Abstract
Background
Parenteral nutrition (PN) provides nutrition intravenously; however, this life-saving therapy is associated with significant liver disease. Recent evidence indicates improvement in PN-associated injury in animals with intact gut treated with enteral bile acid (BA), chenodeoxycholic acid (CDCA), and a gut farnesoid X receptor (FXR) agonist, which drives the gut–liver cross talk (GLCT). We hypothesized that similar improvement could be translated in animals with short bowel syndrome (SBS).
Methods
Using piglets, we developed a novel 90% gut-resected SBS model. Fifteen SBS piglets receiving PN were given CDCA or control (vehicle control) for 2 weeks. Tissue and serum were analyzed posteuthanasia.
Results
CDCA increased gut FXR (quantitative polymerase chain reaction; P = .008), but not downstream FXR targets. No difference in gut fibroblast growth factor 19 (FGF19; P = .28) or hepatic FXR (P = .75), FGF19 (P = .86), FGFR4 (P = .53), or Cholesterol 7 α-hydroxylase (P = .61) was noted. PN resulted in cholestasis; however, no improvement was noted with CDCA. Hepatic fibrosis or immunostaining for Ki67, CD3, or Cytokeratin 7 was not different with CDCA. PN resulted in gut atrophy. CDCA preserved (P = .04 vs control) gut mass and villous/crypt ratio. The median (interquartile range) for gut mass for control was 0.28 (0.17–0.34) and for CDCA was 0.33 (0.26–0.46).
Conclusions
We note that, unlike in animals with intact gut, in an SBS animal model there is inadequate CDCA-induced activation of gut-derived signaling to cause liver improvement. Thus, it appears that activation of GLCT is critically dependent on the presence of adequate gut. This is clinically relevant because it suggests that BA therapy may not be as effective for patients with SBS.
Keywords: bile acids, chenodeoxycholic acid, farnesoid X receptor, fibroblast growth factor 19, gut atrophy, liver injury, parenteral nutrition, SBS animal model, short gut syndrome
Introduction
Parenteral nutrition (PN) therapy involves intravenous (IV) delivery of nutrition, thus bypassing the normal enteral route of feeding.1–3 It is commonly used in patients with bowel resection or impaired gut function.4,5 Unfortunately, this lifesaving PN therapy is associated with significant liver disease that is characterized by an increase in serum bilirubin level, which is a marker for hepatic cholestasis.6,7 About two-thirds of patients receiving PN experience development of chronic cholestasis, with mortality rates of about 20%.8,9 This poses a significant clinical burden in caring for such patients. In addition, PN therapy is also associated with gut atrophy, which is counterproductive to the goals of intestinal rehabilitation.10
In clinical practice, PN is commonly used for prolonged periods in patients with intestinal failure (IF), a diagnosis given when there is not enough functional gut present to sustain the patient’s nutrition needs solely on enteral feeding.9 When this condition results from extensive bowel resection, often secondary to trauma, ischemic injury, inflammatory bowel disease, gastroschisis, or necrotizing enterocolitis, the patients are classified as having short bowel syndrome (SBS).11,12 Patients with extensive bowel resection require PN therapy for survival because enteral delivery of nutrition is not adequate to sustain their caloric and nutrition requirements for growth. Gut resection in such patients can involve resection of a small segment to extensive bowel resection, the latter involving significantly higher PN support and presenting with advanced liver injury.13,14 Preventing liver disease and promoting gut growth remain a major focus in the care of patients with SBS who are receiving PN therapy.
Despite significant ongoing research, the mechanisms of PN-associated injury remain an enigma.15 New evidence points to gut-derived signaling secondary to luminal contents activating gut receptors that regulate the gut–liver cross talk (GLCT), which is known to maintain hepatic homeostasis.10,16
Because PN therapy commonly occurs in the absence of regular enteral feeding, it is postulated that this results in a lack of enterocyte receptor activation in the absence of luminal nutrients, which are known receptor agonists.16,17 This causes an impairment of downstream gut-originated signaling molecules that normally travel via the portal circulation to the liver and, therefore, lead to an interruption of the normal GLCT.18,19
Using a piglet PN animal model with intact gut, we have previously reported that the bile acid (BA) chenodeoxycholic acid (CDCA) delivered enterally can prevent cholestasis and liver disease associated with PN therapy in piglets by activating the farnesoid X receptor (FXR), which is found in the gut.16 This CDCA-mediated gut FXR activation results in enhanced expression of the hepatoprotective fibroblast growth factor 19 (FGF19)20,21 that regulates hepatic Cholesterol 7 α-hydroxylase (CyP7A1),22,23 which is the rate-limiting step of BA synthesis and drives the GLCT.24 In addition, such therapy with CDCA, in animals with intact gut, prevents PN-associated atrophic changes in the gut.16,25
However, given the premise that liver improvement with CDCA is secondary to gut-derived signals, and thus dependent on the presence of at least some portion of the bowel, it needs to be tested whether hepatic and gut protection seen with CDCA in PN piglets with intact gut could bring about similar improvements in SBS piglets receiving PN with extensive bowel resection. Because this model recapitulates the clinically relevant pediatric condition of PN-dependent SBS, we set forth to test the role of CDCA in this model. Therefore, leveraging results from our published studies, we hypothesized that enterally delivered CDCA given to piglets receiving PN with surgically induced SBS could prevent PN-associated injury.
Design and Methods
Neonatal Pig as a Model
Over the past 2 decades there has been widespread use of the pig as an animal model to study human physiology and development.26 Extensive research has demonstrated homology in both form and function between the pig and human with respect to numerous organ systems, especially the liver and the gastrointestinal tract.27,28 In particular, the neonatal pig, unlike rodents, has been shown to be highly comparable with the human neonate in regard to several aspects of metabolism, body composition, organ function, and stages of development.29 This comparison is very relevant in the setting of SBS.30,31 We have developed a new ambulatory PN delivery mechanism to emulate human PN delivery.3 For this study, we used our ambulatory PN delivery method in piglets with the modification of 90% surgical resection of the gut to create a short bowel animal model.
Animal Procurement and Short Bowel Surgery
The protocol for CDCA BA treatment of neonatal pigs (piglets) undergoing 90% bowel resection was approved by the Institutional Animal Care and Use Committee of Saint Louis University (SLU Animal Use Protocol 2657, US Department of Agriculture registration 43-R-011). The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals.32 One-week-old female piglets (n = 15), with a mean weight ± SD of 3.3 ± 0.3 kg were procured from an approved class A vendor and immediately placed in heated cages. As described previously, jugular and duodenal catheters were surgically implanted.
Short bowel surgery
After catheter placement, animals underwent surgery for bowel resection and iatrogenic creation of SBS. Via the abdominal incision, the ligament of Treitz and the ileocecal junction were identified and marked using a sterile silk ribbon placed along the antimesenteric border of the gently stretched small intestine. Boundaries were marked with silk ligatures around the bowel that were then clamped with regular crushing clamps (hemostats). Subsequently, the process of transecting the bowel and removal of the resected segment was performed. Replacement of saline-soaked gauze and repeated flushing of the bowel tissue with sterile warm saline were performed. On the boundaries of the intestinal resection, Doyen forceps were placed on the portion of the intestine that was used to create the anastomosis. Closely opposed to the Doyen forceps, hemostats were placed on the segment to be resected. Using scissors, we transected the intestine between each Doyen/clamp pair. If the proximal and distal lumens were of unequal diameter, the smaller segment was transected at an oblique angle favoring retention of the vasa recta at the mesenteric border. This ensured equal luminal diameter for anastomosis. Electrocautery was then used to cauterize andvv remove the mesentery and associated vasculature in between these sites. Once the resected segment was freed from the mesentery, the Doyen-clamped ends of each remnant bowel section (jejunum and ileum) were brought together. Each segment was measured. The resected bowel length was also measured to ascertain the total bowel length. Our goal was retention of 10% bowel with 90% resection. If either the jejunal or the ileal segment was >5% of the total bowel length, then it was trimmed. Stay sutures at the mesenteric and antimesenteric borders were used to ensure the correct apposition. Luminal continuity of the 2 remnant sections was restored by an end-to-end stapled (Just Right 5-mm stapler) anastomosis. The anastomosis seal was subsequently tested by injecting sterile, warm saline into the lumen using a 25G needle and checked for leakage. The anastomosis was copiously washed in a bowl of warm saline before being returned to the abdomen. The abdominal incision was then closed in 3 layers (absorbable suture/surgical staples), and the incision line and exit site near the scapula were infiltrated with local anesthetic (bupivacaine) for postoperative pain relief. Subsequently, preconditioned jackets and ambulatory pumps for PN delivery were placed.
