Abstract
Objective
To determine whether IgG subclasses of B. burgdorferi antibodies differ from those of 3 Lyme disease (LD)-associated autoantibodies.
Methods
The IgG antibody subclasses were determined by ELISA in serum samples from 215 patients representative of each of the 3 stages of Lyme disease. Antibody and cytokine profiles were measured in matched serum and synovial fluid (SF) samples from Lyme arthritis patients. Synovial tissue from patients with antibiotic-refractory arthritis was examined for histologic features, IgG subclasses of plasma cells, and mRNA subclass expression.
Results
B. burgdorferi antibodies were primarily of the IgG1 and IgG3 subclasses, and the amounts increased as the infection progressed. In contrast, LD-associated autoantibodies were mainly of the IgG2 and IgG4 subclasses, and these responses were found primarily in patients with either antibiotic-refractory or antibiotic-responsive arthritis, particularly in SF. However, compared with the responsive group, the inflammatory milieu in SF in the refractory group was enriched for cytokines representative of innate, Th1, Th2, and Th17 responses. Synovial tissue in a subgroup of patients with refractory arthritis showed marked mRNA expression of IgG4 antibodies and large numbers of IgG4-staining plasma cells. IgG4 autoantibodies in SF to each of the 3 LD-associated autoantigens correlated with the magnitude of obliterative microvascular lesions and fibrosis in the tissue.
Conclusion
The subclasses of IgG antibodies to B. burgdorferi differ from those of LD-associated autoantibodies. Furthermore, the correlation of IgG4 autoantibodies with specific synovial pathology in the refractory group suggests a role for these autoantibodies, either protective or pathologic, in antibiotic-refractory Lyme arthritis.
Lyme disease, which is caused by the tick-transmitted spirochete, Borrelia burgdorferi, usually begins with an expanding skin lesion, erythema migrans (stage 1) 1. Weeks later, patients who do not receive antibiotic therapy may develop neurologic involvement or carditis (stage 2), and months later, untreated patients commonly have Lyme arthritis (stage 3). Patients with each manifestation of the illness usually respond to appropriate antibiotic therapy 2, but post-infectious syndromes may delay recovery 3. For example, a small percentage of Lyme arthritis patients have persistent synovitis for months or several years despite spirochetal killing with 2–3 months of oral and intravenous (IV) antibiotics, called post-infectious, antibiotic-refractory Lyme arthritis 4.
As the infection progresses, IgG antibody responses to B. burgdorferi expand gradually to an increasing array of spirochetal proteins 5, and these responses are important in the control of this large, extracellular pathogen 3,6. In addition, we have identified 4 autoantigens, endothelial cell growth factor (ECGF), matrix metalloproteinase-10 (MMP-10), apolipoprotein B-100 (apo B-100), and annexin A2, which are targets of T and B responses in Lyme disease, particularly in antibiotic-refractory Lyme arthritis 7–10. IgG autoantibodies to annexin A2 may be found in several rheumatic diseases 11, but the other 3 autoantibodies (called here LD-associated autoantibodies) are found primarily or exclusively in LD. For this study, we focused on the 3 Lyme disease-specific autoantibodies.
IgG antibodies are separated into 4 subclasses (IgG1-4) 12. IgG1 is the most common subclass and in healthy individuals, it accounts for about 60% of IgG antibodies in serum. IgG4 is the least abundant subclass, accounting for only 5% of serum IgG antibodies 13. IgG1 and IgG3 antibodies, which engage Fcγ receptors, fix complement, and promote opsonization, are generally activating antibodies 14,15, and IgG3 antibodies are especially potent inducers of antibody-dependent cell cytotoxicity 16. In contrast, IgG2 and IgG4 antibodies often have non-inflammatory effects 15,17. Previous investigators have reported that antibodies to B. burgdorferi were primarily of the IgG1 and IgG3 subclasses 18,19; the levels of IgG2 B. burgdorferi antibodies were generally low, and IgG4 antibodies were below detection level 19. However, the antibody subclasses of LD-associated autoantibodies were not yet known.
Herein, we characterized the IgG subclasses of antibodies to B. burgdorferi and 3 LD autoantigens in each of the 3 stages of Lyme disease. We found that B. burgdorferi antibodies were primarily of the IgG1 and IgG3 subclasses, as reported previously 18,19, whereas the autoantibodies were primarily of the IgG2 and IgG4 subclasses. Moreover, in post-infectious antibiotic-refractory Lyme arthritis, IgG4 autoantibodies to each autoantigen, measured in synovial fluid (SF), correlated directly with the magnitude of obliterative microvascular lesions and fibrosis in synovial tissue, suggesting a role for these autoantibodies in disease pathogenesis.
PATIENTS AND METHODS
Study Patients
Serum samples, collected from 1988 through 2016, were analyzed from 215 patients who had manifestations which were representative of each of the 3 stages of Lyme disease. SF was available for testing in 47 of the 137 patients with Lyme arthritis. All patients with Lyme disease met Centers for Disease Control and Prevention criteria 20. For comparison, serum samples were obtained from 16 healthy control subjects and 9 blood bank donors. The Human Investigation Committees at Tufts Medical Center, Boston, MA (1988 and 2002) and Massachusetts General Hospital (MGH) (2002–2016) approved the study, titled “Immunity in Lyme Disease”, and all patients (including parents of patients ages 12–18) provided written informed consent.
ELISA for antibodies to B. burgdorferi or Lyme disease autoantigens
All antibody responses were determined by ELISA. B. burgdorferi sonicate (strain G39/40) (5 μg/ml), or recombinant human MMP-10 (R&D Systems), human apoB-100 (Millipore), or ECGF (R&D Systems) (in each instance, 2.5 μg/ml) diluted in carbonate coating buffer was added to Immulon 1B ELISA plates (Thermo Scientific), and incubated overnight at 4°C. After washing with PBS with 0.05% Tween 20 (PBST) between each step, the plates were incubated with a 3% bovine serum albumin (BSA; Sigma Aldrich) in PBS blocking buffer, followed by patient serum or synovial fluid samples (1:100), in each instance, for 1 hour on an orbital shaker (200 rpm). The secondary antibodies were horseradish peroxidase (HRP)-conjugated mouse anti-human IgG1, 2, 3, or 4 Fc antibodies (Life Sciences), which were incubated for 2 hours on a shaker, followed by TMB substrate (BD Biosciences). For interplate standardization, one positive patient control and two negative controls were included on each plate. A positive antibody response was defined as >3 SD above the mean value in healthy control subjects.
