Abstract
Nonalcoholic fatty liver disease (NAFLD), which includes nonalcoholic steatohepatitis (NASH), is associated with reduced GH input/signaling, and GH therapy is effective in the reduction/resolution of NAFLD/NASH in selected patient populations. Our laboratory has focused on isolating the direct vs indirect effects of GH in preventing NAFLD/NASH. We reported that chow-fed, adult-onset, hepatocyte-specific, GH receptor knockdown (aHepGHRkd) mice rapidly (within 7 days) develop steatosis associated with increased hepatic de novo lipogenesis (DNL), independent of changes in systemic metabolic function. In this study, we report that 6 months after induction of aHepGHRkd early signs of NASH develop, which include hepatocyte ballooning, inflammation, signs of mild fibrosis, and elevated plasma alanine aminotransferase. These changes occur in the presence of enhanced systemic lipid utilization, without evidence of white adipose tissue lipolysis, indicating that the liver injury that develops after aHepGHRkd is due to hepatocyte-specific loss of GH signaling and not due to secondary defects in systemic metabolic function. Specifically, enhanced hepatic DNL is sustained with age in aHepGHRkd mice, associated with increased hepatic markers of lipid uptake/re-esterification. Because hepatic DNL is a hallmark of NAFLD/NASH, these studies suggest that enhancing hepatocyte GH signaling could represent an effective therapeutic target to reduce DNL and treat NASH.
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of excess (>5%) fat accumulation in the liver (steatosis) without or with hepatocyte ballooning and inflammation in the presence or absence of fibrosis [nonalcoholic steatohepatitis (NASH)]. The prevalence of NAFLD increases with age and is commonly observed in patients with obesity and type 2 diabetes, but it is also observed in patients who are nonobese directly associated with an increase in the risk for cardiovascular disease (1). It is estimated that >60 million people in the United States alone have NAFLD, of whom a quarter progress to NASH (2), with a related per annum health care cost of more than $100 billion (2). Despite the large clinical and economic burden, to date there are no effective medical therapies to reverse NAFLD (2). To develop effective drugs, it is critical to understand the basic mechanisms controlling hepatic fat accumulation and associated liver damage.
Hepatic fatty acids (FAs) come from carbohydrates [de novo lipogenesis (DNL)] or from uptake of dietary FAs or nonesterified FAs (NEFAs) derived from white adipose tissue (WAT) lipolysis. Hepatic FAs can be oxidized as a source of energy or can be esterified to glycerol to be stored as triglycerides (TGs) temporarily in the liver. Hepatic TGs are exported as very low–density lipoproteins (VLDLs) to deliver FAs to peripheral tissues. For the most part, appropriate diet and exercise can keep these processes in balance and prevent hepatic TG accumulation. However, genetic predisposition, age, environmental insults, excess nutrient intake, and reduced physical activity can converge to impair one or more of these processes, leading to NAFLD. In the context of obesity-associated NAFLD, it was originally proposed that excess hepatic fat accumulation is largely due to enhanced dietary fat intake and re-esterification of FAs derived from WAT lipolysis, as the consequence of WAT insulin resistance (3). However, emerging evidence suggests that hepatic DNL is also inappropriately elevated in patients who are obese with NAFLD, compared with patients who are obese without NAFLD/NASH or healthy controls (4, 5). High liver fat was also associated with elevated nighttime NEFA and fasting insulin, but not with flux of free FAs from adipose tissue or production of VLDLs from free FAs (4). Accordingly, inhibition of hepatic DNL has been recently suggested as a potential effective strategy to treat NAFLD/NASH (5–7).
Although the development of NAFLD is a multifactorial process, our laboratory seeks to determine the role that GH plays in the modulation of liver metabolism and health. This interest in GH lies in the fact that circulating GH levels decline with age and weight gain (8–10), and GH levels are negatively associated with NAFLD (11). Additionally, experimental and clinical data suggest that the fatty liver becomes GH resistant, as indicated by reduced levels of plasma IGF-1 (12) and hepatic phosphorylated signal transducer and activator of transcription 5b [STAT5b (13, 14), a GH receptor (GHR)–mediated transcription factor required for IGF-1 production (15)]. The negative association of GH and NAFLD may in fact be causal because genome-wide association data from patients with NAFLD revealed associations with single nucleotide polymorphisms in 18 different genes in the GH signaling pathway, including GH, GHR, Janus kinase 2 (JAK2), and STAT5b (16). Additionally, GH therapy reduces steatosis in pathologies associated with reduced GH secretion (16–21). In animal models, GH treatment (14, 22, 23) or elevated GH signaling (24) decreases diet-induced steatosis, whereas systemic treatment with a GHR antagonist increases steatosis (25). Based on these observations, we hypothesized that selective GH-mediated control of lipid accumulation in hepatocytes may protect against liver disease in adults. To test this hypothesis we have developed a mouse model of adult-onset, hepatocyte-specific, GHR knockdown (aHepGHRkd, also known as aLivGHRkd) and observed that steatosis develops within 7 days of GHR knockdown in chow-fed male mice and was associated with increased hepatic DNL, as measured by heavy water labeling (26) and increased FA ratios indicative of enhanced DNL (27). The increase in hepatic DNL in the aHepGHRkd model was not associated with alterations in hepatic VLDL release, FA uptake, or systemic insulin sensitivity and WAT lipolysis (26). These initial findings may be of clinical relevance given the negative association of GH and NAFLD (28) and the positive association between hepatic DNL and NAFLD (4). In the current study we extended our observations and found that hepatic DNL is sustained with age in chow-fed aHepGHRkd mice. Importantly, chronic hepatic DNL is associated with NASH development in the absence of peripheral metabolic dysfunction. Therefore, enhancing hepatocyte GH signaling could represent a promising therapeutic target to treat NAFLD/NASH without worsening insulin resistance.
Material and Methods
Animal care and induction of aHepGHRkd
These studies were approved by the Institutional Animal Care and Use Committee of the Jesse Brown Veterans Affairs Medical Center. Ghrfl/fl mice (29) were housed in a specific pathogen-free barrier facility maintained at 22 to 24°C, with a 12-hour light:12-hour dark cycle. All mice were fed a standard chow diet (Teklad LM-485; Envigo, Madison, WI). aHepGHRkd mice were generated as previously described (26, 27). Briefly, 10- to 12-week-old male Ghrfl/fl littermates were injected, via the lateral tail vein, with a single dose (1.5 × 1011 genome copies per 100 μL of PBS) of adeno-associated virus serotype 8 (AAV8) bearing a hepatocyte-specific thyroxine-binding globulin promoter (TBGp) driving a Cre recombinase transgene (AAV8-TBGp-Cre; catalog no. AV-8-PV1091, AAV8.TBG.PI.Cre.rBG; Penn Vector Core, University of Pennsylvania, Philadelphia, PA), to generate aHepGHRkd, or a null allele (AAV8-TBGp-Null; catalog no. AV-8-PV0148, AAV8.TBG.PI.Null.bGH), to generate control mice. For this investigation we focused on male mice based on the fact that in humans NAFLD/NASH is more prevalent in males compared with females (30), consistent with our observations that female aHepGHRkd mice are protected from steatosis, despite elevated hepatic DNL (26).
Time-dependent effects of aHepGHRkd on liver phenotype
Subsets of mice were euthanized by decapitation without anesthesia at 1100 hours (food was withdrawn at 0700 hours) at 1, 9, 27, and 55 weeks after aHepGHRkd. Trunk blood was collected in EDTA-containing tubes for determination of alanine aminotransferase (ALT; BioVision, Milpitas, CA) or NEFAs (Wako Diagnostics, Richmond, VA), GH (MilliporeSigma, Burlington, MA), and insulin (Mercodia, Uppsala, Sweden). Livers and adipose depots were weighed and aliquots snap frozen in liquid nitrogen. Frozen liver samples were used for gene expression, protein, and FA composition analysis as described below. Liver aliquots were also fixed in formalin and stored in 70% ethanol to be sectioned and stained with Mayer hematoxylin/eosin Y.
Impact of aHepGHRkd on metabolic function
In a separate set of mice, glucose tolerance tests and insulin tolerance tests were performed at 9 and 27 weeks after aHepGHRkd. Specifically, glucose tolerance tests were performed after an overnight fast, whereas insulin tolerance tests were performed after 4 hours food removal starting at 0800 hours, as we previously reported (31). Blood glucose was assessed by glucometer (Accu-Chek® Aviva Plus; Roche, Basel, Switzerland). At 29 weeks after aHepGHRkd, tail vein blood samples were taken just before lights out (1700 hours) and then mice were fasted overnight and blood was collected at 0900 hours and after 6 hours of refeeding (1500 hours) to assess dynamic changes in circulating NEFA levels. After 30 weeks of aHepGHRkd, whole-body metabolic rate was assessed by indirect calorimetry (based on lean mass assessed by nuclear magnetic resonance; Minispec LF50; Bruker, Rheinstetten, Germany), as well as activity level, and food/water intake was assessed as previously reported (31). At 32 weeks after HepGHRkd, tissue-specific FA uptake was determined by incorporation of boron-dipyrromethene (BODIPY)–C16 (Life Technologies, Carlsbad, CA) (32). Specifically, BODIPY-C16 was dissolved in dimethyl sulfoxide and then prepared for injection at 0.5 µg of BODIPY-C16/g body weight, 1% dimethyl sulfoxide, and 0.25% FA-free BSA and mice were injected IP after 4 hours of food removal starting at 0700 hours. Blood was collected from lateral tail vein prior to (t = 0) and 1, 3, and 5 hours after BODIPY-C16 injections. After the last blood sample (5 hours postinjection), mice were euthanized and tissues collected, weighed, and snap frozen in liquid nitrogen. Blood collected at 0, 1, 3, and 5 hours after BODIPY injection was used to assess GH levels, as described above. Tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer. BODIPY fluorescence was recorded (excitation, 485 nm; emission, 515 nm) using 10 µL of plasma or a dilution of tissue supernatants in black 96-well plates. Fresh adipose tissue was weighed from this group of mice to assess ex vivo WAT lipolysis, and frozen samples were used for Western blot analysis of phosphorylated hormone-sensitive lipase (pHSL), as described below.
