Abstract
Chemokines and chemokine receptors play key roles in cancer progression and metastasis. Although multiple chemokines and chemokine receptors have been investigated, inhibition of CXCR4 emerged as one of the most promising approaches in combination cancer therapy, especially when focused on the metastatic disease. Small RNA molecules, such as siRNA and miRNA, represent new class of therapeutics for cancer treatment through RNA interference-mediated gene silencing. However, the clinical applicability of siRNA and miRNA is severely limited by the lack of effective delivery systems. There is a significant therapeutic potential for CXCR4-targeted nanomedicines in combination with the delivery of siRNA and miRNA in cancer. Recently developed CXCR4-targeted polymeric drugs and nanomedicines, including cyclam- and chloroquine-based polymeric CXCR4 antagonists are introduced here and their ability to deliver functional siRNA and miRNA is discussed.
Graphical/Visual Abstract and Caption
INTRODUCTION
Cancer is a major public health problem and a leading cause of mortality worldwide. Metastasis is the main contributor to cancer-associated deaths.1 The heterogeneity of cancer, combined with multiple gene mutations during tumorigenesis and tumor progression, makes curing cancer a daunting challenge. Recently, combination of chemotherapeutics with RNA interference (RNAi), mainly small interfering RNA (siRNA) and microRNA (miRNA), emerged as an effective strategy in cancer treatment. These combination therapies demonstrate potentially great benefits in targeting multiple cancer-associated pathways, inhibiting metastasis and overcoming adaptive drug resistance.2, 3 However, this combination treatment is limited by a lack of efficacious delivery systems for simultaneous delivery of small-molecule drugs and siRNA/miRNA. Due to the physicochemical differences between the two types of agents, it is a significant challenge to develop delivery systems for combinations of small molecule drugs and siRNA/miRNA. Among the available delivery systems, polymeric nanoparticles have been the most successful delivery approaches in drug/nucleic acid combinations.3, 4 Typical polymeric nanoparticles are composed of pharmacologically inert polymer suitable for encapsulation of both types of therapeutic agents. Recently, alternative approaches have focused on the development of pharmacologically active nanoparticles and polymers to achieve delivery of drug/nucleic acid combinations.5–8
The chemokine receptor CXCR4 is an important emerging target for developing combination delivery strategies for improved cancer therapy.9, 10 CXCR4 is an especially promising target in antimetastatic therapies because of its crucial role in metastatic spread of multiple types of human cancer.11, 12 Mounting evidence also supports the potential of improving chemotherapy and immunotherapy through its combination with CXCR4 antagonists.13–16 As a result, various CXCR4-targeted drug delivery systems, including liposomes, nanoparticles, lipoplexes, and polyplexes have been developed for improved cancer therapy.9 Our group has reported the synthesis of a series of polycations with the ability to simultaneously inhibit CXCR4 and deliver nucleic acids to cancer cells. We have successfully employed these polymeric CXCR4 inhibitors (PCX) to deliver functional siRNA and miRNA for combination cancer therapy.17–20 Recently, we have successfully prepared chloroquine-containing polycations as efficient miRNA delivery vectors with improved endosomal escape and antimigratory activity through CXCR4 inhibition in cancer cells.21 In this review, we will introduce these dual functional CXCR4 targeted polycations and discuss the combination strategies based on CXCR4 targeted nanomedicines for cancer therapy.
CXCR4 AS A THERAPEUTIC TARGET IN CANCER
CXCR4 and its chemokine ligand (CXCL12) are two key factors in the tumor growth, metastasis, angiogenesis, and cancer cell-microenvironment interaction, which make them promising targets for cancer therapy.
CXCR4/CXCL12 axis
Chemokine receptors are a large family of proteins that mediate chemotaxis of cells towards a gradient of chemokines. Based on the location of conserved cysteine residues, chemokine receptors are classified into four groups (C, CC, CXC and CX3C). There are over twenty different chemokine receptors, which all belong to the G-protein coupled receptor family. In tumors, the interplay of chemokines and chemokine receptors modulates the trafficking of cells into and out of the tumor microenvironment and participates in crucial steps of the metastasis of tumor cells. Although different types of cancer have varied expression profiles of chemokine receptors, CXCR4 is the most widely expressed chemokine receptor in human cancers. CXCR4 is a G-protein coupled receptor with a seven-transmembrane structure. CXCR4 exerts its biological effect by binding with its specific ligand CXCL12 (also known as stromal derived factor-1, SDF-1). Through activating multiple downstream signaling pathways (mainly including PI3K, MAPK, and Erk1/2), CXCR4/CXCL12 axis regulates a number of different cellular processes, which includes alteration of gene expression, actin polymerization, cell skeleton rearrangement, cell survival, migration and invasion.22, 23
CXCR4 in cancer and metastasis
CXCR4 expression has been found in more than 20 major human cancer types, including breast, ovarian, prostate, pancreatic, melanoma, and renal cell carcinoma.22 The upregulation of CXCR4 is highly dependent on multiple transcription factors, growth factors, and hypoxia-inducible factors.24, 25 A significant correlation between CXCR4 expression and cancer metastasis has been demonstrated by many preclinical and clinical studies. For example, a clinical study concluded that elevated expression of CXCR4 in primary breast tumors is associated with a higher likelihood of developing bone metastases.26 Another study showed significant correlation between CXCR4 expression and lymph node metastasis.27 High CXCR4 expression also indicates poor survival and enhanced aggressiveness of cancers and can be used as an independent prognostic marker.28 CXCR4 can activate focal adhesion complexes and matrix metalloproteinases, which mediates degradation of extracellular matrix and facilitates the invasion of cancer cells. Then, the CXCL12 concentration gradients drive the movement of CXCR4-expressing cancer cells in the circulation and contribute to the process of extravasation and organ-specific metastasis. For instance, bone marrow, lungs, brain, liver and lymph nodes exhibit elevated expression levels of CXCL12 and represent the most common organs for homing of cancer metastasis in cells that express the CXCR4 receptor.29
CXCR4 as target for cancer therapy
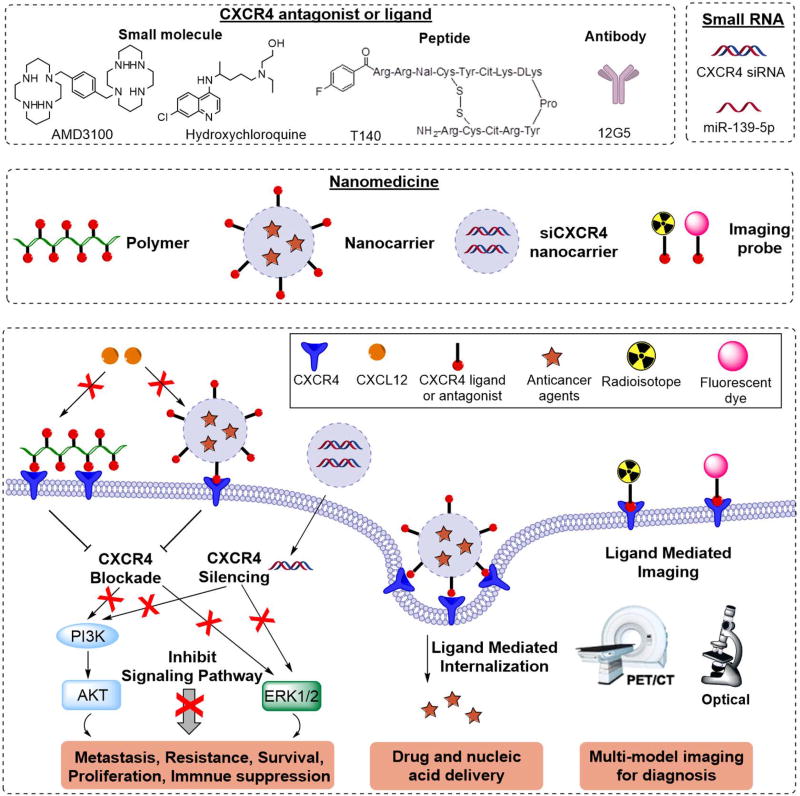
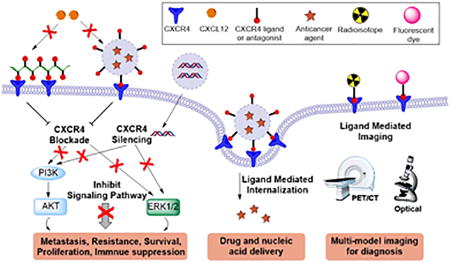
Various strategies have been developed to inhibit CXCR4/CXCL12 axis for anticancer applications. Among them, specific CXCR4 antagonists showed the best effects. A small molecule CXCR4 antagonist Plerixafor (AMD3100) has already been approved by the FDA for clinical use in stem cell mobilization.30 Besides small molecules, CXCR4-binding peptides and siRNA silencing of the CXCR4 gene expression have also been reported to be capable of inhibiting CXCR4-mediated processes in anticancer therapies.31–35 The inhibition of CXCR4 with these therapeutic agents is highly efficacious through inhibiting metastasis, sensitizing tumors to chemotherapy and radiotherapy, and boosting immunotherapy.36–38 In the past decade, development of multiple nanomedicines that target CXCR4 have been also reported (Figure 1). First, CXCR4 can serve as a target for ligand-mediated enhancement of delivery and molecular imaging. CXCR4-binding small molecule organic ligands, CXCR4-binding peptides or anti-CXCR4 antibodies can be attached to the surface of nanoparticles for active targeting to cancer cells for improved therapy and imaging.39, 40 For example, gold nanoclusters functionalized with AMD3100 were used for targeted positron emission tomography (PET) imaging of CXCR4 in primary tumors and metastases in an orthotopic breast cancer model.41 Moreover, inhibition of CXCR4 through CXCR4 siRNA or CXCR4 ligands can be considered as effective approach for cancer therapeutic nanomedicines.35, 42–45 CXCR4-targeted lipid-coated poly(lactic-co-glycolic acid) (PLGA) nanoparticles modified with AMD3100 systemically delivered sorafenib into liver cancer, resulting in effective sensitization of tumors to sorafenib treatment and inhibition of lung metastasis.43
Figure 1.