Cytokine analyses
The levels of 14 cytokines and chemokines associated with innate (IL-6, IL-8, IL-10, TNF) or adaptive Th1 (IFNγ, CXCL10, IL-12p40), Th2 (IL-4, IL-5, IL-13), or TH17 (IL-17A, IL-17F, IL-23, IL-27) immune responses were assessed in serum or SF samples from Lyme arthritis patients using bead-based Luminex multiplex assays (EMD-Millipore). Because sample volumes were limited, the levels of all mediators were determined in one complete experiment.
Immunohistology of synovial tissue
Histologic and immunohistologic analyses were previously performed with synovial tissue from patients with antibiotic-refractory Lyme arthritis 21. For each patient, 13 histologic findings were each ranked from 1 to 13, with 13 being the highest ranked patient 21. This ranking was carried out by two independent pathologists prior to the antibody determinations carried out here. For this study, the absorbance value for each IgG subclass of antibodies to B. burgdorferi, MMP-10, ECGF, and apoB-100 were also ranked from 1 to 13, with 13 being the highest absorbance value. Correlations were then performed between each histologic parameter and autoantibody specificity.
In addition, for this study, remaining synovial tissue, which was available in 10 of the 13 patients, was stained for plasma cells (VS38c, Dako) and for the IgG subclasses of these cells. After blocking, sections were incubated with anti-human IgG1, 2, 3, or 4 rabbit monoclonal antibody (RevMAb Biosciences) for 30 minutes. Optimal concentrations were used according to the company’s specifications. After washing, sections were incubated with biotinylated anti-rabbit secondary antibody, peroxidase-strepavidin, and then diaminobenzidine (DAB) chromogen substrate (Dako). Slides were counterstained with Mayer’s hematoxylin and mounted with permount. Microscopic images were obtained with a Zeiss widefield microscope.
RNA expression of IgG subclasses in synovial tissue
mRNA expression of the IgG subclasses was determined in synovia from 14 patients with antibiotic-refractory Lyme arthritis and from 5 patients each with rheumatoid arthritis or osteoarthritis who underwent synovectomies or joint replacement procedures. Tissue was placed immediately in RNA-later and stored at −20˚C. RNA was recovered from ~100 mg of synovial tissue using the miRNeasy kit (Qiagen), and quality was determined using a Bioanalyzer (Agilent). Ribosomal RNA was depleted using RiboZero (New England Biolabs), and paired-end libraries were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs). Libraries were sequenced to a depth of 25–35 million paired-end, 50 base-pair filtered reads, resulting in ~40% transcriptome coverage (HiSeq PE50 Reagent Kit, Illumina). Library preparation, sequencing, and bioinformatics were performed by the MGH NextGen Sequencing and Bioinformatics Core Facilities.
Statistical analysis
IgG antibodies of each subclass were compared among patients with each of the 3 stages of Lyme disease and between patients with responsive and refractory Lyme arthritis using unpaired t test with Welch’s correction. Subclasses in paired serum and synovial fluid samples were compared using paired t test with Welch’s correction. Correlations were analyzed using Spearman correlation test. All analyses were performed on GraphPad Prism 6. For analysis of high-throughput RNA sequencing, adjusted P values were determined by calculating differential expression between groups, normalizing expression to copies per million base-pair reads.
RESULTS
IgG subclass analysis of each of the 3 stages of Lyme disease
The subclasses of IgG antibodies to B. burgdorferi and 3 LD-associated autoantibodies were determined by ELISA in serum samples from 215 patients with Lyme disease. These included patients with erythema migrans (stage 1), neuroborreliosis or carditis (stage 2), or Lyme arthritis (stage 3), the most common late manifestation of the disease in the United States.
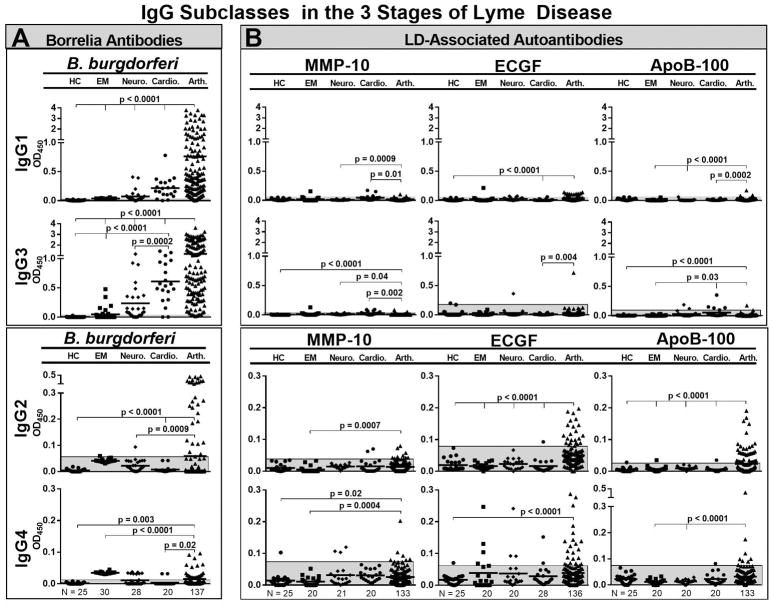
With each disease manifestation, B. burgdorferi antibodies were primarily of the IgG1 and IgG3 subclasses, the subtypes that fix complement and opsonize pathogens. The amounts of these antibodies were low in patients with erythema migrans, intermediate in those with neuroborreliosis or carditis, and highest in those with Lyme arthritis (Figure 1A). In contrast, the levels of IgG2 and IgG4 antibodies to B. burgdorferi were either low or negative in patients with early manifestations of the disease, but were significantly higher in those with Lyme arthritis.