Analytical techniques
Protein analysis
Liver or adipose tissue was homogenized in RIPA buffer supplemented with EDTA, protease and phosphatase inhibitor cocktails (Roche), and a lysine deacetylase inhibitor cocktail (10 mM trichostatin A, 10 mM nicotinamide, and 50 mM butyric acid, all from Sigma-Aldrich, Madison, WI). Protein determination was performed using the bicinchoninic acid assay (Thermo Scientific, Waltham, MA). Adipose tissue was homogenized in RIPA buffer supplemented with phosphatase and protease inhibitor cocktails (Roche) and quantified using Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Hercules, CA). Equal amounts of isolated proteins were separated into precast SDS-PAGE gels in reducing conditions and transferred to nitrocellulose membranes. Membranes were stained with Ponceau to ensure equal loading and incubated overnight at 4°C with nuclear factor κB [NF-κB; catalog no. 1546-1; 1:1000; RRID: AB_562171 (33); Abcam, Cambridge, UK], phosphorylated NF-κB [catalog no. 3033S; 1:1000; RRID: AB_331284 (34)], HSL [catalog no. 4107S; 1:1000; RRID: AB_2296900 (35)], or pHSL [catalog no. 4126S; 1:1000; RRID:AB_490997 (36); Cell Signaling Technology, Danvers, MA] antibodies, followed by a 1-hour incubation at room temperature with a secondary antibody [anti-rabbit IgG, horseradish peroxidase linked; catalog no. 7074S; 1:2000; RRID: AB_2099233 (37); Cell Signaling Technology]. Immunoreactive bands were visualized using the chemiluminescence horseradish peroxidase substrate (EMD Millipore, Billerica, MA) or Clarity Western ECL Substrate (Bio-Rad Laboratories), and densitometry analyses were performed with ImageJ software (National Institutes of Health, Bethesda, MD) or Image Laboratory (Bio-Rad Laboratories).
Malondialdehyde determination
The oxidative degradation of hepatic lipids was determined in liver tissues using a lipid peroxidation [malondialdehyde (MDA)] assay kit (Sigma-Aldrich). Briefly, mouse liver lysate (30 mg of tissue) was incubated with thiobarbituric acid for 60 minutes at 95°C, resulting in the formation of MDA, which was extracted using 300 μL of n-butanol and vacuum dried. The resulting pellet was resuspended in 300 μL of Nanopure water and measured at 532 nm.
Hepatic lipid analysis
Hepatic neutral lipids were extracted with isopropanol as previously described (27) to determine hepatic TG content (Wako Diagnostics). Total hepatic lipids were extracted by the Bligh and Dyer method and transmethylated with BF3-methanol (Sigma-Aldrich) to determine the amount of fatty acyl methyl esters using gas chromatography/mass spectrometry as previously described (27).
Gene expression analysis
Gene expression was assessed by quantitative PCR (qPCR) after reverse transcription of RNA to cDNA, where Pipia, Actb, and Hprt were used as housekeeping genes to calculated a normalization factor, as previously reported (38). The specific sequences of qPCR primers are provided in Table 1.
Table 1.
Sequence of qPCR Primers Used in This Study
| Symbol | Accession No. | Sense Primer (5′→3′) | Antisense Primer (5′→3′) | Product Size, bp |
|---|---|---|---|---|
| Ppia | NM_008907.1 | TGGTCTTTGGGAAGGTGAAAG | TGTCCACAGTCGGAAATGGT | 109 |
| Actb | NM_007393.5 | CTGGGACGACATGGAGAAGA | ACCAGAGGCATACAGGGACA | 205 |
| Hprt | NM_013556.2183 | CAGTCAACGGGGGACATAAA | AGAGGTCCTTTTCACCAGCAA | 183 |
| Ghr | NM_010284.3 | TCTGGAAAGCCTCGATTCAC | TCAGGGCATTCTTTCCATTC | 191 |
| Igf1 | NM_010512.5 | TCGTCTTCACACCTCTTCTACCT | ACTCATCCACAATGCCTGTCT | 202 |
| Socs2 | NM_001168655.1 | CAGCTGTCTGGGACGTGTT | TTCCCCAGTACCATCCTGTTT | 185 |
| Tnfα | NM_013693.2 | TAGCCCACGTCGTAGCAAAC | TGTGGGTGAGGAGCACGTA | 195 |
| Tgfβ1 | NM_011577.1 | CTGAGTGGCTGTCTTTTGACG | CGTGGAGTTTGTTATCTTTGCTG | 123 |
| Col1a1 | NM_007742 | TCAGAGGCGAAGGCAACA | AATGTCCAAGGGAGCCACA | 149 |
| αSma | NM_007392.3 | AACTGGGACGACATGGAAAA | AGGCATAGAGGGACAGCACA | 202 |
| Gck | NM_010292.4 | AAACTACCCCTGGGCTTCAC | ATTGCCACCACATCCATCTC | 179 |
| Khk | NM_008439.3 | GCAGCCTCATGGAAGAGAAG | AAGGACAGTGCAGGAGTTGG | 158 |
| Acc1 | NM_133360.2 | ATCCTGCGAACCTGGATTCT | CCCACCAGAGAAACCTCTCC | 158 |
| Fasn | NM_007988.3 | TGAGCACACTGCTGGTGAAC | CAGGTTCGGAATGCTATCCA | 200 |
| Scd1 | NM_009127.4 | ATCGCCCCTACGACAAGAAC | GTTGATGTGCCAGCGGTACT | 137 |
| Pparg | NM_001127330.1 | AGACCACTCGCATTCCTTTG | CCTGTTGTAGAGCTGGGTCTTT | 214 |
| Cd36 | NM_001159558 | GGAGCCATCTTTGAGCCTTC | TGGATCTTTGTAACCCCACAAG | 201 |
| Mogat1 | NM_026713.3 | TCTGGTTCTGTTTCCCGTTG | ACATTGCCACCTCCATCCTT | 109 |
Abbreviations: Acc1, acetyl–coenzyme A carboxylase 1; Actb, β-actin; Cd36, FA translocase; Col1a1, collage 1a1; Fasn, FA synthase; Gck, glucokinase; Ghr, GH receptor; Hprt, hypoxanthine-guanine phosphoribosyltransferase; Igf1, IGF-1; Khk, ketohexokinase; Mogat1, monoacylglycerol-O-acyltransferase 1; Ppia, peptidylprolyl isomerase; Pparγ, peroxisome proliferator–activated receptor γ; Scd1, stearoyl–coenzyme A desaturase; Socs2, suppressor of cytokine signaling 2; Tgfβ1, TGF-β1; TNFα, TNF-α; αSma, α-smooth muscle actin.
Ex vivo lipolysis in WAT explants
The ex vivo lipolysis protocol was adapted from Vijayakumar et al. (39). Briefly, ∼50 mg of fresh urogenital and subcutaneous adipose tissue was placed in a 24-well plate for 1.5 hours containing 1 mL of ice-cold Krebs-Ringer buffer with HEPES (1 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.4 mM KCl, 130 mM NaCl, 2 mM pyruvate, 2.5% FA-free BSA, 20 mM HEPES). Then, WAT explants were minced with sharp scissors and the buffer was replaced with new prewarmed (37°C) Krebs-Ringer buffer with HEPES with or without 1 µM isoproterenol (Sigma-Aldrich). WAT explants were incubated at 37°C in agitation (114 rpm) for 3 hours. Glycerol content of the media was measured using a free glycerol reagent assay (MilliporeSigma) following the manufacturer’s instructions.
Statistical analysis
Values are represented as means ± SEM. A Student t test was used to assess the effect of aHepGHRkd. Owing to variability in plasma GH levels, values were log transformed prior to analysis. Changes in plasma NEFA and respiratory quotient data were analyzed by two-way ANOVA following by Fisher least significant difference test or a Sidak post hoc test, respectively. Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). A P value <0.05 was considered significant.