Animal Grouping
All animals received IV fluids on day 1 postsurgery. Animals were subsequently started on their randomly assigned groups to receive IV (n = 8) PN plus enterally delivered CDCA (Cat No. C9377; Sigma-Aldrich, St. Louis, MO, USA) via slow bolus infusions at 30 mg/kg/day, divided into 2 daily doses (CDCA group), or (n = 7) PN plus enterally delivered control (vehicle control for CDCA).
Nutrition
As previously described, all animals received a commercially available PN preparation continuously (Clinimix E; Baxter, Deerfield, IL, USA) via the jugular venous catheter. This provided fluids at 260 mL/kg body weight/day with 26 g/kg dextrose, 11.05 g/kg protein, and 5 g/kg fat along with electrolytes, trace minerals, and vitamins for a total of 182 kcal/kg body weight/day (Table 1). The PN was placed in nutrition bags (Medtec Medical, EVA, Product Code 66050) and replaced every 12 hours. Isocaloric nutrition33 was provided to all animals for a period of approximately 2 weeks as previously published.3,16
Table 1.
Nutrition Composition.
| Parenteral Nutrition |
|---|
| Ingredients: |
| Parenteral nutrition: |
| Leucine, isoleucine, valine, lysine, phenylalanine, histidine, threonine, methionine, tryptophan, alanine, arginine, glycine, proline, serine, tyrosine, sodium, potassium, magnesium, calcium, acetate, chloride, phosphate, dextrose) |
| Fat: |
| Soybean oil, egg yolk phospholipids, glycerin, and water |
Animal Care
Animal weights were recorded daily, before nutrition bag changes. Animals were continuously monitored with frequent scheduled (at least 5 times daily) visits by research personnel in accordance with the Institutional Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals.32
Euthanasia and Tissue Collection
The abdomen was opened posteuthanasia, and the liver was removed in its entirety. Subsequently, the remaining small intestine was removed as described previously.3,16 The intestine was flushed with saline, its contents extruded and weighed. Small segments of the liver and the small bowel were sliced and weighed. Tissue was then cut into smaller pieces, snap-frozen in liquid nitrogen, and stored at −80°C for future analysis. At the time of euthanasia, blood was also collected. Serum analysis was done at the Saint Louis University clinical pathology core laboratory.
Histology
Segments of fresh tissue (2–3 cm) from the small intestine and liver were fixed in 4% buffered formalin for 24 hours and then stored in 70% ethanol at room temperature for 24 hours. The tissue was then processed, embedded in paraffin, and stained for hematoxylin and eosin (H&E). Liver tissue was stained with Sirius Red (for evaluating fibrosis). Liver tissue was also stained for Ki67, Cytokeratin-7 (CK-7), and CD3. In addition, gut slides were stained with thymidine analog 5-bromo-2′-deoxyuridine (BrdU). The automated upright microscope system with LED illumination for Life Sciences Leica DM4000 B LED was used along with Q-capture pro digital imaging software. The following subsections describe the specific stains and the methods used for quantification.
Ki67 labeling index
The nuclear antigen Ki67 is expressed in the cell-cycle phases G1, S, G2, and M, however not during G0. The percentage of the Ki67+ nuclei are expressed as a labeling index, which provides an objective cell proliferation index in the tissues.34 Liver tissue in each group was stained via the Ki67 immunohistochemical stain. The slides were then reviewed under a light microscope by a pathologist blinded to group allocation. All of the positive (immunoreactive) hepatic nuclei were calculated and subsequently divided by the total number of cells in 5 nonoverlapping high-power fields. The result was reported as a Ki67 index.
Cytokeratin 7
CK-7 is a low molecular weight cytokeratin, expressed in epithelia lining the cavities of ducts, vessels, and organs. The CK-7 immunohistochemical stain is used to objectively qualify bile duct proliferation.35,36 We performed CK-7 staining on liver tissue from the CDCA and control groups. The slides were reviewed under a light microscope by a pathologist blinded to the group allocation. All of the immunoreactive cells (cytoplasmic and membranous pattern) were calculated and reported in 5 nonoverlapping high-power fields and divided by the total number of cells to compute a final CK-7 score.
CD3
CD3 immunohistochemical stains were performed on the liver tissue to evaluate the inflammatory infiltrate. These provide an objective assessment of hepatic inflammation.37 The slides were reviewed under a light microscope by a pathologist blinded to the group allocation. All of the immunoreactive cells were calculated and subsequently divided by total number of cells in 5 nonoverlapping high-power fields to compute a CD3 score.
Sirius Red staining
Collagen provides structure and support to maintain healthy liver function. However, increased collagen deposition is a known marker for hepatic injury with PN.38 Sirius Red is a strong anionic dye that reacts with the basic functional groups in collagen at a low pH. As a result of these interactions, the elongated dye molecules from Sirius Red attach to the collagen so that their long axes are parallel. This results in increased birefringence in the stained samples.
For this experiment, staining was accomplished by de-paraffinizing neonatal pig liver samples using xylene and rehydrating using decreasing concentrations of ethanol. After this process, the slides were stained with Weigert’s iron hematoxylin solution and then after washing were stained with a Sirius Red/Fast Green solution. Slides were subsequently dehydrated with increasing concentrations of ethanol and xylene.39 Slides were then reviewed by a pathologist blinded to the study groups. The amount of collagen present was determined by calculating the ratio of collagen stained by the Sirius Red as a percentage of the entire sample. Collagen levels were then compared between the groups.
Liver Cholestasis Scoring
The liver H&E slides were reviewed under a light microscope. Bile deposits indicative of cholestasis, which is a known event with PN-associated liver injury,40 were identified by a pathologist blinded to group allocation. These cholestatic foci were counted and reported in 3 nonoverlapping fields to create a liver cholestasis score.
5-Bromo-2′-deoxyuridine
The thymidine analog BrdU is used as an immunohistochemical stain to detect proliferating cells. BrdU is incorporated into newly synthesized DNA of replicating cells, and thus provides an objective assessment of cellular proliferation.41
The BrdU standard operating procedure was approved by the university (SOP 2657). BrdU (Sigma-Aldrich) was administered 6 hours before animal euthanasia via the jugular catheter at a dose of 35mg/kg.
BrdU-stained gut slides were reviewed under a light microscope by a pathologist blinded to the group allocation. Within each high-power field, all BrdU+ cells, identified by a brown nuclear staining pattern, were counted. This number was divided by the total number of cells in 3 nonoverlapping high-power fields. The result was reported as BrdU labeling index.
Morphometric analysis of small-bowel epithelium
Using the small-bowel H&E slides, we quantified the mean villous height and crypt depth in vertically well-oriented villous–crypt columns with the slide reviewer blinded to the treatment to compute a villous/crypt (V/C) ratio for animals in each group.
Confirmation of biological activity of CDCA
To assess the biological activity of CDCA used in this experiment, we established a cell culture model using Human Hepatocellular Carcinoma (HepG2) cells. Upregulation of FXR by CDCA in HepG2 cells is well defined, and thus provides support of CDCA activity.42,43 HepG2 cells were grown in 60-mm Petri dishes with Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal calf serum, penicillin-streptomycin, 15 mm HEPES, and L-glutamine in a humidified incubator at 37°C with 5% CO2 atmosphere. After seeding (65%–70% confluent), cells were treated with CDCA (Cat No. C9377; Sigma-Aldrich) dissolved in dimethyl sulfoxide (DMSO; D2650; Sigma-Aldrich), as given to the piglets and incubated for 24 hours. For control experiments, cells were treated with DMSO only.