Main approaches utilizing CXCR4 in cancer nanomedicine.
SMALL RNA DELIVERY
RNA interference (RNAi) is a natural biological mechanism in which RNA molecules inhibit gene expression or translation by neutralizing target mRNA molecules. RNAi based agents, mainly including siRNA and miRNA, are able to knock down the oncogenes by targeting related mRNA expression, which make them powerful approaches for cancer therapy.46, 47
SiRNA
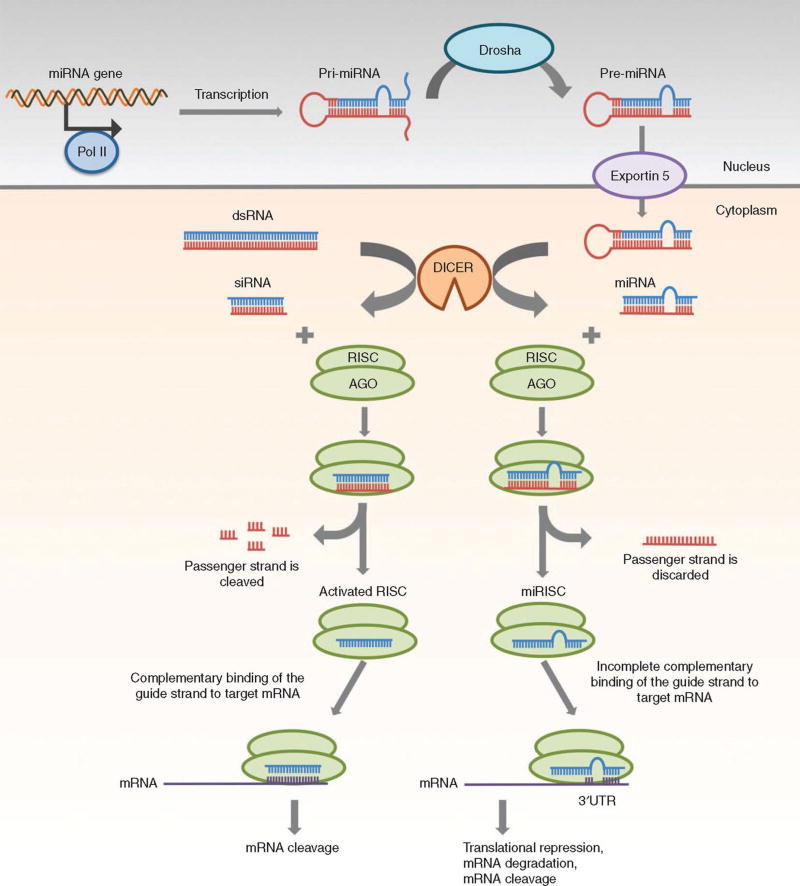
siRNAs are synthetic RNA duplexes (19 to 25 bp in length) with 3' dinucleotide overhangs designed to specifically target a particular mRNA for degradation. SiRNA-mediated RNAi pathway starts with the processing of dsRNA by DICER to siRNA, which is then loaded into the RNA inducing silencing complex (RISC) (Figure 2). The passenger strand of siRNA is cleaved by AGO2, which is a component of RISC. Then, the guide strand of siRNA guides the activated RISC to the target mRNA. Finally, the complete complementary binding between the guide strand and mRNA leads to the cleavage of mRNA of target gene.3, 47, 48 Through silencing of key oncogenes, siRNA is capable of modulating or selectively blocking biological processes that are the defining hallmarks of cancer, which make it potentially an effective therapeutic approach for cancer.49
Figure 2.
Gene silencing mechanisms of siRNA and miRNA. (Reprinted with permission from Ref47)
MiRNA
miRNAs are small (~22 nucleotide, double stranded) noncoding endogenous RNAs that post-transcriptionally regulate gene expression. In the nucleus, the transcription of miRNA gene is carried out by RNA polymerase II to produce pri-miRNA, which is then cleaved by Drosha to form pre-miRNA (Figure 2). Pre-miRNA is transported by Exportin 5 to the cytoplasm. In the cytoplasm, pre-miRNA is processed by Dicer into mature miRNA. Then, miRNA is loaded into RISC. The passenger strand is discarded. The remaining guide strand guides the miRISC to the target mRNA through partially complementary binding. Finally, the target mRNA is inhibited via translational repression, degradation or cleavage.47, 50 Due to the imperfect pairing, a single microRNA is capable of simultaneously targeting different genes, showing the characteristic of multiple targeting. MiRNAs regulate a wide range of cellular pathways and mediate the expression of nearly 30% of all human proteins. Dysregulation of miRNA often results in pathological states such as cancer. MicroRNAs function as tumor suppressors or oncogenes and play an important role in tumorigenesis, tumor growth, angiogenesis, and metastasis.51 Hence, inhibition of overexpressed oncogenic microRNAs or restitution of downregulated tumor-suppressor microRNAs provides a highly promising approach to treat cancer.52
Small RNA delivery
Both siRNA and miRNA are highly effective therapeutic agents for cancer. However, their clinical use is limited by multiple hurdles, such as stability, off-target effects, and poor efficiency of delivery. Although proper chemical modification can improve the stability and reduce off-target effect, poor delivery is still a main challenge in translating therapeutic siRNAs/miRNAs into clinic.53, 54 Since they have similar physicochemical properties (double-stranded RNAs with about 22 nucleotides) and the same intracellular site of action (cytoplasm), similar delivery systems can be utilized for both siRNA and miRNA. An ideal delivery system is expected to sequentially overcome multiple biological barriers, mainly including nucleases degradation, reticuloendothelial system (RES) clearance, poor tumor tissue penetration, intracellular uptake, lysosomal entrapment, and intracellular RNA release.55, 56
Viral vectors and non-viral vectors represent the two main types of delivery technologies for siRNA/miRNA. Non-viral vectors show advantages over viral vectors in terms of safety and represent a potential option for clinical application. Widely used non-viral approaches include polymer-based and lipid-based delivery systems. Cationic polymers (polycations) and cationic lipids bind with anionic siRNA/miRNA through electrostatic interactions which leads to the formation of nanosized polyplexes or lipoplexes, which protect them from degradation and facilitate transport across cellular membranes.47 To meet the requirement of combination RNAi therapy and traditional small molecule therapy, many delivery systems have been reported to co-deliver small molecule agents and RNAi therapeutics in the past decade. Among the available delivery systems, polymeric nanoparticles have been the most successful delivery approaches in drug/RNAi therapeutic combinations. Typical polymeric nanoparticles are composed of pharmacologically inert polymer suitable for encapsulation of both types of therapeutic agents.2 However, the manufacturing complexity and unsatisfactory drug loading ability of the traditional nanoparticles remain a significant hurdle for their clinical translation. Recently, alternative approaches from various labs, including ours, have developed pharmacologically active nanoparticles based on polymeric drugs to achieve delivery of drug/nucleic acid combinations.5 The uniquely designed polymeric drug nanoparticles have several advantages, including simpler formulation and high content of active agents that make them suitable candidates for delivery of drug/RNA combinations. In particular, a series of CXCR4 targeted polymeric drug nanoparticles represent a new generation of drug/RNAi delivery vectors for combination anticancer therapy.