Results
The steatosis that rapidly develops after adult-onset hepatic GH resistance can progress to early NASH-like features, independent of diet-induced obesity
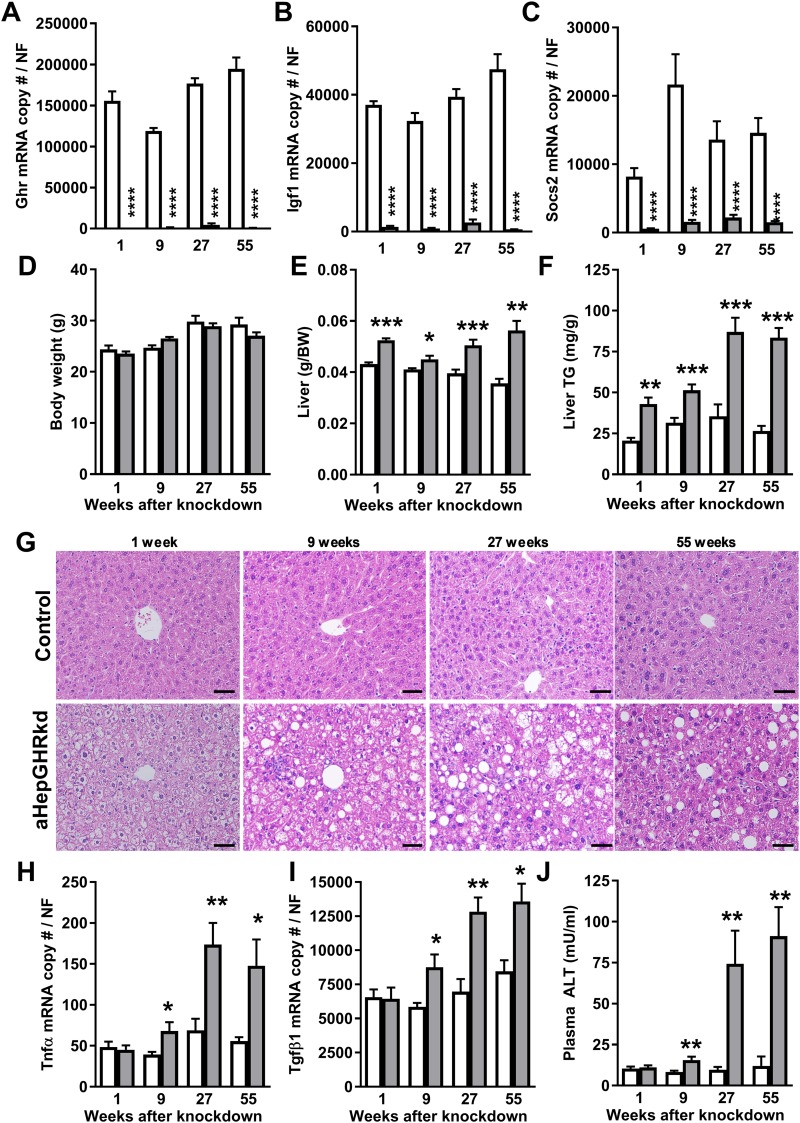
A single dose of AAV8-TBGp-Cre, injected IV in 10- to 12-week-old Ghrfl/fl mice, reduced hepatic Ghr mRNA levels to >98% of AAV8-TBGp-null–treated control Ghrfl/fl mice, up to 55 weeks after AAV8 injection (Fig. 1A). The effective induction of hepatocyte GH resistance was evident by sustained reductions in expression of hepatic GHR/STAT5b target genes, Igf1 and Socs2 (Fig. 1B and 1C).
Figure 1.
aHepGHRkd rapidly (1 wk) leads to steatosis, which progresses to NASH (27 and 55 wk after AAV8 treatment) in chow-fed mice. Hepatic (A) Ghr, (B) Igf1, and (C) Socs2 gene expression. (D) Body weight (BW), (E) liver weight, and (F) TG content. (G) Representative images of hematoxylin and eosin–stained liver sections (scale bars, 50 μm; 20× magnification). Hepatic (H) Tnfα and (I) Tgfβ1 expression. (J) Plasma ALT levels. Open columns represent control mice; filled columns represent aHepGHRkd mice. Data are represented as means ± SEM of four different age groups, that is, 1, 9, 27, and 55 wk after administration of AAV8 vectors. Gene expression is represented as mRNA copy number normalized with a normalization factor (NF) calculated from the expression of three housekeeping genes, Ppia, β-actin, and Hprt. n = 4 to 10 mice per group. Asterisks indicate significant difference between control and aHepGHRkd mice within age group: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
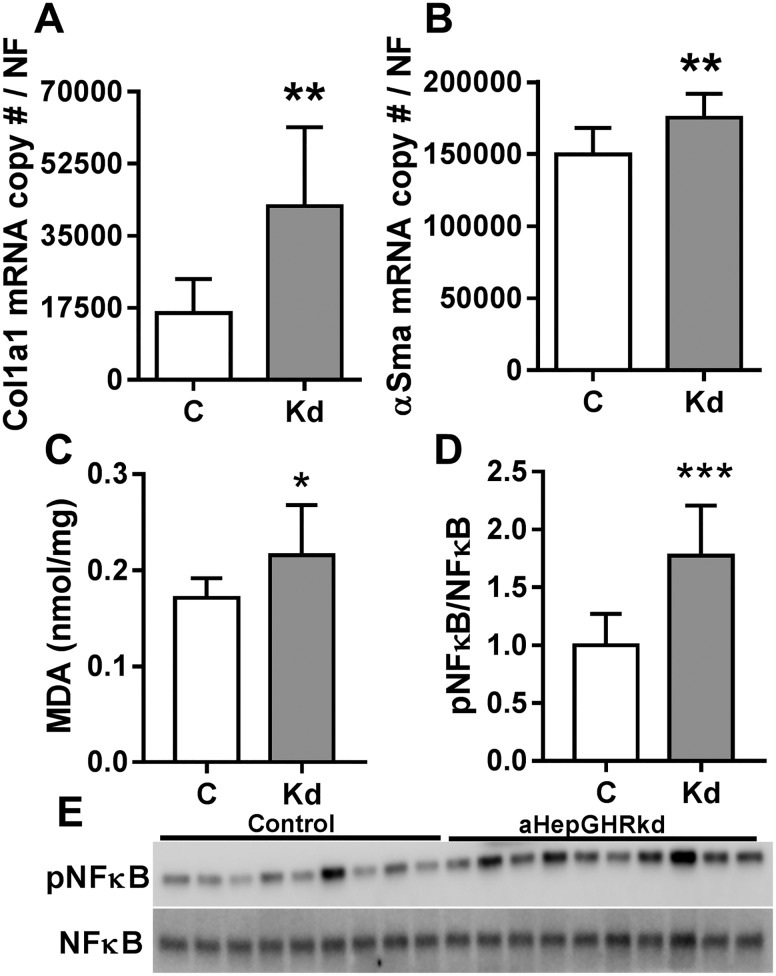
Independent of changes in body weight (Fig. 1D), chow-fed aHepGHRkd mice had increased liver weight (Fig. 1E) and hepatic TG content (Fig. 1F), as compared with those of littermate controls. Of note, after 27 weeks of aHepGHRkd, there was histological evidence of hepatocyte ballooning and inflammation that was associated with an increased hepatic expression of inflammatory markers Tnfα and Tgfβ1 (Fig. 1H and 1I), accompanied by elevated plasma ALT levels (Fig. 1J). Further evaluation of the 27-week cohort revealed an increase in Col1a1 and αSma mRNA (Fig. 2A and 2B), indicative of early signs of fibrosis, as well as an increase in MDA and phosphorylated NF-κB protein levels, indicative of oxidative stress (Fig. 2C–2E). Taken together, the steatosis that rapidly develops after adult-onset hepatic GH resistance can progress to early NASH-like features, independent of diet-induced obesity.
Figure 2.
aHepGHRkd mice (27 wk after AAV8 treatment) show early signs of fibrosis and oxidative stress. Hepatic (A) Col1a1 and (B) αSma expression. Hepatic (C) MDA levels and (D) ratio of phosphorylated NF-κB/NF-κB. (E) Western blot of phosphorylated NF-κB/NF-κB. Open columns indicate control mice (C); filled columns indicate aHepGHRkd mice (Kd). Data are represented as means ± SEM. Gene expression is shown as mRNA copy number divided by a normalization factor (NF) calculated from the expression levels of three housekeeping genes, Ppia, β-actin, and Hprt. n = 9 to 10 mice per group. Asterisks indicate significant difference between control and aHepGHRkd mice within age group: *P < 0.05; **P < 0.01; ***P < 0.001.