Cells were washed with Dulbecco’s phosphate-buffered saline and collected. The total RNA was isolated from the HepG2 cells using TRIzol reagent (Life Technologies). For gene expression analysis, total RNA was reverse transcribed by using Verso cDNA synthesis kit (AB 1453/B-Thermo scientific), and the real-time quantitative polymerase chain reaction (RT-qPCR) analysis was performed per the manufacturer’s instructions (CFX Connect; Bio-Rad).
Tissue: RNA extraction and real-time polymerase chain reaction analysis
RNA was extracted from the liver using Sigma-Aldrich GenElute Mammalian Total RNA Miniprep Kit per protocol (RTN70-1KT) and the terminal ileum using TRIzol (15596018; Thermo Fisher). Isolated RNA (1 ng) was reverse transcribed into complementary DNA using Verso cDNA Synthesis Kit (AB1453B; Thermo Fisher). Primers were designed using Integrated DNA Technologies. Primers for each transcript were validated (Table 2). RT-qPCR was performed in triplicate on the Bio-Rad CFX Connect Real-Time System. Relative mRNA levels were calculated by the comparative threshold cycle method using β-actin as the internal control.
Table 2.
Primer Sequences.
| Primer | Sequence | |
|---|---|---|
| Human | ||
| Farnesoid X Receptor (FXR) | Forward Reverse |
CCGTGAATGAAGACAGTGAAGGTCG ACCCTTTCAGCAAAGCCAATCTGGTC |
| Porcine | ||
| Fibroblast growth factor 19 (FGF19) | Forward Reverse |
ACACCATCTGCCCGTCTCT CCCCTGCCTTTGTACAGC |
| FXR | Forward Reverse |
ACATTCCTCATTCTGGGGCTTT TTTCGGGGTCTTACTCCTTACA |
| FGF19 Receptor: FGFR4 | Forward Reverse |
CGCTCGCGGCCACGCCGCCGT TGCCGCCGCACCGGCGCTCGC |
| Cholesterol 7 α-hydroxylase (CyP7A1) | Forward Reverse |
AGGGTGACGCCTTGAATTT GGGTCTCAGGACAAGTTGGA |
| Small heterodimer partner (SHP) | Forward Reverse |
AGTGCTGCCTGGAGTCCTTA CCTTTCAGGTAGGCGTATTCC |
| Constitutive androstane receptor (CAR) | Forward Reverse |
CCGCCATATGGGCACTATGT GCGAAATGCATGAGCAGAGA |
| Organic solute steroid transporter α (OSTα) | Forward Reverse |
CCTGTTTCTCATCCCTGACG AGCAGCGCTCTCCTCAGA |
| Multidrug-associated protein (MRP) | Forward Reverse |
TCTTGGTGACACACAGCATTC TTCCCACAACCACAATCTCA |
| Peroxisome proliferator-activated receptor (PPAR) | Forward Reverse |
CAGCCTCCAGCCCCTCGTC GCGGTCTCGGCATCTTCTAGG |
| β-Actin | Forward Reverse |
5′-GGACCTGACCGACTACCTCA 5′-GCGACGTAGCAG AGCTTCTC |
Statistical Analysis
GraphPad Prism version 7.03 software was used for statistical analysis. Descriptive statistics on the outcomes were calculated as median and interquartile range (IQR). Mann-Whitney U tests were conducted for the serological markers, histology reads, and relative mRNA expression of the genes. All tests were 2-sided using a significance level of 0.05.
Results
Baseline
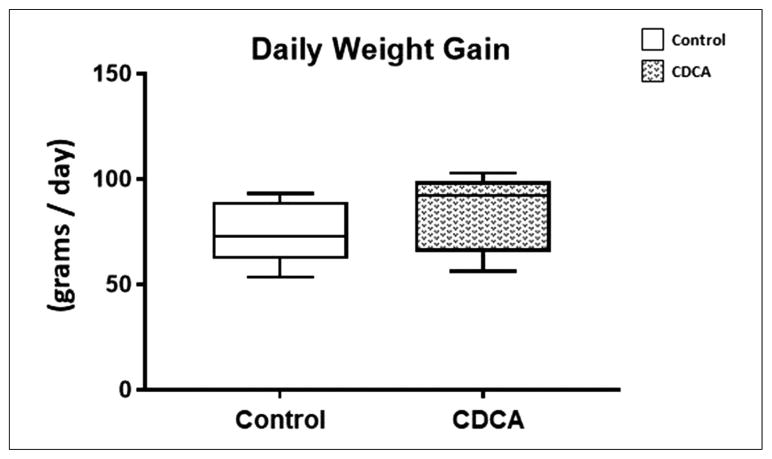
At baseline, both groups were comparable in age and weight. No differences were noted (P = .15) in the daily weight gain in either control or CDCA treatment groups over the course of 14 days of treatment (Figure 1). The median and IQR for daily weight gain for control was 72.78 (62.67–86.48) and for CDCA was 92.19 (70.29–101.1).
Figure 1.
Daily weight gain for control and chenodeoxycholic acid (CDCA)-treated animals. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All test were 2-sided using a significance level of 0.05. Note that there were no differences in daily weight gain between the 2 groups.
CDCA Activity
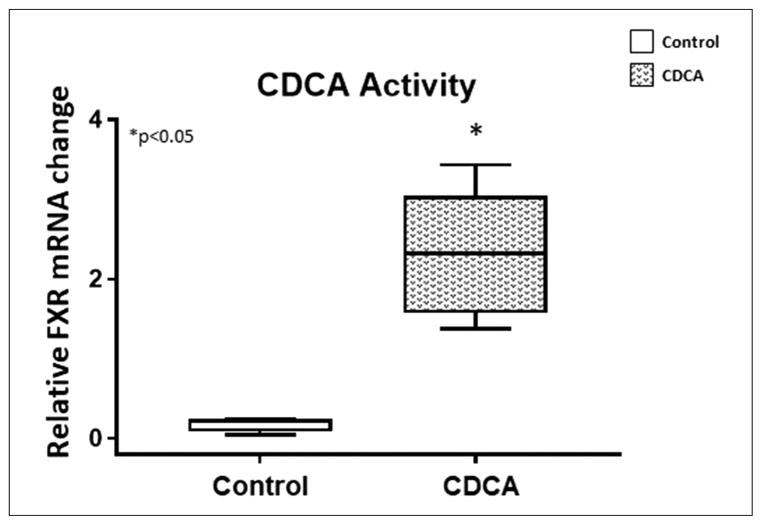
Because CDCA is a known FXR agonist, to confirm activity for the CDCA used in this experiment, we evaluated FXR mRNA expression upon introduction of CDCA in HepG2 cell lines. We dissolved the CDCA in DMSO as given to the piglets and used the same compounded solution to test in HepG2 cells. Confirming CDCA activity, we noted a marked increase (P = .0043) in the mRNA expression of FXR with CDCA treatment (Figure 2). The median and IQR for relative FXR expression for control was 0.22 (0.07–0.28) and for CDCA was 2.32 (1.49–3.17).
Figure 2.
Chenodeoxycholic acid (CDCA) activity: relative farnesoid X receptor (FXR) mRNA expression in HepG2 cells treated with CDCA. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note that there was significant upregulation of FXR with CDCA.
