Supplemental Digital Content is Available in the Text.
The newly designed, pH-dependent opioid agonist NFEPP induced analgesia exclusively through peripheral opioid receptors in models of neuropathic and abdominal pain.
Keywords: NFEPP, Peripheral opioid receptors, Acidic pH, Neuropathic pain, Abdominal pain
Abstract
Recently, (±)-N-(3-fluoro-1-phenethylpiperidine-4-yl)-N-phenyl propionamide (NFEPP), a newly designed μ-opioid receptor (MOR) agonist with a low pKa, has been shown to produce injury-restricted analgesia in models of inflammatory and postoperative pain, without exhibiting typical opioid side effects. Here, we investigated MOR binding of NFEPP in brain and dorsal root ganglia, pH in injured tissues, and the analgesic efficacy of NFEPP compared with fentanyl in a chronic constriction injury model of neuropathic pain, and in the acetic acid–induced abdominal writhing assay in rats. Binding experiments revealed significantly lower affinity of NFEPP compared with fentanyl at pH 7.4. In vivo, pH significantly dropped both at injured nerves after chronic constriction injury and in the abdominal cavity after acetic acid administration. Intravenous NFEPP as well as fentanyl dose-dependently diminished neuropathy-induced mechanical and heat hypersensitivity, and acetic acid–induced abdominal constrictions. In both models, NFEPP-induced analgesia was fully reversed by naloxone methiodide, a peripherally restricted opioid receptor antagonist, injected at the nerve injury site or into the abdominal cavity. Our results indicate that NFEPP exerts peripheral opioid receptor–mediated analgesia exclusively in damaged tissue in models of neuropathic and abdominal pain.
1. Introduction
Both conventional opioids and nonsteroidal analgesics (nonsteroidal anti-inflammatory drugs) produce detrimental side effects. Opioids exert sedation, apnoea, nausea, addiction, and constipation mediated in brain or gut, whereas cyclooxygenase inhibitors can elicit ulcers, bleeding, myocardial infarction, or stroke.4,7,12 Previous strategies in drug development have focused on central opioid receptors in noninjured environments.9,24 However, a large number of painful syndromes (eg, arthritis, neuropathy, and surgery) are driven by peripheral sensory neurons2,27 and are typically accompanied by inflammation with tissue acidosis.1,14,19,35 Under such circumstances, opioid receptors and their signaling pathways in dorsal root ganglion (DRG) neurons are upregulated.33 Targeting peripheral opioid receptors in damaged tissue avoids adverse opioid effects in the brain or gut, as well as detrimental side effects of NSAIDs, as demonstrated in animal models and humans with acute and chronic pain.11,29,32,33,37,41 Moreover, pharmacological, genetic, and clinical studies have shown that a large proportion of opioid analgesia is mediated by peripheral opioid receptors10,15,39 and that such receptors can confer significant anti-inflammatory effects.34
By computer simulations at low pH, a hallmark of injured tissue, we recently designed the novel opioid (±)-N-(3-fluoro-1-phenethylpiperidine-4-yl)-N-phenyl propionamide (NFEPP) which, due to its low acid dissociation constant (pKa = 6.8), selectively activates peripheral μ-opioid receptors (MORs) in inflamed tissue.32 NFEPP showed pH-sensitive binding, G-protein subunit dissociation, and 3′, 5′-cyclic adenosine monophosphate inhibition in MOR-transfected human embryonic kidney 293 cells. It did not produce typical side effects mediated by central or intestinal MOR exposed to normal pH (about 7.4), such as respiratory depression, sedation, addiction, and constipation. Both NFEPP and conventional fentanyl inhibited pain with similar efficacy in rats with complete Freund adjuvant–induced inflammation or incision of one hind paw.32 In this study, we investigated MOR binding of NFEPP in native tissues using membranes of brain and DRG, in vivo pH of damaged tissue, and the analgesic efficacy of NFEPP compared with fentanyl in the chronic constriction injury (CCI) model of neuropathic pain and in the acetic acid–induced abdominal writhing assay in rats.
2. Methods
2.1. Chemicals/drugs
Fentanyl citrate (F3886), naloxone hydrochloride (NLX; N7758), naloxone methiodide (NLXM; N129), and acetic acid were purchased from Sigma-Aldrich (Taufkirchen, Germany), and [³H]-[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin ([3H]-DAMGO; NET902250UC) from Perkin Elmer (Waltham, MA). NFEPP was synthesized according to our design by a contractor (ASCA GmbH, Berlin, Germany).32 NFEPP was dissolved in dimethyl-sulfoxide (DMSO; Sigma-Aldrich) and diluted with 0.9% NaCl or in binding assay buffer. The maximum DMSO concentration for intravenous (i.v.) injections was 0.5%. Fentanyl and NLXM were dissolved in 0.9% NaCl. Control groups were treated with the respective vehicles.
