neuritis and rates of lesion accumulation.
eModels adjusted for sex, age at baseline, disease duration, history of optic neuritis and time since acute optic neuritis (if within 6 months of the baseline MRI).
NA = not applicable. Bold values indicate statistical significance (P < 0.05).
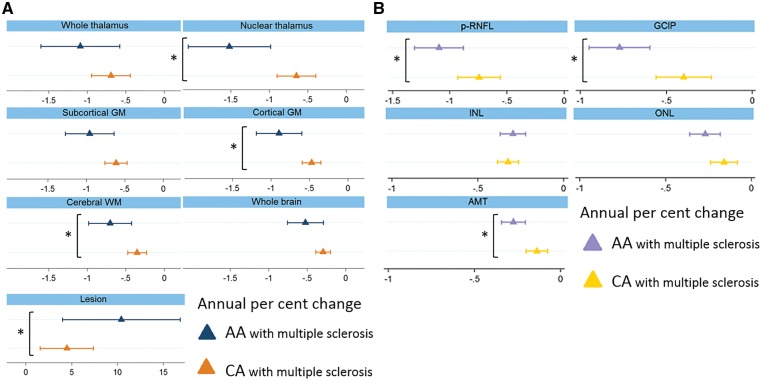
Rates of cortical grey matter (−0.9%/year versus −0.5%/year; P = 0.013) and white matter atrophy (−0.7%/year versus −0.3%/year; P = 0.038) were approximately twice as fast in African Americans as compared to Caucasian Americans. Relative to Caucasian Americans, African Americans exhibited accelerated nuclear thalamic atrophy (−1.5%/year versus −0.7%/year; P = 0.02). Moreover, T2 lesion volume increased at a faster rate of 11.0%/year in African Americans, as compared to 4.7%/year in Caucasian Americans (P = 0.006) (Fig. 2A). Similar results were obtained with all statistical models, suggesting that the observed differences in rates of brain substructure atrophy according to race were independent of T2 lesion accumulation during follow-up or acute optic neuritis at baseline.
Figure 2.
Rates of brain substructure and whole brain volume changes (A) and retinal layer tissue loss (B) (%/year) in African Americans versus Caucasian Americans with multiple sclerosis. Values displayed in per cent change/year. Triangles represent the coefficient and the bars the confidence intervals. Asterisks indicate significant differences. AA = African American; AMT = average macular thickness; CA = Caucasian American; GM = grey matter; WM = white matter.
Differences in retinal layer thicknesses and retina atrophy rates between African and Caucasian Americans
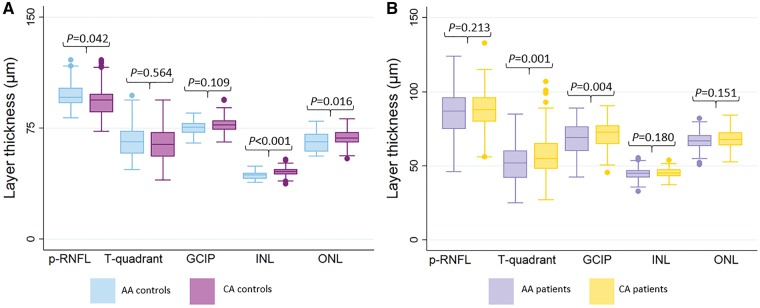
At baseline (Table 4), adjusting for age and sex, average p-RNFL thickness was higher in African American versus Caucasian American healthy controls (97.4 µm versus 93.1 µm; P = 0.042), although temporal quadrant RNFL thickness (thought to predominantly correspond anatomically to the papillomacular bundle) was not significantly different. GCIP thickness was also similar between African American and Caucasian American healthy controls. Interestingly, African American healthy controls exhibited lower average INL (43.1 µm versus 45.6 µm; P < 0.001), ONL (65.8 µm versus 68.8 µm; P = 0.016), and average macular thickness (306.8 µm versus 314.5 µm; P = 0.007) thicknesses as compared to Caucasian American healthy controls (Fig. 3A).
Table 4.
Comparisons of retinal thickness measures at baseline (in µm)a
| African Americans | Caucasian Americans | African Americans versus Caucasian Americans | ||
|---|---|---|---|---|
| Mean (SD) µm | Mean (SD) µm | P-valuea | P-valueb | |
| Healthy controls | n = 31 | n = 61 | ||
| p-RNFL | 97.39 (8.36) | 93.10 (10.37) | 0.039 | NA |
| T-quadrant p-RNFL | 66.21 (10.96) | 64.78 (11.04) | 0.580 | NA |
| GCIP | 75.35 (4.41) | 77.03 (5.36) | 0.110 | NA |
| INL | 43.12 (2.65) | 45.57 (2.67) | <0.001 | NA |
| ONL | 65.78 (6.65) | 68.79 (5.32) | 0.015 | NA |
| AMT | 306.79 (14.48) | 314.54 (12.80) | 0.007 | NA |
| Multiple sclerosis | n = 116 | n = 116 | ||
| p-RNFL | 85.60 (15.28) | 87.87 (12.70) | 0.186 | 0.216 |
| T-quadrant p-RNFL | 51.13 (12.17) | 56.52 (14.16) | 0.001 | 0.001 |
| GCIP | 67.86 (10.57) | 71.15 (8.71) | 0.004 | 0.004 |
| INL | 44.70 (3.55) | 45.19 (3.12) | 0.194 | 0.183 |
| ONL | 66.98 (5.45) | 68.07 (6.42) | 0.145 | 0.152 |
| AMT | 297.86 (18.82) | 304.98 (18.45) | 0.002 | 0.002 |
aDerived from models adjusted for sex and baseline age.
bDerived from models adjusted for sex, age at baseline, disease duration and history optic neuritis.