Both CXCR4 inhibition and siRNA/miRNA delivery are important approaches for cancer therapy. CXCR4 targeted nanomedicines delivering functional siRNA/miRNA represent an effective choice for combinational cancer therapy. These newly developed nanomedicines include polyplexes, lipid nanoparticles, peptide carriers, lipopolymer complexes and fusion proteins (Table 1). Among the CXCR4 targeted nanomedicines, we will focus on polymeric CXCR4 antagonists and polymeric chloroquines.
Table 1.
Example of CXCR4-targeted nanomedicines delivering siRNA/miRNA in cancer therapy.
| CXCR4 targeting moiety |
Delivery system | Delivered cargo | Application | References |
|---|---|---|---|---|
| AMD3100 | Polyplexes (PCX-1) | PLK1 siRNA | Simultaneously inhibit migration through CXCR4 antagonism and kill cells through siPLK1 (in vitro) | 57 |
| AMD3100 | Polyplexes (PCX-1) | NCOA3 siRNA | Increase tumor perfusion by siNCOA3, simultaneously inhibit tumor growth and metastasis (in vivo) | 20 |
| Monocyclam | Polyplexes (PCX-2) | MiR-200c mimic | Combined inhibition of cancer cell invasiveness by CXCR4 antagonism and EMT inhibition (in vitro) | 58 |
| AMD3465 | Polyplexes (P-SS-AMD) | MiR-200c mimic | Combined inhibition of cancer cell migration by CXCR4 antagonism and EMT inhibition (in vitro) | 59 |
| Chloroquine | Polyplexes (PCQ) | MiR-210 inhibitor | Facilitate endosome escape, simultaneously inhibit migration and kill cells (in vitro) | 21 |
| AMD3100 | Lipid nanoparticles | VEGF siRNA | Overcome tumor evasion of antiangiogenic therapy, inhibit tumor growth and metastasis (in vivo) | 60 |
| Peptide | Modular peptide-based carrier | VEGF siRNA | Targeted siRNA delivery into CXCR4-expressing cancer and endothelial cells for inhibition of migration (in vitro) | 61 |
| CXCR4 siRNA | Lipopolymer complexes | CXCR4 siRNA | Decrease CXCR4 expression for acute myeloid leukemia therapy (in vitro) | 62 |
| CXCR4 siRNA | Fusion protein | CXCR4 siRNA | CXCR4 knockdown by siRNA effectively inhibited breast tumor growth and metastasis (in vivo) | 35 |
CYCLAM-BASED POLYMERIC CXCR4 ANTAGONISTS (PCX)
Polymer design and development
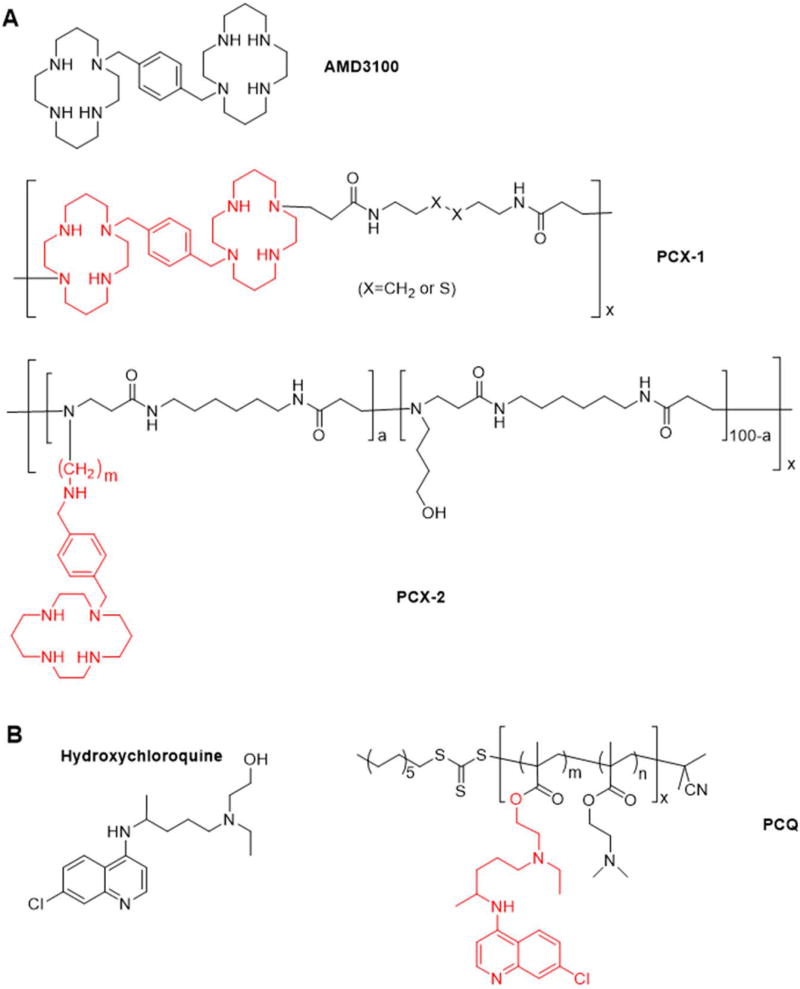
Blockade of CXCR4 with specific antagonists can inhibit metastasis and control the growth of the primary tumors.63 Cyclam derivatives such as AMD3100 are the most widely investigated CXCR4 antagonists which act through inhibiting CXCL12 binding and subsequent CXCR4 signaling.64–66 AMD3100 contains six secondary and two tertiary amines (Figure 3A). Not all of the eight amines are required for binding to the CXCR4 receptor and its pharmacologic function. The redundant amine groups can be used for chemical modification while still maintaining CXCR4 inhibition activity.67 The presence of the protonizable amines provides AMD3100 with positive charge, which makes it a suitable building unit for synthesis of polycations for nucleic acid delivery. Based on this rationale, our group have synthesized the first generation of polymeric CXCR4 antagonists (PCX-1) (Figure 3A), polymeric AMD3100, by direct Michael-like addition polymerization of AMD3100. The synthesized PCX-1 not only retained the CXCR4 inhibitory activity of parent AMD3100 but also successfully delivered nucleic acids to cancer cells.17, 19
Figure 3.
Chemical structure of (A) AMD3100 and cyclam-based polymeric CXCR4 antagonists (PCX) and (B) hydroxychloroquine and chloroquine-based CXCR4 antagonists (PCQ) (Red color indicates the CXCR4-binding repeating unit in the polymers).