2.2. Animals
All experiments were approved by the state animal care committee (Landesamt für Gesundheit und Soziales, Berlin) and performed according to the ARRIVE guidelines.16 Male Wistar rats (200-300 g; Janvier Laboratories, Le Genest-Saint-Isle, France) were kept on a 12-hour light/dark schedule, in groups of 3 in cages lined with ground corncob bedding, with free access to standard laboratory food and tap water. Room temperature was 22 ± 0.5°C and humidity 60% to 65%. Statistical power calculations were performed a priori to determine minimum sample sizes. Rats were handled once per day for 1 to 2 minutes or habituated to the test cages (1-2 times for 15 minutes), starting 4 days before experiments. After completion of experiments, animals were killed with an overdose of isoflurane (AbbVie, Wiesbaden, Germany).
2.3. Isolation of brain, dorsal root ganglion, and membrane preparation
Naive rats were killed by an overdose of isoflurane. Brains without cerebella were removed, collected in ice-cold binding assay buffer (Trizma, 50 mM, pH 7.4), homogenized, and centrifuged at 42,000g for 20 minutes at 4°C. The pellet was resuspended in assay buffer, followed by centrifugation at 42,000g and 4°C for 20 minutes. Protein concentration was determined using the Bradford method.5 Membrane preparation of lumbar and thoracic DRG was performed in the same way except for an additional incubation step at 37°C for 10 minutes after the first centrifugation, and the addition of 1 mM EGTA to the assay buffer, as described by us before.31,42
The corrected half maximal concentration (IC50) of fentanyl or NFEPP necessary to displace 4 nM of the standard MOR ligand [3H]-DAMGO was determined at different pH values (5.5, 6.5, and 7.4), as described previously.32 A protein amount of 100 μg was incubated for 90 minutes at room temperature with the radioligand (53.7 Ci/mmol) and the competing ligands (fentanyl or NFEPP) dissolved in binding assay buffer at the respective pH values. Nonspecific binding was determined by the addition of 10 µM of NLX. Filters were soaked in 0.1% polyethyleneimine solution before use. Bound and free ligands were separated by rapid filtration under vacuum through Whatman GF/B glass fiber filters. Bound radioactivity was assessed by liquid scintillation spectrophotometry with a counting efficiency of 69% for [3H] after overnight extraction of the filters in the scintillation fluid.
2.4. Chronic constriction injury model
Chronic constriction injury of the sciatic nerve was induced under isoflurane anesthesia as described elsewhere.3 Briefly, the sciatic nerve was exposed at the level of the left midthigh, 4 loose 4/0 silk ligatures were placed around the nerve with approximately 1 to 2 mm spacing, and the wound was closed with silk sutures.
2.4.1. Mechanical hyperalgesia (Randall–Selitto test)
Rats were gently restrained under paper wadding and incremental pressure was applied using a wedge-shaped, blunt piston onto the dorsal surface of the hind paws by means of an automated gauge (Ugo Basile, Comerio, Italy). The paw pressure threshold (PPT; cutoff at 250 g) required to elicit paw withdrawal was determined by averaging 3 consecutive trials separated by 15-second intervals. The sequence of paws was alternated between animals to avoid “order” effects, as described previously.32
2.4.2. Mechanical allodynia (von Frey test)
Animals were individually placed in clear Plexiglas cubicles located on a stand with anodized mesh (Model 410; IITC Life Science, Woodland Hills, CA). The plantar surface of each hind paw was stimulated using von Frey filaments (Stoetling, Wood Dale, IL) of increasing force until the filament that produced withdrawal responses to 3 stimuli (paw withdrawal threshold [PWT]) was reached, as described previously.22 The strengths of calibrated von Frey filaments were 0.6, 1, 1.4, 2, 4, 6, 8, 10, 15, and 26 g.
2.4.3. Heat hyperalgesia (Hargreaves test)
Rats were individually placed in clear Plexiglas cubicles positioned on a stand with glass surface. Radiant heat generated by a high-intensity light bulb was applied to the plantar surface of the hind paws from underneath the glass surface, and paw withdrawal latency (PWL) was measured using an electronic timer (Model 390; IITC Life Science), as described earlier.13 Three measurements separated by at least 10 seconds were averaged. The heat intensity was adjusted to obtain a baseline withdrawal latency of about 10 to 12 seconds in uninjured paws, and the cutoff was 20 seconds.
2.5. Writhing model
Animals received 1% acetic acid intraperitoneally (i.p.; 10 mL/kg) under brief isoflurane anesthesia, and were placed individually in transparent cages for observation of abdominal constrictions (“writhing”).38 The total number of writhes between 5 and 35 minutes after acetic acid injection was counted.