Serum Bilirubin and Liver Histology
One of the most important markers for cholestatic liver injury with PN is elevated serum bilirubin level. We have previously noted significant elevation in serum bilirubin level in piglets with intact gut receiving PN.3,16,33,44
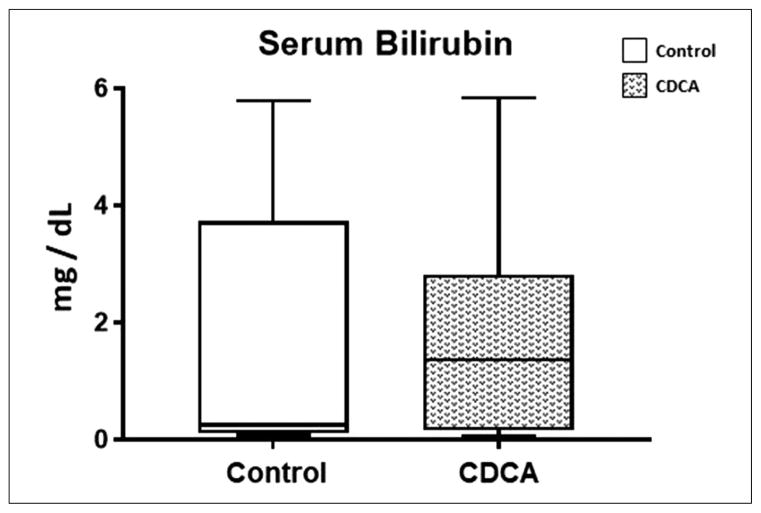
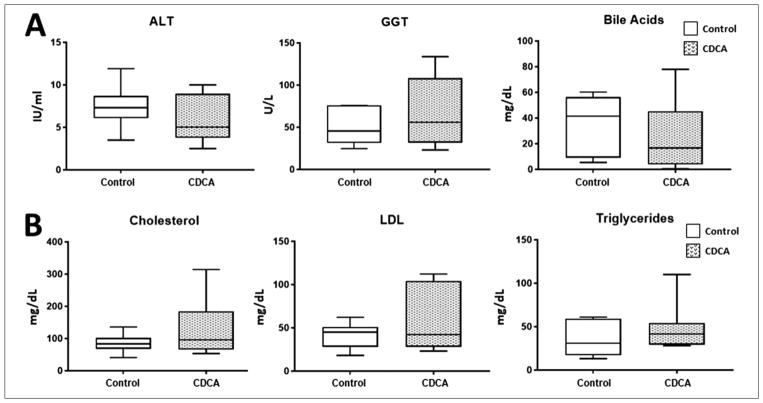
In this study, we also noted that there was a significant elevation in serum bilirubin level in all piglets receiving PN. There was additionally a large variability in the serum bilirubin within each group. In fact, paradoxically, although the median bilirubin was lower in the control group, this did not reach statistical significance (Figure 3). The median and IQR for serum bilirubin for control was 0.25 (−0.26 to 4.01) and for CDCA was 1.37 (0.10–3.43). Thus, unlike in prior publications showing improvement in serum bilirubin with CDCA treatment in piglets with intact gut receiving PN, we did not find any beneficial effect on serum bilirubin with CDCA treatment in comparison with control (P = .93) in our SBS piglets. Given this result, we also evaluated serological markers for liver injury and fat profiles, expecting an improvement with CDCA treatment. However, no statistical differences in serum alanine amino-transferase (P = .39), γ-glutamyl transferase (P = .80), BA (P = .39), cholesterol (P = .46), low-density lipoprotein (P = .64), or triglyceride (P = .51) levels were noted among the groups (Figure 4).
Figure 3.
Serum bilirubin level. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note that there was no statistical difference in serum bilirubin with CDCA treatment.
Figure 4.
(A) Serum alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), and bile acids levels. (B) Serum cholesterol, low-density lipoprotein (LDL), and triglyceride levels. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note that there were no differences in serum ALT, GGT, bile acid, cholesterol, LDL, or triglyceride levels between the groups.
Histologically, hepatic intraparenchymal bile deposition was noted in all animals. Keeping in line with the serum bilirubin results, these cholestatic deposits were not statistically different among the 2 groups, and no improvement was noted with CDCA treatment. Interlobular and periportal fibrosis was noted in piglets from both groups. Although initially the fibrosis appeared less dense with CDCA treatment, on objective quantification this did not reach statistical significance (P = .47) between the 2 groups (Figure 5).
Figure 5.
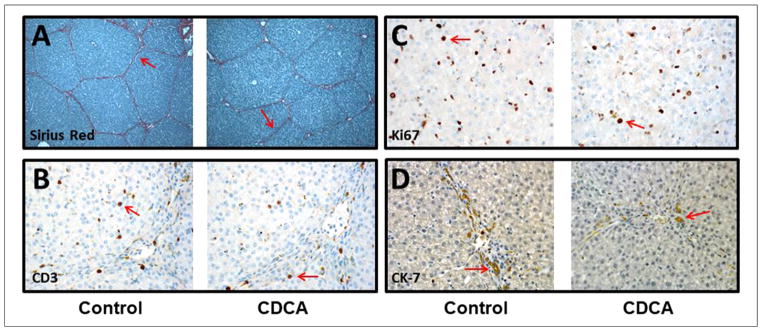
Liver immunohistochemistry: (A) Sirius Red, (B) CD3, (C) Ki67, and (D) Cytokeratin 7 (CK-7). Objective histology scoring was performed, and a Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note that there were no differences in hepatic fibrosis, Ki67, CD3, or CK-7 immunostaining (see arrows) between the groups.
Based on published data demonstrating an alteration of hepatocyte proliferation in animals receiving PN, we calculated the hepatic Ki67 proliferation index in each group.45 Keeping in line with a lack of improvement in liver histology with CDCA treatment, we did not find any statistical difference in the Ki67 index (Figure 5) between the groups (P = .37) to suggest a difference in hepatocyte proliferation.
Previously published data have demonstrated bile duct proliferation and an inflammatory infiltrate on PN therapy.35,37,46 To evaluate differences in bile duct proliferation and inflammatory infiltrate between the groups, we performed the CK-7 and the CD3 immunohistochemistry on the liver slides; however, no statistically significant differences were noted in CK-7 (P = .18) or CD3 score (P = .56) between the 2 groups (Figure 5).
Gut Morphology and Histology
Bowel atrophy on initiation of PN is well described.3,16,47 Although on gross inspection the small bowel of control animals was thin and friable, there was prevention of gut atrophy in piglets receiving CDCA.
Improved bowel weight
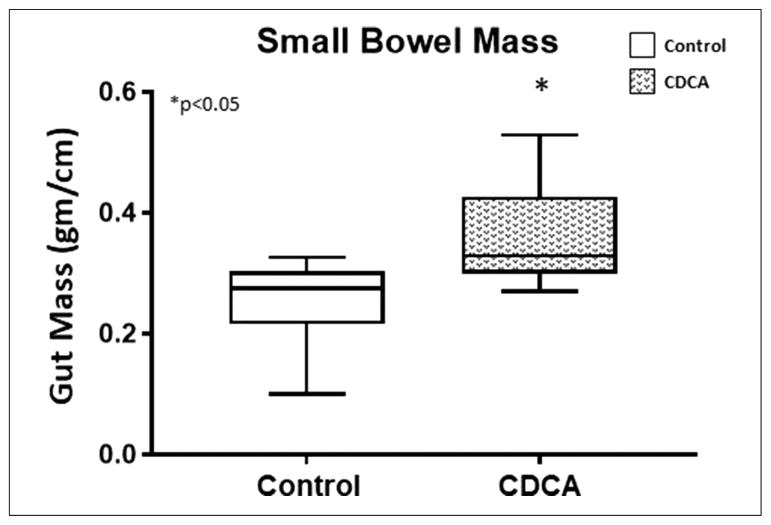
To compare bowel growth among the groups, we calculated the weight per centimeter of the small bowel at the time of animal sacrifice as an estimate of the “gut mass.” Supporting the changes observed on gross examination, in comparison with the CDCA-treated animals, a significant reduction in the gut mass was noted in the small bowel in the control group (Figure 6). CDCA treatment largely prevented the loss of the gut mass (P = .04). The median (IQR) for the gut mass (g/cm) for control was 0.28 (0.17–0.34) and for CDCA was 0.33 (0.26–0.46).
Figure 6.
Gut mass of small bowel. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note the reduction in small bowel gut mass with control and its preservation with chenodeoxycholic acid (CDCA) treatment.