Although PCX-1 was well suited for the proof-of-principle studies, the ability to control the polymerization reaction was severely compromised by the presence of six reactive secondary amines in the chemical structure of AMD3100, which resulted in the generation of highly branched polymers. The random chemical substitution of AMD3100 in PCX-1 also decreased the relative CXCR4 inhibitory activity when compared with the parent AMD3100. To overcome the disadvantage of PCX-1, we then developed the second generation of the poly(amido amine) CXCR4 antagonists (PCX-2) with improved presentation of the CXCR4-binding moieties and better controlled polymerization. Unlike PCX-1, which was based on AMD3100, the linear PCX-2 was prepared by the polymerization of newly synthesized CXCR4-inhibiting monocyclam monomers (Figure 3A). PCX-2 showed improved ability to inhibit CXCR4 when compared with the monomers. PCX-2 inhibited cancer cell invasion in vitro and presented CXCR4 antagonism in vivo to mobilize leukocytes from bone marrow to peripheral blood. Moreover, the dual function PCX-2 was also capable of delivering DNA into cancer cells.18
Chemical modification of the PCX polymers was performed to further improve the activity of PCX to systemically deliver functional nucleic acids for cancer therapy. To improve in vivo applicability, polyplexes are often modified with poly(ethylene glycol) (PEG) to shield the surface charges and improve colloidal stability by steric stabilization.68, 69 Accordingly, PEG modification of PCX was investigated by grafting PEG chains to form PEG-PCX. We found that optimized PEG-PCX retained the desirable CXCR4 antagonism and capability of PCX to inhibit cancer cell invasion, while at the same time allowing to improve safety and colloidal stability of the PCX polyplexes.70 Furthermore, we were able to further balance the polymer hydrophobicity by grafting PCX with cholesterol and prepared amphiphilic Chol-PCX. When compared with simple PCX polyplexes, the optimized Chol-PCX polyplexes increased colloidal stability and greatly improved siRNA transfection in the presence of serum, all the while retaining strong CXCR4 inhibitory activity.57 Overall, PEG-PCX and Chol-PCX were successfully developed as potential vectors for systemic in vivo delivery of nucleic acids.
PCX-mediated delivery of siRNA and miRNA to cancer cells
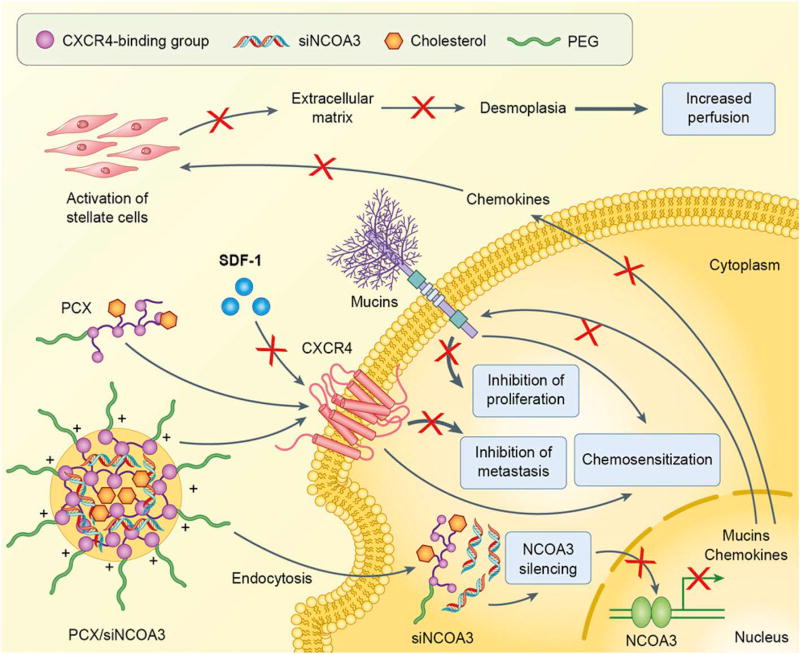
After successful development of the CXCR4 inhibiting polymers PCX, we tested their ability to deliver suitable siRNA or miRNA for combined cancer therapy. In the first example, we focused on PCX/siRNA polyplexes for pancreatic cancer (PC) therapy. PC is one of the most aggressive malignancies with intense desmoplasia, widespread metastasis and inherent chemoresistance. Nuclear receptor co-activator-3 (NCOA3) is a critical modulator of the expression of mucins in PC. Silencing of NCOA3 with siRNA in PC cells downregulates the expression of mainly two mucins, MUC1 and MUC4, which are critical for PC progression.71 Besides mucins, NCOA3 upregulates the expression of multiple chemokines that are responsible for the recruitment of immune cells to pancreatic tumors, perpetuation of pro-inflammatory conditions, and activation of pancreatic stellate cells. As a result, NCOA3 is a suitable target for siRNA nanomedicine design, which aims to modulate PC tumor microenvironment by decreasing desmoplasia, increasing perfusion and enhancing drug delivery to tumor. In addition to the tumor microenvironment modulation by NCOA3 silencing, blockade of CXCR4 is another effective approach to inhibit PC metastasis and progression. We thus combined the two strategies together, using optimized formulation of PCX/siNCOA3 polyplexes to simultaneously target CXCR4 and NCOA3 in PC (Figure 4). Chol-PCX showed maximum CXCR4 antagonism, NCOA3 silencing and inhibition of PC cell migration in vitro. Furthermore, PCX/siNCOA3 polyplexes showed great potential in sensitizing PC cells to chemotherapy. More importantly, the polyplexes showed improved antitumor therapy in an orthotopic mouse model of metastatic PC after systemic delivery. The polyplexes significantly inhibited primary tumor growth, which was because of a decrease in tumor necrosis and increased tumor perfusion. The polyplexes also showed significant antimetastatic effect as demonstrated by effective suppression of metastasis to liver and other organs. Overall, PCX/siNCOA3 polyplexes represent a highly promising combination approach for modulating tumor microenvironment in metastatic PC.20
Figure 4.
Mechanism of action of PCX/siNCOA3 polyplexes in pancreatic cancer therapy. (Reprinted with permission from Ref20)
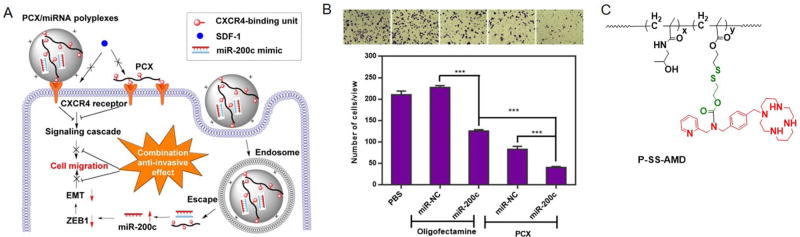
In addition to siRNA delivery, PCX also provide effective delivery activity for therapeutic miRNA as demonstrated in a study focused on delivery of metastasis-regulating miRNA for cooperatively enhanced anti-invasive effect in multiple types of cancer cells. The invasion and metastasis of cancer cells are regulated by multiple factors, which includes not only CXCR4 but also multiple miRNAs. For example, increasing intracellular levels of miR-200c decreased the extent of the epithelial-to-mesenchymal transition (EMT) and inhibited cell migration and invasion in cancer cells.72 Combining the CXCR4 antagonism with the action of miR-200c mimic was thus expected to cooperatively enhance the inhibition of the migration of cancer cells. Based on this rationale, we prepared PCX-2 polyplexes carrying miR-200c mimic (Figure 5). PCX-2 polyplexes effectively delivered miR-200c mimic into cancer cells. By coupling the CXCR4 blockade with miR-200c-induced EMT inhibition, the polyplexes achieved cooperative antimigration activity (Figure 5A and B).58 Moreover, an N-(2-hydroxypropyl)methacrylamide (HPMA)-based self-immolative polymeric prodrug of a CXCR4 antagonist, AMD3465 (P-SS-AMD), also effectively delivered miR-200c and led to the combinational inhibition of cancer cell migration (Figure 5C).59 Overall, PCX/miR-200c treatment is an effective antimetastatic strategy that combines inhibition of two important cell motility pathways.
Figure 5.