2.6. pH measurements
For in vivo measurements, a pH-sensitive glass microelectrode (model IC-401 combination pH electrode; Warner Instruments, Hamden, CT) was calibrated using reference solutions of pH 4.0, 7.0, and 9.2. Measurements were performed at 3, 7, and 14 days after CCI, or before, 5, 15, and 30 minutes after i.p. acetic acid injections, under isoflurane anesthesia. In the CCI model, the microelectrode mounted in the lumen of the 20-gauge needle was inserted close to the sciatic nerve, in an area of 2 to 6 mm around the ligations, or in a similar location on the contralateral uninjured limb. In the writhing model, the needle was introduced until it perforated the skin and moved freely into the abdominal cavity. Stable readings were obtained 2 to 3 minutes after the electrode insertion in both cases. In vitro, we measured the pH of saline (0.9% NaCl; vehicle) and NLXM dissolved in saline (50 µg/100 µL) using a pH-sensitive glass electrode (Mettler Toledo; InLab Routine Pro, Giessen, Germany) calibrated using reference solutions of pH 4.01, 7.0, and 9.21.
2.7. Injections and experimental protocols
Injections of opioid agonists and antagonist were performed i.v. (200 μL), at the nerve injury site (100 μL), or i.p. (100 μL) under brief isoflurane anesthesia. In the CCI model, effects on nociceptive thresholds (PPT, PWT, and PWL) of fentanyl (4-16 μg/kg i.v.) and NFEPP (4-16 μg/kg i.v.) were evaluated before and 15 to 60 minutes after injections, on day 14 after CCI. To examine the contribution of peripheral opioid receptors, we used NLXM, an opioid receptor antagonist that does not readily cross the blood–brain barrier.6 NLXM (50 μg/rat) was injected at the nerve injury site immediately before i.v. injection of agonists, and nociceptive thresholds were measured 15 minutes later.
In the writhing model, fentanyl (4-16 μg/kg i.v.) and NFEPP (4-16 μg/kg i.v.) were injected immediately after acetic acid, and the total number of writhes was counted thereafter within 5 to 35 minutes. NLXM (50 μg/rat i.p.) was injected immediately after injection of acetic acid and right before i.v. injection of agonists, and the number of writhes was counted thereafter within 5 to 35 minutes. All dosages were determined in pilot experiments. The experimenter was blinded to the treatments and dosages.
2.8. Data handling and statistical analyses
All data were assessed for normal distribution and equal variances by Kolmogorov–Smirnov normality tests. In binding experiments, means of values at each agonist concentration and each pH were determined to calculate IC50 by nonlinear regression, which were then subjected to Friedman 1-way analysis of variance (ANOVA) and Dunn tests. All behavioral data were expressed as raw values. Two-sample comparisons were made using paired or unpaired t tests for normally distributed data, or Wilcoxon or Mann–Whitney tests for non-normally distributed data. Two-way repeated-measures ANOVA and Bonferroni test were used to compare 2 parameters over time. To analyze one parameter over time, 1-way repeated-measures ANOVA and Bonferroni test were used. Multiple comparisons at one time point were performed using 1-way ANOVA followed by Bonferroni test for normally distributed data, or Kruskal–Wallis 1-way ANOVA followed by Dunn test for non-normally distributed data. Differences were considered significant if P < 0.05. Prism 5 (GraphPad, San Diego, CA) was used for all tests and graphs, and all data are expressed as mean ± SEM.
3. Results
3.1. NFEPP binding affinity is decreased at pH 7.4
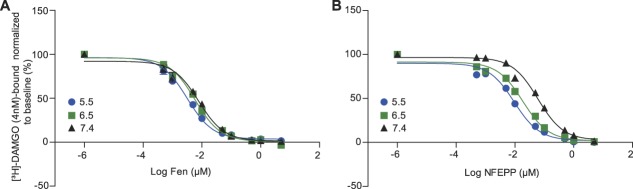
In membranes of brain, the IC50 values to displace DAMGO binding did not significantly differ between fentanyl and NFEPP at pH 5.5 (P > 0.05). However, at pH 6.5 and 7.4, the IC50 values of NFEPP significantly increased (ie, its affinity decreased) compared with fentanyl (P < 0.05) (Fig. 1; Fig. S1, available online as supplemental digital content at http://links.lww.com/PAIN/A612—legend, http://links.lww.com/PAIN/A618; and Table S1, available online as supplemental digital content at http://links.lww.com/PAIN/A615). We also detected impaired binding of NFEPP to MOR at pH 7.4 in membranes of DRG. Due to high variability of the DRG binding values (possibly resulting from the additional incubation step at 37°C), we did not perform statistical analysis, and present descriptive data only (Fig. S2, available online as supplemental digital content at http://links.lww.com/PAIN/A613—legend, http://links.lww.com/PAIN/A618; Fig. S3, available online as supplemental digital content at http://links.lww.com/PAIN/A614—legend, http://links.lww.com/PAIN/A618; and Table S1, available online as supplemental digital content at http://links.lww.com/PAIN/A615).
Figure 1.