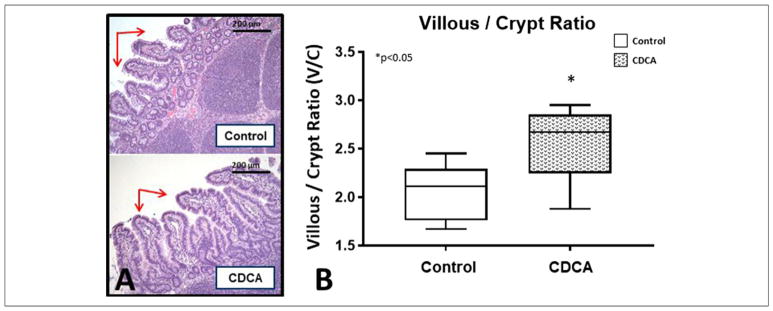
To quantify gut morphometric differences, we evaluated the V/C ratio of the small bowel in each group. We noted significant villous atrophy in all animals with SBS. The median (IQR) for the V/C ratio for control was 2.11 (1.76–2.37) and for CDCA was 2.67 (2.16–2.96). CDCA was able to preserve the V/C ratio (P = .04) in comparison with control piglets (Figure 7), although this preservation was less pronounced than in other published studies using models without gut resection.16,41,48
Figure 7.
(A) Small bowel histology. Note the villous atrophy in control animals (arrows). (B) Small bowel villous/crypt ratio. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note the significantly higher villous/crypt ratio with chenodeoxycholic acid (CDCA) treatment.
BrdU staining
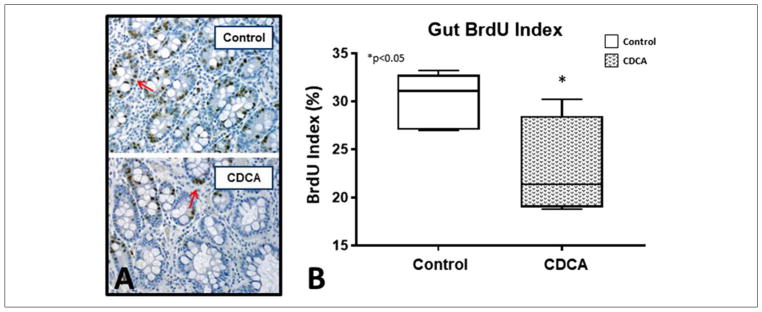
To evaluate differences in gut cellular proliferative activity, we evaluated the BrdU index in each of the animals. The median (IQR) for the BrdU Index for control was 31.09 (27.49–33.19) and for CDCA was 21.39 (14.62–31.28). The BrdU index was significantly lower for CDCA-treated animals (P = .038) compared with their control counterparts (Figure 8).
Figure 8.
(A) Small bowel histology with 5-bromo-2′-deoxyuridine (BrdU) immunostaining (see arrows). (B) Gut BrdU index. Box and whisker plots are shown. Boxes represent the 25th–75th percentile, and central lines represent median values. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note the significantly lower BrdU index with chenodeoxycholic acid (CDCA) treatment.
Key Hepatobiliary Receptors and Transporters
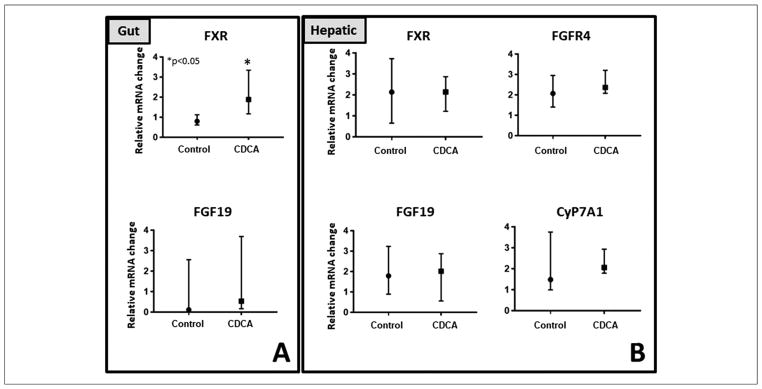
CDCA is known to activate gut FXR with a resultant activation of FGF19. We therefore evaluated gut FXR mRNA expression to ascertain CDCA activation of gut FXR. The median (IQR) for the gut FXR expression for control was 0.79 (0.37–1.45) and for CDCA was 1.88 (−1.38 to 9.82). Thus, an upregulation of gut FXR was noted with CDCA (P = .008) in comparison with control animals (Figure 8). However, in contrast with published studies in animals with intact gut receiving PN, this did not translate into enhanced expression for gut FGF19, and FGF19 was not statistically different between the groups (P = .28). We next tested mRNA expression of multiple key hepatic receptors and transporters that are known to be regulated via the GLCT driven by CDCA activation of gut receptors. No statistical differences in hepatic FGF19 (P = .86), hepatic FGFR4 (P = .53), a receptor for FGF19, hepatic CyP7A1 (P = .61), or hepatic FXR (P = .75) were noted between the 2 groups (Figure 9). In addition, no statistical differences in known CDCA-modulated hepatic receptors, small heterodimer partner49 (SHP), constitutive androstane receptor50 (CAR), organic solute steroid transporter51 (OSTα), multidrug-associated protein52,53 (MRP), or peroxisome proliferator-activated receptor54 (PPAR) were noted between the groups.
Figure 9.
Key bile acid–regulated hepatobiliary receptors and transporters in the (A) gut and (B) the liver. Median and interquartile values are shown. Filled box represents chenodeoxycholic acid, and filled circle represents control. A Mann-Whitney U test was conducted to determine P-value. All tests were 2-sided using a significance level of 0.05. Note the significant upregulation of gut farnesoid X receptor (FXR). Otherwise, no differences in mRNA expression were noted between the groups. CyP7A1, Cholesterol 7 α-hydroxylase; FGF19, fibroblast growth factor 19.
Discussion
PN remains a crucial therapy in patients with gut dysfunction and a variety of debilitating medical conditions. Despite being a critical life-sustaining therapy, enthusiasm for its use is hampered by side effects that carry a high rate of morbidity and mortality. One of the most significant of these side effects is progressive liver disease and gut atrophy.47,55
Although there has been some interest in the role of ω 3–derived fats,56–58 as well as infection prevention59 and strict micronutrient management protocols60,61 for patients receiving PN, to date there are no definitive ameliorative or curative strategies. In addition, mechanisms leading to PN-associated complications remain largely unknown,62 which further complicates therapeutic interventions.6,63,64
Clinical observations of a mitigation of PN-associated injury when some enteral nutrition is provided along with parental nutrition suggest the existence of gut-generated signaling molecules in response to luminal content.65–67 Furthering this idea, emerging data point to an altered GLCT as a potential contributor to PN-associated pathology.10,16,24
Experiments advancing this theory have shown that such gut and hepatoprotective signaling can be recapitulated by delivering luminal BAs in animal PN model systems.68,69 In fact, prevention of gut atrophy and an improvement in hepatic outcomes have been shown to occur on enteral administration of the BA CDCA in animals receiving PN with intact gut.16 Although this idea of using BAs to drive the GLCT as a therapeutic strategy for PN-associated pathology challenges the current treatment paradigm, it is predicated on the existence of an intact bowel.
Clinically, PN therapy is most needed by patients who have had IF or significant bowel resection, a condition called SBS. This cohort represents the most vulnerable of the patient populations to PN-associated injury with high morbidity and mortality.4,70 Interventions that could prevent such injury are urgently called for and present an opportunity for high impact in the care for such patients.11
Inducing a significant 90% surgical short bowel in our well-established PN pig model to recapitulate human SBS, we decided to use enteral BA treatment with CDCA to determine whether we could recapitulate the beneficial effects of such therapy that we have previously published in animals with intact bowel receiving PN.
In this study, as expected, we noted upregulation of hepatic FXR in HepG2 cells, confirming biological activity of our supplied CDCA. However, when enterally administered to animals with significant bowel resection, the same CDCA did not improve hepatic injury.