Evidence supporting a direct action of GH in preventing hepatic lipid accumulation and liver damage
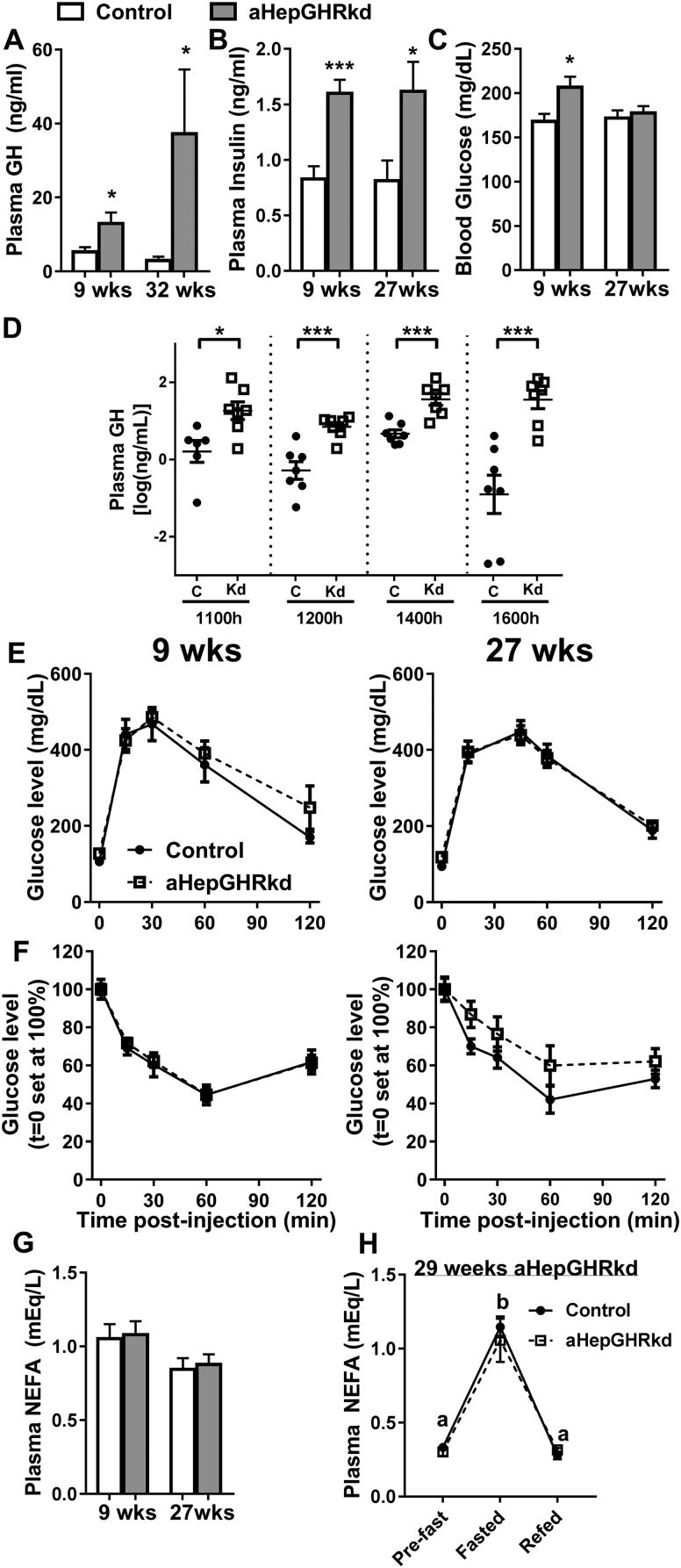
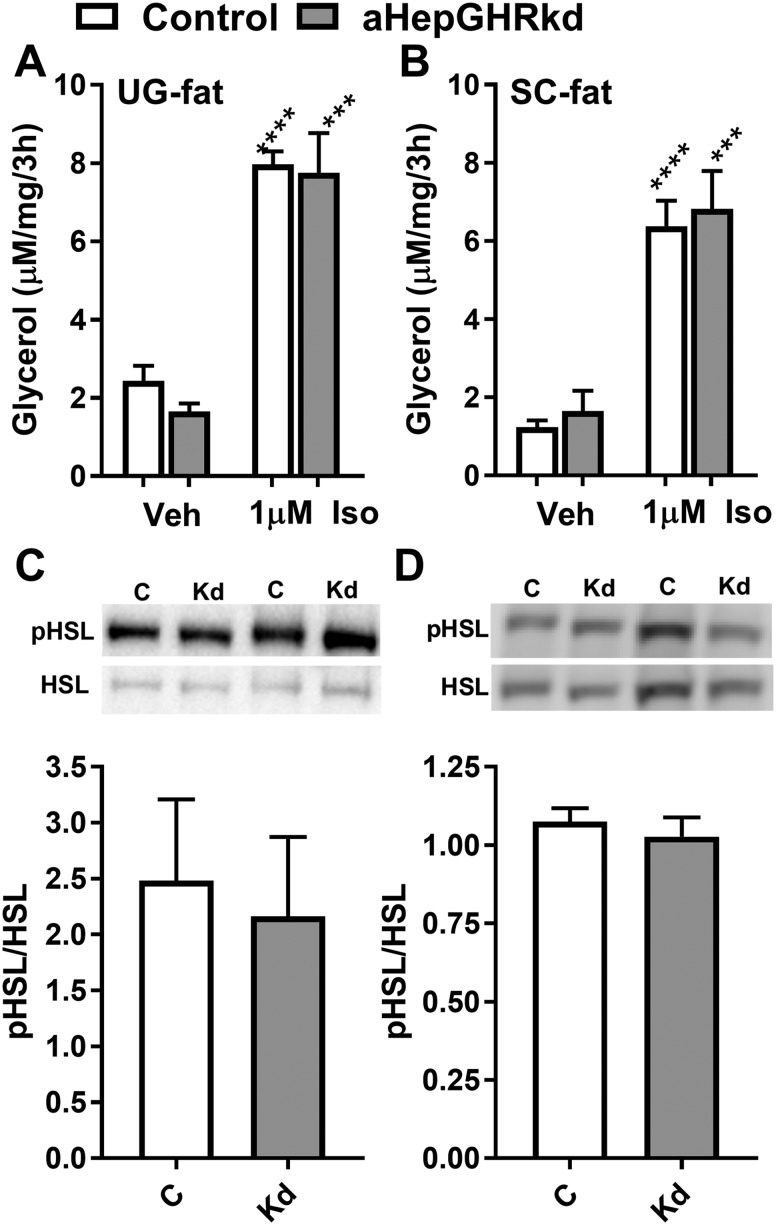
It is commonly accepted that the accumulation of fat in the liver is a benign condition referred to as “simple steatosis,” and that progression from steatosis to NASH requires a “second hit.” The development of the NASH-like phenotype in livers of chow-fed aHepGHRkd mice may be due to the development of systemic insulin resistance and WAT lipolysis (second hit) as a consequence of loss of hepatic GHR-dependent IGF-1 negative feedback on pituitary, which results in the increase of plasma GH levels. GH is considered a diabetogenic and lipolytic hormone (40). In fact, mice with albumin promoter-driven Cre recombinase knockout of hepatocyte Ghr or its downstream signals, Jak2 or Stat5, show a reciprocal shift in circulating IGF-1/GH associated with steatosis, glucose intolerance, systemic insulin resistance, and increased circulating NEFA levels indicative of WAT lipolysis (41–43). Similar to these congenital knockout models, GH and insulin levels are elevated after 9 and 27 weeks of aHepGHRkd, and elevated levels of plasma GH were sustained during the day. However, profound hyperglycemia, glucose intolerance, and whole-body insulin resistance did not develop (Fig. 3A–3F). Consistent with these observations, basal plasma NEFA levels were not elevated 9 and 27 weeks after aHepGHRkd (Fig. 3G). Also, aHepGHRkd mice showed normal elevations in plasma NEFA in response to overnight fasting, which were appropriately suppressed by 6 hours of refeeding (Fig. 3H). Finally, WAT depots from mice 32 weeks after aHepGHRkd exhibited normal ex vivo basal and isoproterenol-induced WAT lipolysis (Fig. 4A and 4B), as well as in vivo pHSL levels (Fig. 4C and 4D).
Figure 3.
aHepGHRkd mice (9 to 32 wk after AAV8 treatment) show elevated plasma GH and insulin levels, but normal glucose and insulin tolerance tests and circulating NEFA. Basal levels (4 h after food removal at 0700 h) of (A) plasma GH, (B) plasma insulin, and (C) blood glucose from mice 9, 27, or 32 wk after aHepGHRkd induction. (D) Plasma GH levels in lateral vein blood samples obtained prior to (1100 h) and after BODIPY-C16 injections (at 1200, 1400, and 1600 h). (E) Glucose tolerance tests (2 g/kg, IP). (F) Insulin tolerance tests (1.5 U/kg, IP). (G and H) Plasma NEFA levels in (G) trunk blood after 4-h food withdrawal starting at 0700 h and from (H) lateral tail vein blood obtained at just prior to lights out (1700 h, pre-fast), after overnight fasting (0900 h, fasted), and after 6-h refeeding with a chow diet (1500 h, refed). Open columns and filled circles with solid lines indicate control mice (C); filled columns and open squares with discontinuous line indicate aHepGHRkd mice (Kd). Data are represented as means ± SEM of groups of mice used at different times after administration of AAV8 vectors. n = 7 to 11 mice per group. Asterisks indicate significant difference between control and aHepGHRkd mice within age group: *P < 0.05; ***P < 0.001. Different letters in (H) indicate significant difference (P < 0.05) between the fed state within group.
Figure 4.
aHepGHRkd mice (32 wk after AAV8 treatment) did not show evidence of increased WAT lipolysis. Ex vivo lipolysis of (A) urogenital fat (UG-fat) and (B) subcutaneous fat (inguinal, SC-fat). Ratio of pHSL/total HSL in (C) UG-fat and (D) SC-fat, with representative Western blots included above graphs. Open columns indicate control mice (C); filled columns indicate aHepGHRkd mice (Kd). Data are represented as means ± SEM. n = 7 mice per group. Asterisks indicate significant difference between vehicle (Veh)-treated and isoproterenol (1µM Iso)-stimulated WAT explants within group: ***P < 0.001; ****P < 0.001.