AMT = average macular thickness; NA = not applicable; T-quadrant = temporal quadrant. Bold values indicate statistical significance (P < 0.05).
Figure 3.
Comparisons of retinal thickness measures at baseline (in µm) in African Americans versus Caucasian Americans. (A) Comparison in healthy controls. (B) Comparison in multiple sclerosis patients. The corresponding P-values are also displayed. AMT = average macular thickness. AA = African American; CA = Caucasian American.
In the multiple sclerosis cohorts, adjusting for age, sex, disease duration, and history of optic neuritis, p-RNFL thickness at baseline (Table 4) was not significantly different between African Americans and Caucasian Americans (85.9 µm versus 88.7 µm; P = 0.173). However, p-RNFL thickness in African Americans was on average >10 µm lower than in African American healthy controls (P < 0.001), whereas p-RNFL thickness in Caucasian American patients was on average only 6 µm lower than in Caucasian American healthy controls (P = 0.002). Temporal quadrant p-RNFL and GCIP thicknesses were both significantly lower in African Americans relative to Caucasian Americans with multiple sclerosis (P = 0.001 and P = 0.003, respectively). Whereas INL and ONL thicknesses did not differ significantly between African Americans and Caucasian Americans with multiple sclerosis, relative to African American healthy controls, INL thickness was on average 1.5 µm higher (P = 0.017) higher in African Americans with multiple sclerosis (Fig. 3B).
Percentage change per year of retinal layers are summarized in Table 5 and in Supplementary Table 5. In addition, absolute thickness changes per year of retinal layers are summarized in Supplementary Table 6, as well as in Fig. 2B. There were no differences between rates of retinal layer atrophy in African American healthy controls as compared to Caucasian American healthy controls. On the other hand, adjusting for age at baseline, sex, disease duration, and history of optic neuritis in patients with multiple sclerosis, rates of p-RNFL (−1.1%/year versus −0.8%/year; P = 0.019), GCIP (−0.8%/year versus −0.4%/year; P = 0.009), and average macular thickness thinning (−0.3%/year versus −0.2%/year; P = 0.003) were all faster in African Americans as compared to Caucasian Americans. Relative to Caucasian Americans, African Americans with multiple sclerosis also exhibited borderline faster rates of ONL atrophy (−0.3%/year versus −0.2%/year; P = 0.077).
Table 5.
Comparisons of retinal layer atrophy (percentage change/year)a
| Retinal layer thickness atrophy (percentage of change/year) | ||||||
|---|---|---|---|---|---|---|
| African Americans | Caucasian Americans | African Americans versus Caucasian Americans P-value | ||||
| %/year | 95% CI | %/year | 95% CI | Model 1b | Model 2c | |
| Healthy controls | n = 17 | n = 33 | ||||
| p-RNFL | −0.42 | (−0.95 to 0.11) | −0.40 | (−0.67 to −0.13) | 0.648 | NA |
| GCIP | −0.37 | (−0.72 to −0.02) | −0.13 | (−0.35 to 0.08) | 0.272 | NA |
| INL | −0.34 | (−0.62 to −0.07) | −0.20 | (−0.36 to −0.03) | 0.383 | NA |
| ONL | −0.18 | (−0.45 to 0.09) | −0.17 | (−0.31 to −0.03) | 0.927 | NA |
| AMT | −0.14 | (−0.34 to 0.06) | −0.08 | (−0.20 to 0.04) | 0.632 | NA |
| Multiple sclerosis | n = 116 | n = 116 | ||||
| p-RNFL | −1.10 | (−1.31 to −0.89) | −0.74 | (−0.93 to −0.56) | 0.019 | 0.018 |
| GCIP | −0.77 | (−0.95 to −0.60) | −0.40 | (−0.56 to −0.24) | 0.009 | 0.009 |
| INL | −0.29 | (−0.36 to −0.22) | −0.32 | (−0.38 to −0.26) | 0.444 | 0.439 |
| ONL | −0.27 | (−0.35 to −0.18) | −0.16 | (−0.23 to −0.08) | 0.076 | 0.076 |
| AMT | −0.30 | (−0.37 to −0.23) | −0.15 | (−0.21 to −0.09) | 0.003 | 0.003 |
aPercentage of change derived by the natural logarithm of each variable obtained in Model 1.
bModels adjusted for sex and baseline age.
cModels adjusted for sex, age at baseline, disease duration and history optic neuritis.