Binding affinity of NFEPP to MOR in brain membranes is reduced at normal pH (7.4). Displacement of bound [3H]-DAMGO (4 nM) by fentanyl (Fen) (A) or NFEPP (B) incubated for 90 minutes at pH 5.5, 6.5, and 7.4. Data are expressed as mean ± SEM; n = 6 independent experiments per group. For IC50 values, see Table S1 (available online as supplemental digital content at http://links.lww.com/PAIN/A615). MOR, μ-opioid receptor.
3.2. pH values
In vivo, compared with contralateral uninjured nerves, the pH values at the site of nerve injury (CCI) were significantly decreased on 3, 7, and 14 days after CCI (7.19 ± 0.007 vs 7.01 ± 0.019; 7.14 ± 0.018 vs 6.91 ± 0.022; and 7.22 ± 0.016 vs 6.98 ± 0.024, respectively; P < 0.001; Fig. 2A). After i.p. injection of 1% acetic acid, intra-abdominal pH values were significantly decreased at 5 minutes (4.52 ± 0.058) and 15 minutes (6.97 ± 0.032) compared with baseline (7.3 ± 0.031) (P < 0.001), and returned to baseline by 30 minutes (P > 0.05; Fig. 2B). The in vitro pH of 0.9% NaCl was 5.36 ± 0.01 and that of NLXM was 5.18 ± 0.05 (n = 3; P = 0.25; Wilcoxon test), consistent with the literature.25
Figure 2.
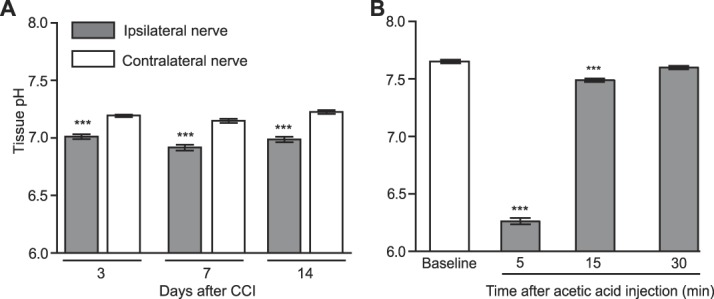
Decreased pH in injured tissue. pH values at sciatic nerves at 3, 7, and 14 days after CCI in ipsilateral and contralateral nerves (A), and in the abdominal cavity before (baseline) and 5, 15, and 30 minutes after intraperitoneal injection of 1% acetic acid (B). ***P < 0.001 vs contralateral nerves, paired t test (A); ***P < 0.001 vs baseline, 1-way repeated-measures ANOVA and Bonferroni test (B). Data are expressed as mean ± SEM; n = 8 to 9 rats per group. ANOVA, analysis of variance; CCI, chronic constriction injury.
3.3. NFEPP produces analgesia selectively through peripheral opioid receptors in the neuropathic pain model
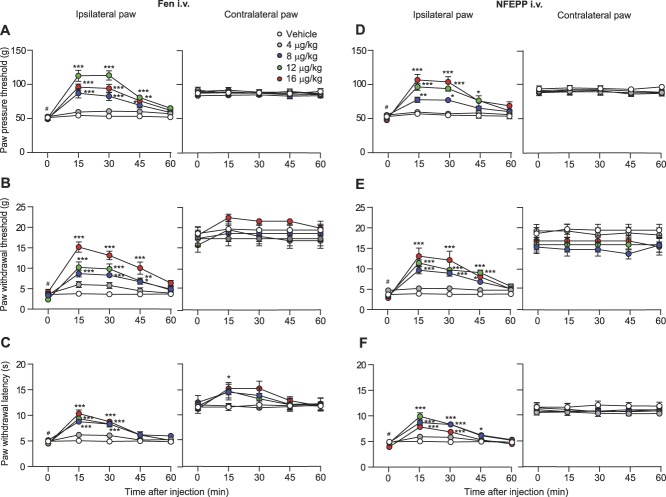
Fourteen days after CCI, rats developed mechanical hyperalgesia (reduced PPT; P < 0.05; Figs. 3A and D), mechanical allodynia (reduced PWT to von Frey filaments; P < 0.05; Figs. 3B and E), and heat hyperalgesia (reduced PWL; P < 0.05; Figs. 3C and F) in ipsilateral compared with contralateral paws. After injection into the tail vein, both fentanyl and NFEPP (4-16 μg/kg) elevated PPT, PWT, and PWL at 15 to 45 minutes in the ipsilateral paw (P < 0.05, P < 0.01, and P < 0.001 depending on the time point; Figs. 3A–F) in a dose-dependent manner (Figs. 4A–C). In contralateral paws, fentanyl significantly increased PWL (P < 0.05; Fig. 3C) but not PPT and PWT (P > 0.05; Figs. 3A and B), whereas NFEPP was not effective (P > 0.05; Fig. 3). In all 3 tests, the analgesic effects induced by fentanyl (12 μg/kg i.v.) were only partially reversed by injection of NLXM (50 μg) at the nerve injury site because the effects were significantly different from baseline levels before injections (P < 0.01; Figs. 4D, F, and H). By contrast, the analgesic effects of NFEPP (12 μg/kg i.v.) were completely reversed by NLXM (50 μg) injected at the nerve injury site to the baseline levels (P > 0.05; Figs. 4E, G, and I). The local injection of 0.9% NaCl (vehicle) (Figs. 4E, G, and I) did not significantly change the analgesic effects of i.v. NFEPP (12 µg/kg) injected alone (Figs. 4A–C) (P > 0.05; t test). These data indicate that NFEPP mediates analgesia in neuropathic pain exclusively through peripheral opioid receptors at the injured nerve.