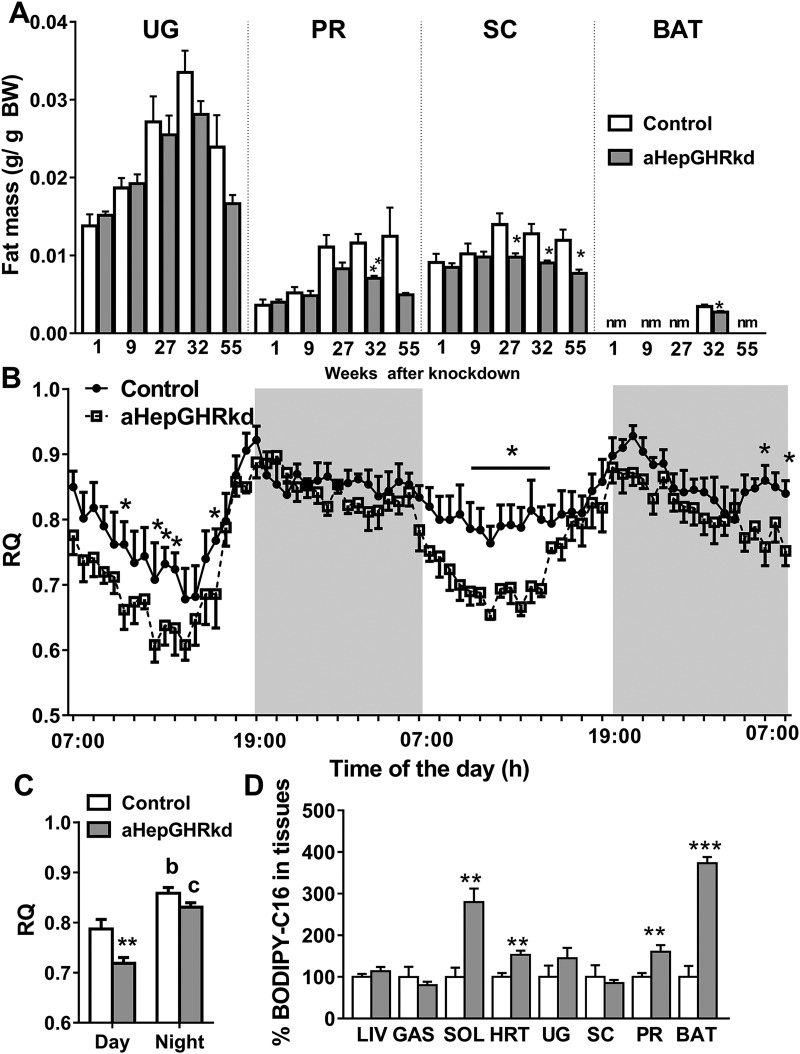
Despite the solid lack of evidence for enhanced WAT lipolysis in aHepGHRkd mice, a modest but significant age-dependent decline in total fat mass was observed, which was associated with a reduction in subcutaneous (inguinal) and perirenal/retroperitoneal but not urogenital WAT depot weight (Fig. 5A). This reduction in fat mass can be explained in part by the observation that 27-week aHepGHRkd mice exhibit an increase in whole-body lipid utilization (oxidation), as indicated by a reduction in the daytime respiratory quotient (Fig. 5B and 5C), which was not observed after short-term 7-day aHepGHRkd. This age-dependent increase in whole-body lipid utilization occurred independent of changes in activity level or food intake. Further support for enhanced systemic lipid utilization, after long-term aHepGHRkd, is the observation that FA uptake was increased in heart and soleus (Fig. 5D), where studies of others demonstrate that GH can work directly on the skeletal muscle and heart to increase FA utilization (39). Additionally, FA uptake was enhanced in perirenal/retroperitoneal fat and brown adipose tissue, but not in the liver (Fig. 5D).
Figure 5.
aHepGHRkd mice show fat depot–selective reduction in weight associated with increased systemic lipid utilization and tissue-specific FA uptake. (A) Fat depot weight of 1- to 55-wk aHepGHRkd mice. (B) Respiratory quotient (RQ) of aHepGHRkd mice 27 wk after AAV injection. Night period (1900 to 0700 h) is indicated by gray background. (C) Average of day and night RQ. (D) Percentage of BODIPY-C16 uptake in different tissues of 32-wk aHepGHRkd mice, where controls were set at 100%. Open columns and filled circles with solid line indicate control mice; filled columns and open squares with discontinuous line indicate aHepGHRkd mice. Data are represented as means ± SEM. n = 5 to 7 mice per group. Asterisks indicate significant difference between control and aHepGHRkd: *P < 0.05; **P < 0.01; ***P < 0.001. Letters indicate significant different between day and night values within group: b, P < 0.01; c, P < 0.001. BAT, brown adipose tissue; GAS, gastrocnemius; HRT, heart; LIV, liver; nm, not measured; PR, perirenal/retroperitoneal fat; SC, subcutaneous fat; SOL, soleus; UG, urogenital fat.
Taken together, the above observations strongly suggest that the sustained steatosis and progressive liver damage observed in the aHepGHRkd model cannot be readily explained by the indirect actions of low IGF-1 and elevated GH on systemic insulin sensitivity and WAT lipolysis. In fact, it would be expected that an enhanced utilization of lipids in extrahepatic tissues should reduce accumulation of lipids in the liver. Therefore, the aHepGHRkd model provides clear evidence that specific GH-mediated effects on hepatocytes prevent excess lipid accumulation and protect against liver damage.
Steatosis observed after long-term adult-onset hepatocyte GH resistance is associated with enhanced hepatic DNL
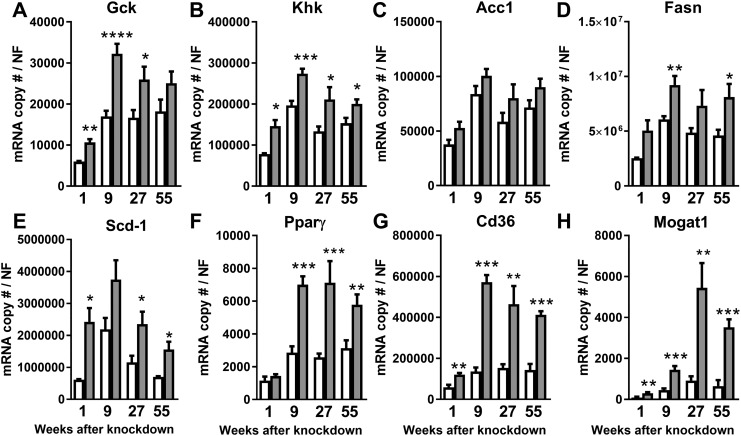
We initially reported that 7 days after induction of aHepGHRkd, steatosis develops owing to enhanced hepatic DNL, associated with hepatic endpoints indicative of an increase in carbohydrate uptake and glycolysis (26). Subsequently, we reported that this increase in DNL was associated with, but independent of, an increase in Pparγ and downstream target genes, Cd36 and Mogat1 (27), which have been shown to be important in hepatic chylomicron remnant and FA uptake (44) and FA re-esterification (45), respectively. We observed hepatic expression of genes involved in glycolysis and DNL (Gck, Khk, Acc1, Fasn, Scd1) (Fig. 6A–6E), as well as genes involved in hepatic lipid uptake and storage (Pparγ, Cd36, Mogat1), remained elevated with age in aHepGHRkd mice (Fig. 6F–6H). This hepatic expression pattern suggests that enhanced DNL continues to contribute to steatosis as aHepGHRkd mice age. Additionally, with time the contribution of extrahepatic FAs to steatosis may increase owing to an enhanced ability of the liver to take up and/or store FAs in a PPARγ-dependent manner (27).
Figure 6.