AMT = average macular thickness; NA = not applicable. Bold values indicate statistical significance (P < 0.05).
Discussion
Results of this study suggest that on average African Americans have accelerated rates of brain and retinal tissue loss over time as compared to Caucasian Americans with multiple sclerosis. Findings from the cohorts included in the current study indicate that relative to Caucasian Americans, in general, African Americans exhibit approximately double the rate of brain substructure atrophy, with cortical grey matter, cerebral white matter and thalamic atrophy all contributing to whole brain atrophy over time. Moreover, African Americans also demonstrate faster rates of T2 lesion accumulation as compared to Caucasian Americans. This study recapitulates that African Americans with multiple sclerosis have accelerated rates of inner (p-RNFL and GCIP) retinal atrophy. Finally, our study findings indicate that African Americans with multiple sclerosis have increased INL thickness at baseline, which has previously been shown to be a predictor of clinical and radiological disease activity, as well as disability progression. The increase in outer retinal layer thicknesses in African Americans likely relates to the marked predilection for MMP noted, and is relevant as MMP has been shown to be a potential marker of disability in multiple sclerosis. In general, our study findings are collectively consistent with a more inflammatory disease course, as well as more profound neurodegeneration, in African Americans and are in accordance with prior suggestions that multiple sclerosis may have a more ominous disease course in the African American population (Kaufman et al., 2003; Cree et al., 2004; Naismith et al., 2006; Weinstock-Guttman et al., 2010; Howard et al., 2012; Kimbrough et al., 2015; Seraji-Bozorgzad et al., 2016; Al-Kawaz et al., 2017). Based on our exploratory statistical analyses, our study findings suggest that, at least in part, the accelerated rates of MRI derived brain substructure atrophy observed in African Americans as compared to Caucasian Americans are independent of overt T2 lesion accumulation.
Our study findings of accelerated brain and retinal atrophy in African Americans corroborate the more rapid clinical progression observed to occur in African Americans with multiple sclerosis. Multiple sclerosis is not the only autoimmune disorder presenting with a worse prognosis in the African American population, with a prior study suggesting that African ancestry predicts stronger auto-inflammatory responses in general (Nédélec et al., 2016). Furthermore, African American patients with systemic lupus erythematous exhibit higher mortality rates (Krishnan and Hubert, 2006), with rheumatoid arthritis show lower rates of remission (Greenberg et al., 2013), and with Graves’ disease demonstrate a higher prevalence of thyrotoxicosis (McLeod et al., 2015). African Americans with multiple sclerosis have a higher CSF IgG index than Caucasian American patients, which is inversely correlated with brain volumes (Rinker et al., 2007; Seraji-Bozorgzad et al., 2017). However, the mechanisms underlying the more severe disease course according to race are poorly understood, with several potential factors proposed to possibly play a role including but not limited to genetic variations, gene–gene interactions, differential gene expression (Oksenberg et al., 2004; Huang et al., 2007; Cree et al., 2009), as well as environmental factors (Ascherio and Munger, 2007) such as lower vitamin D levels (Gelfand et al., 2011). Furthermore, additional factors have also been suggested such as socioeconomic status, insurance coverage, adherence to treatment (Lafata et al., 2008), and health beliefs (Khan et al., 2015; Fiscella and Sanders, 2016). It is clear that future studies are needed to elucidate the mechanisms underlying the more severe disease course occurring in African American patients with multiple sclerosis.
Interestingly, in addition to a higher T2 lesion volume, of the brain substructure volumes assessed at baseline, only thalamic volumes were lower in African Americans relative to Caucasian Americans. This is in contrast to the strikingly accelerated rates of atrophy observed in general across brain substructures (cortical grey matter, cerebral white matter, nuclear thalamus) and to the higher rate of increase in T2 lesion volume in African Americans. Through the utilization of MRI segmentation techniques enabling the measurement of brain substructure volumes, it has become established that grey matter atrophy is a common, early feature of multiple sclerosis that is more strongly associated with disability progression than white matter atrophy (Simon, 2006; Fisniku et al., 2008; Calabrese et al., 2012; Geurts et al., 2012; Parisi et al., 2014). While significant atrophy was observed across brain compartments in both Caucasian and African Americans, highlighting the role of neurodegeneration in general in multiple sclerosis regardless of race, accelerated rates of atrophy across brain compartments was observed in African Americans, consistent with faster degrees of CNS tissue loss than in Caucasian American patients.