Figure 1.
IgG subclasses of B. burgdorferi antibodies and 3 Lyme disease (LD)-associated autoantibodies in serum in patients with early or late manifestations of Lyme disease and in healthy control subjects. The numbers of patients in each group were as follows: erythema migrans (N=20), neuroborreliosis (N=20), carditis (N=20), Lyme arthritis (N = 136), and healthy subjects (N=25). For each IgG subclass, the antibody responses to B. burgdorferi (A) or Lyme disease autoantigens (B) were determined by ELISA. The shaded grey areas correspond to 3 SD above the mean value of healthy control subjects. The antibody values between groups were compared using an unpaired t-test with Welch’s correction. Only significant differences are shown. Matrix metalloproteinase-10 = MMP-10, endothelial cell growth factor = ECGF, apolipoprotein B-100 = apoB-100.
The subclasses of IgG antibodies to the 3 Lyme disease autoantigens were different than those of B. burgdorferi antibodies. The autoantibody responses were primarily of the IgG2 and IgG4 subclasses, though a few patients had low levels of IgG1 and IgG3 autoantibodies (Figure 1B). Small numbers of patients with erythema migrans (stage 1) or neuroborreliosis or carditis (stage 2) had autoantibody responses, whereas larger numbers of Lyme arthritis patients (stage 3) had these responses. The only exception was patients with carditis who had significantly higher levels of IgG3 apoB-100 autoantibodies than those with EM or arthritis. Altogether, anti-B. burgdorferi antibodies were primarily of the IgG1 and IgG3 subclasses throughout the illness, whereas autoantibodies were more often IgG2 and IgG4 subclasses, and these responses were found primarily in Lyme arthritis patients.
Antibodies in responsive or refractory Lyme arthritis
We next determined whether the antibody responses to B. burgdorferi or the 3 autoantigens differed in serum and synovial fluid in patients with responsive or refractory arthritis. Because joint swelling in patients with antibiotic-responsive arthritis resolves with antibiotic treatment, samples in the responsive group were obtained prior to or soon after starting antibiotic therapy when patients were still infected. In contrast, patients in the refractory group were usually referred after antibiotic therapy when few, if any spirochetes remained. Of the 137 patients with Lyme arthritis, 117 (85%) were seen in the most recent decade (2007–2016), and only 12 patients were seen in each of the 2 previous decades. There was no correlation between antibody levels and the decade in which the patient had arthritis.
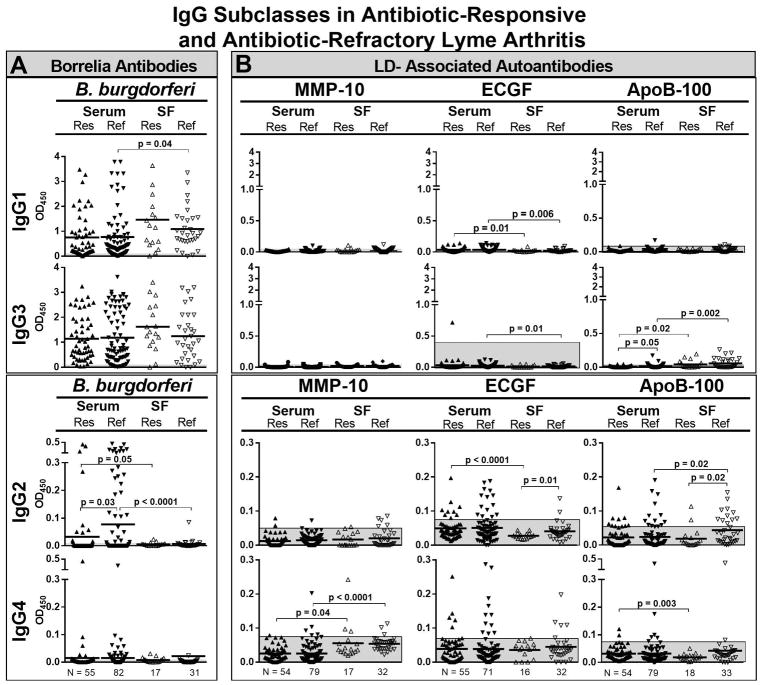
The levels of IgG1 and IgG3 B. burgdorferi antibodies tended to be higher in patients with responsive Lyme arthritis than in those with refractory arthritis, and the levels were higher in SF than in serum (Figure 2A). Conversely, the levels of IgG2 antibodies to B. burgdorferi were significantly higher in patients with refractory arthritis, but these values were higher in serum than in SF. A few patients in the refractory and responsive groups had IgG4 antibodies to B. burgdorferi in serum, but not in SF.
Figure 2.
Total IgG and IgG subclasses of B. burgdorferi antibodies and 3 Lyme disease (LD)-associated autoantibodies in serum and synovial fluid (SF) of patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. The numbers of patients in each group were as follows: responsive arthritis in serum (N= 55) and SF (N= 17), and in refractory arthritis in serum (N=82) and SF (N= 31). For each assay, the antibody responses to B. burgdorferi (A) or Lyme disease autoantigens (B) were determined by ELISA. The shaded grey areas correspond to 3 SD above the mean value of healthy control subjects. The antibody values between groups were compared using an unpaired t-test with Welch’s correction. Only significant differences are shown. Matrix metalloproteinase-10 = MMP-10, endothelial cell growth factor = ECGF, apolipoprotein B-100 = apoB-100.