aHepGHRkd mice (1 to 55 wk after AAV8 treatment) show increased expression of genes important for DNL and lipid uptake/esterification. Hepatic (A) Gck, (B) Khk, (C) Acc1, (D) Fasn, (E) Scd1, (F) Pparγ, (G) Cd36, and (H) Mogat1 gene expression. Open columns indicate control mice; filled columns indicate aHepGHRkd mice. Data are represented as means ± SEM of four different age groups, that is, 1, 9, 27, and 55 wk after administration of AAV8 vectors. Gene expression is represented as mRNA copy number divided by a normalization factor (NF) calculated from the expression level of three housekeeping genes, Ppia, β-actin, and Hprt. n = 3 to 10 mice per group. Asterisks indicate significant difference between control and aHepGHRkd within group: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
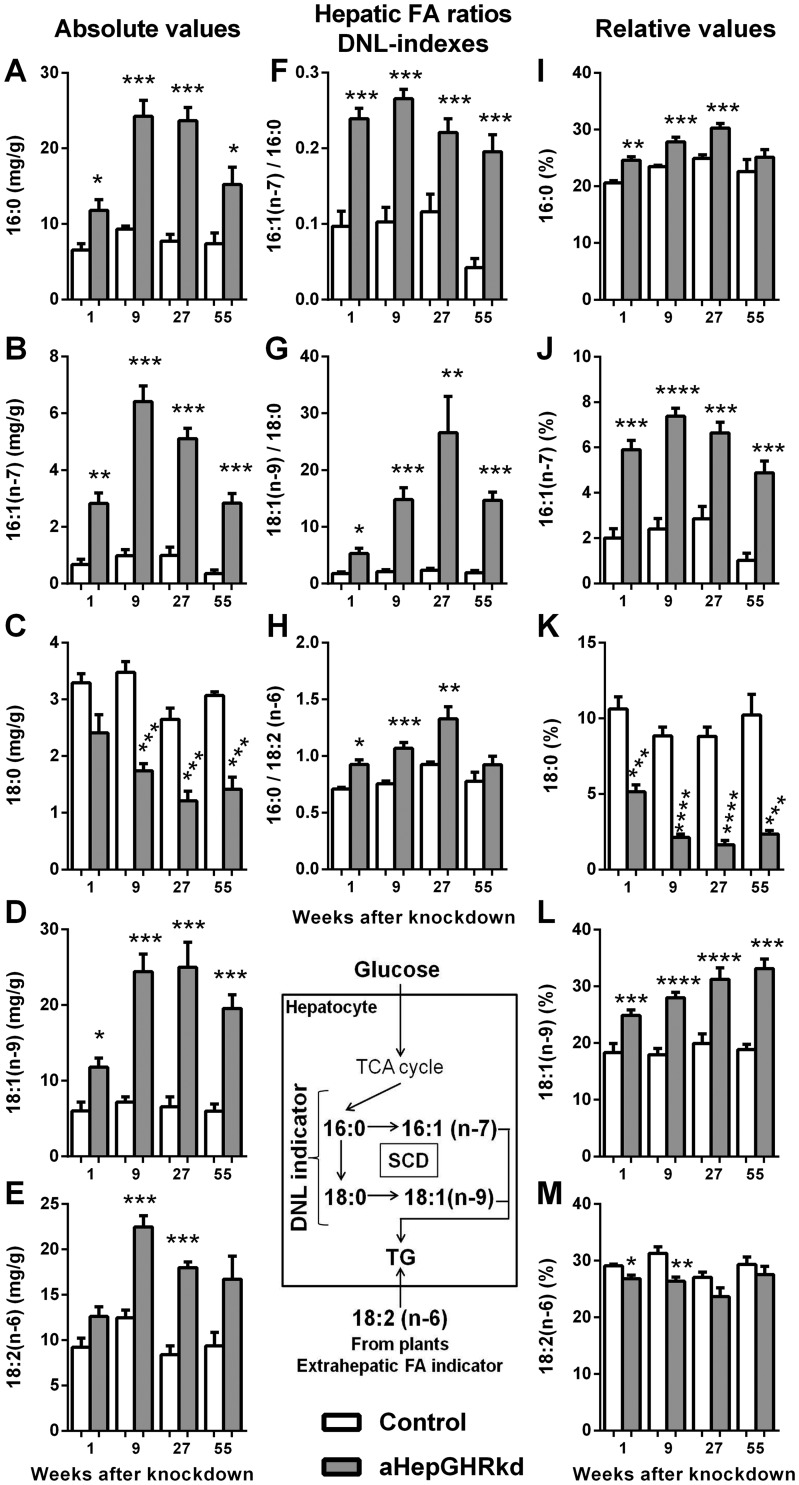
To determine the relative contribution that FAs made de novo relative to those derived from extrahepatic sources, we used gas chromatography/mass spectrometry to assess changes in hepatic FA composition. The absolute levels of palmitic (16:0), palmitoleic [16:1(n-7)], oleic [18:1(n-9)], and linoleic [18:2(n-6)] acids were increased with age in aHepGHRkd livers, compared with littermate controls (Fig. 7A–7E, left column), consistent with the increase in hepatic TG content (Fig. 1F). However, the level of stearic acid (18:0) was reduced, which could be explained by an increase in Scd1 expression (Fig. 6E), where stearoyl–coenzyme A desaturase (SCD)1 desaturates both 16:0 and 18:0, but preferentially converts 18:0 to 18:1(n-9) (46). An increase in desaturase activity is further substantiated by an increase in hepatic ratios of 16:1(n-7)/16:0 and 18:1(n-9)/18:0 (SCD indexes; Fig. 7F–7H, middle column). Expressing individual FAs as a percentage of total hepatic FAs revealed an increased in the relative contribution of 16:0, 16:1(n-7), and 18:1(n-9) to the total hepatic FA pool of aHepGHRkd mice. However, the relative contribution of 18:2(n-6), an essential FA supplied by dietary plant sources, did not increase in aHepGHRkd mice as compared with controls (Fig. 7I–7M). This observation further supports the conclusion that an elevation in hepatic TG content cannot be solely explained by enhanced availability and/or uptake of FAs from diet or WAT lipolysis. However, the fact that the hepatic ratio of 16:0/18:2(n-6) (DNL index) was increased (Fig. 7H), in conjunction with the increase in SCD indexes, strongly suggests that enhanced DNL remains a major contributor to the steatosis observed in aHepGHRkd mice across age (47, 48).
Figure 7.
aHepGHRkd mice (1 to 55 wk after AAV8 treatment) show changes in hepatic FA composition that are indicative of enhanced DNL. Absolute levels of hepatic (A) 16:0, (B) 16:1(n-6), (C) 18:0, (D) 18:1(n-9), and (E) 18:2(n-6). Hepatic (F) SCD index [16:1(n-7)/16:0], (G) SCD index [18:1(n-9)/18:0], and (H) DNL index [16:0/18:2 (n-6)]. Relative hepatic levels (individual FA percentage of total FA) of (I) 16:0, (J) 16:1(n-7), (K) 18:0, (L) 18:1(n-9), and (M) 18:2(n-6). As illustrated in the inset diagram, FAs can be generated de novo from glucose oxidation that is incorporated in the tricarboxylic cycle (TCA cycle). The newly synthetized saturated FAs (16:0 and 18:0) are then desaturated by SCD and subsequently esterified in TGs. SCD activity is closely related with DNL activity in hepatocytes. FAs can also come from extrahepatic sources, where 18:2(n-6), an FA that cannot be synthetized in animal cells (essential FA), is used as an indicator of extrahepatic FA uptake. Therefore, an increase in the ratio of 16:0/18:2(n-6) is also used as an indicator of DNL. Open columns indicate control mice; filled columns indicate aHepGHRkd mice. Data are represented as means ± SEM of four different age groups, that is, 1, 9, 27, and 55 wk after administration of AAV8 vectors. Individual FAs were quantified using an internal standard (13,16,19–docosatrienoic acid; 22:3). n = 4 to 9 mice per group. Asterisks indicate significant difference between control and aHepGHRkd within group: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
The aHepGHRkd model reveals that the steatosis that rapidly develops after adult onset loss of hepatocyte GH signaling (26) is sustained with age and progresses to NASH, associated with enhanced hepatic DNL, without evidence of severe systemic insulin resistance or WAT lipolysis despite the elevation of GH. The phenotype of the aHepGHRkd model differs from that observed with congenital loss of hepatocyte GH signaling, which develops steatosis, systemic glucose intolerance, insulin resistance, and elevated NEFA levels associated with WAT lipolysis (41–43). We could speculate that these differences may be due to the differential impact of the shift of IGF-1/GH on mature tissues (aHepGHRkd model) vs that which occurs during the development of key tissues in metabolism: pancreatic β-cells, adipose tissue, muscle, liver, and brain. Nonetheless, results obtained from mouse models of congenital loss of hepatocyte GH signaling suggest that IGF-1/GH–mediated changes in systemic metabolic function are not solely responsible for the increase in hepatic lipid accumulation. Specifically, blocking the actions of elevated GH in WAT of mice with congenital loss of hepatocyte GH (Jak2) signaling, with adipose-specific Jak2 knockout mice (adipocyte and hepatocyte Jak2KO mice), reduces but does not eliminate steatosis (49). Also, a 2-week continuous treatment of IGF-1 in hepatocyte GHR knockout mice lowers plasma GH levels, but it does not impact hepatic lipid content (41). Additionally, normalization of GH levels in hepatocyte GHR knockout by the expression of a hepatocyte-specific heterologous IGF-1 transgene [Li-GHRKO/HIT (50)] restores systemic metabolic function and reduces but does not eliminate excess hepatic lipid content. Specifically, steatosis observed in Li-GHRKO/HIT mice was associated with FA indexes indicative of elevated DNL, as well as endpoints of liver inflammation (50). These observations, coupled with our current findings, strongly indicate that adult-onset loss of hepatocyte GH signaling can directly promote hepatic lipid accumulation, independent of severe systemic insulin resistance and WAT lipolysis.
In the context of aHepGHRkd, the lack of enhanced WAT lipolysis in the presence of elevated GH at first seems counter to the well-accepted lipolytic action of GH. However, the ability of GH to induce lipolysis is dependent on the level of insulin and tissue sensitivity to insulin. Specifically, in healthy humans overnight fasting raises GH levels; however, blocking this rise had no impact on WAT lipolysis, where a lipolytic action of GH is only evident after prolonged fasting (2 to 3 days), which reduces insulin by 80% (51–53). The lack of an effect of elevated GH on WAT lipolysis, in the context of normal feeding cycles, is also indicated in another mouse model of elevated endogenous GH, HiGH mice (31). Similar to aHepGHRkd mice, this model shows modest elevations in insulin levels, with normal or reduced circulating NEFAs (31, 54). Also, similar to the aHepGHRkd mice, HiGH mice exhibit enhanced whole-body lipid utilization during the resting state (day), as assessed by indirect calorimetry (31). This increase in lipid utilization may be in part due to the action of GH on skeletal muscle, because muscle-specific knockout of the GHR, under chow-fed conditions, led to a reduction in lipid utilization under resting conditions and during exercise (39). In the aHepGHRkd mice, the enhanced systemic lipid utilization would be anticipated to protect against excess hepatic fat accumulation by the oxidation of fat in peripheral tissues, further supporting the conclusion that the steatosis and liver injury that occurs in aHepGHRkd mice is directly due to loss of hepatocyte GHR signaling.