Figure 3.
Time course of analgesic effects after intravenous (i.v.) injections of fentanyl and NFEPP in the neuropathic pain model. Effects of fentanyl (Fen) (A–C) and NFEPP (D–F) on mechanical hyperalgesia (A and D), mechanical allodynia (B and E), and heat hyperalgesia (C and F) in ipsilateral (left panels) and contralateral paws (right panels) at 14 days after CCI. *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle, 2-way repeated-measures ANOVA and Bonferroni test; #P < 0.05 vs contralateral paws, Wilcoxon test. Data are expressed as mean ± SEM; n = 6 to 9 rats per group. ANOVA, analysis of variance; CCI, chronic constriction injury.
Figure 4.
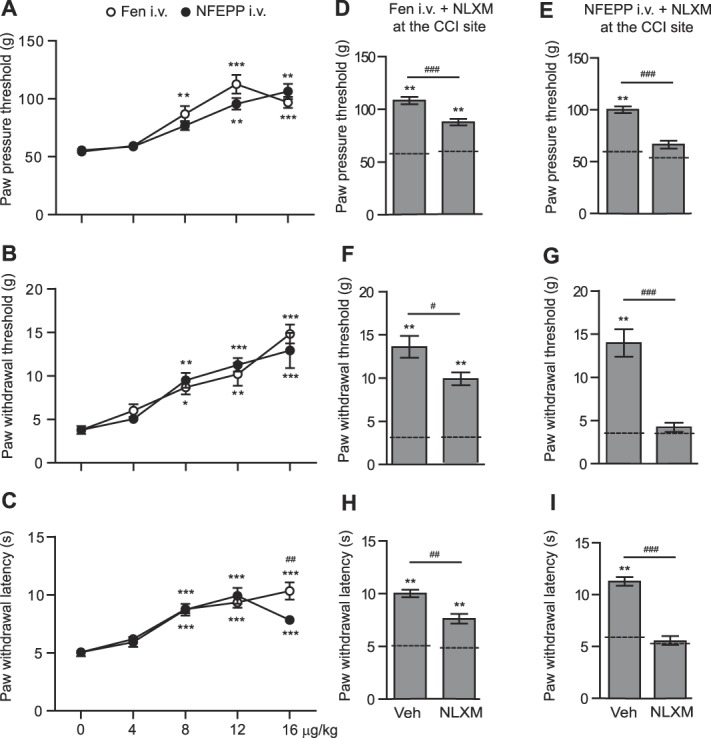
Contribution of peripheral opioid receptors to analgesic effects of intravenous (i.v.) fentanyl and NFEPP in the neuropathic pain model. (A–C) Effects at 15 minutes after i.v. fentanyl (Fen) or NFEPP on mechanical hyperalgesia (A), mechanical allodynia (B), and heat hyperalgesia (C) in ipsilateral paws at 14 days after CCI. *P < 0.05, **P < 0.01, ***P < 0.001 vs control (0 µg/kg), Kruskal–Wallis 1-way ANOVA followed by Dunn test (A and B), or 1-way ANOVA followed by Bonferroni test (C); ##P < 0.01 Fen vs NFEPP, unpaired t test (C). The data are the same as those in Figure 3 measured at 15 minutes in ipsilateral paws. (D–I) Effects of NLXM (50 µg) or vehicle injected at the nerve injury site (CCI site) on analgesic effects of Fen (D, F, and H) and NFEPP (E, G, and I) (both at 12 µg/kg i.v.) in mechanical hyperalgesia (D and E), mechanical allodynia (F and G), and heat hyperalgesia (H and I). NLXM was injected immediately before agonists and the effects were assessed 15 minutes later. **P < 0.01 vs corresponding baseline threshold/latencies (dashed lines) evaluated 14 days after CCI, but before any injections, Wilcoxon test; #P < 0.05, ##P < 0.01, ###P < 0.001, Mann–Whitney test. Data are expressed as mean ± SEM; n = 6 to 9 rats per group. ANOVA, analysis of variance; CCI, chronic constriction injury.