Consistent with the MRI findings, and in accordance with a prior overlapping publication by our group (Kimbrough et al., 2015), our OCT study findings, although not necessarily representing independent confirmation, provide further support that African Americans with multiple sclerosis have faster rates of p-RNFL and GCIP thinning as compared to Caucasian Americans. This is clinically relevant since rates of GCIP thinning have been shown to be accelerated in people with multiple sclerosis exhibiting clinical (non-ocular relapses) and/or radiological evidence of disease activity, as well as disability progression (Ratchford et al., 2013). Moreover, rates of GCIP atrophy have been found to mirror rates of whole brain and in particular cortical grey matter atrophy in multiple sclerosis (Saidha et al., 2015). It is interesting that at baseline, unlike GCIP thicknesses, p-RNFL thicknesses were not statistically significantly different between African American and Caucasian American patients. While this could relate to a higher degree of variability in p-RNFL measurements requiring more statistical power to detect a difference, in order to interpret this perceived ‘lack of difference’ at a single point in time requires knowledge of whether these measures are approximately similar or not in healthy controls stratified by race. Indeed, p-RNFL thickness was significantly greater in African American healthy controls as compared to Caucasian American healthy controls. In this context, p-RNFL thickness in African American patients was on average >10 µm lower than in African American healthy controls, while p-RNFL thickness in Caucasian American patients was on average approximately only 5 µm lower than in Caucasian American healthy controls, suggesting that at baseline there was in fact a greater degree of p-RNLF tissue loss in African American patients. This being said, GCIP thicknesses were very similar between African American and Caucasian American healthy controls, and therefore GCIP thickness may represent a more optimal OCT outcome measure in studies including both Caucasian Americans and African Americans, since GCIP thicknesses are comparable (i.e. there are no significant differences between both African American and Caucasian American healthy controls), and as an assessment of outcome GCIP measures therefore does not require adjustment or calibration for African American or Caucasian American status, as is needed for p-RNFL thickness.
There are a number of major differences between the current and prior assessments of effect of race on rates of retinal atrophy in multiple sclerosis (Kimbrough et al., 2015; Seraji-Bozorgzad et al., 2016) with the current study being larger, tracking participants for a longer period of time, and being performed at a single centre (potentially reducing measurement variability). Moreover, the current study also assessed differences in thicknesses of the INL and ONL according to race at baseline and over time in multiple sclerosis. At baseline, African American healthy controls exhibited lower INL and ONL thicknesses relative to Caucasian American healthy controls. Again, while INL and ONL thicknesses were not ‘statistically different’ between African American and Caucasian American patients, in the context of interpretation relative to findings in healthy controls stratified by race, INL and ONL thicknesses were higher in African American patients as compared to African American healthy controls, which in part may relate to the greater incidence of MMP identified in the African Americans with multiple sclerosis. Interestingly, over time, African Americans also exhibited borderline faster rates of ONL thinning. Moreover, there was a striking predilection for MMP among African Americans relative to Caucasian Americans with multiple sclerosis, not just in the OCT, but also the MRI cohorts. The pathophysiological basis for INL and ONL changes in multiple sclerosis remain unclear with numerous potential mechanisms proposed including retrograde trans-synaptic neurodegeneration and/or plasticity, secondary glial activation triggered by neurodegeneration occurring in the neighbouring GCIP, and/or primary retinal inflammation (Saidha et al., 2012; Barboni et al., 2013; Al-Louzi et al., 2016). Nonetheless, it has become increasingly apparent that changes occurring in the INL and ONL in multiple sclerosis may be clinically relevant and reflect different aspects of the multiple sclerosis disease process to those reflected by changes in the p-RNFL and GCIP (Saidha et al., 2011a; Kaushik et al., 2013; Wolf et al., 2013). Changes in the INL, particularly INL thickening in multiple sclerosis may reflect more widespread inflammatory aspects of the multiple sclerosis disease process (Saidha et al., 2012). On the other hand, ONL thickness reduction has been proposed as being a potentially broad marker of neurodegeneration in general in multiple sclerosis (Saidha et al., 2011a). Finally, the presence of MMP in multiple sclerosis eyes has also been proposed as a potential biomarker of disability.
This study has a number of limitations worthy of discussion. Most participants in this study had relapsing remitting multiple sclerosis, and the study was underpowered to study the impact of race on rates of brain substructure and retinal layer atrophy in the progressive forms of multiple sclerosis. Therefore, the findings of the current study cannot be generalized or extended to secondary progressive or primary progressive multiple sclerosis, and future, larger studies including greater numbers of progressive multiple sclerosis patients are needed to study the differential effects of race in progressive multiple sclerosis. Moreover, the current study lacked MRI data in healthy controls. In addition, relative to the longitudinal OCT multiple sclerosis cohort, the longitudinal MRI multiple sclerosis cohort included in the current study was comparably less well powered, potentially increasing the risk for type I errors (false positives). Furthermore, as compared to Caucasian Americans, fewer African Americans with multiple sclerosis included in the cross-sectional MRI cohorts continued to undergo longitudinal MRI monitoring, which may reflect a degree of self-selection bias. With respect to race, the current study relied on the classification of patients and healthy controls as being either African Americans or Caucasian Americans, based on individual self-reporting, which in itself is prone to inaccuracy and does not account for the effect of admixture(s). African ancestry is combined in one group for census purposes in the USA, but includes large ethnically distinct populations from the Caribbean, East or West Africa for example. Furthermore, the current study was restricted to a single geographical region within a single country, and therefore caution must be exerted in generalizing the current study findings to all patients of African race, particularly in countries other than the USA. Patients were recruited in a tertiary referral multiple sclerosis centre, and there is therefore a possibility that the participants included in the current study may have been biased towards having more severe multiple sclerosis. Moreover, information regarding the socioeconomic status of the participants in this study was not collected/recorded, and since this has been postulated to play a role in the multiple sclerosis disease course (Lafata et al., 2008), future studies assessing differences in multiple sclerosis according to race should address this factor. In addition, the current study represents a targeted study of the effect of African American or Caucasian American race on neurodegeneration in multiple sclerosis. Future studies should continue to study the impact of other ethnicities on the course of multiple sclerosis. Another limitation to consider, in light of retrospective design, is the lack of data regarding the frequency of relapses and gadolinium-enhancing lesions on MRI in the multiple sclerosis patients included in the study. Finally, this study included brain and retinal imaging, but did not include spinal cord imaging. Because of the higher incidence of transverse myelitis and earlier pyramidal involvement known to affect African American patients, future studies should investigate differences in MRI-derived quantitative spinal cord measures, including cross-sectional spinal cord area, according to race. In addition, a topic for future studies should be studying whether effects of DMTs on rates of regional brain atrophy varies by race, with the purpose of understanding if African American patients could benefit more from one treatment over another. Due to the retrospective design of the current study and heterogeneous nature of the study cohorts, it was not possible to interrogate treatment effects in this study.