(A) Proposed mechanism of action of PCX/miR-200c polyplexes. (B) Combined inhibition of cancer cell migration. (Reprinted with permission from Ref58) (C) Chemical structure of an HPMA-based self-immolative polymeric prodrug of a CXCR4 antagonist, AMD3465 (P-SS-AMD). (Reprinted with permission from Ref59)
Mechanism of action of PCX polyplexes
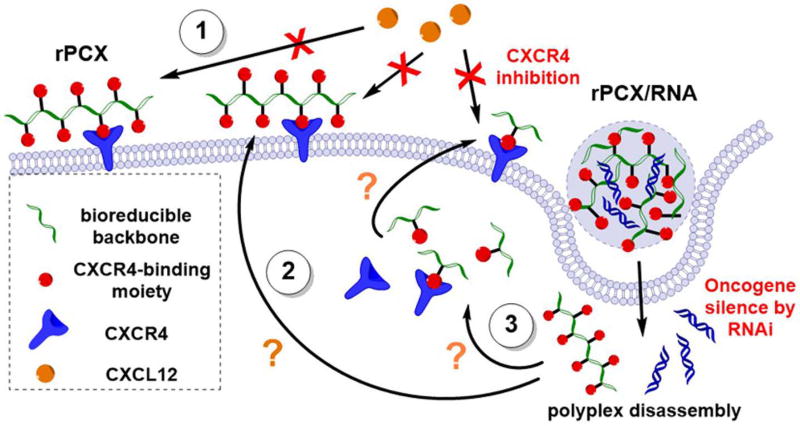
Even though we have experimentally confirmed the dual function of PCX/RNA polyplexes to inhibit CXCR4 and deliver small RNA57, the specific mechanism of action remained unclear because of the seemingly conflicting demands on the system (siRNA delivery vs. CXCR4 inhibition). We have formulated three hypotheses depicted in Figure 6 to explain the mechanism of action. First, as all polyplexes, PCX/RNA are prepared with excess PCX and it is the excess polymer that is responsible for the immediate CXCR4 antagonism. This has been shown by increased CXCR4-dependent anti-migration activity with increasing PCX/siRNA w/w ratio.57 Second, PCX bound to siRNA is released after intracellular siRNA delivery and polyplex disassembly. This mode of action results in delayed CXCR4 inhibition effect, either via binding intracellular CXCR4 during recycling or via PCX excretion from the cells and binding the plasma membrane CXCR4 on cancer cells. Third, in case of intracellularly degradable PCX, the small molecule degradation products containing the CXCR4-binding cyclam moieties may further contribute to the CXCR4 inhibition. Improved understanding of the mechanism of action will contribute to further improvement of the antitumor and antimetastatic activity of the PCX polyplexes.
Figure 6.
Proposed mechanisms of PCX/RNA polyplexes. (1) Excess PCX of the polyplexes formulation is responsible for the immediate CXCR4 antagonism. (2) The disassembly of polyplex releases both small RNA and PCX. Functional small RNA silences oncogene through RNAi mechanism. These released PCX results in delayed CXCR4 inhibition effect via binding intracellular CXCR4 during recycling or via PCX excretion from the cells and binding the plasma membrane CXCR4 on cancer cells. (3) In case of intracellularly bioreducible PCX (rPCX), the small molecule degradation products containing the CXCR4-binding cyclam moieties further contribute to the CXCR4 inhibition.
Another concern in developing PCX has been related to optimizing properties of the formulations with two active agents (CXCR4 inhibitor and siRNA/miRNA). Single formulation of two active agents is often technically challenging, and in many cases, it may be easier to use two single-agent formulations. However, several unique properties of PCX greatly simplify the process of optimizing pharmacologic activity of the dual PCX/siRNA polyplexes and justify their development. The most important one is a broad therapeutic window of PCX, which gives us great leeway in optimizing the formulation for effective siRNA/miRNA delivery without significant concerns about the CXCR4 activity. For example, the effective dose (EC50) of the most active PCX is only 0.021 µg/mL, while its toxic dose (LD50) is more than 8,000-times higher (171 µg/mL).18 In a typical siRNA (10 nM) silencing experiment with polyplexes formulated at PCX/siRNA w/w ratio of 5, the PCX concentration would be 0.665 µg/mL (i.e., 30-times above EC50). Thus, at the anticipated siRNA concentration range, changes in PCX concentrations will have minimal effect on its CXCR4 activity.
CHLOROQUINE-BASED CXCR4 ANTAGONISTS (PCQ)
Polymer development
Chloroquine (CQ) is a widely used antimalarial drug. In recent years, the potential benefits of CQ in anticancer therapies have been also reported mainly due to its effects on autophagy and cholesterol metabolism.73–76 Recently, the ability of CQ to inhibit CXCR4/CXCL12 axis has also been reported and successfully used in the treatment of solid tumors.77 CQ was able to inhibit CXCL12-mediated invasion and proliferation of PC cells and improve survival of tumor-bearing mice when combined with gemcitabine treatment.78 Despite its promise, CQ is a poor (mM) inhibitor of CXCR4 when compared with existing specific nM inhibitors like AMD3100. Thus, we aimed to improve the CXCR4-inhibiting activity of CQ by taking advantage of the multivalency effect by conjugating multiple CQ molecules to a polymeric carrier. We have synthesized CQ-containing copolymers (PCQ) by copolymerization of methacryloylated hydroxy-CQ (HCQ) and HPMA. The PCQ-1 enhanced inhibition of cancer cell migration and invasion in vitro, and improved antimetastatic activity in vivo with lower toxicity when compared with the parent HCQ. The effective inhibition of the CXCR4/CXCL12 axis has been confirmed as one mechanism of the PCQ antimetastatic activity.79, 80 Besides HPMA-based PCQ, our group reported another PCQ drug, chloroquine-modified hydroxyethyl starch (CQ-HES) which was synthesized by conjugation of HES with HCQ by a carbonyldiimidazole coupling. CQ-HES was able to target CXCR4 signaling and improve inhibition of migration and invasion of PC cells when compared with HCQ.81 Overall, PCQ represents a new generation of safe and effective CXCR4 inhibitors for metastatic cancer therapy.
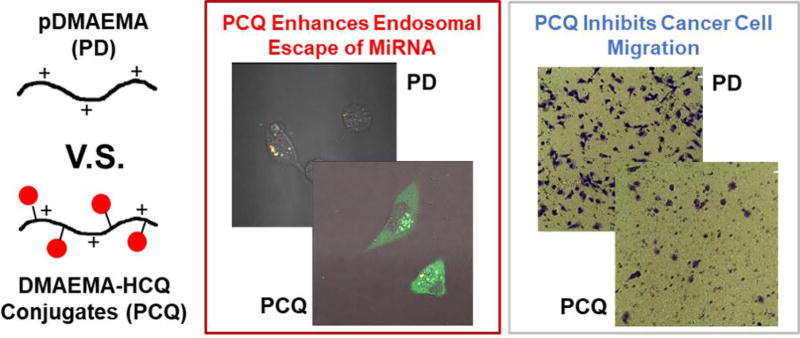
Besides CXCR4 antagonism, CQ is also a widely used endosomolytic agent to improve in vitro transfection of polyplexes. Simple co-transfection of polyplexes with free CQ enhances the cytoplasmic delivery of nucleic acids.82 However, CQ co-transfection is difficult to use in vivo because in order to achieve the functional levels, toxic doses of CQ are required. To overcome the limitations of CQ as endosomal agent in vivo, covalent conjugation of CQ to the nanoparticles improved siRNA delivery in vivo by enhancing endosomal escape.83 Thus, conjugation of CQ to polycations was also expected to improve endosomal escape of polyplexes. Hence, we aimed to prepare CQ-conjugated polycation for both CXCR4 antagonism and improved endosomal escape. We reported the synthesis of CQ-containing 2-(dimethylamino)ethyl methacrylate (DMAEMA) copolymers (PCQ-2) by reversible addition–fragmentation chain-transfer polymerization. After careful optimization of both polymer molecular weight and CQ content in the polymer, the best performing PCQ-2 polyplexes presented the expected dual function through not only inhibiting the migration of cancer cells but also facilitating the endosomal escape for cytoplasm delivery of miRNA (Figure 7).21
Figure 7.