3.4. NFEPP produces analgesia selectively through peripheral opioid receptors in the abdominal pain model
Within 5 to 35 minutes after i.p. injection of 1% acetic acid, rats demonstrated abdominal constrictions (writhes) indicative of abdominal pain. Intravenous fentanyl (4-16 μg/kg) dose-dependently inhibited the writhes (Fig. 5A). NFEPP (4-16 μg/kg i.v.) also dose-dependently but only partially (by about 51%) decreased the number of writhes (Fig. 5A). Analgesic effects induced by i.v. fentanyl (12 μg/kg) were partially attenuated by i.p. NLXM (50 μg) (P < 0.001) because the effects were significantly different from control animals treated with acetic acid and vehicle (P < 0.001; Fig. 5B), implying the activation of both peripheral and central opioid receptors. By contrast, analgesic effects of i.v. NFEPP (12 μg/kg) were fully reversed by i.p. NLXM (50 μg) (P < 0.001) because there was no significant difference compared with control animals treated with acetic acid and vehicle (P > 0.05; Fig. 5C). This indicates that NFEPP produces analgesia through abdominal peripheral opioid receptors.
Figure 5.
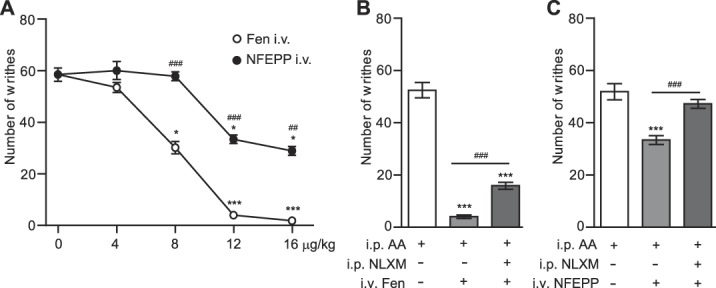
Contribution of peripheral opioid receptors to analgesic effects of intravenous (i.v.) fentanyl and NFEPP in the abdominal pain model. (A) Dose-dependent analgesic effects of i.v. fentanyl (Fen) and NFEPP. Agonists were injected immediately after 1% acetic acid (i.p.) and the total number of writhes was counted at 5 to 35 minutes thereafter. *P < 0.05, ***P < 0.01 vs control (0 µg/kg), Kruskal–Wallis 1-way ANOVA and Dunn test; ##P < 0.01, ###P < 0.001, NFEPP vs Fen, Mann–Whitney test. (B and C) Effects of NLXM (50 μg i.p.) on analgesia induced by Fen (B) and NFEPP (C) (both at 12 µg/kg i.v.). NLXM was injected i.p. immediately after acetic acid (AA) injection and right before i.v. injections of agonists, and the total number of writhes was counted at 5 to 35 minutes thereafter. ***P < 0.001 vs control groups receiving acetic acid and vehicle (white bars); ###P < 0.001, 1-way ANOVA and Bonferroni test. Data are expressed as mean ± SEM; n = 9 rats per group. ANOVA, analysis of variance; i.p.,intraperitoneal.
4. Discussion
Previous attempts to develop new analgesics without side effects have met various obstacles.40 In contrast to other strategies (eg, blockade of individual excitatory ion channels or receptors on sensory neurons),27 the activation of peripheral opioid receptors on DRG neurons presents advantages such as reduced tolerance development and simultaneous modulation of multiple ion currents.33 We took advantage of injury-specific MOR–ligand interactions at low pH and designed an opioid agonist (NFEPP) with low pKa and decreased receptor activation at normal pH, by combining quantum–chemical simulations with classic computational modeling.32 In complete Freund adjuvant–induced paw inflammation and in the Brennan model of postoperative pain, NFEPP produced analgesia of similar efficacy to fentanyl by selective activation of peripheral MOR in inflamed tissue, but did not act in healthy central or peripheral compartments. Therefore, the actions of NFEPP were devoid of typical opioid side effects such as reward, sedation, motor impairment, respiratory depression, and constipation.32
We now extended our studies to examine binding of NFEPP to MOR in native membranes of brain and DRG, and its analgesic efficacy in 2 additional models involving inflammation and pain transmission through DRG neurons.1,3,19,20,26,38 In line with our previous findings in human embryonic kidney 293 cells (Table S1, available online as supplemental digital content at http://links.lww.com/PAIN/A615, and Ref. 32), NFEPP binding to MOR at pH 7.4 was about one order of magnitude lower than that of conventional fentanyl, whereas the 2 ligands had similar affinities at pH 5.5, both in the brain and DRG. In the CCI model, pH values at the site of nerve injury were decreased in comparison with noninjured nerves for at least 2 weeks, in line with previously demonstrated inflammatory reactions in damaged nerves.1,19,21,23 Similar to our previous report,32 the largest pH difference measured between noninjured tissue (pH 7.22) and injured tissue (pH 6.98) reflects a 1.74-fold increase in proton concentration, which is apparently sufficient for improved binding and selective activation of peripheral opioid receptors by NFEPP. Furthermore, functional opioid receptors are upregulated both in sensory fibers and immune cells at the CCI site.8,17,30,36 This can account for the substantial analgesia induced by i.v. NFEPP in both mechanical and heat sensitivity. Our results (Fig. 4) demonstrate that the local injection of saline (vehicle) at the CCI site does not change the effects of i.v. NFEPP, whereas NLXM fully reverses those effects. Because the pH values of both solutions are below the pKa of NFEPP,32 the local injection of NLXM will not deprotonate NFEPP and, therefore, should not influence MOR binding and efficacy of NFEPP. This indicates that the reversal of the analgesic effects by local NLXM is indeed due to blocking of peripheral opioid receptors and not to deprotonation of NFEPP.