In summary, our study findings provide compelling evidence that African Americans have accelerated rates of atrophy across brain substructures, particularly of the thalamus, relative to Caucasian Americans with multiple sclerosis. Moreover, African Americans also exhibit more pronounced atrophy of the inner retina over time, higher presence of MMP, and a relative thickening of the INL at baseline, as well as possibly faster rates of ONL atrophy. Our study findings are in line with the body of literature over the past several years suggesting a more severe multiple sclerosis disease course on average in African American patients, and accordingly raise the importance of a greater recruitment of African Americans in future studies of multiple sclerosis to better evaluate differences in mechanisms and treatment responses, which may account for individual propensity for worse outcomes. While it is hypothesized that genetic, immunological, environmental and socio-economic factors may contribute to the more aggressive disease course observed in African Americans with multiple sclerosis, the mechanisms underlying this observation remain unclear. Future studies that more precisely elucidate the basis for variations in disease course according to race may play a critical role in helping to advance our understanding of the pathobiology of multiple sclerosis, not only for specific ethnic populations, but for multiple sclerosis in general.
Supplementary Material
Acknowledgements
The authors would like to thank the patients who made this study possible and all those who helped in carrying out this project.
Glossary
Abbreviations
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- GCIP
ganglion cell plus inner plexiform layer
- INL
inner nuclear layer
- MMP
microcystoid macular pathology
- OCT
optical coherence tomography
- ONL
outer nuclear layer
- p-RNFL
peripapillary retinal nerve fibre layer
Funding
This study was funded by the NIH/NINDS [R01NS082347 (to P.A.C.), National MS Society [RG-1606-08768 (to S.S.), TR-3760-A-3 (to P.A.C.) and RG-4212-A-4 (to L.J.B. subcontracted to P.A.C.), RG-1507-05243 (to D.P.)], Race to Erase MS (to S.S.), Intramural Research Program (to D.S.R.), and Walters Foundation (to E.M.F., P.A.C., and L.J.B).
Competing interests
S.S. has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen-Idec, Genzyme, Genentech Corporation, EMD Serono and Novartis. He is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec, and received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer. He is also the site investigator of a trial sponsored by MedDay Pharmaceuticals. T.F. has received speaker and consulting fees from Acorda, Genzyme, and Novartis. E.F. has received speaker and consulting fees from Genzyme, Acorda, Novartis, and TEVA. L.B. has received consulting fees from Biogen. J.O. has received personal honoraria for consulting from Biogen-Idec, EMD-Serono, Novartis, Sanofi-Genzyme, and Roche. She is a PI on research grants to St. Michael’s Hospital from Biogen-Idec. E.M. receives free medication for a clinical trial, of which she is PI, from Teva Neuroscience. She is PI of investigator-initiated studies sponsored by Biogen and Genzyme. She is site PI of studies sponsored by Biogen and, within the last 12 months, by Sun Pharma. She receives royalties for editorial duties for UpToDate. D.S.R. is PI on a Cooperative Research and Development Agreement with Vertex Pharmaceuticals. P.C. has received personal honoraria for consulting from Biogen and Disarm Therapeutics. He is PI on research grants to Johns Hopkins from MedImmune, Annexon, and Genzyme. N.G.C., E.S.S., B.D., N.C., J.G., K.F., J.N., A.R., E.O., N.F., S.F., O.K., H.R., D.K., C.C., D.P., and J.P. report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- Agosta F, Rovaris M, Pagani E, Sormani MP, Comi G, Filippi M. Magnetization transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain 2006; 129 (Pt 10): 2620–7. [DOI] [PubMed] [Google Scholar]
- Al-louzi O, Bhargava P, Newsome SD, Balcer LJ, Frohman EM, Crainiceanu C et al. . Outer retinal changes following acute optic neuritis. Mult Scle 2016; 22 : 362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-louzi O, Button J, Newsome SD, Calabresi PA, Saidha S. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: a case series. Mult Scler 2017; 23: 1035–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kawaz M, Monohan E, Morris E, Perumal JS, Nealon N, Vartanian T et al. . Differential impact of multiple sclerosis on cortical and deep gray matter structures in African Americans and Caucasian Americans. J Neuroimaging 2017; 27: 333–8. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol 2007; 61: 504–13. [DOI] [PubMed] [Google Scholar]
- Barboni P, Carelli V, Savini G, Carbonelli M, La Morgia C, Sadun AA. Microcystic macular degeneration from optic neuropathy: not inflammatory, not trans-synaptic degeneration, Brain 2013; 136 (Pt7): e239. [DOI] [PubMed] [Google Scholar]