In contrast with B. burgdorferi antibodies, IgG2 and IgG4 were the prominent subclasses of each of the 3 LD-associated autoantibodies. IgG4 antibodies to MMP-10 were significantly greater in SF than in serum in patients with refractory or responsive arthritis (Figure 2B). For ECGF, the levels of each IgG subclass were greater in serum, except for IgG4 antibodies which were similar in serum and SF in both the responsive and refractory groups. For apoB-100, the levels of each IgG subclass were greater in SF in patients with refractory arthritis than in those with responsive arthritis, particularly IgG2 and IgG4 antibodies, and the values in SF tended to be higher than those in serum, especially in the refractory group.
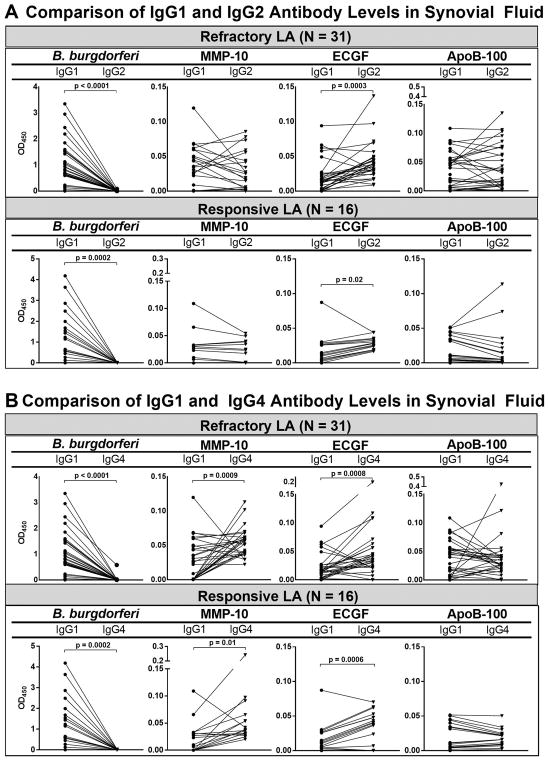
To emphasize the differences in antibody responses to B. burgdorferi and the Lyme disease autoantigens in refractory and responsive arthritis, we plotted IgG1 versus IgG4 absorbance values and IgG1 versus IgG2 values in SF in the 31 patients with refractory arthritis and in the 16 patients with responsive arthritis (Figure 3). With B. burgdorferi, the amount of IgG1 antibodies was significantly greater than that of IgG4 antibodies (P<0.0001), and the values in the responsive and refractory groups were similar (Figure 3A). In contrast, the amounts of IgG4 antibodies to each of the 3 autoantigens were similar or greater than IgG1 autoantibodies, and these differences were statistically significant for MMP-10 (P=0.009) and ECGF (P=0.003). Although the mean levels of IgG1 or IgG4 autoantibodies in patients with refractory or responsive arthritis were not significantly different, several patients in the refractory group had particularly high levels of IgG4 ECGF or apoB-100 autoantibodies.
Figure 3.
IgG1 and IgG4 B. burgdorferi antibodies and 3 Lyme disease autoantibodies in synovial fluid from 31 patients with antibiotic-refractory Lyme arthritis (LA). The values in the same patient are connected by lines. The differences between IgG1 and IgG4 antibody absorbance values were determined by paired t-test with Welch’s correction. Only significant differences are shown. Matrix metalloproteinase-10 = MMP-10, endothelial cell growth factor = ECGF, apolipoprotein B-100 = apoB-100.
As with IgG1 and IgG4 values, the levels of IgG1 B. burgdorferi antibodies were significantly greater than the levels of IgG2 antibodies to the organism in both the refractory and responsive groups (Figures 3B). However, for ECGF, the levels of IgG2 autoantibodies in SF were significantly greater than IgG1 levels in both the responsive and refractory groups, and for MMP-10 and apoB-100, the levels of IgG1 and IgG2 autoantibodies in SF were similar in the responsive and refractory groups. Thus, although the differences were greater for the IgG1 and IgG4 autoantibody comparisons, there was still reversal of the normal ratio of the IgG1 and IgG2 antibodies.
When IgG4 autoantibody values in SF were correlated with those of the other subclasses, only limited correlations were observed. For ECGF, IgG4 levels correlated with IgG1 values (r=0.4, P=0.01) and with IgG2 values (r=0.7, P<0.0001) (data not shown). However, for MMP-10 and apoB-100, there were no correlations between IgG4 autoantibodies and those of the other subclasses. This suggests that IgG subclass responses to specific autoantigens are variable and are not simply a reflection of total IgG autoantibody levels.
Cytokine/chemokine levels in serum and synovial fluid
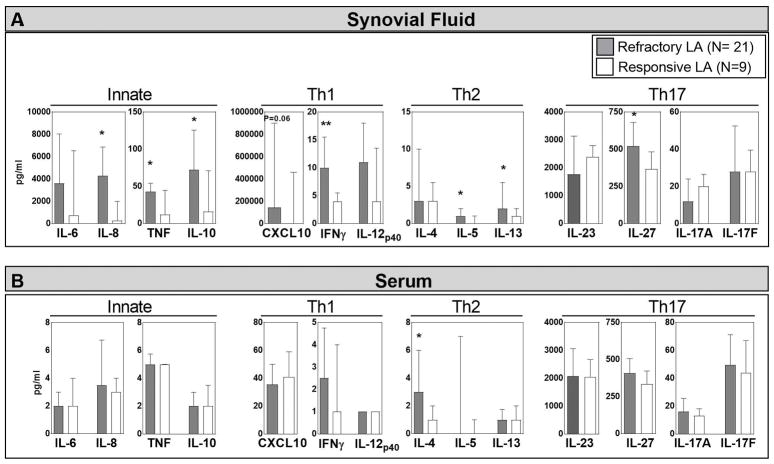
Because antibody subclasses may be determined by the cytokine environment, the levels of 14 cytokines/chemokines representative of innate, Th1, Th2 and Th17 responses were measured in serum and SF from 21 patients with refractory arthritis and 9 patients with responsive arthritis in whom enough sample remained. In SF, the levels of most mediators were greater in the refractory group than in the responsive group, and these differences reached statistical significance for a number of innate, Th1, Th2, and Th17 mediators (Figure 4A). In contrast, the levels of most mediators in serum were much lower than in SF, and the values were similar in the responsive and refractory groups (Figure 4B). The only exception was significantly higher serum levels of IL-4 in the refractory group. Thus, SF in patients with antibiotic-refractory arthritis was highly polarized toward Th1 and innate cytokine responses, but most of these patients also had moderate levels of Th17 mediators and low levels of Th2 cytokines, which could presumably still support the development of IgG4 antibody responses.