Chloroquine containing polycation for improving endosome escape of delivered miRNA and inhibiting cell migration. (Reprinted with permission from Ref21)
RNA delivery by PCQ
After successful preparation of PCQ-2, we then aimed to deliver suitable siRNA or miRNA for cancer therapy. Intratumoral hypoxia is a hallmark of cancer due to a structurally and functionally disturbed microcirculation, with deterioration of the diffusion geometry, and of tumor-associated anemia.84 As a key factor in tumor progression, hypoxia induces cancer metastasis and increases the resistance of cancer cells to chemotherapy, radiotherapy and photodynamic therapy.85–87 Hypoxia is also able to induce and stabilize CXCR4 expression in cancer.88, 89 MiR-210 is a major hypoxia-inducible miRNA which is overexpressed in multiple types of cancers.90, 91 MiR-210 controls a wide range of biological processes, including cell proliferation, apoptosis, differentiation, DNA repair, cell metabolism, metastasis, and antitumor immune responses.92, 93 Hence, miR-210 inhibition with anti-miR-210 provides a valid target for the treatment of cancer. Accordingly, we prepared PCQ polyplexes to deliver anti-miR-210. Besides retaining the antimigration activity by CXCR4 antagonism, PCQ polyplexes improved the delivery of anti-miR-210 to cancer cells by facilitating endosomal escape. Moreover, though inhibition of miR-210 function, PCQ/anti-miR-210 polyplexes improved anticancer activity by inducing significant cell killing in cancer cells. These results further validate the use of PCQ as a efficacious polymeric drug platform in combination anti-metastatic and anticancer miRNA therapeutic strategies.21
CXCR4-TARGETED NANOPARTICLES
CXCR4 antagonist nanoparticles
Besides PCX and PCQ, other CXCR4 targeted nanoparticles have also been reported for RNAi cancer therapy. These nanoparticles were typically formed by the physical addition of small molecular CXCR4 inhibitor like AMD3100 to the formulation. AMD3100 can act as both cancer targeting ligand for improved nanoparticles delivery and CXCR4 antagonism for cancer therapy.42, 45, 60 Chen’s group reported a series of CXCR4-targeted nanoparticles with the ability to deliver both functional siRNA and small drugs for the treatment of liver cancer and liver fibrosis.43, 94, 95 For example, a CXCR4-targeted lipid-based nanoparticles (NP) were reported to specifically deliver vascular endothelial growth factor (VEGF) siRNA as an antiangiogenic treatment of liver cancer. AMD3100 was added to the nanoparticles to serve as both a targeting moiety and to enhance the antiangiogenic effect. These AMD3100-modified NPs efficiently delivered VEGF siRNAs in liver cancer and downregulated VEGF expression in vitro and in vivo. The combination of CXCR4 inhibition by AMD3100 and VEGF siRNA induced synergistic antiangiogenic effects and suppressed primary tumor growth and distant metastasis in orthotopic liver cancer model.60
CXCR4-silencing nanoparticles
SiRNA silencing of CXCR4 also represents an additional approach to target CXCR4/CXCL12 axis. Multiple studies reported the delivery of CXCR4 siRNA using nanoparticles.35, 62, 96–98 For example, a lipid-modified polymeric carrier was developed for CXCR4 siRNA delivery to acute myeloid leukemia (AML) cells. CXCR4 siRNA was successfully delivered to mononuclear cells derived from AML patients, which resulted in significant CXCR4 silencing in tested samples. Decreasing CXCR4 expression via lipopolymer/siRNA nanocarriers was proven as a potential option for AML therapy.62 In another study, a fusion protein containing an anti-HER2 single-chain antibody fragment was reported to deliver CXCR4 siRNA for HER2+ breast cancer treatment. CXCR4 knockdown by siRNA effectively inhibited breast tumor growth and metastasis both in vitro and in vivo.35
Conclusion
The benefits of both CXCR4 inhibition and siRNA/miRNA delivery for improved cancer therapies are clearly established. This review provided an overview of emerging nanomedicines, which use CXCR4-targeted nanocarriers to deliver functional siRNA/miRNA for combination therapy. The dual function polymeric CXCR4 antagonists simultaneously block the CXCR4/CXCL12 axis and deliver functional siRNA and miRNA to cancer cells, which normally result in the inhibition of primary tumor growth and reduction of metastasis. Using CQ as an alternative CXCR4 inhibitor allows to advantageously combine CXCR4 antagonism with endosomolytic properties of CQ to enhance cytoplasmic delivery of siRNA and miRNA. Besides the PCX and PCQ polymers, CXCR4 inhibitors or CXCR4 siRNA can also be encapsulated into suitable nanocarriers for CXCR4 inhibition. These CXCR4 targeted nanomedicines carrying functional siRNA/miRNA represent a promising choice for combinational cancer therapy especially metastasis inhibition. Despite the great potential, there are still many challenges for the clinical use of the discussed nanomedicines. Future directions will focus on improving their systemic delivery to tumors. For example, recent studies reported that fluorination of polyplexes is able to improve nucleic acids delivery activity while reducing toxicity mainly by increasing stability and membranes transport properties.99–101 Optimized fluorination of PCX is thus expected to improve their in vivo efficiency. Other approaches include zwitterionic modifications,102, 103 and tumor targeting and penetrating moiety conjugation104, 105 are also expected to enhance systemic delivery of these nanomedicines. In addition to improving delivery, we also aim to combine the CXCR4 targeted nanomedicines with other therapies like immunotherapy. For example, recent studies confirm that CXCR4 inhibition is able to boost the PD-1/PD-L1 pathway blockade immunotherapy by facilitating T cell infiltration.15, 16, 106 Intervention of PD-1/PD-L1 pathway via RNA interference (RNAi) also represents an effective approach to boost immunotherapy.107, 108 Accordingly, use of CXCR4 targeted nanomedicine to deliver PD-L1 siRNA is a potent strategy for improved checkpoints blockade immunotherapy.
Acknowledgments
This work was supported by the University of Nebraska Medical Center and by the National Institutes of Health (EB015216, EB020308). Support from China Scholarship Council (Y.X. and Y.W.) is gratefully acknowledged.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Wang Y, Zhu Y, Oupický D. Recent advances in delivery of drug–nucleic acid combinations for cancer treatment. J Control Release. 2013;172:589–600. doi: 10.1016/j.jconrel.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Maciel D, Rodrigues Jo, Shi X, Tom00E1;s H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem Rev. 2015;115:8564–8608. doi: 10.1021/cr500131f. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Yu F, Chen Y, Oupický D. Polymeric drugs: Advances in the development of pharmacologically active polymers. J Control Release. 2015;219:369–382. doi: 10.1016/j.jconrel.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Murray-Stewart T, Wang Y, Yu F, Li J, Marton LJ, Casero RA, Oupický D. Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J Control Release. 2017;246:110–119. doi: 10.1016/j.jconrel.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Wang W, Guo S, Wang Y, Miao L, Xiong Y, Huang L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun. 2016;7:11822. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao X, Wang W, Wang C, Wang Y, Zhou J, Ding Y, Wang X, Jin Y. A chitosan-graft-PEI-candesartan conjugate for targeted co-delivery of drug and gene in anti-angiogenesis cancer therapy. Biomaterials. 2014;35:8450–8466. doi: 10.1016/j.biomaterials.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Xie Y, Oupický D. Potential of CXCR4/CXCL12 chemokine axis in cancer drug delivery. Curr Pharmacol Rep. 2016;2:1–10. doi: 10.1007/s40495-015-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleightholm RL, Neilsen BK, Li J, Steele MM, Singh RK, Hollingsworth MA, Oupický D. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol Ther. 2017;179:158–170. doi: 10.1016/j.pharmthera.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Guo F, Wang Y, Liu J, Mok S, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35:816–826. doi: 10.1038/onc.2015.139. [DOI] [PubMed] [Google Scholar]