- Bazin PL, Pham DL. Preserving tissue classification of magnetic resonance brain images. IEEE Trans Med Imaging 2007; 26: 487–96. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing when and how? J Clin Epidemiol 2001; 54: 343–9. [DOI] [PubMed] [Google Scholar]
- Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006; 5: 158–70. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Lang A, Al-Louzi O, Carass A, Prince J, Calabresi PA, Saidha S. Applying an open-source segmentation algorithm to different oct devices in multiple sclerosis patients and healthy controls: implications for clinical trials. Mult Scler Int 2015; 136295: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, et al. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 2004; 127: 34–44. [DOI] [PubMed] [Google Scholar]
- Button J, Al-Louzi O, Lang A, Bhargava P, Newsome SD, Frohman T et al. . Disease-modifying therapies modulate retinal atrophy in multiple sclerosis A retrospective study. Neurology 2017; 88: 525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese M, Poletto V, Favaretto A, Alessio S, Bernardi V, Romualdi. et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012; 135: 2952–61. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Worrall JT1, Mahad DJ. The central role of mitochondria in axonal degeneration in multiple sclerosis. Mult Scler 2014; 20: 1806–13. [DOI] [PubMed] [Google Scholar]
- Cettomai D, Pulicken M, Gordon-Lipkin E, Salter A, Frohman TC, Conger A et al. . reproducibility of optical coherence tomography in multiple sclerosis. Arch Neurol 2008; 65: 1218–22. [DOI] [PubMed] [Google Scholar]
- Cree BA, Khan O, Bourdette D, Goodin DS, Cohen JA, Marrie RA et al. . Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 2004; 63: 2039–45. [DOI] [PubMed] [Google Scholar]
- Cree BA, Al-Sabbagh A, Bennett R, Goodin D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch Neurol 2005; 62: 1681–3. [DOI] [PubMed] [Google Scholar]
- Cree BA, Reich DE, Khan O, De Jager PL, Nakashima I, Takahashi T et al. . Modification of multiple sclerosis phenotypes by African ancestry at HLA. Arch Neurol 2009; 66: 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criste G, Trapp B, Dutta R. Axonal loss in multiple sclerosis: causes and mechanisms. In: Goodin DS. editor. Multiple sclerosis and related disorders. Elsevier; 2014; 122 (Suppl C): 405–25. [DOI] [PubMed] [Google Scholar]
- Filippi M. MRI measures of neurodegeneration in multiple sclerosis?: implications for disability, disease monitoring, and treatment. J Neurol 2015; 262: 1–6. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health 2016; 37: 375–94. [DOI] [PubMed] [Google Scholar]
- Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA et al. . Gray matter atrophy is related to long- term disability in multiple sclerosis. Ann Neurol 2008; 64: 247–54. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Cree BA, McElroy J, Oksenberg J, Green R, Mowry EM et al. . Vitamin D in African Americans with multiple sclerosis. Neurology 2011; 76: 1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts JJG, Calabrese M, Fisher E, Rudick RA. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol 2012; 11: 1082–92. [DOI] [PubMed] [Google Scholar]
- Glaister J, Carass A, Stough JV, Calabresi PA, Prince JL. Thalamus parcellation using multi-modal feature classification and thalamic nuclei priors. Proc SPIE Int Soc Opt Eng 2016; 9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-López JJ, Rebolleda G, Leal M, Oblanca N, Muñoz-Negrete FJ, Costa-Frossard L. et al. Comparative diagnostic accuracy of ganglion cell-inner plexiform and retinal nerve fiber layer thickness measures by cirrus and spectralis optical coherence tomography in relapsing-remitting multiple sclerosis. Biomed Res Int 2014; 2014: 128517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JD, Spruill TM, Shan Y, Reed G, Kremer JM, Potter J et al. . Racial and ethnic disparities in disease activity in rheumatoid arthritis patients. Am J Med 2013; 126: 1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Pham DL, Tosun D, Rettmann ME, Xu C, Prince JL. CRUISE?: Cortical reconstruction using implicit surface evolution. Neuroimage 2004; 23: 997–1012. [DOI] [PubMed] [Google Scholar]
- Harrison DM, Shiee N, Bazin PL, Newsome SD, Ratchford JN, Pham D et al. . Tract-specific quantitative MRI better correlates with disability than conventional MRI in multiple sclerosis. J Neurol 2013; 260: 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol 2010; 225: 9–17. [DOI] [PubMed] [Google Scholar]
- Horakova D, Kalincik T, Dusankova JB, Dolezal O. Clinical correlates of grey matter pathology in multiple sclerosis. BMC Neurol 2012; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Battaglini M, Babb JS, Arienzo D, Holst B, Omari M et al. . MRI Correlates of disability in African-Americans with multiple sclerosis. PLoS One 2012; 7: e43061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W et al. . Optical coherence tomography. Science 1991; 254: 1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Hughes M, Mobley S, Lanham I, Poduslo SE. APOE genotypes in African American female multiple sclerosis patients. Neurosci Lett 2007; 414: 51–6. [DOI] [PubMed] [Google Scholar]