Figure 4.
Cytokines/chemokines representative of innate, Th1, Th2, and Th17 immune responses in synovial fluid (panel A) or serum (panel B) in 21 patients with antibiotic-refractory Lyme arthritis (LA) and in 9 patients with antibiotic-responsive LA. The median value is shown with the bar and the third interquartile value is shown with the I-bar. Stars indicates significant differences between the refractory and responsive groups, *P=0.05,**P=0.01.
Correlations of SF autoantibodies with histologic findings in synovial tissue
In 13 patients with refractory Lyme arthritis in whom synovectomies were performed, synovial tissue was available for correlation of antibody levels with synovial pathology. In these patients, the surgical procedure was usually performed >1 year after oral and IV antibiotic treatment for B. burgdorferi infection. Synovial tissue was not available in the responsive group because their arthritis resolves with antibiotic therapy, and therefore, these patients do not undergo synovectomies.
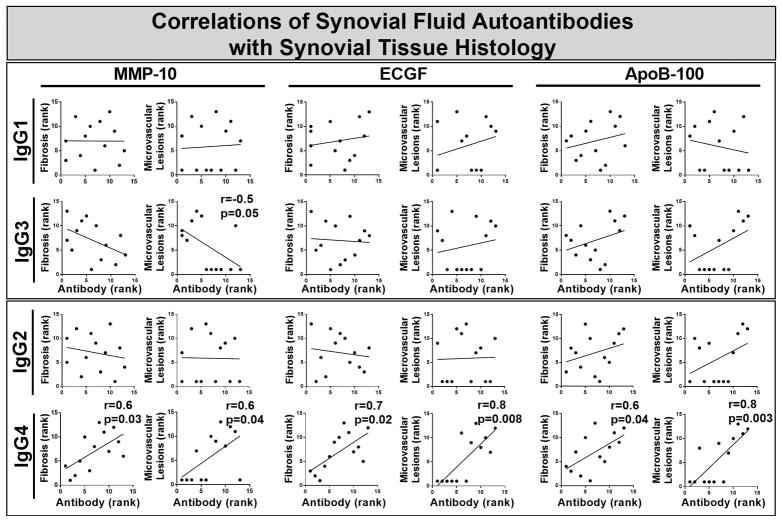
When the levels of each of the 3 LD-specific autoantibodies in SF were correlated with the rankings for each histologic finding in synovial tissue, IgG4 antibody levels to each of the 3 autoantigens correlated directly with the magnitude of fibrosis and obliterative microvascular lesions in the tissue (Figure 5). IgG4 antibody levels did not correlate with 11 other histologic findings, including lining layer thickness, global cellular infiltration, lymphoid aggregates, sublining layer vascularity, and the numbers of plasma cells, B cells, macrophages, T cells, endothelial cells, myeloid dendritic cells, or fibroblasts. Additionally, autoantibody levels of the other IgG subclasses did not correlate directly with any histologic finding. Moreover, each subclass of B. burgdorferi antibodies did not correlate with any histologic finding (data not shown). Finally, there was no correlation between serum autoantibody responses and synovial histology. Thus, the only significant correlations were between IgG4 autoantibody levels to MMP-10, ECGF, and apoB-100 in SF and the magnitude of fibrosis and obliterative microvascular lesions in synovial tissue.
Figure 5.
Correlations of 3 Lyme disease autoantibody absorbance values in serum with histologic findings in synovial tissue in 13 patients with antibiotic-refractory Lyme arthritis (LA) in whom synovectomies were performed. (Synovial fluid was not available in all patients.) The autoantibody absorbance values and histologic findings were each ranked by independent observers from 1 to 13, with 13 being the highest rank. The ranks of absorbance values and histologic findings were correlated using Spearman’s correlation test. Significant correlations are shown with bold type. IgG4 antibodies to each of the 3 autoantigens correlated directly with fibrosis staining and obliterative microvascular lesions, whereas IgG3 antibodies to obliterative microvascular lesions correlated inversely. Matrix metalloproteinase-10 = MMP-10, endothelial cell growth factor = ECGF, apolipoprotein B-100 = apoB-100.
RNA expression and histologic analysis of IgG subclasses in synovial tissue
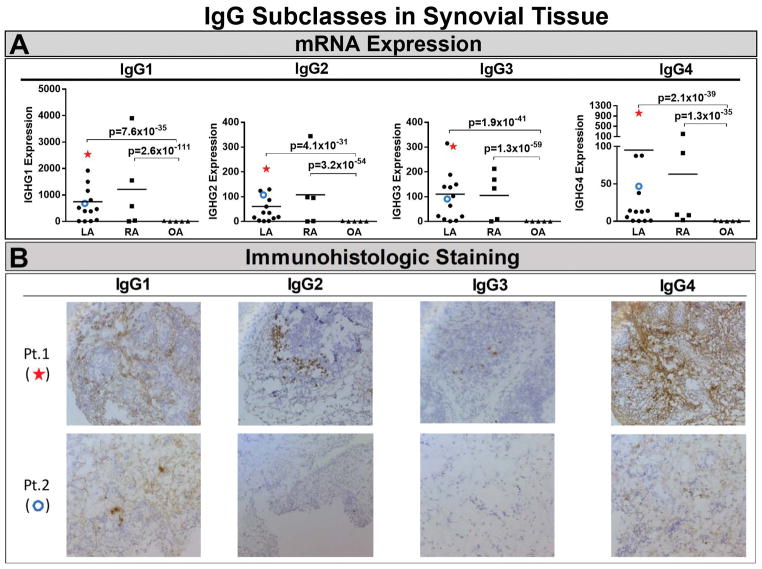
mRNA expression of IgG antibody subclasses was determined in synovial tissue from 14 patients with refractory Lyme arthritis and for comparison, from 5 patients each with rheumatoid arthritis or osteoarthritis. Rather than responses to specific antigens, this analysis measured the total expression of all IgG heavy chains, which determines the antibody subclass. Of the 14 patients, 10 had high expression of IgG1 heavy chains, with lower expression of IgG2 and IgG3 heavy chains (Figure 6A). Although expression of IgG4 heavy chains was low in most patients, several patients had especially high IgG4 expression. mRNA expression of each subclass was similar in patients with Lyme arthritis or rheumatoid arthritis, the prototypic form of chronic inflammatory arthritis, whereas osteoarthritis patients, a minimally inflammatory form of arthritis, had very low expression of each IgG subclass.