- 12.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 13.Zeng Z, Shi YX, Samudio IJ, Wang R-Y, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Srivastava S, Bhardwaj A, Owen L, Singh A. CXCL12–CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671–1679. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61:1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Zhu Y, Hazeldine ST, Li C, Oupický D. Dual-function CXCR4 antagonist polyplexes to deliver gene therapy and inhibit cancer cell invasion. Angew Chem Int Ed. 2012;51:8740–8743. doi: 10.1002/anie.201203463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Hazeldine ST, Li J, Oupický D. Development of functional poly (amido amine) CXCR4 antagonists with the ability to mobilize leukocytes and deliver nucleic acids. Adv Healthc Mater. 2015;4:729–738. doi: 10.1002/adhm.201400608. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Oupický D. Effect of biodegradability on CXCR4 antagonism, transfection efficacy and antimetastatic activity of polymeric Plerixafor. Biomaterials. 2014;35:5572–5579. doi: 10.1016/j.biomaterials.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Kumar S, Rachagani S, Sajja BR, Xie Y, Hang Y, Jain M, Li J, Boska MD, Batra SK. Polyplex-mediated inhibition of chemokine receptor CXCR4 and chromatin-remodeling enzyme NCOA3 impedes pancreatic cancer progression and metastasis. Biomaterials. 2016;101:108–120. doi: 10.1016/j.biomaterials.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Yu F, Tang W, Alade BO, Peng ZH, Wang Y, Li J, Oupický D. Synthesis and evaluation of chloroquine-containing DMAEMA copolymers as efficient anti-miRNA delivery vectors with improved endosomal escape and antimigratory activity in cancer cells. Macromol Biosci. 2018;18:1700194. doi: 10.1002/mabi.201700194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 24.Kubic JD, Lui JW, Little EC, Ludvik AE, Konda S, Salgia R, Aplin AE, Lang D. PAX3 and FOXD3 promote CXCR4 expression in melanoma. J Biol Chem. 2015;290:21901–21914. doi: 10.1074/jbc.M115.670976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. J Biol Chem. 2005;280:22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 26.Andre F, Xia W, Conforti R, Wei Y, Boulet T, Tomasic G, Spielmann M, Zoubir M, Berrada N, Arriagada R, et al. CXCR4 Expression in Early Breast Cancer and Risk of Distant Recurrence. Oncologist. 2009;14:1182–1188. doi: 10.1634/theoncologist.2009-0161. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:R144. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 29.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burger J, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 32.Hassan S, Buchanan M, Jahan K, Aguilar-Mahecha A, Gaboury L, Muller WJ, Alsawafi Y, Mourskaia AA, Siegel PM, Salvucci O. CXCR4 peptide antagonist inhibits primary breast tumor growth, metastasis and enhances the efficacy of anti-VEGF treatment or docetaxel in a transgenic mouse model. Int J Cancer. 2011;129:225–232. doi: 10.1002/ijc.25665. [DOI] [PubMed] [Google Scholar]
- 33.Galsky MD, Vogelzang NJ, Conkling P, Raddad E, Polzer J, Roberson S, Stille JR, Saleh M, Thornton D. A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clin Cancer Res. 2014;20:3581–3588. doi: 10.1158/1078-0432.CCR-13-2686. [DOI] [PubMed] [Google Scholar]
- 34.Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang K, Li J, Yin J, Ma Q, Yan B, Zhang X, Wang L, Wang L, Liu T, Zhang Y. Targeted delivery of CXCR4-siRNA by scFv for HER2+ breast cancer therapy. Biomaterials. 2015;59:77–87. doi: 10.1016/j.biomaterials.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, Masui T, Fujimoto K, Tamamura H, Hiramatsu K. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 38.Mei L, Liu Y, Zhang Q, Gao H, Zhang Z, He Q. Enhanced antitumor and anti-metastasis efficiency via combined treatment with CXCR4 antagonist and liposomal doxorubicin. J Control Release. 2014;196:324–331. doi: 10.1016/j.jconrel.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Gallo J, Kamaly N, Lavdas I, Stevens E, Nguyen QD, Wylezinska-Arridge M, Aboagye EO, Long NJ. CXCR4-targeted and MMP-responsive iron oxide nanoparticles for enhanced magnetic resonance imaging. Angew Chem Int Ed. 2014;53:9550–9554. doi: 10.1002/anie.201405442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Torre C, Casanova I, Acosta G, Coll C, Moreno MJ, Albericio F, Aznar E, Mangues R, Royo M, Sancenón F. Gated mesoporous silica nanoparticles using a double-role circular peptide for the controlled and target-preferential release of doxorubicin in CXCR4-expresing lymphoma cells. Adv Funct Mater. 2015;25:687–695. [Google Scholar]
- 41.Zhao Y, Detering L, Sultan D, Cooper ML, You M, Cho S, Meier SL, Luehmann H, Sun G, Rettig M. Gold nanoclusters-doped with 64Cu for CXCR4 positron emission tomography imaging of breast cancer and metastasis. ACS nano. 2016;10:5959. doi: 10.1021/acsnano.6b01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Gong S, Wu J, Li H, Oupicky D, Sun M. CXCR4-targeted and redox responsive dextrin nanogel for metastatic breast cancer therapy. Biomacromolecules. 2017;18:1793–1802. doi: 10.1021/acs.biomac.7b00208. [DOI] [PubMed] [Google Scholar]
- 43.Gao D-Y, Lin T-T, Sung Y-C, Liu YC, Chiang W-H, Chang C-C, Liu J-Y, Chen Y. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials. 2015;67:194–203. doi: 10.1016/j.biomaterials.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Wang K, Yang X, Zhou Y, Ping Q, Oupicky D, Sun M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017;53:399–413. doi: 10.1016/j.actbio.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Yang X, Zhou Z, Wang K, Li C, Qiao H, Oupicky D, Sun M. Near-infrared light-triggered drug release from a multiple lipid carrier complex using an all-in-one strategy. J Control Release. 2017;261:126–137. doi: 10.1016/j.jconrel.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Daka A, Peer D. RNAi-based nanomedicines for targeted personalized therapy. Adv Drug Deliv Rev. 2012;64:1508–1521. doi: 10.1016/j.addr.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Lam JK, Chow MY, Zhang Y, Leung SW. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson BL, McCray PB. Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devi G. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 50.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 51.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Conde J, Artzi N. Are RNAi and miRNA therapeutics truly dead? Trends Biotechnol. 2015;33:141–144. doi: 10.1016/j.tibtech.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Chakraborty C, Sharma A, Sharma G, Doss CGP, Lee S-S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 56.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Li J, Chen Y, Oupický D. Balancing polymer hydrophobicity for ligand presentation and siRNA delivery in dual function CXCR4 inhibiting polyplexes. Biomater Sci. 2015;3:1114–1123. doi: 10.1039/C5BM00003C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Y, Wehrkamp CJ, Li J, Wang Y, Wang Y, Mott JL, Oupický D. Delivery of miR-200c mimic with poly (amido amine) CXCR4 antagonists for combined inhibition of cholangiocarcinoma cell invasiveness. Mol Pharm. 2016;13:1073–1080. doi: 10.1021/acs.molpharmaceut.5b00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Z-H, Xie Y, Wang Y, Li J, Oupick00FD; D. Dual-Function Polymeric HPMA prodrugs for the delivery of miRNA. Mol Pharm. 2017;14:1395–1404. doi: 10.1021/acs.molpharmaceut.6b00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J-Y, Chiang T, Liu C-H, Chern G-G, Lin T-T, Gao D-Y, Chen Y. Delivery of siRNA using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol Ther. 2015;23:1772–1782. doi: 10.1038/mt.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egorova A, Shubina A, Sokolov D, Selkov S, Baranov V, Kiselev A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int J Pharm. 2016;515:431–440. doi: 10.1016/j.ijpharm.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 62.Landry B, Gül-Uludağ H, Plianwong S, Kucharski C, Zak Z, Parmar MB, Kutsch O, Jiang H, Brandwein J, Uludağ H. Targeting CXCR4/SDF-1 axis by lipopolymer complexes of siRNA in acute myeloid leukemia. J Control Release. 2016;224:8–21. doi: 10.1016/j.jconrel.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 63.Rhodes LV, Short SP, Neel NF, Salvo VA, Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71:603–613. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 65.Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3:47–75. doi: 10.7150/thno.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong D, Korz W. Translating an antagonist of chemokine receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 67.Bridger GJ, Skerlj RT, Hernandez-Abad PE, Bogucki DE, Wang Z, Zhou Y, Nan S, Boehringer EM, Wilson T, Crawford J. Synthesis and structure− activity relationships of azamacrocyclic CXC chemokine receptor 4 antagonists: Analogues containing a single azamacrocyclic ring are potent inhibitors of T-cell tropic (X4) HIV-1 replication. J Med Chem. 2009;53:1250–1260. doi: 10.1021/jm901530b. [DOI] [PubMed] [Google Scholar]