- Huo Y, Plassard AJ, Carass A, Resnick SM, Pham DL, Prince JL et al. . NeuroImage consistent cortical reconstruction and multi-atlas brain segmentation. Neuroimage 2016; 138: 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Sabuncu MR. Multi-atlas segmentation of biomedical images?: a survey. Med Image Anal 2015; 24: 205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta F. Zimmerman Hm. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurol 1976; 26: 26–28. [DOI] [PubMed] [Google Scholar]
- Inglese M, Oesingmann N, Casaccia P, Fleysher L. Progressive multiple sclerosis and gray matter pathology: an MRI perspective. Mt Sinai J Med 2011; 78: 258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M, Grossman RI, Filippi M. Magnetic resonance imaging monitoring of multiple sclerosis lesion evolution. J Neuroimaging 2005; 15 (4Suppl): 22S–29S. [DOI] [PubMed] [Google Scholar]
- Joy JE, Johnston RB, Jr, editors. Institute of medicine; board on neuroscience and behavioral health; committee on multiple sclerosis: current status and strategies for the future. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- Kaufman MD, Johnson SK, Moyer D, Bivens J, Norton HJ. Multiple sclerosis severity and progression rate in African Americans compared with whites. Am J Phys Med Rehabil 2003; 82: 582–90. [DOI] [PubMed] [Google Scholar]
- Kaushik M, Wang CY, Barnett MH, Garrick R, Parratt J, Graham SL et al. . Inner nuclear layer thickening is inversley proportional to retinal ganglion cell loss in optic neuritis. PLoS One 2013; 8: e78341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, Williams MJ, Amezcua L, Javed A, Larsen KE, Smrtka JM. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract 2015; 5: 132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough DJ, Sotirchos ES, Wilson JA, Al-Louzi O, Conger A, Conger D et al. . Retinal damage and vision loss in African-American multiple sclerosis patients. Ann Neurol 2015; 77: 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kister I, Chamot E, Bacon JH, Niewczyk PM, De Guzman RA, Apatoff B et al. . Rapid disease course in African Americans with multiple sclerosis. Neurology 2010; 75: 217–24. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Los Angeles: David L Schriger; 2008. [Google Scholar]
- Knier B, Schmidt P, Aly L, Buck D, Berthele A, Mühlau M et al. . Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016; 139: 2855–63. [DOI] [PubMed] [Google Scholar]
- Krishnan E, Hubert HB. Ethnicity and mortality from systemic lupus erythematous in the US. Ann Rheum Dis 2006; 65: 1500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafata JE, Cerghet M, Dobie E, Schultz L, Tunceli K, Reuther J et al. . Measuring adherence and persistence to disease-modifying agents among patients with relapsing remitting multiple sclerosis. J Am Pharm Assoc 2008; 48: 752–7. [DOI] [PubMed] [Google Scholar]
- Lang A, Carass A, Hauser M, Sotirchos ES, Calabresi PA, Yin HS et al. . Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express 2013; 4: 1133–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013; 80: 1734–39. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol 2010; 225: 2–8. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology 1996; 46: 907–11. [DOI] [PubMed] [Google Scholar]
- Maldonado RS, Mettu P, El-Dairi M, Bhatti MT. The application of optical coherence tomography in neurologic diseases. Neurol. Clin. Pract 2015. 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Does multiple sclerosis–associated disability differ between races. Neurology 2006; 66: 1235–40. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Cooper DS, Ladenson PW, Whiteman DC, Jordan SJ. Race/Ethnicity and the prevalence of thyrotoxicosis in young Americans. Thyroid 2015; 25: 621–8. [DOI] [PubMed] [Google Scholar]
- Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics 2015; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 1998; 121 (Pt1): 3–24. [DOI] [PubMed] [Google Scholar]
- Miller DH. Biomarkers and surrogate outcomes in neurodegenerative disease: lessons from multiple sclerosis. NeuroRx 2004; 1: 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Barnett MH, Benedict RH, Pelletier D, Pirko I, Sahraian MA et al. . The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 2013; 80: 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HE, Gao W, Balcer LJ, Joslin CE. Association of race with visual outcomes following acute optic neuritis?: an analysis of the Optic Neuritis Treatment Trial. JAMA Ophthalmol 2014; 132: 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult Scler 2006; 12: 775–81. [DOI] [PubMed] [Google Scholar]
- Napoli SQ, Bakshi R. Magnetic resonance imaging in multiple sclerosis. Rev Neurol Dis 2005; 2: 109–16. [PubMed] [Google Scholar]
- Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A et al. . Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 2016; 167: 657–69. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Barcellos LF, Cree BA, Baranzini SE, Bugawan TL, Khan O et al. . Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet 2004; 74: 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi L, Rocca MA, Mattioli F, Riccitelli GC, Capra R, Stampatori C et al. . Gray matter atrophy is related to long-term disability in multiple sclerosis. J Neurol 2014; 261: 1715–25. [DOI] [PubMed] [Google Scholar]