It is clear that the development of steatosis and progression to NASH are commonly associated with obesity-related insulin resistance (55), where it is difficult to determine the relative contribution of extrahepatic vs intrahepatic changes to NASH progression. The aHepGHRkd model demonstrates that early signs of NASH can develop independent of obesity and overt systemic metabolic dysfunction in chow-fed mice. The age-dependent development of steatosis and liver injury in aHepGHRkd mice is associated with an increase in hepatic DNL and expression of hepatic genes critical for DNL, as well as an increase of genes that favor hepatic lipid uptake and FA re-esterification. Many of these genes, including GCK (56), KHK (57), PPARγ (58), and CD36 (59), have been shown to be upregulated in liver biopsies from patients with NAFLD/NASH. Unexpectedly, hepatic uptake of free palmitate was not increased in aHepGHRkd mice, which appears inconsistent with the increase in CD36 expression. However, it is possible that the steatogenic role of hepatic CD36 may be related with uptake of chylomicron remnants (60).
It remains to be determined whether hepatic FAs formed by de novo vs those derived from extrahepatic sources are more or less deleterious to liver health. However, evidence is emerging suggesting that enhanced hepatic DNL is a key player in disease progression. Specifically, a seminal paper by Parks and colleagues (4) reported that the relative contribution of FAs derived from WAT lipolysis and diet were equal in body mass index–matched patients who are obese without or with NAFLD, whereas those with NAFLD showed a fourfold increase in the relative contribution of FAs derived from DNL. Since then, others have supported this finding and extended it to demonstrate that hepatic DNL is also increased in patients with NASH (5). Based on these studies, a clinical trial is underway to inhibit DNL, using a liver-targeted allosteric inhibitor of acetyl–coenzyme A carboxylase (GS-0976), with initial (short-term) results indicating it is highly effective at reducing liver fat and endpoints of liver injury (5). Additional studies are testing the effectiveness of inhibiting FA synthase activity (6, 7).
As discussed previously, clinical and experimental studies have demonstrated that reduced GH secretion is associated with NAFLD/NASH, which can be reversed by GH therapy (20, 21). Our data demonstrate that a reduction in GH input to the liver directly promotes hepatic DNL, steatosis, and NASH development. Although highly associated, it remains to be determined whether the enhanced DNL observed after hepatocyte loss of GH signaling is the primary driver of liver injury, or whether other direct actions of GH contribute to this process. For example, it has been shown that GH impacts the expression of multiple genes important in xenobiotic clearance (61) and may play a role in hepatocyte regeneration (62). In addition to the direct effects of GH on liver metabolism and health, hepatic GH signaling is also required to maintain circulating IGF-1 levels. Whereas hepatocytes do not appreciably express the IGF-1 receptor, other hepatic cell types, that is, hepatic stellate cells and Kupffer cells, do express the IGF-1 receptor (63).
Nonetheless, the direct effects of GH on hepatocyte function may explain in part the previous clinical observations showing that GH therapy reduces steatosis in pathologies associated with reduced GH secretion, including young men with abdominal obesity (28), patients with primary GH deficiency (19–21), and patients who have HIV with lipodystrophy due to antiviral treatment (18). In fact, the reduction in steatosis, observed in GH-treated patients with HIV, was associated with a decrease in hepatic DNL (18). Additionally, acromegaly is associated with low liver fat that increases after controlling GH levels by surgical removal of the pituitary tumor and/or octreotide therapy combined with a GHR antagonist (64). These positive actions of GH have promoted clinical trials to examine the impact of low-dose GH therapy (to avoid the anti-insulin actions of GH) to treat NASH (NCT02726542, NCT02217345, NCT03375788). In addition to this systemic approach, our current results suggest that hepatocyte-specific GH signaling could represent a future drug target to treat NASH.
Acknowledgments
We thank Neena Majumdar for breeding the Ghrfl/fl mice and assisting in the analysis of samples/mice; Dr. Rafael de Cabo (Experimental Gerontology Section, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, MD) for assistance in the protein analysis of the aHepGHRkd livers; Dr. John J. Kopchick (Edison Biotechnology Institute and Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH) for providing the Ghrfl/fl mouse model; and Dr. Natalia Nieto (University of Illinois at Chicago, Chicago, IL) for key discussions of the hepatic pathophysiology of aHepGHRkd mice. Part of this work was presented at the 2017 and 2018 Endocrine Society’s Annual meetings in Orlando, FL, and Chicago, IL, respectively.
Financial Support: This work was supported by National Institutes of Health Grant K01DK115525 (to J.C.-C.); the Intramural Research Program of the National Institutes of Health, National Institute on Aging (to A.D.-R.); US Department of Veterans Affairs, Office of Research and Development Merit Award BX001090 (to P.V.S.); National Institutes of Health Grants R21AT008457 and S10OD010660 (to P.V.S); and by US Department of Veterans Affairs, Office of Research and Development Merit Award BX001114 (to R.D.K.).
Author Contributions: J.C.-C., A.S.-C., and R.D.K. designed the study, performed the experiments, analyzed the data, and wrote the manuscript; M.d.R.-M. and A.D.-R. performed experiments related to protein analysis; and P.V.S. provided technical assistance on the use of gas chromatography/mass spectrometry, as well as data interpretation. All authors have discussed the results and revised and approved the final version of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 16:0
palmitic acid
- 16:1(n-7)
palmitoleic acid
- 18:0
stearic acid
- 18:1(n-9)
oleic acid
- 18:2(n-6)
linoleic acid
- AAV8
adeno-associated virus serotype 8
- aHepGHRkd
adult-onset, hepatocyte-specific, GH receptor knockdown
- ALT
alanine aminotransferase
- BODIPY
boron-dipyrromethene
- DNL
de novo lipogenesis
- FA
fatty acid
- GHR
GH receptor
- HSL
hormone-sensitive lipase
- JAK2
Janus kinase 2
- MDA
malondialdehyde
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor κB
- pHSL
phosphorylated hormone-sensitive lipase
- qPCR
quantitative PCR
- RIPA
radioimmunoprecipitation assay
- SCD
stearoyl–coenzyme A desaturase
- STAT5b
signal transducer and activator of transcription 5b
- TBGp
thyroxine-binding globulin promoter
- TG
triglyceride
- VLDL
very low–density lipoprotein
- WAT
white adipose tissue
References
- 1. Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. [DOI] [PubMed]
- 3. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawitz EJ, Coste A, Poordad F, Alkhouri N, Loo N, McColgan BJ, Tarrant JM, Nguyen T, Han L, Chung C, Ray AS, McHutchison JG, Subramanian GM, Myers RP, Middleton MS, Sirlin C, Loomba R, Nyangau E, Fitch M, Li K, Hellerstein M. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2018;S1542-3565(18)30404-X. [DOI] [PubMed]
- 6. Parks EJ, Manrique CM, Syed-Abdul MM, Gaballah AH, Hammoud GM, Buckley D, Duke G, McCulloch W, Kemble G. Pharmacologic inhibition of FASN reverses diet-induced steatohepatitis in mice and inhibits lipogenesis in humans. Hepatology. In: The Liver Meeting of the American Association for the Study of Liver Diseases; 20–24 October 2017; Washington, DC. Abstract 1045A. [Google Scholar]
- 7. Duke G, Wagman A, Buckley D, McCulloch W, Feigh M, Skovgaard Veidal S, Kemble G.. Fatty acid synthase inhibitor TVB-3664 reduces collagen accumulation in bleomycin-induced murine skin fibrosis and reverses multiple components of diet-induced and biopsy-confirmed nonalcoholic steatohepatitis in mice treated with or without co-administered pirfenidone. In: The Liver Meeting of the American Association for the Study of Liver Diseases; 20–24 October 2017; Washington, DC. Abstract 1056A. [Google Scholar]
- 8. De Marinis L, Bianchi A, Mancini A, Gentilella R, Perrelli M, Giampietro A, Porcelli T, Tilaro L, Fusco A, Valle D, Tacchino RM. Growth hormone secretion and leptin in morbid obesity before and after biliopancreatic diversion: relationships with insulin and body composition. J Clin Endocrinol Metab. 2004;89(1):174–180. [DOI] [PubMed] [Google Scholar]
- 9. Scacchi M, Pincelli AI, Cavagnini F. Growth hormone in obesity. Int J Obes Relat Metab Disord. 1999;23(3):260–271. [DOI] [PubMed] [Google Scholar]
- 10. Shalet SM, Toogood A, Rahim A, Brennan BM. The diagnosis of growth hormone deficiency in children and adults. Endocr Rev. 1998;19(2):203–223. [DOI] [PubMed] [Google Scholar]
- 11. Xu L, Xu C, Yu C, Miao M, Zhang X, Zhu Z, Ding X, Li Y. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One. 2012;7(8):e44136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Völzke H, Nauck M, Rettig R, Dorr M, Higham C, Brabant G, Wallaschofski H. Association between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sample. Eur J Endocrinol. 2009;161(5):705–713. [DOI] [PubMed]