With CDCA treatment, although we noted increased mRNA expression of gut FXR, this did not translate into alterations in the key downstream hepatobiliary receptors or transporters, which is known to occur in animals with intact gut. In addition, serum bilirubin did not improve with CDCA treatment. Downstream gut FXR-regulated hepatic targets did not show any statistical difference between the study groups. There was no difference in gut FXR-regulated, FGF19 expression levels or in the hepatic FGF19 receptor FGFR4. Similarly, CYP7A1, the rate-limiting step of BA synthesis that is known to be regulated by FGF19 signaling,71,72 was not different between the groups. In addition, SHP, CAR, OSTα, MRP, and PPAR, which are key hepatobiliary transporters and receptors in the GLCT, did not show any statistical change with CDCA.
Prior studies have noted changes in fat profiles with gut FXR agonists.73,74 With our model of significant gut resection, we did not find differences in the fat panel with CDCA treatment. Histologically, cholestasis and periportal fibrosis were noted in both groups with no improvement with CDCA.
Findings from the gut in this study interestingly did not mirror those of the liver. Although we did not note improvement in liver injury with CDCA treatment, a striking observation was a preservation of PN-induced gut atrophy in CDCA-treated animals in comparison with the control group. This was noted both in gut mass comparisons and histological morphometrics. The gut density, as well as the V/C ratio critical for gut function, was improved with CDCA treatment, but this response appeared tempered in comparison with published studies evaluating luminal receptor agonists in PN with intact gut.48,75,76 This observation thus challenges our current understanding and calls for further research into the mechanistic pathways triggered by luminal content that drive gut growth.
Although we were expecting a higher cell proliferation in CDCA-treated animals, as has been noted in other studies,77 our BrdU index data indicated a lower proliferation activity with CDCA. We postulate that this may have been because of potentially greater ongoing gut reparative processes in our control animals with greater gut atrophy as compared with the CDCA-treated animals, where CDCA treatment preserved the atrophic changes.
Thus, although at first our results may seem counterintuitive to published data of hepatic improvement with CDCA treatment in animals receiving PN with intact gut,16 they nevertheless are clinically very relevant and provide key data crucial for translational science. Therefore, although the altered gut–liver signaling can be modulated by enteral BA therapy, patients with significant gut resection, as occurring with SBS, may not benefit from such therapy. It also provides significant support to our published novel theory that there are indeed gut-derived signals that regulate systemic health, and an absence of gut translates to an absence of these signals.
However, we also noted significant protections from PN-associated gut atrophic changes in these animals as shown by a preservation of the gut density, as well as the V/C ratio. Mechanisms into such gut-protective effect with CDCA remain intriguing. Certainly the TGR5-GLP axis,28,33,78 as well as the role of other trophic factors including epidermal growth factor,79 insulin-like growth factor,80 keratinocyte growth factor,81 and the role of the gut microbiota,44 need critical evaluation in future studies. Further research may also provide insights into other relevant mechanistic pathways driving gut growth and remain the focus of our ongoing studies.
Although this study provides an interesting observation regarding the role of CDCA in SBS, there were some obvious limitations. First, the animals used in this study are not pure-bred, and therefore could present individual variations in responses, thus skewing our results. This, however, would be representative of patient populations with SBS who again are not genetically identical.
A second theoretical limitation would be potential subclinical infections in these animals with significant surgical procedures and the presence of long-term indwelling catheters, which could influence our results. Although all animals were rigorously monitored by the study staff and board-certified veterinarians, clinical signs of infection could have lagged pathology. To mitigate this potential issue, we did perform blood cultures; however, we did not find any obvious signs of infection among either group.
A third limitation could be length of treatment of our piglets. Even though we did not see initial differences in terms of hepatic protection via CDCA, it is possible that with sustained gut adaptation driven by CDCA, we could over time achieve adequate gut–liver signaling to prevent hepatic damage. Dose and frequency variation in CDCA treatment could also potentially alter gut signaling, which could be the focus of future studies.
Nevertheless, our results do bring up the very important question that if enteral BA therapy is not helpful in a model of SBS with extensive bowel resection, where should we direct future research to help this condition in these patients? Could IV delivered downstream signaling molecules generated on BA-mediated gut receptor activation prove to be of therapeutic utility in PN-associated injury in SBS? We speculate that such targeted therapy could potentially translate into better outcomes for patients with SBS. Equally crucial would be knowledge as to the bowel segment or the percentage of bowel needed to drive the GLCT. Thus, further studies with differing bowel segment or percentages of bowel resection could help answer that question.
Such focused research could ultimately advance our understanding of the mechanistic pathways involved in the GLCT and potentially bring to fruition ameliorative or therapeutic intervention for patients with SBS.
Conclusion
This study evaluating the role of CDCA in SBS is clinically very relevant and provides novel evidence that the GLCT is impaired with significant bowel resection. Our data thus demonstrate that the gut is integral in mediating the cross talk with the liver. Thus, “No gut results in no gain!” Our results challenge the current paradigm and prompt the thought-provoking idea that despite the phenomenal interest in enteral BA treatment for a variety of hepatic disorders, this treatment may not be effective in patients receiving PN with extensive bowel resection. Instead, future research focusing on IV delivered downstream molecules/signals normally generated upon luminal content could act as a surrogate in driving the GLCT and mitigate PN-associated complication in patients with SBS.
Clinical Relevancy Statement.
Parenteral Nutrition (PN) involves providing nutrition via the intravenous route in patients with bowel resection or impaired gut function. Unfortunately this therapy is associated with significant liver disease and mortality. New evidence points to gut derived signaling secondary to luminal contents that regulates the Gut-Liver cross talk (GLCT) essential for hepatic health. We have previously published improvement in PN associated injury in animals with intact gut treated enterally by chenodeoxycholic acid (CDCA) which is a gut Farnesoid X Receptor (FXR) agonist. CDCA a bile acid (BA) causes secretion of the hepato-protective fibroblast growth factor 19 (FGF19) which acts as a signaling molecule mediating the GLCT. We thus hypothesized that similar improvement could be translated in the clinically relevant condition of short bowel syndrome (SBS), which results from extensive bowel resection. However we noted that that in an animal model of SBS there is inadequate activation of gut derived signaling with CDCA which precludes hepatic improvement. Therefore modulating the GLCT is intimately related to the existence of some bowel. Thus no gut no gain! This is clinically very relevant as it provides novel information cautioning the recent excitement that BA therapy has generated for a variety of liver disorders targeting the GLCT. Thus trialing intravenously administered downstream signaling molecules along the GLCT pathway could be the focus of future SBS studies. Additionally, mechanisms for gut preservation with CDCA despite significant gut resection remain unknown and further studies will be needed to evaluate those pathways.
Footnotes
Conflicts of interest: None declared.
Statement of Authorship
A. K. Jain contributed to conception/design of the research; G. Villalona, A. Price, K. Blomenkamp, C. Manithody, S. Saxena, T. Ratchford, M. Westrich, V. Kakarla, S. Pochampally, W. Phillips, N. Heafner, N. Korremla, J. Greenspon, M. Guzman, and A. K. Jain contributed to acquisition, analysis, or interpretation of the data; G. Villalona, A. Price, K. Blomenkamp, C. Manithody, S. Saxena, T. Ratchford, M. Westrich, V. Kakarla, S. Pochampally, W. Phillips, N. Heafner, N. Korremla, J. Greenspon, M. Guzman, and A. K. Jain drafted the manuscript; A. K. Jain, T. Ratchford, G. Villalona, and M. Guzman critically revised the manuscript; and G. Villalona, A. Price, K. Blomenkamp, C. Manithody, S. Saxena, T. Ratchford, M. Westrich, V. Kakarla, S. Pochampally, W. Phillips, N. Heafner, N. Korremla, J. Greenspon, M. Guzman, and A. K. Jain agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Financial disclosure: AKJ received funding from the National Institutes of Health (grant K08DK098623) as Principal Investigator. Additional funding was provided to AKJ via the Saint Louis University internal grant mechanism.