In the writhing model, the injection of 1% acetic acid provoked a large reduction of intra-abdominal pH, in line with the literature.28 The transient acidosis (15 minutes; this study) and inflammatory response26 as well as a lack of upregulation of opioid receptors on peripheral terminals of sensory neurons in the peritoneum18 may explain the moderate analgesic efficacy of NFEPP in this short-lasting (30 minutes) pain assay. Nevertheless, also in this model, NEFPP exerted its actions solely through peripheral opioid receptors, supported by the complete antagonism by i.p. NLXM.
By contrast, the analgesic effects of fentanyl were only partially reversed by NLXM in both models. In line with our previous studies, these data suggest that fentanyl acts at both central and peripheral opioid receptors (both at normal and low pH), whereas NFEPP selectively activates peripheral opioid receptors in injured tissues at low pH.32 This is consistent with the fact that the acid dissociation constant (pKa) of fentanyl (and other conventional opioid ligands) is above 7.4, whereas that of NFEPP is 6.8.32 Consequently, fentanyl is protonated and activates MOR at both normal (eg, in brain) and low pH, whereas NFEPP is only protonated at low pH.32 It is noteworthy that fentanyl and NFEPP exert similar analgesic effects in the CCI model; however (at least at 14 days), central sensitization should be present and opioid effects in the spinal cord or brain might contribute to analgesia. However, because the effects of NFEPP were abolished (and those of fentanyl were significantly reduced) by locally administered NLXM, it seems that peripheral opioid receptors mediate a large part of the overall analgesic effects, similar to clinical reports.15 This is consistent with the notion that continuing input from primary sensory neurons is essential for the maintenance of central sensitization, and that blocking those neurons (eg, by peripheral opioid receptor activation, local anesthetics, or capsaicin) can effectively reduce even chronic neuropathic pain (reviewed in Ref. 2,17,27).
In summary, compared with fentanyl, NFEPP showed markedly diminished MOR binding in native tissues at pH 7.4 and produced analgesia exclusively through peripheral opioid receptors in models of neuropathic and abdominal pain.
Conflict of interest statement
C. Stein is listed as inventor on patents US 14/239,461 and EP 2,801,046. The remaining authors have no conflicts of interest to declare.
This work was supported by Bundesministerium für Bildung und Forschung (VIP 0272/03V0364).
Supplementary Material
Acknowledgements
The authors thank N. Vogel for technical assistance.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A612, http://links.lww.com/PAIN/A613, http://links.lww.com/PAIN/A614, http://links.lww.com/PAIN/A615, and http://links.lww.com/PAIN/A618.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 2010;229:26–50. [DOI] [PubMed] [Google Scholar]
- [2].Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol 2013;74:630–6. [DOI] [PubMed] [Google Scholar]
- [3].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. PAIN 1988;33:87–107. [DOI] [PubMed] [Google Scholar]
- [4].Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- [6].Brown DR, Goldberg LI. The use of quaternary narcotic antagonists in opiate research. Neuropharmacology 1985;24:181–91. [DOI] [PubMed] [Google Scholar]
- [7].Califf RM, Woodcock J, Ostroff S. A proactive response to prescription opioid abuse. N Engl J Med 2016;374:1480–5. [DOI] [PubMed] [Google Scholar]
- [8].Celik MO, Labuz D, Henning K, Busch-Dienstfertig M, Gaveriaux-Ruff C, Kieffer BL, Zimmer A, Machelska H. Leukocyte opioid receptors mediate analgesia via Ca(2+)-regulated release of opioid peptides. Brain Behav Immun 2016;57:227–42. [DOI] [PubMed] [Google Scholar]
- [9].Dosen-Micovic L, Ivanovic M, Micovic V. Steric interactions and the activity of fentanyl analogs at the mu-opioid receptor. Bioorg Med Chem 2006;14:2887–95. [DOI] [PubMed] [Google Scholar]
- [10].Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, Maldonado R, Kieffer BL. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. PAIN 2011;152:1238–48. [DOI] [PubMed] [Google Scholar]
- [11].Gonzalez-Rodriguez S, Quadir MA, Gupta S, Walker KA, Zhang X, Spahn V, Labuz D, Rodriguez-Gaztelumendi A, Schmelz M, Joseph J, Parr MK, Machelska H, Haag R, Stein C. Polyglycerol-opioid conjugate produces analgesia devoid of side effects. Elife 2017;6:e27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grosser T, Ricciotti E, FitzGerald GA. The cardiovascular pharmacology of nonsteroidal anti-inflammatory drugs. Trends Pharmacol Sci 2017;38:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. PAIN 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- [14].Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol 2009;194:283–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jagla C, Martus P, Stein C. Peripheral opioid receptor blockade increases postoperative morphine demands–a randomized, double-blind, placebo-controlled trial. PAIN 2014;155:2056–62. [DOI] [PubMed] [Google Scholar]