- 13. Owen C, Lees EK, Mody N, Delibegović M. Regulation of growth hormone induced JAK2 and mTOR signalling by hepatic protein tyrosine phosphatase 1B. Diabetes Metab. 2015;41(1):95–101. [DOI] [PubMed] [Google Scholar]
- 14. Qin Y, Tian YP. Preventive effects of chronic exogenous growth hormone levels on diet-induced hepatic steatosis in rats. Lipids Health Dis. 2010;9(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rotwein P. Mapping the growth hormone–Stat5b–IGF-I transcriptional circuit. Trends Endocrinol Metab. 2012;23(4):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen QR, Braun R, Hu Y, Yan C, Brunt EM, Meerzaman D, Sanyal AJ, Buetow K. Multi-SNP analysis of GWAS data identifies pathways associated with nonalcoholic fatty liver disease. PLoS One. 2013;8(7):e65982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, Grinspoon SK. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwarz JM, Mulligan K, Lee J, Lo JC, Wen M, Noor MA, Grunfeld C, Schambelan M. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87(2):942. [DOI] [PubMed] [Google Scholar]
- 19. Hazlehurst JM, Tomlinson JW. Non-alcoholic fatty liver disease in common endocrine disorders. Eur J Endocrinol. 2013;169(2):R27–R37. [DOI] [PubMed]
- 20. Takahashi Y, Iida K, Takahashi K, Yoshioka S, Fukuoka H, Takeno R, Imanaka M, Nishizawa H, Takahashi M, Seo Y, Hayashi Y, Kondo T, Okimura Y, Kaji H, Kitazawa R, Kitazawa S, Chihara K. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132(3):938–943. [DOI] [PubMed] [Google Scholar]
- 21. Nishizawa H, Iguchi G, Murawaki A, Fukuoka H, Hayashi Y, Kaji H, Yamamoto M, Suda K, Takahashi M, Seo Y, Yano Y, Kitazawa R, Kitazawa S, Koga M, Okimura Y, Chihara K, Takahashi Y. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol. 2012;167(1):67–74. [DOI] [PubMed]
- 22. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52(8):1647–1655. [DOI] [PubMed] [Google Scholar]
- 23. Tateno C, Kataoka M, Utoh R, Tachibana A, Itamoto T, Asahara T, Miya F, Tsunoda T, Yoshizato K. Growth hormone-dependent pathogenesis of human hepatic steatosis in a novel mouse model bearing a human hepatocyte-repopulated liver. Endocrinology. 2011;152(4):1479–1491. [DOI] [PubMed] [Google Scholar]
- 24. Greenhalgh CJ, Rico-Bautista E, Lorentzon M, Thaus AL, Morgan PO, Willson TA, Zervoudakis P, Metcalf D, Street I, Nicola NA, Nash AD, Fabri LJ, Norstedt G, Ohlsson C, Flores-Morales A, Alexander WS, Hilton DJ. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J Clin Invest. 2005;115(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang T, Householder LA, Lubbers ER, List EO, Troike K, Vesel C, Duran-Ortiz S, Kopchick JJ, Berryman DE. Growth hormone receptor antagonist transgenic mice are protected from hyperinsulinemia and glucose intolerance despite obesity when placed on a HF diet. Endocrinology. 2015;156(2):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cordoba-Chacon J, Majumdar N, List EO, Diaz-Ruiz A, Frank SJ, Manzano A, Bartrons R, Puchowicz M, Kopchick JJ, Kineman RD. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015;64(9):3093–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kineman RD, Majumdar N, Subbaiah PV, Cordoba-Chacon J. Hepatic PPARγ is not essential for the rapid development of steatosis after loss of hepatic GH signaling, in adult male mice. Endocrinology. 2016;157(5):1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bredella MA, Gerweck AV, Lin E, Landa MG, Torriani M, Schoenfeld DA, Hemphill LC, Miller KK. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98(9):3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524–535. [DOI] [PMC free article] [PubMed]
- 30. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gahete MD, Córdoba-Chacón J, Anadumaka CV, Lin Q, Brüning JC, Kahn CR, Luque RM, Kineman RD. Elevated GH/IGF-I, due to somatotrope-specific loss of both IGF-I and insulin receptors, alters glucose homeostasis and insulin sensitivity in a diet-dependent manner. Endocrinology. 2011;152(12):4825–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalifeh-Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, Turner SM, Chawla A, Stahl A, Atabai K. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med. 2014;20(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RRID: AB_562171.
- 34. RRID: AB_331284.
- 35. RRID: AB_2296900.
- 36. RRID: AB_490997.
- 37. RRID: AB_2099233.
- 38. Cordoba-Chacon J, Gahete MD, McGuinness OP, Kineman RD. Differential impact of selective GH deficiency and endogenous GH excess on insulin-mediated actions in muscle and liver of male mice. Am J Physiol Endocrinol Metab. 2014;307(10):E928–E934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vijayakumar A, Wu Y, Buffin NJ, Li X, Sun H, Gordon RE, Yakar S, LeRoith D. Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One. 2012;7(9):e44777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. [DOI] [PubMed] [Google Scholar]
- 41. Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937–19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sos BC, Harris C, Nordstrom SM, Tran JL, Balázs M, Caplazi P, Febbraio M, Applegate MA, Wagner KU, Weiss EJ. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest. 2011;121(4):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46(2):504–513. [DOI] [PubMed]
- 44. Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, Dyck JR. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–2871. [DOI] [PubMed] [Google Scholar]
- 45. Lee YJ, Ko EH, Kim JE, Kim E, Lee H, Choi H, Yu JH, Kim HJ, Seong JK, Kim KS, Kim JW. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci USA. 2012;109(34):13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43(2):91–104. [DOI] [PubMed] [Google Scholar]
- 47. Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87(4):817–823. [DOI] [PubMed] [Google Scholar]
- 48. Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Königsrainer A, Königsrainer I, Häring HU, Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem. 2009;55(12):2113–2120. [DOI] [PubMed] [Google Scholar]
- 49. Nordstrom SM, Tran JL, Sos BC, Wagner KU, Weiss EJ. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol Endocrinol. 2013;27(8):1333–1342. [DOI] [PMC free article] [PubMed]
- 50. Liu Z, Cordoba-Chacon J, Kineman RD, Cronstein BN, Muzumdar R, Gong Z, Werner H, Yakar S. Growth hormone control of hepatic lipid metabolism. Diabetes. 2016;65(12):3598–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K, Barkan A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab. 2008;93(7):2755–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rabinowitz D, Zierler KL. A metabolic regulating device based on the actions of human growth hormone and of insulin, singly and together, on the human forearm. Nature. 1963;199(4896):913–915. [DOI] [PubMed] [Google Scholar]
- 53. Moller L, Norrelund H, Jessen N, Flyvbjerg A, Pedersen SB, Gaylinn BD, Liu J, Thorner MO, Moller N, Lunde Jorgensen JO. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J Clin Endocrinol Metab. 2009;94(11):4524–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cordoba-Chacon J, Majumdar N, Pokala NK, Gahete MD, Kineman RD. Islet insulin content and release are increased in male mice with elevated endogenous GH and IGF-I, without evidence of systemic insulin resistance or alterations in β-cell mass. Growth Horm IGF Res. 2015;25(4):189–195. [DOI] [PubMed] [Google Scholar]
- 55. Utzschneider KM, Kahn SE. The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753–4761. [DOI] [PubMed] [Google Scholar]
- 56. Peter A, Stefan N, Cegan A, Walenta M, Wagner S, Königsrainer A, Königsrainer I, Machicao F, Schick F, Häring HU, Schleicher E. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab. 2011;96(7):E1126–E1130. [DOI] [PubMed] [Google Scholar]
- 57. Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pettinelli P, Videla LA. Up-regulation of PPAR-γ mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96(5):1424–1430. [DOI] [PubMed] [Google Scholar]
- 59. Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. [DOI] [PubMed] [Google Scholar]
- 60. Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, Koseki M, Matsuura F, Nishida M, Kawamoto T, Ishigami M, Hori M, Shimomura I, Yamashita S. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res. 2009;50(5):999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collin de l’Hortet A, Zerrad-Saadi A, Prip-Buus C, Fauveau V, Helmy N, Ziol M, Vons C, Billot K, Baud V, Gilgenkrantz H, Guidotti JE. GH administration rescues fatty liver regeneration impairment by restoring GH/EGFR pathway deficiency. Endocrinology. 2014;155(7):2545–2554. [DOI] [PubMed] [Google Scholar]
- 63. Takahashi Y. The role of growth hormone and insulin-like growth factor-I in the liver. Int J Mol Sci. 2017;18(7):1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Madsen M, Krusenstjerna-Hafstrøm T, Møller L, Christensen B, Vendelbo MH, Pedersen SB, Frystyk J, Jessen N, Hansen TK, Stødkilde-Jørgensen H, Flyvbjerg A, Jørgensen JO. Fat content in liver and skeletal muscle changes in a reciprocal manner in patients with acromegaly during combination therapy with a somatostatin analog and a GH receptor antagonist: a randomized clinical trial. J Clin Endocrinol Metab. 2012;97(4):1227–1235. [DOI] [PubMed] [Google Scholar]