[This article was corrected on 2018-05-26 after initial online publication to add the funding information.]
References
- 1.Wales PW, Allen N, Worthington P, et al. A.S.P.E.N. Clinical Guidelines: support of pediatric patients with intestinal failure at risk of parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr. 2014;38(5):538–557. doi: 10.1177/0148607114527772. [DOI] [PubMed] [Google Scholar]
- 2.Chowdary KV, Reddy PN. Parenteral nutrition: revisited. Indian J Anaesth. 2010;54(2):95–103. doi: 10.4103/0019-5049.63637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain AK, Wen JX, Arora S, et al. Validating hyperbilirubinemia and gut mucosal atrophy with a novel ultramobile ambulatory total parenteral nutrition piglet model. Nutr Res. 2015;35(2):169–174. doi: 10.1016/j.nutres.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Ekema G, Milianti S, Boroni G. Total parenteral nutrition in patients with short bowel syndrome. Minerva Pediatr. 2009;61(3):283–291. [PubMed] [Google Scholar]
- 5.Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol. 2011;17(4):229–235. doi: 10.4103/1319-3767.82573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly DA. Preventing parenteral nutrition liver disease. Early Hum Dev. 2010;86(11):683–687. doi: 10.1016/j.earlhumdev.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Naini BV, Lassman CR. Total parenteral nutrition therapy and liver injury: a histopathologic study with clinical correlation. Hum Pathol. 2012;43(6):826–833. doi: 10.1016/j.humpath.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132(7):525–532. doi: 10.7326/0003-4819-132-7-200004040-00003. [DOI] [PubMed] [Google Scholar]
- 9.Choi SJ, Lee KJ, Choi JS, et al. Poor prognostic factors in patients with parenteral nutrition-dependent pediatric intestinal failure. Pediatr Gastroenterol Hepatol Nutr. 2016;19(1):44–53. doi: 10.5223/pghn.2016.19.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar JA, Teckman JH. Controversies in the mechanism of total parenteral nutrition induced pathology. Children (Basel) 2015;2(3):358–370. doi: 10.3390/children2030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganousse-Mazeron S, Lacaille F, Colomb-Jung V, et al. Assessment and outcome of children with intestinal failure referred for intestinal transplantation. Clin Nutr. 2015;34(3):428–435. doi: 10.1016/j.clnu.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Nucci A, Burns RC, Armah T, et al. Interdisciplinary management of pediatric intestinal failure: a 10-year review of rehabilitation and transplantation. J Gastrointest Surg. 2008;12(3):429–435. doi: 10.1007/s11605-007-0444-0. discussion 435–436. [DOI] [PubMed] [Google Scholar]
- 13.Gouttebel MC, Saint-Aubert B, Astre C, Joyeux H. Total parenteral nutrition needs in different types of short bowel syndrome. Dig Dis Sci. 1986;31(7):718–723. doi: 10.1007/BF01296449. [DOI] [PubMed] [Google Scholar]
- 14.Platell CF, Coster J, McCauley RD, Hall JC. The management of patients with the short bowel syndrome. World J Gastroenterol. 2002;8(1):13–20. doi: 10.3748/wjg.v8.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int. 2012;11(6):586–593. doi: 10.1016/s1499-3872(12)60229-x. [DOI] [PubMed] [Google Scholar]
- 16.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2012;302(2):G218–G224. doi: 10.1152/ajpgi.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claudel T, Staels B, Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25(10):2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 18.Schaap FG. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2012;15(4):386–391. doi: 10.1097/MCO.0b013e3283547171. [DOI] [PubMed] [Google Scholar]
- 19.Boesjes M, Brufau G. Metabolic effects of bile acids in the gut in health and disease. Curr Med Chem. 2014;21(24):2822–2829. doi: 10.2174/0929867321666140303142053. [DOI] [PubMed] [Google Scholar]
- 20.Stroeve JH, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest. 2010;90(10):1457–1467. doi: 10.1038/labinvest.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avila MA, Moschetta A. The FXR-FGF19 gut-liver axis as a novel “hepatostat”. Gastroenterology. 2015;149(3):537–540. doi: 10.1053/j.gastro.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859–1864. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walters JR, Appleby RN. A variant of FGF19 for treatment of disorders of cholestasis and bile acid metabolism. Ann Transl Med. 2015;3(suppl 1):S7. doi: 10.3978/j.issn.2305-5839.2015.03.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015;33(3):327–331. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Gerspach AC, Steinert RE, Keller S, Malarski A, Schulte FH, Beglinger C. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans. J Clin Endocrinol Metab. 2013;98(8):3351–3358. doi: 10.1210/jc.2012-4109. [DOI] [PubMed] [Google Scholar]
- 26.Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care. 2008;11(5):601–606. doi: 10.1097/MCO.0b013e32830b5b15. [DOI] [PubMed] [Google Scholar]
- 27.Groenen MAM, Archibald AL, Uenishi H, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491:393. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burrin D, Stoll B, Moore D. Digestive physiology of the pig symposium: intestinal bile acid sensing is linked to key endocrine and metabolic signaling pathways. J Anim Sci. 2013;91(5):1991–2000. doi: 10.2527/jas.2013-6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci. 2013;91(10):4713–4729. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim DW, Turner JM, Wales PW. Emerging piglet models of neonatal short bowel syndrome. JPEN J Parenter Enteral Nutr. 2015;39(6):636–643. doi: 10.1177/0148607114554621. [DOI] [PubMed] [Google Scholar]
- 31.Sangild PT, Ney DM, Sigalet DL, Vegge A, Burrin D. Animal models of gastrointestinal and liver diseases. Animal models of infant short bowel syndrome: translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;307(12):G1147–G1168. doi: 10.1152/ajpgi.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 33.Jain AK, Wen JX, Blomenkamp KS, et al. Oleanolic acid improves gut atrophy induced by parenteral nutrition. JPEN J Parenter Enteral Nutr. 2016;40(1):67–72. doi: 10.1177/0148607115583536. [DOI] [PubMed] [Google Scholar]
- 34.Stoll B, Horst DA, Cui L, et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr. 2010;140(12):2193–2200. doi: 10.3945/jn.110.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SJ, Park JB, Kim KH, et al. Immunohistochemical study for the origin of ductular reaction in chronic liver disease. Int J Clin Exp Pathol. 2014;7(7):4076–4085. [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst LM, Spinner NB, Piccoli DA, Mauger J, Russo P. Interlobular bile duct loss in pediatric cholestatic disease is associated with aberrant cytokeratin 7 expression by hepatocytes. Pediatr Dev Pathol. 2007;10(5):383–390. doi: 10.2350/06-09-0171.1. [DOI] [PubMed] [Google Scholar]
- 37.Shinkai M, Shinkai T, Puri P, Stringer MD. Increased CXCR3 expression associated with CD3-positive lymphocytes in the liver and biliary remnant in biliary atresia. J Pediatr Surg. 2006;41(5):950–954. doi: 10.1016/j.jpedsurg.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 38.Fiel MI, Sauter B, Wu HS, et al. Regression of hepatic fibrosis after intestinal transplantation in total parenteral nutrition liver disease. Clin Gastroenterol Hepatol. 2008;6(8):926–933. doi: 10.1016/j.cgh.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Vogel B, Siebert H, Hofmann U, Frantz S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX. 2015;2:124–134. doi: 10.1016/j.mex.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkharfy TM, Ba-Abbad R, Hadi A, Sobaih BH, AlFaleh KM. Total parenteral nutrition-associated cholestasis and risk factors in preterm infants. Saudi J Gastroenterol. 2014;20(5):293–296. doi: 10.4103/1319-3767.141688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology. 2005;146(1):22–32. doi: 10.1210/en.2004-1119. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim ZS. Chenodeoxycholic acid increases the induction of CYP1A1 in HepG2 and H4IIE cells. Exp Ther Med. 2015;10(5):1976–1982. doi: 10.3892/etm.2015.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noh K, Kim YM, Kim YW, Kim SG. Farnesoid X receptor activation by chenodeoxycholic acid induces detoxifying enzymes through AMP-activated protein kinase and extracellular signal-regulated kinase 1/2-mediated phosphorylation of CCAAT/enhancer binding protein beta. Drug Metab Dispos. 2011;39(8):1451–1459. doi: 10.1124/dmd.111.038414. [DOI] [PubMed] [Google Scholar]