- [16].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Labuz D, Machelska H. Stronger antinociceptive efficacy of opioids at the injured nerve trunk than at its peripheral terminals in neuropathic pain. J Pharmacol Exp Ther 2013;346:535–44. [DOI] [PubMed] [Google Scholar]
- [18].Labuz D, Mousa SA, Schäfer M, Stein C, Machelska H. Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res 2007;1160:30–8. [DOI] [PubMed] [Google Scholar]
- [19].Labuz D, Schmidt Y, Schreiter A, Rittner HL, Mousa SA, Machelska H. Immune cell-derived opioids protect against neuropathic pain in mice. J Clin Invest 2009;119:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Machelska H. Dual peripheral actions of immune cells in neuropathic pain. Arch Immunol Ther Exp (Warsz) 2011;59:11–24. [DOI] [PubMed] [Google Scholar]
- [21].Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004;129:767–77. [DOI] [PubMed] [Google Scholar]
- [22].Nockemann D, Rouault M, Labuz D, Hublitz P, McKnelly K, Reis FC, Stein C, Heppenstall PA. The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol Med 2013;5:1263–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pannell M, Labuz D, Celik MÖ, Keye J, Batra A, Siegmund B, Machelska H. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation 2016;13:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pogozheva ID, Przydzial MJ, Mosberg HI. Homology modeling of opioid receptor-ligand complexes using experimental constraints. AAPS J 2005;7:E434–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reddi BA. Why is saline so acidic (and does it really matter?). Int J Med Sci 2013;10:747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 2000;387:111–8. [DOI] [PubMed] [Google Scholar]
- [27].Richards N, McMahon SB. Targeting novel peripheral mediators for the treatment of chronic pain. Br J Anaesth 2013;111:46–51. [DOI] [PubMed] [Google Scholar]
- [28].Roos A, Boron WF. Regulation of intracellular pH in barnacle muscle. Kroc Found Ser 1981;15:205–19. [PubMed] [Google Scholar]
- [29].Roques BP, Fournie-Zaluski MC, Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discov 2012;11:292–310. [DOI] [PubMed] [Google Scholar]
- [30].Schmidt Y, Gaveriaux-Ruff C, Machelska H. mu-opioid receptor antibody reveals tissue-dependent specific staining and increased neuronal mu-receptor immunoreactivity at the injured nerve trunk in mice. PLoS One 2013;8:e79099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shaqura MA, Zöllner C, Mousa SA, Stein C, Schäfer M. Characterization of mu opioid receptor binding and G protein coupling in rat hypothalamus, spinal cord, and primary afferent neurons during inflammatory pain. J Pharmacol Exp Ther 2004;308:712–8. [DOI] [PubMed] [Google Scholar]
- [32].Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 2017;355:966–9. [DOI] [PubMed] [Google Scholar]
- [33].Stein C. Opioid receptors. Annu Rev Med 2016;67:433–51. [DOI] [PubMed] [Google Scholar]
- [34].Stein C. Targeting pain and inflammation by peripherally acting opioids. Front Pharmacol 2013;4:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013;339:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol 2003;53:366–75. [DOI] [PubMed] [Google Scholar]
- [37].Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag 2011;7:55–68. [DOI] [PubMed] [Google Scholar]
- [38].Vyklicky L. Techniques for the study of pain in animals. In: Advances in pain research and therapies. Bonica JJ, Liebeskind JC, Albe-Fessard DG, editors. New York: Raven Press, 1979. p. 727–745. [Google Scholar]
- [39].Weibel R, Reiss D, Karchewski L, Gardon O, Matifas A, Filliol D, Becker JA, Wood JN, Kieffer BL, Gaveriaux-Ruff C. Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice. PLoS One 2013;8:e74706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 2017;16:545–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zeng C, Gao SG, Cheng L, Luo W, Li YS, Tu M, Tian J, Xu M, Zhang FJ, Jiang W, Wei LC, Lei GH. Single-dose intra-articular morphine after arthroscopic knee surgery: a meta-analysis of randomized placebo-controlled studies. Arthroscopy 2013;29:1450–8.e2. [DOI] [PubMed] [Google Scholar]
- [42].Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M. Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol 2003;64:202–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.