- 68.Oupicky D, Ogris M, Howard KA, Dash PR, Ulbrich K, Seymour LW. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol Ther. 2002;5:463–472. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 69.Ogris M, Brunner S, Schüller S, Kircheis R, Wagner E. PEGylated DNA/transferrin–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Li J, Oupický D. Polymeric plerixafor: effect of PEGylation on CXCR4 antagonism, cancer cell invasion, and DNA transfection. Pharm Res. 2014;31:3538–3548. doi: 10.1007/s11095-014-1440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar S, Das S, Rachagani S, Kaur S, Joshi S, Johansson S, Ponnusamy M, Jain M, Batra S. NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene. 2015;34:4879–4889. doi: 10.1038/onc.2014.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, Zhao X, Andersen JB, Ye QH, Jia HL. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792–1803. doi: 10.1002/hep.25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eng CH, Wang Z, Tkach D, Toral-Barza L, Ugwonali S, Liu S, Fitzgerald SL, George E, Frias E, Cochran N. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc Natl Acad Sci USA. 2016;113:182–187. doi: 10.1073/pnas.1515617113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King M, Ganley I, Flemington V. Inhibition of cholesterol metabolism underlies synergy between mTOR pathway inhibition and chloroquine in bladder cancer cells. Oncogene. 2016;35:4518–4528. doi: 10.1038/onc.2015.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 76.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 77.Kim J, Yip MR, Shen X, Li H, Hsin L-YC, Labarge S, Heinrich EL, Lee W, Lu J, Vaidehi N. Identification of anti-malarial compounds as novel antagonists to chemokine receptor CXCR4 in pancreatic cancer cells. PLoS One. 2012;7:e31004. doi: 10.1371/journal.pone.0031004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balic A, Sørensen MD, Trabulo SM, Sainz B, Cioffi M, Vieira CR, Miranda-Lorenzo I, Hidalgo M, Kleeff J, Erkan M. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther. 2014;13:1758–1771. doi: 10.1158/1535-7163.MCT-13-0948. [DOI] [PubMed] [Google Scholar]
- 79.Yu F, Xie Y, Wang Y, Peng Z-H, Li J, Oupický D. Chloroquine-containing HPMA copolymers as polymeric inhibitors of cancer cell migration mediated by the CXCR4/SDF-1 chemokine axis. ACS Macro Lett. 2016;5:342–345. doi: 10.1021/acsmacrolett.5b00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu F, Li J, Xie Y, Sleightholm RL, Oupický D. Polymeric chloroquine as an inhibitor of cancer cell migration and experimental lung metastasis. J Control Release. 2016;244:347–356. doi: 10.1016/j.jconrel.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sleightholm R, Yang B, Yu F, Xie Y, Oupický D. Chloroquine-modified hydroxyethyl starch as a polymeric drug for cancer therapy. Biomacromolecules. 2017;18:2247–2257. doi: 10.1021/acs.biomac.7b00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 83.Perche F, Yi Y, Hespel L, Mi P, Dirisala A, Cabral H, Miyata K, Kataoka K. Hydroxychloroquine-conjugated gold nanoparticles for improved siRNA activity. Biomaterials. 2016;90:62–71. doi: 10.1016/j.biomaterials.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 84.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 85.Vaupel P, Kelleher DK, Höckel M. Oxygenation status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 86.Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci USA. 2014;111:E5429–E5438. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le Q-T, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pulkkinen K, Malm T, Turunen M, Koistinaho J, Ylä-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo. FEBS Lett. 2008;582:2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 92.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: The master hypoxamir. Microcirculation. 2012;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C-H, Chan K-M, Chiang T, Liu J-Y, Chern G-G, Hsu F-F, Wu Y-H, Liu Y-C, Chen Y. Dual-functional nanoparticles targeting CXCR4 and delivering antiangiogenic siRNA ameliorate liver fibrosis. Mol Pharm. 2016;13:2253–2262. doi: 10.1021/acs.molpharmaceut.5b00913. [DOI] [PubMed] [Google Scholar]
- 95.Sung Y-C, Liu Y-C, Chao P-H, Chang C-C, Jin P-R, Lin T-T, Lin J-A, Cheng H-T, Wang J, Lai CP. Combined delivery of sorafenib and a MEK inhibitor using CXCR4-targeted nanoparticles reduces hepatic fibrosis and prevents tumor development. Theranostics. 2018;8:894–905. doi: 10.7150/thno.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang Y-P, Hung C-M, Hsu Y-C, Zhong C-Y, Wang W-R, Chang C-C, Lee M-J. Suppression of breast cancer cell migration by small interfering RNA delivered by polyethylenimine-functionalized graphene oxide. Nanoscale Res Lett. 2016;11:247–254. doi: 10.1186/s11671-016-1463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abedini F, Ismail M, Hosseinkhani H, Ibrahim TAT, Omar AR, Chong PP, Bejo MH, Domb AJ. Effects of CXCR4 siRNA/dextran-spermine nanoparticles on CXCR4 expression and serum LDH levels in a mouse model of colorectal cancer metastasis to the liver. Cancer Manag Res. 2011;3:301–309. doi: 10.2147/CMR.S11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abedini F, Hosseinkhani H, Ismail M, Domb AJ, Omar AR, Chong PP, Hong P-D, Yu D-S, Farber I-Y. Cationized dextran nanoparticle-encapsulated CXCR4-siRNA enhanced correlation between CXCR4 expression and serum alkaline phosphatase in a mouse model of colorectal cancer. Int J Nanomedicine. 2012;7:4159. doi: 10.2147/IJN.S29823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen G, Wang K, Hu Q, Ding L, Yu F, Zhou Z, Zhou Y, Li J, Sun M, Oupický D. Combining Fluorination and Bioreducibility for Improved siRNA Polyplex Delivery. ACS Appl Mater Interfaces. 2017;9:4457–4466. doi: 10.1021/acsami.6b14184. [DOI] [PubMed] [Google Scholar]
- 100.Wang M, Liu H, Li L, Cheng Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat Commun. 2014;5:3053. doi: 10.1038/ncomms4053. [DOI] [PubMed] [Google Scholar]
- 101.Wang LH, Wu DC, Xu HX, You YZ. High DNA-binding affinity and gene-transfection efficacy of bioreducible cationic nanomicelles with a fluorinated core. Angew Chem Int Ed. 2016;128:765–769. doi: 10.1002/anie.201508695. [DOI] [PubMed] [Google Scholar]
- 102.Jackson MA, Werfel TA, Curvino EJ, Yu F, Kavanaugh TE, Sarett SM, Dockery MD, Kilchrist KV, Jackson AN, Giorgio TD. Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS nano. 2017;11:5680–5696. doi: 10.1021/acsnano.7b01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang P, Sun F, Tsao C, Liu S, Jain P, Sinclair A, Hung H-C, Bai T, Wu K, Jiang S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc Natl Acad Sci USA. 2015;112:12046–12051. doi: 10.1073/pnas.1512465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Xie Y, Li J, Peng Z-H, Sheinin Y, Zhou J, Oupický D. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS nano. 2017;11:2227–2238. doi: 10.1021/acsnano.6b08731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu X, Wu J, Liu Y, Yu M, Zhao L, Zhu X, Bhasin S, Li Q, Ha E, Shi J. Ultra-pH-responsive and tumor-penetrating nanoplatform for targeted siRNA delivery with robust anti-cancer efficacy. Angew Chem Int Ed. 2016;55:7091–7094. doi: 10.1002/anie.201601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miao L, Li J, Liu Q, Feng R, Das M, Lin CM, Goodwin TJ, Dorosheva O, Liu R, Huang L. Transient and local expression of chemokine and immune checkpoint traps to treat pancreatic cancer. ACS nano. 2017;11:8690–8706. doi: 10.1021/acsnano.7b01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hobo W, Maas F, Adisty N, de Witte T, Schaap N, van der Voort R, Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen–specific CD8+ T cells. Blood. 2010;116:4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 108.Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, Zhou F, Fu Y, Yin Q, Zhang P. Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 2016;16:5503–5513. doi: 10.1021/acs.nanolett.6b01994. [DOI] [PubMed] [Google Scholar]