- 44.Jain AK, Sharma A, Arora S, et al. Preserved gut microbial diversity accompanies upregulation of TGR5 and hepatobiliary transporters in bile acid-treated animals receiving parenteral nutrition. JPEN J Parenter Enteral Nutr. 2017;41:198–207. doi: 10.1177/0148607116661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tazuke Y, Wildhaber BE, Yang H, Washburn J, Teitelbaum DH. Total parenteral nutrition leads to alteration of hepatocyte cell cycle gene expression and proliferation in the mouse. Dig Dis Sci. 2007;52(4):920–930. doi: 10.1007/s10620-006-9364-1. [DOI] [PubMed] [Google Scholar]
- 46.Chou YH, Yau KI, Hsu HC, Chang MH. Total parenteral nutrition-associated cholestasis in infants: clinical and liver histologic studies. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1993;34(4):264–271. [PubMed] [Google Scholar]
- 47.Niinikoski H, Stoll B, Guan X, et al. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134(6):1467–1474. doi: 10.1093/jn/134.6.1467. [DOI] [PubMed] [Google Scholar]
- 48.Conour JE, Ganessunker D, Tappenden KA, Donovan SM, Gaskins HR. Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets. Am J Physiol Gastrointest Liver Physiol. 2002;283(5):G1185–G1196. doi: 10.1152/ajpgi.00097.2002. [DOI] [PubMed] [Google Scholar]
- 49.Kim YC, Byun S, Zhang Y, et al. Liver ChIP-seq analysis in FGF19-treated mice reveals SHP as a global transcriptional partner of SREBP-2. Genome Biol. 2015;16:268. doi: 10.1186/s13059-015-0835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banerjee M, Robbins D, Chen T. Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discov Today. 2015;20(5):618–628. doi: 10.1016/j.drudis.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front Biosci (Landmark Ed) 2009;14:2829–2844. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Lu H, Lu YF, et al. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci. 2014;141(2):538–546. doi: 10.1093/toxsci/kfu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kast HR, Goodwin B, Tarr PT, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277(4):2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 54.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17(2):259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 55.Tomar BS. Hepatobiliary abnormalities and parenteral nutrition. Indian J Pediatr. 2000;67(9):695–701. doi: 10.1007/BF02762189. [DOI] [PubMed] [Google Scholar]
- 56.Pradelli L, Mayer K, Muscaritoli M, Heller AR. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care. 2012;16(5):R184. doi: 10.1186/cc11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klek S. Omega-3 fatty acids in modern parenteral nutrition: a review of the current evidence. J Clin Med. 2016;5(3):E34. doi: 10.3390/jcm5030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nandivada P, Carlson SJ, Chang MI, Cowan E, Gura KM, Puder M. Treatment of parenteral nutrition-associated liver disease: the role of lipid emulsions. Adv Nutr. 2013;4(6):711–717. doi: 10.3945/an.113.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliveira C, Nasr A, Brindle M, Wales PW. Ethanol locks to prevent catheter-related bloodstream infections in parenteral nutrition: a meta-analysis. Pediatrics. 2012;129(2):318–329. doi: 10.1542/peds.2011-1602. [DOI] [PubMed] [Google Scholar]
- 60.Jin J, Mulesa L, Carrilero Rouillet M. Trace elements in parenteral nutrition: considerations for the prescribing clinician. Nutrients. 2017;9(5):E440. doi: 10.3390/nu9050440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burjonrappa SC, Miller M. Role of trace elements in parenteral nutrition support of the surgical neonate. J Pediatr Surg. 2012;47(4):760–771. doi: 10.1016/j.jpedsurg.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2007;4(5):277–287. doi: 10.1038/ncpgasthep0796. [DOI] [PubMed] [Google Scholar]
- 63.Lauriti G, Zani A, Aufieri R, et al. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr. 2014;38(1):70–85. doi: 10.1177/0148607113496280. [DOI] [PubMed] [Google Scholar]
- 64.Kumpf VJ. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract. 2006;21(3):279–290. doi: 10.1177/0115426506021003279. [DOI] [PubMed] [Google Scholar]
- 65.Fallon EM, Mitchell PD, Nehra D, et al. Neonates with short bowel syndrome: an optimistic future for parenteral nutrition independence. JAMA Surg. 2014;149(7):663–670. doi: 10.1001/jamasurg.2013.4332. [DOI] [PubMed] [Google Scholar]
- 66.Dodge ME, Bertolo RF, Brunton JA. Enteral feeding induces early intestinal adaptation in a parenterally fed neonatal piglet model of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2012;36(2):205–212. doi: 10.1177/0148607111417447. [DOI] [PubMed] [Google Scholar]
- 67.Javid PJ, Collier S, Richardson D, et al. The role of enteral nutrition in the reversal of parenteral nutrition-associated liver dysfunction in infants. J Pediatr Surg. 2005;40(6):1015–1018. doi: 10.1016/j.jpedsurg.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 68.Koelfat KVK, Schaap FG, Hodin C, et al. Parenteral nutrition dys-regulates bile salt homeostasis in a rat model of parenteral nutrition-associated liver disease. Clin Nutr. 2017;36(5):1403–1410. doi: 10.1016/j.clnu.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Lim DW, Wales PW, Mi S, et al. Glucagon-like peptide-2 alters bile acid metabolism in parenteral nutrition–associated liver disease. JPEN J Parenter Enteral Nutr. 2016;40(1):22–35. doi: 10.1177/0148607115595596. [DOI] [PubMed] [Google Scholar]
- 70.Wessel JJ, Kocoshis SA. Nutritional management of infants with short bowel syndrome. Semin Perinatol. 2007;31(2):104–111. doi: 10.1053/j.semperi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49(1):297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu T, Kim YC, Byun S, et al. FXR primes the liver for intestinal FGF15 signaling by transient induction of beta-Klotho. Mol Endocrinol. 2016;30(1):92–103. doi: 10.1210/me.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cariou B, Staels B. FXR: a promising target for the metabolic syndrome? Trends Pharmacol Sci. 2007;28(5):236–243. doi: 10.1016/j.tips.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Jiao Y, Lu Y, Li XY. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol Sin. 2015;36(1):44–50. doi: 10.1038/aps.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drucker DJ. Gut adaptation and the glucagon-like peptides. Gut. 2002;50(3):428–435. doi: 10.1136/gut.50.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suri M, Turner JM, Sigalet DL, et al. Exogenous glucagon-like peptide-2 improves outcomes of intestinal adaptation in a distal-intestinal resection neonatal piglet model of short bowel syndrome. Pediatr Res. 2014;76(4):370–377. doi: 10.1038/pr.2014.97. [DOI] [PubMed] [Google Scholar]
- 77.Burrin DG, Stoll B, Jiang R, et al. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol. 2000;279(6):G1249–G1256. doi: 10.1152/ajpgi.2000.279.6.G1249. [DOI] [PubMed] [Google Scholar]
- 78.Cipriani S, Mencarelli A, Chini MG, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PloS one. 2011;6(10):e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rowland KJ, Choi PM, Warner BW. The role of growth factors in intestinal regeneration and repair in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22(2):101–111. doi: 10.1053/j.sempedsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuemmerle JF. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol Metab Clin North Am. 2012;41(2):409–423. vii. doi: 10.1016/j.ecl.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krishnan K, Arnone B, Buchman A. Intestinal growth factors: potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm Bowel Dis. 2011;17(1):410–422. doi: 10.1002/ibd.21316. [DOI] [PubMed] [Google Scholar]