- Pérez-Cerdá F, Sánchez-Gómez MV, Matute C. The link of inflammation and neurodegeneration in progressive multiple sclerosis. Mult Scler Demyelinating Disord 2016; 1: 1–8. [Google Scholar]
- Phillips PH, Newman NJ, Lynn MJ. Optic neuritis in African Americans. Arch Neurol 1998; 55: 186–92. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C et al. . Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013; 80: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebolleda G, Diez-Alvarez L, Casado A, Sánchez-Sánchez C, de Dompablo E, González-López JJ et al. . OCT?: New perspectives in neuro-ophthalmology. Saudi J Ophthalmol 2015; 29: 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Smith SA, Gordon-Lipkin EM, Ozturk A, Caffo BS, Balcer LJ. et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol 2009; 66: 998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JR, Trinkaus K, Naismith RT, Cross AH. Higher IgG index found in African Americans versus caucasians with multiple sclerosis. Neurology 2007; 69: 68–72. [DOI] [PubMed] [Google Scholar]
- Romeo M, Martinelli-Boneschi F, Rodegher M, Esposito F, Martinelli V, Comi G. Clinical and MRI predictors of response to interferon-beta and glatiramer acetate in relapsing–remitting multiple sclerosis patients. Eur J Neurol 2013; 20: 1060–7. [DOI] [PubMed] [Google Scholar]
- Roy S, He Q, Sweeney E, Carass A, Reich DS, Prince JL et al. . subject specific sparse dictionary learning for atlas based brain mri segmentation. IEEE J Biomed Health Inform 2015; 19: 1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD et al. . Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 2015; 78: 801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK et al. . Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 2011a; 134 (Pt 2): 518–33. [DOI] [PubMed] [Google Scholar]
- Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA et al. . Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness, Mult Scler 2011b; 17: 1449–63. [DOI] [PubMed] [Google Scholar]
- Saidha S, Sotirchos S, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ et al. . Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 2012; 11: 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Calabresi PA. “Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis?: yes. Mult Scler 2014; 20: 1296–8. [DOI] [PubMed] [Google Scholar]
- Seraji-Bozorgzad N, Reed S, Bao F, Santiago C, Tselis A, Bernitsas E et al. . Characterizing retinal structure injury in African-Americans with multiple sclerosis. Mult Scler Relat Disord 2016; 7: 16–20. [DOI] [PubMed] [Google Scholar]
- Seraji-Bozorgzad N, Khan O, Cree BAC, Bao F, Caon C, Zak I et al. . Cerebral gray matter atrophy is associated with the CSF IgG index in African American with multiple sclerosis. J Neuroimaging 2017; 27: 476–80. [DOI] [PubMed] [Google Scholar]
- Sicotte NL. Magnetic resonance imaging in multiple sclerosis: the role of conventional imaging. Neurol. Clin Neurol Clin 2011; 29: 343–56. [DOI] [PubMed] [Google Scholar]
- Simon JH. Brain atrophy in multiple sclerosis?: what we know and would like to know. Mult Scler 2006; 12: 679–87. [DOI] [PubMed] [Google Scholar]
- Simon JH. MRI outcomes in the diagnosis and disease course of multiple sclerosis In: Goodin DS. editor. Multiple sclerosis and related disorders. Vol. 122 (supplC). Elsevier; 2014. p. 405–25. [DOI] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A et al. . Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet 2005; 76: 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S et al. . The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012; 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint D, Perier O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol 1983; 3: 211–20. [PubMed] [Google Scholar]
- Wallin TM, Culpepper WJ, Coffman P, Pulaski S, Maloni H, Mahan CM et al. . The gulf war era multiple sclerosis cohort?: age and incidence rates by race, sex and service. Brain 2012; 135 (Pt 6): 1778–85. [DOI] [PubMed] [Google Scholar]
- Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol (suppl) 2015; 25: 157–65. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Ramanathan M, Hashmi K, Abdelrahman N, Hojnacki D, Dwyer MG et al. . Increased tissue damage and lesion volumes in African Americans with multiple sclerosis. Neurology 2010; 74: 538–44. [DOI] [PubMed] [Google Scholar]
- Wolff B, Basdekidou C, Vasseur V, Mauget-Faÿsse M, Sahel JA, Vignal C. Retinal inner nuclear layer microcystic changes in optic nerve atrophy: a novel spectral-domain OCT finding. Retina 2013; 33: 2133–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author and approval from the Institutional Review Board. The data are not publicly available due to them containing information that could compromise participants’ privacy and consent.