Figure 6.
RNA expression of IgG subclasses in synovial tissue and immunohistologic staining of the tissue for IgG subclasses in patients with antibiotic-refractory Lyme arthritis (LA). RNA expression of each subclass was determined in 14 patients with antibiotic-refractory LA, and for comparison in 5 patients each with rheumatoid arthritis (RA) or osteoarthritis (OA) (panel A). Immunoglobulin heavy chains for each IgG subclass were determined by high-throughput RNA sequencing (RNA-Seq). In panel B, immunohistochemical staining of synovial tissue for IgG subclasses is shown from 2 representative patients with antibiotic-refractory Lyme arthritis Patient 1 had high RNA expression of each IgG subclass (shown with a star in panel A), particularly IgG4, and staining of plasma cells for each IgG subclass, especially IgG4 (panel B). Patient 2 had low RNA expression of each IgG subclass (shown with an open circle in panel A), and staining of small numbers of plasma cells, particularly IgG4 antibodies (panel B). Brown indicates specific staining of cells for IgG subclasses and purple is the counter stain (hematoxylin). Images are 20x magnification.
Histologic staining of synovial tissue for antibodies of each subclass was performed in 10 patients with antibiotic-refractory Lyme arthritis. Consistent with the mRNA expression data, there was a range of plasma cell abundance, but plasma cell staining was found in the same areas as IgG subclass staining (data not shown). In Figure 6B, immunohistologic staining for all 4 IgG subclasses is shown for two patients, one (patient 1) with especially large numbers of IgG4-staining cells and the other (patient 2) with small numbers of IgG1 and IgG4-staining cells.
DISCUSSION
In this study, we found that the IgG antibody subclasses to B. burgdorferi were distinct from those of 3 LD-associated autoantibodies. B. burgdorferi antibodies were primarily of the IgG1 and IgG3 subclasses, which are antibodies important for the opsonization of pathogens 13. In contrast, the LD autoantibodies were mainly of the IgG2 and IgG4 subclasses, and these responses were found primarily in patients with Lyme arthritis, especially in SF, implying that some of these antibodies may be produced locally in synovial tissue. In support of this idea, a subgroup of patients with refractory arthritis had marked mRNA expression of IgG4 antibodies and large numbers of IgG4-staining plasma cells in synovial tissue. Because Lyme arthritis patients usually had undetectable levels of IgG4 antibodies to B. burgdorferi in SF, it seems likely that IgG4-staining plasma cells were producing primarily autoantibodies. It was not possible to compare these findings with those in patients with antibiotic-responsive arthritis, since they do not undergo synovectomies.
The only exception to these generalizations were the significantly higher levels of IgG3 apoB-100 autoantibodies in the serum of patients with Lyme carditis compared with those in patients with other manifestations of Lyme disease. Several factors may play a role. First, since B. burgdorferi acquires essential membrane components, such as cholesterol, from its host 22, immune processing of the organism early in the disease may lead to cross-reactive IgG3 autoantibody responses to apoB-100. But why is this finding prominent only in patients with Lyme carditis? In murine Lyme carditis, the heart muscle has a macrophage predominance unlike that in affected joints 23. Based on experience with atherosclerosis 24, myeloperoxidases from macrophages, and to a lesser degree from neutrophils, oxidize low-density lipoproteins (LDL), of which apoB-100 is a part, and oxidized LDL may contribute to apoB-100 antigenicity.
We have previously reported total IgG antibody responses to B. burgdorferi 25 and the 3 known LD autoantigens 7–9 in patients with each of the 3 stages of Lyme disease, as was done here with IgG subclasses. Both total and subclass IgG antibody responses to B. burgdorferi, showed gradually increasing antibody levels over the course of the disease. In addition, with both total and subclass determinations, the most prominent autoantibody responses were in SF in patients with Lyme arthritis. In previous studies, autoantibody-positive patients with erythema migrans usually had only B cell responses to a given autoantigen, without detectable T cell responses. In contrast, patients with Lyme arthritis, particularly those with refractory arthritis, often had both T and B cell responses to the antigen 7–9, which is a further indication of maturation of the autoimmune response in late disease.
In patients with antibiotic-refractory Lyme arthritis, we previously showed that total IgG autoantibodies to the 3 Lyme disease autoantigens correlated with obliterative microvascular lesions, fibrosis, or fibroblast proliferation in synovial tissue 8,9,21. We found here that it was specifically the IgG4 subclass of these autoantibodies that correlated directly with the magnitude of obliterative microvascular lesions and the degree of fibrosis. Autoantibodies of the other subclasses did not have this association. Obliterative vascular lesions appear to be a unique feature of the synovial tissue in Lyme arthritis and are not found in other forms of chronic inflammatory arthritis, including rheumatoid arthritis 21.
The overall profile of IgG antibody subclass responses in antibiotic-refractory Lyme arthritis has similarities with that in rheumatoid arthritis. The finding of increased levels of IgG antibodies in some rheumatoid arthritis patients was first noted years ago 26, but interest in the role of IgG4 autoantibodies in rheumatic diseases has increased recently27–29. In RA, IgG1 antibodies are most prominent, as might be expected, but IgG4 antibodies are next most prominent 27,30, which is surprising given that they are normally the least abundant subclass 13. As was seen here with synovial tissue in Lyme arthritis, antibodies of each IgG subclass were found in RA synovial tissue, and in some patients, IgG4 antibodies were particularly prominent 28. The subgroup of RA patients with elevated levels of serum IgG4 antibodies had higher disease activity, higher levels of rheumatoid factor and anti-citrullinated protein autoantibodies (ACPA), and a poor response to disease modifying anti-rheumatic drugs (DMARDs) 28, suggesting that serum IgG4 antibodies define a specific clinical phenotype with more severe disease. However, the specificities of these autoantibodies are different in RA than in Lyme arthritis. In RA, IgG4 autoantibodies to rheumatoid factor, anti-citrullinated protein antibodies (ACPA), or anti-carbamylated protein antibodies have been noted in almost half of patients 26,29,30. In contrast, these RA-associated autoantibodies are not features of the autoantibody response in Lyme arthritis, and autoantibodies to the 3 Lyme disease autoantigens studied here are found rarely, if at all, in RA 7–9.
The recently described “IgG4-related disease” has further increased interest in the role of this IgG subclass in rheumatic diseases. The histologic hallmarks of IgG4-related disease include lymphoplasmacytic infiltrates, storioform (“woven mat”) fibrosis, and predominance of IgG4-expressing plasma cells in target tissues 31–33. This description is broadly reminiscent of findings in synovial tissue in certain patients with refractory Lyme arthritis. In IgG4-related disease, CD4+SLAMF7+ cytotoxic lymphocytes (CTL) are thought to be the principal drivers of the disease 34. In contrast, the role of IgG4 antibodies is less clear. IgG4 antibodies are poor binders to Fc receptors and complement and they are not usually able to crosslink antigen or form immune complexes due to Fab-arm exchange. Therefore, for the most part, they are thought to blunt the inflammatory response resulting from chronic antigenic exposure by competing with other IgG subclasses to bind antigen at sites of inflammation 35.
On the other hand, in a murine model of IgG4-related disease, passive transfer of either patients’ IgG4 or IgG1 antibodies to neonatal mice resulted in pancreatic and salivary gland injuries reminiscent of those in human IgG4-related disease 36. Additionally, in pemphigus, IgG4 autoantibodies to desmoglein have a direct role in disruption of the epithelial cell surface, leading to the blistering lesions of the disease 37. Moreover, in lymphatic filariasis, IgG4 antibodies from asymptomatic individuals suppressed granulocytes, whereas IgG4 antibodies from symptomatic patients did not 38. Thus, under certain circumstances, the usual anti-inflammatory effects of IgG4 antibodies may be altered resulting in loss of their usual suppressive function, or these antibodies may develop a direct pathogenic role.
In antibiotic-refractory Lyme arthritis, the marked pro-inflammatory cytokine milieu in joints, which is enriched for innate, Th1, Th2, and Th17 mediators, may alter the function of IgG4 autoantibodies. As shown previously and again here, the levels of pro-inflammatory cytokines, such as IL-6, TNF-α and IFN-γ, are greater in the SF of patients with antibiotic-refractory arthritis 39,40, and these cytokines are present in their synovial tissue 41. In addition, we previously found that total IgG ECGF autoantibody levels in SF correlated strongly with SF IL-17 and IL-23 values in patients with refractory arthritis, whereas in patients with responsive arthritis, ECGF antibody levels correlated with IL-10 values 42. Similarly, in the current study, the subclasses of ECGF autoantibodies (IgG1, IgG2 and IgG4) in SF correlated strongly with Th17 cytokine mediators in the refractory group, but not in the responsive group. Moreover, the levels of several Th2 mediators, although low, may still be adequate to support IgG4 antibody production or effector functions. To gain further insights as to whether IgG4 autoantibodies may have a protective or pathologic role in refractory LA, it will be important to compare the affinity and function of LD-associated IgG4 autoantibodies in patients with responsive or refractory arthritis 15.
In summary, an awareness of IgG4 autoantibodies is increasing in rheumatic diseases. In Lyme disease, the IgG subclasses of LD-associated autoantibodies were distinct from those of B. burgdorferi antibodies. Moreover, LD-associated IgG4 autoantibodies in patients with refractory arthritis correlated with specific synovial pathology, suggesting that these autoantibodies may have a functional role in this post-infectious complication of Lyme disease. Increased levels of IgG4 antibodies are not found in all patients with Lyme arthritis or rheumatoid arthritis, and thus, they are not an invariant component of disease pathogenesis. However, they may have an additive role in inflammatory processes resulting in more severe disease phenotypes.
Acknowledgments
The authors thank Fei Ji (MGH) and Ruslan Sadreyev (MGH) for RNA sequencing and for bioinformatics analysis; John Aversa (Yale University) and Dennis Burke (MGH) for help in obtaining synovial tissue from patients with Lyme arthritis, rheumatoid arthritis, or osteoarthritis; and the many patients and control subjects who participated in this study.
Supported by NIH (National Institute of Allergy and Infectious Diseases [NIAID] grant R01-AI-101175, the English, Bonter, Mitchell Foundation, the Eshe Fund, and Massachusetts General Hospital (Lyme Disease and Arthritis Fund). Dr. Lochhead’s work was supported by National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) grant T32-AR-007258, NIAID grant F32-AI-125764, and by Massachusetts General Hospital (post-doctoral fellowship from the Executive Committee on Research). Dr. Strle was supported by NIAMS grant K01-AR-062098 from the NIH.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Ms. Sulka and Dr. Steere had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sulka, Strle, Crowley, Anthony, Steere
Acquisition of data. Sulka, Strle, Crowley, Lochhead
Analysis and interpretation of data. Sulka, Strle, Crowley, Lochhead, Anthony, Steere
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steere AC, Angelis SM. Therapy for Lyme arthritis; strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–85. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis. 2008;47:188–95. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drouin EE, Seward RJ, Strle K, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013;65:186–96. doi: 10.1002/art.37732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley JT, Strle K, Drouin EE, et al. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J Autoimmun. 2016;69:24–37. doi: 10.1016/j.jaut.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley JTDE, Pianta A, Strle K, Wang Q, McHugh G, Costello C, Steere AC. A highly expressed human protein, apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with Lyme disease. J Infect Dis. 2015 doi: 10.1093/infdis/jiv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pianta A, Drouin EE, Crowley JT, et al. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin Immunol. 2015;160:336–41. doi: 10.1016/j.clim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salle V, Maziere JC, Smail A, et al. Anti-annexin II antibodies in systemic autoimmune diseases and antiphospholipid syndrome. J Clin Immunol. 2008;28:291–7. doi: 10.1007/s10875-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 12.Terry WD, Fahey JL. Subclasses of human gamma-2-globulin based on differences in the heavy polypeptide chains. Science. 1964;146:400–1. doi: 10.1126/science.146.3642.400. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimmerjahn F, Gordan S, Lux A. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015;36:325–36. doi: 10.1016/j.it.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 18.Hechemy KE, Harris HL, Duerr MJ, Benach JL, Reimer CB. Immunoglobulin G subclasses specific to Borrelia burgdorferi in patients with Lyme disease. Annals NY Acad Sci. 1988;539:162–9. doi: 10.1111/j.1749-6632.1988.tb31849.x. [DOI] [PubMed] [Google Scholar]
- 19.Widhe M, Ekerfelt C, Forsberg P, Bergstrom S, Ernerudh J. IgG subclasses in Lyme borreliosis: a study of specific IgG subclass distribution in an interferon-gamma-predominated disease. Scand J Immunol. 1998;47:575–81. [PubMed] [Google Scholar]
- 20.CDC. Case definitions for public health surveillance. Morb Mort Wkly Reports. 1990;39(RR-13):1–43. [PubMed] [Google Scholar]
- 21.Londono D, Cadavid D, Drouin EE, et al. Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory lyme arthritis. Arthritis Rheumatol. 2014;66:2124–33. doi: 10.1002/art.38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfine H. Bacterial membranes and lipid packing theory. J Lipid Res. 1984;25:1501–7. [PubMed] [Google Scholar]
- 23.Ruderman EM, Kerr JS, Telford SR, 3rd, Spielman A, Glimcher LH, Gravallese EM. Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J Infect Dis. 1995;171:362–70. doi: 10.1093/infdis/171.2.362. [DOI] [PubMed] [Google Scholar]
- 24.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–44. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steere AC, McHugh G, Suarez C, Hoitt J, Damle N, Sikand VJ. Prospective study of coinfection in patients with erythema migrans. Clin Infect Dis. 2003;36:1078–81. doi: 10.1086/368187. [DOI] [PubMed] [Google Scholar]
- 26.Cohen PL, Cheek RL, Hadler JA, Yount WJ, Eisenberg RA. The subclass distribution of human IgG rheumatoid factor. J Immunol. 1987;139:1466–71. [PubMed] [Google Scholar]
- 27.Lin G, Li J. Elevation of serum IgG subclass concentration in patients with rheumatoid arthritis. Rheumatol Int. 2010;30:837–40. doi: 10.1007/s00296-009-1330-8. [DOI] [PubMed] [Google Scholar]
- 28.Chen LF, Mo YQ, Ma JD, Luo L, Zheng DH, Dai L. Elevated serum IgG4 defines specific clinical phenotype of rheumatoid arthritis. Mediators Inflamm. 2014;2014:635293. doi: 10.1155/2014/635293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Delft MAM, Verheul MK, Burgers LE, et al. The isotype and IgG subclass distribution of anti-carbamylated protein antibodies in rheumatoid arthritis patients. Arthritis Res Ther. 2017;19:190. doi: 10.1186/s13075-017-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapuy-Regaud S, Nogueira L, Clavel C, Sebbag M, Vincent C, Serre G. IgG subclass distribution of the rheumatoid arthritis-specific autoantibodies to citrullinated fibrin. Clin Exp Immunol. 2005;139:542–50. doi: 10.1111/j.1365-2249.2004.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annual Rev Pathol. 2014;9:315–47. doi: 10.1146/annurev-pathol-012513-104708. [DOI] [PubMed] [Google Scholar]
- 33.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–71. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 34.Mattoo H, Mahajan VS, Maehara T, et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016;138:825–38. doi: 10.1016/j.jaci.2015.12.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perugino CA, Mattoo H, Mahajan VS, et al. Emerging treatment models in rheumatology: IgG4-related disease: insights into human immunology and targeted therapies. Arthritis Rheumatol. 2017;69:1722–32. doi: 10.1002/art.40168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiokawa M, Kodama Y, Kuriyama K, et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016;65:1322–32. doi: 10.1136/gutjnl-2015-310336. [DOI] [PubMed] [Google Scholar]
- 37.Rock B, Martins CR, Theofilopoulos AN, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 38.Prodjinotho UF, von Horn C, Debrah AY, et al. Pathological manifestations in lymphatic filariasis correlate with lack of inhibitory properties of IgG4 antibodies on IgE-activated granulocytes. PLoS Negl Trop Dis. 2017;11:e0005777. doi: 10.1371/journal.pntd.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–39. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–35. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 42.Strle K, Sulka KB, Pianta A, et al. T-helper 17 cell cytokine responses in Lyme disease correlate with Borrelia burgdorferi antibodies during early infection and with autoantibodies late in the illness in patients with antibiotic-refractory Lyme arthritis. Clin Infect Dis. 2017;64:930–8. doi: 10.1093/cid/cix002. [DOI] [PMC free article] [PubMed] [Google Scholar]